Abstract
Simple Summary
Pheochromocytoma and paraganglioma (PPGL) are rare neuroendocrine cancers which carry the risk of metastatic disease. Pathogenic variants in the succinate dehydrogenase subunit A gene (SDHA) have been shown to cause metastatic disease, occurring in various regions of the body. Imaging is an early and vital step in the diagnosis and clinical care of these patients. The study here identifies which imaging modality among positron emission tomography (PET), computed tomography (CT), and magnetic resonance imaging (MRI) performs better in localizing metastatic PPGL lesions related to SDHA. The study identified that 68Ga-DOTATATE PET/CT performed best at overall lesion detection; however, 18F-FDG PET/CT performed better in certain anatomic regions of the body. A combined approach with 68Ga-DOTATATE and 18F-FDG would optimize care and guide clinicians in selecting the appropriate interventions and therapies.
Abstract
The study identifies the importance of positron emission tomographic (PET) and anatomic imaging modalities and their individual performances in detecting succinate dehydrogenase A (SDHA)-related metastatic pheochromocytoma and paraganglioma (PPGL). The detection rates of PET modalities—68Ga-DOTATATE, 18F-FDG, and 18F-FDOPA—along with the combination of computed tomography (CT) and magnetic resonance imaging (MRI) are compared in a cohort of 11 patients with metastatic PPGL in the setting of a germline SDHA mutation. The imaging detection performances were evaluated at three levels: overall lesions, anatomic regions, and a patient-by-patient basis. 68Ga-DOTATATE PET demonstrated a lesion-based detection rate of 88.6% [95% confidence interval (CI), 84.3–92.5%], while 18F-FDG, 18F-FDOPA, and CT/MRI showed detection rates of 82.9% (CI, 78.0–87.1%), 39.8% (CI, 30.2–50.2%), and 58.2% (CI, 52.0–64.1%), respectively. The study found that 68Ga-DOTATATE best detects lesions in a subset of patients with SDHA-related metastatic PPGL. However, 18F-FDG did detect more lesions in the liver, mediastinum, and abdomen/pelvis anatomic regions, showing the importance of a combined approach using both PET modalities in evaluating SDHA-related PPGL.
Keywords: pheochromocytoma, paraganglioma, metastatic, SDHA, PET, CT, MRI, 68Ga-DOTATATE, 18F-FDG
1. Introduction
Succinate dehydrogenase is a critical enzyme participating in cellular aerobic respiration. The enzyme is comprised of four subunits (collectively known as complex II), which converts succinate to fumarate via oxidation. These subunits are represented by the alphabetic characters: A, B, C, and D, where each subunit is expressed by a respective SDH-A, B, C, or D tumor suppressor gene [1]. Pathogenic variants in these genes can predispose a patient to pheochromocytoma and paraganglioma (PPGL), among other cancers. An international consortium of experts has found that SDHx-related pathogenic variants cause disease in 20% of patients with PPGLs [2]. However, patterns of the disease, including clinical manifestations, anatomic location, penetrance, and malignant potential, vary according to the afflicted subunit [3,4]. Identification of these phenotypic patterns, in conjunction with the pathogenic genetic variant in this largely heritable cancer, is critical in the care of patients and their families.
PPGLs are rare neuroendocrine tumors that occur in the adrenal medulla and in sympathetic and parasympathetic ganglia. These tumors can be life-threatening and thus require a thorough clinical workup to prevent morbidity and mortality. Biallelic SDHA germline mutations were first identified in a heritable neurodegenerative condition known as Leigh Syndrome [5,6]. Monoallelic pathogenic SDHA variants have been associated with tumors such as renal cell carcinoma, gastrointestinal stromal tumor, neuroblastoma, and lung cancers, as well as the focus of this study: PPGLs. The first reported case of SDHA-related paraganglioma (PGL) was in 2010 [7]; since then, there have been several studies and observations detailing the potential risk of metastasis [8,9]. While one study using a Bayesian inference predicted a 1.7% penetrance of PPGL for patients with pathogenic variants in SDHA, the risk of metastatic disease in those with SDHA-related PPGL is up to 66% [10,11].
The clinical picture of metastatic SDHA-related PPGL has been reported and observed to be similar in aggressiveness to SDHB-related PPGL [8,12]. Since publishing our initial data on ten patients which identified clinical characteristics in metastatic SDHA-related PPGL from 2010 to 2018, our group has had one additional patient in this cohort illustrating the rarity of the patients [7]. Although the SDHA variants are rare in an already rare condition, the morbidity and mortality related to this genetic predisposition to PPGL are worrisome for patients, their families, and treating clinicians.
In conjunction with biochemical and clinical workup, radiologic imaging pinpoints the anatomic location of PPGLs and whether metastatic disease is present. Imaging can directly translate to therapy (“theranostics”), specifically when functional imaging is correlated to a radiotherapeutic counterpart in patients with inoperable disease. The proposed algorithm from the European Association of Nuclear Medicine/Society of Nuclear Medicine and Molecular Imaging (EANM/SNMMI) in 2019 recommends 68Ga-DOTA(0)-Tyr(3)-octreotate (68Ga-DOTATATE) as the radiotracer of choice, followed by 18F-fluorodeoxyglucose (18F-FDG) and 18F-fluorodihydroxyphenylalanine (18F-FDOPA), in the functional imaging of metastatic PPGL and SDHx-related PPGL [13].
In congruence with the EANM/SNMMI guidelines, our study will be the first head-to-head study comparing functional and anatomic imaging modalities in the SDHA cohort of PPGL patients. The objective of this study is to extrapolate upon the imaging guidelines by comparing diagnostic performances of 68Ga-DOTATATE, 18F-FDOPA, 18F-FDG, and CT/MRI in SDHA-related metastatic PPGL.
