Abstract
The NLRP inflammasome is a multi-protein complex which mainly consists of the nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain. Its activation is linked to microglial-mediated neuroinflammation and partial neuronal degeneration. Many neuropsychiatric illnesses have increased inflammatory responses as both a primary cause and a defining feature. The NLRP inflammasome inhibition delays the progression and alleviates the deteriorating effects of neuroinflammation on several neuropsychiatric disorders. Evidence on the central effects of the NLRP inflammasome potentially provides the scientific base of a promising drug target for the treatment of neuropsychiatric disorders. This review elucidates the classification, composition, and functions of the NLRP inflammasomes. It also explores the underlying mechanisms of NLRP inflammasome activation and its divergent role in neuropsychiatric disorders, including Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, depression, drug use disorders, and anxiety. Furthermore, we explore the treatment potential of the NLRP inflammasome inhibitors against these disorders.
Keywords: NLRP inflammasome, neuropsychiatric disorders, neuroinflammation, pharmacological treatment
1. Introduction
Neuropsychiatric disorders have a significant impact on human health and quality of life, causing a huge socio-economic burden on society and overstretching the healthcare system. Therefore, studies investigating the mechanisms of neuropsychiatric disorders for effective therapies have increased. Activated inflammatory responses are a major cause and common feature of numerous neuropsychiatric disorders [1,2]. Nucleotide-binding oligomerization domain (NOD) and leucine-rich repeat (LRR)-containing receptors or NOD-like receptors (NLRs) are inflammasomes that are critical to initiate innate immune responses to host-derived danger signals [3]. Activations of many prototypic NLRs, including a NLR with a pyrin domain (NLRP) containing NLRP1, NLRP3, and NLRP4, result in the maturation and release of different pro-inflammatory cytokines (IL-1β and IL-18) [4]. The process has been suggested to be of great importance to the occurrence of programmed cell death, which is called pyroptosis [5]. As a part of the innate immune system, the NLRP inflammasome regulates the host’s defense against harmful threats. Its activation is implicated in microglial-mediated neuroinflammation and partial neuronal degeneration [6]. In addition, it modulates the pathogenesis of various neuropsychiatric disorders, including Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, Amyotrophic lateral sclerosis (ALS), traumatic brain injury (TBI), drug use disorder, depression, and anxiety [7]. This review explores the significance and therapeutical implications of NLRP inflammasomes in neuropsychiatric disorders, geared towards providing a basis for exploring common pathway-based treatments for different neuropsychiatric diseases.
2. NLRP Inflammasome
NLRP inflammasome is a three-part multi-protein complex which senses danger signals via the nucleotide-binding oligomerization domain (NOD) such as receptors (NLR) containing a CARD (C-terminal caspase-recruitment domain), and controls the activation of Caspase (Casp-1) [8]. The NLRP inflammasome family consists of more than 20 species, including NLRP1, NLRP3, and NLRP4. Studies on neuropsychiatric disorders primarily focus on NLRP1 and NLRP3 inflammasome [9]. NLRP1 inflammasome, majorly expressed in the microglia and neurons of the brain, was the first member of the NLRP family to be identified [10]. This inflammasome comprises a receptor protein (NLRP1), an adaptor protein (ASC), and an effector protein (pro-caspase-1) [11]. NLRP3 inflammasome, primarily localized in the microglia, was the first inflammasome to be investigated in the brain. NLRP3 inflammasome is composed of NLRP3, ASC, and pro-caspase-1. As depicted in Figure 1, the structure of the NLRP inflammasome includes ASC, NLRPs, pro-caspase-1. The adaptor ASC has two protein interaction domains, an N-terminal PYD and a CARD [12]. Most inflammatory vesicles are activated by only one or a few highly specific agonists, but NLRP1 and NLRP3 can be activated by many agonists with a Toll-like receptor agonist (lipopolysaccharide (LPS), nigericin, monosodium urate crystals, and adenosine-triphosphate), pathogens (bacteria, fungi and viruses), or a proinflammatory cytokine (tumor necrosis factor, TNF) [13]. It can also be activated by various signalings specifically associated with neuropsychiatric disorders, including K+ or Cl−, Ca2+, lysosomal disruption, mitochondrial dysfunction, metabolic changes and trans-Golgi catabolism [12,14]. In microglia, NLRP3 inflammasome becomes activated when these cells sense proteins such as misfolded or aggregated amyloid-β, α-synuclein and prion protein or superoxide dismutase, ATP and members of the complement pathway, and results in the maturation and release of various pro-inflammatory cytokines (e.g., IL-1β, and IL-18) [7]. IL-1β promotes inflammatory responses including leukocyte infiltrations, lymphocyte activation and acute phase protein induction. Moreover, after binding to IL-1β receptors, it induces the secretion of large amounts of inflammatory factors and chemokines [15,16]. IL-18 exerts its pro-inflammatory effects by stimulating the production of nitric oxide and reactive oxygen species. NLRP3 inflammasome activation results in caspase-1 activation, in turn causing cleavage of pro-IL-1β and gasdermin D (GSDMD). Active GSDMD aggregates and forms pores in the cell membrane, resulting in cell swelling and a programmed cell death which is called pyroptosis [17]. Pyroptosis is implicated in the pathogenesis of several neuropsychiatric disorders, such as multiple sclerosis (MS), Alzheimer’s disease, TBI, drug use disorder, and depression [18,19]. In addition to microglia, NLRP1 and NLRP3 can also be found in myeloid cells in the central nervous system and may also contribute to the modulation of central innate immunity [20,21].
Figure 1.
Structure of NLRP inflammasome.
The mechanisms leading to NLRP inflammasome activation are intensely debated. Some of the detailed signaling pathways involved in NLRP1/NLRP3 inflammasome activation are described in this article, such as elevated ROS, K+/Ca2+ imbalance and autophagic inhibition in activating NLRP inflammasome in neuropsychiatric disorders. The mechanisms of action in NLRP inflammasomes in neuropsychiatric disorders are mainly depicted in Figure 2. Recent reports have revealed complex interactions between the inflammasome and ROS pathways. Chronic cerebral hypoperfusion (CCH) induces ROS accumulation and promotes the activation of NLRP3 inflammasomes and the release of IL-1β. However, URB597 (URB) alleviated autophagy and mitochondrial impairment by reducing the mitochondrial ROS as well as restoring the lysosomal function, which further inhibited the NLRP3-CASP1 pathway activation in the rat hippocampus [22]. Acetoxychavicol acetate (ACA) inhibited NLRP3 agonists (e.g., nigericin, MSU crystals, and ATP) in mouse bone marrow-derived macrophages and NLRP3 inflammasome activation in human THP-1 monocytes by suppressing the production of mitochondrial reactive oxygen species (ROS) [5]. In addition, it inhibited the oligomerization of adapter molecules, ASC and the cleavage of the cystein-1 mediated pyroptosis actuator Gasdermin D [5]. K+/Ca2+ imbalance plays an important role in the activation of NLRP3 inflammasomes. Ca2+ influx and K+ efflux promote NLRP3 inflammasome activation in mice. In addition, aldose reductase (AR) regulates NLRP3 inflammation-mediated innate immune responses by altering the ROS/LYN/SYK/PI3K/Ca2+/K+ signaling pathway [23]. Contact of the P2X7 purinoceptor with extracellular ATP induces transmembrane K+/Ca2+ imbalance, leading to activation of NLRP1 and NLRP3 inflammasomes in LPS-stimulated macrophages. This evidence suggests that both potassium efflux and calcium influx are necessary for the generation of mitochondrial ROS and to trigger NLRP inflammation [24]. Autophagic activity is maintained at relatively low levels under steady-state conditions, but is effectively induced by various cellular stresses, such as organelle damage and pathogen infection [25]. Recent studies have shown that autophagy, an intracellular degradation system associated with the maintenance of cellular homeostasis, plays a key role in the inactivation of the inflammasome. Notably, autophagy deficiency caused by genetic mutations can disrupt organelle elimination, thereby inducing aberrant activation of the inflammasome and leading to severe tissue damage [26]. Blocked autophagy and mitochondrial flux also enhanced the activation of NLRP3-CASP1 pathways. Autophagy inhibition can lead to lysosomal damage, resulting in the cytoplasmic release of lysosomal contents which (e.g.,) activate NLRP inflammasome [27]. Mechanistically, Kaempferol (KA) promotes macrophage/autophagy in microglia and promotes neuroinflammatory suppressive effects through the cooperation of ubiquitination and autophagy, leading to the reduced expression of NLRP3 proteins and consequently to the deactivation of NLRP3 inflammasomes in mice [28]. The blockade of autophagy by genetic ablation of the autophagy regulators Atg16L1 or Atg7 makes LPS-dependent inflammasome activation possible in the central nervous system (CNS) in mice [29]. In conclusion, NLRP inflammasome activators play an important role in the activation of inflammatory vesicles by triggering multiple cellular and molecular events including potassium-calcium ion imbalance, mitochondrial dysfunction and lysosomal rupture, especially alterations in intracellular ion levels that link different signal transductions; however, the detailed mechanisms of inflammatory vesicle activation by each activation signal still deserve more in-depth investigation. Information about the chemical structure of NLRP inflammatory vesicle inhibitors, models of drug treatment of disease, and clinical advances in drug treatment of neuropsychiatric disorders are described mainly in Table 1.
