Abstract
In patients affected by Parkinson’s disease (PD), the most common neurodegenerative movement disorder, the brain is characterized by the loss of dopaminergic neurons in the nigrostriatal system, leading to dyshomeostasis of the basal ganglia network activity that is linked to motility dysfunction. PD mostly arises as an age-associated sporadic disease, but several genetic forms also exist. Compelling evidence supports that synaptic damage and dysfunction characterize the very early phases of either sporadic or genetic forms of PD and that this early PD synaptopathy drives retrograde terminal-to-cell body degeneration, culminating in neuronal loss. The Ras-associated binding protein (Rab) family of small GTPases, which is involved in the maintenance of neuronal vesicular trafficking, synaptic architecture and function in the central nervous system, has recently emerged among the major players in PD synaptopathy. In this manuscript, we provide an overview of the main findings supporting the involvement of Rabs in either sporadic or genetic PD pathophysiology, and we highlight how Rab alterations participate in the onset of early synaptic damage and dysfunction.
Keywords: Rab proteins, Parkinson’s disease, synaptopathy, autophagy, alpha-synuclein, GBA1, LRRK2
1. Introduction
Parkinson’s disease (PD) is the most common neurodegenerative disorder with motor symptoms. It mostly arises as an age-associated sporadic disease, but several genetic forms also exist [1,2]. The brain of affected patients is characterized by the prominent loss of nigrostriatal dopaminergic neurons [3] and the presence of Lewy bodies (LB) and Lewy neurites (LN). These are insoluble proteinaceous deposits that form in neuronal cell bodies and processes, respectively, and are mainly composed of alpha-synuclein (aSyn) [4,5], a protein involved in the regulation of synaptic functions [6,7,8]. Indeed, small synaptic alpha-synuclein aggregates have also been reported in synaptic terminals in patients with synucleinopathies [9,10]. The loss of nigrostriatal dopaminergic neurons is the main culprit in the onset of motor symptoms in PD patients. Interestingly, human brain imaging studies highlighted that in both sporadic and genetic cases of PD, the loss of nigrostriatal neurons proceeds retrogradely, first involving putaminal synaptic terminals and projections and later affecting nigral cell bodies [11,12,13]. It has been estimated that, given the massive arborization of striatal axonal projections, each nigral dopaminergic neuron gives rise to about 2 million synapses in the putamen [14]. Therefore, the retrograde degeneration pattern of this neuronal population can account for the fact that the large number of connections established by nigrostriatal neurons guarantees a very high threshold of resilience to terminal loss. Along this line, a large body of evidence supports that motor disturbances appear when 80% of putaminal dopaminergic terminals are lost [12]. It is thus clear that synaptopathy plays a key role in triggering nigrostriatal dopaminergic neuronal loss in PD.
Interestingly, the analysis of post-mortem brains of patients affected by PD highlighted that the amount of aSyn deposited within synaptic microaggregates is several orders of magnitude higher than the actual amount of the protein within LB and LN [10]. This is in agreement with the fact that aSyn levels are enriched at synaptic terminals even in physiological conditions [4,6,7,12]. Abnormal accumulation of aSyn at synaptic terminals may thus promote pathological aSyn aggregates, initiating synaptic damage. Consistently, age-related dysfunctions in the homeostatic control of aSyn levels at synapses, such as alterations in the ubiquitin–proteasome system (UPS) and the autophagy–lysosome pathway (ALP), may prompt aSyn accumulation at synaptic terminals and consequently its pathological aggregation [15,16,17]. Moreover, multiplications of the aSyn gene (SNCA) cause the onset of a rare form of early-onset PD with rapid progression [18]. These findings support that the progressive accumulation of aSyn microaggregates at synaptic terminals represents a main toxic event contributing to neurodegeneration and disease progression in PD, while LB and LN may be a later evolutionary state of the microaggregates, deriving from the progression of the pathological deposition of the protein and the consequent impairment of proper aSyn trafficking at synapses. The main contribution of synaptic aSyn microaggregates to neuronal damage is also in line with the trans-synaptic spreading hypothesis of disease-relevant aSyn strains, which are believed to propagate pathology and disease symptoms in PD and other synucleinopathies [19]. Indeed, even though the mechanisms involved in the trans-synaptic conveyance of aSyn strains remain to be elucidated, it is feasible that the released toxic polymorphs could propagate neuronal damage either by affecting the integrity of outer synaptic membranes or by promoting aSyn aggregation in recipient neurons.
This notwithstanding, a plethora of genetic forms of PD linked to gene loci that are completely unrelated to aSyn also exist [20]. In some cases, these forms do not present LB or LN, and the incurring disease is defined as parkinsonism in that, neuropathologically, PD is defined by the presence of LB. Some of the genes mutated in PD encode for proteins involved in synaptic functions, such as: Ras-associated binding protein 39b (Rab39b), synaptojanin 1 (SYNJ1), Leucine-rich repeat kinase 2 (LRRK2), synphilin-1 (SYPH1) and transmembrane protein 230 (TMEM230) [21,22,23,24,25,26,27,28,29,30,31,32,33]. On the other hand, several PD-associated genes, including those encoding for some of the aforementioned proteins (RAB39B, SYPH1, SYNJ1 and TMEM230, in addition to GBA1, Parkin, PINK-1, ATP13A2, FBXO7 and SYT11), have been linked to dysregulations of ALP [31,32,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52].
This scenario suggests that intracellular trafficking problems characterize PD, and they can be linked to synaptic derangement or ALP dysfunction [53]. Functionally intact anterograde and retrograde intracellular trafficking is crucial for ensuring and sustaining synaptic activity, and this is predicted to be especially relevant in nigrostriatal dopaminergic neurons, which are long-projecting and bear extensive arborization. Interestingly, alterations in several members of the Rab family of small GTPases (Rabs), key regulators of intracellular trafficking [54,55], have been reported in PD brains or in experimental models of the disease [53]. For instance, Rab39b, whose gene mutation is associated with familial PD, controls anterograde trafficking in the cell body [29]. Several other Rabs, such as Rab1, Rab3, Rab5, Rab7Aa, Rab8a, Rab8b, Rab10, Rab11a, Rab13, Rab29, Rab32 and Rab35, can affect aSyn aggregation, propagation and clearance, co-localize with aSyn, LRRK2, PTEN-induced kinase 1 (PINK1), Parkin and TMEM230 or participate in their function [56,57]. Here, we present a perspective on how Rab alterations can contribute to the onset of synaptopathy in either sporadic or genetic PD.
2. Rabs and Synaptic Function
Rabs are essential regulators of membrane trafficking and orchestrate cell physiology by spatially and temporally controlling vesicle sorting, fission, tethering, docking and fusion through interactions with multiple effector proteins [58,59,60,61,62]. In particular, they play an essential role in defining the identity of subcellular membranes, thus governing membrane trafficking [61,63]. The key role of Rabs in cell physiology is also supported by the fact that more than 60 Rabs have been described in eukaryotes.
Rab proteins are activated upon GTP binding, while they are inactive when they associate with GDP. GTPase-activating proteins (GAP) and guanine nucleotide exchange factor (GEF) control the exchange of GDP with GTP and GTP hydrolysis, thus regulating Rab activation and inactivation. Moreover, small GTPases carrying a C-terminal farnesyl or geranylgeranyl group, guanine dissociation inhibitors (GDIs), can form soluble complexes with small GTPases to combine the cytosol/membrane and GDP/GTP alternation [64]. Rab-GDI dissociation is then mediated by the GDI displacement factor (GDF) [65]. Importantly, GTP binding activates the Rab protein by leading to a major conformational modification in the so-called switch I and switch II regions, which mediate the binding to effector proteins [66].
Numerous studies have described the involvement of Rabs in the regulation of neurotransmitter release, ranging from the generation of synaptic vesicles (SVs), the control of their size and trafficking toward the synaptic cleft, and the mobilization, docking and recycling of SV pools at terminals to the retrograde transport of SVs toward the endosomal/lysosomal system. These processes can be regulated by a specific subset of Rabs, which include Rab3, Rab5, Rab11, Rab22, Rab26, Rab27b, Rab33 and Rab35 [67,68]. In particular, the control of SV release appears to be specifically regulated by Rab3a and Rab27b [69,70], both of which localize to SVs, bear elevated structural and functional redundancy and share common sets of regulators [71,72,73,74]. In agreement, Rab3-GTP can bind to SV membranes and dissociates from them upon GTP hydrolysis during neurotransmitter release [69]. Furthermore, Rab27b and Rab3a synergistically promote excitatory transmission in mammals [75].
Interestingly, rabphilin and Rab3-interacting molecule (RIM) represent the main Rab effectors in the control of SV exocytosis [58,71], though only the latter appears to play a relevant role in the regulation of neurotransmitter release [76,77], which is not significantly perturbed by rabphilin deficiency [78]. Indeed, RIM localizes to the active zone, where it forms a multimolecular complex with Rab3 and Munc13 [79,80]. While the RIM-Munc13 complex controls SV docking and priming and Ca2+-evoked release, RIM can also regulate both SV localization and release probability by a Munc13-independent-mechanism [81].
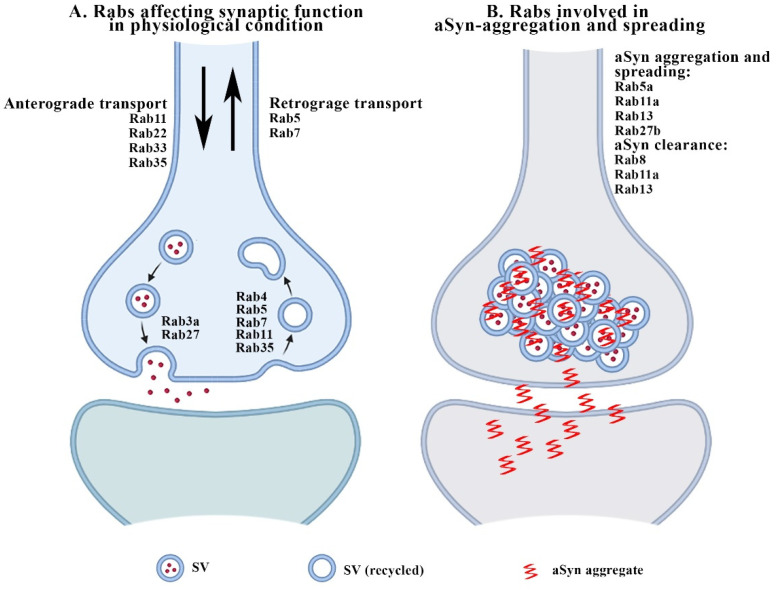
Rab4, Rab5, Rab7, Rab11 and Rab35 control SV recycling [55]. In particular, Rab5 plays a primary role, as it controls the size and retrieval of SVs [82,83] and associates with the synaptic endosomal compartment [84,85]. While Rab11, Rab22, Rab33 and Rab35 are involved in the anterograde axonal transport of receptors, Rab5 and Rab7 play a major role in the control of retrograde axonal trafficking [68]. Rabphilin can interact with the Glutamate N2A receptor subunit (GluN2A)/postsynaptic density protein 95 (PSD-95) complex to retain N-methyl-D-aspartate (NMDA) receptors at synaptic sites, thus impinging on long-term potentiation (LTP)-dependent spine remodeling [86,87]. Rab 11 is involved in dendritic spine formation [88]. Finally, Rab26 and Rab33 have been proposed to be pivotal in SV degradation and turnover, as they are involved in the formation of the autophagosome [89,90] or in anterograde vesicular transport [91]. For a detailed summary of the role of Rabs in the modulation of synaptic function, please see Figure 1A.
