Abstract
Simple Summary
This review summarizes reports from the latest clinical trials assessing the safety and clinical effectiveness of new biological drugs stimulating the immune system to fight cancer. The aim of this study is to show the enormous therapeutic potential of monoclonal antibodies in the treatment of cancer, in particular triple negative breast cancer (TNBC). Introduction of these innovative drugs to the standard clinical cancer therapies, including TNBC, allows for an increase in the response rate to the applied treatment, and consequently extending the lives of patients suffering from cancer. We hope to draw attention to the extremely difficult-to-treat TNBC, as well as the importance of the development of clinical trials evaluating drugs modulating the immune system in TNBC therapy.
Abstract
Triple-Negative Breast Cancer is a subtype of breast cancer characterized by the lack of expression of estrogen receptors, progesterone receptors, as well as human epidermal growth factor receptor 2. This cancer accounts for 15–20% of all breast cancers and is especially common in patients under 40 years of age, as well as with the occurring BRCA1 mutation. Its poor prognosis is reflected in the statistical life expectancy of 8–15 months after diagnosis of metastatic TNBC. So far, the lack of targeted therapy has narrowed therapeutic possibilities to classic chemotherapy. The idea behind the use of humanized monoclonal antibodies, as inhibitors of immunosuppressive checkpoints used by the tumor to escape from immune system control, is to reduce immunotolerance and direct an intensified anti-tumor immune response. An abundance of recent studies has provided numerous pieces of evidence about the safety and clinical benefits of immunotherapy using humanized monoclonal antibodies in the fight against many types of cancer, including TNBC. In particular, phase three clinical trials, such as the IMpassion 130, the KEYNOTE-355 and the KEYNOTE-522 resulted in the approval of immunotherapeutic agents, such as atezolizumab and pembrolizumab by the US Food and Drug Administration in TNBC therapy. This review aims to present the huge potential of immunotherapy using monoclonal antibodies directed against immunosuppressive checkpoints—such as atezolizumab, avelumab, durvalumab, pembrolizumab, nivolumab, cemiplimab, tremelimumab, ipilimumab—in the fight against difficult to treat TNBCs as monotherapy as well as in more advanced combination strategies.
Keywords: triple-negative breast cancer, immunotherapy
1. Introduction
It is estimated that in 2020 there were approximately 2.3 million patients diagnosed with breast cancer (BC) worldwide and 685,000 died of BC [1]. It is supposed that triple negative breast cancer (TNBC) may account for 10–15% of diagnosed breast cancer [2]. This subtype of tumor is often found in patients under 40 years of age and/or with an occurring BRCA1 mutation [3]. Extensive research was conducted to better recognize the molecular phenotypes of breast cancer, which helps adjust treatment and develop new therapeutic opportunities. The clinical classification of BC includes hormone receptor-positive (HR+) tumors with the expression of estrogen (ER) and/or progesterone (PR) receptors, human epidermal receptor 2 (HER2) -enriched tumors with overexpression of HER2 in the absence of HR expression as well as triple-negative tumors without the expression of these three receptors [4]. Furthermore, BC can be categorized into molecular subtypes based on the immunohistochemical markers and complementary DNA (cDNA) microarrays:
Luminal A (ER and PR positive and HER2 negative);
Luminal B (ER and PR positive and HER2 positive or negative);
Basal-like (ER and PR negative, HER2 positive or negative);
HER2 overexpressing (ER and PR negative and HER2 positive) [5].
As TNBC do not express ER, PR, and HER2, TNBC is more diverse than other types in terms of worse outcomes, narrow therapeutic possibilities, and malignant characteristics such as rapid growth and formation of metastases [6]. Its aggressive characteristics reflect the dismal prognosis: the median overall survival of metastatic TNBC is 8–15 months [7]. Because of the actionable molecular targets, chemotherapy remains the main option in TNBC treatment. However, systemic chemotherapy provokes adverse effects and most patients quickly develop resistance. Furthermore, TNBC tends to relapse frequently, which creates the necessity to develop new therapeutic strategies [8].
2. Role of the Immune System
The immune system is a very important factor in the fight against cancer. Its role is to prevent the growth of neoplasm by destroying cancer cells as well as decreasing the possibility to metastasize. However, it can promote tumor progression [9]. The immune system is under self-control by immune checkpoints to protect the body’s natural, healthy cells from immune-mediated death, a process called peripheral tolerance [10]. The basis of this process is the recognition and binding of a T-cell receptor (TCR) to an antigen presented in the major histocompatibility complex (MHC) on the surface of an antigen-presented cell (APC). The cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), programmed cell death-1 (PD-1), and its ligands (PD-L1) are involved in the suppression of the T-cell immune response. It is supposed that the CTLA-4 and PD-1 pathways play roles at different stages of immune system activity. CTLA-4 is responsible for inhibiting a potentially autoaggressive T-cell at the early stages, especially in the lymph nodes. While the PD-1 pathway provides self-tolerance by regulating already activated T-cells at the later stages of an immune response—mostly in peripheral tissue [11].
Immunotherapy has shown high efficacy in the treatment of some types of tumors—such as melanoma, kidney and non-small cell lung cancer (NSCLC)—including immune checkpoint inhibitors (ICIs) as indications for treatment [12,13,14]. In TNBC with a lack of ER, PR and HER2, the use of monoclonal antibodies alone or with other therapeutic options such as chemotherapy, radiotherapy as well as select targeted therapies seems hopeful in early or advanced stages of TNBC. In this review, we aim to shed a light on immunotherapeutic opportunities using monoclonal antibodies approved by the U.S. Food and Drug Administration (FDA) in the treatment of TNBCs, such as atezolizumab and pembrolizumab, We also discuss additional immune checkpoint inhibitors (ICIs), such as nivolumab, avelumab, durvalumab, cemiplimab, tremelimumab and ipilimumab—currently undergoing clinical trials for evaluation of their safety and efficacy in patients with TNBC.
3. CTLA-4 Pathway
CTLA-4 is an immune checkpoint receptor expressed on the surface of activated T-cells. It is a CD28 homolog, however, it binds significantly stronger to B7-1 (CD80) or B7-2 (CD86) molecules presented on the APC [15]. Activation of the T-cells is possible due to the interaction of CD28 with B7-1/2. This leads to the proliferation of T-cells, increased T-cell survival and differentiation due to interleukin-2 production (as a growth factor), as well as increased energy metabolism and upregulation of cell survival genes. Nevertheless, the effect of interaction between CTLA-4 and B7 does not provide stimulation and it is suggested, that suppressive signals may be the result of this process [16]. This is particularly important when the immune system is overactive. Thus, the binding ratio of CD28 and B7 to CTLA-4 and B7 determines whether the T-cells will be activated or inhibited [17]. Furthermore, CTLA-4 may contribute to immune system control through other mechanisms. The expression of CTLA-4 on the surface of regulatory T-cells (Tregs) is constant. Tregs are responsible for regulating the activity of the effector T-cells by downregulating B7 ligands on APCs, leading to limited CD28 costimulation [18]. As a result, of the impairment of CD28:B7-mediated costimulation, there is a limitation of T-cell proliferation and their effector functions [19].
4. PD-1/PD-L1 Pathway
Programmed cell death protein 1 (PD-1) is a transmembrane protein from the B7-CD28 family and is referred to as an inhibitory immune checkpoint that affects T-cell regulation. The basis of this process is the interaction of PD-1 with its ligand—PD-L1 or PD-L2. It is thought that the PD-1 pathway contributes to the inhibition of T-cells proliferation, decreased production of interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and interleukine-2 (IL-2), as well as reduced T-cell survival [20].
PD-1 expression is noticed during T-cell activation and is present on the surface of all subsets of T-cells as well as other immune-related cells—such as B cells, natural killer (NK) cells, and myeloid cells [21]. Interestingly, PD-1 is expressed significantly higher on tumor-infiltrating T-cells [22]. It has been shown that higher expression of PD-1 is related to exhausted T-cells leading to an impaired antitumor response [23].
Binding T-cells by their TCR to an antigen expressed on the cell surface, leads to T-cell activation, proliferation, PD-1 upregulation, and inflammatory cytokine production. Their secretion may contribute to PD-L1 expression on the cancer cell surface, and subsequently after PD-1 and PD-L1 binding, results in suppression of TCR-mediated immune response [24]. Sustained expression of PD-1 and its ligands is usually noticeable during certain conditions—such as chronic infections or cancer. Thus, improvement of T-cell functions and reduction of tumor burden may be achieved by blocking the PD-1 pathway [25,26].
Moreover, it is thought that vascular endothelial growth factor A (VEGF-A)—a proangiogenic molecule produced from cancer cells—is a meaningful factor in the development of an immunosuppressive microenvironment. The basis of this process is the accumulation of myeloid-derived suppressor cells, the inhibition of dendritic cell maturation as well as the induction of regulatory T-cells. A recent study demonstrated that VEGF-A produced in the tumor microenvironment increased the expression of PD-1 and other inhibitory checkpoints involved in CD8+ T-cell exhaustion, which could be counteracted by antiangiogenic agents targeting VEGF-A-VEGFR [27].
5. Immunotherapy Using PD-1/PD-L1 Checkpoint Inhibitors
Cancer cells very often acquire the features that enable them to evade the immune system’s responses through immune checkpoint pathways or by forming an immunosuppressive microenvironment that causes tumor recognition impairment and disease development [28].
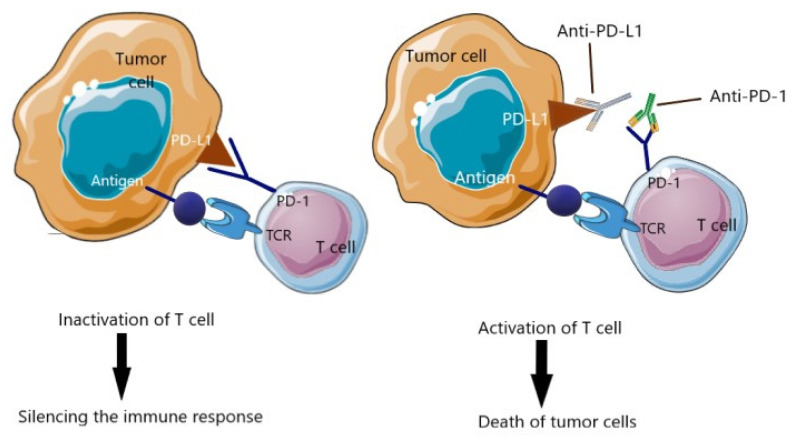
The immune checkpoints pathway is essential to protect normal tissue from immune system hyperactivity. The expression of PD-L1 in TNBC is about 20%, which is correlated with the development of resistance to specific CD8+ T-cells and higher tumorigenesis [29]. T-cells, B cells, natural killer (NK) T-cells express PD-1 receptor on their surface and it is correlated with the silencing of the immune system by the tumor. The purpose of using ICIs is to elicit an immune response by suppressing the inhibitory pathways (Figure 1) [30]. Thus, these features suggest a promising therapeutic target for this disease.
Figure 1.
Immune checkpoint inhibitors. The expression of PD-L1 allows tumor cells to switch T-cells off and keep the cancer cells from being killed. The use of immune checkpoint inhibitors permits boosting the immune response to kill the cancer cells. TCR, T-cell receptor; PD-1, programmed death protein 1; PD-L1, programmed death-ligand 1 [Own illustration designed on the basis of [4]].
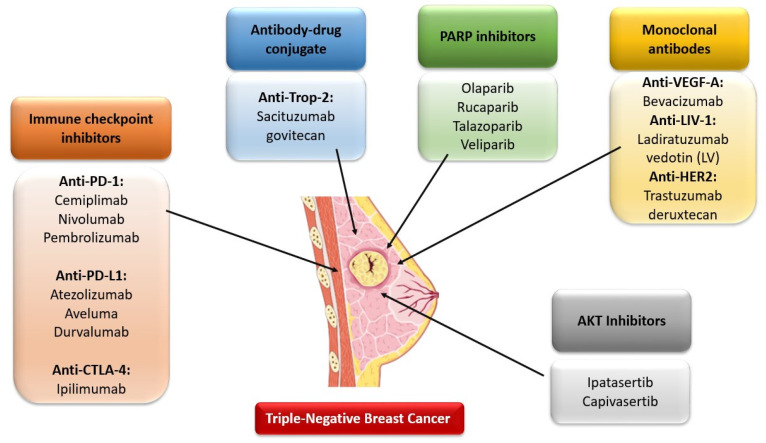
Drugs including immunotherapeutic agents that are approved for TNBC therapy such as atezolizumab and pembrolizumab as well as other drugs such as AKT inhibitors, monoclonal antibodies, Poly ADP-Ribose Polymerase (PARP) inhibitors and antibody-drug conjugate currently being investigated in TNBC treatment are shown in Figure 2.