Figure 2.
Mechanisms of NLRP inflammasomes in neuropsychiatric disorders.
Table 1.
Basic information on NLRP inflammasome inhibitors for the treatment of neuropsychiatric disorders.
| Name | Structure | Model | Findings | Clinical Advance | References |
|---|---|---|---|---|---|
| Rg1 |
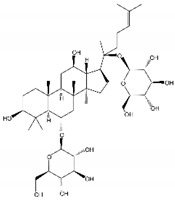
|
APP/PS1 mice | hippocampus and cortex of mice Aβ↓. | [30,31] | |
| JC-124 |

|
CRND8 APP transgenic mice (TgCRND8) | Aβ, Aβ1-42↓ | [32,33,34] | |
| MCC950 (CRID3) |
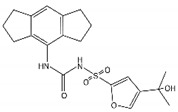
|
In vitro cell culture model of Alzheimer’s disease (SH-APP cells); METH use disorder. |
caspase-1 and IL-1β levels, tau, Aβ↓; Capase-1, lysosomal histone B activity, ROS↓. |
[35] | |
| Osthole |

|
APP/PS1 double transfected mice | hippocampal tau↓ | [36,37] | |
| Probenecid |

|
Aged rats | Inflammasome activation↓ spatial learning performance↑. |
[38,39] | |
| EA (Ellagic acid) |
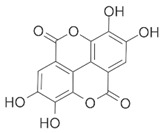
|
LPS induced rat DA neuronal (MN9D, BV-2 cell line) damage model. NLRP3 siRNA. |
Neuroprotection. | [40] | |
| SAFE (Safflower Flavonoid Extract) |
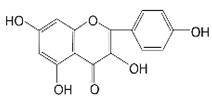
|
6-Hydroxydopamine (6-OHDA)-induced rat model of Parkinson’s disease | NLRP3 inflammasome activation ↓ | [41] | |
| NAR(Naringin) |

|
Rat nigral stereotaxic injection of lipopolysaccharide (LPS). NLRP3 siRNA. | Neuroprotection. | [42] | |
| ECH(Echinacoside) |

|
Mice model of Parkinson’s disease | NLRP3/Caspase-1/IL-1β signaling pathway↓. | [43] | |
| Laquinimod |

|
Parkinson’s disease | [44] | ||
| PAP(Papaverine) |
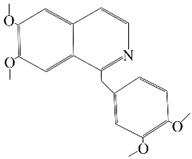
|
Mice model mice of Huntington’s disease | Regulation of NF-κB and CREB signaling pathways to inhibit NLRP3. | [45] | |
| RRx-001 |

|
Hunting’s disease | NLRP3↓. | [46,47] | |
| DSF(Disulfiram) |

|
Alcohol use disorder | ROS and NLRP3↓ | Alcohol use disorder | [48] |
| Cod liver oil |
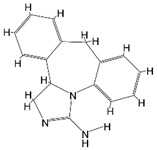
|
Alcohol consumption↓ | Alcohol use disorder | [49] |
3. Roles of NLRP Inflammasome in Neuropsychiatric Disorders
Neuroinflammation is a vital factor in the pathogenesis of psychiatric illnesses, including Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, drug use disorders, depression, and anxiety. In addition, it is involved in sickness behaviors, diminished cognition, and memory [38].
3.1. Alzheimer’s Disease
Alzheimer’s disease is a neurodegenerative disease with the characteristics of memory loss and cognitive decreases. It is also associated with progressive atrophy and extensive neuronal death in the temporal lobe, hippocampus, frontal cortex, and other brain areas [50,51]. Alzheimer’s disease develops from the accumulation of beta-amyloid (Aβ) and neuronal tangles comprising hyperphosphorylated tau proteins within neural progenitor fibers. Subsequent neurodegeneration and microglial activation mediate neuroinflammation in the brain [52,53,54,55,56,57]. Oxidative stress, neuroinflammation, and Ca2+ overload are significant in the development of Alzheimer’s disease. NLRP inflammasome promotes oxidative stress and inflammation in the brain [58].
There is a significant link between NLRP inflammasome and the pathogenesis of Alzheimer’s disease [59,60]. Aβ plaques not only induce oxidative stress and damage neurons, but also activate NLRP3 inflammasomes further releasing IL-1β to trigger neuroinflammation in patients with Alzheimer’s disease [61]. In neurons, NLRP1 inflammasome levels increase by approximately 25–30 fold in the patients with Alzheimer’s disease [62]. Neurotoxic effects of Aβ open the cellular ion channels, causing the inward and outward flow of calcium and potassium. An imbalance of K+/Ca2+ activates NLRP1 inflammasome in neurons, hence upregulating the expression of NLRP1 inflammasome, caspase-1, and IL-1β in LPS-primed macrophages [13]. Additionally, chronic glucocorticoid exposure is associated with neuronal degeneration, subsequently accelerating the deleterious progression in mice. Chronic glucocorticoid exposure in mice activates NLRP1 inflammasome-signaling pathways [13,63]. NLRP1 inflammasome inhibition by Ginsenoside Rg1 suppressed chronic glucocorticoid exposure-induced neuronal degenerations in mice [30,31]. Recent studies indicate that pyroptosis could also promote the development of Alzheimer’s disease. Chronic Aβ treatment significantly decreased PC12 cell viabilities and activated NLRP-1/caspase-1/GSDMD pathways, which was followed by the increased extracellular release of IL-18 and IL-1β [64].
NLRP3 inflammasome plays a role in destructive inflammatory responses by producing active forms of inflammatory cytokines. NLRP3 inflammasome activation modulates neuroinflammation, tissue damage, and cognitive impairment commonly found in the mouse model of Alzheimer’s disease [52]. Intestinal bacteria in the patients with Alzheimer’s disease mediate neuroinflammation via NLRP3 inflammasome activation [65]. Deposition and aggregation of Aβ in the brain of APP/PS1 mice stimulate NLRP3 inflammation, caspase-1, and IL-Iβ. In APP/PS1 mice, the process appears to be critical for the development of neuroinflammatory responses. In contrast, improved cognition using the Morris water maze (MWM) model and increased Aβ level was observed in NLRP3-knockout mice [66]. Primary microglia stimulation in vitro by fibrillar Aβ activates the production of NLRP3 inflammasome, caspase-1, causing the increased secretion of IL-1β in the animal models of Alzheimer’s disease [37]. Additionally, autophagy regulates the Aβ-induced activation of NLRP3 inflammasome via the LRP1/AMPK and the AMPK/mTOR/ULK1 pathway in Aβ-induced BV-2 cells and APP/PS1 mice [67,68]. Impaired autophagic processes in the microglia cause dysregulated Aβ clearance and severe deposition via the PRKAA1 pathway, potentially exacerbating inflammatory responses and NLRP3 inflammasome activation in mice [66]. The intraperitoneal injection of NLRP3 inflammasome inhibitor (JC-124) significantly improves the Aβ load in the brain of mice, suppressing neuroinflammatory responses and thereby producing neuroprotective effects [32]. A small molecule, MCC950, can inhibit NLRP3 inflammasome, repressing IL-1β and IL-18 secretion in mice [69]. The abnormal expression of tau proteins activates NLRP3 inflammasome, causing the subsequent release of IL-1β from the microglia [52,70,71]. NLRP3 inflammasome induces tau protein aggregation and hyperphosphorylation, promoting neuronal degeneration in mice [72,73,74]. Considering the non-negligible role of NLRP3/ASC/caspase-1 axis-mediated inflammation in Alzheimer’s disease, Alzheimer’s disease transgenic mice with selective suppression of NLRP3 inflammasome or caspase-1 expressions in the brain revealed significantly improvement cognitive functions [75]. The total Aβ volume significantly decreases in the hippocampus and cortex in NLRP3 or caspase-1-knockdown mice. Therefore, NLRP3/ASC/caspase-1 signaling pathways are implicated in the neuroinflammatory effects of Alzheimer’s disease [52,76,77,78].
Rats exhibited an improvement in spatial learning when treated with an anti-inflammatory drug (probenecid), which reduced NLRP1 inflammasome activation-mediated IL-1β and IL-18 secretion [38,39]. NLRP3 inflammasome inhibitors (CRID3) reduced tau hyperphosphorylation and aggregation by regulating tau kinase and phosphatase, improving spatial memory deficits in mice with Alzheimer’s symptoms [33,35]. Rats displayed significantly improved spatial memory after treatment with phosphatidylcholine (EPA-PC), inhibiting Aβ-induced toxicity by reducing NLRP3 inflammasome activation and increasing autophagy [79]. Osthole (OST) reduced hippocampal Aβ deposition and improved cognitive dysfunctions in the rat model of Alzheimer’s disease by NLRP3 inflammasome suppression via a PI3K/Akt/GSK-3β signaling pathway [36,80]. NLRP inflammasome is a potential treatment target for the progression of Alzheimer’s disease.
