Abstract
Purpose
Current guidelines for surveillance strategy in cervical cancer are rigid, recommending the same strategy for all survivors. The aim of this study was to develop a robust model allowing for individualised surveillance based on a patient’ risk profile.
Methods
Data of 4,343 early-stage cervical cancer patients treated between 2007–2016 were obtained from international SCCAN (Surveillance in Cervical CANcer) consortium. Cox proportional hazards model predicting disease-free survival (DFS) was developed and internally validated. Risk score, derived from regression coefficients of the model, stratified the cohort into significantly distinctive risk groups. On its basis, the annual recurrence risk model (ARRM) was calculated.
Results
Five variables were included in the prognostic model: maximal pathologic tumour diameter, tumour histotype, grade, number of positive pelvic lymph nodes, and lymphovascular space invasion. Five risk groups significantly differing in prognosis were identified: with five-year DFS of 97.5%, 94.7%, 85.2%, and 63.3% in increasing risk groups, while two-year DFS in the highest risk group equalled 15.4%. Based on ARRM, the annual recurrence risk in the lowest risk group was below 1% since the beginning of follow-up and declined below 1% at years three, four, and >5 in the medium-risk groups. In the whole cohort, 26% of recurrences appeared at the first year of the follow-up, 48% by year two, and 78% by year five.
Conclusion
ARRM represents a potent tool for tailoring the surveillance strategy in early-stage cervical cancer patients based on the patient’ risk status and respective annual recurrence risk. It can easily be utilised in routine clinical settings internationally.
Keywords: cervical cancer, surveillance, prognostic model, annual recurrence risk
Introduction
Surveillance of cancer survivors consumes a significant proportion of the overall costs of cancer treatment and considerably adds to the workload of specialists, especially in diseases with generally good prognoses, such as cervical cancer [1]. The concept of surveillance in cervical cancer survivors is based on the assumption that an early recurrence diagnosis may prolong survival and increase the chance for curative treatment [2]. However, unlike for other types of cancer (e.g., breast cancer), cervical cancer surveillance guidelines are not based on prospective evidence but on retrospective studies and expert opinions [3].
While the risk of recurrence is clearly associated with prognostic risk markers [4], current international clinical practice guidelines uniformly recommend an identical surveillance strategy for all patients after the treatment. Guidelines published by the European Society of Gynaecological Oncology (ESGO), European Society for Medical Oncology (ESMO), and National Comprehensive Cancer Network (NCCN) do not contain any individualisation of the surveillance, indicating a lack of agreement on the best post-treatment follow-up [5–7]. Society of Gynecologic Oncology (SGO) guidelines stratify patients into two groups, low- and high-risk. However, the high-risk group is only vaguely defined as “advanced stage or high risk histologies”; also, the strategy is not clearly differentiated, only suggesting that an increased frequency of follow-up visits be considered [8, 9]. The aim of our study was to develop a comprehensive model that will allow for surveillance tailoring based on prognostic factors in early-stage cervical cancer patients that were referred for surgical treatment with curative intent.
Methods
Study design and participants
The SCCAN (Surveillance in Cervical CANcer) is an international, multicentre, retrospective study that evaluated recurrence patterns in cervical cancer survivors. The SCCAN study consortium consisted of 20 tertiary centres of excellence with a large volume of cervical cancer cases located in Europe, Asia, North America, or Latin America. In order for a centre to join the trial, the following requirements had to be fulfilled: (i) a minimum of 100 patients eligible for the trial; (ii) one of the modern imaging modalities routinely used in clinical staging (magnetic resonance imaging MRI, expert ultrasound, computed tomography, or positron emission tomography–computed tomography); (iii) all cases discussed by a multidisciplinary team; (iv) surgery performed by a surgeon with experience in gynae-oncology; (v) pathology performed by pathologist with experience in gynae-oncology; (vi) institutional follow-up performed by physicians; and (vii) availability of an institutional prospectively collected database of cases.
Patients were retrospectively included if they met the following inclusion criteria: (i) histologically confirmed cervical cancer treated between 2007 and 2016; (ii) TNM stage T1a-T2b (based on the preoperative assessment; American Joint Committee on Cancer – Cervix Uteri Cancer Staging); (iii) primary surgical management, including fertility-sparing procedures/surgical treatment following neoadjuvant chemotherapy; (iv) and at least one year of follow-up data availability (patient underwent surgery ≥1 years before last or follow-up visit or was not lost to follow-up during the first year post-surgery). Patients were eligible irrespective of adjuvant treatment, neoadjuvant chemotherapy, tumour type, lymph node status, or lymph node staging.
Patients were not eligible if they had precancer disease (including CIN 3 neoplasia), they were treated with definitive radiotherapy/chemoradiation, primary surgical treatment was abandoned intra-operatively, or they were lost to follow-up within the first-year post-surgery.
The protocol was approved by the institutional review board of the lead institution (General University Hospital in Prague, Czech Republic) in 2016. Institutional review board approval at the participating sites was a prerequisite for participation. Due to the retrospective nature of the study, the need for informed consent was waived by the Institutional Review board. The study was performed in accordance with the Declaration of Helsinki.
Data collection
The principal investigator at each institution identified eligible patients, anonymised the data, and transferred the data using a web-based system to ensure consistent data collection, which ended in November 2020. The following data about treatment were collected: type of uterine procedure, type of parametrectomy, surgical approach, lymph node (LN) staging and its extent, type of neoadjuvant therapy, and type of adjuvant treatment. The type of parametrectomy was classified using the Querleu–Morrow modified classification system [10]. Regarding disease characteristics, we collected data about the type and size of the tumour (pathologically confirmed), depth of stromal invasion, pathologic TNM stage, number, and size of removed/positive lymph nodes, parametrial involvement, lymphovascular space invasion, and grade. Histological types of the tumours were classified according to WHO classification and were consequently clustered into six main groups: adenocarcinoma, adenosquamous cancer, squamous cell carcinoma, neuroendocrine cancer, and a cluster of others. In relapsing patients, the data about the precise location of the recurrence, presence of symptoms, and recurrence treatment modality were collected.
After the patient’ data were received, the database was cleaned. Patients with missing information (N = 242) on key predictor variables, such as tumour and surgery characteristics (tumour type, tumour size), and details about the follow up (date of the last visit, disease status at the last visit, and date of recurrence/death) were excluded.
Data analyses
The length of the follow-up period was calculated from the surgery date to the last recorded follow-up visit. Standard descriptive statistics were used to summarise the data: categorical variables were described by absolute and relative frequencies; continuous variables were described by mean with standard deviation and median with interquartile range. Missing values of grade (27.6% patients), which were, according to the univariable analysis, expected to be a significant predictor in multivariable analyses, were for multivariable analysis imputed on the basis of other predictors (age, number of positive pelvic lymph nodes, tumour diameter, LVSI, histotype, pT, adjuvant therapy). In total, five different data sets were created by multiple imputation and, therefore, the subsequent results had to be pooled. The imputation was performed using SPSS 25.0.0.1 and R-3.6.1.