2. Methods
2.1. Selection of Patients
Between 2014 and 2021, 11 consecutive patients (5 female and 6 male) identified with SDHA-related metastatic PPGL were prospectively evaluated at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) at the National Institutes of Health (NIH). All patients had clinically proven metastatic PPGLs based on surgically resected PPGLs, biochemical diagnosis, and anatomic and functional imaging. Patient enrollment into the study began in January 2014 when 68Ga-DOTATATE PET/CT was available as a research scan and transitioned to a clinical scan in July 2021 (Clinical Trial Government Identifier: NCT00004847). The protocol was approved by the institutional review board of the Eunice Kennedy Shriver NICHD. Informed consent was obtained from all participants and guardians for minors as a part of the investigation.
2.2. Patient Cohort and Disease Characteristics
The age of the patients ranged from 16 to 67, with a mean age of 41.9 ± 19.9 years. The mean age at primary PPGL diagnosis was 36.3 ± 18.4 years. The average interval between the first primary PPGL diagnosis and referral to the NIH was 3.3 ± 3.9 years. Nonsense mutations in SDHA were most common among these patients, seen in 9 of 11 patients (82%). Eight of 11 patients (73%) had the c.91C>T variant, resulting in a stop gain at codon 31 (p.Arg31*). The other nonsense mutation was seen in patient 3 (c.1534C>T) with a stop gain at codon 512 (p.Arg512*). Full gene deletion was seen in one (patient 8). Patient 6 was the only one with a missense variant (c.1334C>T), changing serine to leucine at codon 445 (p.S445L). Different interpretations of this missense variant are reported in ClinVar as either a variant of uncertain significance (VUS) or a likely pathogenic variant [14]. Additional cohort characteristics, including sex, age when primary PPGL diagnosed, age when imaging scans were performed, location of primary PPGL, biochemical phenotype, time to metastatic disease progression, location of metastatic PPGL, treatments received, Ki-67 percent on histology, and deceased status are summarized in Table 1.
Table 1.
Clinical Characteristics of SDHA Patients in the study.
| PT ID i | Sex | SDHA Mutation | Age (d) ii | Age (s) iii | Primary Tumor | Biochemical Phenotype/s iv | Time to Metastasis | Metastatic Location/s |
Treatment/s | Ki-67 v | Deceased |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | f | c.91C>T (p.Arg31*) | 11 | 23 | Left Vagale PGL | None | meta, 12 mo |
Bones, Neck, Abdomen, Lung | Surgery of Primary, Surgery of Recurrence | Not available | No |
| 2 | m | c.91C>T (p.Arg31*) | 57 | 61 | Paraaortic PGL and Left Carotid Body PGL | ADR, DA | meta, 7 mo |
Neck and Mediastinum | Surgery | Not available | No |
| 3 | f | c.1534C>T (p.Arg512*) | 53 | 63 | Paraaortic PGL | NA, DA | syn | Bone, Neck, Mediastinum, Abdomen, Pelvis | Partial resection of Primary; SSA; 90Y-DOTATOC; 177Lu-DOTATOC; CVD; bortezomib and clofarabine; combination capecitabine and TMZ | 15% in focal areas of periaortic PGL | Yes |
| 4 | m | c.91C>T (p.Arg31*) | 20 | 23 | Aortocaval PGL | None | syn, 2 mo vi |
Bones and Abdomen | Surgery | Not available | No |
| 5 | m | c.91C>T (p.Arg31*) | 14 | 16 | Paracaval PGL | ADR, NA | syn | Bone | Surgery of Primary, 90Y-DOTATOC, SSA, ONC201 | 3.5% in PGL biopsy | No |
| 6 | m | c.1334C>T (p.S445L) VUS | 53 | 59 | Mediastinal PGL | NA, DA | meta, 48 mo |
Bones and Mediastinum | Surgery, SSA, TMZ | 15–20% in T10 met | No |
| 7 | m | c.91C>T (p.Arg31*) | 56 | 67 | Left Adrenal PHEO | ADR, NA, DA | meta, 120 mo |
Bones, Lung, Liver, Neck | Surgery of Primary, EBRT, 123I-MIBG, CVD | Not available | Yes |
| 8 | f | 5′UTR_3′ UTRdel | 29 | 33 | Porta Hepatis/Right Adrenal PPGL | NA | meta, 20 mo |
Bones, Mediastinum, Lungs | Surgery of Primary, Surgery of Recurrence, EBRT, 177Lu-DOTATATE | 20–30% in T7 epidural Met | Yes |
| 9 | m | c.91C>T (p.Arg31*) | 44 | 45 | Paraaortic PGL | None | syn | Bones, Lung, Mediastinum, Abdomen | Surgical decompression of Spine Met, 123I-MIBG, TMZ | 10–15% Primary | Yes |
| 10 | f | c.91C>T (p.Arg31*) | 46 | 54 | Aortocaval PGL | ADR, NA | meta, 78 mo |
Bone, Mediastinum, Liver, Abdomen, Pelvis, Neck | Surgery of Primary, EBRT, 177Lu-DOTATATE, CVD, Liver embolization, Liver trisegmentectomy | >20% in Liver Mets | Yes |
| 11 | f | c.91C>T (p.Arg31*) | 16 | 17 | Mediastinal PGL | None | meta, 7 mo |
Bone | Resection of Primary, resection of recurrent bed | Not available | No |
abbreviations: f—female, m—male, PHEO—pheochromocytoma, PGL—paraganglioma, ADR—adrenergic, NA—Noradrenergic, DA—Dopaminergic, meta—metachronous, syn—synchronous, mo—months, SSA—somatostatin analogs, TMZ—temozolomide, VUS—variant of unknown significance, EBRT—external beam radiation therapy, CVD—chemotherapeutic regimen cyclophosphamide-vincristine-dacarbazine. i PT ID is the patient identification number in the cohort. ii Age (d) is Age of PPGL diagnosis in years. iii Age (s) is Age at time of imaging scans in years. iv Biochemical elevation at time of Scans, with adrenergic, noradrenergic, and dopaminergic referring to elevations in epinephrine and/or metanephrine, norepinephrine and/or normetanephrine, and dopamine and/or 3-methoxytyramine, respectively. v Ki-67 is the cellular proliferative index staining on histopathology. vi Patient has synchronous metastases and did not have full body imaging until seen in our study.