3.2. Parkinson’s Disease
Parkinson’s disease is one of the most common neurodegenerative disorders and the classical motor symptoms include bradykinesia, tremors, rigid movements, and dementia [81]. One of the primary causes of Parkinson’s disease is a gradual loss of dopaminergic (DA) neurons in the substantial nigra pars compacta (SNPC) [82]. Lewy bodies (LB) formation primarily comprising fibrillar alpha-synuclein (a-Syn) is also evident in patients with Parkinson’s disease [83]. Peripheral immune cell infiltrations and the activation of microglia and astrocytes have been reported in patients with Parkinson’s disease. These changes cause neuroinflammation [84,85]. Central and peripheral inflammation occurs in the prodromal stage of Parkinson’s disease, and remains sustained throughout the disease’s progression [81]. The abnormal regulation of a-Syn activates microglia to produce inflammatory factors and damage neurons in mice models of Parkinson’s disease [81]. Production of these cytokines is primarily regulated by the nuclear factor kappa B (NF-kB) and multi-protein inflammasome complexes, including NLRP1 inflammasome, NLRP3 inflammasome, and caspase-1 [86]. A clinical study collected serum samples from 12 untreated patients with Parkinson’s disease (aged 63–78 years) and detected a significantly upregulated expression of NLRP3 inflammasome, caspase-1, and IL-1β levels [87]. In BV2 cells, α-Syn activates NLRP3 inflammasome, followed by the release of caspase-1 and IL-1β. Increased caspase-1 and IL-1β levels cause neuroinflammation, subsequently damaging dopaminergic neurons [88,89,90,91,92,93,94,95,96]. The inhibition of the hepatic Nlrp3 protects dopaminergic neurons by attenuating systemic inflammation in a MPTP/p mouse model of Parkinson’s disease [97]. Based on recent studies, the inflammasome spontaneously assembles in mice and human DA neurons when parkin function is lost, which causes DA neuron death and the symptoms of Parkinson’s disease in the animals [98].
NLRP3 inflammasome-mediated neuroinflammation exerts a significant effect on the pathogenesis of Parkinson’s disease. NLRP3 inflammasome inhibitors promote the survival of DA neurons. NLRP3 inflammasome blockade significantly prevents α-syn-induced microglial activation and IL-1β production, preventing neuronal damage of midbrain DA neurons, ultimately improving the symptoms in patients with Parkinson’s disease [99]. Ellagic acid (EA) harbors profound implications in protecting DA neurons by inhibiting NLRP3 inflammasome activation in the microglia [40]. Safflower flavonoid extract (SAFE) reduced the level of plasma inflammatory factors, inhibiting NLRP3 inflammasome activation in mice, exhibiting significant anti-Parkinson’s disease effects [41]. Naringin (NAR) inhibited microglial NLRP3 inflammasome signaling activation and pro-inflammatory factor release in rats and protected DA neuron viabilities in rats [42]. Echinacoside (ECH) promoted the survival of DA neurons and inhibited microglial-mediated NLRP3/Caspase1/IL-Iβ inflammatory signaling pathway activation in the substantia nigra (SN) in mice [43]. These results highlight the neuroprotective effects of NLRP3 inflammasome inhibitors in the occurrence and progression of Parkinson’s disease [43].
3.3. Huntington’s Disease
Huntington’s disease is an autosomal dominant neurodegenerative disease caused by expansions of triplet repeats encoded by polyglutamine sequences in the N-terminal region of the proteins associated with Huntington’s disease (mHTTs). Moreover, Huntington’s disease is characterized by impaired motor and cognitive functions, brain atrophy, weight loss, and reduced life expectancy [100].
Aggregation of mutant huntingtin culminates in neuronal dysfunction and death in patients with Huntington’s disease. In patients with Huntington’s disease and mice modeling Huntington’s disease, mHTTs were highly expressed in both neurons and microglia, whereas mutated mHTT aggregate to form inclusion bodies. Mutated mHTT expression activates NLRP3 inflammasome, causing neuroinflammatory responses, disrupting brain cell functions, and causing ultimate neuronal dysfunctions or even death [97]. Unlike in healthy individuals, NLRP3 inflammasome levels significantly increase in peripheral blood mononuclear cells (PBMCs) in patients with Huntington’s disease [101]. Galectin (Gal), which includes the carbohydrate recognition structural domain of β-galactosidase, affects the development of comorbid cognitive impairment in patients with Huntington’s disease. Gal3 damaged neurons by improving the secretion of NLRP3 inflammasome and IL-1β in the mice model of Huntington’s disease [97]. R6/2 mice (transgenic mice of Huntington’s disease) with Gal3 gene mutation in the striatum reduced the number of mHTT and inhibited NLRP3 inflammasome activation in the microglia, mitigating the effects of neuronal damage [97,102].
NLRP3 inflammasome inhibition or the use of other immunosuppressive agents reduces the pathophysiological changes in Huntington’s disease. PAP (papaverine) suppresses NLRP3 inflammasome activation by regulating NF-κB and CREB signaling pathways in mice, hence inhibiting microglial activation and neuronal cell death [45]. Tail vein injections of LV3-siNLRP3 in mice suppress hepatic pro-inflammatory cytokine production, down-regulate hepatic NLRP3 protein expression, and inhibit NLRP3 inflammasome activation, subsequently alleviating midbrain DA neuronal damage [103]. MIF (macrophage migration inhibitory factor) reduces the expression of NLRP3 inflammasome and inflammatory factors induced by MPP+ (1-methyl-4-phenylpyridinium) in microglia, reducing DA neuronal damage and exerting protective effects against neuroinflammation induced by Parkinson’s disease [104]. NLRP3 inflammasome inhibition by MCC950 decreases microglial inflammatory vesicle activation in mice, protects DA neurons in the substantia nigra, and suppresses motor dysfunctions in the mice model of Huntington’s disease [94,105]. RRx-001, the NLRP3 inflammasome inhibitors, reduces IL-1β and IL-18 expressions, inducing a decrease in T cell initiation and T cell trafficking to the brain and improving the course of experimental allergic encephalomyelitis (EAE) [46,47].
3.4. Depression
Abnormalities in cytokines and innate immunity receptors, including NLRP inflammasome, have been observed in the postmortem brains of depressed individuals. The protein and mRNA expressions of NLRP1, NLRP3, NLRP6 inflammasome, caspase-3, and ASC are significantly increased in individuals with major depression in the prefrontal cortex [106]. Since proinflammatory cytokine levels in the serum of people with major depression were significantly increased [106], inflammatory abnormalities have been involved in the pathophysiology of depression. NLRP1 inflammasome is significantly activated undergoing chronic stress in the hippocampus of rats [38,107]. NLRP1 inflammasome inhibition attenuates the depression-like behaviors and inhibits the secretion of mature IL-1β in the hippocampus of rats via the PKR/NLRP1 inflammasome pathway [107].
3.5. Drug Use Disorder
Drug use disorder is a chronic brain disorder with devastating consequences for individuals and society [108]. Drugs interact with the neuroimmune system to change neuroimmune gene expression and signaling, resulting in neurotoxicity. Chronic drug exposure causes compulsive drug use behaviors and long-lasting cravings, along with severe cognitive dysfunctions [109].
Neuroinflammation is a major underlying mechanism of methamphetamine (METH)-induced cognitive deficits. Increased levels of hippocampal NLRP1 and NLRP3 inflammasome expression as well as the induction of inflammation and apoptosis were found in 11 patients with METH drug use disorder [110]. METH promotes NLRP inflammasome release, and upregulates caspase-1 expressions, causing the aggregation of apoptosis-associated ASC proteins from rat cortical microglial. These outcomes are followed by the maturation and secretion of IL-Iβ, eventually resulting in neuroinflammation and neurotoxicity [34,111]. METH causes toxic effects in primary rat striatal neurons, cortical neurons, and PC12 cells, resulting in apoptosis and autophagy. The toxic effects of METH were accompanied by a significant increase in NLRP1 inflammasome expression, suppressed by knocking down the NLRP1 inflammasome gene [112]. METH further causes microglia to participate in neuroinflammatory effects by activating NLRP inflammasome [113]. In BV2 cells, NLRP3 inflammasome blockade by MCC950 significantly inhibits METH-induced increases in iNOS (a marker of activated microglia) expression, reducing microglial activation and cytotoxicity [34]. Mice treated with intraperitoneal injections of METH showed increased NLRP3 inflammasome levels and caspase-1 activation in the hippocampal brain regions [114]. NLRP1 inflammasome and downstream NLRP1/Caspase-1/GSDMD signaling pathways have important roles in the METH-induced cognitive function in rats. Using the new object recognition test, METH induced significant cognitive impairment and increased activity of NLRP1, cleaved-Caspase-11, IL-1β and TNF-α in the rat hippocampus. These phenomena were attenuated by aspirin-triggered-lipoxin A4 (ATL), a potent anti-inflammatory mediator [115]. In various cells, including cardiomyocytes, microglia, and neurons, METH induces apoptosis and pyroptosis through the NLRP-Caspase1-GSDMD pathway [116]. Therefore, METH exposures increased NLRP1 and NLRP3 inflammasome levels as well as neuroinflammation responses in the brain, followed by neurotoxicity and substantial damage to the brain.