The relationship between patient, tumour, and treatment characteristics and the analysed endpoint (disease-free survival) was evaluated by univariable and multivariable Cox proportional hazard models and described by hazard ratios, their 95% confidence intervals, and statistical significance. A backward stepwise algorithm and Akaike information criterion (AIC) were used to choose the optimal multivariable model from predictors which were found to have a significant impact on disease-free survival in univariable analyses. The discrimination ability of the model was assessed using the Harrell’s C-index. A ten-fold cross-validation was performed within each of five imputed datasets to obtain estimates of model performance that are adjusted for in-sample optimism.
A risk score was derived from regression coefficients (β) of the final model, which were weighted to the maximum sum of 100 points. The results of the model were expressed by Kaplan-Meier curves based on a stratified risk score (25-point intervals with the exception of the first category corresponding to the absence of risk predictors: 0, 1–25, 26–50, 51–75, 76–100). The chi-squared test for trend in proportions was used to assess the relationship between risk score and recurrence localisation and presence of symptoms. The annual risk of recurrence was assessed by conditional survival analysis. The conditional survival was estimated by calculating the survival probabilities with different landmark starting points: zero-year, one year, two years, three years, and four years. Only the patients who survived until the beginning of the interval were included for the estimation of recurrence probability in the certain year derived as 1 minus 1-year survival. Analysis was computed using SPSS 25.0.0.1 for data pre-processing and R-3.6.1 for data imputation (the mice package) and model building (the survival package).
Results
Cohort characteristics
We analysed the data from 4,343 patients with histologically confirmed cervical cancer who underwent primary treatment between January 2007 and December 2016. Each of the involved SCCAN study consortium centres contributed at least 100 cases, while the largest of them submitted data from more than 400 individuals. The proportion of patients with different tumour size categories varied among the institutions, reflecting national and institutional selection criteria for the management of early-stage cervical cancer, especially regarding patients with larger tumours which are in certain regions/institutions referred for primary (chemo)radiation (Supplementary Fig. 1). The main patient characteristics are summarised in Table 1. The majority of the patients had squamous cell carcinoma (68.4%) or adenocarcinoma (24.7%). In all, 80.2% of the patients underwent radical hysterectomy, followed by simple hysterectomy (9.2%), and conisation (6.5%). The prevailing surgical approach was laparotomy in 59.5% of cases.
Table 1.
Data summary (N = 4,343)
| Parameter | Description* | |
|---|---|---|
|
| ||
| Age at surgery | 46 (± 12); 44 (37–54) | |
|
| ||
| Type of uterine/cervical procedure | Conization | 281 (6.5%) |
| Radical hysterectomy | 3,480 (80.2%) | |
| Radical parametrectomy | 10 (0.2%) | |
| Radical trachelectomy | 146 (3.4%) | |
| Simple hysterectomy | 398 (9.2%) | |
| Trachelectomy | 25 (0.6%) | |
|
| ||
| Not available (% of total) | 3 (0.1%) | |
|
| ||
| Type of parametrial resection | A | 702 (16.9%) |
| B | 774 (18.6%) | |
| C1 | 1,742 (41.9%) | |
| C2 | 935 (22.5%) | |
|
| ||
| Not available (% of total) | 190 (4.4%) | |
|
| ||
| Surgical approach | Open | 2,578 (59.5%) |
| Laparoscopic | 964 (22.2%) | |
| Robotic | 428 (9.9%) | |
| Vaginal | 219 (5.1%) | |
| Combined | 146 (3.4%) | |
|
| ||
| Not available (% of total) | 8 (0.2%) | |
|
| ||
| Neoadjuvant therapy | No | 4,164 (95.9%) |
| Yes | 179 (4.1%) | |
|
| ||
| Sentinel lymph node biopsy performed (including ultrastaging) | No | 3,342 (77.0%) |
| Yes | 1,001 (23.0%) | |
|
| ||
| Pelvic lymphadenectomy performed | No | 567 (13.1%) |
| Yes | 3,776 (86.9%) | |
|
| ||
| Paraaortic lymphadenectomy performed | No | 3,655 (84.2%) |
| Yes | 688 (15.8%) | |
|
| ||
| Number of pelvic LN retrieved | (if > 0, n = 3,771) | 25 (± 13); 23 (16–32) |
|
| ||
| Number of paraaortic LN retrieved | (if > 0, n = 632) | 11 (± 8); 9 (5–14) |
| Number of positive pelvic LN | (if > 0, n = 643) | 3 (± 3); 2 (1–3) |
|
| ||
| Number of positive paraaortic LN | (if > 0, n = 50) | 5 (± 7); 2 (1–6) |
|
| ||
| Number of positive pelvic + paraaortic LN | (if > 0, n = 650) | 3 (± 5); 2 (1–3) |
|
| ||
| Largest type of metastasis in LN (if number of positive LN > 0; n = 650) | Isolated tumour cells | 27 (4.2%) |
| Micrometastasis | 89 (13.7%) | |
| Macrometastasis | 534 (82.2%) | |
|
| ||
| Pathologic T stage (pT) | 1a1 | 550 (12.7%) |
| 1a2 | 421 (9.7%) | |
| 1b1 | 2,709 (62.4%) | |
| 1b2 | 321 (7.4%) | |
| 2a1 | 155 (3.6%) | |
| 2a2 | 39 (0.9%) | |
| 2b | 148 (3.4%) | |
|
| ||
| Maximal pathologic tumour diameter | < 0.5 cm | 711 (16.4%) |
| 0.5–1.99 cm | 1,723 (39.7%) | |