All eleven patients underwent 68Ga-DOTATATE, 18F-FDG, and either CT or MRI. Additionally, seven patients underwent 18F-FDOPA, and six patients underwent both CT and MRI.
2.3. Imaging Modality Techniques
Screening CT scans of the neck, chest, abdomen, and pelvis were performed on a multidetector CT device supplied by Siemens (SOMATOM Force and Definition), with the administration of nonionic, low osmolarity iodinated vascular contrast material. Screening MR imaging studies, including the neck, chest, abdomen, and pelvis were performed with either 1.5 or 3.0 Tesla scanners supplied by Philips (Achieva, manufactured Andover, MA, USA) and Siemens (Aera and Verio, manufactured Malvern, PA, USA), using a gadolinium-based contrast agent, and multiple sequences including fat-suppressed and non-fat-suppressed T2-weighted images, in-phase and out-of-phase T1-weighted images, and fat-suppressed sequences following contrast administration.
The PET/CT imaging was performed from the top of the skull to the mid-thighs using time-of-flight and obtained in a 3D mode reconstructed on a matrix suggested by the device manufacturer, Siemens. These scans were performed with a low-dose CT without contrast that was primarily used for anatomical localization. The average lapsed time between imaging and the mean administered radiotracer activity were approximately 60 min and 5.06 mCi for 68Ga-DOTATATE, 60.5 min and 7.38 mCi for 18F-FDG, and 30.5 min and 12.42 mCi of 18F-FDOPA. A 200-mg dose of carbidopa was administered 60 min prior to i.v. 18F-FDOPA administration. 68Ga-DOTATATE and 18F-FDOPA were formulated in the PET Department at the National Institutes of Health as stated in the investigational new drugs application.
2.4. Analysis of Data
The CT and MR studies were reviewed by a board-certified diagnostic radiologist (author Ling) with 35 years of experience, including 14 years in PPGL evaluation. All 68Ga-DOTATATE, 18F-FDG, and 18F-DOPA PET/CTs were reviewed by a board-certified nuclear medicine physician with 35 years of experience, including 21 years in PPGL evaluation (author Carrasquillo). Both experts were blinded to clinical information, except for diagnosis, sex, and age. Lesions were considered positive on the PET scans when areas of non-physiologic focal uptake displayed higher maximal standardized uptake values (SUVmax) than the surrounding tissue.
Imaging studies were performed within a mean duration of 14 ± 19 days of each other. The following analyses were conducted within each imaging study: lesion, region, and patient. In the patient analysis, a patient was determined positive by the presence of a single positive lesion on a particular imaging modality. Similarly, in region analysis, a region was determined positive by the presence of a single positive lesion on a particular imaging modality in the following areas: head and neck, bones, lungs, mediastinum, liver, adrenal glands, and abdomen and pelvis compartments (excluding the liver and adrenal glands). An imaging comparator was constructed from a composite of all the imaging modalities, where a true lesion was deemed to be present if positive on at least two functional modalities or one functional and one anatomic modality. However, if a lesion was only positive on one functional modality or one anatomic modality, the lesion was not considered positive. The composite method was chosen as the reference standard, as a substitute for histologic diagnosis for metastatic lesions, as biopsy and surgical resection for each lesion was not clinically feasible. This imaging comparator was applied in all three analyses: total lesion, anatomic region, and patient-to-patient analyses.
2.5. Statistical Analysis
Detection rates are reported as ratios and percentages for lesion detection by each modality with a 95% confidence interval calculated using the GraphPad QuikCalcs website (https://www.graphpad.com/quickcalcs/confInterval1/ accessed on 10 July 2022). McNemar’s tests were performed to compare lesion detection rates between 68Ga-DOTATATE and the other functional and anatomic (combined CT/MRI) imaging modalities. A p-value < 0.05 shows that a likely difference exists in the detection rates of the modalities being compared. Contingency tables comparing imaging modalities are presented in Supplementary Figures S1 and S2 with a p-value calculated using GraphPad QuikCalcs website (https://www.graphpad.com/quickcalcs/mcNemar1/ accessed on 10 July 2022).
3. Results
3.1. Lesion Analysis
Eleven patients with SDHA-related PPGL were identified and analyzed for lesions. 68Ga-DOTATATE PET/CT had a total lesion detection rate of 88.6% (95% CI, 84.3–92.1), identifying 249 of the 281 composite positive lesions, which was higher than 18F-FDG, 18F-FDOPA, and CT/MRI (shown in Table 2). Ten primary lesions were found in 8 patients at the time of scanning, and the detection performance on imaging is shown in Table 2. Three patients (Patients 7, 10, and 11) did not have primary lesions imaged at the time of scanning due to resection of these tumors. Three patients (Patients 1, 5, and 8) had imaging of their primary tumors despite interim resections of these tumors due to recurrence of the primary mass. These primary lesions were determined based on tumor anatomical location where chromaffin tissue is physiologically present in conjunction with clinical history. Metastatic lesions were clinically determined based on the following criteria: location in the bones, lungs, or liver; locally invasive recurrent tumors; and presence of tumors where chromaffin tissue is not physiologically present. The detection rate of metastatic lesions by 68Ga-DOTATATE outperformed the three other modalities (shown in Table 2).
Table 2.
