Abstract
A DNA fragment containing the Pseudomonas aeruginosa fabD (encoding malonyl-coenzyme A [CoA]:acyl carrier protein [ACP] transacylase), fabG (encoding β-ketoacyl-ACP reductase), acpP (encoding ACP), and fabF (encoding β-ketoacyl-ACP synthase II) genes was cloned and sequenced. This fab gene cluster is delimited by the plsX (encoding a poorly understood enzyme of phospholipid metabolism) and pabC (encoding 4-amino-4-deoxychorismate lyase) genes; the fabF and pabC genes seem to be translationally coupled. The fabH gene (encoding β-ketoacyl-ACP synthase III), which in most gram-negative bacteria is located between plsX and fabD, is absent from this gene cluster. A chromosomal temperature-sensitive fabD mutant was obtained by site-directed mutagenesis that resulted in a W258Q change. A chromosomal fabF insertion mutant was generated, and the resulting mutant strain contained substantially reduced levels of cis-vaccenic acid. Multiple attempts aimed at disruption of the chromosomal fabG gene were unsuccessful. We purified FabD as a hexahistidine fusion protein (H6-FabD) and ACP in its native form via an ACP-intein-chitin binding domain fusion protein, using a novel expression and purification scheme that should be applicable to ACP from other bacteria. Matrix-assisted laser desorption–ionization spectroscopy, native polyacrylamide electrophoresis, and amino-terminal sequencing revealed that (i) most of the purified ACP was properly modified with its 4′-phosphopantetheine functional group, (ii) it was not acylated, and (iii) the amino-terminal methionine was removed. In an in vitro system, purified ACP functioned as acyl acceptor and H6-FabD exhibited malonyl-CoA:ACP transacylase activity.
Fatty acid biosynthesis in eukaryotes is catalyzed by a multienzyme complex encoded by a single gene and known as the type I system. In contrast, eubacteria contain a type II or dissociated Fab (fatty acid biosynthesis) system, where the reactions are carried out by proteins encoded by multiple genes (for a review, see reference 6). Bacterial fatty acid biosynthesis necessitates a three-carbon precursor, malonyl-coenzyme A (CoA), which is derived from acetyl-CoA by the action of acetyl-CoA carboxylase. The malonyl-CoA is transferred to the acyl carrier protein (ACP) by malonyl-CoA:ACP acyltransferase (FabD). Fatty acid elongation involves four reactions: (i) a condensation reaction catalyzed by one of three β-ketoacyl-ACP synthases, FabB, FabF, or FabH; (ii) a reduction involving a NADPH-dependent β-ketoacyl-ACP reductase (FabG); (iii) a dehydration reaction catalyzed by either FabA or FabZ, both of which are β-hydroxyacyl-ACP dehyratases with broad, overlapping chain length specificities (15); and (iv) a second reduction reaction catalyzed by NADH-dependent enoyl-ACP reductase (FabI).
In Escherichia coli (28) and other bacteria (9, 25, 38), the genes acpP, fabD, fabF, fabG, and fabH, encoding ACP, FabD, FabF, FabG, and FabH, respectively, are contained in a fab gene cluster. In Bacillus subtilis, fabH is not part of the major fab gene cluster (25).
Besides the role of ACP in phospholipid (6) and rhamnolipid (3, 27) synthesis, ACPs play central roles in a broad range of other biosynthetic pathways that depend on acyl transfer reactions, including polyketide (35), nonribosomal peptide (1), and depsipeptide biosynthesis (31), as well as in the transacylation of oligosaccharides (8, 11) and proteins (22). More recently, acyl-ACPs derived from the Fab pathway have been proposed to be the acyl donors for synthesis of acylated homoserine lactones (AHLs) (26, 32, 40). The AHLs (or autoinducers) have received considerable attention in recent years since they are required for a regulatory phenomenon termed quorum sensing (10). Characterization of the two known AHL-producing systems of Pseudomonas aeruginosa, rhl and las, has shown that the respective autoinducers, N-butyryl homoserine lactone (C4-HSL) and N-(3-oxo)-dodecanoyl homoserine lactone (3-oxo-C12-HSL), are required for expression of a multitude of virulence factors and secondary metabolites (41).
We recently began characterization of the unique aspects of the P. aeruginosa Fab system (20), and in this report we describe the characterization of the P. aeruginosa fabD-fabG-acpP-fabF gene cluster, as well as the purification and characterization of FabD and ACP.
Cloning and characterization of the acpP-containing region.
When we initiated these experiments, the sequence of only a fragmented acpP-containing region was available from the P. aeruginosa genome database, and it contained no intact acpP gene. This acpP-containing gene cluster was cloned into M13, which led to expression of toxic levels of apo-ACP. Previous attempts to clone intact acpP from E. coli (42), as well as from a number of other bacteria (25, 36), into multicopy plasmids were hampered by the toxic effects of overexpressed apo-ACP (23). We therefore chose to reclone the chromosomal acpP-containing region into the low copy-number pWSK vectors (typically exhibiting only five to eight copies per cell [44]). A 147-bp sequence containing a partial acpP coding sequence was PCR amplified from P. aeruginosa chromosomal DNA by using the two primers ACP1 and ACP2 (Table 1). These partially degenerate primers were modeled after conserved amino acid sequence regions found in the E. coli, Haemophilus influenzae and Vibrio harveyi ACP homologs. PCR was performed on a PTC-100 PCR system thermocycler (MJ Research, Watertown, Mass.), using Taq polymerase from Qiagen (Santa Clarita, Calif.) and previously described conditions (19). The PCR fragment was cloned into pGEM-T (Promega, Madison, Wis.) to form pPS831. Nucleotide sequence analysis revealed the presence of a partial acpP gene on the PCR fragment. This fragment was used as a probe to generate a partial restriction map of the PAO1 chromosomal acpP region, which revealed a 3.4-kb EcoRV-BamHI fragment which was cloned into the low-copy-number cloning vector pWKS30 by using previously described strategies (17, 20), yielding pPS840 (Fig. 1A).