| 2–3.99 cm | 1,349 (31.1%) | |
| ≥ 4 cm | 560 (12.9%) | |
|
| ||
| Tumour histotype | Adenocancer | 1,072 (24.7%) |
| Adenosquamous | 206 (4.8%) | |
| Neuroendocrine | 43 (1.0%) | |
| Squamous | 2,966 (68.4%) | |
| Other | 49 (1.2%) | |
|
| ||
| Not available (% of total) | 7 (0.2%) | |
|
| ||
| Grade | 1 | 599 (19.1%) |
| 2 | 1,628 (51.8%) | |
| 3 | 917 (29.2%) | |
|
| ||
| Not available (% of total) | 1,199 (27.6%) | |
|
| ||
| LVSI | No | 1,949 (55.6%) |
| Yes | 1,556 (44.4%) | |
|
| ||
| Not available (% of total) | 838 (19.3%) | |
|
| ||
| Adjuvant therapy | No | 2,821 (65.0%) |
| Yes | 1,522 (35.0%) | |
|
| ||
| Adjuvant therapy type (N = 1,522) | CRT | 743 (48.8%) |
| CT | 68 (4.5%) | |
| NACT/RT and CT/outback | 7 (0.5%) | |
| RT | 704 (46.3%) | |
|
| ||
| Cervical cancer recurrence | No | 3,815 (87.8%) |
| Yes | 528 (12.2%) | |
|
| ||
| Recurrence type (if recurrence = yes; n = 528) | Isolated | 322 (61.9%) |
| Multiple | 198 (38.1%) | |
|
| ||
| Not available (% of n) | 8 (1.5%) | |
|
| ||
| Recurrence localization (if recurrence = yes; n = 528) | Pelvic | 240 (46.3%) |
| Distant | 149 (28.8%) | |
| Combined | 129 (24.9%) | |
|
| ||
| Not available (% of n) | 10 (1.9%) | |
|
| ||
| Recurrence diagnosis (if recurrence = yes; n = 528) | Follow-up visit | 338 (72.7%) |
| Unscheduled | 127 (27.3%) | |
|
| ||
| Not available (% of n) | 63 (11.9%) | |
|
| ||
| Symptoms at recurrence (if recurrence = yes; n = 528) | Asymptomatic | 189 (40.9%) |
| Symptomatic | 273 (59.1%) | |
|
| ||
| Not available (% of n) | 66 (12.5%) | |
|
| ||
| Recurrence treatment modality (if recurrence = yes; n = 528) | CRT | 115 (24.0%) |
| CT | 180 (37.6%) | |
| RT | 43 (9.0%) | |
| Surgery | 37 (7.7%) | |
| Surgery + RT/CRT/CT | 77 (16.1%) | |
| No treatment | 23 (4.8%) | |
|
| ||
| Not available (% of n) | 43 (10.1%) | |
|
| ||
| Disease status at last FU visit | Alive with disease | 144 (3.3%) |
| Died of other cause | 46 (1.1%) | |
| Died of disease | 251 (5.8%) | |
| No evidence of disease | 3,902 (89.8%) | |
Categorical variables are described by absolute and relative frequencies (% are based on available data only), mean (± SD) and median (25th–75th percentile) are shown for continuous variables.
CRT: chemoradiotherapy; CT: chemotherapy; LN: lymph node; NACT: neoadjuvant chemotherapy; RT: radiotherapy.
LN staging was performed in 86.9% of the patients, while 64.9% underwent systematic pelvic lymph node dissection (PLND), 22.0% PLND in combination with sentinel lymph node biopsy (SLN), and 1% SLN biopsy only. Paraaortic lymphadenectomy was performed in 15.8% of patients. The median number of removed pelvic lymph nodes among patients who underwent PLND ± SLN was 23 (25th–75th percentile: 16–32). Positive pelvic LNs were found in 15.0% of cases: 82.2% had macrometastasis (metastasis larger than ≥2 mm), 13.7% micrometastasis (MIC: 0.2–2 mm), and 4.2% isolated tumour cells (ITC: cell clusters smaller than 0.2 mm), as the largest determined LN metastasis. MIC and ITC were reported only amongst patients with SLN processed by ultrastaging. The median number of positive LNs per impacted patient was two (5th–95th percentile: 1–8). Fifty patients (1.2%) also had positive paraaortic lymph nodes, out of which seven had negative pelvic LNs (SLN biopsy was not performed among those patients).
Thirty-five percent of the patients received adjuvant treatment, from which the majority underwent chemoradiation (48.8%) or combined radiotherapy (46.3%). Out of the patients belonging to the intermediate-risk group (N0 and tumour size 2–4 cm + LVSI OR tumour size ≥4 cm), 60% received adjuvant treatment. Only 8.7% of the patients from the high-risk group (N1 OR parametrial invasion OR positive surgical margins) did not receive any type of adjuvant treatment.
Recurrence pattern and oncological outcome
The median follow-up period in the cohort was 4.8 years (25th–75th percentile: 3.0–6.7). During the last follow-up visit, 3,902 patients (89.8%) had no evidence of disease, 144 were alive with disease (3.3%), 251 patients died of disease or treatment-related complications (5.8%), and 46 patients died of other causes (1.1%).
Out of 528 patients with recurrence, 322 (61.9%) had isolated recurrence and 198 (38.1%) experienced recurrence localised on multiple sites, while eight had recurrence of unknown location. From the patients with isolated recurrence, 144 had a central pelvic recurrence, 48 relapsed in lateral pelvis; meanwhile, 88 of the patients developed isolated distant recurrence, mainly located in the thorax/lungs (42 patients) or in the liver (eight patients). In patients with multiple recurrence sites, 107 (54%) developed combined relapses located in the pelvis and distantly.
Disease-free survival (DFS) in the whole cohort reached 87.7% (95% CI: 86.7%; 88.7%) at five years after the surgery (Fig. 1). The DFS curve did not have any clear inflection point, and there was a low but continuous frequency of new recurrences five–ten years post-surgery: DFS ten-years post-surgery declined to 84.2% (95% CI: 82.6%; 85.7%).
Fig 1.