Cocaine use disorder has been demonstrated to increase the level of oxidative stress and induce neuroinflammation which produces detrimental effects on the central nervous system in cocaine-disorder co-occurring AIDS patients [117]. Cocaine exposure was associated with the increased expression of various pro-inflammatory cytokines, NLRP1 inflammasome as well as adhesion molecules [118]. The expression of NLRP3 inflammasome in the cortical brain tissues of cocaine-dependent patients was significantly higher than that of the control participants [119]. Microglial activation mediated by cocaine is suggested to be involved with both ROS and NLRP3 inflammasome [120]. Studies using an NLRP3 blocker (MCC950) and siNLRP3 have also demonstrated the essential role of NLRP3 inflammasome in cocaine-mediated activation of inflammasomes and microglial activation in mice in both the striatum and the cortical regions [119]. NLRP3 inflammasome is a potential therapeutic target for relieving cocaine-mediated neuroinflammation.
Excessive ethanol consumption causes neurotoxicity via oxidative stress, inflammation, and cell death of the brain tissues in male C57BL/6 mice [121]. NLRP inflammasome is also involved in the neurotoxicity of alcohol [122]. Alcohol exposure significantly upregulates the expression of NLRP3 inflammasome and caspase-1. Alcohol increases caspase-1 and IL-1β expression in the central nervous system of wild-type mice, but not in NLRP3 or ASC knockout mice. Caspase-1 is subsequently activated, causing mitochondrial dysfunction in mice [123,124]. Previous studies have shown the role of NLRP3 inflammasome in alcohol-induced astrocyte inflammation and associated its activation with the production of ROS. This suggests that the activation of NLRP3 inflammatory in astrocytes is caused by alcohol exposure, eventually exacerbating the accumulation of mitochondrial ROS and the occurrence of cell death [125,126,127]. This suggests a critical role for NLRP3 inflammasome in regulating the effects of alcohol-induced neuroinflammation and neurotoxicity [128]. Calcium overload by alcohol has been shown to promote NLRP1, and NLRP3 inflammasome formation in rat cortical neurons and human neuroblastoma cells via the CaMKII/JNK1 pathway [129,130]. ROCK2 inhibition decreases the expression of NLRP3 inflammasome in astrocytes and attenuates alcohol-induced neuronal damage. This shows that ROCK2 downregulation suppresses the activation of NLRP3 inflammatory bodies [131]. Disulfiram (DSF), by reducing oxidative stress and blocking NLRP3 inflammasome, is an FDA-approved drug to treat chronic alcohol-use disorder. Additional studies indicate that DSF treatment alleviates the function of the lowered left heart and the apoptosis of myocardial cells by suppressing the activation of NLRP3 inflammasome in mice [48]. Considering the abstinence and cardiac protection impacts of DSF via the inhibition of NLRP inflammasome, the clinical application of NLRP inflammasome inhibitors represents a novel approach to protecting and treating disease-induced inflammation.
Significant NLRP3 inflammasome activation was observed in the prefrontal cortex and peripheral blood of morphine-treated mice [132]. Repeated exposures to morphine in wild-type mice increased the level of inflammation-related signals including NLRP3 inflammasome via the TLR4/NF-κB/NLRP3 pathway [133]. Procyanidins inhibit the morphine-induced activation of NLRP3 inflammasome and inflammatory responses in the microglia [134]. The different neuropsychiatric disorders and NLRP inflammasome inhibitors are summarized in Table 2.
Table 2.
Different neuropsychiatric disorders and NLRP inflammasome inhibitors.
| Diseases | Drugs | Pathways | Pathological Changes | Performance | References | |
|---|---|---|---|---|---|---|
| Alzheimer’s disease | Rg1, JC-124, CR2D3, EPA-PC, OST, Probenecid | K+/Ca2+-NLRP-Caspase1-IL-1β | Aβ deposition, tau protein entanglement. | Loss of memory function and cognitive decline. | [51,53,56,63,65] | |
| Parkinson’s disease | EA, SAFE, NAR, ECH, Laquinimod | a-Syn-NLRP-Caspase1 | Decrease in dopaminergic neurons and formation of Lewy bodies. | The Campaign Triad. | [83,89,95,97,100] | |
| Huntington’s disease | PAP, MCC950 | Gal3-NLRP3-IL-1β | Inclusion body formation, brain atrophy. | Motor, cognitive impairment. | [101,102,104,105] | |
| Depression | caspase3-NLRP-IL-1β | Inflammatory lesion. | Persistent depression and loss of interest. | [109,110] | ||
| Drug use disorder |
METH | MCC950 | NLRP-Caspase-1-IL-1β | ASC protein aggregation and increased autophagosomes. | Cognitive dysfunction, schizophrenia. | [114,115,117,119] |
| Cocaine | MCC950 | ROS/NLRP | Oxidative stress and neuroinflammation. | Nervous and mental injury. | [120,123] | |
| Alcohol | Cod liver oil, VX765 | CaMKII/JNK1-NLRP1 | Lysosomal and mitochondrial damage. | Central nervous system lesions, behavioral disorders. | [125,127] | |
4. Outlook
Many studies have demonstrated the basis for the identification of novel therapeutic approaches for neuropsychiatric disorders. Several studies presented in this review highlighted the importance of regulating NLRP inflammasome to delay the progression and outcomes of neuroinflammation in neuropsychiatric disorders. Clinical trials have also investigated the therapeutic effects of NLRP inflammasome inhibitors on alcohol-use disorder [135,136]. More clinical trials would be critically needed, in order to provide more solid evidence about the potential benefits of NLRP inhibitors. Furthermore, it appears that different psychiatric disorders share a common mechanism, neuroinflammation. The relationship between NLRP inflammasome and other neuropsychiatric disorders, including schizophrenia, autism, and obsessive-compulsive disorder, remains largely unknown. NLRP inflammasome inhibitors may potentially have a common therapeutic effect on different neuropsychiatric disorders.
Author Contributions
Conceptualization, Y.S. and L.Q.; resources, H.L.; writing—original draft preparation, Y.S. and L.Q.; writing—review and editing, Y.S., R.F., Z.S. and L.Q.; supervision, Y.S., X.L., Y.R., Y.C. and L.L.; project administration, Y.S., X.L. and H.L.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81971247), Zhejiang Provincial Key R & D Plan 2019 (2020C03064), Science and Technology Plan of Ningbo (2022S075), “One-health” Plan of Ningbo University, National Social Science Foundation Key Programs (18ZDA215).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Amor S., Peferoen L.A.N., Vogel D.Y.S., Breur M., van der Valk P., Baker D., van Noort J.M. Inflammation in neurodegenerative diseases—An update. Immunology. 2014;142:151–166. doi: 10.1111/imm.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephenson J., Nutma E., van der Valk P., Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154:204–219. doi: 10.1111/imm.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan M., Jiang Z., Bi G., Nomura K., Liu M., Wang Y., Cai B., Zhou J.-M., He S.Y., Xin X.-F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature. 2021;592:105–109. doi: 10.1038/s41586-021-03316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toldo S., Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat. Rev. Cardiol. 2018;15:203–214. doi: 10.1038/nrcardio.2017.161. [DOI] [PubMed] [Google Scholar]
- 5.Sok S.P.M., Ori D., Wada A., Okude H., Kawasaki T., Momota M., Nagoor N.H., Kawai T. 1′-Acetoxychavicol acetate inhibits NLRP3-dependent inflammasome activation via mitochondrial ROS suppression. Int. Immunol. 2021;33:373–386. doi: 10.1093/intimm/dxab016. [DOI] [PubMed] [Google Scholar]
- 6.Walsh J.G., Muruve D.A., Power C. Inflammasomes in the CNS. Nat. Rev. Neurosci. 2014;15:84–97. doi: 10.1038/nrn3638. [DOI] [PubMed] [Google Scholar]
- 7.Heneka M.T., McManus R.M., Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2018;19:610–621. doi: 10.1038/s41583-018-0055-7. [DOI] [PubMed] [Google Scholar]
- 8.Moloudizargari M., Moradkhani F., Asghari N., Fallah M., Asghari M.H., Moghadamnia A.A., Abdollahi M. NLRP inflammasome as a key role player in the pathogenesis of environmental toxicants. Life Sci. 2019;231:116585. doi: 10.1016/j.lfs.2019.116585. [DOI] [PubMed] [Google Scholar]