TABLE 1.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Relevant properties or sequencea | Reference or origin |
|---|---|---|
| E. coli | ||
| BL21(DE3) | E. coli B; F−ompT rB− mB− (λDE3) | 37 |
| SA1503(DE3) | lon-100 his-87 ompT::KmrrelA1 rpsL781 spoT1 thi-1 (λDE3) | 19a |
| P. aeruginosa | ||
| PAO1 | Prototroph | B. H. Holloway |
| PAO198 | PAO1 with fabF::Gmr | This study |
| PAO204 | PAO1 with fabD(Ts) | This study |
| Plasmids | ||
| pEX100T | AprsacB+; gene replacement vector | 34 |
| pUCGM | Apr Gmr; source of Gmr cassette | 33 |
| pWSK29 and pWKS30 | Apr; low-copy-number cloning and T7 expression vectors | 44 |
| pCYB1 | Apr; intein-chitin binding domain expression vector | New England Biolabs |
| pET-15b | Apr; hexahistidine fusion and expression vector | Novagen |
| pGEM-T | Apr; TA-cloning vector | Promega |
| pNam | Apr; low-copy-number intein-chitin binding domain expression vector | 19a |
| pT7-7 | Apr; T7 promoter expression vector | 39 |
| pPS671 | AprfabF+ pabC+ (ligation of chromosomal 4-kb EcoRI-HindIII fragment between the same sites of pWSK29) | This study |
| pPS681 | AprfabD+ fabG+ acpP+ fabF+ pabC+ (ligation of 3.4-kb BamHI-EcoRV fragment from pPS840 between the BamHI and XbaI [blunt-ended] sites of pPS671) | This study |
| pPS831 | Apr; PCR-amplified 147-bp genomic segment from PAO1 cloned into pGEM-T | This study |
| pPS840 | AprfabD+ fabG+ acpP+ (3.7-kb chromosomal EcoRV-BamHI fragment cloned between the same sites of pWSK29) | This study |
| pPS966 | Apr; ligation of 1.8-kb NdeI-PstI fragment from pPS981 between the same sites of pT7-7 | This study |
| pPS981 | ApracpP+; ligation of NdeI-SapI-digested PCR fragment between the same sites of pCYB1 | This study |
| pPS1096 | ApracpP+ (ligation of 735-bp HindIII-XbaI fragment from pPS966 between the same sites of pNam) | This study |
| Primers | ||
| ACP1 | ATCGAGGAGCG(A/C/T/G)GT(G/C)AAGAAGAT(A/C)AT | |
| ACP2 | GTCGAACTCCTC(G/C)TC(A/C/T/G)AG(G/C)GCCAT | |
| FabD1 | TGGATGCTCTCCACCtgGCGACCCGGGCTGT | |
| ACP-Nde | NdeI-TGAAAACAACATatgAGCACCATCGAAGAACGCGTT | |
| ACP-Sap | SapI-ATCCGGCTCTTCCGCAttgCTGGGGAGC | |
| FabD-Nde | NdeI-GGGACCTATcatATGTCTGCATCCCTCGCATTCGTC | |
| FabD-Bam | BamHI-CCCAGGatCCCCAGTTCCAGCGCAATCGCC |
Primer sequences are printed 5′ to 3′; lowercase letters indicate nonmatching oligonucleotides used to either form the indicated motif as underlined or introduce other nonmotif changes indicated in lowercase letters.
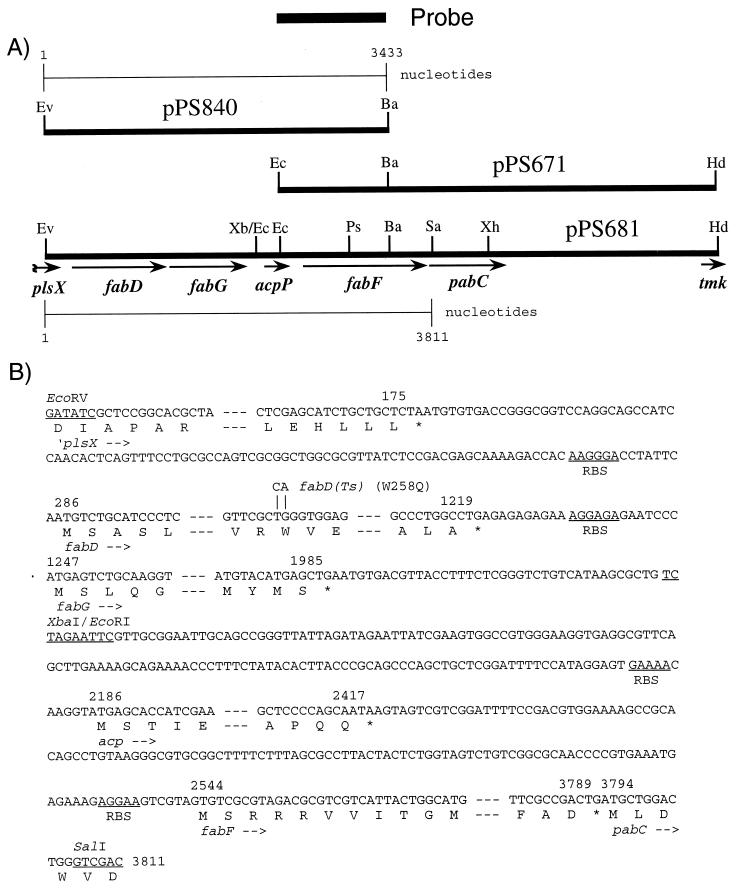
FIG. 1.