Figure 2.
Drugs including immunotherapeutic agents that are currently being investigated in TNBC treatment [Own illustration designed on the basis of [8]].
5.1. Atezolizumab
Atezolizumab is an Fc-engineered, humanized, monoclonal IgG1 antibody that selectively binds to programmed death-ligand 1 (PD-L1) and inhibits its interactions with PD-1 and B7.1 receptors causing T-cells more sensitive to the tumor. In a phase one of the clinical trial, atezolizumab as a single agent was evaluated in terms of safety and antitumor activity and showed good clinical outcomes and potential benefits in patients with metastatic TNBC especially in earlier lines of treatment or those with higher levels of tumor-infiltrating immune cells (ICs) and PD-L1 positive ICs [31].
The IMpassion130 was carried out to investigate the antitumor effects and clinical benefits of combining atezolizumab with taxane chemotherapeutic agent—paclitaxel in the form of nanoparticle albumin-bound (nab-paclitaxel)—in patients with unresectable, locally advanced or metastatic TNBC. In the first interim analysis, this combined therapy increased progression-free survival (PFS) compared with placebo plus nab-paclitaxel—(7.2 months compared to 5.5 months, respectively). Furthermore, higher differences were noticed among patients with PD-L1-positive tumors. The median PFS was 7.5 months in the atezolizumab group and 5.0 months in the placebo groups. Interestingly, therapy with atezolizumab prolonged overall survival (OS), particularly in groups with PD-L1 expression. There were 3.7 months of difference between the atezolizumab and control groups (21.3 vs. 17.6 months). In addition, there was a ten-month difference in median overall survival between the PD-L1 groups receiving atezolizumab and placebo (25.0 vs. 15.5 months properly). There were no significant differences between races. The most meaningful benefits occurred in the oldest patient TNBC group (≥65 years of age) [32].
The next interim overall survival analysis of IMpassion130 confirmed the previous results of improvement in PFS and OS. Thus, these findings clearly indicate antitumor activity as well as clinical benefits of combination therapy with atezolizumab and nab-paclitaxel, particularly for patients with PD-L1 immune cell-positive metastatic TNBC [33]. Based on the findings from the IMpassion130 clinical trial, the U.S. Food and Drug Administration and the European Commission have approved atezolizumab plus nab-paclitaxel for the treatment of adult patients with unresectable locally advanced or metastatic TNBC whose tumors have PD-L1 expression ≥1% and who have not received prior chemotherapy for metastatic disease [34].
The safety and efficacy of therapy with atezolizumab and nab-paclitaxel were also evaluated in a Japanese subgroup. The outcomes were consistent with those received from IMpassion130. This therapy prolonged PFS and showed a high objective response rate (ORR) even higher than the ORR announced in the IMpassion130 trial. Objectively, atezolizumab had a demonstrated safety profile and was well-tolerated in the Japanese population however, there were adverse events such as alopecia, peripheral sensory neuropathy, decreased neutrophil count, nasopharyngitis and decreased white blood cell count occurring more frequently than in the overall Impasssion130 population. These findings were limited by the small study population but can be contributed to the adjustment of combined therapy with the use of atezolizumab and nab-paclitaxel to the standards of treatment available in Japan [35].
5.2. Pembrolizumab
Pembrolizumab is a humanized, monoclonal IgG4 antibody that selectively binds to programmed death-1 (PD-1) receptor and inhibits interaction with its ligands—PD-L1 and PD-L2. The KEYNOTE-012 was the first study evaluating the safety and anti-cancer efficacy of pembrolizumab as a monotherapy in 32 enrolled, previously treated with chemotherapy patients with PD-L1-positive, advanced TNBC. In this 1b cohort study, this single agent showed an encouraging overall response rate (ORR) of 18.5% and with a demonstrated safety profile consistent with previous findings [36]. However, in the phase two of the KEYNOTE-086 study (cohort A) pembrolizumab did not provide significant outcomes in patients with mTNBC. ORR was 5.3% in a total group of 170 previously treated PD-L1-unselected patients [37]. Interestingly, in cohort B of the same investigation, ORR was 21.4% in patients with untreated PD-L1-positive tumors. Moreover, pembrolizumab demonstrated a clinically relevant median duration of response of 10.4 months and was well-tolerated. Thus, these results indicate that pembrolizumab has greater efficacy in the treatment of earlier metastatic stages [38].
In addition, some results point to the immunomodulatory potential of chemotherapy to improve anticancer response to ICIs [39]. A clinically significant antitumor effect of pembrolizumab combined with chemotherapy was demonstrated in the KEYNOTE-355 phase three clinical trial. In this randomized clinical trial—with 1372 patients enrolled—the combined therapy with this monoclonal antibody prolonged PFS as much as 4.1 months compared with placebo plus chemotherapy (9.7 months in the pembrolizumab plus chemotherapy group and 5.6 months in the chemotherapy alone group) among mTNBC patients with PD-L1 expression in whom a combined positive score (CPS) was ≥10. Furthermore, this trial included not only one chemotherapeutic option, such as presented in the IMPASSION-130 clinical trial (atezolizumab and nab-paclitaxel), but investigated several therapeutic standards—such as nab-paclitaxel, paclitaxel, or gemcitabine plus carboplatin—expanding the spectrum of possibilities. There were no new safety concerns. Adverse effects were noticed in 68% of the pembrolizumab group and 67% of the placebo group [40].
Pembrolizumab and Radiotherapy
Consistent with the previously noted findings concerning the immunomodulatory effects of radiotherapy (RT) in combination with ICIs, based on increasing cytotoxic T-cell levels and activation of apoptosis pathways, a phase two clinical trial demonstrated promising clinical benefits from the combination of RT and pembrolizumab. This study included a small group of 17 TNBC patients showing poor prognosis. The women were not divided into groups in terms of PD-L1 expression. The median ORR was 17.6%, similar to the findings with pembrolizumab as a single agent in pretreated patients with PD-L1-positive mTNBC. Furthermore, this combined therapy seemed to be safe with-low grade adverse effects. This study was limited to a small single-arm group and more future clinical trials are needed to confirm the clinical effectiveness [41].
5.3. Immunotherapy Combined with Antiangiogenic Factor
The impaired function of the tumor blood vessels may contribute to the decreasing levels of cytotoxic T-cells (involved in the fight against cancer) and increasing levels of myeloid-derived suppressor cells (MDSC) and regulatory T-cells (involved in inhibiting of the immune response). In addition, some research indicates that antiangiogenic therapy leads to the augmentation of PD-L1 expression as well as CD8+ T-cell infiltration in the tumor microenvironment. Thus, a reduction of the immunosuppression, as well as sensitizing the tumor to the immune response could be achieved by restoring proper perfusion [42].
Consistent with these promising findings, camrelizumab (PD-1 inhibitor) in combination with apatinib (tyrosine kinase inhibitor, which selectively inhibits vascular endothelial growth factor receptor 2 (VEGFR2)) was was investigated in patients with advanced TNBC. In this two phase clinical trial, this therapy showed a significant ORR of 43.3% and was well-tolerated, irrespective of PD-L1 expression. However, this open-label study had several limitations and large clinical trials with randomization are needed to confirm these results as to compare ICIs in combination with VEGFR2 inhibitors with ICIs plus placebo [7].
5.4. Durvalumab with Neoadjuvant Therapy
As previously mentioned, ICIs showed promising therapeutic benefits, especially in combination with chemotherapy. In the GeparNuevo phase two clinical trial, the combination of durvalumab (PD-1 inhibitor) with standard neoadjuvant chemotherapy was evaluated in early TNBC. In this study, the patients were randomized in terms of stromal tumor-infiltrating lymphocytes. Two weeks before starting treatment with chemotherapy, the patients received durvalumab or a placebo in monotherapy. Subsequently, the patients were treated with durvalumab or placebo (every four weeks) and nab-paclitaxel (weekly) for 12 weeks, followed by durvalumab or placebo and epirubicin/cyclo- phosphamide (EC) (four cycles). The use of durvalumab in combined therapy with nab-paclitaxel and EC demonstrated a higher pCR rate than the placebo group (53.4% vs. 44.2% respectively), however without statistical significance (p = 0.287). Nevertheless, the patients who had received durvalumab as a monotherapy two weeks before the main treatment showed a higher pCR rate than the placebo group (61.0% vs. 41.4%, p = 0.035). Thus, these findings suggest the potential benefits derived from using durvalumab with anthracycline/taxane-based therapy, particularly in patients pretreated with durvalumab as a single agent before the main therapy [43].
5.5. Nivolumab
Nivolumab is a human monoclonal antibody directed against the PD-1 immunoregulatory receptor located on the surface of T-cells. Despite the numerous indications for solid tumor treatment (melanoma, non-small cell lung cancer, malignant pleural mesothelioma, renal cell carcinoma, squamous cell cancer of the head and neck, urothelial cancer, colorectal cancer, oesophageal squamous cell carcinoma), research concerning the use of nivolumab in TNBC therapy is limited.
Recently, in the first stage of phase two TONIC clinical trial, nivolumab had shown high clinical efficacy among patients with metastatic TNBC. In this trial, 67 patients were divided into five cohorts, the first one was a control group with a two-week waiting period, and the other four included patients who received the induction treatment consisting of irradiation, low-dose cyclophosphamide, cisplatin, or doxorubicin for two weeks. After this short-term treatment, the therapy was continued for eight weeks with three cycles of nivolumab. In all cohorts, the objective response rate was 20% after full treatment. Additionally, the median progression-free survival was 1.9 months. The best results were noticed in the cisplatin and doxorubicin cohorts, where the ORR was 23% and 35%, respectively. Furthermore, treatment with cisplatin and doxorubicin was associated with the upregulation of immune-related genes involved in the PD-1/PD-L1 and T-cell cytotoxicity pathways. Thus, consistent with these findings pretreatment with the use of immunomodulating chemotherapeutic agents—such as cisplatin and doxorubicin—promotes a more favorable tumor microenvironment and provides a high and durable response to nivolumab, as well as the clinical benefits derived from this induction strategies therapy [44].
5.6. Avelumab
Avelumab is another immune checkpoint inhibitor. This human IgG1 monoclonal antibody directed against PD-L1 blocks the PD-1/PD-L1 pathway but does not affect PD-1/PD-L2 interaction [45]. The specific feature that distinguishes this agent is its second mechanism of action. In the preclinical studies, avelumab showed an additional advantage consisting of the ability to destroy human cancer cells by inducing antibody-dependent cell-mediated cytotoxicity (ADCC) [46]. Furthermore, an in vitro study was carried out to evaluate the avelumab-mediated ADCC on TNBC line cells, whose PD-L1 expression was changed with the use of peripheral blood mononuclear cells (PBMC) or purified NK cells from healthy donors. In this research, avelumab showed antitumor activity against TNBC cells independent of the PD-1/PD-L1 pathway. Avelumab increased TNBC cell death through an NK-mediated cytotoxicity mechanism. Additionally, higher expression of PD-L1 on tumor cells was related to increasing sensitivity to avelumab-mediated ADCC. Interestingly, the addition of IL-2 and IL-15 to the tumor cells contributed to the enhancement of the lysis of tumor cells through increased NK cell activity [47]. However, in clinical conditions in patients with metastatic breast cancer, avelumab in monotherapy did not show significant results. The phase one clinical trial JAVELIN enrolled 168 patients with various subtypes of breast cancer. This population included 58 patients with TNBC. The pretreatment of all patients had been a median of three prior therapies for metastatic disease. Among patients with TNBC, 50% of them had been previously treated with ≥2 prior lines of therapy for metastatic disease. The overall objective response rate was 3.0%, and 5.2% in the subgroup with TNBC. Furthermore, the percentage of patients in the overall population with PD-L1-positive tumors achieved a much higher ORR relative to the patients without PD-L1 expression (16.7% vs. 1.6% respectively and 22.2% vs. 2.6% respectively, in the TNBC subgroup). Additionally, avelumab as a single agent was well-tolerated and showed a safety profile consistent with previous findings [48].