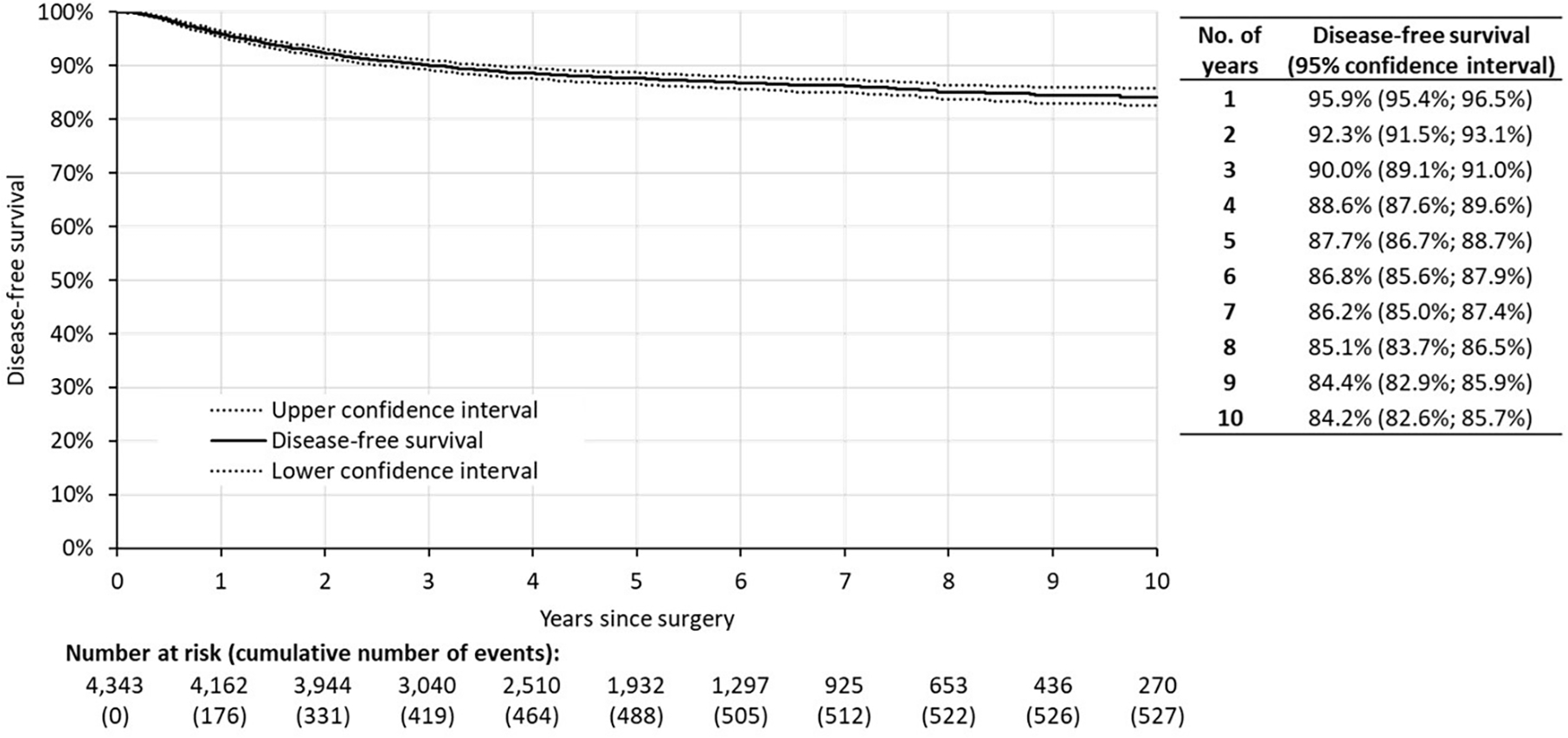
Disease-free survival of the whole cohort (N = 4343). Time 0 represents date of surgery.
Based on the Kaplan-Meier estimates calculated for the period of ten years of follow-up, the cumulative proportion of observed recurrences reached 25.6% at one year, 48.4% at two years, and 77.7% at five years post-surgery. The remaining 22.3% of recurrences occurred between six to ten years after surgery (Supplementary Table 1).
Prognostic model development and validation
Ten traditional prognostic markers were evaluated in univariable analysis for predicting DFS (Supplementary Table 2). Only surgical approach (open vs minimally invasive) ceased to be significant. The highest prognostic risk, described as hazard ratio (HR), was found for disease stage, maximum tumour size, tumour histotype, presence of positive paraaortic LNs, and positive pelvic LNs. The depth of stromal invasion (DSI) was also analysed, though no significance in the univariable analysis was found. However, we decided to exclude this parameter from the final analyses due to extremely heterogenous DSI assessment methods (mathematical calculation of DSI from the largest tumour size and cervical size; evaluation of DSI in one slice only or in transversal diameter only, etc). Sequential landmark analysis describing the annual probability of recurrence according to the respective factor was performed (heatmap, Supplementary Table 2).
All significant markers related to the DFS in univariable analyses were included in multivariable analyses using the Cox proportional hazards model. The final prognostic model consists of five parameters: (i) maximal pathologic tumour diameter; (ii) tumour histotype; (iii) grade; (iv) number of positive pelvic LNs; and (v) presence of LVSI (Table 2).
Table 2.
Multivariable model for the risk of recurrence prediction
| Predictor | β | SE(P) | HR (95% CI) | P-value | Risk points (max. 100) | |
|---|---|---|---|---|---|---|
|
| ||||||
| Histotype | Squamous cell | Reference | 0 | |||
| Adenocarcinoma | 0.342 | 0.116 | 1.408 (1.120; 1.771) | 0.003 | 7 | |
| Adenosquamous | 0.598 | 0.164 | 1.819 (1.317; 2.513) | < 0.001 | 11 | |
| Neuroendocrine | 1.741 | 0.246 | 5.704 (3.514; 9.260) | < 0.001 | 33 | |
| Other | 1.145 | 0.270 | 3.144 (1.848; 5.349) | < 0.001 | 22 | |
|
| ||||||
| Tumour diameter | < 0.5 cm | Reference | 0 | |||
| 0.5–1.99 cm | 0.501 | 0.237 | 1.651 (1.035; 2.634) | 0.035 | 10 | |
| 2–3.99 cm | 1.115 | 0.236 | 3.051 (1.915; 4.858) | < 0.001 | 21 | |
| ≥ 4 cm | 1.556 | 0.245 | 4.738 (2.925; 7.674) | < 0.001 | 30 | |
|
| ||||||
| Grade | 1 | Reference | 0 | |||
| 2 | 0.260 | 0.214 | 1.297 (0.852; 1.976) | 0.235 | 5 | |
| 3 | 0.457 | 0.247 | 1.579 (0.970; 2.570) | 0.085 | 9 | |
|
| ||||||
| Positive pelvic LN | 0 / not assessed | Reference | 0 | |||
| 1 | 0.255 | 0.154 | 1.291 (0.953; 1.748) | 0.098 | 5 | |
| 2 | 0.482 | 0.170 | 1.619 (1.158; 2.264) | 0.005 | 9 | |
| ≥ 3 | 0.939 | 0.144 | 2.557 (1.927; 3.394) | < 0.001 | 18 | |
|
| ||||||
| LVSI | No / not assessed | Reference | 0 | |||
| Yes | 0.538 | 0.106 | 1.713 (1.390; 2.111) | < 0.001 | 10 | |
β: beta coefficient; CI: confidence interval; LN: lymph node; LVSI: lymphovascular space invasion; SE: standard error.
The beta coefficients of the multivariable model were consequently converted into the risk points (Table 2). The Harrell’s concordance statistic factor (C-statistics) of the resulting model is 0.735 (95% CI: 0.713; 0.757). After performing the ten-fold cross-validation within each imputed dataset, the average AUC of 0.732 was obtained.