The P. aeruginosa acpP region. (A) Maps of plasmids containing a fab gene cluster and flanking genes. The chromosomal inserts of pPS840 and pPS671 were cloned individually, and fusion of these two clones at the common BamHI site yielded pPS681, containing a continuous sequence of this region. Abbreviations: Ba, BamHI; Ec, EcoRI; Ev, EcoRV; Hd, HindIII; Ps, PstI; Sa, SalI; Xb, XbaI; Xh, XhoI. The following genes and their products were identified by nucleotide sequencing: plsX, encoding a poorly understood protein involved in phospholipid biosynthesis; fabD, malonyl-CoA:ACP transacylase; fabG, β-ketoacyl-ACP reductase; acpP, ACP; fabF, β-ketoacyl-ACP synthase II; pabC, 4-amino-deoxychorismate lyase; tmk, thymidylate kinase. (B) Partial nucleotide sequences of the P. aeruginosa plsX, fabD, fabG, acpP, fabF, and pabC genes. Most of the sequences within the structural genes are omitted, as indicated by dashes. The deduced amino acid sequences are given in one-letter code below the nucleotide sequence. Putative ribosome binding sites (RBS) are labeled. Numbers above the sequence mark the first nucleotides of the initiation and last codons, and stop codons are marked with asterisks. The TG-to-CA nucleotide changes in fabD that were introduced by site-directed mutagenesis and resulted in a W258Q change and a FabD(Ts) phenotype are indicated above the nucleotide sequence.
The entire nucleotide sequence of both strands of a 3,433-bp chromosomal EcoRV-BamHI fragment was determined (relevant segments of the sequence are shown in Fig. 1B). BLAST searches revealed the presence of the 3′ end of plsX, complete sequences of fabD, fabG, and acpP, and the 5′ end of fabF (Fig. 1A). To obtain the 3′ end of fabF, a 1.1-kb BamHI-EcoRI fragment from pPS840 was used as a probe to identify a ∼4-kb EcoRI-HindIII fragment, which was cloned into pWSK29 to generate pPS671 (Fig. 1A).
Sequence analyses of pPS671 and several subclones derived from it established the entire sequence of both strands from the BamHI site within fabF to the SalI site immediately downstream of fabF (Fig. 1A). The fab gene cluster contains fabF, acpP, fabG, and fabD but not fabH. Many bacteria, i.e., B. subtilis (25), V. harveyi (36), H. influenzae (9), and Streptomyces glaucescens (38), contain similarly organized fab gene clusters, but P. aeruginosa provides the first example of a gram-negative bacterium where the fabH gene is absent from the acpP-containing cluster; the only other known example is B. subtilis (25).
The fab cluster is delimited by pabC and plsX homologs, encoding 4-amino-deoxychorismate lyase and a protein of unknown function in phospholipid synthesis, respectively. We recently confirmed that pabC encodes P. aeruginosa aminodeoxychorismate lyase (13), which is required for p-aminobenzoic acid (PABA) synthesis, since pabC mutants are PABA auxotrophs (18). Unlike E. coli, the ATG start codon of the P. aeruginosa pabC gene overlaps the TGA stop codon of fabF. Although the two genes are translationally coupled, it is unclear whether both genes are cotranscribed from a single promoter upstream of fabF. The fabF mutant PAO198 (Table 1), containing a nonpolar gentamicin resistance (Gmr) cassette in a transcriptional orientation opposite fabF and pabC, was not a PABA auxotroph, and we have not yet isolated mutants containing polar fabF mutations.
After our studies were completed, a contig appeared in the Pseudomonas genome project database; albeit not annotated, this entry confirmed our results and the gene order plsX-fabD-fabG-acpP-fabF-pabC, as well as the absence of fabH from this cluster. Searches of this database revealed several possible fabH homologs, which are located elsewhere in the chromosome, and we are in the process of characterizing the most likely fabH homolog.
The deduced amino acid sequences of the fabD, fabG, acpP, and fabF genes were analyzed, and, in general, found to be most similar to the respective E. coli homologs. The identities were 66% for FabF, 65% for FabG, and 56% for FabD. However, other bacterial Fab proteins also exhibited considerable similarities to the deduced P. aeruginosa protein sequences.
The high degree of primary amino acid sequence conservation was especially evident with ACP. P. aeruginosa ACP was 90% identical to E. coli ACP, 83% identical to H. influenzae ACP, and 80% identical to V. harveyi ACP. All ACPs consisted of between 76 to 78 amino acids, and they contained (i) the consensus sequence, DSLD, for attachment of the 4′-phosphopantetheine and (ii) a large proportion of acidic amino acids. The calculated isoelectric point of PAO1 ACP (pH 3.8) confirmed the acidic nature this protein.
Characteristics of fab genes and their products.
Several lines of evidence indicated that the fabF, fabD, and fabG genes cloned in this work encode β-ketoacyl-ACP synthase II (FabF), malonyl-CoA:ACP transacylase (FabD), and β-ketoacyl-ACP reductase (FabG), respectively.