Lesion analysis displaying detection rates and confidence intervals by imaging modalities categorized by total, primary, and metastatic lesions in 11 patients. Further subcategorization of which region the primary and metastatic lesions were located, and their detection rates are shown.
| 68Ga-DOTATATE | 18F-FDG | 18F-FDOPA | CT/MRI | |
|---|---|---|---|---|
| Total Lesions | 249/281 88.6 (84.3–92.1) |
233/281 82.9 (78.0–87.1) |
39/102 39.8 (30.2–50.2) |
157/270 58.2 (52.0–64.1) |
| Primary Lesions | 9/10 90.0 (55.5–99.8) |
9/10 90.0 (55.5–99.8) |
3/7 42.9 (9.9–81.6) |
7/10 70.0 (34.8–93.3) |
| Mediastinum | 1/1 | 1/1 | - vii | 0/1 |
| Adrenal | 1/1 | 1/1 | 0/1 | 1/1 |
| Abdomen/Pelvis | 5/6 | 6/6 | 2/5 | 6/6 |
| Head/Neck | 2/2 | ½ | 1/1 | 0/2 |
| Metastatic Lesions | 240/271 88.6 (84.2–92.1) |
225/271 83.0 (78.0–87.3) |
36/95 37.9 (28.1–48.4) |
150/260 57.7 (51.4–63.8) |
| Bone | 208/223 | 188/223 | 27/64 | 110/212 |
| Lungs | 11/19 | 15/19 | 3/10 | 19/19 |
| Mediastinum | 4/7 | 5/7 | 3/7 | 4/7 |
| Adrenal | 1/1 | 1/1 | 0/1 | 1/1 |
| Liver | 4/7 | 7/7 | 0/4 | 7/7 |
| Abdomen/Pelvis | 6/7 | 6/7 | 2/5 | 4/7 |
| Head/Neck | 6/7 | 3/7 | ¼ | 5/7 |
vii Patient did not receive 18F-FDOPA Imaging due logistics of scheduling.
McNemar’s tests comparing total lesion detection rates found the following two-sided p-values: 0.085 for 68Ga-DOTATATE vs. 18F-FDG, <0.0001 for 68Ga-DOTATATE vs. 18F-FDOPA, and <0.0001 for 68Ga-DOTATATE vs. CT/MRI. The p-value less than 0.05 rejects the hypothesis that the modalities’ detection rates are equal, and a difference in performance is evident. Contingency tables comparing lesion detection between modalities, on which McNemar’s tests were conducted are shown in Supplementary Figure S1.
3.2. Region Analysis
Region analysis was performed in the following anatomic areas: bones, lungs, mediastinum, adrenals, liver, abdomen and pelvis, and head and neck. 68Ga-DOTATATE PET/CT and 18F-FDG identified 32 of 36 (88.9%, CI 73.9–96.9) possible positive anatomic regions across the eleven patients. CT/MRI identified lesions in 30 of 36 (83.3%, CI 67.2–93.6) and 18F-FDOPA in 14 of 24 (58.3%, CI 36.6–77.9) anatomic regions. 68Ga-DOTATATE demonstrated the highest region detection rates in bone and head/neck lesions, 18F-FDG PET/CT was the highest in the mediastinum and abdominopelvic compartment, and CT/MRI was the highest in the lungs. There was a 100% detection rate in the adrenal regions, which consisted of two adrenal gland lesions in two patients by 68Ga-DOTATATE, 18F-FDG, and CT/MRI. 18F-FDG and CT/MRI were also superior in detecting liver lesions. 18F-FDOPA showed inferior detection rates compared to the other modalities across all anatomic regions. Detection rates in these regions by the imaging modalities are shown in Table 3.
Table 3.
Imaging modality detection of a positive lesion in all anatomic regions and individual anatomic regions.
| 68Ga-DOTATATE | 18F-FDG | 18F-FDOPA | CT/MRI | |
|---|---|---|---|---|
| All Regions | 32/36 | 32/36 | 14/24 | 30/36 |
| Bone | 9/10 | 10/10 | 5/6 | 8/10 |
| Lungs | 4/4 | 4/4 | 1/2 | 4/4 |
| Mediastinum | 5/6 | 4/6 | 3/5 | 3/6 |
| Liver | 2/2 | 2/2 | 0/1 | 2/2 |
| Adrenal | 2/2 | 2/2 | 0/2 | 2/2 |
| Abdomen/Pelvis | 6/7 | 7/7 | 3/5 | 7/7 |
| Head/Neck | 4/5 | 3/5 | 2/3 | 4/5 |
3.3. Patient Analysis
On patient analysis, imaging performance found 68Ga-DOTATATE and 18F-FDG did not detect disease in one out of the eleven patients (different patient in either modality). 18F-FDOPA did not miss detection in any of the seven patients. CT/MRI did not detect in two of the eleven patients. Patient level results are detailed in Table 4.
Table 4.
Ratios, detection rates (DR), and confidence intervals (CI) of imaging modalities at patient level, lesion level, and region level.
| 68Ga-DOTATATE | 18F-FDG | 18F-FDOPA | CT/MRI | |||||
|---|---|---|---|---|---|---|---|---|
| Ratio | DR (CI) | Ratio | DR (CI) | Ratio | DR (CI) | Ratio | DR (CI) | |
| Patient Level | 10/11 | 90.9% (58.7–99.8) |
10/11 | 90.9% (58.7–99.8) |
7/7 | 100% (59.0–100.0) |
9/11 | 81.8% (48.2–97.7) |
| Region Level | 32/36 | 88.9% (73.9–96.9) |
32/36 | 88.9% (73.9–96.9) |
14/24 | 58.3% (36.6–77.9) |
30/36 | 83.3% (67.2–93.6) |
| Lesion Level | 249/281 | 88.6% (84.3–92.1) |
233/281 | 82.9% (78.0–87.1) |
39/98 | 39.8% (30.2–50.2) |
159/270 | 58.9% (52.8–64.8) |
3.4. Summary of Patient, Region, and Lesion Analyses
The performances of imaging modalities in detection of SDHA-related metastatic lesions on a patient level, region level, and lesion level are summarized in Table 4.
3.5. Lesions outside the Reference Standard
Other lesions were noted on evaluation, which did not qualify for the reference standard using the imaging comparator. 68Ga-DOTATATE identified 70 additional lesions—68 in bones, 1 in the abdomen/pelvis, and 1 in the mediastinum. 18F-FDG identified 13 additional lesions to the imaging comparator—7 in bones, 3 in the abdomen/pelvis, 2 in the head/neck, and 1 in the mediastinum. CT/MRI identified 10 additional lesions—4 in lungs, 3 in the liver, 2 in the adrenals, and 1 in the abdomen/pelvis. 18F-FDOPA identified 12 additional lesions—11 in bones and 1 in the head/neck.