5.7. Cemiplimab
Cemiplimab is an IgG4 monoclonal antibody blocking the PD-1 pathway. In a clinical trial among patients with metastatic cutaneous squamous cell carcinoma (CSCC), Cemiplimab showed a safety profile and high efficacy. Consistent with these findings, this agent was approved by the U.S. FDA for patients with metastatic CSCC or locally advanced CSCC [49]. However, to date, studies evaluating the safety and efficacy of TNBC therapy are ongoing and results are not yet available.
5.8. Tremelimumab
Tremelimumab is one of the first CTLA-4-pathway immune checkpoint inhibitors. This IgG2 monoclonal antibody has antitumor activity potential by inhibiting the CTLA-4 receptor expressed on activated T-cells, preventing the interaction between the antigen-presenting cell ligands B7-1 (CD80), B7-2 (CD86), and CTLA4. Disabling the CTLA-4-mediated inhibition pathway leads to binding the CD28 protein presented on T-cells with its B7-1/2 ligand on APCs. Thus, the use of this agent allows for increasing the activation of T-cells, as well as their effector functions to kill the cancer cells [50,51]. Currently, its safety and antitumor activity have been evaluated in many types of cancer. To date, tremelimumab has no indications for the treatment of any type of breast cancer. Its safety and activity were assessed in patients with HR-positive metastatic BC in combination with a steroidal aromatase inhibitor—exemestane. In this clinical trial, tremelimumab in combined therapy was well-tolerated with stable responses for ≥12 weeks in 42% of patients. Additionally, the immunomodulatory activity of tremelimumab was observed and associated with the augmented expression of inducible costimulator (ICOS) on CD4+ and CD8+ T-cells as well as a decreased number of FoxP3+ Treg cells [51,52]. Furthermore, a phase one clinical trial was conducted to investigate the safety and efficacy of tremelimumab with radiotherapy in patients with metastatic breast cancer. This study enrolled six patients—five with HR+ metastatic BC and one with TNBC. Tremelimumab in this strategy was safe and well-tolerated with the frequent adverse events such as lymphopenia, fatigue and rash. Objective responses were not observed. The most meaningful response was stable disease. Progression-free survival was 1.5 months, and overall survival was 50.8 months [52,53].
5.9. Ipilimumab
Ipilimumab is the second CTLA-4-pathway antagonist. It is a human IgG1 monoclonal antibody selectively binding with CTLA-4 receptor presented on the activated T-lymphocytes. To date, ipilimumab is approved by the U.S. Food and Drug Administration for the treatment of melanoma, renal cell carcinoma, colorectal cancer, hepatocellular carcinoma, non-small cell lung cancer and malignant pleural mesothelioma [53,54]. Despite many clinical trials in the treatment of solid tumors, studies focused on the efficacy and toxicity of ipilimumab in the treatment of breast cancer, especially in TNBC, are less abundant. An in vitro study showed that the inhibition of CTLA-4 receptors by ipilimumab has an impact on the release of IL-2 by tumor cells simultaneously regulating the tumor microenvironment and increasing the immune response [55]. In a clinical study in a group of 29 patients with relapsed malignancy, after a single infusion of ipilimumab, enhanced intracellular CTLA-4 expression (probably due to T-cell activation) as well as increased levels of activated T lymphocytes were observed without significant changes in the regulatory T-cell population [56]. In addition, ipilimumab was evaluated in a pilot study in patients with breast cancer. Nineteen patients were enrolled in this clinical trial, 12 of them received ipilimumab—six patients received ipilimumab in monotherapy and six patients received ipilimumab with cryoablation. After the use of combination immunotherapy, an increase in T helper type 1 cytokines, ICOS+Ki67+CD4+, and ICOS+Ki67+CD8+ T-cells was observed as well as an increased CD8+ Tcell/FoxP3+ Treg ratio within the tumor. Thus, consistent with these findings, the combination of Ipilimumab with cryoablation may contribute to the anti-tumor benefits derived from this strategy [57,58].
6. Biomarkers
Because not all patients respond to the administered immunotherapy, there is a strong need to identify prognostic and predictive biomarkers of response to the applied treatment, which would allow the selection of patients only to the group that would respond to the applied treatment. As it turns out, the implementation of ICIs for mTNBC therapy in patients with high tumor-infiltrating lymphocytes (TILs) shows encouraging results, which indicates the potential benefits of the applied immunotherapy for these patients [59]. Some authors, for instance, Xiao, Y., et al., in their studies, suggest the use of TILs and immune checkpoint molecules as biomarkers providing information about the effectiveness of treatment using ICIs in patients with TNBC [60]. Interestingly, the selection based on PD-L1 is quite controversial due to the lack of a consistent assessment standard. Additionally, new biomarkers like immune gene signatures liquid biopsy, and gut microbiome are still under research and cannot yet be used in clinical practice. Therefore, it is necessary to develop complex methods and further research into new and already known potential biomarkers of the response to immunotherapy in patients with TNBC [59].
7. Immune-Related Adverse Events (IRAEs)
The activation of the immune system using ICIs is also associated with the occurrence of characteristic side effects that differ from chemotherapy due to the mechanism of immune-related toxicity, a delayed onset and prolonged duration. IRAEs usually develop within weeks to months of starting immunotherapy. Nevertheless, they can appear at any time, even after the use of ICIs has been discontinued. The most common IRAEs include dermatologic toxicities, endocrine toxicities, gastrointestinal toxicities and hepatitis. Rarely developing IRAEs involve the cardiovascular, pulmonary, musculoskeletal, ocular, and central nervous systems. Nevertheless, these toxicities can develop in almost any organ. Reducing IRAEs very often requires a comprehensive approach and the involvement of a multidisciplinary medical team. Corticosteroids are most commonly used to mitigate the effects of ICIs, but other measures may also be required, such as additional immunosuppression, delay, or discontinuation of treatment. Therefore, it is also extremely important to educate both healthcare professionals and patients to identify IRAEs early and manage treatment appropriately [61].
8. Conclusions
Immunotherapy is without a doubt a groundbreaking achievement having a big impact on the improvement of clinical conditions and could be a life-changing opportunity for patients struggling with a highly aggressive type of breast cancer. Immune checkpoints are the most often used by cancer to switch T-cells off and keep the cancer cells from being killed. The use of immune checkpoint inhibitors permits boosting the immune response to kill the cancer cells. Current studies clearly indicate the necessity to combine the ICIs in various therapeutic regimens in order to increase their effectiveness and clinical benefits (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8). Furthermore, there is a strong need to implement immunotherapy in the early-stage setting of metastatic treatment to increase the overall response rate [58].
Table 1.
Atezolizumab.
| NCT Number | Status | Co-Treatment/Intervention | Phase | Participants | Results/Conclusions | References |
|---|---|---|---|---|---|---|
| NCT03125902 | Active, not recruiting | Paclitaxel, Placebo | III | 651 | Combining atezolizumab with paclitaxel did not improve PFS or OS versus paclitaxel alone | [62] |
| NCT04148911 | Recruiting | Nab-Paclitaxel | III | 180 | Estimated Primary Completion Date: 29 October 2024 | [63] |
| NCT02425891 | Completed | Nab-Paclitaxel, Placebo | III | 902 | Atezolizumab plus nab-paclitaxel prolonged progression-free survival among patients with metastatic triple-negative breast cancer in both the intention-to-treat population and the PD-L1-positive subgroup. | [32,64,65] |
| NCT03281954 | Active, not recruiting | Neoadjuvant chemotherapy with Atezolizumab or Placebo followed by adjuvant continuation of atezolizumab or placebo | III | 1520 | Estimated Primary Completion Date: 31 December 2023 | [66] |
| NCT03164993 | Recruiting | Chemotherapy (pegylated liposomal doxorubicin + cyclophosphamide), Placebo for atezolizumab | II | 75 | Estimated Primary Completion Date: 31 December 2023 | [67] |
| NCT04690855 | Recruiting | Talazoparib, Radiation | II | 23 | Estimated Primary Completion Date: June 2022 | [68] |
| NCT04584112 | Recruiting | Tiragolumab, Nab-Paclitaxel, Carboplatin, Doxorubicin, Cyclophosphamide, Granulocyte colony-stimulating factor (G-CSF), Granulocyte-Macrophage colony-stimulating factor (GM-CSF) | I | 80 | Estimated Primary Completion Date: 15 March 2022 | [69] |
| NCT03498716 | Recruiting | Chemotherapy (Paclitaxel, Dose-dense Doxorubicin/Epirubicin, Cyclophosphamide) | III | 2300 | Estimated Primary Completion Date: 31 May 2024 | [70] |
| NCT03197935 | Active, not recruiting | Nab-Paclitaxel, Doxorubicin, Cyclophosphamide, Filgastrim, Pegfilgastrim, Placebo | III | 333 | In patients with early-stage TNBC, neoadjuvant treatment with atezolizumab in combination with nab-paclitaxel and anthracycline-based chemotherapy significantly improved pathological complete response rates with an acceptable safety profile. | [71] |
| NCT04177108 | Active, not recruiting | Ipatasertib, Paclitaxel, Placebo for Atezolizuamb, Placebo for Ipatasertib | III | 242 | Estimated Primary Completion Date: 10 October 2025 | [72] |
| NCT03256344 | Active, not recruiting | Talimogene Laherparepvec | I | 36 | Estimated Study Completion Date: 25 August 2022 | [73] |
| NCT03292172 | Terminated | RO6870810 | I | 36 | The study has stopped early and will not start again. Participants are no longer being examinated or treated. | [74] |
| NCT04739670 | Not yet recruiting | Bevacizumab, Carboplatin, Gemcitabine | II | 31 | Estimated Primary Completion Date: September 2025 | [75] |
| NCT03371017 | Recruiting | Chemotherapy (Gemcitabine, Capecitabine, Carboplatin), Placebo for atezolizumab | III | 572 | Estimated Primary Completion Date: 31 January 2023 | [76] |
| NCT04770272 | Recruiting | Atezolizumab 840 MG in 14 ML Injection, Atezolizumab 1200 MG in 20 ML Injection, Carboplatin, Paclitaxel, Epirubicin, Cyclophosphamide, Biopsy Arm A, Biopsy Arm B, Surgery | II | 458 | Estimated Primary Completion Date: 1 August 2023 | [77] |
| NCT03206203 | Active, not recruiting | Carboplatin | II | 106 | Estimated Primary Completion Date: 30 November 2022 | [78] |
| NCT03756298 | Recruiting | Capecitabine, Capecitabine in monotherapy | II | 284 | Estimated Primary Completion Date: 31 January 2024 | [79] |