The evidence for FabF includes the following: (i) there is a high degree of similarity or identity to the well-characterized homologs from E. coli; (ii) translation of both E. coli and P. aeruginosa FabF coding sequences is apparently initiated at a GTG because the first Met codon found in the P. aeruginosa FabF protein is not preceded by a suitable Shine-Dalgarno sequence and the first 10 NH2-terminal amino acids of the P. aeruginosa FabF protein (MSRRRVVITG) are 80% identical to those of the E. coli FabF protein (MSKRRVVVTG), which has a GTG start (GenBank accession no. AE000210); and (iii) a P. aeruginosa fabF mutant was constructed by deletion of a 597-bp NcoI fragment from within the fabF gene and replacing it with a 830-bp blunt-ended Gmr encoding cassette from plasmid pUCGM (33). The mutated fabF sequence was returned to the PAO1 chromosome via the gene replacement vector pEX100T (34). Gas chromatography-mass spectrometric (12) analysis of the fatty acid content in cell extracts of the fabF knockout mutant PAO198 (fabF::Gmr) demonstrated eightfold lower levels of cis-vaccenic acid compared to wild-type PAO1 (data not shown). This compares favorably with a 10-fold reduction of cis-vaccenic acid levels seen in an E. coli fabF mutant (12).
The following evidence suggests that the fabD gene encodes malonyl-CoA:ACP transacylase (FabD). (i) FabD not only is 56% identical (78% similar) to its counterpart from E. coli but also contains the critical catalytic domain, the conserved pentapeptide GHSLG, which is the GXSXG signature motif of serine-dependent acylhydrolases (2). Characterization of an E. coli fabD(Ts) mutant showed that the temperature-sensitive (Ts) phenotype resulted from a mutation that led to a W257Q substitution (43). We changed a conserved Trp258 to a Gln residue in the P. aeruginosa FabD protein by mutating two nucleotides (Fig. 1B) by site-specific mutagenesis using the Altered Sites mutagenesis system (Promega). Primer FabD1 (Table 1) introduced a double TG-to-CA mismatch that resulted in a W258Q change in the FabD amino acid sequence, which was confirmed by nucleotide sequence analysis. Return of the fabD(Ts) allele to the PAO1 chromosome was achieved by using a previously described strategy (20). FabD(Ts) mutants, including PAO204, were obtained at a frequency of 23%, demonstrating the essentiality of FabD in P. aeruginosa. (iii) A hexahistidine (H6)-tagged FabD protein was overexpressed and purified (Fig. 2A). Its observed molecular mass of ∼33 kDa matches closely that for FabD (32,442 Da) plus the H6-containing NH2-terminal extension (2,181 Da). (iv) The purified FabD protein exhibits malonyl-CoA:ACP transacylase activity (see below).
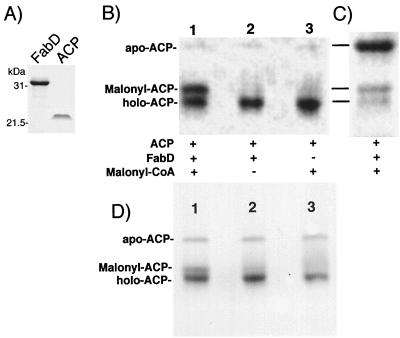
FIG. 2.
Purification of ACP and FabD, and assay of FabD activity. (A) The FabD protein was expressed as an H6-FabD protein and purified by Ni2+-agarose affinity chromatography. ACP was expressed as an ACP-intein-CBD fusion protein which was adsorbed to a chitin-agarose affinity column, from which the ACP was eluted after self-cleavage of the fusion protein by DTT. Each protein (3 μg) was analyzed by electrophoresis on a 0.1% SDS–10% polyacrylamide gel, and protein bands were visualized by Coomassie blue staining. The positions of two molecular weight markers, carbonic anhydrase (31 kDa) and trypsin inhibitor (21.5 kDa), are indicated on the left. (B) Acylation of ACP by H6-FabD. The reaction mixtures (20 μl) contained ACP (2 μg), H6-FabD (0.25 μg), and 0.1 mM malonyl-CoA in the indicated combinations. The products were analyzed by electrophoresis on 20% native polyacrylamide gels followed by staining with Coomassie blue. (C) The acylation reaction and detection were performed as described for panel B except that the ACP was purified from cultures grown under conditions that did not allow for proper modification of apo-ACP with 4′-phosphopantetheine (see text for details). (D) The same experiment as shown in panel B except that the ACP was purified from a low-copy-number expression construct.
Besides the high homology to other bacterial FabG proteins and the presence of the amino acids of the NADPH binding signature AX2GXGX2AX6G near the NH2 terminus, other evidence suggests its essential role in the cycles of fatty acid elongation in P. aeruginosa. First, despite repeated attempts, we were unable to isolate a fabG knockout mutation, indicating the gene’s essential nature. Second, the purified FabG protein is essential for fatty acid elongation in a reconstituted in vitro Fab synthesis system (21). A separate NADPH-dependent β-ketoacyl-ACP reductase, RhlG, that is specifically involved in rhamnolipid synthesis has recently been described (3), although no biochemical data supporting its presumed function were presented.
Expression and purification of ACP.