3.6. Patients with Four Imaging Modalities
Additionally, the total lesion detection in seven patients for whom all four imaging modalities were conducted found that 68Ga-DOTATATE identified 80 of 102 (78.4%, CI 69.2–86.0), 18F-FDG identified 81 of 102 (79.4%, CI 70.3–86.8), 18F-FDOPA identified 39 of 102 (38.2%, CI 28.8–48.4), and CT/MRI identified 55 of 102 (53.9%, CI 43.8–63.8). The results are detailed in Table 5. Detection of lesions by 68Ga-DOTATATE and 18F-FDG performed similarly in these seven patients. McNemar’s tests conducted to compare lesion detection rates found the following two-sided p-values: 1.00 for 68Ga-DOTATATE vs. 18F-FDG, less than 0.0001 for 68Ga-DOTATATE vs. 18F-FDOPA, and 0.001 for 68Ga-DOTATATE vs. CT/MRI (Contingency Tables shown in Supplementary Figure S2).
Table 5.
Lesion analysis in patients in which all four imaging modalities were performed displaying detection rates and confidence intervals in total SDHA lesions, primary SDHA lesions, and metastatic SDHA lesions.
| 68Ga-DOTATATE | 18F-FDG | 18F-FDOPA | CT/MRI | |
|---|---|---|---|---|
| Total Lesions | 80/102 78.4 (69.2–90.0) |
81/102 79.4 (70.3–86.8) |
39/102 39.8 (30.2–50.2) |
55/102 53.9 (43.8–63.8) |
| Primary Lesions | 6/7 85.7 (42.1–99.6) |
6/7 85.7 (42.1–99.6) |
3/7 42.9 (9.9–81.6) |
6/7 85.7 (42.1–99.6) |
| Metastatic Lesions | 74/95 77.9 (68.2–85.8) |
75/95 79.0 (69.4–86.6) |
36/95 37.9 (28.1–48.4) |
49/95 51.6 (41.1–62.0) |
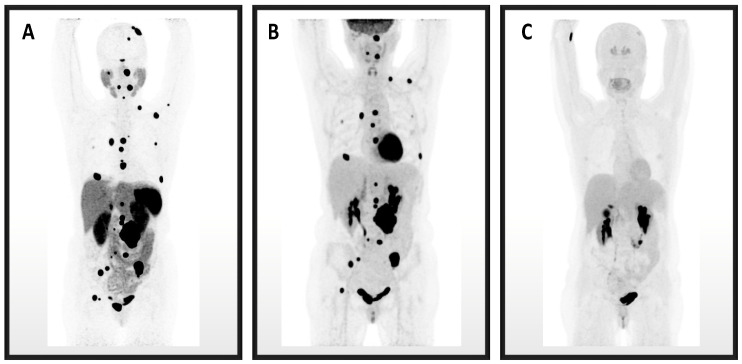
An imaging comparison of 68Ga-DOTATATE, 18F-FDG, and 18F-FDOPA PET images is depicted in Figure 1.
Figure 1.
These are maximum intensity projection image PET/CTs for Patient 3. (A) 68Ga-DOTATATE identified the most PPGL lesions, followed by (B) 18F-FDG, and the fewest lesions were identified on (C) 18F-FDOPA.
4. Discussion
The study determines the performance of 68Ga-DOTATATE, 18F-FDG, 18F-FDOPA, and CT/MRI in a small cohort of patients with SDHA-related metastatic PPGL, which have been found to be aggressive in their clinical course in our institutional experience [8]. The study is important in the clinical care of patients since imaging can localize the disease burden which translates to the urgency in therapeutic selection and intervention. Analyzing how imaging modalities perform in detecting SDHA-related PPGL may translate to optimized clinical management. EANM/SNMMI outlined recommendations in 2019, prioritizing 68Ga-DOTATATE in metastatic SDHx-related PPGL, while our study corroborates these guidelines; based on our results, there are certain additional suggestions in imaging recommendations for SDHA patients with metastatic PPGL [13].
The EANM/SNMMI guidelines recommend 68Ga-DOTATATE as first-line for extra-adrenal sympathetic, metastatic, multifocal, and SDHx-related PPGLs with both 18F-FDG and 18F-FDOPA recommended as unequivocal second-line options. Our study identifies that 68Ga-DOTATATE displayed an overall lesion detection rate of 88.6%, where 18F-FDG had a rate of 82.9%, 18F-FDOPA of 39.8%, and CT/MRI of 58.9%. Upon evaluating detection rates among the PET modalities by anatomic regions, 68Ga-DOTATATE performed best in detecting bone and head/neck lesions, 18F-FDG was best in detecting mediastinal and abdominal/pelvic lesions, and 18F-FDOPA was inferior in all anatomic regions. In a head-to-head comparison, 18F-FDG outperformed 68Ga-DOTATATE in soft tissue organ lesions, apart from head and neck lesions (Table 2). The performance of 18F-FDG in detecting lesions of the lungs, mediastinum, liver, and abdomen/pelvis should inform clinicians to consider using this modality when SDHA-related metastatic disease is suspected in these regions.
To put in perspective the performance of 18F-FDG in the lungs, mediastinum, liver, and abdomen/pelvis, the results were compared to the performance of 68Ga-DOTATATE on an anatomic region basis. The ratio of regions detected by 68Ga-DOTATATE were 4/4 lung, 5/6 mediastinal, 2/2 liver, and 6/7 abdomen/pelvis (Table 2). Despite the better performance of 18F-FDG on a per lesion basis in these soft tissue regions, 68Ga-DOTATATE was able to detect a lesion in all regions but two—one mediastinum and one abdomen/pelvis—where there were only single lesions identified by the reference standard. Comparatively, CT/MRI region performance detected 4/4 lung, 3/6 mediastinal, 2/2 liver, and 7/7 abdomen/pelvis.