| NCT02530489 | Active, not recruiting | Atezolizumab + Nab-Paclitaxel | II | 37 | Estimated Primary Completion Date: 28 February 2022 | [80] |
| NCT04408118 | Recruiting | Paclitaxel, Bevacizumab | II | 100 | Estimated Primary Completion Date: October 2022 | [81] |
| NCT03853707 | Suspended | Capecitabine, Carboplatin, Ipatasertib, Paclitaxel | I, II | 40 | Estimated Primary Completion Date: 21 June 2023 | [82] |
| NCT03483012 | Active, not recruiting | Stereotactic radiosurgery (SRS) | II | 45 | Estimated Primary Completion Date: 30 September 2021 | [83] |
| NCT03101280 | Completed | Rucaparib | I | 29 | No Study Results Posted | [84] |
| NCT01898117 | Recruiting | Carboplatine and Cyclophosphamide, Carboplatine and Cyclophosphamide in monotherapy, Paclitaxel, Paclitaxel in monotherapy | II | 304 | Estimated Primary Completion Date: December 2024 | [85] |
| NCT03464942 | Recruiting | Stereotactic Ablative Body Radiotherapy (SABR) | II | 52 | Estimated Study Completion Date: April 2022 | [86] |
| NCT02883062 | Active, not recruiting | Carboplatin, Lumpectomy, Mastectomy, Paclitaxel | II | 72 | Estimated Primary Completion Date: 1 July 2022 | [87] |
| NCT04249167 | Active, not recruiting | Cryosurgery, Nab-Paclitaxel | I | 5 | Estimated Primary Completion Date: 31 December 2021 | [88] |
| NCT02322814 | Completed | Cobimetynib, Paclitaxel, Nab-Paclitaxel, Placebo | II | 169 | Percentage of Participants with Confirmed Overall Response (OR): Cohort II (Cobimetinib, Paclitaxel, Atezolizumab): 34.4%, Cohort III (Cobimetinib, Nab-Paclitaxel, Atezolizumab): 29%, Serious Adverse Events: Cohort II: 46.88%, Cohort III: 43.33% | [89] |
| NCT04434040 | Recruiting | Sacituzumab govitecan | II | 40 | Estimated Primary Completion Date: 30 December, 2023 | [90] |
| NCT03800836 | Active, not recruiting | Ipatasertib, Paclitaxel, Nab-Paclitaxel, AC (Doxorubicin and Cyclophosphamide) | I | 140 | Estimated Primary Completion Date: 29 October 2022 | [91] |
| NCT03961698 | Recruiting | IPI-549 (eganelisib), Nab-Palitaxel, Bevacizumab | II | 90 | Estimated Primary Completion Date: 1 August 2022 | [92] |
| NCT02620280 | Active, not recruiting | Carboplatin, Nab-Paclitaxel, Anthra-AC or EC (adriamycin or epirubicin and cyclophosphamide or FEC (fluorouracil, epirubicin and cyclophosphamide) | III | 278 | Estimated Primary Completion Date: May 2022 | [93] |
| NCT04849364 | Recruiting | Capecitabine, Talazoparib, Inavolisib, | II | 197 | Estimated Primary Completion Date: January 2024 | [94] |
| NCT04639245 | Recruiting | Atezolizumab, Cyclophosphamide, Fludarabine, MAGE-A1-specific T-cell Receptor-transduced Autologous T-cells, PD1 Inhibitor | I, II | 18 | Estimated Primary Completion Date: 1 December 2024 | [95] |
| NCT02708680 | Unknown | Etinostat, Placebo for atezolizumab | I, II | 88 | No Study Results Posted | [96] |
| NCT03915678 | Recruiting | BDB001, Radiotherapy | II | 247 | Estimated Primary Completion Date: September 2023 | [97] |
| NCT03424005 | Recruiting | Capecitabine, Ipatasertib, SGN-LIV1A, Bevacizumab, Chemotherapy (Gemcitabine, Carboplatin or Eribulin), Selicrelumab, Tocilizumab, Nab-Paclitaxel, Sacituzumab Govitecan | I, II | 280 | Estimated Primary Completion Date: 3 January 2023 | [98] |
| NCT03289962 | Recruiting | Autogene cevumeran | I | 770 | Estimated Primary Completion Date: 1 February 2024 | [99] |
| NCT05001347 | Not yet recruiting | TJ004309 | II | 60 | Estimated Primary Completion Date: October 2024 | [100] |
| NCT03829501 | Recruiting | KY1044 and KY1044 as a single agent | I, II | 412 | Estimated Primary Completion Date: May 2023 | [101] |
| NCT02543645 | Terminated | Varlilumab | I | 18 | The study has stopped early and will not start again. Participants are no longer being examined or treated. No Study Results Posted | [102] |
| NCT03170960 | Recruiting | Cabozantinib | I, II | 1732 | Estimated Primary Completion Date: December 2021 | [103] |
| NCT04638751 | Recruiting | Immunotherapy and Chemotherapeutic agent | Observational study | 4000 | Estimated Primary Completion Date: December 2022 | [104] |
| NCT03232593 | Recruiting | Monotherapy | Phase 4, surveillance study | 3000 | Estimated Primary Completion Date: 11 January 2023 | [105] |
| NCT03952325 | Terminated | Pembrolizumab, Tesetaxel, Nivolumab | II | 294 | The Sponsor has discontinued the development of tesetaxel. No Study results Posted | [106] |
| NCT04102618 | Recruiting | Trastuzumab, Pelareoreb, Letrazole | I | 38 | No Study Results Posted | [107] |
| NCT04954599 | Not yet recruiting | CP-506, Carboplatin, Immune checkpoint inhibitor (including atezolizumab) | I, II | 126 | Estimated Primary Completion Date: September 2024 | [108] |
| NCT05069935 | Not yet recruiting | FT538, Cyclophosphamide, Fludarabine, Combination Product: Monoclonal antibody (including Atezolizumab)- Dose Escalation Combination Product: Monoclonal antibody (including Atezolizumab)—Dose Expansion | I | 189 | Estimated Primary Completion Date: 5 September 2023 | [109] |
Table 2.
Pembrolizumab.
| NCT Number | Status | Co-Treatment/Intervention | Phase | Participants | Results/Conclusions | References |
|---|---|---|---|---|---|---|
| NCT04683679 | Recruiting | Olaparib, Radiation | II | 56 | Estimated Primary Completion Date: January 2025 | [110] |
| NCT04095689 | Recruiting | Docetaxel, IL-12 gene therapy, L-NMMA | II | 30 | Estimated Primary Completion Date: January 2024 | [111] |
| NCT04427293 | Recruiting | Lenvatinib, | I | 12 | Estimated Primary Completion Date: July 2026 | [112] |
| NCT04024800 | Active, not recruiting | AE37 Peptide vaccine | II | 29 | Estimated Primary Completion Date: 30 June 2023 | [113] |
| NCT02977468 | Recruiting | Intraoperative radiation therapy (IORT) | I | 15 | Estimated Primary Completion Date: 31 December 2022 | [114] |
| NCT03362060 | Active, not recruiting | PVX-410 | I | 20 | Estimated Primary Completion Date: 31 December 2022 | [115] |
| NCT04191135 | Active, not recruiting | Olaparib, Carboplatin, Gemcitabine | II, III | 1225 | Estimated Primary Completion Date: 26 January 2026 | [116] |
| NCT02768701 | Active, not recruiting | Cyclophosphamide | II | 40 | Estimated Primary Completion Date: 15 March 2023 | [117] |
| NCT02622074 | Completed | Nab-paclitaxel, Anthracycline (doxorubicin), Cyclophosphamide, Carboplatin, Palictaxel | I | 60 | Combination neoadjuvant chemotherapy and pembrolizumab for high-risk, early-stage TNBC showed manageable toxicity and promising antitumor activity. In an exploratory analysis, the pCR rate showed a positive correlation with tumor PD-L1 expression and sTIL levels | [118] |
| NCT03720431 | Active, not recruiting | TTAC-0001 | I | 11 | Estimated Study Completion Date: 26 February 2022 | [119] |
| NCT03121352 | Active, not recruiting | Nac-paclitaxel, Carboplatin | II | 30 | Estimated Primary Completion Date: 6 February 2022 | [120] |
| NCT03036488 | Active, not recruiting | Carboplatin. Paclitaxel, Doxorubicin, Epirubicin, Cyclophosphamide, Granulocyte colony stimulating factor: Filgrastim or Pegfilgrastim, Placebo for pembrolizumab | III | 1174 | Estimated Primary Completion Date: 30 September 2025 | [121] |
| NCT03145961 | Active, not recruiting | Monotherapy | II | 208 | Estimated Study Completion Date: 1 December 2022 | [122] |
| NCT02819518 | Active, not recruiting | Nab-Paclitaxel, Paclitaxel, Gemcitabine, Carboplatin, Normale Saline Solution (placebo) | III | 882 | Pembrolizumab-chemotherapy showed a significant and clinically meaningful improvement in progression-free survival versus placebo-chemotherapy among patients with metastatic triple-negative breast cancer with CPS of 10 or more. These findings suggest a role for the addition of pembrolizumab to standard chemotherapy for the first-line treatment of metastatic triple-negative breast cancer. | [40] |
| NCT03567720 | Recruiting | Tavokinogene telseplasmid, Immunopulse (electroporation), Nab-paclitaxel | II | 65 | Estimated Primary Completion Date December 2023 | [123] |
| NCT02555657 | Completed | Capecitabine, Eribulin, Gemcitabine, Vinorelbine | III | 622 | Pembrolizumab did not significantly improve overall survival in patients with previously treated metastatic triple-negative breast cancer versus chemotherapy. | [124] |
| NCT03639948 | Recruiting | Carboplatin, Docetaxel, Pegfilgrastim | II | 100 | Estimated Primary Completion Date: November 2021 | [125] |
| NCT03184558 | Terminated | Bemcentinib | II | 29 | Disease Control Rate (DCR): 3.4% of participants Progression-free Survival (PFS): 13.1 weeks Overall Survival (OS): 32.0 weeks |
[126] |
| NCT04986852 | Not yet recruiting | Olinvacimab | II | 36 | Estimated Primary Completion Date: 28 February 2025 | [127] |
| NCT04468061 | Recruiting | Sacituzumab govitecan | II | 110 | Estimated Primary Completion Date: 1 June 2023 | [128] |
| NCT02734290 | Active, not recruiting | Paclitaxel, Capecitabine | I, II | 29 | Estimated Completion Date: May 2022 | [129] |
| NCT03752723 | Recruiting | GX-I7, Cyclophosphamide | I, II | 83 | Estimated Study Completion Date: December 2021 | [130] |
| NCT02981303 | Completed | Imprime PGG | II | 64 | No Study Results Posted | [131] |
| NCT03644589 | Withdrawn | Cisplatin | II | 0 | Withdrawn (No participants enrolled). | [132] |
| NCT02755272 | Recruiting | Carboplatin, Gemcitabine | II | 87 | Estimated Primary Completion Date: October 2022 | [133] |
| NCT04373031 | Recruiting | IRX 2 | II | 30 | Estimated Primary Completion Date: June 2024 | [134] |
| NCT02971761 | Active, not recruiting | Enobosarm, Laboratory Biomarker Analysis | II | 29 | Results Submitted—Quality Control (QC) Review Has Not Concluded | [135] |
| NCT02513472 | Completed | Eribulin mesylate | I, II | 258 | ORR 25.8% in participants with mTNBC who were never treated with systemic anticancer therapy in the metastatic setting, 21.8% in participants with mTNBC previously treated with 1 to 2 lines of systemic anticancer therapy in the metastatic setting, 23.4% in participants with mTNBC who were never treated with systemic anticancer therapy and previously treated with 1 to 2 lines of systemic anticancer therapy in the metastatic setting | [136] |
| NCT03310957 | Recruiting | Ladiratuzumab vedotin | I, II | 161 | Estimated Primary Completion Date: 28 February 2022 | [137] |
| NCT02447003 | Completed | Monotherapy | II | 254 | Safe, durable antitumor activity in a subset of patients with previously treated mTNBC. Safe, durable antitumor activity as first-line therapy for patients with PD-L1-positive mTNBC. |
[36,37,38] |
| NCT03599453 | Active, not recruiting | Chemokine Modulation Therapy, Celecoxib, Recombinant Interferon Alfa-2b, Rintatolimod | I | 8 | Estimated Study Completion Date: 6 July 2022 | [138] |
| NCT03106415 | Recruiting | Binimetynib, Laboratory Biomarker Analysis | I, II | 38 | Estimated Primary Completion Date: 15 November 2022 | [139] |