The ACP was overexpressed and purified by using the intein-chitin binding domain (CDB) system. An ACP-intein-CBD fusion protein was constructed according the basic protocol provided by New England Biolabs (Beverly, Mass.). Two PCR primers, ACP-Nde and ACP-Sap, were designed to introduce an NdeI site at the ACP initiation codon and a SapI site immediately downstream of the last codon of acpP. These primers were used to amplify a ∼270-bp fragment by using pPS681 (Table 1) DNA as the template and standard PCR conditions (18). The PCR fragment was cloned into pCYB1 DNA. This procedure yielded pPS981. Using pPS981, various E. coli host strains, and different induction conditions, we detected no expression of ACP-intein-CBD unless the lac promoter was replaced with the more powerful T7 promoter from pT7-7 (39), a step that yielded pPS966. Optimization of the expression conditions by using E. coli BL21(DE3)/pPS966 revealed that an overnight induction and growth at room temperature (RT) in Luria-Bertani (LB) medium (Gibco-BRL, Gaithersburg, Md.) led to substantial overproduction of the ∼66,000-Mr ACP-intein-CBD fusion protein.
For purification of ACP-intein-CBD from a high-copy-number expression vector, 4-liter LB-ampicillin (100 μg/ml) cultures of BL21(DE3)/pPS966 were grown at 37°C to log phase (A600 of ∼1.0), and gene expression was induced by addition of 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG). The cells were shaken at RT and harvested after a 9-h induction period. The cells were resuspended in 4 liters of fresh LB medium without IPTG and further incubated for 1.5 h at 37°C before they were harvested. During this recovery period, most of the apo-ACP was converted to holo-ACP by ligation of the 4′-phosphopantetheine. When this period was omitted and the cells were harvested after a 13-h incubation at RT in the presence of IPTG, ∼90 to 95% of the ACP preparation was apo-ACP which did not serve as a FabD substrate. The cell pellet was suspended in 50 ml of column buffer (20 mM Tris-HCl [pH 8.0], 500 mM NaCl, 0.1 mM EDTA, 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride) and disrupted by French press treatment (19,000 lb/in2). All subsequent steps were performed at 4°C. Cell debris was removed by ultracentrifugation for 1 h at 260,000 × g. The cell extract was applied to a 10-ml bed volume of chitin beads (New England Biolabs) in a 30-ml column and washed with 15 volumes of column buffer. The column buffer was exchanged with cleavage buffer (20 mM Tris-HCl [pH 8.0], 50 mM NaCl, 0.1 mM EDTA, 30 mM dithiothreitol [DTT]), and cleavage of the fusion protein on the column was achieved by overnight incubation. The cleaved ACP was eluted with 30 ml of cleavage buffer without DTT, and fractions were collected. The ACP content of the fractions was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (24), and protein concentrations were determined by using the Bradford dye binding assay (Bio-Rad Laboratories) and bovine serum albumin as the standard.
For purification of ACP from E. coli cells containing the low-copy-number expression vector pPS1096, 250-ml LB-ampicillin cultures of E. coli SA1503(DE3)/pPS1096 were grown at 37°C to log phase (A600 of ∼1.0) and gene expression was induced by addition of 0.5 mM IPTG. The cells were shaken at RT for 12 h, and ACP was purified as described above. The low-copy-number expression vector allowed for a simpler induction scheme and yielded an ACP preparation with a holo-ACP content of ∼95%. This amount could not be boosted by growing the cells in the presence of pantothenic acid. In contrast to expression experiments using the high-copy-number expression construct, the low-copy-number expression vector did not lead to an observable cessation of cell growth.
SDS-PAGE analysis revealed that ACP had been purified to near homogeneity (Fig. 2A). On this gel, ACP migrated at the position of a 22.5-kDa protein, significantly larger than its calculated molecular mass of 8.7 kDa. The anomolous migration of PAO1 ACP on SDS-PAGE is consistent with observations for ACPs from other bacteria (25, 28, 35) and has been attributed to the protein’s high charge-to-mass ratio (with ACP being highly acidic; calculated pI 3.8) as well as its low hydrophobic amino acid content, two factors that have a considerable influence on SDS binding (30). When the same ACP preparation was analyzed on a 0.1% SDS–13% polyacrylamide gel containing 5 M urea, we observed a single protein band of ∼9 kDa (data not shown), a value that closely matches its calculated mass of 8.7 kDa.
Characterization of ACP.
Matrix-assisted laser desorption–ionization mass spectrometric analysis (4) (performed at the Colorado State University Macromolecular Resource Facility) of the purified ACP fraction revealed three species with molecular masses of 8,583 Da (minor peak), 8,934 Da (major peak), and 17,844 Da (minor peak) (data not shown). These values correspond to the three ACP species commonly found in ACP preparations, apo-ACP (without 4′-phosphopantetheine), holo-ACP (with 4′-phosphopantetheine), and the ACP dimer. ACP dimerization was an artifact of the precipitation step used in matrix-assisted laser desorption-ionization sample preparation, as no dimers were present in our ACP preparation, as judged by native PAGE (data not shown). The data confirm that the majority of our recombinant ACP (the major peak at 8,934 Da) contains the 4′-phosphopantetheine group required for its activity, presumably attached to Ser36, and that the NH2-terminal methionine of ACP is posttranslationally removed by an aminopeptidase (16), as has been observed with other ACPs (25, 28, 35). The latter was verified by NH2-terminal amino acid sequence analysis of purified P. aeruginosa ACP (performed at the Peptide Sequencing Facility at the University of Victoria, Victoria, British Columbia, Canada), which revealed the sequence STIEE, corresponding to amino acids 2 to 6 deduced from the nucleotide sequence (Fig. 1B).