Multivariable prognostic model outcomes
Using accumulated risk score of the patients, five groups stratifying the patients according to the risk score were created: (i) zero points; (ii) 1–25 points; (iii) 26–50 points; (iv) 51–75 points; and (v) 76–100 points. Pairwise comparison of the groups proved a significant difference in DFS between the groups (P < 0.001). The only exception was the absence of significance between the two lowest-risk groups, zero points and 1–25 points, where P = 0.076. Since the group with zero points represents a cohort of 213 patients with excellent prognosis (only four recurrences were observed), we kept the two groups separate. The Kaplan-Meier disease-free survival curve for the respective risk-score groups is shown in Fig. 2.
Fig. 2.
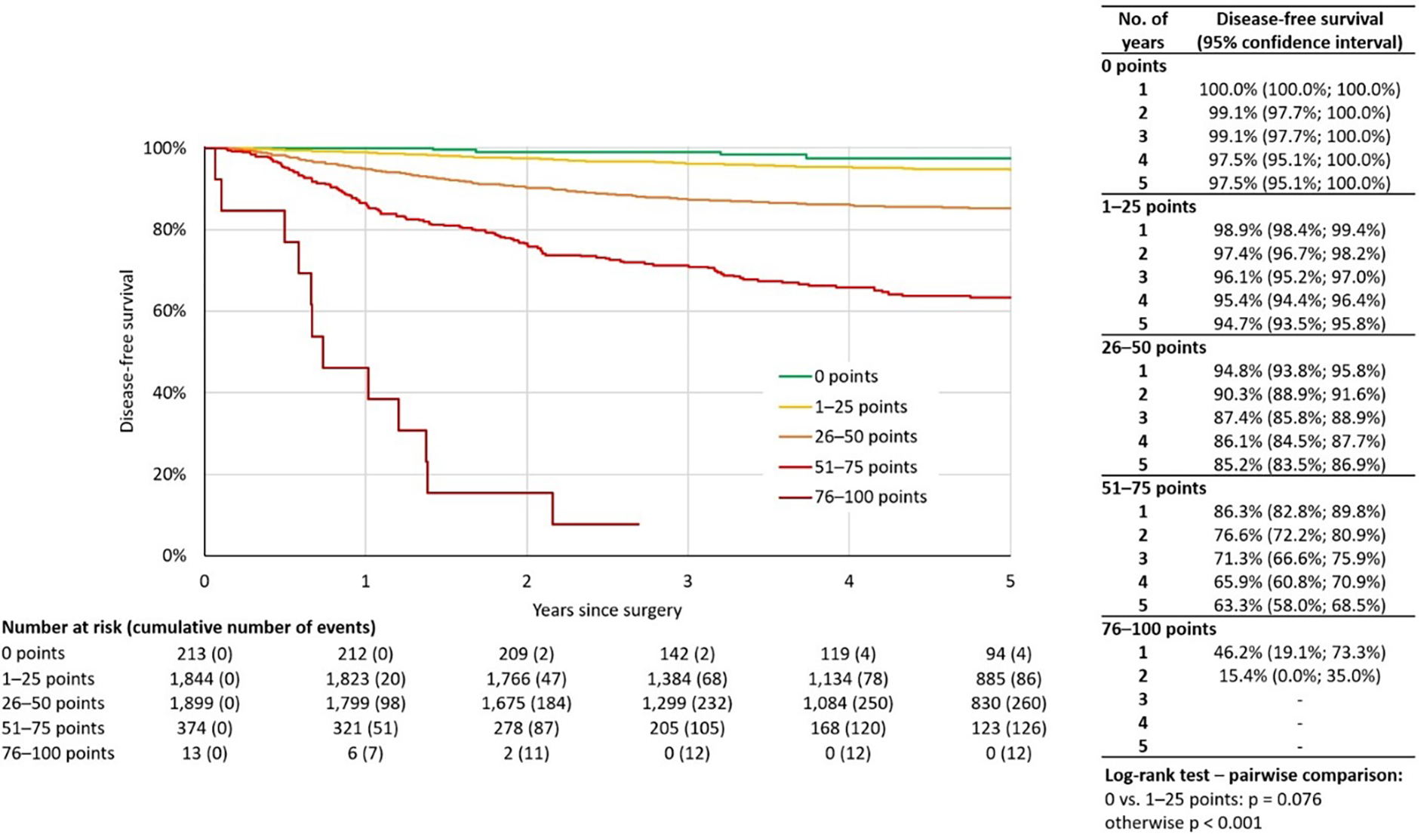
Disease-free survival of all cases stratified by risk score (N = 4343).
Annual recurrence risk model (ARRM)
Based on the previously described patient stratification into groups according to risk score, a landmark model of the annual recurrence risk was established (ARRM) (Fig 3).
Fig. 3.
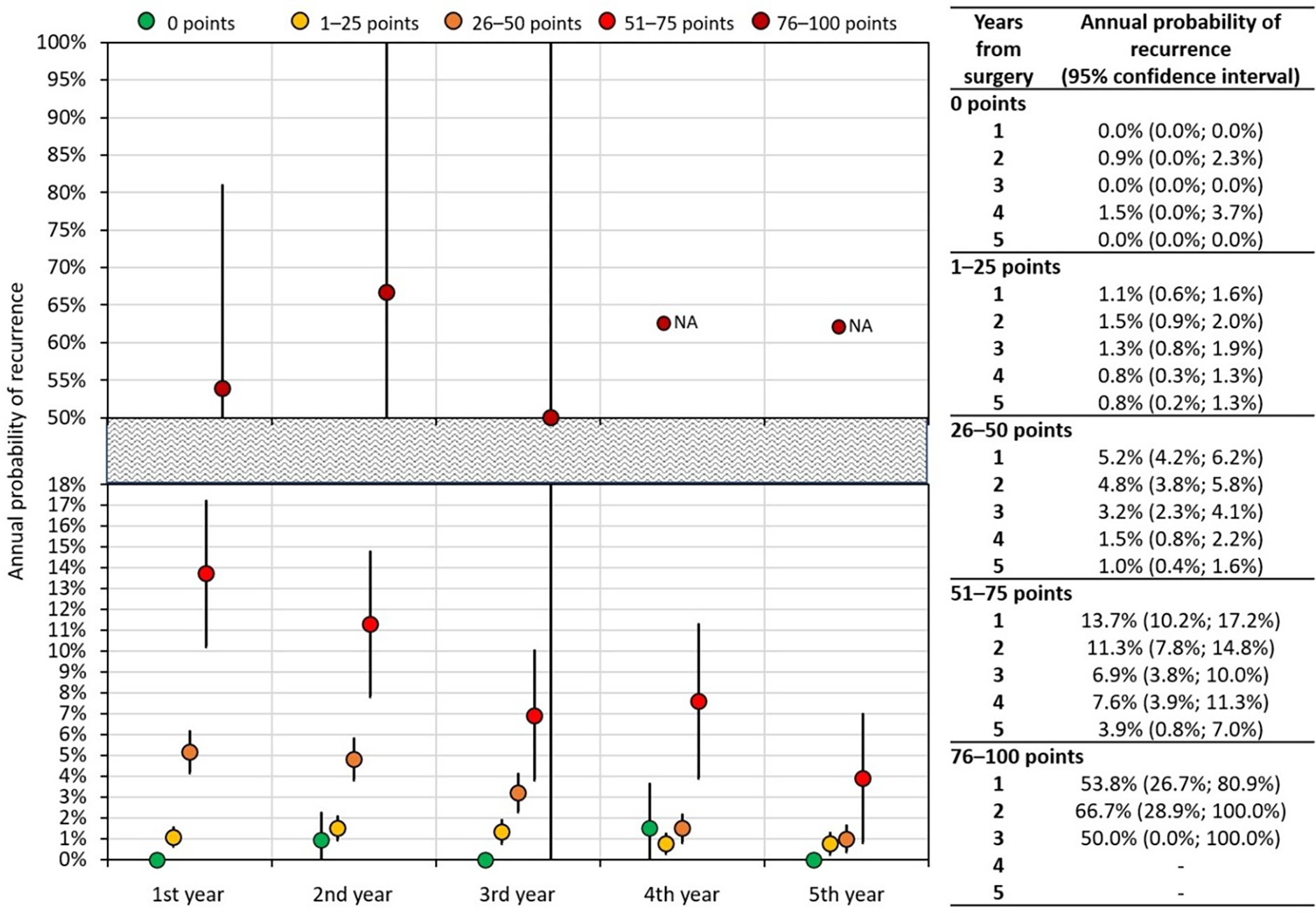
ARRM (annual recurrence risk model): Landmark analysis of the annual probability of recurrence after surgery. N/A: not analysed.
