Abstract
Background
Trials evaluating efficacy of omega-3 highly unsaturated fatty acids (HUFAs) in major depressive disorder report discrepant findings.
Aims
To establish the reasons underlying inconsistent findings among randomised controlled trials (RCTs) of omega-3 HUFAs for depression and to assess implications for further trials.
Method
A systematic bibliographic search of double-blind RCTs was conducted between January 1980 and July 2014 and an exploratory hypothesis-testing meta-analysis performed in 35 RCTs including 6665 participants receiving omega-3 HUFAs and 4373 participants receiving placebo.
Results
Among participants with diagnosed depression, eicosapentaenoic acid (EPA)-predominant formulations (>50% EPA) demonstrated clinical benefits compared with placebo (Hedge’s G = 0.61, P<0.001) whereas docosahexaenoic acid (DHA)-predominant formulations (>50% DHA) did not. EPA failed to prevent depressive symptoms among populations not diagnosed for depression.
Conclusions
Further RCTs should be conducted on study populations with diagnosed or clinically significant depression of adequate duration using EPA-predominant omega-3 HUFA formulations.
Many randomised controlled trials (RCTs) have reported beneficial effects for omega-3 highly unsaturated fatty acids (HUFAs) in bipolar and major depressive disorder, but others have reported essentially no effect.1–35 Since Ross and colleagues, in 2007,36 initially explored the reasons for discrepant findings, subsequent meta-analyses have also identified these possible explanatory factors: (a) that only eicosapentaenoic acid (EPA)-predominant formulations of omega-3 HUFA have an antidepressant effect;37,38 and (b) that the putative antidepressant effects of omega-3 HUFAs only occur in episodes of diagnosed clinical depression.39,40 In contrast, the meta-analysis in 2012 by Bloch & Hannestad41 and Bloch42 attributed the evidence for benefit of omega-3 HUFAs in prior meta-analyses to publication and other biases, and concluded that the small to negligible effect on depressive symptoms did not justify further funding for large clinical trials as a result of the heterogeneity of results and publication bias. It is important to note that heterogeneity in meta-analyses can be mistakenly attributed to publication bias, including inappropriate study populations or inclusion of ineffective treatment formulations. Consequently, we examined whether omega-3 HUFAs have efficacy for the treatment of depression with specific attention to evaluating potential sources of heterogeneity, for example differing compositions of EPA in the intervention agents, that could account for the discrepancy in results in an attempt to determine whether there is sufficient evidence to justify further clinical trials, and if so, how such trials would be best conceptualised, designed and performed. There are substantial biological differences between docosahexaenoic acid (DHA) and EPA, EPA having a greater anti-inflammatory effect in the brain compared with DHA, which may contribute to its putative greater antidepressant effect.43 Furthermore, it has been postulated that unopposed EPA (the modest excess of EPA compared with DHA) is the mechanism for its putative antidepressant effect.38 We consider this in detail in the Discussion.