| NCT02730130 | Active, not recruiting | Radiotherapy | II | 17 | Complete Response in 3 participants (17.6%), and Duration of Response: 4.5 months, Time to Response 2.8 months | [140] |
| NCT04634747 | Not yet recruiting | PVX-410 chemotherapy | II | 53 | Estimated Primary Completion Date: 1 April 2023 | [141] |
| NCT03225547 | Active, not recruiting | Mifepristone | II | 74 | Estimated Primary Completion Date: September 2022 | [142] |
| NCT02657889 | Completed | Niraparib | I, II | 122 | Combination niraparib plus pembrolizumab provides promising antitumor activity in patients with advanced or metastatic TNBC, with numerically higher response rates in those with tumor BRCA mutations. The combination therapy was safe with a tolerable safety profile, warranting further investigation. | [143] |
| NCT03012230 | Recruiting | Ruxolitinib Phosphate, Laboratory Biomarker Analysis | I | 18 | Estimated Primary Completion Date: 1 March 2022 | [144] |
| NCT02411656 | Recruiting | Laboratory Biomarker Analysis | II | 35 | Estimated Primary Completion Date: 31 December 2023 | [145] |
| NCT01676753 | Active, not recruiting | Dinaciclib, | I | 32 | Estimated Study Completion Date: 31 December 2022 | [146] |
| NCT04301011 | Recruiting | TBio-6517 | I, II | 114 | Estimated Primary Completion Date: 20 August 2022 | [147] |
| NCT04348747 | Not yet recruiting | Anti-HER2/HER3 Dendritic Cell Vaccine, Celecoxib, Recombinant Interferon Alfa-2b, Rintatolimod | II | 23 | Estimated Primary Completion Date: 1 December 2023 | [148] |
| NCT04879849 | Recruiting | TAK-676, radiotherapy | I | 46 | Estimated Primary Completion Date: 18 January 2024 | [149] |
| NCT05082259 | Not yet recruiting | ASTX660 | I | 48 | Estimated Primary Completion Date: 16 March 2026 | [150] |
| NCT04230109 | Active, not recruiting | Sacituzumab Govitecan | II | 51 | Estimated Primary Completion Date: 30 October 2024 | [151] |
| NCT03197389 | Completed | Monotherapy | I | 54 | Among patients with TNBC, administration of single dose of pembrolizumab was not correlated with PD-1 expression in patients with or without neoadjuvant chemotherapy. | [152] |
| NCT04443348 | Recruiting | Radiation Therapy Boost, Paclitaxel, Carboplatin, Cyclophosphamide, Doxorubicin, Capecitabine | II | 120 | Estimated Primary Completion Date: 1 June 2023 | [153] |
| NCT05112536 | Recruiting | Pembrolizumab, Trilaciclib, Cylophosphamide, Doxorubicin, Paclitaxel, Carboplatin | II | 30 | Estimated Primary Completion Date: 20 August 2022 | [154] |
| NCT03775850 | Completed | EDP1503 | I, II | 69 | No Study Results Posted | [155] |
| NCT03289819 | Completed | Nab-paclitaxel Epirubicin Cyclophosphamide | II | 53 | No Study Results Posted | [156] |
| NCT04432857 | Recruiting | AN0025 | I | 84 | Estimated Primary Completion Date: December 2023 | [157] |
| NCT03396445 | Recruiting | MK-5890, Pemetrexed, Carboplatin, Nab-paclitaxel | I | 202 | Estimated Primary Completion Date: 25 October 2024 | [158] |
| NCT01986426 | Completed | LTX-315 | I | 80 | No Study Results Posted | [159] |
| NCT03761914 | Recruiting | Galinpepimut-S | I, II | 90 | Estimated Primary Completion Date: 31 January 2024 | [160] |
| NCT03797326 | Active, not recruiting | Lenvatinib | II | 590 | Estimated Primary Completion Date: 22 December 2023 | [161] |
| NCT04265872 | Recruiting | Bortezomib, and cisplatin injections--bortezomib followed by pembro/cis | Early phase I | 20 | Estimated Primary Completion Date: 1 October 2023 | [162] |
| NCT04332653 | Recruiting | Efineptakin alfa | I, II | 178 | Estimated Primary Completion Date: 30 June 2022 | [163] |
| NCT04429542 | Recruiting | BCA101 | I | 292 | Estimated Primary Completion Date: 31 December 2022 | [164] |
| NCT05082610 | Not yet recruiting | HMBD-002 | I | 240 | Estimated Primary Completion Date: October 2024 | [165] |
| NCT02644369 | Active, not recruiting | Monotherapy | II | 100 | No Study Results Posted | [166] |
| NCT05094804 | Recruiting | OR2805, Nivolumab | I, II | 130 | Estimated Primary Completion Date: 15 April 2024 | [167] |
| NCT05070247 | Not yet recruiting | TAK-500 | I | 106 | Estimated Primary Completion Date: 8 April 2025 | [168] |
| NCT04725331 | Recruiting | BT-001 | I, II | 48 | Estimated Primary Completion Date: 30 November 2024 | [169] |
| NCT03454451 | Recruiting | Ciforadenant, CPI-006 | I | 378 | Estimated Primary Completion Date: March 2022 | [170] |
| NCT03849469 | Recruiting | XmAb®22841 | I | 242 | Estimated Primary Completion Date: June 2024 | [171] |
| NCT04234113 | Recruiting | SO-C101 | I | 96 | Estimated Primary Completion Date: December 2023 | [172] |
| NCT02178722 | Completed | Epacadostat | I, II | 444 | Ammong patients with TNBC Safety, ORR: 11.1% | [173] |
| NCT05007106 | Recruiting | Vibostolimab Co-Formulation, Lenvatinib, 5-Fluorouracil, Cisplatin, Paclitaxel | II | 480 | Estimated Primary Completion Date: 19 February 2025 | [174] |
| NCT03621982 | Recruiting | ADCT-301 | I | 95 | Estimated Primary Completion Date: 15 November 2022 | [175] |
| NCT04348916 | Recruiting | ONCR-177 | I | 132 | Estimated Primary Completion Date: January 2025 | [176] |
| NCT01042379 | Recruiting | Standard Therapy, AMG 386 with or without Trastuzumab, AMG 479 (Ganitumab) plus Metformin, MK-2206 with or without Trastuzumab, AMG 386 and Trastuzumab, T-DM1 and Pertuzumab, Pertuzumab and Trastuzumab, Ganetespib, ABT-888, Neratinib, PLX3397, Pembrolizumab- 4 cycle, Talazoparib plus Irinotecan, Patritumab and Trastuzumab, Pembrolizumab-8 cycle, SGN-LIV1A, Durvalumab plus Olaparib, SD-101 + Pembrolizumab, Tucatinib plus trastuzumab and pertuzumab, Cemiplimab, Cemiplimab plus REGN3767, Trilaciclib with or without trastuzumab + pertuzumab, SYD985 ([vic-]trastuzumab duocarmazine), Oral Paclitaxel + Encequidar + Dostarlimab (TSR-042) + Carboplatin with or without trastuzumab, Oral Paclitaxel + Encequidar + Dostarlimab (TSR-042) with or without trastuzumab, Amcenestrant, Amcenestrant + Abemaciclib Amcenestrant + Letrozole |
II | 4000 | Estimated Primary Completion Date: December 2030 | [177] |
| NCT04060342 | Recruiting | GB1275D, Nab-paclitaxel and gemcitabine |
I, II | 242 | Estimated Primary Completion Date: March 2023 | [178] |
| NCT03366844 | Recruiting | Radiotherapy | I, II | 60 | Estimated Primary Completion Date: 21 January 2022 | [179] |
| NCT04148937 | Active, not recruiting | LY3475070 | I | 150 | Estimated Primary Completion Date: 20 December 2021 | [180] |
| NCT03277352 | Terminated | Epacadostat | I, II | 10 | The study was terminated due to emergent data from another study and unrelated to safety. Treatment-Emergent Adverse Events: 100% ORR: 30% |
[181] |
| NCT05069935 | Not yet recruiting | FT538, Cyclophosphamide, Fludarabine, Monoclonal antibody including pembrolizumab—Dose Escalation, Monoclonal antibody (including pembrolizumab)—Dose Expansion | I | 189 | Estimated Primary Completion Date 5 September 2023 | [109] |
| NCT03952325 | Terminated (The Sponsor has discontinued the development of tesetaxel) | Atezolizumab, Nivolumab, Tesetaxel | II | 294 | The study has stopped early and will not start again. Participants are no longer being examined or treated. | [106] |
| NCT04954599 | Not yet recruiting | CP-506 Carboplatin Immune checkpoint inhibitor (including pembrolizumab) | I, II | 126 | Estimated Primary Completion Date: September 2024 | [108] |
Table 3.
Nivolumab.
| NCT Number | Status | Co-Treatment/Intervention | Phase | Participants | Results/Conclusions | References |
|---|---|---|---|---|---|---|
| NCT04331067 | Recruiting | Paclitaxel, Carboplatin, Cabiralizumab, Tumor biopsy, Bone marrow, Blood draw | I, II | 50 | Estimated Primary Completion Date: 31 December 2022 | [182] |
| NCT02393794 | Active, not recruiting | Romidepsin, Cisplatin | I, II | 51 | Estimated Primary Completion Date: July 2022 | [183] |
| NCT03818685 | Recruiting | Ipilimumab, Capecitabine | II | 114 | Estimated Primary Completion Date: 1 February 2022 | [184] |
| NCT03487666 | Active, not recruiting | Capecitabine | II | 45 | Estimated Primary Completion Date: December 2021 | [185] |
| NCT03414684 | Active, not recruiting | Carboplatin | II | 78 | Estimated Primary Completion Date: 30 December 2021 | [186] |
| NCT03316586 | Completed | Cabozantinib | II | 18 | Results Submitted—Quality Control (QC) Review Has Not Concluded | [187] |
| NCT02499367 | Active, not recruiting | Radiation therapy, Low dose doxorubicin, Cyclophosphamide, Cisplatin | II | 84 | Estimated Primary Completion Date December 2021 | [188] |
| NCT03098550 | Complted | Daratumumab | I, II | 105 | Among patients with TNBC: Adverse events occurred in 100% and serious adverse events in 70.7%. ORR was 4.9% and PFS was 1.22 months | [189] |
| NCT04159818 | Recruiting | Cisplatin, Low dose doxorubicin | II | 52 | Estimated Primary Completion Date: 15 December 2022 | [190] |
| NCT04142931 | Recruiting | ImmunicomAIAC | I | 30 | Estimated Primary Completion Date: 30 December 2021 | [191] |
| NCT02834247 | Terminated | TAK-659 | I | 41 | Insufficient efficacy of drug; no safety concern | [192] |
| NCT03546686 | Recruiting | Ipilimumab, Core Biopsy/Cryoablation, Breast Surgery | II | 80 | Estimated Primary Completion Date: June 2022 | [193] |
| NCT05094804 | Recruiting | Pembrolizumab, OR2805 | I, II | 130 | Estimated Primary Completion Date: 15 April 2024 | [167] |
| NCT03435640 | Active, not recruiting | Bempegaldesleukin, NKTR-262 | I, II | 64 | Estimated Primary Completion Date: July 2022 | [194] |
| NCT02637531 | Active, not recruiting | IPI-549 (eganelisib) | I | 219 | Estimated Study Completion Date: October 2022 | [195] |
| NCT03829436 | Recruiting | Part 1 TPST-1120 Part 2 TPST-1120 + nivolumab Part 3 TPST-1120 Part 4 TPST-1120 + nivolumab |
I | 138 | Estimated Primary Completion Date: 18 February 2022 | [196] |
| NCT04423029 | Recruiting | DF6002 | I, II | 380 | Estimated Primary Completion Date: September 2022 | [197] |
| NCT03667716 | Recruiting | COM701 co-treatment and COM701 monotherapy, | I | 140 | Estimated Primary Completion Date: December 2022 | [198] |
| NCT04561362 | Recruiting | BT8009 | I, II | 146 | Estimated Primary Completion Date: June 2023 | [199] |
| NCT04638751 | Recruiting | Immunotherapy (Including Nivolumab) Chemotherapeutic Agent | Observational | 4000 | Estimated Primary Completion Date: December 2022 | [104] |
| NCT05069935 | Not yet recruiting | FT538, Cyclophosphamide, Fludarabine, Combination Product: Monoclonal antibody (including Nivolumab)- Dose Escalation Combination Product: Monoclonal antibody (including Nivolumab)—Dose Expansion | I | 189 | Estimated Primary Completion Date: 5 September 2023 | [109] |
| NCT03952325 | Terminated | Pembrolizumab, Tesetaxel, Nivolumab | II | 294 | The Sponsor has discontinued the development of tesetaxel. No Study results Posted | [106] |
| NCT04954599 | Not yet recruiting | CP-506, Carboplatin, Immune checkpoint inhibitor (including Nivolumab) | I, II | 126 | Estimated Primary Completion Date: September 2024 | [108] |
Table 4.