- 9.Kepp O., Galluzzi L., Kroemer G. Mitochondrial control of the NLRP3 inflammasome. Nat. Immunol. 2011;12:199–200. doi: 10.1038/ni0311-199. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J., Hu Z., Han X., Wang D., Jiang Q., Ding J., Xiao M., Wang C., Lu M., Hu G. Dopamine D2 receptor restricts astrocytic NLRP3 inflammasome activation via enhancing the interaction of β-arrestin2 and NLRP3. Cell Death Differ. 2018;25:2037–2049. doi: 10.1038/s41418-018-0127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Rivero Vaccari J.P., Dietrich W.D., Keane R.W. Activation and regulation of cellular inflammasomes: Gaps in our knowledge for central nervous system injury. J. Cereb. Blood Flow Metab. 2014;34:369–375. doi: 10.1038/jcbfm.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanson K.V., Deng M., Ting J.P.Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaron J.R., Gangaraju S., Rao M.Y., Kong X., Zhang L., Su F., Tian Y., Glenn H.L., Meldrum D.R. K(+) regulates Ca(2+) to drive inflammasome signaling: Dynamic visualization of ion flux in live cells. Cell Death Dis. 2015;6:e1954. doi: 10.1038/cddis.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Dinarello C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 16.Sims J.E., Smith D.E. The IL-1 family: Regulators of immunity. Nat. Rev. Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 17.Ulrich C., Kneser L., Fiedler R., Beckert J., Wildgrube S., Seibert E., Fick S., Schafer C., Markau S., Trojanowicz B., et al. Pyroptosis: A Common Feature of Immune Cells of Haemodialysis Patients. Toxins. 2021;13:839. doi: 10.3390/toxins13120839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burdette B.E., Esparza A.N., Zhu H., Wang S. Gasdermin D in pyroptosis. Acta Pharm. Sin. B. 2021;11:2768–2782. doi: 10.1016/j.apsb.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenzie B.A., Dixit V.M., Power C. Fiery Cell Death: Pyroptosis in the Central Nervous System. Trends Neurosci. 2020;43:55–73. doi: 10.1016/j.tins.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Hohsfield L.A., Najafi A.R., Ghorbanian Y., Soni N., Hingco E.E., Kim S.J., Jue A.D., Swarup V., Inlay M.A., Green K.N. Effects of long-term and brain-wide colonization of peripheral bone marrow-derived myeloid cells in the CNS. J. Neuroinflamm. 2020;17:279. doi: 10.1186/s12974-020-01931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothhammer V., Borucki D.M., Tjon E.C., Takenaka M.C., Chao C.-C., Ardura-Fabregat A., de Lima K.A., Gutiérrez-Vázquez C., Hewson P., Staszewski O., et al. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557:724–728. doi: 10.1038/s41586-018-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su S.-H., Wu Y.-F., Lin Q., Wang D.-P., Hai J. URB597 protects against NLRP3 inflammasome activation by inhibiting autophagy dysfunction in a rat model of chronic cerebral hypoperfusion. J. Neuroinflamm. 2019;16:260. doi: 10.1186/s12974-019-1668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pal P.B., Sonowal H., Shukla K., Srivastava S.K., Ramana K.V. Aldose Reductase Mediates NLRP3 Inflammasome-Initiated Innate Immune Response in Hyperglycemia-Induced Thp1 Monocytes and Male Mice. Endocrinology. 2017;158:3661–3675. doi: 10.1210/en.2017-00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pétrilli V., Papin S., Dostert C., Mayor A., Martinon F., Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 25.Ktistakis N.T., Tooze S.A. Digesting the Expanding Mechanisms of Autophagy. Trends Cell Biol. 2016;26:624–635. doi: 10.1016/j.tcb.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Takahama M., Akira S., Saitoh T. Autophagy limits activation of the inflammasomes. Immunol. Rev. 2018;281:62–73. doi: 10.1111/imr.12613. [DOI] [PubMed] [Google Scholar]
- 27.Man S.M., Kanneganti T.-D. Regulation of lysosomal dynamics and autophagy by CTSB/cathepsin B. Autophagy. 2016;12:2504–2505. doi: 10.1080/15548627.2016.1239679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han X., Sun S., Sun Y., Song Q., Zhu J., Song N., Chen M., Sun T., Xia M., Ding J., et al. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: Implications for Parkinson disease. Autophagy. 2019;15:1860–1881. doi: 10.1080/15548627.2019.1596481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y., Zhou D., Ren Y., Zhang Z., Guo X., Ma M., Xue Z., Lv J., Liu H., Xi Q., et al. Mir223 restrains autophagy and promotes CNS inflammation by targeting ATG16L1. Autophagy. 2019;15:478–492. doi: 10.1080/15548627.2018.1522467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Hu W., Zhang B., Yin Y., Zhang J., Huang D., Huang R., Li W., Li W. Ginsenoside Rg1 protects against neuronal degeneration induced by chronic dexamethasone treatment by inhibiting NLRP-1 inflammasomes in mice. Int. J. Mol. Med. 2017;40:1134–1142. doi: 10.3892/ijmm.2017.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H., Su Y., Sun Z., Chen M., Han Y., Li Y., Dong X., Ding S., Fang Z., Li W., et al. Ginsenoside Rg1 alleviates Aβ deposition by inhibiting NADPH oxidase 2 activation in APP/PS1 mice. J. Ginseng Res. 2021;45:665–675. doi: 10.1016/j.jgr.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin J., Zhao F., Chojnacki J.E., Fulp J., Klein W.L., Zhang S., Zhu X. NLRP3 Inflammasome Inhibitor Ameliorates Amyloid Pathology in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018;55:1977–1987. doi: 10.1007/s12035-017-0467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atluri V.S.R., Tiwari S., Rodriguez M., Kaushik A., Yndart A., Kolishetti N., Yatham M., Nair M. Inhibition of Amyloid-Beta Production, Associated Neuroinflammation, and Histone Deacetylase 2-Mediated Epigenetic Modifications Prevent Neuropathology in Alzheimer’s Disease Model. Front. Aging Neurosci. 2019;11:342. doi: 10.3389/fnagi.2019.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu E., Liu J., Liu H., Wang X., Xiong H. Inflammasome Activation by Methamphetamine Potentiates Lipopolysaccharide Stimulation of IL-1β Production in Microglia. J. Neuroimmune Pharmacol. 2018;13:237–253. doi: 10.1007/s11481-018-9780-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurent C., Dorothee G., Hunot S., Martin E., Monnet Y., Duchamp M., Dong Y., Legeron F.P., Leboucher A., Burnouf S., et al. Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain. 2017;140:184–200. doi: 10.1093/brain/aww270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y., Chen X., Gong Q., Shi J., Li F. Osthole improves cognitive function of vascular dementia rats: Reducing Aβ deposition via inhibition NLRP3 inflammasome. Biol. Pharm. Bull. 2020;43:1315–1323. doi: 10.1248/bpb.b20-00112. [DOI] [PubMed] [Google Scholar]
- 37.Shao Q.H., Zhang X.L., Yang P.F., Yuan Y.H., Chen N.H. Amyloidogenic proteins associated with neurodegenerative diseases activate the NLRP3 inflammasome. Int. Immunopharmacol. 2017;49:155–160. doi: 10.1016/j.intimp.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 38.Singhal G., Jaehne E.J., Corrigan F., Toben C., Baune B.T. Inflammasomes in neuroinflammation and changes in brain function: A focused review. Front. Neurosci. 2014;8:315. doi: 10.3389/fnins.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mawhinney L.J., de Rivero Vaccari J.P., Dale G.A., Keane R.W., Bramlett H.M. Heightened inflammasome activation is linked to age-related cognitive impairment in Fischer 344 rats. BMC Neurosci. 2011;12:123. doi: 10.1186/1471-2202-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He X.M., Zhou Y.Z., Sheng S., Li J.J., Wang G.Q., Zhang F. Ellagic Acid Protects Dopamine Neurons via Inhibition of NLRP3 Inflammasome Activation in Microglia. Oxid. Med. Cell Longev. 2020;2020:2963540. doi: 10.1155/2020/2963540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lei H., Ren R., Sun Y., Zhang K., Zhao X., Ablat N., Pu X. Neuroprotective Effects of Safflower Flavonoid Extract in 6-Hydroxydopamine-Induced Model of Parkinson’s Disease May Be Related to its Anti-Inflammatory Action. Molecules. 2020;25:5206. doi: 10.3390/molecules25215206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C., Wei Y.Z., He X.M., Li D.D., Wang G.Q., Li J.J., Zhang F. Naringenin Produces Neuroprotection Against LPS-Induced Dopamine Neurotoxicity via the Inhibition of Microglial NLRP3 Inflammasome Activation. Front. Immunol. 2019;10:936. doi: 10.3389/fimmu.2019.00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao M., Wang M., Jia Y., Tian D., Liu A., Wang W., Yang L., Chen J., Yang Q., Liu R., et al. Echinacoside protects dopaminergic neurons by inhibiting NLRP3/Caspase-1/IL-1β signaling pathway in MPTP-induced Parkinson’s disease model. Brain Res. Bull. 2020;164:55–64. doi: 10.1016/j.brainresbull.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 44.Comi G., Jeffery D., Kappos L., Montalban X., Boyko A., Rocca M.A., Filippi M. Placebo-controlled trial of oral laquinimod for multiple sclerosis. N. Engl. J. Med. 2012;366:1000–1009. doi: 10.1056/NEJMoa1104318. [DOI] [PubMed] [Google Scholar]