There are several reasons why studies should be differentiated by the severity of participant symptoms. Antidepressants for example have greater therapeutic efficacy for individuals with moderate to severe depression compared with mild depression.44 This is potentially because of the larger placebo response, ‘floor effects’, or regression to the mean, observed in studies of mild depression.44,45 Studies of primary or secondary prevention have additional methodological requirements, including adequate statistical power (see Discussion). Here we focused solely on studies designed to prevent or treat depressive symptoms in the context of affective disorders (major depressive disorder or bipolar disorder), as other meta-analyses have not demonstrated therapeutic efficacy for depressive symptoms in the context of other disorders such as schizophrenia, autism and attention-deficit hyperactivity disorder.36,40
Our principal hypothesis in this exploratory hypothesis-testing meta-analysis is that, compared with placebo, EPA-predominant formulations would demonstrate superior efficacy among participants with operationally diagnosed clinical acute depression (for example DSM-III-R/DSM-IV or ICD-10). In contrast, no efficacy would be demonstrated among participants with non-clinical depressive symptoms, regardless of the DHA or EPA formulation. This hypothesis-testing meta-analysis is a substantially different form of analysis from a traditional meta-analysis. The can be illustrated best as a tree diagram; the trunk includes all studies, testing one primary hypothesis for no efficacy v. efficacy with analysis then only continuing in the branches demonstrating efficacy (see Fig. 2 in the Results section). We first evaluated whether EPA-predominant formulations (EPA >50% of omega-3 HUFA formulation) would demonstrate greater efficacy for depressive symptoms compared with DHA-predominant formulations (hypothesis 1). The branch demonstrating efficacy was evaluated in hypothesis 2, which predicted that EPA formulations would be efficacious only among patients with a diagnosed clinical depression. The branch demonstrating efficacy was evaluated in hypothesis 3, which predicted that selectively enriched EPA (>80% EPA) (potentially resulting in ‘unopposed EPA’) would have a greater antidepressant effect compared with mixed EPA–DHA formulations (<80% EPA). Hypothesis 4 predicted that omega-3 HUFA supplementation given as an adjunct to antidepressants would display greater efficacy for patients with clinical depression than when given as monotherapy. Hypothesis 5 predicted that longer treatment trials of omega-3 HUFAs would produce a more significant antidepressant effect, and hypothesis 6 predicted that omega-3 HUFAs would have a more substantial antidepressant effect for participants with major depressive disorder compared with bipolar depression. We then examined whether publication bias would account for some of the reported antidepressant efficacy of omega-3 HUFAs.