Figure 1.
The cartoon summarizes the Rabs involved in the modulation of synaptic function in physiological conditions (A) and in aSyn aggregation and spreading (B).
3. Major Rab Alterations in PD and Other Forms of Parkinsonism: A Focus on aSyn-, GBA1- and LRRK2-Associated Synaptopathy
3.1. Rabs and aSyn-Related Synaptopathy
In 2014, Wilson and co-authors described that the c.503C > A missense mutation in RAB39B causes X-linked intellectual disability and early-onset PD with aSyn pathology as well as extensive dopaminergic neuronal loss in the substantia nigra [92]. By using both in silico modeling and site-directed mutagenesis, the authors found that this mutation destabilizes Rab39b, supporting its loss of function. Interestingly, they also described that the short-hairpin (sh)-RNA-mediated knockdown of RAB39B reduced the density of aSyn levels and immunoreactive puncta in the dendritic processes of cultured neurons. Moreover, the study reported the presence of axonal spheroids, structures reminiscent of the Wallerian-like degeneration observed in neurodegenerative diseases with impaired axonal transport [93], in the white matter tracts of the basal ganglia of the post-mortem brain of a patient bearing the c.503C > A RAB39B mutation [92]. This is in agreement with the involvement of Rab39b in endocytic retrograde and/or early-stage anterograde secretory transport [32].
Subsequently, other RAB39B mutations were reported to be associated with the onset of parkinsonism [94,95,96,97,98,99]. These can result in the total loss of Rab39B expression or in reduced Rab39b protein stability or altered function. Moreover, marked accumulation of Rab39b in Amyloid β (Aβ) plaques and a subset of LB in parallel with a marked reduction in Rab39b in the white matter regions were reported in the brains of patients affected by dementia with LB (DLB) [31].
Abnormal interaction of aSyn with rabphilin and the loss of rabphilin/Rab3a coupling have also been detected in PD brains [100]. Since aSyn, Rab3a and rabphilin play a relevant role in the regulation of SV release, it can be inferred that such alterations may contribute to impairing neurotransmitter exocytosis in LB diseases. Furthermore, the serum levels of Rab35 positively correlate with those of aSyn in PD patients, and the combined assessment of Rab35 and aSyn is a better predictive biomarker for discriminating PD patients from those affected by atypical parkinsonism or from healthy subjects [101], emphasizing the critical interplay between Rabs and aSyn. Rabs, particularly Rab5 with its effector Rabaptin-5, have been found in glial cytoplasmic inclusions (GCI), characteristic aggregates in the brains of multiple system atrophy (MSA) patients, also composed of aSyn [102,103].
The interaction between Rabs and aSyn is also supported by results from a plethora of studies on experimental models, where aSyn accumulation has been found to negatively impact Rab-mediated ER–Golgi homeostasis and SV fusion by affecting Rab1 and Rab3, respectively [104,105,106]. Moreover, overexpression of human A30P mutant aSyn in mice results in abnormal aSyn–Rab interaction [107].
On the other hand, Rabs were found to control the aggregation, toxicity and secretion of aSyn. Indeed, a recent microscopy-based large-scale RNA interference study showed that Rab8b, Rab11a, Rab13 and Rab39b modulate aSyn aggregation, toxic potential and levels [108]. In particular, Rab8b, Rab11a and Rab13 were found to promote the clearance of aSyn inclusions and rescue aSyn-induced toxicity. Rab11a and Rab13 expression also improved aSyn secretion and endocytic recycling in cells accumulating aSyn inclusions [108], while Rab3a recycling machinery and synaptic activity appear to control wild-type aSyn membrane association [109], and Rab5a-dependent aSyn endocytosis can induce neuronal death [110]. Finally, Rab27b has been reported to be relevant for aSyn aggregation, toxicity and spreading [111]. The Rabs possibly involved in aSyn aggregation and spreading at synaptic sites are listed in Figure 1B. A detailed summary of the alterations of Rabs related to aSyn synaptopathy is reported in Table 1.
Table 1.
Alterations of Rabs in aSyn-related synaptopathy.
| Rab Alterations and aSyn-Related Synaptopathy | ||
| Rab | Alteration Type | References |
| Mutations Identified in Rabs | ||
| RAB39B | c.503C > A missense mutation | [92] |
| RAB39B | c.557G > A missense mutation | [94] |
| RAB39B | c.574G > A missense mutation | [95] |
| RAB39B | c.432delA single base pair deletion | [96] |
| RAB39B | c. 536dupA duplication | [97] |
| RAB39B | c.543A > G; c.215 + 61G > ; c.215 + 39C > G | [98] |
| RAB39B | c.137dupT; c.371delA | [99] |
| Activity or pathological changes | ||
| Rab3a | Loss of coupling with rabphilin | [100] |
| Rab35 | Increased levels in PD patient serum | [101] |
| Rab5 | Accumulation in GCI | [102,103] |
| Rab1 | Increased levels protect against aSyn-mediated neuron loss | [104] |
| Rab3a | Protects against aSyn-mediated neuron loss | [105] |
| Rab8a | Protects against aSyn-mediated neuron loss | [105] |
| Rab3a | Aberrant interaction with A30P aSyn | [107] |
| Rab5 | Aberrant interaction with A30P aSyn | [107] |
| Rab8 | Aberrant interaction with A30P aSyn | [107] |
| Rab8b | Promotes aSyn aggregate clearance | [108] |
| Rab11a | Promotes aSyn aggregate clearance/aSyn secretion | [108] |
| Rab13 | Promotes aSyn aggregate clearance/aSyn secretion | [108] |
| Rab3a | Regulates aSyn binding to presynaptic membranes | [109] |
| Rab5a | Mediates aSyn endocytosis (spreading?) | [110] |
| Rab27b | Reduces aSyn spreading via nonexosomal pathways | [111] |
3.2. Critical Involvement of Rabs in LRRK2-Associated Parkinsonism
Numerous mutations in the LRRK2 gene have been associated with late-onset autosomal-dominant PD [112]. Among them, the G2019S variant residing in the kinase domain is among the most common, together with R1441G, located in the ROC domain, and G2385R in the WD40 domain [113,114,115,116]. Rab1, Rab3, Rab5, Rab8, Rab10, Rab12, Rab29, Rab35 and Rab43 are among the most important LRRK2 substrates and mediate multiple LRRK2 functions [117]. Moreover, Rab29 recruits LRRK2 to the trans-Golgi to activate its kinase activity [118,119]. This in turn leads to Rab8, Rab10 and Rab12 phosphorylation [120,121]. However, since the basal phosphorylation of Rab10 and Rab12 by LRRK2 is not affected by the knockout or overexpression of Rab29 [122], it is unlikely that the encoded protein is among the mediators of basal or pathogenic LRRK2 phosphorylation activity.
Rab10 phosphorylation is differentially affected by the R1441C- and G2019S-LRRK2 mutations, with the former inducing an increase and the latter causing a decrease in the process [123]. The overexpression of pathological LRRK2 in LRRK2-mutant cells and fibroblasts from G2019S-LRRK2 PD patients decreases Rab7 activity and results in delayed endosomal trafficking and impaired epidermal growth factor receptor (EGFR) degradation, which can be reversed upon LRRK2 inhibition and overexpression of Rab7 [124]. However, Rab7 is not an LRRK2 phosphorylation substrate [117,125], and G2019S-LRRK2 patients do not exhibit alterations in Rab7 levels [124].
Moreover, concerning the Rabs involved in endolysosomal trafficking, it has been described that the loss of Rab8 mimics deficits in endolysosomal function and impairs EGFR degradation, similarly to Rab7 activity reduction. The loss of Rab8 also reduces Rab7 activity, suggesting a relationship between the two and indicating that Rab7 activity is located downstream from Rab8. Notably, in contrast to what has been observed for Rab7, the expression of active Rab8 or the up-regulation of the Rab11-rabin8 cascade rescues deficits in endolysosomal membrane trafficking mediated by G2019S-LRRK2 [126]. Rab35 is involved in endocytic recycling and is another substrate of LRRK2 [127]. It has been described that increased LRRK2 kinase activity enhances aSyn propagation through LRRK2-mediated Rab35 phosphorylation [128]. Consistently, the functional inhibition of LRRK2 kinase activity reduced both Rab35 and aSyn levels [128]. Whether the reduction in Rab35 levels results from an increase in its degradation or a reduction in its synthesis due to LRRK2 inhibition is not yet clear. Rab32 can directly interact with LRRK2, affecting retromer trafficking in the trans-Golgi [129,130]. LRRK2, Rab5 and Rab11 have been found to be involved in SV recycling in the Drosophila model [131].
Given the essential role exerted by LRRK2 in the regulation of synaptic function [21,132] and its interplay with Rabs, it is easily foreseeable that these proteins may together contribute to pathogenic LRRK2-associated synaptopathy. Indeed, LRRK2 kinase activity regulates SV trafficking, neurotransmitter release and synaptic morphology [26,133], and LRRK2 kinase inhibition also promotes anterograde axonal transport and presynaptic targeting of aSyn [134]. G2019S- or R1441C-LRRK2 mutants assist the activity of glutamatergic synapses thus inducing excitotoxic dendritic degeneration [135]. The G2019S-LRRK2 mutation induces dopaminergic nigral neuron dysfunction, affects corticostriatal long-term depression and also promotes tau spreading in the mouse brain [136,137]. Furthermore, though different LRRK2 kinase inhibitors do not perturb basal dopamine release or firing in nigrostriatal neurons in wild-type mice, GNE-7915, an LRRK2 inhibitor, enhanced dopamine release and SV mobilization and recycling in BAC LRRK2 hG2019S and hR1441G transgenic mice [138]. It can thus be speculated that since Rab5 and Rab11 are LRRK2 kinase substrates regulating SV recycling [131], they may be directly implicated in this effect and thus in pathogenic LRRK2-associated synaptopathy. It has been demonstrated that PD-associated LRRK2 mutants influence the degree of phosphorylation of different Rab proteins [139]. Among them, levels of Rab10 phosphorylation at threonine 73 (pT73) were found to be increased in peripheral blood mononuclear cells derived from heterozygous LRRK2 G2019S variant carriers [140] and in neutrophils and monocytes from patients carrying different LRRK2 mutations [141]. Moreover, Rab10 pT73 levels in urine exhibited a positive association with PD disease progression [142], suggesting that this could represent a valid target engagement biomarker for LRRK2 inhibitors in the clinic [143]. A decrease in Rab29 phosphorylation was also reported in urinary exosomes from patients with idiopathic and aSyn mutation-related PD [140]. Nevertheless, the indirect involvement of all LRRK2 kinase Rab substrates may also be predicted. Table 2 provides a summary of the involvement of Rabs in LRRK2 trafficking as well as in LRRK2-associated parkinsonism.