Analysis revealed that the annual RR for the zero-point group was close to 0% for all five years. The RR of the 1–25 point group also did not significantly change during the five-year follow-up, oscillating close to 1% RR. As for the 26–50 point group, the RR for the first year of follow-up was 5.2% (95% CI: 4.2%; 6.2%) and steadily decreased from year three (3.2% (95% CI: 2.3%; 4.1%) to year five (1.0% (95% CI: 0.4%; 1.6%)). The higher-risk 51–75 point group had recurrence risk equalling 13.7% (95% CI: 10.2%; 17.2%) at year one, which significantly decreased by year five, when it reached 3.9% (95% CI: 0.8%; 7.0%). The landmark analysis for the group with the highest risk (76–100 points) was only performed until year three of follow-up, due to the limited number of cases (13 patients) and high recurrence rate in the first three years (cumulatively, 12 recurrences). The analysis ceased to be reliable after this point. The probability of recurrence in years one and two equalled 53.8% (95% CI: 26.7%; 80.9%) and 66.7% (95% CI: 28.9%; 100%), respectively.
The proportion of asymptomatic recurrences at the diagnosis decreased with the increasing number of risk points (Supplementary Table 3) (P = 0.003). Also, with the increasing risk score, the proportion of pelvic recurrences was declining (P < 0.001), and the proportion of distant recurrences was increasing (P = 0.002). The proportion of combined recurrences does not follow any risk-score related trend (Supplementary Table 3). The landmark analysis of risk of annual recurrence was also separately performed for isolated pelvic recurrence (Supplementary Fig. 2).
Discussion
The aim of this retrospective international multicentre study was to develop a model for a tailored surveillance strategy for individual subgroups of patients and to analyse recurrence patterns, especially recurrence risk and localisation, at individual years following primary surgical treatment.
The main outcomes of the study are the analysis of annual recurrence risk (ARRM) and annual pelvic recurrence risk, both based on multivariable model for the risk of recurrence, which may serve for tailoring surveillance strategy in early-stage cervical cancer patients after surgical treatment. The model is based on five traditional risk markers that allow for stratification of five groups with significantly different prognosis and recurrence patterns.
To date, no prospective studies have addressed the efficacy and strategy of surveillance of early-stage cervical cancer patients. Although prognostic factors are well recognised [7, 11], the majority of the current international guidelines recommend uniform surveillance strategy for all patients irrespective of the risk groups. Neither ESGO, ESMO, nor NCCN guidelines stratify patients according to their risk status. Population-based surveillance is recommended beyond the five-year mark [5, 6, 12]. Unsurprisingly, the professional community is also divided in opinions about surveillance intensity and frequency. A survey performed among 375 ESGO and NSGO members revealed that 29% of the respondents considered a conventional protocol for follow-up adequate, 25% would welcome a less-intensive hospital follow-up for all patients, and 46% considered less-intensive follow-up adequate only for low-risk patients [13]. A significant determinant in the responses was the economic status of the country in which the responder practised—higher-income countries were inclined to a less-intensive strategy (P = 0.006). This is not surprising, as the costs of surveillance in those countries can easily reach or even exceed the costs of the treatment itself.
A systematic review of 17 retrospective trials showed that 89–99% of cervical cancer relapses occur by year five post-treatment [14], while 70–80% of recurrences are reported to be diagnosed during the first two years after the treatment [15–17]. However, the majority of the previously published studies reported the absolute proportion of cumulative recurrences, not taking into account the significant drop-out of followed at-risk patients with an increasing interval since treatment. In order to avoid this bias, we employed Kaplan-Meier proportion-based methodology that should minimise the stated bias and allow for better prediction of general recurrence distribution. In all patients kept in the follow-up, it is estimated that only 48.4% of recurrences occur in the first two years, 77.7% occur by year five, and the remaining 22.3% are diagnosed six to ten years post-surgery.
Numerous previous studies have analysed prognostic factors for cervical cancer recurrence and developed prognostic models for the risk of recurrence. The majority used small cohorts or included only specific subgroups of patients [18–28]. Amongst studies based on large groups, the Korean Gynaecologic Oncology Group developed a DFS prediction model based on 1,441 early-stage cervical cancer patients which consisted of para-aortic lymph node status, tumour histotype, LVSI, depth of invasion, pelvic lymph node status, and pre-treatment serum haemoglobin level [29]. A Danish model for the risk of recurrence in 1,415 1A1-1B1 cervical cancer patients included the age of the patient, 1B1 stage, and LVSI [17]. Another prognostic nomogram was established by a Chinese group and based on the largest dataset of 8,202 patients obtained from the Surveillance, Epidemiology, and End Results (SEER) database. Their model was rather complicated and entailed age at diagnosis, race, marital status, tumour grade, FIGO stage, histological type, tumour size, and log ratio between number of positive and negative lymph nodes [30].