Native PAGE is a powerful means for characterization of modified and unmodified ACP (29). It can be used to assess ACP dimer content and the ratio of apo-ACP to holo-ACP-SH. Since acylation alters the Stokes radius of ACP, it can also be used to assess the fraction of acyl-ACP in ACP preparations (29). Using native PAGE, we showed that our ACP preparation (i) contained ∼95% holo-ACP and ∼5% apo-ACP and (ii) contained no detectable acyl-ACPs (Fig. 2B). We do not know whether nonacylated ACP is due to masking of the 4′-phosphopantetheine prosthetic group in the ACP-intein-CBD fusion protein or due to efficient removal of acyl groups by the DTT used for cleavage of the fusion protein.
The identity of the holo-ACP was confirmed in a malonyl-CoA:ACP transferase assay by incubation of our ACP preparation with malonyl-CoA and purified FabD. For purification of FabD, a H6-FabD expression vector was constructed. The fabD coding sequence was PCR amplified from PAO1 genomic DNA with primers FabD-Nde, creating a NdeI site at the fabD ATG initiation codon, and FabD-Bam, which creates a BamHI site immediately downstream of fabD. The PCR fragment was cloned into pET-15b (Novagen, Madison, Wis.) to form pPS979, which was then transformed into E. coli BL21(DE3). Expression of H6-FabD, cell lysis, and purification of the soluble fusion protein on a Ni2+-agarose column (Qiagen) were performed as previously described (14) except that the cells were grown in LB-ampicillin medium. Purified FabD was used in acylation reaction mixtures (20 μl) that contained 2 μg of ACP, 0.25 μg of FabD, and 0.1 mM malonyl-CoA in 20 mM Tris-HCl (pH 7.2)–100 mM NaCl–10% glycerol–1 mM DTT–2 mM EDTA–25 mM MgSO4–0.1 mM FeSO4. The mixtures were incubated at RT for 5 min, and products were analyzed by electrophoresis on 20% native polyacrylamide gels and followed by staining with Coomassie blue R-250 as previously described (29). In this assay, at least 50% of the holo-ACP was converted to malonyl-ACP by FabD (Fig. 2B, lane 1); conversion was not complete since the reaction catalyzed by FabD is reversible. This reaction required FabD and malonyl-CoA since neither FabD alone (lane 2) nor malonyl-CoA alone (lane 1) led to formation of malonyl-ACP after a 5-min incubation period. Longer incubation times and higher malonyl-CoA concentrations resulted in some spontaneous acylation of ACP by malonyl-CoA alone. As shown in Fig. 2C, apo-ACP did not serve as a FabD substrate. Although of the ∼10% holo-ACP present about half was converted to malonyl-ACP, the ACP preparation contained ∼90% apo-ACP which did not serve as a FabD substrate. Similar results were obtained with ACP purified from a strain carrying a low-copy-number ACP overproducer (Fig. 2D).
Conclusions.
The ACP purification procedure described herein has several distinct advantages over other purification methods (7, 8). (i) It is rapid, and yields are comparable to those obtained by other methods (15, 25, 29). We routinely isolate in excess of 5 mg of ACP from 4 liter of induced culture, containing either low- or high-copy-number expression vectors, using a single 5- to 10-ml chitin-agarose affinity column on which all washing steps and the cleavage step are performed. We only know of one other method that yields more ACP when expressed from an overproducer, but it is much more involved with respect to both labor and instrumentation requirements (15). (ii) Our purification procedure yields >95% holo-ACP, whereas other procedures relying on expression constructs yield mostly apo-ACP and little to almost no holo-ACP (7, 8). Although apo-ACP can efficiently be converted into holo-ACP by purified holo-ACP synthase (8), this step contaminates the ACP preparation, and thus additional steps are required to purify the holo-ACP before further use. (iii) ACP purified by our procedure is not acylated and therefore does not require the deacylation steps prior to its use as holo-ACP in the synthesis of defined acyl-ACP substrates, compared to ACP obtained with more traditional purification procedures (5). (iv) Our purification method is inexpensive and can be performed in laboratories that have neither the expertise nor the equipment necessary for traditional protein purification schemes.
We have successfully used the P. aeruginosa ACP prepared in this manner for the synthesis of defined acyl-ACP substrates (19); together with purified H6-FabD and H6-FabG, it functions in a reconstituted enzyme system leading to synthesis of biologically active 3-oxo-C12-HSL from simple metabolic precursors (21).
Nucleotide sequence accession number.
The complete sequence of the 3,811-bp EcoRV-SalI fragment (Fig. 1A) was deposited in GenBank and assigned accession no. U91631.
Acknowledgments
This work was supported by Public Health Service grant GM56685 from the National Institutes of Health and by a grant from the CSU College of Veterinary Medicine and Biomedical Sciences. A.J.K. was supported by a graduate teaching assistantship from the Department of Microbiology.
A.J.K. and T.T.H. contributed equally to this work.