Table 2.
Alterations in Rabs in LRRK2-associated parkinsonism and the effect of Rabs on LRRK2 trafficking.
| Rab Alterations in LRRK2-Associated Parkinsonism | ||
| Rab | Alteration Type | References |
| Phosphorylation/activity changes | ||
| Rab8a | Aberrant phosphorylation by G2019S-LRRK2 | [119] |
| Rab7L1 | Aberrant phosphorylation by R1441C, Y1699C and G2019S-LRRK2 | [120] |
| Rab10 | Phosphorylation increase (R1441C)/decrease (G2019S) | [123] |
| Rab7 | Decrease in protein activity induced by expression of PD-associated LRRK2 mutants | [124] |
| Rab8a | Impaired function mediated by G2019S-LRRK2 | [126] |
| Rab35 | Increased phosphorylation mediated by G2019S-LRRK2 | [128] |
|
Rab impact on physiological and pathological
LRRK2 trafficking | ||
| Trafficking changes | ||
| Rab29 | Abnormal recruitment of R1441G/C and Y1699C-LRRK2 to the Golgi without affecting LRRK2 phosphorylation activity | [121,122] |
| Rab32 | Regulates LRRK2 late endosomal transport | [130] |
3.3. Involvement of Rabs in Other Genetic Forms of Parkinsonism
It has been described that several of the protein products of genetic parkinsonism-linked genes interact with Rabs. SYNJ1 regulates the endosomal trafficking of synaptic proteins by affecting Rab7 [144]. TMEM230, which plays a role in vesicle formation and trafficking, co-localizes with SVs and is also found in vesicular structures located in the perinuclear region, and it interacts with Rab5a, Rab7 and Rab11a [145]. TMEM230 is also required for Rab8a-mediated secretory vesicle and retromer trafficking [34].
Heterozygous mutations in the GBA1 gene, leading to glucocerebrosidase (GCase) dysfunction, are the most common genetic risk factor for PD [146,147]. Of note, reduced GCase activity can decrease lysosomal-mediated aSyn degradation, while aSyn inhibits GCase lysosomal activity, supporting that GCase deficiency and aSyn may set up a positive feedback loop propagating PD [148,149]. In agreement, reduced GCase activity can also occur in the brains of PD patients without GBA1 mutations and is associated with increased levels of phosphorylated aSyn, which is considered a marker of advanced LB pathology [149,150]. A reduction in GCase activity has also been shown to promote aSyn accumulation in different in vivo and in vitro models of PD [151,152]. For example, midbrain organoids derived from genetically engineered induced pluripotent stem cells with GBA1 deletion and SNCA overexpression develop LB-like inclusions [153]. However, it is also possible that a reduction in GCase activity may increase neuronal susceptibility to pre-existing aSyn aggregation [154]. Indeed, most of the subjects bearing either heterozygous or homozygous GBA1 mutations do not develop PD or aSyn aggregate deposition, supporting that other factors participate in linking reduced GCase activity to aSyn pathology [155,156]. For instance, GCase reduction can also impact the lipid compositions of late endosomal membranes and may thus consequently impair aSyn degradation through altered endosomal microautophagy or trigger aSyn oligomerization [157,158]. Indeed, the degradation of aSyn is reduced in lysosomes with reduced GCase enzymatic activity [159]. Furthermore, glycosphingolipid-induced aSyn accumulation can trigger cellular degeneration in human iPSC-derived midbrain neurons from patients with or without GBA1 mutations. On the contrary, glycosphingolipid-reducing agents improve synaptic localization of aSyn, reducing toxic aSyn assemblies in neuronal cultures [160]. It is also worth considering that different GBA1 mutations cause autophagy dysfunction [161,162], and Rabs are involved in various stages of autophagy (please see below for further details) [89,163]. In addition, Rab8 has also been suggested to promote the storage of lipids and lipid droplets [164]. It is thus plausible that Rab alterations are associated with GCase deficiency-related aSyn pathology and synaptopathy by modulating autophagy and/or lipid composition, two mechanisms implicated in GBA1 mutation-related PD synaptopathy [165]. In agreement, it has been reported that neuroinflammation and aSyn accumulation derived from GCase deficiency in mice are accompanied by synaptic dysfunction [166], which is also believed to be the starting point for disease progression in lysosomal storage disorders [167].
4. Interplay between Rabs and Autophagic Defects in PD Synaptopathy
Autophagy is a process leading to the self-degradation of unwanted or toxic macromolecules and organelles that are sequestered and delivered to the lysosome to generate raw materials (proteins, lipids, carbohydrates and nucleic acids) to be used in metabolic processes. Macroautophagy, chaperone-mediated autophagy (CMA) and microautophagy are the three main types of autophagy. CMA is a selective autophagic pathway that degrades cytosolic proteins with a particular pentapeptide motif upon their recognition by the chaperone Hsc70 and their subsequent entrance into lysosomes through the transmembrane protein lysosomal-associated membrane protein 2A (Lamp2A). Macroautophagy requires the formation of autophagosomes, double-membrane structures engulfing cytosolic components and degrading them through the generation of autophagolysosomes, which result from the fusion of autophagosomes and lysosomes. Finally, microautophagy degrades cytosolic components simply by invaginations in the lysosomal membrane. In post-mitotic neurons, autophagy is important for maintaining normal cellular homeostasis, particularly the critical turnover of misfolded proteins and damaged organelles [15,16].
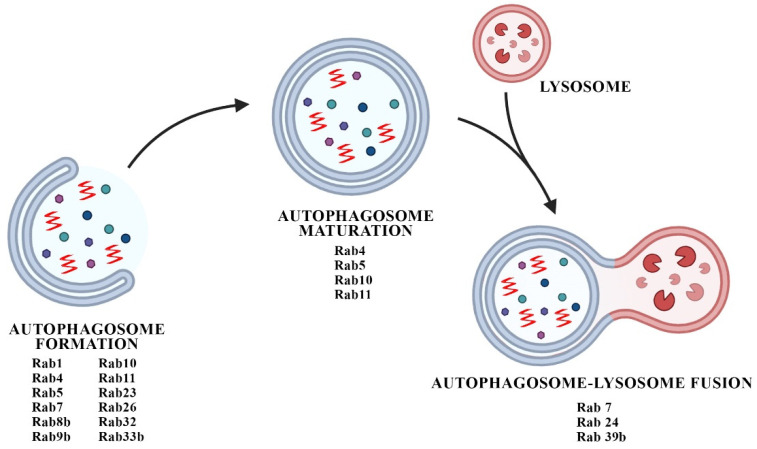
Rabs control different steps of autophagic processes but are mainly involved in the control of macroautophagy (Figure 2). Rab1, Rab5, Rab7, Rab9a, Rab11, Rab23, Rab32 and Rab33b participate in autophagosome formation, Rab9 is involved in non-canonical autophagy, and Rab7, Rab8b and Rab24 control autophagosome maturation [163]. Of note, the presence of large Rab7a-positive endosomes and an increase in Rab7a protein levels were detected in the post-mortem brains of patients affected by DLB [168]. Rab7 was also found to decrease aSyn pathology, probably by regulating autophagosome transport and fusion with lysosomes [169].
Figure 2.
The cartoon summarizes the Rabs participating in the different phases of macroautophagy, from autophagosome formation to its fusion with the lysosome.
PARK2/Parkin mutations also affect the activity of Rabs by activating the effector proteins TBC1D15 and TBC1D17, which are required for routing damaged mitochondria to autophagosomes through Rab7 [170].
PINK1 was found to phosphorylate Ser111 of Rab8a, Rab8b and Rab13 in a Parkin-independent manner, thus impairing the interaction with GEF and consequently hampering Rab activation [171]. Parkin and PINK1 have proven involvement in the induction of mitophagy, the selective autophagic-based degradation of mitochondria [172,173], but recent evidence has also highlighted that PINK1, SYPH1 and seven in absentia homolog 1 (SIAH-1) complex constitutes a novel Parkin-independent mitophagy pathway [37], in which the possible involvement of Rabs can be postulated.
ATP13A2 encodes for a late endosomal/lysosomal ATPase, which modulates autophagy by regulating another PD-associated gene, synaptotagmin 11 (SYT11) [49]. The latter mediates a vesicle trafficking pathway that is essential for development and synaptic plasticity [174] and is also a mediator of Parkin-associated neurotoxicity [175]. Therefore, its interplay with Rabs and autophagy can be expected. GBA1 and LRRK2 mutations can affect Rabs and autophagy, as explained above, and RAB39B mutations can also impact autophagy [30,31].
Although the link between some PD pathological proteins and genes, Rabs, autophagy and synaptopathy is not fully clarified, autophagy is implicated in the control of synaptic function, and its alterations are expected to significantly impact synaptic homeostasis [176,177]. Synaptic proteins and SVs, postsynaptic receptors and synaptic mitochondria are known to be degraded by autophagy, thereby contributing to the remodeling of synapses [177]. Autophagy also regulates synaptic development [178], and autophagy modulation appears to be required for neurotransmission, different forms of synaptic plasticity and memory formation [176]. Not by chance, autophagosome formation is prominent at synaptic terminals, and neuronal autophagy is regulated in a compartment-specific fashion [176,179]. In turn, synaptic activity has been found to control dendritic autophagic vacuole motility and function [180]. There is an interdependency between autophagy and SV trafficking in the regulation of dopamine release, and this could involve Rab3 and Rab27 [181]. Finally, Rab26 links SVs to autophagic pathways [90].
Therefore, autophagy and synaptic dysfunction may promote a self-propagating disease pathway that compromises neuronal resilience, where Rabs can serve as critical mediators. Table 3 summarizes the involvement of Rabs in autophagy or autophagy-related synaptic alterations.
Table 3.
Involvement of Rabs in autophagy and autophagy-related synaptic alterations.
| Rabs in Autophagy and Autophagy-Related Synaptic Alterations | ||
|---|---|---|
| Rab | ROLE | Reference |
| Rab39b | Autophagy activation and fusion of autophagosomes with lysosomes | [30] |
| Rab26 | Directs SVs into pre-autophagosomal structures | [90] |
| Rab1 | Autophagosome formation | [163] |
| Rab5 | Autophagosome formation | [163] |
| Rab7 | Autophagosome formation | [163] |
| Rab7 | Fusion of autophagosomes with lysosomes | [163] |
| Rab8b | Autophagosome formation (non-canonical autophagy) | [163] |
| Rab8b | Autophagy-based unconventional secretory pathway | [163] |
| Rab9a | Autophagosome formation | [163] |
| Rab11 | Amphisome formation | [163] |
| Rab23 | Autophagosome formation | [163] |
| Rab24 | Fusion of autophagosomes with lysosomes | [163] |
| Rab32 | Autophagosome formation | [163] |
| Rab33b | Autophagosome formation | [163] |
| Rab7 | Involved in autophagosome formation during mitophagy | [170] |
| Rab8a | Downstream target of PINK1 | [171] |
| Rab8b | Downstream target of PINK1 | [166] |
| Rab13 | Downstream target of PINK1 | [171] |
| Rab5 | Directs SVs to autophagy | [181] |
| Rab35 | Directs SVs to autophagy | [181] |
| Rab4 | Autophagosome formation and maturation | [181] |
| Rab5 | Autophagosome formation and maturation | [181] |
| Rab10 | Autophagosome formation and maturation | [181] |
| Rab11 | Autophagosome formation and maturation | [181] |
5. Conclusions
Altogether, these findings support that Rabs play a crucial role as direct or indirect (through autophagic involvement) mediators of synaptopathy in PD. Even though the involvement of Rabs in the induction of synaptic alterations concerning aSyn and LRRK2 is quite delineated, more studies are needed to decipher the interplay between many other PD genes, Rab alterations and synaptic pathologies. In particular, we envisage that more detailed investigations on experimental models of GBA1-, SYNJ1-, SYPH1-, TMEM230-, Parkin-, PINK1-, ATP13A2-, FBXO7- and SYT11-associated parkinsonism could provide new insight into the pathophysiological mechanisms leading to PD that may involve Rab dysfunction. Developing novel therapeutic strategies targeting Rabs could help to restore autophagic dysfunction and synaptopathy, thus counteracting the key mechanisms of neurodegeneration in PD.