The CT fused with the PET imaging is a low-dose modality performed without contrast in this study used for the purpose of anatomic localization and attenuation correction. The fusion CT is not of diagnostic quality, unlike the CT used in the study for detection of lesions. Contrast-enhanced CT fused with PET or PET/MRI could provide an optimized and effective single diagnostic imaging modality. In lesion detection, CT/MRI identified 24 lesions which were negative on 68Ga-DOTATATE for a combined detection rate of 273/281 (97.2%). On 18F-FDG, CT/MRI identified 42 negative lesions for a combined detection rate of 275/281 (97.9%). While 11 lesions were not imaged by CT/MRI and were by 68Ga-DOTATATE and 18F-FDG, the improvement in lesion detection rates by combining the two modalities shows that contrast-enhanced PET/CT or PET/MRI could be a preferred modality in SDHA-related metastatic PPGL [15].
There were 32 lesions not identified by 68Ga-DOTATATE PET/CT compared to the reference standard. The missed lesions were distributed in the following regions across eight patients: 15 bone lesions (of which 14 were identified by 18F-FDG, 8 of possible 9 by 18F-FDOPA, and 8 by CT/MRI), 3 mediastinal (of which 3 were identified by 18F-FDG, 2 by 18F-FDOPA, and 2 by CT/MRI), 8 lung (of which 8 were identified by 18F-FDG, 2 of possible 6 by 18F-FDOPA, and 8 by CT/MRI), 3 liver (of which 3 were identified by 18F-FDG, 0 of possible 2 by 18F-FDOPA, and 3 by CT/MRI), 2 abdominopelvic (of which 1 was identified by 18F-FDG, 2 by 18F-FDOPA, and 2 by CT/MRI), and 1 head/neck (of which 1 was identified by 18F-FDG and 1 by CT/MRI).
Comparatively, 48 lesions were not identified by 18F-FDG PET/CT distributed in the following anatomic regions: 35 bone, 2 mediastinal, 4 lung, 1 abdominopelvic, and 6 head/neck across nine patients. There were 111 lesions not identified on CT/MRI and 63 lesions not identified on 18F-FDOPA PET/CT.
Limitations of this study include a small number of patients, given that this is a rare condition; however, it is the first and largest study in SDHA-related metastatic PPGLs. A limitation of targeting by 18F-FDG is its less specific mechanism of tumor uptake, which may identify a large variety of benign and malignant processes compared to 68Ga-DOTATATE and 18F-FDOPA. The detection of PPGLs by these PET modalities occurs through the following mechanisms: 68Ga-DOTATATE targets overexpressed somatostatin transmembrane receptors (SSTRs), 18F-FDOPA enters cells through large amino acid transport channels (LAT), and 18F-FDG enters cells via glucose transporters (GLUTs) [16]. 18F-FDG rapidly enters cells using GLUTs in which high rates of metabolism are occurring, such as the cerebrum, myocardium, cancers, and inflammatory processes [17]. Therefore, the low specificity and high sensitivity of 18F-FDG present a possibility of identifying processes unrelated to SDHA-related PPGL.
Patients with metastatic PPGL bone lesions have less morbidity compared to metastatic lesions in liver, retroperitoneum, and lungs [18,19,20,21]. The approach for therapy, in turn, may differ for exclusively osseous metastases versus soft tissue metastases. Locoregional therapies, such as surgery and radiation, and systemic therapies, such as chemotherapy, peptide receptor radionuclide therapy (PRRT), and receptor analogs, are all potential treatment options in any type of metastases. In addition to these approaches, patients with PPGL-related bone metastases may also benefit from therapies such as bisphosphonates or a RANKL inhibitor [22].
In our cohort of SDHA patients, bone metastases were the most abundant lesions compared to all other types of metastases combined, which is consistent with findings in all PPGL-related metastatic disease [23]. 68Ga-DOTATATE outperformed the other imaging modalities at detecting bone metastases, which compromised 223 of the 281 potential lesions. The sizes of these bone metastases were not measured on PET/CT, due to the difficulty of delineating tumor margins on a low-dose non-contrast fusion CT. However, the smaller sizes of numerous bone lesion may contribute to the detection differences we observed between functional and anatomic imaging. From the perspective of identifying more aggressive lesions than bone, 18F-FDG detected more in the lungs and liver, supporting that 18F-FDG may be utilized in combination with 68Ga-DOTATATE for this cohort of patients, rather than a second-line modality.
In terms of detecting disease on a patient-by-patient basis, both 68Ga-DOTATATE and 18F-FDG did not detect a lesion in one patient (different patient in each modality). Patient 4 had disease that was not detected by initial 68Ga-DOTATATE imaging; however, in subsequent annual follow-up scans, the abdominal lesion and bone lesions were avid on the modality. From a clinical viewpoint, detection on 68Ga-DOTATATE in any SDHA-related PPGL metastases translates to the opportunity of using PRRT to target overexpressed transmembrane receptors on tumors.
Advanced metastatic PPGL is uncurable, leading patients and clinicians to seek therapies that stabilize disease, minimize progression, and reduce symptoms. 177Lu-DOTATATE (Lutathera®), which is approved in other NETs, awaits phase II trial (NCT03206060) results in PPGL, offering an approved treatment option in 68Ga-DOTATATE-avid PPGL [24]. In addition to 177Lutetium (177Lu), patients with SSTR-avid disease can be considered for 90Yttrium (90Y)-based PRRTs [25]. In our cohort, Patients 8 and 10 received Lutathera®, Patient 5 received 90Y-DOTATOC, and Patient 3 received 90Y-DOTATOC followed by 177Lu-DOTATOC. Patient 3 had a rapidly deteriorating clinical course in which lesions erupted after PRRT [26].
In our analysis, the results could not determine the impact of advanced metastatic PPGL and somatostatin receptor differentiation on the tumors. In the four deceased patients with high Ki-67 proliferative indices (greater than 10%), 68Ga-DOTATATE performed better in Patients 3 and 8, while 18F-FDG performed better in Patients 9 and 10.
Larger studies are difficult to organize in an SDHA cohort of PPGL patients due to the low occurrence and penetrance rates in the population [12,27,28]. A multicenter approach to accumulate a sizable cohort of SDHA-related patients could support these findings and help draw more accurate conclusions on the role of each imaging modality in these patients.