Fig. 2. Tree diagram.
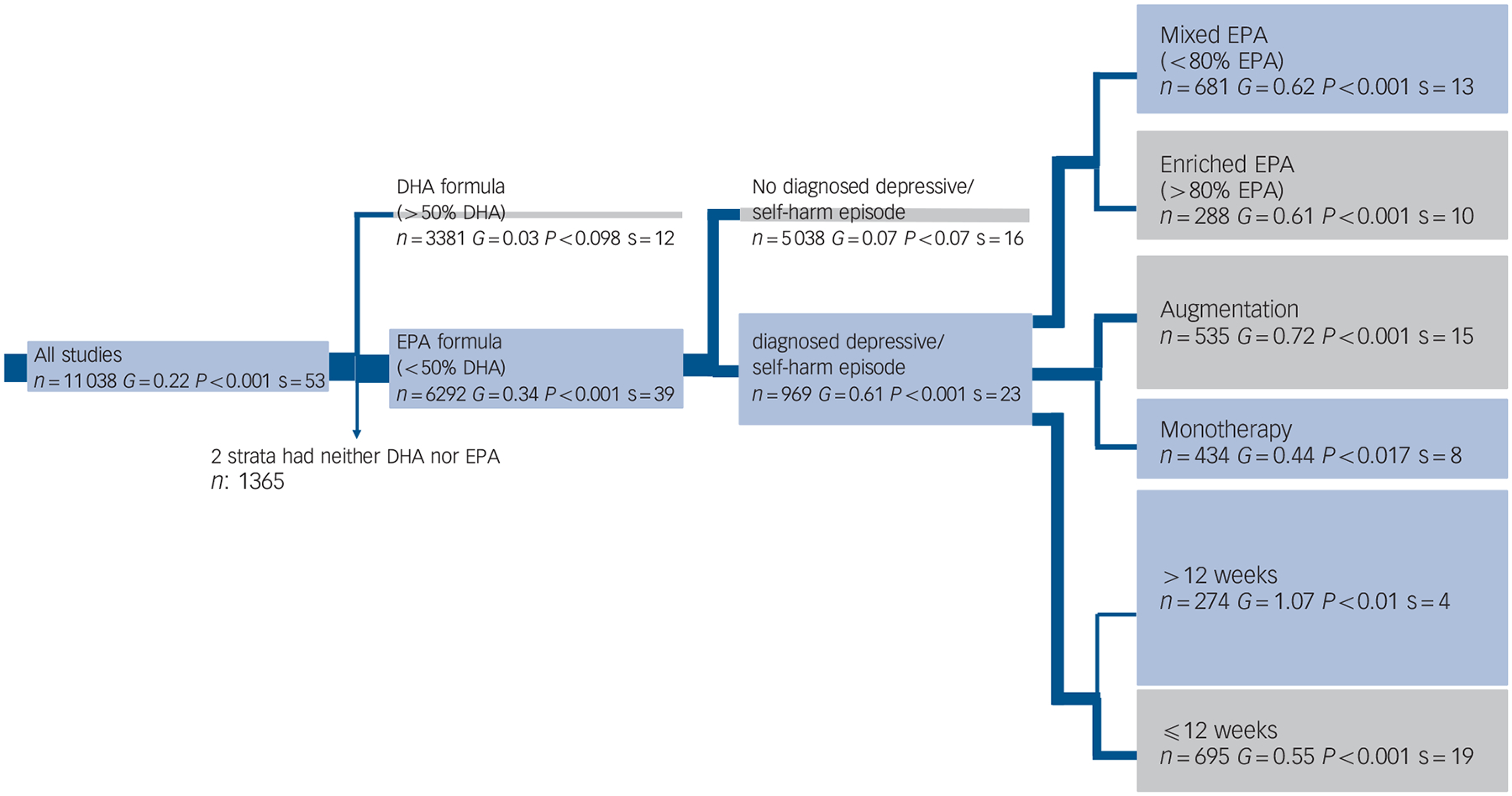
When a group’s effect size (G) or number of strata (s) approaches zero no further analysis occurs. The width of the blue line is proportional to the number of trials in the following group. The area of the box under a group is proportional to its effect size. The grey boxes contain groups hypothesised to decrease effect size and the blue boxes contain groups hypothesised to increase effect size. Two strata had neither docosahexaenoic acid (DHA) nor eicosapentaenoic acid (EPA, n = 1365).
The logic of this model is to analyse just on a branch that demonstrated efficacy. If both branches demonstrated efficacy, or if there were not enough studies in a branch to continue this model, a conventional meta-analysis was performed where the remaining hypotheses were tested separately. We also undertook extensive sensitivity analysis with alternate definitions of important indices or attributes to ascertain whether findings would be robust under different definitions or assumptions, including testing hypothesis 2 before hypothesis 1, and testing whether there was a dose–response for omega-3 HUFAs or EPA in hypothesis 1 and 3. This study including predefined hypotheses, and meta-analysis design were previously presented at the 49th American College of Neuropsychopharmacology (ACNP) 2010 meeting in Florida, USA.46
Method
Data sources
We conducted a systematic bibliographic search for studies utilising omega-3 HUFAs in mood disorders from the following databases: Cochrane Central Register of Controlled Trials (CENTRAL), Medline, PsycINFO and EMBASE. We searched for articles published between January 1980 and July 2014 without language restriction, using medical subject heading key words: depression OR depressive disorder OR bipolar affective disorder OR bipolar depression OR bipolar illness OR mood disorder OR affective disorder OR mania OR hypomania AND omega-3 fatty acids, OR omega-3 polyunsaturated fatty acids (PUFA) OR n-3 polyunsaturated fatty acids OR highly unsaturated fatty acid (HUFA) OR eicosapentaenoic acid (EPA) OR docosahexaenoic acid (DHA) OR fish oil OR nutritional supplement. We also searched by hand the above references from the papers identified, relevant reviews, Trials Central (http://www.trialscentral.org), Current Controlled Trials (http://controlled-trials.com), Clinical Trials (http://clinicaltrials.gov) and contacted several experts in the field to find any published or unpublished studies.
Study selection
We included double-blind, placebo-controlled studies of adults and children, that examined the antidepressant effect of omega-3 HUFAs either as a monotherapy or when augmented to psychotropic agents in: (a) participants with an operationally diagnosed depressive episode, or in the depressive pole of bipolar disorder or a depressive episode comorbid with an episode of self-harm; and in (b) non-clinical populations at risk for depression in which a subgroup had symptoms of depression.