Author Contributions
Conceptualization, A.B. and M.G.S.; writing—original draft preparation, A.B., F.L. and M.G.S.; writing—review and editing, A.B., F.L. and M.G.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
A.B. is grateful to the Michael J Fox Foundation for Parkinson’s Research, NY, USA, Fall 2021 RFP: Accelerating Early Proof-of-Concept Testing of Promising PD Therapies, Grant ID: MJFF-021179, to the Italian MIUR PRIN 2017-1065 and to Lions Club Porcia (PN), Italy. M.G.S. is grateful to the Michael J Fox Foundation for Parkinson’s Research, NY, USA and to Parkinson’s UK.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bologna M., Truong D., Jankovic J. The etiopathogenetic and pathophysiological spectrum of parkinsonism. J. Neurol. Sci. 2022;433:120012. doi: 10.1016/j.jns.2021.120012. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic J., Tan E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry. 2020;91:795–808. doi: 10.1136/jnnp-2019-322338. [DOI] [PubMed] [Google Scholar]
- 3.Hornykiewicz O. 50 years of levodopa. Mov. Disord. 2015;30:1008. doi: 10.1002/mds.26240. [DOI] [PubMed] [Google Scholar]
- 4.Spillantini M.G., Goedert M. Neurodegeneration and the ordered assembly of alpha-synuclein. Cell Tissue Res. 2018;373:137–148. doi: 10.1007/s00441-017-2706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spillantini M.G., Schmidt M.L., Lee V.M.-Y., Trojanowski J.Q., Jakes R., Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 6.Burré J., Sharma M., Südhof T.C. Definition of a Molecular Pathway Mediating -Synuclein Neurotoxicity. J. Neurosci. 2015;35:5221–5232. doi: 10.1523/JNEUROSCI.4650-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burré J., Sharma M., Südhof T.C. Cell Biology and Pathophysiology of α-Synuclein. Cold Spring Harb. Perspect. Med. 2017;8:a024091. doi: 10.1101/cshperspect.a024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longhena F., Faustini G., Spillantini M.G., Bellucci A. Living in Promiscuity: The Multiple Partners of Alpha-Synuclein at the Synapse in Physiology and Pathology. Int. J. Mol. Sci. 2019;20:141. doi: 10.3390/ijms20010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reitboeck P.G., Anichtchik O., Bellucci A., Iovino M., Ballini C., Fineberg E., Ghetti B., Della Corte L., Spano P., Tofaris G.K., et al. SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain. 2010;133:2032–2044. doi: 10.1093/brain/awq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulz-Schaeffer W.J. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010;120:131–143. doi: 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellucci A., Antonini A., Pizzi M., Spano P. The End Is the Beginning: Parkinson’s Disease in the Light of Brain Imaging. Front. Aging Neurosci. 2017;9:330. doi: 10.3389/fnagi.2017.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellucci A., Mercuri N.B., Venneri A., Faustini G., Longhena F., Pizzi M., Missale C., Spano P., Bellucci A., Mercuri N.B., et al. Review: Parkinson’s disease: From synaptic loss to connectome dysfunction. Neuropathol. Appl. Neurobiol. 2016;42:77–94. doi: 10.1111/nan.12297. [DOI] [PubMed] [Google Scholar]
- 13.Andica C., Kamagata K., Hatano T., Okuzumi A., Saito A., Nakazawa M., Ueda R., Motoi Y., Kamiya K., Suzuki M., et al. Neurite orientation dispersion and density imaging of the nigrostriatal pathway in Parkinson’s disease: Retrograde degeneration observed by tract-profile analysis. Park. Relat. Disord. 2018;51:55–60. doi: 10.1016/j.parkreldis.2018.02.046. [DOI] [PubMed] [Google Scholar]
- 14.Pissadaki E.K., Bolam J.P. The energy cost of action potential propagation in dopamine neurons: Clues to susceptibility in Parkinson’s disease. Front. Comput. Neurosci. 2013;7:13. doi: 10.3389/fncom.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelender S., Stefanis L., Oddo S., Bellucci A. Can We Treat Neurodegenerative Proteinopathies by Enhancing Protein Degradation? Mov. Disord. 2022;37:1346–1359. doi: 10.1002/mds.29058. [DOI] [PubMed] [Google Scholar]
- 16.Stefanis L., Emmanouilidou E., Pantazopoulou M., Kirik D., Vekrellis K., Tofaris G.K. How is alpha-synuclein cleared from the cell? J. Neurochem. 2019;150:577–590. doi: 10.1111/jnc.14704. [DOI] [PubMed] [Google Scholar]
- 17.Murphy K.E., Halliday G.M. Glucocerebrosidase deficits in sporadic Parkinson disease. Autophagy. 2014;10:1350–1351. doi: 10.4161/auto.29074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devine M.J., Ryten M., Vodicka P., Thomson A.J., Burdon T., Houlden H., Cavaleri F., Nagano M., Drummond N.J., Taanman J.-W., et al. Parkinson’s disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat. Commun. 2011;2:440. doi: 10.1038/ncomms1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson M.X., Henrich M.T., Geibl F.F., Oertel W.H., Brundin P., Surmeier D.J. The roles of connectivity and neuronal phenotype in determining the pattern of α-synuclein pathology in Parkinson’s disease. Neurobiol. Dis. 2022;168:105687. doi: 10.1016/j.nbd.2022.105687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim C.Y., Alcalay R.N. Genetic Forms of Parkinson’s Disease. Semin. Neurol. 2017;37:135–146. doi: 10.1055/s-0037-1601567. [DOI] [PubMed] [Google Scholar]
- 21.Lee S., Imai Y., Gehrke S., Liu S., Lu B. The synaptic function of LRRK2. Biochem. Soc. Trans. 2012;40:1047–1051. doi: 10.1042/BST20120113. [DOI] [PubMed] [Google Scholar]
- 22.Arranz A.M., Delbroek L., van Kolen K., Guimarães M.R., Mandemakers W., Daneels G., Matta S., Calafate S., Shaban H., Baatsen P., et al. LRRK2 functions in synaptic vesicle endocytosis through a kinase-dependent mechanism. J. Cell Sci. 2015;128:541–552. doi: 10.1242/jcs.158196. [DOI] [PubMed] [Google Scholar]
- 23.Beccano-Kelly D.A., Kuhlmann N., Tatarnikov I., Volta M., Munsie L.N., Chou P., Cao L.-P., Han H., Tapia L., Farrer M.J., et al. Synaptic function is modulated by LRRK2 and glutamate release is increased in cortical neurons of G2019S LRRK2 knock-in mice. Front. Cell. Neurosci. 2014;8:301. doi: 10.3389/fncel.2014.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhlmann N., Milnerwood A.J. A Critical LRRK at the Synapse? The Neurobiological Function and Pathophysiological Dysfunction of LRRK2. Front. Mol. Neurosci. 2020;13:153. doi: 10.3389/fnmol.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belluzzi E., Gonnelli A., Cirnaru M.-D., Marte A., Plotegher N., Russo I., Civiero L., Cogo S., Carrion M.P., Franchin C., et al. LRRK2 phosphorylates pre-synaptic N-ethylmaleimide sensitive fusion (NSF) protein enhancing its ATPase activity and SNARE complex disassembling rate. Mol. Neurodegener. 2016;11:1. doi: 10.1186/s13024-015-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cirnaru M.D., Marte A., Belluzzi E., Russo I., Gabrielli M., Longo F., Arcuri L., Murru L., Bubacco L., Matteoli M., et al. LRRK2 kinase activity regulates synaptic vesicle trafficking and neurotransmitter release through modulation of LRRK2 macro-molecular complex. Front. Mol. Neurosci. 2014;7:49. doi: 10.3389/fnmol.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris T.W., Hartwieg E., Horvitz H.R., Jorgensen E.M. Mutations in Synaptojanin Disrupt Synaptic Vesicle Recycling. J. Cell Biol. 2000;150:589–600. doi: 10.1083/jcb.150.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geng J., Wang L., Lee J.Y., Chen C.-K., Chang K.T. Phosphorylation of Synaptojanin Differentially Regulates Endocytosis of Functionally Distinct Synaptic Vesicle Pools. J. Neurosci. 2016;36:8882–8894. doi: 10.1523/JNEUROSCI.1470-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mignogna M.L., Giannandrea M., Gurgone A., Fanelli F., Raimondi F., Mapelli L., Bassani S., Fang H., van Anken E., Alessio M., et al. The intellectual disability protein RAB39B selectively regulates GluA2 trafficking to determine synaptic AMPAR composition. Nat. Commun. 2015;6:6504. doi: 10.1038/ncomms7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niu M., Zheng N., Wang Z., Gao Y., Luo X., Chen Z., Fu X., Wang Y., Wang T., Liu M., et al. RAB39B Deficiency Impairs Learning and Memory Partially Through Compromising Autophagy. Front. Cell Dev. Biol. 2020;8:598622. doi: 10.3389/fcell.2020.598622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koss D.J., Bondarevaite O., Adams S., Leite M., Giorgini F., Attems J., Outeiro T.F. RAB39B is redistributed in dementia with Lewy bodies and is sequestered within aβ plaques and Lewy bodies. Brain Pathol. 2021;31:120–132. doi: 10.1111/bpa.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koss D.J., Campesan S., Giorgini F., Outeiro T.F. Dysfunction of RAB39B-Mediated Vesicular Trafficking in Lewy Body Diseases. Mov. Disord. 2021;36:1744–1758. doi: 10.1002/mds.28605. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro C.S., Carneiro K., Ross C.A., Menezes J.R.L., Engelender S. Synphilin-1 Is Developmentally Localized to Synaptic Terminals, and Its Association with Synaptic Vesicles Is Modulated by α-Synuclein. J. Biol. Chem. 2002;277:23927–23933. doi: 10.1074/jbc.M201115200. [DOI] [PubMed] [Google Scholar]
- 34.Kim M.J., Deng H.-X., Wong Y.C., Siddique T., Krainc D. The Parkinson’s disease-linked protein TMEM230 is required for Rab8a-mediated secretory vesicle trafficking and retromer trafficking. Hum. Mol. Genet. 2017;26:729–741. doi: 10.1093/hmg/ddw413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang B.L. RAB39B’s role in membrane traffic, autophagy, and associated neuropathology. J. Cell. Physiol. 2021;236:1579–1592. doi: 10.1002/jcp.29962. [DOI] [PubMed] [Google Scholar]
- 36.Casadei N., Pöhler A.-M., Tomás-Zapico C., Torres-Peraza J., Schwedhelm I., Witz A., Zamolo I., De Heer R., Spruijt B., Noldus L.P., et al. Overexpression of synphilin-1 promotes clearance of soluble and misfolded alpha-synuclein without restoring the motor phenotype in aged A30P transgenic mice. Hum. Mol. Genet. 2014;23:767–781. doi: 10.1093/hmg/ddt467. [DOI] [PubMed] [Google Scholar]