- 45.Leem Y.-H., Park J.-S., Park J.-E., Kim D.-Y., Kim H.-S. Papaverine Exerts Neuroprotective Effect by Inhibiting NLRP3 Inflammasome Activation in an MPTP-Induced Microglial Priming Mouse Model Challenged with LPS. Biomol. Ther. 2021;29:295. doi: 10.4062/biomolther.2021.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inoue M., Chen P.-H., Siecinski S., Li Q.-J., Liu C., Steinman L., Gregory S.G., Benner E., Shinohara M.L. An interferon-β-resistant and NLRP3 inflammasome-independent subtype of EAE with neuronal damage. Nat. Neurosci. 2016;19:1599–1609. doi: 10.1038/nn.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y., He H., Lin B., Chen Y., Deng X., Jiang W., Zhou R. RRx-001 ameliorates inflammatory diseases by acting as a potent covalent NLRP3 inhibitor. Cell Mol. Immunol. 2021;18:1425–1436. doi: 10.1038/s41423-021-00683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei S., Xiao Z., Huang J., Peng Z., Zhang B., Li W. Disulfiram inhibits oxidative stress and NLRP3 inflammasome activation to prevent LPS-induced cardiac injury. Int. Immunopharmacol. 2022;105:108545. doi: 10.1016/j.intimp.2022.108545. [DOI] [PubMed] [Google Scholar]
- 49.Larose T.L., Chen Y., Camargo C.A., Langhammer A., Romundstad P., Mai X.-M. Factors associated with vitamin D deficiency in a Norwegian population: The HUNT Study. J. Epidemiol. Community Health. 2014;68:165–170. doi: 10.1136/jech-2013-202587. [DOI] [PubMed] [Google Scholar]
- 50.Weiner H.L., Frenkel D. Immunology and immunotherapy of Alzheimer’s disease. Nat. Rev. Immunol. 2006;6:404–416. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- 51.Meyer-Luehmann M., Spires-Jones T.L., Prada C., Garcia-Alloza M., de Calignon A., Rozkalne A., Koenigsknecht-Talboo J., Holtzman D.M., Bacskai B.J., Hyman B.T. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halle A., Hornung V., Petzold G.C., Stewart C.R., Monks B.G., Reinheckel T., Fitzgerald K.A., Latz E., Moore K.J., Golenbock D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanchez-Mejias E., Navarro V., Jimenez S., Sanchez-Mico M., Sanchez-Varo R., Nunez-Diaz C., Trujillo-Estrada L., Davila J.C., Vizuete M., Gutierrez A., et al. Soluble phospho-tau from Alzheimer’s disease hippocampus drives microglial degeneration. Acta Neuropathol. 2016;132:897–916. doi: 10.1007/s00401-016-1630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prinz M., Priller J., Sisodia S.S., Ransohoff R.M. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 2011;14:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 55.Ising C., Heneka M.T. Functional and structural damage of neurons by innate immune mechanisms during neurodegeneration. Cell Death Dis. 2018;9:120. doi: 10.1038/s41419-017-0153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lucin K.M., Wyss-Coray T. Immune Activation in Brain Aging and Neurodegeneration: Too Much or Too Little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ismael S., Sakata K., McDonald M.P., Liao F.-F., Ishrat T. ER stress associated TXNIP-NLRP3 inflammasome activation in hippocampus of human Alzheimer’s disease. Neurochem. Int. 2021;148:105104. doi: 10.1016/j.neuint.2021.105104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Z., Li X., Yang L., Dong X., Han Y., Li Y., Luo J., Li W. SOCE-mediated NFAT1-NOX2-NLRP1 inflammasome involves in lipopolysaccharide-induced neuronal damage and Aβ generation. Mol. Neurobiol. 2022;59:3183–3205. doi: 10.1007/s12035-021-02717-y. [DOI] [PubMed] [Google Scholar]
- 59.Sims R., van der Lee S.J., Naj A.C., Bellenguez C., Badarinarayan N., Jakobsdottir J., Kunkle B.W., Boland A., Raybould R., Bis J.C., et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat. Genet. 2017;49:1373–1384. doi: 10.1038/ng.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Griciuc A., Serrano-Pozo A., Parrado A.R., Lesinski A.N., Asselin C.N., Mullin K., Hooli B., Choi S.H., Hyman B.T., Tanzi R.E. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang T., Zhang L., Shang Y., Zhu Z., Jin S., Guo Z., Wang X. Concurrent suppression of Aβ aggregation and NLRP3 inflammasome activation for treating Alzheimer’s disease. Chem. Sci. 2022;13:2971–2980. doi: 10.1039/D1SC06071F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaushal V., Dye R., Pakavathkumar P., Foveau B., Flores J., Hyman B., Ghetti B., Koller B.H., LeBlanc A.C. Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ. 2015;22:1676–1686. doi: 10.1038/cdd.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun L., Chen Y., Shen X., Xu T., Yin Y., Zhang H., Ding S., Zhao Y., Zhang Y., Guan Y., et al. Inhibition of NOX2-NLRP1 signaling pathway protects against chronic glucocorticoids exposure-induced hippocampal neuronal damage. Int. Immunopharmacol. 2019;74:105721. doi: 10.1016/j.intimp.2019.105721. [DOI] [PubMed] [Google Scholar]
- 64.Jia J., Zhang X., Xu G., Zeng X., Li L. Thioredoxin-1 inhibits amyloid-β-induced activation of NLRP1/caspase-1/GSDMD pyroptotic pathway in PC12 cells. Mol. Biol. Rep. 2022;49:3445–3452. doi: 10.1007/s11033-022-07177-8. [DOI] [PubMed] [Google Scholar]
- 65.Zhang X., Wang R., Hu D., Sun X., Fujioka H., Lundberg K., Chan E.R., Wang Q., Xu R., Flanagan M.E., et al. Oligodendroglial glycolytic stress triggers inflammasome activation and neuropathology in Alzheimer’s disease. Sci. Adv. 2020;6:eabb8680. doi: 10.1126/sciadv.abb8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou W., Xiao D., Zhao Y., Tan B., Long Z., Yu L., He G. Enhanced Autolysosomal Function Ameliorates the Inflammatory Response Mediated by the NLRP3 Inflammasome in Alzheimer’s Disease. Front. Aging Neurosci. 2021;13:629891. doi: 10.3389/fnagi.2021.629891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiu W.-Q., Pan R., Tang Y., Zhou X.-G., Wu J.-M., Yu L., Law B.Y.-K., Ai W., Yu C.-L., Qin D.-L., et al. Lychee seed polyphenol inhibits Aβ-induced activation of NLRP3 inflammasome via the LRP1/AMPK mediated autophagy induction. Biomed. Pharmacother. 2020;130:110575. doi: 10.1016/j.biopha.2020.110575. [DOI] [PubMed] [Google Scholar]
- 68.Xiong R., Zhou X.-G., Tang Y., Wu J.-M., Sun Y.-S., Teng J.-F., Pan R., Law B.Y.-K., Zhao Y., Qiu W.-Q., et al. Lychee seed polyphenol protects the blood-brain barrier through inhibiting Aβ(25-35)-induced NLRP3 inflammasome activation via the AMPK/mTOR/ULK1-mediated autophagy in bEnd.3 cells and APP/PS1 mice. Phytother. Res. 2021;35:954–973. doi: 10.1002/ptr.6849. [DOI] [PubMed] [Google Scholar]
- 69.Bakhshi S., Shamsi S. MCC950 in the treatment of NLRP3-mediated inflammatory diseases: Latest evidence and therapeutic outcomes. Int. Immunopharmacol. 2022;106:108595. doi: 10.1016/j.intimp.2022.108595. [DOI] [PubMed] [Google Scholar]
- 70.Shafiei S.S., Guerrero-Munoz M.J., Castillo-Carranza D.L. Tau Oligomers: Cytotoxicity, Propagation, and Mitochondrial Damage. Front. Aging Neurosci. 2017;9:83. doi: 10.3389/fnagi.2017.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Usenovic M., Niroomand S., Drolet R.E., Yao L., Gaspar R.C., Hatcher N.G., Schachter J., Renger J.J., Parmentier-Batteur S. Internalized Tau Oligomers Cause Neurodegeneration by Inducing Accumulation of Pathogenic Tau in Human Neurons Derived from Induced Pluripotent Stem Cells. J. Neurosci. 2015;35:14234–14250. doi: 10.1523/JNEUROSCI.1523-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhaskar K., Konerth M., Kokiko-Cochran O.N., Cardona A., Ransohoff R.M., Lamb B.T. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68:19–31. doi: 10.1016/j.neuron.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sheedy F.J., Grebe A., Rayner K.J., Kalantari P., Ramkhelawon B., Carpenter S.B., Becker C.E., Ediriweera H.N., Mullick A.E., Golenbock D.T., et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ising C., Venegas C., Zhang S., Scheiblich H., Schmidt S.V., Vieira-Saecker A., Schwartz S., Albasset S., McManus R.M., Tejera D., et al. NLRP3 inflammasome activation drives tau pathology. Nature. 2019;575:669–673. doi: 10.1038/s41586-019-1769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tan M.S., Tan L., Jiang T., Zhu X.C., Wang H.F., Jia C.D., Yu J.T. Amyloid-beta induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis. 2014;5:e1382. doi: 10.1038/cddis.2014.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walker J.M., Fowler S.W., Miller D.K., Sun A.Y., Weisman G.A., Wood W.G., Sun G.Y., Simonyi A., Schachtman T.R. Spatial learning and memory impairment and increased locomotion in a transgenic amyloid precursor protein mouse model of Alzheimer’s disease. Behav. Brain Res. 2011;222:169–175. doi: 10.1016/j.bbr.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 77.Kanneganti T.-D., Özören N., Body-Malapel M., Amer A., Park J.-H., Franchi L., Whitfield J., Barchet W., Colonna M., Vandenabeele P., et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 78.Li P., Allen H., Banerjee S., Franklin S., Herzog L., Johnston C., Seshadri T. Mice Deficient in IL-lp-Converting Enzyme Are Defective in Production of Mature IL-lp and Resistant to Endotoxic Shock. Cell Death Dis. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 79.Wen M., Ding L., Zhang L., Zhang T., Teruyoshi Y., Wang Y., Xue C. Eicosapentaenoic Acid-Enriched Phosphatidylcholine Mitigated Abeta1-42-Induced Neurotoxicity via Autophagy-Inflammasome Pathway. J. Agric. Food Chem. 2019;67:13767–13774. doi: 10.1021/acs.jafc.9b05947. [DOI] [PubMed] [Google Scholar]