5. Conclusions
In conclusion, our study in SDHA-related metastatic PPGL supports current guidelines recommending 68Ga-DOTATATE as the agent of choice, as well as the use of 18F-FDG as a second-line agent. In addition, we show that for SDHA patients with extensive lesions in the soft tissue regions (i.e., lungs, mediastinum, liver, and abdomen/pelvis), a dual approach of 68Ga-DOTATATE and 18F-FDG should be utilized for maximal sensitivity in evaluation of disease. Of note, in contrast with recommended guidelines, we show that 18F-FDOPA performs poorly in SDHA patients and should not be relied upon in this subgroup. Early detection of SDHA-related PPGL benefits the patient by leading to individualized management and therapy selection, which may reduce morbidity related to disease. Clinical decisions and the care of patients are often guided by imaging, especially in the case of 68Ga-DOTATATE, which can help clinicians identify whether PRRT is the appropriate option for the patient.
Acknowledgments
The authors would like to acknowledge Paul Wakim and Xiaobai Li of the Biostatistics and Clinical Epidemiology Service at the NIH for their assistance in statistical approaches in the study. The authors would like to acknowledge Erina He of the Medical Arts at the NIH for her assistance in preparing the graphical abstract. Lastly, Mayank Patel would like to acknowledge fellow lab members Thanh Huynh, Tamara Prodanov, Ondrej Uher, and Katerina Hadrava Vanova for their support and ongoing research in pheochromocytoma and paraganglioma.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14163886/s1, Figure S1: Contingency Tables for All 11 Patients; Figure S2: Contingency Tables for 7 Patient Subset. Contingency Tables compare lesions detected on 68Ga-DOTATATE to the other imaging modalities for the Patients with SDHA-related PPGL.
Author Contributions
There were several authors integral to the process of this research study and manuscript. The specific contributions of the authors are as follows: Conceptualization, M.P., A.J., C.C.C., J.A.C. and K.P.; methodology, M.P., A.J., C.C.C., J.A.C. and K.P.; formal analysis, M.P., A.J., C.C.C., J.A.C. and K.P.; investigation, M.P., A.J. and K.P.; data curation and validation, all authors; writing—original draft preparation, M.P., A.J. and K.P; writing—M.P., A.J., C.C.C., D.T., J.A.C. and K.P.; supervision, K.P.; funding acquisition, K.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the National Institutes of Health (00-CH-0093 approved on 22 March 2000).
Informed Consent Statement
Informed consent was obtained from the participants and/or their guardians in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the protection of patient identification.
Conflicts of Interest
The authors declare no perceived conflict of interest.
Funding Statement
This work was supported by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development and funded by National Institutes of Health (grant number: Z1AHD008735).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jochmanova I., Pacak K. Pheochromocytoma: The First Metabolic Endocrine Cancer. Clin. Cancer Res. 2016;22:5001–5011. doi: 10.1158/1078-0432.CCR-16-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amar L., Pacak K., Steichen O., Akker S.A., Aylwin S.J.B., Baudin E., Buffet A., Burnichon N., Clifton-Bligh R.J., Dahia P.L.M., et al. International consensus on initial screening and follow-up of asymptomatic SDHx mutation carriers. Nat. Rev. Endocrinol. 2021;17:435–444. doi: 10.1038/s41574-021-00492-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crona J., Taieb D., Pacak K. New Perspectives on Pheochromocytoma and Paraganglioma: Toward a Molecular Classification. Endocr. Rev. 2017;38:489–515. doi: 10.1210/er.2017-00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alrezk R., Suarez A., Tena I., Pacak K. Update of Pheochromocytoma Syndromes: Genetics, Biochemical Evaluation, and Imaging. Front Endocrinol. 2018;9:515. doi: 10.3389/fendo.2018.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourgeron T., Rustin P., Chretien D., Birch-Machin M., Bourgeois M., Viegas-Pequignot E., Munnich A., Rotig A. Mutation of a nuclear succinate dehydrogenase gene results in mitochondrial respiratory chain deficiency. Nat. Genet. 1995;11:144–149. doi: 10.1038/ng1095-144. [DOI] [PubMed] [Google Scholar]
- 6.Horvath R., Abicht A., Holinski-Feder E., Laner A., Gempel K., Prokisch H., Lochmuller H., Klopstock T., Jaksch M. Leigh syndrome caused by mutations in the flavoprotein (Fp) subunit of succinate dehydrogenase (SDHA) J. Neurol. Neurosurg. Psychiatry. 2006;77:74–76. doi: 10.1136/jnnp.2005.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnichon N., Briere J.J., Libe R., Vescovo L., Riviere J., Tissier F., Jouanno E., Jeunemaitre X., Benit P., Tzagoloff A., et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum. Mol. Genet. 2010;19:3011–3020. doi: 10.1093/hmg/ddq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jha A., de Luna K., Balili C.A., Millo C., Paraiso C.A., Ling A., Gonzales M.K., Viana B., Alrezk R., Adams K.T., et al. Clinical, Diagnostic, and Treatment Characteristics of SDHA-Related Metastatic Pheochromocytoma and Paraganglioma. Front. Oncol. 2019;9:53. doi: 10.3389/fonc.2019.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tufton N., Ghelani R., Srirangalingam U., Kumar A.V., Drake W.M., Iacovazzo D., Skordilis K., Berney D., Al-Mrayat M., Khoo B., et al. SDHA mutated paragangliomas may be at high risk of metastasis. Endocr. Relat. Cancer. 2017;24:L43–L49. doi: 10.1530/ERC-17-0030. [DOI] [PubMed] [Google Scholar]
- 10.Benn D.E., Zhu Y., Andrews K.A., Wilding M., Duncan E.L., Dwight T., Tothill R.W., Burgess J., Crook A., Gill A.J., et al. Bayesian approach to determining penetrance of pathogenic SDH variants. J. Med. Genet. 2018;55:729–734. doi: 10.1136/jmedgenet-2018-105427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nolting S., Bechmann N., Taieb D., Beuschlein F., Fassnacht M., Kroiss M., Eisenhofer G., Grossman A., Pacak K. Personalized management of pheochromocytoma and paraganglioma. Endocr. Rev. 2021;43:199–239. doi: 10.1210/endrev/bnab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bausch B., Schiavi F., Ni Y., Welander J., Patocs A., Ngeow J., Wellner U., Malinoc A., Taschin E., Barbon G., et al. Clinical Characterization of the Pheochromocytoma and Paraganglioma Susceptibility Genes SDHA, TMEM127, MAX, and SDHAF2 for Gene-Informed Prevention. JAMA Oncol. 2017;3:1204–1212. doi: 10.1001/jamaoncol.2017.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taieb D., Hicks R.J., Hindie E., Guillet B.A., Avram A., Ghedini P., Timmers H.J., Scott A.T., Elojeimy S., Rubello D., et al. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:2112–2137. doi: 10.1007/s00259-019-04398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Center for Biotechnology Information [(accessed on 27 July 2022)]; Available online: https://www.ncbi.nlm.nih.gov/clinvar/variation/VCV000472322.8.