The prognostic model presented in our study consists of five commonly used prognostic markers, including maximal pathologic tumour diameter, tumour histotype, grade, number of positive pelvic lymph nodes, and presence of LVSI. It stratified five risk-groups with significantly different risk of recurrence, with five-year DFS reaching 97.5% in the zero-point group and, on the other hand, two-year DFS of only 15.4% in the 76–100 point group.
The prognostic model was consequently used to calculate the annual recurrence risk model (ARRM), the main outcome of our study. As far as we are aware, such model has not been developed by any previous study in a population of early-stage cervical cancer patients. ARRM shows the risk of recurrence at individual years after the surgery for five prognostically different cohorts. We showed that recurrence pattern, both the interval since surgery and recurrence localisation, differ among the identified risk groups. Should we consider 1% annual risk of recurrence as a threshold for institutional follow-up, patients from the 1–25 point group should be followed for three years, patients in the 26–50 points group for four years, and patients from 51–75 for at least 5 years after primary surgery. As for the lowest risk group with excellent prognosis, no regular follow-up is needed. On the other hand, 76–100 point group will likely not benefit from any surveillance strategy due to prevailing distant metastases and expectedly very poor disease-specific survival.
Using the ARRM, the threshold for the follow-up can be set at different levels, based on many criteria, such as regional or institutional guidelines, personal and financial resources in a region, or the patient’ individual preferences and expectations. It should be kept in mind that the main burden of the disease is currently in countries with limited resources. Since ARRM is based on the combination of commonly used and easily accessible prognostic risk markers, it can be used in routine clinical practice anywhere in the world.
Moreover, as the prognosis of the recurrent disease depends largely on the site of recurrence, prediction of recurrence localisation is another important aspect for surveillance strategy planning. Previous studies reported recurrences site in the central pelvis (i.e., vaginal apex or pelvis without sidewall involvement) in 22–56% of cases, in the lateral pelvis in 28–37%, and distantly or on multiple sites in 15–61% [17, 31–33]. The wide variety in prevalence is likely attributed to the different risk profiles of the patients. In our study, pelvic recurrences were significantly more frequent in lower-risk groups while a proportion of distant recurrences increased with a higher risk score. Additionally, we also observed the time-dependent trend in recurrence localisation: the proportion of pelvic recurrences decreased while the proportion of distant recurrences increased with the interval since the primary surgery.
Our study presents the first analysis of annual recurrence pattern in early-stage cervical cancer patients. We utilised a large dataset composed of validated data from carefully selected tertiary centres of excellence with high volumes of cervical cancer patients geographically distributed on four continents. The complete data on the mandatory variables from 4,343 cervical cancer patients were included and analysed. The scale of patients was sufficient for analysis of the prognostic significance of a large number of prognostic markers. Furthermore, the discrimination ability of the model was internally validated using cross-validation and performance was assessed by C-statistics (=0.732), indicating good prognostic accuracy of our model. The advantage of ARRM is that the modeĺs parameters are commonly used and easily accessible in routine practice. Surveillance strategy can be consequently adopted individually using the preferred threshold for an acceptable annual risk. The major limitation of this study is its retrospective design; therefore, our analysis may have biases connected to patient selection, since only patients with complete data availability were registered to the study. Also, since the grade was missing for 27.6% of patients, these cases were not excluded but missing values were imputed on the basis of other predictors. Finally, the different composition of patients between sites reflects regional standards of treatment and approaches of attending physicians in referring patients to either surgical treatment or primary (chemo)radiation.
Conclusions
In conclusion, we developed the ARRM model showing the annual recurrence pattern in five years following the surgical treatment of cervical cancer and stratifying patients according to their risk profile. It is based on a robust prognostic model, combining five traditional and easily accessible prognostic markers, and was internally validated to ensure its accuracy. This model allowed us to stratify patients into five risk groups significantly differing in their prognosis and, as such, represents a tool for individualisation of the appropriate surveillance strategy. ARRM is easy to use and can be utilised quickly in a routine clinical setting. Furthermore, it could also serve as a base for international recommendations and guidelines and for planning future prospective studies.
Supplementary Material
Highlights:
Recurrence risk model in cervical cancer was composed of 5 prognostic factors
Developed ARRM model stratifies cohort into 5 significantly distinctive risk groups
ARRM represents a powerful tool for tailoring of appropriate surveillance strategy
ARRM can easily be utilised in routine clinical settings internationally
Acknowledgement
We would like to acknowledge all medical specialists, data and case managers, and study coordinators, from all 20 sites participating in the SCCAN study.
Funding
This work was supported by Charles University in Prague (UNCE 204065 and PROGRES Q28/LF1) and the NIH/NCI Cancer Center Support Grant (P30 CA008748). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Conflict of interest statement
The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval and consent to participate
The protocol was approved by the institutional review board of the lead institution (General University Hospital in Prague, Czech Republic) in 2016. Institutional review board approval at the participating sites was a prerequisite for participation. Due to the retrospective nature of the study, the need for informed consent was waived by the Institutional Review board. The study was performed in accordance with the Declaration of Helsinki.
Appendix A. Supplementary data
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Insinga RP, Ye X, Singhal PK, Carides GW. Healthcare resource use and costs associated with cervical, vaginal and vulvar cancers in a large U.S. health plan. Gynecol Oncol. 2008;111:188–96. [DOI] [PubMed] [Google Scholar]
- [2].Wright JD. Management of recurrent or metastatic cervical cancer. UpToDate; 2020. [Google Scholar]
- [3].Zola P, Macchi C, Cibula D, Colombo N, Kimmig R, Maggino T, et al. Follow-up in Gynecological Malignancies: A State of Art. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2015;25:1151–64. [DOI] [PubMed] [Google Scholar]
- [4].Sartori E, Pasinetti B, Carrara L, Gambino A, Odicino F, Pecorelli S. Pattern of failure and value of follow-up procedures in endometrial and cervical cancer patients. Gynecologic oncology. 2007;107:S241–7. [DOI] [PubMed] [Google Scholar]
- [5].Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology. 2017;28:iv72–iv83. [DOI] [PubMed] [Google Scholar]
- [6].NCCN guidelines version 2.2020. 2020.