Avelumab.
| NCT Number | Status | Co-Treatment/Intervention | Phase | Participants | Results/Conclusions | References |
|---|---|---|---|---|---|---|
| NCT02926196 | Not yet recruiting | Monotherapy | III | 474 | Estimated Primary Completion Date: December 2021 | [200] |
| NCT04360941 | Recruiting | Palbociclib | I | 45 | Estimated Primary Completion Date: 1 January 2024 | [201] |
| NCT04188119 | Not yet recruiting | Aspirin, Lansoprazole | II | 42 | Estimated Primary Completion Date: 30 August 2022 | [202] |
| NCT03971409 | Recruiting | Anti-OX40 Antibody PF-04518600, Binimetinib, Utomilumab, Liposomal Doxorubicin, Sacituzumab Govitecan | II | 150 | Estimated Primary Completion Date: 30 July 2023 | [203] |
| NCT03387085 | Unknown | Aldoxorubicin HCl, N-803, ETBX-011, ETBX-051, ETBX-061, GI-4000, GI-6207, GI-6301, haNK for Infusion, Bevacizumab, Capecitabine, Cisplatin, Cyclophosphamide, 5-Fluorouracil, Leucovorin, nab-Paclitaxel, SBRT | I, II | 79 | No study results posted | [204] |
| NCT03861403 | Terminated | TRX518, Cyclophosphamide | I, II | 10 | No study results posted | [205] |
| NCT02630368 | Recruiting | Cyclophosphamide and JX-594 dose escalation, Cyclophosphamide and JX-594, Cyclophosphamide as a single agent, Avelumab and JX-594 and Cyclophosphamide | I, II | 197 | Estimated Primary Completion Date: May 2023 | [206] |
| NCT04638751 | Recruiting | Immunotherapy (including avelumab), Chemotherapeutic agent | Observational | 4000 | Estimated Primary Completion Date: December 2022 | [104] |
| NCT02554812 | Active, not recruiting | Utomilumab, PF-04518600, PD 0360324, CMP-001 | II | 398 | Estimated Primary Completion Date: 29 April 2022 | [207] |
| NCT05069935 | Not yet recruiting | FT538, Cyclophosphamide, Fludarabine, Combination Product: Monoclonal antibody (including Avelumab)—Dose EscalationCombination Product: Monoclonal antibody (including Avelumab)—Dose Expansion | I | 189 | Estimated Primary Completion Date: 5 September 2023 | [109] |
| NCT04551885 | Active, not recruiting | FT516, Fludarabine, Cyclophosphamide, Il-2 | I | 12 | Estimated Primary Completion Date: August 2022 | [208] |
| NCT02222922 | Completed | PF-06647020 Q3W, Fluconazole, PF-06647020 Q2W, PF-06647020 combined with Avelumab | I | 138 | No study results for avelumab co-treatment | [209] |
| NCT04954599 | Not yet recruiting | CP-506 Carboplatin Immune checkpoint inhibitor (including pembrolizumab) | I, II | 126 | Estimated Primary Completion Date: September 2024 | [108] |
| NCT01772004 | Completed | Monotherapy | I | 1756 | The anti-PD-L1 antibody avelumab has a safety profle that is considered generally manageable and tolerable, and showed modest clinical activity in a heavily pretreated population of patients with metastatic BC. Durable clinical beneft can be achieved with anti-PD-1/PD-L1 monotherapy in a subset of patients with metastatic BC, particularly TNBC. | [48] |
Table 5.
Cemiplimab.
| NCT Number | Status | Co-Treatment/Intervention | Phase | Participants | Results/Conclusions | References |
|---|---|---|---|---|---|---|
| NCT04243616 | Recruiting | Paclitaxel, Carboplatin (not mandatory), Doxorubicin, Cyclophosphamide | II | 36 | Estimated Primary Completion Date: 15 March 2022 | [210] |
| NCT04638751 | Recruiting | Immunotherapy (including cemiplimab) Chemotherapeutic Agent | Observational study | 4000 | Estimated Primary Completion Date: December 2022 | [104] |
| NCT01042379 | Recruiting | Standard Therapy, AMG 386 with or without Trastuzumab, AMG 479 (Ganitumab) plus Metformin, MK-2206 with or without Trastuzumab, AMG 386 and Trastuzumab, T-DM1 and Pertuzumab, Pertuzumab and Trastuzumab, Ganetespib, ABT-888, Neratinib, PLX3397, Pembrolizumab- 4 cycle, Talazoparib plus Irinotecan, Patritumab and Trastuzumab, Pembrolizumab-8 cycle, SGN-LIV1A, Durvalumab plus Olaparib, SD-101 + Pembrolizumab, Tucatinib plus trastuzumab and pertuzumab, Cemiplimab, Cemiplimab plus REGN3767, Trilaciclib with or without trastuzumab + pertuzumab, SYD985 ([vic-]trastuzumab duocarmazine), Oral Paclitaxel + Encequidar + Dostarlimab (TSR-042) + Carboplatin with or without trastuzumab, Oral Paclitaxel + Encequidar + Dostarlimab (TSR-042) with or without trastuzumab, Amcenestrant, Amcenestrant + Abemaciclib Amcenestrant + Letrozole |
II | 4000 | Estimated Primary Completion Date: December 2030 | [177] |
| NCT04954599 | Not yet recruiting | CP-506, Carboplatin, Immune checkpoint inhibitor (including cemiplimab) | I, II | 126 | Estimated Primary Completion Date: September 2024 | [108] |
Table 6.
Durvalumab.
| NCT Number | Status | Co-Treatment/Intervention | Phase | Participants | Results/Conclusions | References |
|---|---|---|---|---|---|---|
| NCT03199040 | Active, not recruiting | Neoantigen DNA vaccine | I | 10 | Estimated Study Completion Date: 3 December 2022 | [211] |
| NCT03167619 | Active, not recruiting | Olaparib | II | 50 | Estimated Study Completion Date: 30 June 2022 | [212] |
| NCT02826434 | Active, not recruiting | PVX-410 Hiltonol |
I | 22 | Estimated Study Completion Date: September 2022 | [213] |
| NCT02489448 | Active, not recruiting | Nab-paclitaxel, Tremelimumab | I, II | 71 | Estimated Study Completion Date: December 2021 | [214] |
| NCT02527434 | Active, not recruiting | Monotherapy, Tremelimumab combination therapy | II | 64 | 1 patient completed, 11 not completed (death 6, withdrawal by subject 3, lost to follow-up 2) | [215] |
| NCT03982173 | Active, not recruiting | combination with tremelimumab | II | 88 | Estimated Study Completion Date: April 2023 | [216] |
| NCT03616886 | Recruiting | Paclitaxel Carboplatin Oleclumab (MEDI9447) |
I, II | 171 | Estimated Study Completion Date: October 2023 | [217] |
| NCT03356860 | Recruiting | Paclitaxel Epirubicin Cyclophosphamide |
I, II | 57 | Estimated Study Completion Date: January 2022 | [218] |
| NCT03742102 | Recruiting | Capivasertib Oleclumab Paclitaxel Trastuzumab deruxtecan Datopotamab deruxtecan |
I, II | 200 | Estimated Study Completion Date: 13 February 2023 | [219] |
| NCT04176848 | Recruiting | CFI-400945 | II | 28 | Estimated Study Completion Date: 31 December 2022 | [220] |
| NCT03801369 | Recruiting | Olaparib | II | 28 | Estimated Study Completion Date: 31 December 2026 | [221] |
| NCT03606967 | Recruiting | Carboplatin Gemcitabine Hydrochloride Nab-paclitaxel Personalized Synthetic Long Peptide Vaccine Poly ICLC Tremelimumab |
II | 70 | Estimated Study Completion Date: 31 December 2021 | [222] |
| NCT03740893 | Recruiting | AZD6738 Olaparib |
II | 81 | Estimated Study Completion Date: December 2025 | [223] |
| NCT03739931 | Recruiting | mRNA-2752 | I | 264 | Estimated Study Completion Date: 30 January 2023 | [224] |
| NCT04504669 | Recruiting | AZD8701 | I | 123 | Estimated Study Completion Date: 7 September 2023 | [225] |
| NCT03983954 | Recruiting | Naptumomab estafenatox (ABR-217620; NAP) Obinutuzumab pretreatment (Gazyva®) |
I | 50 | Estimated Study Completion Date: 28 July 2022 | [226] |
| NCT04638751 | Recruiting | Chemotherapeutic Agent | Prospective study | 4000 | Estimated Study Completion Date: December 2024 | [104] |
| NCT01042379 | Recruiting | In combination with Olaparib | II | 4000 | Estimated Study Completion Date: December 2031 | [177] |
| NCT04556773 | Recruiting | In combination with Trastuzumab deruxtecan and Paclitaxel |
I | 185 | Estimated Study Completion Date: December 2031 | [227] |
| NCT04954599 | Not yet recruiting | CP-506 Carboplatin Immune checkpoint inhibitor |
I, II | 126 | Estimated Study Completion Date: October 2024 | [108] |
| NCT03544125 | Completed | Olaparib | I | 3 | No Study Results Posted | [228] |
| NCT02685059 | Completed | Placebo, Nab-Paclitaxel, Epirubicin, Cyclophosphamide | II | 174 | The addition of durvalumab to anthracycline-/taxane-based NACT increases pCR rate particularly in patients treated with durvalumab alone before start of chemotherapy. | [43] |
| NCT02628132 | Completed | Paclitaxel | I, II | 22 | Safety of therapy. The confirmed objective response rate (ORR) was observed in five patients with a median duration of 10.0 months. Median Progression-free survival (PFS) and overall survival (OS) were 5 and 20.7 months, respectively. | [229] |
| NCT02658214 | Completed | Gemcitabine + carboplatin, Nab-paclitaxel (paclitaxel-albumin) + carboplatin, Tremelimumab, | I | 32 | No Study Results Posted | [230] |
| NCT03872505 | Withdrawn | Radiation Therapy, Carboplatin, Paclitaxel | II | 140 | Lack of funding | [231] |
Table 7.
Ipilimumab.
| NCT Number | Status | Co-Treatment/Intervention | Phase | Participants | Results/Conclusions | References |
|---|---|---|---|---|---|---|
| NCT03818685 | Recruiting | Radiotherapy and Capecitabine Nivolumab |
II | 114 | Estimated Study Completion Date: 1 December 2022 | [184] |
| NCT03546686 | Recruiting | Nivolumab Core biopsy and cryoablation |
II | 80 | Estimated Study Completion Date: 1 December 2024 | [193] |
| NCT04638751 | Recruiting | Chemotherapeutic Agent | Obser-vational study | 4000 | Estimated Study Completion Date: December 2024 | [104] |
| NCT03752398 | Recruiting | XmAb®23104 | I | 234 | Estimated Study Completion Date: December 2024 | [232] |
| NCT03606967 | Recruiting | Nab-Paclitaxel + Durvalumab (MEDI4736) + Tremelimumab + Neoantigen Vaccine vs. Nab-Paclitaxel + Durvalumab + Tremelimumab | II | 70 | No Study Results Posted | [222] |
| NCT04954599 | Not yet recruiting | CP-506 Carboplatin Immune checkpoint inhibitor |
I, II | 126 | No Study Results Posted | [108] |
| NCT04434560 | Terminated | Nivolumab | II | 1 | Poor enrollment | [233] |
| NCT02983045 | Active, not recruiting | Combination of NKTR-214 + nivolumab | I, II | 557 | Estimated Primary Completion Date November 2021 | [234] |
| NCT03126110 | Active, not recruiting | INCAGN01876 | I, II | 145 | No Study Results Posted | [235] |
| NCT03241173 | Completed | INCAGN01949 | I, II | 52 | No Results Posted | [236] |
| NCT04879888 | Completed | Peptide pulsed Dendritic cell | I | 9 | Restoring the responsiveness of T-cells by increasing the frequency and activation in peripheral blood of tumor specific T-cells present in the tumor | [237,238] |
Table 8.