REFERENCES
- 1.Baldwin J E, Bird J W, Field R A, O’Callaghan N M, Schofield C J, Willis A C. Isolation and partial characterization of ACV synthetase from Cephalosporium acremonium and Streptomyces clavuligerus. Evidence for the presence of phosphopantothenate in ACV synthase. J Antibiot. 1991;44:241–248. doi: 10.7164/antibiotics.44.241. [DOI] [PubMed] [Google Scholar]
- 2.Brenner S. The molecular evolution of genes and proteins: a tale of two serines. Nature. 1988;334:528–530. doi: 10.1038/334528a0. [DOI] [PubMed] [Google Scholar]
- 3.Campos-Garcia J, Caro A D, Najera R, Miller-Maier R M, Al-Tahhan R A, Soberon-Chavez G. The Pseudomonas aeruginosa rhlG gene encodes an NADPH-dependent β-ketoacyl reductase which is specifically involved in rhamnolipid synthesis. J Bacteriol. 1998;180:4442–4451. doi: 10.1128/jb.180.17.4442-4451.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cottrell J S, Sutton C W. The identification of electrophoretically separated proteins by peptide mass fingerprinting. In: Walker J M, editor. Protein protocols on CD-ROM. Totowa, N.J: Humana Press; 1998. pp. 1–16. [DOI] [PubMed] [Google Scholar]
- 5.Cronan J E, Klages A L. Chemical synthesis of acyl thioesters of acyl carrier protein with native structure. Proc Natl Acad Sci USA. 1981;78:5440–5444. doi: 10.1073/pnas.78.9.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cronan J E, Rock C O. Biosynthesis of membrane lipids. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 612–636. [Google Scholar]
- 7.Crosby J, Sherman D H, Bibb M J, Revill W P, Hopwood D A, Simpson T J. Polyketide synthase acyl carrier proteins from Streptomyces: expression in Escherichia coli, purification and partial characterization. Biochim Biophys Acta. 1995;1251:32–42. doi: 10.1016/0167-4838(95)00053-w. [DOI] [PubMed] [Google Scholar]
- 8.Epple G, Van der Drift K M G M, Thomas-Oates J E, Geiger O. Characterization of a novel acyl carrier protein, RkpF, encoded by an operon involved in capsular polysaccharide biosynthesis in Sinorhizobium meliloti. J Bacteriol. 1998;180:4950–4954. doi: 10.1128/jb.180.18.4950-4954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudeck D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 10.Fuqua W C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 11.Geiger O, Spaink H P, Kennedy E P. Isolation of the Rhizobium leguminosarum NodF nodulation protein: NodF carries a 4′-phosphopantetheine prosthetic group. J Bacteriol. 1991;173:2872–2878. doi: 10.1128/jb.173.9.2872-2878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelman E P, Cronan J E. Mutant of Escherichia coli deficient in the synthesis of cis-vaccenic acid. J Bacteriol. 1972;112:381–387. doi: 10.1128/jb.112.1.381-387.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green J M, Merkel W K, Nichols B P. Characterization and sequence of Escherichia coli pabC, the gene encoding aminodeoxychorismate lyase, a pyridoxal phosphate-containing enzyme. J Bacteriol. 1992;174:5317–5323. doi: 10.1128/jb.174.16.5317-5323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heath R J, Rock C O. Roles of the FabA and FabZ β-hydroxyacyl-acyl carrier protein dehydratase in Escherichia coli fatty acid biosynthesis. J Biol Chem. 1996;271:27795–27801. doi: 10.1074/jbc.271.44.27795. [DOI] [PubMed] [Google Scholar]
- 15.Hill R B, MacKenzie K R, Flanagan J M, Cronan J E, Prestegard J H. Overexpression, purification and characterization of Escherichia coli acyl carrier protein and two mutant proteins. Protein Expression Purif. 1995;6:394–400. doi: 10.1006/prep.1995.1052. [DOI] [PubMed] [Google Scholar]
- 16.Hirel P H, Schmitter J M, Dessen P, Fayat K, Banque T S. Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc Natl Acad Sci USA. 1989;86:8274–8251. doi: 10.1073/pnas.86.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoang T, Williams S, Schweizer H P. Molecular genetic analysis of the region containing the essential Pseudomonas aeruginosa asd gene encoding aspartate-β-semialdehyde dehydrogenase. Microbiology. 1997;143:899–907. doi: 10.1099/00221287-143-3-899. [DOI] [PubMed] [Google Scholar]
- 18.Hoang T T, Karkhoff-Schweizer R R, Kutchma A J, Schweizer H P. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212:77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 19.Hoang T T, Schweizer H P. Characterization of Pseudomonas aeruginosa enoyl-acyl carrier protein reductase: a target for the antimicrobial triclosan and its role in acylated homoserine lactone synthesis. J Bacteriol. 1999;181:5489–5497. doi: 10.1128/jb.181.17.5489-5497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Hoang, T. T., Y. Ma, R. J. Stern, M. R. McNeil, and H. P. Schweizer. Construction and use of low-copy number T7 expression vectors for purification of problem proteins: purification of Mycobacterium tuberculosis RmlD and Pseudomonas aeruginosa LasI and RhlI, and functional analysis of purified RhlI. Gene, in press. [DOI] [PubMed]
- 20.Hoang T T, Schweizer H P. Fatty acid biosynthesis in Pseudomonas aeruginosa: cloning and characterization of the fabAB operon encoding β-hydroxyacyl-acyl carrier protein dehydratase (fabA) and β-ketoacyl-acyl carrier protein synthase I (fabB) J Bacteriol. 1997;179:5326–5332. doi: 10.1128/jb.179.17.5326-5332.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoang T T, Schweizer H P. Abstracts of the 99th General Meeting of the American Society for Microbiology, 1999. Washington, D.C: American Society for Microbiology; 1999. Synthesis of the Pseudomonas aeruginosa autoinducer N-[3-oxo-dodecanoyl]-l-homoserine lactone from metabolic precursors in a reconstituted enzyme system, abstr. B/D309; p. 90. [Google Scholar]