- 15.El-Rabadi K., Weber M., Mayerhofer M., Nakuz T., Scherer T., Mitterhauser M., Dudczak R., Hacker M., Karanikas G. Clinical Value of 18F-fluorodihydroxyphenylalanine Positron Emission Tomography/Contrast-enhanced Computed Tomography (18F-DOPA PET/CT) in Patients with Suspected Paraganglioma. Anticancer Res. 2016;36:4187–4193. [PubMed] [Google Scholar]
- 16.Carrasquillo J.A., Chen C.C., Jha A., Ling A., Lin F.I., Pryma D.A., Pacak K. Imaging of Pheochromocytoma and Paraganglioma. J. Nucl. Med. 2021;62:1033–1042. doi: 10.2967/jnumed.120.259689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belhocine T., Spaepen K., Dusart M., Castaigne C., Muylle K., Bourgeois P., Bourgeois D., Dierickx L., Flamen P. 18FDG PET in oncology: The best and the worst (Review) Int. J. Oncol. 2006;28:1249–1261. doi: 10.3892/ijo.28.5.1249. [DOI] [PubMed] [Google Scholar]
- 18.Ayala-Ramirez M., Palmer J.L., Hofmann M.C., de la Cruz M., Moon B.S., Waguespack S.G., Habra M.A., Jimenez C. Bone metastases and skeletal-related events in patients with malignant pheochromocytoma and sympathetic paraganglioma. J. Clin. Endocrinol. Metab. 2013;98:1492–1497. doi: 10.1210/jc.2012-4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilanchezhian M., Jha A., Pacak K., Del Rivero J. Emerging Treatments for Advanced/Metastatic Pheochromocytoma and Paraganglioma. Curr. Treat. Options Oncol. 2020;21:85. doi: 10.1007/s11864-020-00787-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pacak K., Tella S.H. Pheochromocytoma and Paraganglioma. In: Feingold K.R., Anawalt B., Boyce A., Chrousos G., de Herder W.W., Dungan K., Grossman A., Hershman J.M., Hofland H.J., Kaltsas G., et al., editors. Endotext. MDText.com, Inc.; South Dartmouth, MA, USA: 2000. [Google Scholar]
- 21.Jha A., Patel M., Saboury B., Millo C., Ling A., Shah R., Chen C., Meuter L., Talvacchio S., Knue M., et al. Superiority of 68Ga-DOTATATE PET/CT compared to 18F-FDG PET/CT and MRI of the spine in the detection of spinal bone metastases in metastatic pheochromocytoma and/or paraganglioma. J. Nucl. Med. 2020;61:125. [Google Scholar]
- 22.Van Loon K., Zhang L., Keiser J., Carrasco C., Glass K., Ramirez M.T., Bobiak S., Nakakura E.K., Venook A.P., Shah M.H., et al. Bone metastases and skeletal-related events from neuroendocrine tumors. Endocr. Connect. 2015;4:9–17. doi: 10.1530/EC-14-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamidi O., Young W.F., Jr., Iniguez-Ariza N.M., Kittah N.E., Gruber L., Bancos C., Tamhane S., Bancos I. Malignant Pheochromocytoma and Paraganglioma: 272 Patients Over 55 Years. J. Clin. Endocrinol. Metab. 2017;102:3296–3305. doi: 10.1210/jc.2017-00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strosberg J., El-Haddad G., Wolin E., Hendifar A., Yao J., Chasen B., Mittra E., Kunz P.L., Kulke M.H., Jacene H., et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taieb D., Jha A., Treglia G., Pacak K. Molecular imaging and radionuclide therapy of pheochromocytoma and paraganglioma in the era of genomic characterization of disease subgroups. Endocr. Relat. Cancer. 2019;26:R627–R652. doi: 10.1530/ERC-19-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf K.I., Jha A., van Berkel A., Wild D., Janssen I., Millo C.M., Janssen M.J.R., Gonzales M.K., Timmers H., Pacak K. Eruption of Metastatic Paraganglioma After Successful Therapy with (177)Lu/(90)Y-DOTATOC and (177)Lu-DOTATATE. Nucl. Med. Mol. Imaging. 2019;53:223–230. doi: 10.1007/s13139-019-00579-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korpershoek E., Favier J., Gaal J., Burnichon N., van Gessel B., Oudijk L., Badoual C., Gadessaud N., Venisse A., Bayley J.P., et al. SDHA immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J. Clin. Endocrinol. Metab. 2011;96:E1472–E1476. doi: 10.1210/jc.2011-1043. [DOI] [PubMed] [Google Scholar]
- 28.van der Tuin K., Mensenkamp A.R., Tops C.M.J., Corssmit E.P.M., Dinjens W.N., van de Horst-Schrivers A.N.A., Jansen J.C., de Jong M.M., Kunst H.P.M., Kusters B., et al. Clinical Aspects of SDHA-Related Pheochromocytoma and Paraganglioma: A Nationwide Study. J. Clin. Endocrinol. Metab. 2018;103:438–445. doi: 10.1210/jc.2017-01762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the protection of patient identification.