Data extraction
Four reviewers (I.T.M., S.G., J.M.D. and B.H.) independently assessed and extracted relevant data including participants’ clinical characteristics, type and dose of compound administered, trial duration, mean psychometric scores and standard deviations or the risk differences, risk ratios or odd ratios of depressive symptoms. Our primary analysis selected the following hierarchy of psychometric instruments: (a) the Hamilton Rating Scale for Depression (HRSD) (n = 17); (b) the Montgomery–Åsberg Depression Rating Scale (MADRS) (n = 6); (c) the Beck Depression Inventory (BDI) (n = 2); (d) the Geriatric Depression Scale (GDS) (n = 5); or (e) other depression scales as appropriate (n = 7) (online Table DS1). Sensitivity analyses were based on the second highest psychometric instrument in the hierarchy.
DHA-predominant trials were defined as those providing higher quantities of DHA (>50%) compared with other omega-3 HUFAs. EPA-predominant trials were categorised as either ‘mixed EPA’ denoting EPA-predominant formulations containing at least 20% DHA; or ‘selectively enriched EPA’, denoting formulations containing at least 80% EPA and less than 20% DHA (online Fig. DS1).
Our primary dichotomous categorisation of severity was to identify studies enrolling participants with a diagnosed depressive/self-harm episode, defined as: (a) being treated for a depressive episode;7,9,11,13,15,16,19,21–25,27,29,32–34,47,48 (b) in the depressed pole of a bipolar disorder;2–4,6,31 or (c) with a depressive episode comorbid with self-harm (94% of individuals fulfilled criteria for major depressive disorder and all patients had a BDI >19).12 Participants attained a diagnosis of depressive episodes from international diagnostic criteria, such as DSM/ICD, or in one case2 from a well-validated diagnostic interview (Composite International Diagnostic Interview, CIDI). A second categorisation was used for sensitivity analysis. We also determined depression severity on a four-point scale with ratings undertaken masked by B.H. and J.M.D. with a rank correlation of 0.9 attained (see online Table DS1).
When a study did not separately present data regarding augmentation and monotherapy in individual strata, we classified it with the category used for the majority of the patients in the study. We stratified trial duration at ⩽12 weeks v. >12 weeks and the dose of EPA as ⩽0.8 g v. >0.8 g, and total dose of omega-3 HUFA as ⩽1.5 g v. >1.5 g.
Study quality was measured utilising two measures, the Cochrane risk of bias tool, as modified by Corbett & Woolacott,49 which examines eight potential sources of bias namely: (a) sequence generation, (b) allocation concealment, (c) masking of participants, (d) masking of personnel, (e) masking of outcome assessor, (f) incomplete outcome data, (g) selective outcome reporting and (h) other sources of bias (presented in the online supplement DS1), and the Jadad scale examining randomisation, double blinding and the description of study withdrawals (online Table DS1).
Statistical analysis
We calculated effect sizes for continuous data by attaining the mean (s.d.) and sample size (n) of the omega-3 HUFA and placebo groups. When standard deviations were not available, we estimated these based on the other statistical parameters reported in the study. When continuous data were not available, we evaluated the dichotomous data, calculated odds ratios, which were converted to the Hedge’s G effect-size statistic (G). For studies using multiple arms of the drug and one arm of the placebo (for example several omega-3 HUFA doses compared with placebo) the letter ‘n’ entered for each stratum of dose was reduced by dividing by the number of strata (for example if three dosages were utilised, a third of the total n of the placebo group was allocated to each stratum, rounding down when not a whole number).6,10,14,22,25,30,35,48,50 We used Duval & Tweedie’s trim and fill test, using a random-effects model on ‘clinical’ studies utilising EPA-predominant formulations to assess for publication bias.
‘Comprehensive Meta-Analysis’, Version 2, was used to evaluate any treatment effect between the treatment groups to ascertain the random-model treatment effect size (G), 95% confidence intervals and standard errors (s.e.) for each study and were carried out a number of sensitivity analyses to ratify our results. In the most important sensitivity analysis, we calculated the effect size using the next-highest psychometric instrument from the predefined hierarchy described above. We assessed heterogeneity of intervention using the Cochrane Q, I2 and tau-square statistics.