Tremelimumab.
| NCT Number | Status | Co-Treatment/Intervention | Phase | Participants | Results/Conclusions | References |
|---|---|---|---|---|---|---|
| NCT02527434 | Active, not recruiting | Monotherapy Combination therapy with MEDI4736 |
II | 12 | 1 patient completed, 11 not completed (death 6, withdrawal by subject 3, lost to follow-up 2) | [215] |
| NCT03982173 | Active, not recruiting | Durvalumab | II | 88 | Estimated Study Completion Date April 2023 | [216] |
| NCT02489448 | Active, not recruiting | Nab-Paclitaxel, Durvalumab (MEDI4736) | I, II | 71 | Estimated Study Completion Date December 2021 | [214] |
| NCT03606967 | Recruiting | Carboplatin Durvalumab Gemcitabine Hydrochloride Nab-paclitaxel Personalized Synthetic Long Peptide Vaccine Poly ICLC |
II | 70 | No Study Results Posted | [222] |
| NCT02658214 | Completed | Platinum Durvalumab |
I | 32 | No Study Results Posted | [230] |
| NCT03674827 | Completed | A vaccine-based immunotherapy regimen (VBIR-2) (PF-06936308), Sasanlimab | I | 36 | No Study Results Posted | [239] |
Author Contributions
Conceptualization, J.W. and A.T.-K.; writing—original draft preparation, J.W. and A.T.-K.; writing—review and editing, J.W., A.T.-K. and D.P.; figures preparation, J.W.; supervision, D.P.; funding acquisition, A.T.-K. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the Medical University of Bialystok, Poland (grant number SUB/2/DN/22/001/2228).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. [(accessed on 15 October 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
- 2.Keenan T.E., Tolaney S.M. Role of Immunotherapy in Triple-Negative Breast Cancer. J. Natl. Compr. Cancer. Netw. 2020;18:479–489. doi: 10.6004/jnccn.2020.7554. [DOI] [PubMed] [Google Scholar]
- 3.Irvin W.J.J., Carey L.A. What is triple-negative breast cancer? Eur. J. Cancer. 2008;44:2799–2805. doi: 10.1016/j.ejca.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Thomas R., Al-Khadairi G., Decock J. Immune Checkpoint Inhibitors in Triple Negative Breast Cancer Treatment: Promising Future Prospects. Front. Oncol. 2021;10:600573. doi: 10.3389/fonc.2020.600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar P., Aggarwal R. An overview of triple-negative breast cancer. Arch. Gynecol. Obs. 2016;293:247–269. doi: 10.1007/s00404-015-3859-y. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society: Triple-Negative Breast Cancer. [(accessed on 15 October 2021)]. Available online: https://www.cancer.org/cancer/breast-cancer/about/types-of-breast-cancer/triple-negative.html.
- 7.Liu J., Liu Q., Li Y., Li Q., Su F., Yao H., Su S., Wang Q., Jin L., Wang Y., et al. Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: An open-label phase II trial. J. Immunother. Cancer. 2020;8:e000696. doi: 10.1136/jitc-2020-000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Cancer Society: Treatment of Triple-Negative Breast Cancer. [(accessed on 15 October 2021)]. Available online: https://www.cancer.org/cancer/breast-cancer/treatment/treatment-of-triple-negative.html.
- 9.Disis M.L. Immune regulation of cancer. J. Clin. Oncol. 2010;28:4531–4538. doi: 10.1200/JCO.2009.27.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ElTanbouly M.A., Noelle R.J. Rethinking peripheral T-cell tolerance: Checkpoints across a T-cell’s journey. Nat. Rev. Immunol. 2021;21:257–267. doi: 10.1038/s41577-020-00454-2. [DOI] [PubMed] [Google Scholar]
- 11.Fife B.T., Bluestone J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 12.Gettinger S., Horn L., Jackman D., Spigel D., Antonia S., Hellmann M., Powderly J., Heist R., Sequist L.V., Smith D.C., et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J. Clin. Oncol. 2018;36:1675–1684. doi: 10.1200/JCO.2017.77.0412. [DOI] [PubMed] [Google Scholar]
- 13.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Rutkowski P., Grob J.J., Cowey C.L., Lao C.D., Wagstaff J., Schadendorf D., Ferrucci P.F., et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S., Tykodi S.S., Sosman J.A., Procopio G., Plimack E.R., et al. CheckMate 025 Investigators. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins A.V., Brodie D.W., Gilbert R.J., Iaboni A., Manso-Sancho R., Walse B., Stuart D.I., van der Merwe P.A., Davis S.J. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–210. doi: 10.1016/S1074-7613(02)00362-X. [DOI] [PubMed] [Google Scholar]
- 16.Chen L., Flies D.B. Molecular mechanisms of T-cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krummel M.F., Allison J.P. CD28 and CTLA-4 have opposing effects on the response of T-cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qureshi O.S., Zheng Y., Nakamura K., Attridge K., Manzotti C., Schmidt E.M., Baker J., Jeffery L.E., Kaur S., Briggs Z., et al. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T-cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 20.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharpe A.H., Pauken K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018;18:153–167. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 22.Han Y., Liu D., Li L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020;10:727–742. [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmadzadeh M., Johnson L.A., Heemskerk B., Wunderlich J.R., Dudley M.E., White D.E., Rosenberg S.A. Tumor antigen-specific CD8 T-cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchbinder E.I., Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016;39:98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barber D.L., Wherry E.J., Masopust D., Zhu B., Allison J.P., Sharpe A.H., Freeman G.J., Ahmed R. Restoring function in exhausted CD8 T-cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 26.Pauken K.E., Wherry E.J. Overcoming T-cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voron T., Colussi O., Marcheteau E., Pernot S., Nizard M., Pointet A.L., Latreche S., Bergaya S., Benhamouda N., Tanchot C., et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T-cells in tumors. J. Exp. Med. 2015;212:139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 29.Mittendorf E.A., Philips A.V., Meric-Bernstam F., Qiao N., Wu Y., Harrington S., Su X., Wang Y., Gonzalez-Angulo A.M., Akcakanat A., et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayraktar S., Batoo S., Okuno S., Glück S. Immunotherapy in breast cancer. J. Carcinog. 2019;18:2. doi: 10.4103/jcar.JCar_2_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emens L.A., Cruz C., Eder J.P., Braiteh F., Chung C., Tolaney S.M., Kuter I., Nanda R., Cassier P.A., Delord J.P., et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019;5:74–82. doi: 10.1001/jamaoncol.2018.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmid P., Adams S., Rugo H.S., Schneeweiss A., Barrios C.H., Iwata H., Diéras V., Hegg R., Im S.A., Shaw Wright G., et al. IMpassion130 Trial Investigators. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 33.Schmid P., Rugo H.S., Adams S., Schneeweiss A., Barrios C.H., Iwata H., Diéras V., Henschel V., Molinero L., Chui S.Y., et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 34.Narayan P., Wahby S., Gao J.J., Amiri-Kordestani L., Ibrahim A., Bloomquist E., Tang S., Xu Y., Liu J., Fu W., et al. FDA Approval Summary: Atezolizumab Plus Paclitaxel Protein-bound for the Treatment of Patients with Advanced or Metastatic TNBC Whose Tumors Express PD-L1. Clin. Cancer Res. 2020;26:2284–2289. doi: 10.1158/1078-0432.CCR-19-3545. [DOI] [PubMed] [Google Scholar]
- 35.Iwata H., Inoue K., Kaneko K., Ito Y., Tsugawa K., Hasegawa A., Nakagawa S., Kuratomi H., Tamura K. Subgroup analysis of Japanese patients in a Phase 3 study of atezolizumab in advanced triple-negative breast cancer (IMpassion130) Jpn. J. Clin. Oncol. 2019;49:1083–1091. doi: 10.1093/jjco/hyz135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nanda R., Chow L.Q., Dees E.C., Berger R., Gupta S., Geva R., Pusztai L., Pathiraja K., Aktan G., Cheng J.D., et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 2016;34:2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams S., Schmid P., Rugo H.S., Winer E.P., Loirat D., Awada A., Cescon D.W., Iwata H., Campone M., Nanda R., et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019;30:397–404. doi: 10.1093/annonc/mdy517. [DOI] [PubMed] [Google Scholar]
- 38.Adams S., Loi S., Toppmeyer D., Cescon D.W., De Laurentiis M., Nanda R., Winer E.P., Mukai H., Tamura K., Armstrong A., et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: Cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 2019;30:405–411. doi: 10.1093/annonc/mdy518. [DOI] [PubMed] [Google Scholar]
- 39.Ramakrishnan R., Gabrilovich D.I. Mechanism of synergistic effect of chemotherapy and immunotherapy of cancer. Cancer Immunol. Immunother. 2011;60:419–423. doi: 10.1007/s00262-010-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cortes J., Cescon D.W., Rugo H.S., Nowecki Z., Im S.A., Yusof M.M., Gallardo C., Lipatov O., Barrios C.H., Holgado E., et al. KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–1828. doi: 10.1016/S0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 41.Ho A.Y., Barker C.A., Arnold B.B., Powell S.N., Hu Z.I., Gucalp A., Lebron-Zapata L., Wen H.Y., Kallman C., D’Agnolo A., et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer. 2020;126:850–860. doi: 10.1002/cncr.32599. [DOI] [PubMed] [Google Scholar]
- 42.Li Q., Wang Y., Jia W., Deng H., Li G., Deng W., Chen J., Kim B.Y.S., Jiang W., Liu Q., et al. Low-Dose Anti-Angiogenic Therapy Sensitizes Breast Cancer to PD-1 Blockade. Clin. Cancer Res. 2020;26:1712–1724. doi: 10.1158/1078-0432.CCR-19-2179. [DOI] [PubMed] [Google Scholar]
- 43.Loibl S., Untch M., Burchardi N., Huober J., Sinn B.V., Blohmer J.U., Grischke E.M., Furlanetto J., Tesch H., Hanusch C., et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019;30:1279–1288. doi: 10.1093/annonc/mdz158. [DOI] [PubMed] [Google Scholar]
- 44.Voorwerk L., Slagter M., Horlings H.M., Sikorska K., van de Vijver K.K., de Maaker M., Nederlof I., Kluin R.J.C., Warren S., Ong S., et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019;25:920–928. doi: 10.1038/s41591-019-0432-4. [DOI] [PubMed] [Google Scholar]
- 45.Heery C.R., O’Sullivan-Coyne G., Madan R.A., Cordes L., Rajan A., Rauckhorst M., Lamping E., Oyelakin I., Marté J.L., Lepone L.M., et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): A phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017;18:587–598. doi: 10.1016/S1470-2045(17)30239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyerinas B., Jochems C., Fantini M., Heery C.R., Gulley J.L., Tsang K.Y., Schlom J. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti-PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunol. Res. 2015;3:1148–1157. doi: 10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Juliá E.P., Amante A., Pampena M.B., Mordoh J., Levy E.M. Avelumab, an IgG1 anti-PD-L1 Immune Checkpoint Inhibitor, Triggers NK Cell-Mediated Cytotoxicity and Cytokine Production Against Triple Negative Breast Cancer Cells. Front. Immunol. 2018;9:2140. doi: 10.3389/fimmu.2018.02140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dirix L.Y., Takacs I., Jerusalem G., Nikolinakos P., Arkenau H.T., Forero-Torres A., Boccia R., Lippman M.E., Somer R., Smakal M., et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018;167:671–686. doi: 10.1007/s10549-017-4537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Migden M.R., Chandra S., Rabinowits G., Chen C.I., Desai J., Seluzhytsky A., Sasane M., Campanelli B., Chen Z., Freeman M.L., et al. CASE (CemiplimAb-rwlc Survivorship and Epidemiology) study in advanced cutaneous squamous cell carcinoma. Future Oncol. 2020;16:11–19. doi: 10.2217/fon-2019-0762. [DOI] [PubMed] [Google Scholar]
- 50.National Cancer Institute. [(accessed on 10 December 2021)]; Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/tremelimumab.
- 51.Vonderheide R.H., LoRusso P.M., Khalil M., Gartner E.M., Khaira D., Soulieres D., Dorazio P., Trosko J.A., Rüter J., Mariani G.L., et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T-cells. Clin. Cancer Res. 2010;16:3485–3494. doi: 10.1158/1078-0432.CCR-10-0505. [DOI] [PubMed] [Google Scholar]
- 52.Jiang D.M., Fyles A., Nguyen L.T., Neel B.G., Sacher A., Rottapel R., Wang B.X., Ohashi P.S., Sridhar S.S. Phase I study of local radiation and tremelimumab in patients with inoperable locally recurrent or metastatic breast cancer. Oncotarget. 2019;10:2947–2958. doi: 10.18632/oncotarget.26893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaddepally R.K., Kharel P., Pandey R., Garje R., Chandra A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers. 2020;12:738. doi: 10.3390/cancers12030738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yervoy FDA Approval History. [(accessed on 10 December 2021)]. Available online: https://www.drugs.com/history/yervoy.html.