- 37.Szargel R., Shani V., Abd Elghani F., Mekies L.N., Liani E., Rott R., Engelender S. The PINK1, synphilin-1 and SIAH-1 complex constitutes a novel mitophagy pathway. Hum. Mol. Genet. 2016;25:3476–3490. doi: 10.1093/hmg/ddw189. [DOI] [PubMed] [Google Scholar]
- 38.Zaarur N., Meriin A.B., Gabai V.L., Sherman M.Y. Triggering Aggresome Formation. Dissecting aggresome-targeting and aggregation signals in synphilin 1. J. Biol. Chem. 2008;283:27575–27584. doi: 10.1074/jbc.M802216200. [DOI] [PubMed] [Google Scholar]
- 39.Engelender S. Ubiquitination of α-synuclein and autophagy in Parkinson’s disease. Autophagy. 2008;4:372–374. doi: 10.4161/auto.5604. [DOI] [PubMed] [Google Scholar]
- 40.Pan P.-Y., Zhu J., Rizvi A., Zhu X., Tanaka H., Dreyfus C.F. Synaptojanin1 deficiency upregulates basal autophagosome formation in astrocytes. J. Biol. Chem. 2021;297:100873. doi: 10.1016/j.jbc.2021.100873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanhauwaert R., Kuenen S., Masius R., Bademosi A., Manetsberger J., Schoovaerts N., Bounti L., Gontcharenko S., Swerts J., Vilain S., et al. The SAC 1 domain in synaptojanin is required for autophagosome maturation at presynaptic terminals. EMBO J. 2017;36:1392–1411. doi: 10.15252/embj.201695773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manzoni C., Lewis P.A. LRRK2 and Autophagy. Adv. Neurobiol. 2017;14:89–105. doi: 10.1007/978-3-319-49969-7_5. [DOI] [PubMed] [Google Scholar]
- 43.Bravo-San Pedro J.M., Niso-Santano M., Gomez-Sanchez R., Pizarro-Estrella E., Aiastui-Pujana A., Gorostidi A., Climent V., Lopez de Maturana R., Sanchez-Pernaute R., Lopez de Munain A., et al. The LRRK2 G2019S mutant exacerbates basal autophagy through activation of the MEK/ERK pathway. Cell Mol. Life Sci. 2013;70:121–136. doi: 10.1007/s00018-012-1061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Narendra D., Tanaka A., Suen D.-F., Youle R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parganlija D., Klinkenberg M., Dominguez-Bautista J.A., Hetzel M., Gispert S., Chimi M.A., Dröse S., Mai S., Brandt U., Auburger G., et al. Loss of PINK1 Impairs Stress-Induced Autophagy and Cell Survival. PLoS ONE. 2014;9:e95288. doi: 10.1371/journal.pone.0095288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janda E., Isidoro C., Carresi C., Mollace V. Defective Autophagy in Parkinson’s Disease: Role of Oxidative Stress. Mol. Neurobiol. 2012;46:639–661. doi: 10.1007/s12035-012-8318-1. [DOI] [PubMed] [Google Scholar]
- 47.Schöndorf D.C., Aureli M., McAllister F.E., Hindley C.J., Mayer F., Schmid B., Sardi S.P., Valsecchi M., Hoffmann S., Schwarz L.K., et al. iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat. Commun. 2014;5:4028. doi: 10.1038/ncomms5028. [DOI] [PubMed] [Google Scholar]
- 48.Dasari S.K., Schejter E., Bialik S., Shkedy A., Levin-Salomon V., Levin-Zaidman S., Kimchi A. Death by over-eating: The Gaucher disease associated gene GBA1, identified in a screen for mediators of autophagic cell death, is necessary for developmental cell death in Drosophila midgut. Cell Cycle. 2017;16:2003–2010. doi: 10.1080/15384101.2017.1380134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bento C.F., Ashkenazi A., Jimenez-Sanchez M., Rubinsztein D.C. The Parkinson’s disease-associated genes ATP13A2 and SYT11 regulate autophagy via a common pathway. Nat. Commun. 2016;7:11803. doi: 10.1038/ncomms11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson M.P., Shacka J.J. Autophagy Modulation in Disease Therapy: Where Do We Stand? Curr. Pathobiol. Rep. 2013;1:239–245. doi: 10.1007/s40139-013-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sesar A., Cacheiro P., López-López M., Camiña-Tato M., Quintáns B., Monroy-Jaramillo N., Alonso-Vilatela M.-E., Cebrián E., Yescas-Gómez P., Ares B., et al. Synaptotagmin XI in Parkinson’s disease: New evidence from an association study in Spain and Mexico. J. Neurol. Sci. 2016;362:321–325. doi: 10.1016/j.jns.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 52.Pu J., Lin Z., Zheng R., Yan Y., Xue N., Yin X., Zhang B. Association analysis of SYT11, FGF20, GCH1 rare variants in Parkinson’s disease. CNS Neurosci. Ther. 2021;28:175–177. doi: 10.1111/cns.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh P.K., Muqit M.M.K. Parkinson’s: A Disease of Aberrant Vesicle Trafficking. Annu. Rev. Cell Dev. Biol. 2020;36:237–264. doi: 10.1146/annurev-cellbio-100818-125512. [DOI] [PubMed] [Google Scholar]
- 54.Müller M.P., Goody R.S. Molecular control of Rab activity by GEFs, GAPs and GDI. Small GTPases. 2018;9:5–21. doi: 10.1080/21541248.2016.1276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mignogna M.L., D’Adamo P. Critical importance of RAB proteins for synaptic function. Small GTPases. 2018;9:145–157. doi: 10.1080/21541248.2016.1277001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonet-Ponce L., Cookson M.R. The role of Rab GTPases in the pathobiology of Parkinson’ disease. Curr. Opin. Cell Biol. 2019;59:73–80. doi: 10.1016/j.ceb.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi M.-M., Shi C.-H., Xu Y.-M. Rab GTPases: The Key Players in the Molecular Pathway of Parkinson’s Disease. Front. Cell. Neurosci. 2017;11:81. doi: 10.3389/fncel.2017.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol. Life Sci. 2008;65:2801–2813. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kennedy M.J., Ehlers M.D. Organelles and trafficking machinery for postsynaptic plasticity. Annu. Rev. Neurosci. 2006;29:325–362. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 61.Zerial M., McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 62.Hutagalung A.H., Novick P.J. Role of Rab GTPases in Membrane Traffic and Cell Physiology. Physiol. Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barr F.A. Review series: Rab GTPases and membrane identity: Causal or inconsequential? J. Cell Biol. 2013;202:191–199. doi: 10.1083/jcb.201306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cherfils J., Zeghouf M. Regulation of Small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 2013;93:269–309. doi: 10.1152/physrev.00003.2012. [DOI] [PubMed] [Google Scholar]
- 65.Pfeffer S., Aivazian D. Targeting Rab GTPases to distinct membrane compartments. Nat. Rev. Mol. Cell Biol. 2004;5:886–896. doi: 10.1038/nrm1500. [DOI] [PubMed] [Google Scholar]
- 66.Vetter I.R., Wittinghofer A. The Guanine Nucleotide-Binding Switch in Three Dimensions. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- 67.Binotti B., Jahn R., Chua J.J.E. Functions of Rab Proteins at Presynaptic Sites. Cells. 2016;5:7. doi: 10.3390/cells5010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Veleri S., Punnakkal P., Dunbar G.L., Maiti P. Molecular Insights into the Roles of Rab Proteins in Intracellular Dynamics and Neurodegenerative Diseases. NeuroMolecular Med. 2018;20:18–36. doi: 10.1007/s12017-018-8479-9. [DOI] [PubMed] [Google Scholar]
- 69.Fischer Von Mollard G., Südhof T.C., Jahn R. A small GTP-binding protein dissociates from synaptic vesicles during exocytosis. Nature. 1991;349:79–81. doi: 10.1038/349079a0. [DOI] [PubMed] [Google Scholar]
- 70.Stahl B., von Mollard G.F., Walch-Solimena C., Jahn R. GTP cleavage by the small GTP-binding protein Rab3A is associated with exocytosis of synaptic vesicles induced by alpha-latrotoxin. J. Biol. Chem. 1994;269:24770–24776. doi: 10.1016/S0021-9258(17)31458-8. [DOI] [PubMed] [Google Scholar]
- 71.Fukuda M. Distinct Rab Binding Specificity of Rim1, Rim2, Rabphilin, and Noc2. Identification of a critical determinant of Rab3A/Rab27A recognition by Rim2. J. Biol. Chem. 2003;278:15373–15380. doi: 10.1074/jbc.M212341200. [DOI] [PubMed] [Google Scholar]
- 72.Yu E., Kanno E., Choi S., Sugimori M., Moreira J.E., Llinás R.R., Fukuda M. Role of Rab27 in synaptic transmission at the squid giant synapse. Proc. Natl. Acad. Sci. USA. 2008;105:16003–16008. doi: 10.1073/pnas.0804825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pavlos N.J., Grønborg M., Riedel D., Chua J.J.E., Boyken J., Kloepper T.H., Urlaub H., Rizzoli S.O., Jahn R. Quantitative Analysis of Synaptic Vesicle Rabs Uncovers Distinct Yet Overlapping Roles for Rab3a and Rab27b in Ca2+-Triggered Exocytosis. J. Neurosci. 2010;30:13441–13453. doi: 10.1523/JNEUROSCI.0907-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pavlos N.J., Jahn R. Distinct yet overlapping roles of Rab GTPases on synaptic vesicles. Small GTPases. 2011;2:77–81. doi: 10.4161/sgtp.2.2.15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arias-Hervert E.R., Xu N., Njus M., Murphy G.G., Hou Y., Williams J.A., Lentz S.I., Ernst S.A., Stuenkel E.L. Actions of Rab27B-GTPase on mammalian central excitatory synaptic transmission. Physiol. Rep. 2020;8:e14428. doi: 10.14814/phy2.14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schoch S., Castillo P.E., Jo T., Mukherjee K., Geppert M., Wang Y., Schmitz F., Malenka R.C., Südhof T.C. RIM1α forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- 77.Schoch S., Mittelstaedt T., Kaeser P.S., Padgett D., Feldmann N., Chevaleyre V., Castillo P., Hammer R.E., Han W., Schmitz F., et al. Redundant functions of RIM1α and RIM2α in Ca2+-triggered neurotransmitter release. EMBO J. 2006;25:5852–5863. doi: 10.1038/sj.emboj.7601425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schlüter O.M., Schnell E., Verhage M., Tzonopoulos T., Nicoll R.A., Janz R., Malenka R.C., Geppert M., Südhof T.C. Rabphilin Knock-Out Mice Reveal That Rabphilin Is Not Required for Rab3 Function in Regulating Neurotransmitter Release. J. Neurosci. 1999;19:5834–5846. doi: 10.1523/JNEUROSCI.19-14-05834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deng L., Kaeser P.S., Xu W., Südhof T.C. RIM Proteins Activate Vesicle Priming by Reversing Autoinhibitory Homodimerization of Munc13. Neuron. 2011;69:317–331. doi: 10.1016/j.neuron.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dulubova I., Lou X., Lu J., Huryeva I., Alam A., Schneggenburger R., Südhof T.C., Rizo J. A Munc13/RIM/Rab3 tripartite complex: From priming to plasticity? EMBO J. 2005;24:2839–2850. doi: 10.1038/sj.emboj.7600753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zarebidaki F., Camacho M., Brockmann M.M., Trimbuch T., Herman M.A., Rosenmund C. Disentangling the Roles of RIM and Munc13 in Synaptic Vesicle Localization and Neurotransmission. J. Neurosci. 2020;40:9372–9385. doi: 10.1523/JNEUROSCI.1922-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimizu H., Kawamura S., Ozaki K. An essential role of Rab5 in uniformity of synaptic vesicle size. J. Cell Sci. 2003;116:3583–3590. doi: 10.1242/jcs.00676. [DOI] [PubMed] [Google Scholar]