- 22.Issartel J P, Koronakis V, Hughes C. Activation of Escherichia coli prohaemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature. 1991;351:759–761. doi: 10.1038/351759a0. [DOI] [PubMed] [Google Scholar]
- 23.Keating D H, Carey M R, Cronan J E. The unmodified (apo) form of Escherichia coli acyl carrier protein is a potent inhibitor of cell growth. J Biol Chem. 1995;270:22229–22235. doi: 10.1074/jbc.270.38.22229. [DOI] [PubMed] [Google Scholar]
- 24.Makowski G S, Ramsby M L. pH modification to enhance the molecular sieving properties of sodium dodecyl sulfate-10% polyacrylamide gel. Anal Biochem. 1993;212:283–285. doi: 10.1006/abio.1993.1324. [DOI] [PubMed] [Google Scholar]
- 25.Morbidoni H R, De Mendoza D, Cronan J E. Bacillus subtilis acyl carrier protein is encoded in a cluster of lipid biosynthesis genes. J Bacteriol. 1996;178:4794–4800. doi: 10.1128/jb.178.16.4794-4800.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moré M I, Finger D, Stryker J L, Fuqua C, Eberhard A, Winans S C. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science. 1996;272:1655–1658. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- 27.Ochsner U A, Fiechter A, Reiser J. Isolation, characterization, and expression in Escherichia coli of the Pseudomonas aeruginosa rhlAB genes encoding a rhamnosyltransferase involved in rhamnolipid biosurfactant synthesis. J Biol Chem. 1994;269:19787–19795. [PubMed] [Google Scholar]
- 28.Rawlings M, Cronan J E. The gene encoding Escherichia coli acyl carrier protein lies within a cluster of fatty acid biosynthetic genes. J Biol Chem. 1992;267:5751–5754. [PubMed] [Google Scholar]
- 29.Rock C O, Cronan J E. Acyl carrier protein from Escherichia coli. Methods Enzymol. 1981;71:341–351. doi: 10.1016/0076-6879(81)71043-7. [DOI] [PubMed] [Google Scholar]
- 30.Rock C O, Cronan J E. Re-evaluation of the solution structure of acyl carrier protein. J Biol Chem. 1979;254:9778–9785. [PubMed] [Google Scholar]
- 31.Rusnak F, Sakaitani M, Drueckhammer D, Reichert J, Walsh C T. Biosynthesis of the Escherichia coli siderophore enterobactin: sequence of the entF gene, expression and purification of EntF, and analysis of covalent phosphopantetheine. Biochemistry. 1991;30:2916–2927. doi: 10.1021/bi00225a027. [DOI] [PubMed] [Google Scholar]
- 32.Schaefer A L, Val D L, Hanzelka B L, Cronan J E, Greenberg E P. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc Natl Acad Sci USA. 1996;93:9505–9509. doi: 10.1073/pnas.93.18.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schweizer H P. Small broad-host-range gentamycin resistance cassettes for site-specific insertion and deletion mutagenesis. BioTechniques. 1993;15:831–833. [PubMed] [Google Scholar]
- 34.Schweizer H P, Hoang T. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene. 1995;158:15–22. doi: 10.1016/0378-1119(95)00055-b. [DOI] [PubMed] [Google Scholar]
- 35.Shen B, Summers R G, Gramajo H, Bibb M J, Hutchinson C R. Purification and characterization of the acyl carrier protein of the Streptomyces glaucescens tetracenomycin C polyketide synthase. J Bacteriol. 1992;174:3818–3821. doi: 10.1128/jb.174.11.3818-3821.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen Z, Byers D M. Isolation of Vibrio harveyi acyl carrier protein and the fabG, acpP, and fabF genes involved in fatty acid biosynthesis. J Bacteriol. 1996;178:571–573. doi: 10.1128/jb.178.2.571-573.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 38.Summers R G, Ali A, Shen B, Wessel W A, Hutchinson C R. Malonyl-coenzyme A:acyl carrier protein acyltransferase of Streptomyces glaucescens: a possible link between fatty acid and polyketide biosynthesis. Biochemistry. 1995;34:9389–9402. doi: 10.1021/bi00029a015. [DOI] [PubMed] [Google Scholar]
- 39.Tabor S. Expression using the T7 RNA polymerase/promoter system. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Short protocols in molecular biology, 3 ed. New York, N.Y: John Wiley & Sons; 1995. pp. 16.2–16.9. [Google Scholar]
- 40.Val D L, Cronan J E. In vivo evidence that S-adenosylmethionine and fatty acid intermediates are the substrates for the LuxI family of autoinducer synthases. J Bacteriol. 1998;180:2644–2651. doi: 10.1128/jb.180.10.2644-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Delden C, Iglewski B H. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis. 1998;4:551–560. doi: 10.3201/eid0404.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Den Boom T, Cronan J E. Genetics and regulation of bacterial lipid metabolism. Annu Rev Microbiol. 1989;43:317–343. doi: 10.1146/annurev.mi.43.100189.001533. [DOI] [PubMed] [Google Scholar]
- 43.Verwoert I G S, Verhagen E F, van der Linden K H, Verbree H, Nijkamp J J, Stuitje A R. Molecular characterization of an Escherichia coli mutant with a temperature-sensitive malonyl coenzyme A:acyl carrier protein transacylase. FEBS Lett. 1994;348:311–316. doi: 10.1016/0014-5793(94)00630-x. [DOI] [PubMed] [Google Scholar]
- 44.Wang R F, Kushner S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]