Results
Literature search
A copy of the PRISMA diagram, outlining the search strategy of the literature is presented in Fig. 1. This literature search yielded a total of 1255 potentially relevant articles. The titles and abstracts were reviewed and clearly irrelevant articles were discarded. Consequently, 107 articles were examined in depth, with 43 RCTs selected as they satisfied inclusion criteria. Eight studies could not be included because of insufficient meaningful rating data.20,50–54 Two strata of a study10 utilised α-linoleic acid (α-LNA) as the predominant omega-3 HUFA administered, and these were only included in the omega-3 HUFA compared with placebo analysis. Consequently, there were 35 studies divided into 53 strata investigating the therapeutic effect of omega-3 HUFAs in 6665 participants compared with placebo in 4373 participants. These studies are detailed in Table DS1, with design issues and any potential threats to validity described. Thirteen strata compared DHA-predominant formulations with placebo,5,8,11,16,18,19,22,26,27,29,30,47 17 strata compared selectively enriched EPA formulations with placebo,2,6,9,13–15,21–23,25,48 and 22 strata compared mixed-EPA formulations to placebo.4,12,24,28,32 Eighteen strata evaluated studies for stand-alone major depressive disorder,9,11,13,15,19,21–25,29,32,47,48 3 strata evaluated bipolar disorder,6,31,55 6 strata examined depressive symptoms in the perinatal/postnatal period,5,7,16,18,27,33 12 strata examined depressive symptoms in elderly people8,14,28,30,34,35 9 strata examined participants with depression and comorbid cerebrovascular or coronary heart disease,1,3,10,26 2 strata evaluated depression in Parkinson’s Disease,4 1 stratum evaluated depression in participants with self-harm (suicide attempts)12 and 1 stratum evaluated depression in diabetes mellitus.2
Fig. 1. Flow chart of literature search and study selection.
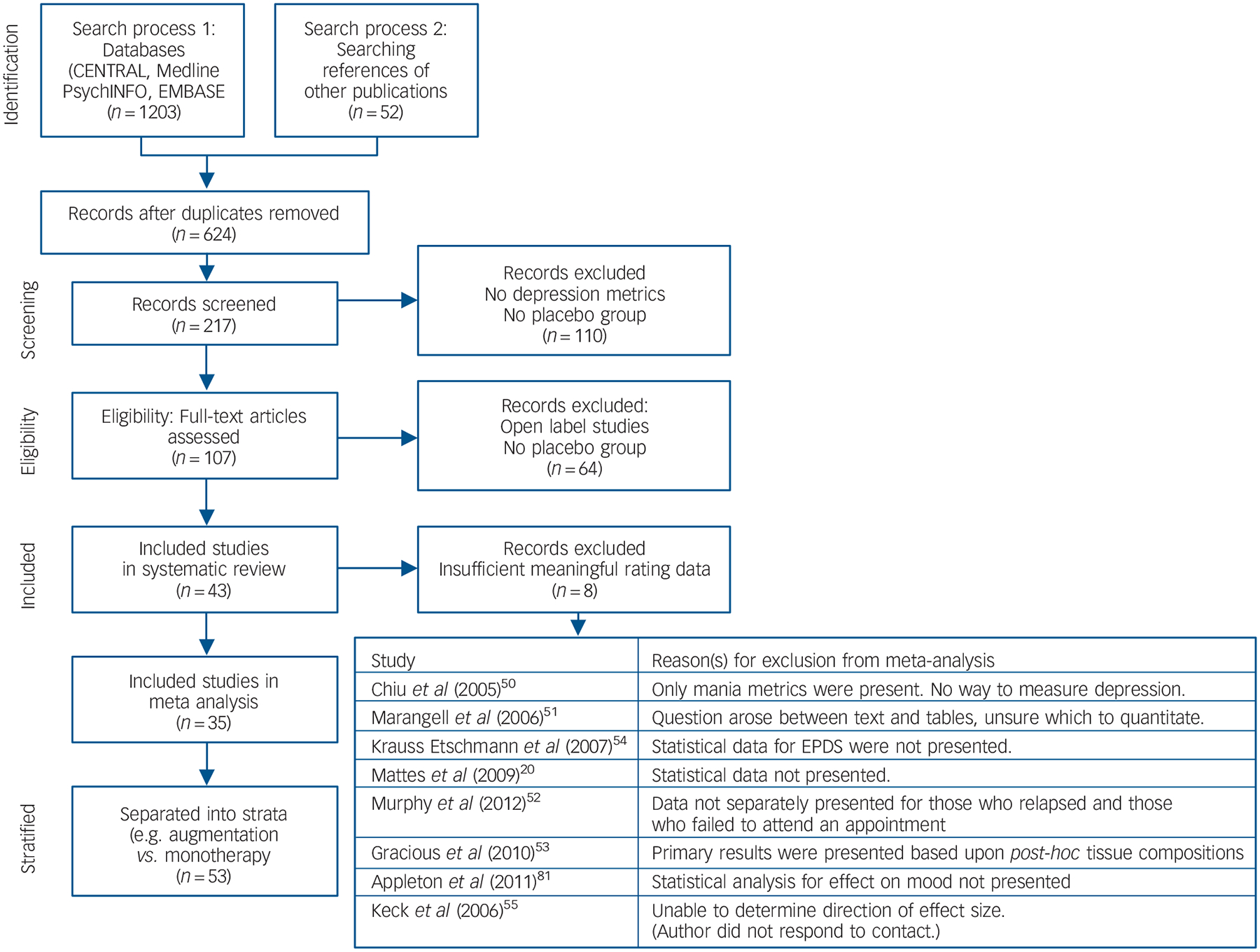
EPDS, Edinburgh Post-Natal Depression Scale.