- 83.Wucherpfennig T., Wilsch-Bräuninger M., González-Gaitán M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Hoop M.J., Huber L.A., Stenmark H., Williamson E., Zerial M., Parton R.G., Dotti C.G. The involvement of the small GTP-binding protein Rab5a in neuronal endocytosis. Neuron. 1994;13:11–22. doi: 10.1016/0896-6273(94)90456-1. [DOI] [PubMed] [Google Scholar]
- 85.Fischer von Mollard G., Stahl B., Walch-Solimena C., Takei K., Daniels L., Khoklatchev A., de Camilli P., Südhof T.C., Jahn R. Localization of Rab5 to synaptic vesicles identifies endosomal intermediate in synaptic vesicle recycling pathway. Eur. J. Cell Biol. 1994;65:319–326. [PubMed] [Google Scholar]
- 86.Franchini L., Stanic J., Barzasi M., Zianni E., Mauceri D., Diluca M., Gardoni F. Rabphilin-3A Drives Structural Modifications of Dendritic Spines Induced by Long-Term Potentiation. Cells. 2022;11:1616. doi: 10.3390/cells11101616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stanic J., Carta M., Eberini I., Pelucchi S., Marcello E., Genazzani A.A., Racca C., Mulle C., Di Luca M., Gardoni F. Rabphilin 3A retains NMDA receptors at synaptic sites through interaction with GluN2A/PSD-95 complex. Nat. Commun. 2015;6:10181. doi: 10.1038/ncomms10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nishino H., Saito T., Wei R., Takano T., Tsutsumi K., Taniguchi M., Ando K., Tomomura M., Fukuda M., Hisanaga S.-I. The LMTK1-TBC1D9B-Rab11A Cascade Regulates Dendritic Spine Formation via Endosome Trafficking. J. Neurosci. 2019;39:9491–9502. doi: 10.1523/JNEUROSCI.3209-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Szatmári Z., Sass M. The autophagic roles of Rab small GTPases and their upstream regulators: A review. Autophagy. 2014;10:1154–1166. doi: 10.4161/auto.29395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Binotti B., Pavlos N.J., Riedel D., Wenzel D., Vorbrüggen G., Schalk A.M., Kühnel K., Boyken J., Erck C., Martens H., et al. The GTPase Rab26 links synaptic vesicles to the autophagy pathway. eLife. 2015;4:e05597. doi: 10.7554/eLife.05597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakazawa H., Sada T., Toriyama M., Tago K., Sugiura T., Fukuda M., Inagaki N. Rab33a Mediates Anterograde Vesicular Transport for Membrane Exocytosis and Axon Outgrowth. J. Neurosci. 2012;32:12712–12725. doi: 10.1523/JNEUROSCI.0989-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilson G.R., Sim J.C., McLean C., Giannandrea M., Galea C.A., Riseley J.R., Stephenson S.E., Fitzpatrick E., Haas S.A., Pope K., et al. Mutations in RAB39B Cause X-Linked Intellectual Disability and Early-Onset Parkinson Disease with α-Synuclein Pathology. Am. J. Hum. Genet. 2014;95:729–735. doi: 10.1016/j.ajhg.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coleman M.P., Freeman M.R. Wallerian degeneration, wld(s), and nmnat. Annu Rev. Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lesage S., Bras J., Cormier-Dequaire F., Condroyer C., Nicolas A., Darwent L., Guerreiro R., Majounie E., Federoff M., Heutink P., et al. Loss-of-function mutations in RAB39B are associated with typical early-onset Parkinson disease. Neurol. Genet. 2015;1:e9. doi: 10.1212/NXG.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mata I.F., Jang Y., Kim C.H., Hanna D.S., Dorschner M.O., Samii A., Agarwal P., Roberts J.W., Klepitskaya O., Shprecher D.R., et al. The RAB39B p.G192R mutation causes X-linked dominant Parkinson’s disease. Mol. Neurodegener. 2015;10:50. doi: 10.1186/s13024-015-0045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Güldner M., Schulte C., Hauser A.-K., Gasser T., Brockmann K. Broad clinical phenotype in Parkinsonism associated with a base pair deletion in RAB39B and additional POLG variant. Park. Relat. Disord. 2016;31:148–150. doi: 10.1016/j.parkreldis.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 97.Shi C.-H., Zhang S.-Y., Yang Z.-H., Yang J., Shang D.-D., Mao C.-Y., Liu H., Hou H.-M., Shi M.-M., Wu J., et al. A novel RAB39B gene mutation in X-linked juvenile parkinsonism with basal ganglia calcification. Mov. Disord. 2016;31:1905–1909. doi: 10.1002/mds.26828. [DOI] [PubMed] [Google Scholar]
- 98.Löchte T., Brüggemann N., Vollstedt E.-J., Krause P., Domingo A., Rosales R., Lee L.V., Hopfner F., Westenberger A., Kühn A., et al. RAB39B mutations are a rare finding in Parkinson disease patients. Park. Relat. Disord. 2016;23:116–117. doi: 10.1016/j.parkreldis.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 99.Ciammola A., Carrera P., Di Fonzo A., Sassone J., Villa R., Poletti B., Ferrari M., Girotti F., Monfrini E., Buongarzone G., et al. X-linked Parkinsonism with Intellectual Disability caused by novel mutations and somatic mosaicism in RAB39B gene. Park. Relat. Disord. 2017;44:142–146. doi: 10.1016/j.parkreldis.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 100.Dalfó E., Barrachina M., Rosa J.L., Ambrosio S., Ferrer I. Abnormal α-synuclein interactions with rab3a and rabphilin in diffuse Lewy body disease. Neurobiol. Dis. 2004;16:92–97. doi: 10.1016/j.nbd.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 101.Wang H.-L., Lu C.-S., Yeh T.-H., Shen Y.-M., Weng Y.-H., Huang Y.-Z., Chen R.-S., Liu Y.-C., Cheng Y.-C., Chang H.-C., et al. Combined Assessment of Serum Alpha-Synuclein and Rab35 is a Better Biomarker for Parkinson’s Disease. J. Clin. Neurol. 2019;15:488–495. doi: 10.3988/jcn.2019.15.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakamura S., Kawamoto Y., Nakano S., Akiguchi I. Expression of the endocytosis regulatory proteins Rab5 and Rabaptin-5 in glial cytoplasmic inclusions from brains with multiple system atrophy. Clin. Neuropathol. 2000;19:51–56. [PubMed] [Google Scholar]
- 103.Dalfó E., Ferrer I. α-Synuclein binding to rab3a in multiple system atrophy. Neurosci. Lett. 2005;380:170–175. doi: 10.1016/j.neulet.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 104.Cooper A.A., Gitler A.D., Cashikar A., Haynes C.M., Hill K.J., Bhullar B., Liu K., Xu K., Strathearn K.E., Liu F., et al. α-Synuclein Blocks ER-Golgi Traffic and Rab1 Rescues Neuron Loss in Parkinson’s Models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gitler A.D., Bevis B.J., Shorter J., Strathearn K.E., Hamamichi S., Su L.J., Caldwell K.A., Caldwell G.A., Rochet J.-C., McCaffery J.M., et al. The Parkinson’s disease protein α-synuclein disrupts cellular Rab homeostasis. Proc. Natl. Acad. Sci. USA. 2008;105:145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Z., McCloskey A., Cheng S., Wu M., Xue C., Yu Z., Fu J., Liu Y., Luo Z.-Q., Liu X. Regulation of the small GTPase Rab1 function by a bacterial glucosyltransferase. Cell Discov. 2018;4:5. doi: 10.1038/s41421-018-0055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dalfó E., Gómez-Isla T., Rosa J.L., Nieto Bodelón M., Cuadrado Tejedor M., Barrachina M., Ambrosio S., Ferrer I. Abnormal α-Synuclein Interactions with Rab Proteins in α-Synuclein A30P Transgenic Mice. J. Neuropathol. Exp. Neurol. 2004;63:302–313. doi: 10.1093/jnen/63.4.302. [DOI] [PubMed] [Google Scholar]
- 108.Gonçalves S.A., Macedo D., Raquel H., Simões P.D., Giorgini F., Ramalho J., Barral D.-C., Ferreira Moita L., Outeiro T.F. shRNA-Based Screen Identifies Endocytic Recycling Pathway Components That Act as Genetic Modifiers of Alpha-Synuclein Aggregation, Secretion and Toxicity. PLoS Genet. 2016;12:e1005995. doi: 10.1371/journal.pgen.1005995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen R.H.C., Wislet-Gendebien S., Samuel F., Visanji N., Zhang G., Marsilio D., Langman T., Fraser P.E., Tandon A. α-Synuclein Membrane Association Is Regulated by the Rab3a Recycling Machinery and Presynaptic Activity*. J. Biol. Chem. 2013;288:7438–7449. doi: 10.1074/jbc.M112.439497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sung J.Y., Kim J., Paik S.R., Park J.H., Ahn Y.S., Chung K.C. Induction of Neuronal Cell Death by Rab5A-dependent Endocytosis of α-Synuclein. J. Biol. Chem. 2001;276:27441–27448. doi: 10.1074/jbc.M101318200. [DOI] [PubMed] [Google Scholar]
- 111.Underwood R., Wang B., Carico C., Whitaker R.H., Placzek W.J., Yacoubian T.A. The GTPase Rab27b regulates the release, autophagic clearance, and toxicity of α-synuclein. J. Biol. Chem. 2020;295:8005–8016. doi: 10.1074/jbc.RA120.013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cookson M.R. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat. Rev. Neurosci. 2010;11:791–797. doi: 10.1038/nrn2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haugarvoll K., Rademakers R., Kachergus J.M., Nuytemans K., Ross O.A., Gibson J.M., Tan E.-K., Gaig C., Tolosa E., Goldwurm S., et al. Lrrk2 R1441C parkinsonism is clinically similar to sporadic Parkinson disease. Neurology. 2008;70:1456–1460. doi: 10.1212/01.wnl.0000304044.22253.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Healy D.G., Wood N.W., Schapira A. Test for LRRK2 mutations in patients with Parkinson’s disease. Pract. Neurol. 2008;8:381–385. doi: 10.1136/jnnp.2008.162420. [DOI] [PubMed] [Google Scholar]