- 80.Yao Y., Wang Y., Kong L., Chen Y., Yang J. Osthole decreases tau protein phosphorylation via PI3K/AKT/GSK-3β signaling pathway in Alzheimer’s disease. Life Sci. 2019;217:16–24. doi: 10.1016/j.lfs.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 81.Kip E., Parr-Brownlie L. Reducing neuroinflammation via therapeutic compounds and lifestyle to prevent or delay progression of Parkinson’s disease.Prevention of neuroinflammation in Parkinson’s disease. Ageing Res. Rev. 2022;78:101618. doi: 10.1016/j.arr.2022.101618. [DOI] [PubMed] [Google Scholar]
- 82.Massano J., Bhatia K.P. Clinical approach to Parkinson’s disease: Features, diagnosis, and principles of management. Cold Spring Harb. Perspect Med. 2012;2:a008870. doi: 10.1101/cshperspect.a008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Faustini G., Longhena F., Bruno A., Bono F., Grigoletto J., La Via L., Barbon A., Casiraghi A., Straniero V., Valoti E., et al. Alpha-synuclein/synapsin III pathological interplay boosts the motor response to methylphenidate. Neurobiol. Dis. 2020;138:104789. doi: 10.1016/j.nbd.2020.104789. [DOI] [PubMed] [Google Scholar]
- 84.Gasser T. Molecular pathogenesis of Parkinson disease: Insights from genetic studies. Expert Rev. Mol. Med. 2009;11:e22. doi: 10.1017/S1462399409001148. [DOI] [PubMed] [Google Scholar]
- 85.Schapira A.H., Bezard E., Brotchie J., Calon F., Collingridge G.L., Ferger B., Hengerer B., Hirsch E., Jenner P., Le Novere N., et al. Novel pharmacological targets for the treatment of Parkinson’s disease. Nat. Rev. Drug Discov. 2006;5:845–854. doi: 10.1038/nrd2087. [DOI] [PubMed] [Google Scholar]
- 86.Kicik A., Tuzun E., Erdogdu E., Bilgic B., Tufekcioglu Z., Ozturk-Isik E., Hanagasi H., Gurvit H. Neuroinflammation Mediators are Reduced in Sera of Parkinson’s Disease Patients with Mild Cognitive Impairment. Noro Psikiyatr. Ars. 2020;57:15–17. doi: 10.29399/npa.23624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cuttitta G., Cibella F., Bellia V., Grassi V., Cossi S., Bucchieri S., Bonsignore G. Changes in FVC During Methacholine-Induced Bronchoconstriction in Elderly Patients With Asthma: Bronchial Hyperresponsiveness and Aging. Chest. 2001;119:1685–1690. doi: 10.1378/chest.119.6.1685. [DOI] [PubMed] [Google Scholar]
- 88.Devine M.J., Gwinn K., Singleton A., Hardy J. Parkinson’s disease and alpha-synuclein expression. Mov. Disord. 2011;26:2160–2168. doi: 10.1002/mds.23948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang W., Wang T., Pei Z., Miller D.S., Wu X., Block M.L., Wilson B., Zhang W., Zhou Y., Hong J.S., et al. Aggregated alpha-synuclein activates microglia: A process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 90.Zhou Y., Lu M., Du R.H., Qiao C., Jiang C.Y., Zhang K.Z., Ding J.H., Hu G. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol. Neurodegener. 2016;11:28. doi: 10.1186/s13024-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo H., Callaway J.B., Ting J.P.Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Panicker N., Sarkar S., Harischandra D.S., Neal M., Kam T.I., Jin H., Saminathan H., Langley M., Charli A., Samidurai M., et al. Fyn kinase regulates misfolded alpha-synuclein uptake and NLRP3 inflammasome activation in microglia. J. Exp. Med. 2019;216:1411–1430. doi: 10.1084/jem.20182191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Codolo G., Plotegher N., Pozzobon T., Brucale M., Tessari I., Bubacco L., de Bernard M. Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PLoS ONE. 2013;8:e55375. doi: 10.1371/journal.pone.0055375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gordon R., Albornoz E.A., Christie D.C., Langley M.R., Kumar V., Mantovani S., Woodruff T.M. Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 2018;10:eaah4066. doi: 10.1126/scitranslmed.aah4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Litteljohn D., Mangano E., Clarke M., Bobyn J., Moloney K., Hayley S. Inflammatory Mechanisms of Neurodegeneration in Toxin-Based Models of Parkinson’s Disease. Parkinson’s Dis. 2011;2011:1–18. doi: 10.4061/2011/713517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferrari C.C., Pott Godoy M.C., Tarelli R., Chertoff M., Depino A.M., Pitossi F.J. Progressive neurodegeneration and motor disabilities induced by chronic expression of IL-1beta in the substantia nigra. Neurobiol. Dis. 2006;24:183–193. doi: 10.1016/j.nbd.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 97.Siew J., Chen H., Chen H., Chen H., Chen C., Soong B., Wu Y., Chang C., Chan Y., Lin C., et al. Galectin-3 is required for the microglia-mediated brain inflammation in a model of Huntington’s disease. Nat. Commun. 2019;10:3473. doi: 10.1038/s41467-019-11441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Panicker N., Kam T.-I., Wang H., Neifert S., Chou S.-C., Kumar M., Brahmachari S., Jhaldiyal A., Hinkle J.T., Akkentli F., et al. Neuronal NLRP3 is a parkin substrate that drives neurodegeneration in Parkinson’s disease. Neuron. 2022;110:2422–2437.e9. doi: 10.1016/j.neuron.2022.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Piancone F., Saresella M., La Rosa F., Marventano I., Meloni M., Navarro J., Clerici M. Inflammatory Responses to Monomeric and Aggregated alpha-Synuclein in Peripheral Blood of Parkinson Disease Patients. Front. Neurosci. 2021;15:639646. doi: 10.3389/fnins.2021.639646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.MacDonald M.E., Ambrose C.M., Duyao M.P., Myers R.H., Lin C., Srinidhi L., Harper P.S. A Novel Gene Containing a Trinucleotide Repeat That Is Expanded and Unstable on Huntington’s Disease Chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-E. [DOI] [PubMed] [Google Scholar]
- 101.Glinsky G.V. SNP-guided microRNA maps (MirMaps) of 16 common human disorders identify a clinically accessible therapy reversing transcriptional aberrations of nuclear import and inflammasome pathways. Cell Cycle. 2008;7:3564–3576. doi: 10.4161/cc.7.22.7073. [DOI] [PubMed] [Google Scholar]
- 102.Chen Y.-J., Wang S.-F., Weng I.C., Hong M.-H., Lo T.-H., Jan J.-T., Hsu L.-C., Chen H.-Y., Liu F.-T. Galectin-3 Enhances Avian H5N1 Influenza A Virus–Induced Pulmonary Inflammation by Promoting NLRP3 Inflammasome Activation. Am. J. Pathol. 2018;188:1031–1042. doi: 10.1016/j.ajpath.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 103.Qiao C., Zhang Q., Jiang Q., Zhang T., Chen M., Fan Y., Ding J., Lu M., Hu G. Inhibition of the hepatic Nlrp3 protects dopaminergic neurons via attenuating systemic inflammation in a MPTP/p mouse model of Parkinson’s disease. J. Neuroinflamm. 2018;15:193. doi: 10.1186/s12974-018-1236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang H., Gao Y., Nie K., Wang L. Macrophage migration inhibitory factor meditates MPP+/MPTP-induced NLRP3 inflammasome activation in microglia cells. Nan Fang Yi Ke Da Xue Xue Bao = J. South. Med. Univ. 2021;41:972–979. doi: 10.12122/j.issn.1673-4254.2021.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marcellino D., Suárez-Boomgaard D., Sánchez-Reina M.D., Aguirre J.A., Yoshitake T., Yoshitake S., Hagman B., Kehr J., Agnati L.F., Fuxe K., et al. On the role of P2X7 receptors in dopamine nerve cell degeneration in a rat model of Parkinson’s disease: Studies with the P2X7 receptor antagonist A-438079. J. Neural Transm. 2010;117:681–687. doi: 10.1007/s00702-010-0400-0. [DOI] [PubMed] [Google Scholar]
- 106.Pandey G.N., Zhang H., Sharma A., Ren X. Innate immunity receptors in depression and suicide: Upregulated NOD-like receptors containing pyrin (NLRPs) and hyperactive inflammasomes in the postmortem brains of people who were depressed and died by suicide. J. Psychiatry Neurosci. 2021;46:E538–E547. doi: 10.1503/jpn.210016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Q., Liu S., Zhu X., Mi W., Maoying Q., Wang J., Yu J., Wang Y. Hippocampal PKR/NLRP1 Inflammasome Pathway Is Required for the Depression-Like Behaviors in Rats with Neuropathic Pain. Neuroscience. 2019;412:16–28. doi: 10.1016/j.neuroscience.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 108.Volkow N.D., Boyle M. Neuroscience of Addiction: Relevance to Prevention and Treatment. Am. J. Psychiatry. 2018;175:729–740. doi: 10.1176/appi.ajp.2018.17101174. [DOI] [PubMed] [Google Scholar]