- 55.Navarrete-Bernal M.G.C., Cervantes-Badillo M.G., Martínez-Herrera J.F., Lara-Torres C.O., Gerson-Cwilich R., Zentella-Dehesa A., Ibarra-Sánchez M.J., Esparza-López J., Montesinos J.J., Cortés-Morales V.A., et al. Biological Landscape of Triple Negative Breast Cancers Expressing CTLA-4. Front. Oncol. 2020;10:1206. doi: 10.3389/fonc.2020.01206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou J., Bashey A., Zhong R., Corringham S., Messer K., Pu M., Ma W., Chut T., Soiffer R., Mitrovich R.C., et al. CTLA-4 blockade following relapse of malignancy after allogeneic stem cell transplantation is associated with T-cell activation but not with increased levels of T regulatory cells. Biol. Blood Marrow Transplant. 2011;17:682–692. doi: 10.1016/j.bbmt.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McArthur H.L., Diab A., Page D.B., Yuan J., Solomon S.B., Sacchini V., Comstock C., Durack J.C., Maybody M., Sung J., et al. A Pilot Study of Preoperative Single-Dose Ipilimumab and/or Cryoablation in Women with Early-Stage Breast Cancer with Comprehensive Immune Profiling. Clin. Cancer Res. 2016;22:5729–5737. doi: 10.1158/1078-0432.CCR-16-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marra A., Viale G., Curigliano G. Recent advances in triple negative breast cancer: The immunotherapy era. BMC Med. 2019;17:90. doi: 10.1186/s12916-019-1326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang J., Tian Q., Zhang M., Wang H., Wu L., Yang J. Immune-related biomarkers in triple-negative breast cancer. Breast Cancer. 2021;28:792–805. doi: 10.1007/s12282-021-01247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao Y., Ma D., Zhao S., Suo C., Shi J., Xue M.Z., Ruan M., Wang H., Zhao J., Li Q., et al. Multi-Omics Profiling Reveals Distinct Microenvironment Characterization and Suggests Immune Escape Mechanisms of Triple-Negative Breast Cancer. Clin. Cancer Res. 2019;25:5002–5014. doi: 10.1158/1078-0432.CCR-18-3524. [DOI] [PubMed] [Google Scholar]
- 61.Gumusay O., Callan J., Rugo H.S. Immunotherapy toxicity: Identification and management. Breast Cancer Res. Treat. 2022;192:1–17. doi: 10.1007/s10549-021-06480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miles D., Gligorov J., André F., Cameron D., Schneeweiss A., Barrios C., Xu B., Wardley A., Kaen D., Andrade L., et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021;32:994–1004. doi: 10.1016/j.annonc.2021.05.801. [DOI] [PubMed] [Google Scholar]
- 63.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04148911?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=3.
- 64.Wang H., Ma H., Sové R.J., Emens L.A., Popel A.S. Quantitative systems pharmacology model predictions for efficacy of atezolizumab and nab-paclitaxel in triple-negative breast cancer. J. Immunother. Cancer. 2021;9:e002100. doi: 10.1136/jitc-2020-002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adams S., Diéras V., Barrios C.H., Winer E.P., Schneeweiss A., Iwata H., Loi S., Patel S., Henschel V., Chui S.Y., et al. Patient-reported outcomes from the phase III IMpassion130 trial of atezolizumab plus nab-paclitaxel in metastatic triple-negative breast cancer. Ann. Oncol. 2020;31:582–589. doi: 10.1016/j.annonc.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 66.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT03281954?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=2.
- 67.Kyte J.A., Røssevold A., Falk R.S., Naume B. ALICE: A randomized placebo-controlled phase II study evaluating atezolizumab combined with immunogenic chemotherapy in patients with metastatic triple-negative breast cancer. J. Transl. Med. 2020;18:252. doi: 10.1186/s12967-020-02424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04690855?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=11.
- 69.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT04584112?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=5.
- 70.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT03498716?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=6.
- 71.Mittendorf E.A., Zhang H., Barrios C.H., Saji S., Jung K.H., Hegg R., Koehler A., Sohn J., Iwata H., Telli M.L., et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet. 2020;396:1090–1100. doi: 10.1016/S0140-6736(20)31953-X. [DOI] [PubMed] [Google Scholar]
- 72.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04177108?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=10.
- 73.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03256344?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=12.
- 74.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03292172?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=14.
- 75.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT04739670?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=15.
- 76.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03371017?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=7.
- 77.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04770272?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=13.
- 78.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03206203?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=16.
- 79.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03756298?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=17.
- 80.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT02530489?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=27&view=results.
- 81.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04408118?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=18.
- 82.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03853707?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=28.
- 83.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03483012?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=23.
- 84.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03101280?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=20.
- 85.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT01898117?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=25.
- 86.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03464942?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=24.
- 87.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT02883062?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=22.
- 88.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04249167?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=21.
- 89.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT02322814?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=26.
- 90.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04434040?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=19.
- 91.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03800836?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=29.
- 92.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03961698?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=31.
- 93.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT02620280?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=32.
- 94.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04849364?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=30.
- 95.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04639245?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=35.
- 96.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT02708680?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=37.
- 97.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03915678?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=34.
- 98.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03424005?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=33.
- 99.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03289962?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=36.
- 100.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT05001347?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=38.
- 101.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT03829501?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=40.
- 102.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT02543645?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=42.
- 103.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03170960?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=41.
- 104.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04638751?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=43.
- 105.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03232593?term=NCT03232593&draw=2&rank=1.
- 106.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03952325?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=47.
- 107.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04102618?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=48.
- 108.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04954599?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=49.
- 109.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT05069935?term=atezolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=46.
- 110.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04683679?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=1.
- 111.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04095689?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=2.
- 112.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04427293?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=3.
- 113.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04024800?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=4.
- 114.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT02977468?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=5.
- 115.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT03362060?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=5.
- 116.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04191135?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=7.
- 117.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT02768701?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=8.
- 118.Schmid P., Salgado R., Park Y.H., Muñoz-Couselo E., Kim S.B., Sohn J., Im S.A., Foukakis T., Kuemmel S., Dent R., et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: Results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann. Oncol. 2020;31:569–581. doi: 10.1016/j.annonc.2020.01.072. [DOI] [PubMed] [Google Scholar]
- 119.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT03720431?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=10.
- 120.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT03121352?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=11.
- 121.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03036488?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=12.
- 122.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT03145961?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=13.
- 123.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03567720?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=15.
- 124.Winer E.P., Lipatov O., Im S.A., Goncalves A., Muñoz-Couselo E., Lee K.S., Schmid P., Tamura K., Testa L., Witzel I., et al. KEYNOTE-119 investigators. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:499–511. doi: 10.1016/S1470-2045(20)30754-3. [DOI] [PubMed] [Google Scholar]
- 125.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03639948?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=17.
- 126.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT03184558?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=18.
- 127.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04986852?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=19.
- 128.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04468061?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=20.
- 129.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT02734290?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2.
- 130.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT03752723?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2.
- 131.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT02981303?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=23.
- 132.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT03644589?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=24.
- 133.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT02755272?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=25.
- 134.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT04373031?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2.
- 135.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT02971761?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=28.
- 136.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT02513472?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=29.
- 137.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03310957?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=30.
- 138.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT03599453?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=32.
- 139.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT03106415?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=33.
- 140.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT02730130?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=34.
- 141.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04634747?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=35.
- 142.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03225547?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=36.
- 143.Vinayak S., Tolaney S.M., Schwartzberg L., Mita M., McCann G., Tan A.R., Wahner-Hendrickson A.E., Forero A., Anders C., Wulf G.M., et al. Open-label Clinical Trial of Niraparib Combined With Pembrolizumab for Treatment of Advanced or Metastatic Triple-Negative Breast Cancer. JAMA Oncol. 2019;5:1132–1140. doi: 10.1001/jamaoncol.2019.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03012230?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=38.
- 145.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT02411656?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=39.
- 146.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT01676753?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=40.
- 147.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT04301011?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=41.
- 148.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04348747?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=42.
- 149.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04879849?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=43.
- 150.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT05082259?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=44.
- 151.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04230109?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=45.
- 152.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT03197389?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=46.
- 153.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT04443348?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=47.
- 154.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT05112536?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=48.
- 155.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT03775850?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=49.
- 156.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT03289819?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2.
- 157.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT04432857?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=51.
- 158.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT03396445?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=52.
- 159.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT01986426?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=53.
- 160.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT03761914?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=54.
- 161.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT03797326?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=55.
- 162.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT04265872?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=56.
- 163.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT04332653?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=57.
- 164.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04429542?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=58.
- 165.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT05082610?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=59.
- 166.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT02644369?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=60.
- 167.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT05094804?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=61.
- 168.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT05070247?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=63.
- 169.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04725331?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=64.
- 170.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03454451?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=65.
- 171.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03849469?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=66.
- 172.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT04234113?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=67.
- 173.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT02178722?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=68.
- 174.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT05007106?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=70.
- 175.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03621982?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=71.
- 176.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04348916?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=73.
- 177.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT01042379?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=74.
- 178.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT04060342?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=75.
- 179.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT03366844?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=76.
- 180.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT04148937?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=77.
- 181.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT03277352?term=pembrolizumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=78.
- 182.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/record/NCT04331067?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=1.
- 183.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/study/NCT02393794?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=2.
- 184.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/record/NCT03818685?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=3.
- 185.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/record/NCT03487666?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=4.
- 186.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/record/NCT03414684?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=5.
- 187.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT03316586?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=6.
- 188.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/study/NCT02499367?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=7.
- 189.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT03098550?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=8.
- 190.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/study/NCT04159818?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=9.
- 191.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT04142931?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=10.
- 192.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT02834247?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=11.
- 193.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/record/NCT03546686?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=12.
- 194.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/record/NCT03435640?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=14.
- 195.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/study/NCT02637531?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=15.
- 196.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/record/NCT03829436?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=16.
- 197.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/record/NCT04423029?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=17.
- 198.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/record/NCT03667716?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=18.
- 199.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/study/NCT04561362?term=Nivolumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=19.
- 200.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/record/NCT02926196?term=avelumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=1.
- 201.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/record/NCT04360941?term=avelumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=2.
- 202.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/study/NCT04188119?term=avelumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=3.
- 203.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/record/NCT03971409?term=avelumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=5.
- 204.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT03387085?term=avelumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=7.
- 205.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/study/NCT03861403?term=avelumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=9.
- 206.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/study/NCT02630368?term=avelumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=10.
- 207.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/record/NCT02554812?term=avelumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=12.
- 208.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/study/NCT04551885?term=avelumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=14.
- 209.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT02222922?term=avelumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=15.
- 210.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT04243616?term=cemiplimab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=1.
- 211.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03199040?term=Durvalumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=1.
- 212.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03167619?term=Durvalumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=2.
- 213.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT02826434?term=NCT02826434&draw=2&rank=1.
- 214.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02489448?term=Tremelimumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=4.
- 215.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT02527434?term=Tremelimumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=1.
- 216.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03982173?term=Tremelimumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=3.
- 217.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03616886?term=Durvalumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=4.
- 218.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03356860?term=Durvalumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=5.
- 219.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03742102?term=NCT03742102&draw=2&rank=1.
- 220.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04176848?term=NCT04176848&draw=2&rank=1.
- 221.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03801369?term=Durvalumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=9.
- 222.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03606967?term=ipilimumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=10.
- 223.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03740893?term=Durvalumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=14.
- 224.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03739931?term=Durvalumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=17.
- 225.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04504669?term=NCT04504669&draw=2&rank=1.
- 226.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03983954?term=NCT03983954&draw=2&rank=1.
- 227.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04556773?term=NCT04556773&draw=2&rank=1.
- 228.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT03544125?term=NCT03544125&draw=2&rank=1.
- 229.Ghebeh H., Al-Sayed A., Eiada R., Cabangon L., Ajarim D., Suleman K., Tulbah A., Al-Tweigeri T. Weekly Paclitaxel given concurrently with Durvalumab has a favorable safety profile in triple-negative metastatic breast cancer. Sci. Rep. 2021;11:19154. doi: 10.1038/s41598-021-98113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02658214?term=Tremelimumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=5.
- 231.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT03872505?term=durvalumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=3.
- 232.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03752398?term=ipilimumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=7.
- 233.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04434560?term=ipilimumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=5.
- 234.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/record/NCT02983045?term=ipilimumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=6.
- 235.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT03126110?term=ipilimumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=8.
- 236.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT03241173?term=ipilimumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=9.
- 237.Bernal-Estévez D., Sánchez R., Tejada R.E., Parra-López C. Chemotherapy and radiation therapy elicits tumor specific T-cell responses in a breast cancer patient. BMC Cancer. 2016;16:591. doi: 10.1186/s12885-016-2625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04879888?term=ipilimumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=11.
- 239.Clinical Trials. [(accessed on 7 January 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03674827?term=Tremelimumab&cond=TNBC+-+Triple-Negative+Breast+Cancer&draw=2&rank=6.