- 115.Simón-Sánchez J., Martí-Massó J., Sánchez-Mut J.V., Paisán-Ruíz C., Martínez-Gil A., Ruiz-Martínez J., Sáenz A., Singleton A.B., Lopez de Munain A., Pérez-Tur J. Parkinson’s disease due to the R1441G mutation in Dardarin: A founder effect in the basques. Mov. Disord. 2006;21:1954–1959. doi: 10.1002/mds.21114. [DOI] [PubMed] [Google Scholar]
- 116.Ho D.H., Jang J., Joe E.-H., Son I., Seo H., Seol W. G2385R and I2020T Mutations Increase LRRK2 GTPase Activity. BioMed Res. Int. 2016;2016:7917128. doi: 10.1155/2016/7917128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Steger M., Diez F., Dhekne H.S., Lis P., Nirujogi R.S., Karayel O., Tonelli F., Martinez T.N., Lorentzen E., Pfeffer S.R., et al. Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. eLife. 2017;6:e31012. doi: 10.7554/eLife.31012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tucci A., Nalls M.A., Houlden H., Revesz T., Singleton A.B., Wood N.W., Hardy J., Paisán-Ruíz C. Genetic variability at the PARK16 locus. Eur. J. Hum. Genet. 2010;18:1356–1359. doi: 10.1038/ejhg.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Madero-Pérez J., Fernández B., Ordóñez A.J.L., Fdez E., Lobbestael E., Baekelandt V., Hilfiker S. RAB7L1-Mediated Relocalization of LRRK2 to the Golgi Complex Causes Centrosomal Deficits via RAB8A. Front. Mol. Neurosci. 2018;11:417. doi: 10.3389/fnmol.2018.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu Z., Bryant N., Kumaran R., Beilina A., Abeliovich A., Cookson M.R., West A.B. LRRK2 phosphorylates membrane-bound Rabs and is activated by GTP-bound Rab7L1 to promote recruitment to the trans-Golgi network. Hum. Mol. Genet. 2018;27:385–395. doi: 10.1093/hmg/ddx410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Purlyte E., Dhekne H.S., Sarhan A.R., Gomez R., Lis P., Wightman M., Martinez T.N., Tonelli F., Pfeffer S.R., Alessi D.R. Rab29 activation of the Parkinson’s disease-associated LRRK2 kinase. EMBO J. 2018;37:1–18. doi: 10.15252/embj.201798099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kalogeropulou A.F., Freemantle J.B., Lis P., Vides E.G., Polinski N.K., Alessi D.R. Endogenous Rab29 does not impact basal or stimulated LRRK2 pathway activity. Biochem. J. 2020;477:4397–4423. doi: 10.1042/BCJ20200458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Iannotta L., Biosa A., Kluss J.H., Tombesi G., Kaganovich A., Cogo S., Plotegher N., Civiero L., Lobbestael E., Baekelandt V., et al. Divergent Effects of G2019S and R1441C LRRK2 Mutations on LRRK2 and Rab10 Phosphorylations in Mouse Tissues. Cells. 2020;9:2344. doi: 10.3390/cells9112344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gómez-Suaga P., Rivero-Ríos P., Fdez E., Blanca Ramírez M., Ferrer I., Aiastui A., Lopez de Munain A., Hilfiker S. LRRK2 delays degradative receptor trafficking by impeding late endosomal budding through decreasing Rab7 activity. Hum. Mol. Genet. 2014;23:6779–6796. doi: 10.1093/hmg/ddu395. [DOI] [PubMed] [Google Scholar]
- 125.Steger M., Tonelli F., Ito G., Davies P., Trost M., Vetter M., Wachter S., Lorentzen E., Duddy G., Wilson S., et al. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. eLife. 2016;5:e12813. doi: 10.7554/eLife.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rivero-Ríos P., Romo-Lozano M., Madero-Pérez J., Thomas A.P., Biosa A., Greggio E., Hilfiker S. The G2019S variant of leucine-rich repeat kinase 2 (LRRK2) alters endolysosomal trafficking by impairing the function of the GTPase RAB8A. J. Biol. Chem. 2019;294:4738–4758. doi: 10.1074/jbc.RA118.005008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kouranti I., Sachse M., Arouche N., Goud B., Echard A. Rab35 Regulates an Endocytic Recycling Pathway Essential for the Terminal Steps of Cytokinesis. Curr. Biol. 2006;16:1719–1725. doi: 10.1016/j.cub.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 128.Bae E.-J., Kim D.-K., Kim C., Mante M., Adame A., Rockenstein E., Ulusoy A., Klinkenberg M., Jeong G.R., Bae J.R., et al. LRRK2 kinase regulates α-synuclein propagation via RAB35 phosphorylation. Nat. Commun. 2018;9:3465. doi: 10.1038/s41467-018-05958-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Waschbüsch D., Hübel N., Ossendorf E., Lobbestael E., Baekelandt V., Lindsay A.J., McCaffrey M.W., Khan A.R., Barnekow A. Rab32 interacts with SNX6 and affects retromer-dependent Golgi trafficking. PLoS ONE. 2019;14:e0208889. doi: 10.1371/journal.pone.0208889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Waschbüsch D., Michels H., Strassheim S., Ossendorf E., Kessler D., Gloeckner C.J., Barnekow A. LRRK2 Transport Is Regulated by Its Novel Interacting Partner Rab32. PLoS ONE. 2014;9:e111632. doi: 10.1371/journal.pone.0111632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Inoshita T., Arano T., Hosaka Y., Meng H., Umezaki Y., Kosugi S., Morimoto T., Koike M., Chang H.-Y., Imai Y., et al. Vps35 in cooperation with LRRK2 regulates synaptic vesicle endocytosis through the endosomal pathway in Drosophila. Hum. Mol. Genet. 2017;26:2933–2948. doi: 10.1093/hmg/ddx179. [DOI] [PubMed] [Google Scholar]
- 132.Albanese F., Domenicale C., Volta M., Morari M. Modeling Parkinson’s disease in LRRK2 mice: Focus on synaptic dysfunction and the autophagy-lysosomal pathway. Biochem. Soc. Trans. 2022;50:621–632. doi: 10.1042/BST20211288. [DOI] [PubMed] [Google Scholar]
- 133.Lee B.D., Shin J.-H., VanKampen J., Petrucelli L., West A.B., Ko H.S., Lee Y.-I., Maguire-Zeiss K.A., Bowers W.J., Federoff H.J., et al. Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson’s disease. Nat. Med. 2010;16:998–1000. doi: 10.1038/nm.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brzozowski C.F., Hijaz B.A., Singh V., Gcwensa N.Z., Kelly K., Boyden E.S., West A.B., Sarkar D., Volpicelli-Daley L.A. Inhibition of LRRK2 kinase activity promotes anterograde axonal transport and presynaptic targeting of α-synuclein. Acta Neuropathol. Commun. 2021;9:180. doi: 10.1186/s40478-021-01283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Plowey E.D., Johnson J.W., Steer E., Zhu W., Eisenberg D.A., Valentino N.M., Liu Y.-J., Chu C.T. Mutant LRRK2 enhances glutamatergic synapse activity and evokes excitotoxic dendrite degeneration. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014;1842:1596–1603. doi: 10.1016/j.bbadis.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chou J.-S., Chen C.-Y., Chen Y.-L., Weng Y.-H., Yeh T.-H., Lu C.-S., Chang Y.-M., Wang H.-L. (G2019S) LRRK2 causes early-phase dysfunction of SNpc dopaminergic neurons and impairment of corticostriatal long-term depression in the PD transgenic mouse. Neurobiol. Dis. 2014;68:190–199. doi: 10.1016/j.nbd.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 137.Nguyen A.P.T., Daniel G., Valdés P., Islam M.S., Schneider B.L., Moore D.J. G2019S LRRK2 enhances the neuronal transmission of tau in the mouse brain. Hum. Mol. Genet. 2018;27:120–134. doi: 10.1093/hmg/ddx389. [DOI] [PubMed] [Google Scholar]
- 138.Qin Q., Zhi L.-T., Li X.-T., Yue Z.-Y., Li G., Zhang H. Effects of LRRK2 Inhibitors on Nigrostriatal Dopaminergic Neurotransmission. CNS Neurosci. Ther. 2017;23:162–173. doi: 10.1111/cns.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nirujogi R.S., Tonelli F., Taylor M., Lis P., Zimprich A., Sammler E., Alessi D.R. Development of a multiplexed targeted mass spectrometry assay for LRRK2-phosphorylated Rabs and Ser910/Ser935 biomarker sites. Biochem. J. 2021;478:299–326. doi: 10.1042/BCJ20200930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Petropoulou-Vathi L., Simitsi A., Valkimadi P.-E., Kedariti M., Dimitrakopoulos L., Koros C., Papadimitriou D., Papadimitriou A., Stefanis L., Alcalay R.N., et al. Distinct profiles of LRRK2 activation and Rab GTPase phosphorylation in clinical samples from different PD cohorts. Npj Park. Dis. 2022;8:73. doi: 10.1038/s41531-022-00336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mir R., Tonelli F., Lis P., Macartney T., Polinski N.K., Martinez T.N., Chou M.-Y., Howden A.J.M., König T., Hotzy C., et al. The Parkinson’s disease VPS35[D620N] mutation enhances LRRK2-mediated Rab protein phosphorylation in mouse and human. Biochem. J. 2018;475:1861–1883. doi: 10.1042/BCJ20180248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang S., Unnithan S., Bryant N., Chang A., Rosenthal L.S., Pantelyat A., Dawson T.M., Al-Khalidi H.R., West A.B. Elevated Urinary Rab10 Phosphorylation in Idiopathic Parkinson Disease. Mov. Disord. 2022;37:1454–1464. doi: 10.1002/mds.29043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Atashrazm F., Hammond D., Perera G., Bolliger M.F., Matar E., Halliday G.M., Schüle B., Lewis S.J.G., Nichols R.J., Dzamko N. LRRK2-mediated Rab10 phosphorylation in immune cells from Parkinson’s disease patients. Mov. Disord. 2019;34:406–415. doi: 10.1002/mds.27601. [DOI] [PubMed] [Google Scholar]
- 144.George A.A., Hayden S., Holzhausen L.C., Ma E.Y., Suzuki S.C., Brockerhoff S.E. Synaptojanin 1 Is Required for Endolysosomal Trafficking of Synaptic Proteins in Cone Photoreceptor Inner Segments. PLoS ONE. 2014;9:e84394. doi: 10.1371/journal.pone.0084394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Deng H.-X., Shi Y., Yang Y., Ahmeti K.B., Miller N., Huang C., Cheng L., Zhai H., Deng S., Nuytemans K., et al. Identification of TMEM230 mutations in familial Parkinson’s disease. Nat. Genet. 2016;48:733–739. doi: 10.1038/ng.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sidransky E., Samaddar T., Tayebi N., Nichols W.C., Pankratz N., Foroud T. Mutations in gba are associated with FAMILIAL parkinson disease susceptibility and age at onset. Neurology. 2009;73:1424–1426. doi: 10.1212/WNL.0b013e3181b28601. [DOI] [PubMed] [Google Scholar]