- 109.Northrop N.A., Halpin L.E., Yamamoto B.K. Peripheral ammonia and blood brain barrier structure and function after methamphetamine. Neuropharmacology. 2016;107:18–26. doi: 10.1016/j.neuropharm.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mahmoudiasl G.R., Abbaszadeh H.A., Rezaei-Tavirani M., Abdollahifar M.A., Khoramgah M.S., Niknazar S., Darabi S., Roozbahany N.A. Nod-like receptor protein 3 and nod-like receptor protein 1 inflammasome activation in the hippocampal region of postmortem methamphetamine chronic user. Bratisl. Lek. Listy. 2019;120:769–776. doi: 10.4149/BLL_2019_129. [DOI] [PubMed] [Google Scholar]
- 111.Salter M.W., Stevens B. Microglia emerge as central players in brain disease. Nat. Med. 2017;23:1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 112.Xu X., Huang E., Tai Y., Zhao X., Chen X., Chen C., Chen R., Liu C., Lin Z., Wang H., et al. Nupr1 Modulates Methamphetamine-Induced Dopaminergic Neuronal Apoptosis and Autophagy through CHOP-Trib3-Mediated Endoplasmic Reticulum Stress Signaling Pathway. Front. Mol. Neurosci. 2017;10:203. doi: 10.3389/fnmol.2017.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chao J., Zhang Y., Du L., Zhou R., Wu X., Shen K., Yao H. Molecular mechanisms underlying the involvement of the sigma-1 receptor in methamphetamine-mediated microglial polarization. Sci. Rep. 2017;7:11540. doi: 10.1038/s41598-017-11065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Du L., Shen K., Bai Y., Chao J., Hu G., Zhang Y., Yao H. Involvement of NLRP3 inflammasome in methamphetamine-induced microglial activation through miR-143/PUMA axis. Toxicol. Lett. 2019;301:53–63. doi: 10.1016/j.toxlet.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 115.Fan R., Shen Y., Li X., Luo H., Zhang P., Liu Y., Si Z., Zhou W., Liu Y. The effect of the NLRP1 inflammasome on methamphetamine-induced cognitive impairment in rats. Drug Alcohol. Depend. 2022;237:109537. doi: 10.1016/j.drugalcdep.2022.109537. [DOI] [PubMed] [Google Scholar]
- 116.Liu Y., Wen D., Gao J., Xie B., Yu H., Shen Q., Zhang J., Jing W., Cong B., Ma C. Methamphetamine induces GSDME-dependent cell death in hippocampal neuronal cells through the endoplasmic reticulum stress pathway. Brain Res. Bull. 2020;162:73–83. doi: 10.1016/j.brainresbull.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 117.Dahal S., Chitti S.V.P., Nair M.P.N., Saxena S.K. Interactive effects of cocaine on HIV infection: Implication in HIV-associated neurocognitive disorder and neuroAIDS. Front. Microbiol. 2015;6:931. doi: 10.3389/fmicb.2015.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thangaraj A., Periyasamy P., Guo M.-L., Chivero E.T., Callen S., Buch S. Mitigation of cocaine-mediated mitochondrial damage, defective mitophagy and microglial activation by superoxide dismutase mimetics. Autophagy. 2020;16:289–312. doi: 10.1080/15548627.2019.1607686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chivero E.T., Thangaraj A., Tripathi A., Periyasamy P., Guo M.-L., Buch S. NLRP3 Inflammasome Blockade Reduces Cocaine-Induced Microglial Activation and Neuroinflammation. Mol. Neurobiol. 2021;58:2215–2230. doi: 10.1007/s12035-020-02184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.López-Pedrajas R., Ramírez-Lamelas D.T., Muriach B., Sánchez-Villarejo M.V., Almansa I., Vidal-Gil L., Romero F.J., Barcia J.M., Muriach M. Cocaine promotes oxidative stress and microglial-macrophage activation in rat cerebellum. Front. Cell Neurosci. 2015;9:279. doi: 10.3389/fncel.2015.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Amirshahrokhi K., Niapour A. Methylsulfonylmethane protects against ethanol-induced brain injury in mice through the inhibition of oxidative stress, proinflammatory mediators and apoptotic cell death. Int. Immunopharmacol. 2022;106:108638. doi: 10.1016/j.intimp.2022.108638. [DOI] [PubMed] [Google Scholar]
- 122.Kane C.J., Drew P.D. Inflammatory responses to alcohol in the CNS: Nuclear receptors as potential therapeutics for alcohol-induced neuropathologies. J. Leukoc. Biol. 2016;100:951–959. doi: 10.1189/jlb.3MR0416-171R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Blednov Y.A., Benavidez J.M., Black M., Leiter C.R., Osterndorff-Kahanek E., Johnson D., Borghese C.M., Hanrahan J.R., Johnston G.A., Chebib M., et al. GABAA receptors containing rho1 subunits contribute to in vivo effects of ethanol in mice. PLoS ONE. 2014;9:e85525. doi: 10.1371/journal.pone.0085525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sun B., Wang X., Ji Z., Wang M., Liao Y.P., Chang C.H., Li R., Zhang H., Nel A.E., Xia T. NADPH Oxidase-Dependent NLRP3 Inflammasome Activation and its Important Role in Lung Fibrosis by Multiwalled Carbon Nanotubes. Small. 2015;11:2087–2097. doi: 10.1002/smll.201402859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Narayanan K.B., Park H.H. Purification and analysis of the interactions of caspase-1 and ASC for assembly of the inflammasome. Appl. Biochem. Biotechnol. 2015;175:2883–2894. doi: 10.1007/s12010-014-1471-4. [DOI] [PubMed] [Google Scholar]
- 126.Proell M., Gerlic M., Mace P.D., Reed J.C., Riedl S.J. The CARD plays a critical role in ASC foci formation and inflammasome signalling. Biochem. J. 2013;449:613–621. doi: 10.1042/BJ20121198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Poyet J.L., Srinivasula S.M., Tnani M., Razmara M., Fernandes-Alnemri T., Alnemri E.S. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J. Biol. Chem. 2001;276:28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 128.Lippai D., Bala S., Petrasek J., Csak T., Levin I., Kurt-Jones E.A., Szabo G. Alcohol-induced IL-1β in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J. Leukoc. Biol. 2013;94:171–182. doi: 10.1189/jlb.1212659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yan J., Bare D., DeSantiago J., Zhao W., Mei Y., Chen Z., Ginsburg K., Solaro R., Wolska B., Bers D., et al. JNK2, a Newly-Identified SERCA2 Enhancer, Augments an Arrhythmic [Ca] Leak-Load Relationship. Circ. Res. 2021;128:455–470. doi: 10.1161/CIRCRESAHA.120.318409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lim J., Lee H., Jung Y., Kim J., Chae C., Kim S., Han H. Ethanol-activated CaMKII signaling induces neuronal apoptosis through Drp1-mediated excessive mitochondrial fission and JNK1-dependent NLRP3 inflammasome activation. Cell Commun. Signal. CCS. 2020;18:123. doi: 10.1186/s12964-020-00572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li X., Tong J., Liu J., Wang Y. Downregulation of ROCK2 attenuates alcohol-induced inflammation and oxidative stress in astrocytes. Int. J. Neurosci. 2020;132:521–530. doi: 10.1080/00207454.2020.1825421. [DOI] [PubMed] [Google Scholar]
- 132.Liu Q., Su L.-Y., Sun C., Jiao L., Miao Y., Xu M., Luo R., Zuo X., Zhou R., Zheng P., et al. Melatonin alleviates morphine analgesic tolerance in mice by decreasing NLRP3 inflammasome activation. Redox Biol. 2020;34:101560. doi: 10.1016/j.redox.2020.101560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang H., Huang M., Wang W., Zhang Y., Ma X., Luo L., Xu X., Xu L., Shi H., Xu Y., et al. Microglial TLR4-induced TAK1 phosphorylation and NLRP3 activation mediates neuroinflammation and contributes to chronic morphine-induced antinociceptive tolerance. Pharmacol. Res. 2021;165:105482. doi: 10.1016/j.phrs.2021.105482. [DOI] [PubMed] [Google Scholar]
- 134.Cai Y., Kong H., Pan Y.-B., Jiang L., Pan X.-X., Hu L., Qian Y.-N., Jiang C.-Y., Liu W.-T. Procyanidins alleviates morphine tolerance by inhibiting activation of NLRP3 inflammasome in microglia. J. Neuroinflamm. 2016;13:53. doi: 10.1186/s12974-016-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang C., Wang Y., Wang D., Zhang J., Zhang F. NSAID Exposure and Risk of Alzheimer’s Disease: An Updated Meta-Analysis From Cohort Studies. Front. Aging Neurosci. 2018;10:83. doi: 10.3389/fnagi.2018.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Morris R., Armbruster K., Silva J., Widell D.J., Cheng F. The Association between the Usage of Non-Steroidal Anti-Inflammatory Drugs and Cognitive Status: Analysis of Longitudinal and Cross-Sectional Studies from the Global Alzheimer’s Association Interactive Network and Transcriptomic Data. Brain Sci. 2020;10:961. doi: 10.3390/brainsci10120961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.