- [7].Cibula D, Potter R, Planchamp F, Avall-Lundqvist E, Fischerova D, Haie Meder C, et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2018;127:404–16. [DOI] [PubMed] [Google Scholar]
- [8].Salani R, Khanna N, Frimer M, Bristow RE, Chen LM. An update on post-treatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncology (SGO) recommendations. Gynecologic oncology. 2017;146:3–10. [DOI] [PubMed] [Google Scholar]
- [9].Lanceley A, Fiander A, McCormack M, Bryant A. Follow-up protocols for women with cervical cancer after primary treatment. Cochrane Database Syst Rev. 2013:CD008767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Querleu D, Cibula D, Abu-Rustum NR. 2017 Update on the Querleu-Morrow Classification of Radical Hysterectomy. Annals of surgical oncology. 2017;24:3406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cibula D, Slama J, Dostálek L, Fischerová D, Germanova A, Frühauf F, et al. Tumour-free distance: a novel prognostic marker in patients with early-stage cervical cancer treated by primary surgery. British journal of cancer. 2021;124:1121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology. 2017;28 Suppl 4:iv72–iv83. [DOI] [PubMed] [Google Scholar]
- [13].Vistad I, Cvancarova M, Salvesen HB. Follow-up of gynecological cancer patients after treatment - the views of European experts in gynecologic oncology. Acta obstetricia et gynecologica Scandinavica. 2012;91:1286–92. [DOI] [PubMed] [Google Scholar]
- [14].Elit L, Fyles AW, Devries MC, Oliver TK, Fung-Kee-Fung M, Gynecology Cancer Disease Site G. Follow-up for women after treatment for cervical cancer: a systematic review. Gynecol Oncol. 2009;114:528–35. [DOI] [PubMed] [Google Scholar]
- [15].Vistad I, Bjorge L, Solheim O, Fiane B, Sachse K, Tjugum J, et al. A national, prospective observational study of first recurrence after primary treatment for gynecological cancer in Norway. Acta obstetricia et gynecologica Scandinavica. 2017;96:1162–9. [DOI] [PubMed] [Google Scholar]
- [16].Hillesheim I, Limone GA, Klimann L, Monego H, Appel M, de Souza A, et al. Cervical Cancer Posttreatment Follow-up: Critical Analysis. Int J Gynecol Cancer. 2017;27:1747–52. [DOI] [PubMed] [Google Scholar]
- [17].Taarnhoj GA, Christensen IJ, Lajer H, Fuglsang K, Jeppesen MM, Kahr HS, et al. Risk of recurrence, prognosis, and follow-up for Danish women with cervical cancer in 2005–2013: A national cohort study. Cancer. 2018;124:943–51. [DOI] [PubMed] [Google Scholar]
- [18].Smiley LM, Burke TW, Silva EG, Morris M, Gershenson DM, Wharton JT. Prognostic factors in stage IB squamous cervical cancer patients with low risk for recurrence. Obstet Gynecol. 1991;77:271–5. [DOI] [PubMed] [Google Scholar]
- [19].Ishikawa H, Nakanishi T, Inoue T, Kuzuya K. Prognostic factors of adenocarcinoma of the uterine cervix. Gynecol Oncol. 1999;73:42–6. [DOI] [PubMed] [Google Scholar]
- [20].Odell EW, Jani P, Sherriff M, Ahluwalia SM, Hibbert J, Levison DA, et al. The prognostic value of individual histologic grading parameters in small lingual squamous cell carcinomas. The importance of the pattern of invasion. Cancer. 1994;74:789–94. [DOI] [PubMed] [Google Scholar]
- [21].Kristensen GB, Abeler VM, Risberg B, Trop C, Bryne M. Tumor size, depth of invasion, and grading of the invasive tumor front are the main prognostic factors in early squamous cell cervical carcinoma. Gynecologic oncology. 1999;74:245–51. [DOI] [PubMed] [Google Scholar]
- [22].Tsai CS, Lai CH, Wang CC, Chang JT, Chang TC, Tseng CJ, et al. The prognostic factors for patients with early cervical cancer treated by radical hysterectomy and postoperative radiotherapy. Gynecol Oncol. 1999;75:328–33. [DOI] [PubMed] [Google Scholar]
- [23].Gulseren V, Kocaer M, Cakir I, Ozdemir IA, Sanci M, Gungorduk K. Postoperative nomogram for the prediction of disease-free survival in lymph node-negative stage I-IIA cervical cancer patients treated with radical hysterectomy. J Obstet Gynaecol. 2020;40:699–704. [DOI] [PubMed] [Google Scholar]
- [24].Sevin B-U, Lu Y, Bloch DA, Nadji M, Koechli OR, Averette HE. Surgically defined prognostic parameters in patients with early cervical carcinoma: A multivariate survival tree analysis. Cancer. 1996;78:1438–46. [DOI] [PubMed] [Google Scholar]
- [25].Polterauer S, Grimm C, Hofstetter G, Concin N, Natter C, Sturdza A, et al. Nomogram prediction for overall survival of patients diagnosed with cervical cancer. Br J Cancer. 2012;107:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kim M-K, Jo H, Kong H-J, Kim HC, Kim JW, Kim Y-M, et al. Postoperative Nomogram Predicting Risk of Recurrence After Radical Hysterectomy for Early-Stage Cervical Cancer. International Journal of Gynecologic Cancer. 2010;20:1581–6. [PubMed] [Google Scholar]
- [27].Obrzut B, Kusy M, Semczuk A, Obrzut M, Kluska J. Prediction of 10-year Overall Survival in Patients with Operable Cervical Cancer using a Probabilistic Neural Network. J Cancer. 2019;10:4189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yang Z, Hou Y, Lyu J, Liu D, Chen Z. Dynamic prediction and prognostic analysis of patients with cervical cancer: a landmarking analysis approach. Annals of epidemiology. 2020;44:45–51. [DOI] [PubMed] [Google Scholar]
- [29].Paik ES, Lim MC, Kim MH, Kim YH, Song ES, Seong SJ, et al. Prognostic Model for Survival and Recurrence in Patients with Early-Stage Cervical Cancer: A Korean Gynecologic Oncology Group Study (KGOG 1028). Cancer Res Treat. 2020;52:320–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang C, Yang C, Wang W, Xia B, Li K, Sun F, et al. A Prognostic Nomogram for Cervical Cancer after Surgery from SEER Database. Journal of Cancer. 2018;9:3923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bodurka-Bevers D, Morris M, Eifel PJ, Levenback C, Bevers MW, Lucas KR, et al. Posttherapy surveillance of women with cervical cancer: an outcomes analysis. Gynecol Oncol. 2000;78:187–93. [DOI] [PubMed] [Google Scholar]
- [32].Zola P, Fuso L, Mazzola S, Piovano E, Perotto S, Gadducci A, et al. Could follow-up different modalities play a role in asymptomatic cervical cancer relapses diagnosis? An Italian multicenter retrospective analysis. Gynecologic oncology. 2007;107:S150–4. [DOI] [PubMed] [Google Scholar]
- [33].Morice P, Deyrolle C, Rey A, Atallah D, Pautier P, Camatte S, et al. Value of routine follow-up procedures for patients with stage I/II cervical cancer treated with combined surgery-radiation therapy. Ann Oncol. 2004;15:218–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