- 147.Stoker T.B., Camacho M., Winder-Rhodes S., Liu G., Scherzer C.R., Foltynie T., Evans J., Breen D.P., Barker R.A., Williams-Gray C.H. Impact of GBA1 variants on long-term clinical progression and mortality in incident Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2020;91:695–702. doi: 10.1136/jnnp-2020-322857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mazzulli J.R., Xu Y.-H., Sun Y., Knight A.L., McLean P.J., Caldwell G.A., Sidransky E., Grabowski G.A., Krainc D. Gaucher Disease Glucocerebrosidase and α-Synuclein Form a Bidirectional Pathogenic Loop in Synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gegg M.E., Burke D., Heales S.J.R., Cooper J.M., Hardy J., Wood N.W., Schapira A.H.V. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann. Neurol. 2012;72:455–463. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gündner A.L., Duran-Pacheco G., Zimmermann S., Ruf I., Moors T., Baumann K., Jagasia R., van de Berg W.D.J., Kremer T. Path mediation analysis reveals GBA impacts Lewy body disease status by increasing α-synuclein levels. Neurobiol. Dis. 2019;121:205–213. doi: 10.1016/j.nbd.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 151.Cullen V., Sardi S.P., Ng J., Xu Y.-H., Sun Y., Tomlinson J.J., Kolodziej P., Kahn I., Saftig P., Woulfe J., et al. Acid β-glucosidase mutants linked to gaucher disease, parkinson disease, and lewy body dementia alter α-synuclein processing. Ann. Neurol. 2011;69:940–953. doi: 10.1002/ana.22400. [DOI] [PubMed] [Google Scholar]
- 152.Yang S.-Y., Gegg M., Chau D., Schapira A. Glucocerebrosidase activity, cathepsin D and monomeric α-synuclein interactions in a stem cell derived neuronal model of a PD associated GBA1 mutation. Neurobiol. Dis. 2020;134:104620. doi: 10.1016/j.nbd.2019.104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jo J., Yang L., Tran H., Yu W., Sun A.X., Chang Y.Y., Jung B.C., Lee S., Saw T.Y., Xiao B., et al. Lewy Body–like Inclusions in Human Midbrain Organoids Carrying Glucocerebrosidase and α-Synuclein Mutations. Ann. Neurol. 2021;90:490–505. doi: 10.1002/ana.26166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Henderson M.X., Sedor S., McGeary I., Cornblath E.J., Peng C., Riddle D.M., Li H.L., Zhang B., Brown H.J., Olufemi M.F., et al. Glucocerebrosidase Activity Modulates Neuronal Susceptibility to Pathological α-Synuclein Insult. Neuron. 2019;105:822–836. doi: 10.1016/j.neuron.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alcalay R.N., Dinur T., Quinn T., Sakanaka K., Levy O., Waters C., Fahn S., Dorovski T., Chung W.K., Pauciulo M., et al. Comparison of Parkinson Risk in Ashkenazi Jewish Patients With Gaucher Disease and GBA Heterozygotes. JAMA Neurol. 2014;71:752–757. doi: 10.1001/jamaneurol.2014.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Do J., McKinney C., Sharma P., Sidransky E. Glucocerebrosidase and its relevance to Parkinson disease. Mol. Neurodegener. 2019;14:36. doi: 10.1186/s13024-019-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fecchio C., Palazzi L., de Laureto P.P. α-Synuclein and Polyunsaturated Fatty Acids: Molecular Basis of the Interaction and Implication in Neurodegeneration. Molecules. 2018;23:1531. doi: 10.3390/molecules23071531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rivero-Ríos P., Romo-Lozano M., Fasiczka R., Naaldijk Y., Hilfiker S. LRRK2-Related Parkinson’s Disease Due to Altered Endolysosomal Biology With Variable Lewy Body Pathology: A Hypothesis. Front. Neurosci. 2020;14:556. doi: 10.3389/fnins.2020.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Klein A.D., Mazzulli J.R. Is Parkinson’s disease a lysosomal disorder? Brain. 2018;141:2255–2262. doi: 10.1093/brain/awy147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zunke F., Moise A.C., Belur N.R., Gelyana E., Stojkovska I., Dzaferbegovic H., Toker N.J., Jeon S., Fredriksen K., Mazzulli J.R. Reversible Conformational Conversion of α-Synuclein into Toxic Assemblies by Glucosylceramide. Neuron. 2018;97:92–107. doi: 10.1016/j.neuron.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Morén C., Juárez-Flores D.L., Chau K.-Y., Gegg M., Garrabou G., González-Casacuberta I., Guitart-Mampel M., Tolosa E., Martí M.J., Cardellach F., et al. GBA mutation promotes early mitochondrial dysfunction in 3D neurosphere models. Aging. 2019;11:10338–10355. doi: 10.18632/aging.102460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li H., Ham A., Ma T.C., Kuo S.-H., Kanter E., Kim D., Ko H.S., Quan Y., Sardi S.P., Li A., et al. Mitochondrial dysfunction and mitophagy defect triggered by heterozygous GBA mutations. Autophagy. 2019;15:113–130. doi: 10.1080/15548627.2018.1509818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ao X., Zou L., Wu Y. Regulation of autophagy by the Rab GTPase network. Cell Death Differ. 2014;21:348–358. doi: 10.1038/cdd.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yu M., Arshad M., Wang W., Zhao D., Xu L., Zhou L. LRRK2 mediated Rab8a phosphorylation promotes lipid storage. Lipids Health Dis. 2018;17:34. doi: 10.1186/s12944-018-0684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Imbriani P., Schirinzi T., Meringolo M., Mercuri N.B., Pisani A. Centrality of Early Synaptopathy in Parkinson’s Disease. Front. Neurol. 2018;9:103. doi: 10.3389/fneur.2018.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ginns E.I., Mak S.K.-K., Ko N., Karlgren J., Akbarian S., Chou V.P., Guo Y., Lim A., Samuelsson S., LaMarca M.L., et al. Neuroinflammation and α-synuclein accumulation in response to glucocerebrosidase deficiency are accompanied by synaptic dysfunction. Mol. Genet. Metab. 2014;111:152–162. doi: 10.1016/j.ymgme.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 167.Pará C., Bose P., Pshezhetsky A.V. Neuropathophysiology of Lysosomal Storage Diseases: Synaptic Dysfunction as a Starting Point for Disease Progression. J. Clin. Med. 2020;9:616. doi: 10.3390/jcm9030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Higashi S., Moore D., Minegishi M., Kasanuki K., Fujishiro H., Kabuta T., Togo T., Katsuse O., Uchikado H., Furukawa Y., et al. Localization of MAP1-LC3 in Vulnerable Neurons and Lewy Bodies in Brains of Patients With Dementia With Lewy Bodies. J. Neuropathol. Exp. Neurol. 2011;70:264–280. doi: 10.1097/NEN.0b013e318211c86a. [DOI] [PubMed] [Google Scholar]
- 169.Szegö E.M., van den Haute C., Höfs L., Baekelandt V., van der Perren A., Falkenburger B.H. Rab7 reduces α-synuclein toxicity in rats and primary neurons. Exp. Neurol. 2022;347:113900. doi: 10.1016/j.expneurol.2021.113900. [DOI] [PubMed] [Google Scholar]
- 170.Yamano K., Fogel A.I., Wang C., van der Bliek A.M., Youle R.J. Mitochondrial Rab GAPs govern autophagosome biogenesis during mitophagy. eLife. 2014;3:e01612. doi: 10.7554/eLife.01612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lai Y., Kondapalli C., Lehneck R., Procter J.B., Dill B.D., Woodroof H.I., Gourlay R., Peggie M., Macartney T.J., Corti O., et al. Phosphoproteomic screening identifies Rab GTP ases as novel downstream targets of PINK 1. EMBO J. 2015;34:2840–2861. doi: 10.15252/embj.201591593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Agarwal S., Muqit M.M.K. PTEN-induced kinase 1 (PINK1) and Parkin: Unlocking a mitochondrial quality control pathway linked to Parkinson’s disease. Curr. Opin. Neurobiol. 2022;72:111–119. doi: 10.1016/j.conb.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 173.Narendra D.P. Managing risky assets–mitophagy in vivo. J. Cell Sci. 2021;134:jcs240465. doi: 10.1242/jcs.240465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shimojo M., Madara J., Pankow S., Liu X., Yates J., 3rd, Südhof T.C., Maximov A. Synaptotagmin-11 mediates a vesicle trafficking pathway that is essential for development and synaptic plasticity. Genes Dev. 2019;33:365–376. doi: 10.1101/gad.320077.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang C., Kang X., Zhou L., Chai Z., Wu Q., Huang R., Xu H., Hu M., Sun X., Sun S., et al. Synaptotagmin-11 is a critical mediator of parkin-linked neurotoxicity and Parkinson’s disease-like pathology. Nat. Commun. 2018;9:81. doi: 10.1038/s41467-017-02593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Decet M., Verstreken P. Presynaptic Autophagy and the Connection with Neurotransmission. Front. Cell Dev. Biol. 2021;9:790721. doi: 10.3389/fcell.2021.790721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liang Y. Emerging Concepts and Functions of Autophagy as a Regulator of Synaptic Components and Plasticity. Cells. 2019;8:34. doi: 10.3390/cells8010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shen D.-N., Zhang L.-H., Wei E.-Q., Yang Y. Autophagy in synaptic development, function, and pathology. Neurosci. Bull. 2015;31:416–426. doi: 10.1007/s12264-015-1536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hill S.E., Colón-Ramos D.A. The Journey of the Synaptic Autophagosome: A Cell Biological Perspective. Neuron. 2020;105:961–973. doi: 10.1016/j.neuron.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 180.Kulkarni V.V., Anand A., Herr J.B., Miranda C., Vogel M.C., Maday S. Synaptic activity controls autophagic vacuole motility and function in dendrites. J. Cell Biol. 2021;220:e202002084. doi: 10.1083/jcb.202002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Limanaqi F., Biagioni F., Gambardella S., Ryskalin L., Fornai F. Interdependency Between Autophagy and Synaptic Vesicle Trafficking: Implications for Dopamine Release. Front. Mol. Neurosci. 2018;11:299. doi: 10.3389/fnmol.2018.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.