Figure 2 emphasises the most important statistical parameter: the change in effect size in the tree branches. Our focus was on whether the hypothesis is confirmed by a clinically meaningful effect size (G) v. an essentially zero effect size. The shaded areas indicate visually the effect size, which is stated within the box. As a result of the ordered nature of the hypotheses, there are fewer studies in every subsequent branch, and effect sizes may increase or decrease. Past the third branching, there were too few studies to justify further branching. Therefore, we conducted conventional meta-analyses for the remaining hypotheses. The distribution of omega-3 HUFA used in these studies was trimodal, (online Fig. DS1). All but two studies9,47 fell within the following 3 groups; 0–30% EPA (mean 15%), 55–75% EPA (mean 61%) and 85–100% EPA (mean 96%).
Hypothesis 1: EPA- v. DHA-predominant samples
EPA-predominant formulations demonstrated a superior antidepressant efficacy compared with placebo (G = 0.34, 95% CI 0.21–0.47, P<0.001, I2 = 61%). In contrast, DHA-predominant preparations consistently demonstrated no benefit over placebo (G = 0.03, 95% CI −0.12 to 0.19, P<0.66, I2 = 35%). All the fixed and mixed output of both the primary analyses are presented in the online supplement.
Hypothesis 2: diagnosed depression v. undiagnosed samples
Among populations with a diagnosed depressive episode, EPA-predominant formulations demonstrated a significant benefit compared with placebo (G = 0.61, 95% CI 0.38–0.85, P<0.001, I2 = 61%), with no benefit consistently demonstrated for the populations without a formal diagnosis of depression (G = 0.08, 95% CI −0.01 to 0.17, P<0.07, I2 = 5%). The diagnosed depression group was more heterogeneous than the non-diagnosed group. These findings were corroborated by both sensitivity analyses. Our meta-regression found that higher rating of severity was associated with high efficacy compared with placebo (z = 4.7, P<0.001). The forest diagram (Fig. 3) shows virtually the same results. Effect sizes for studies utilising DHA-predominant formulations in both diagnosed depression and undiagnosed depression are also presented in Fig. 3, with no evidence for benefit over placebo demonstrated for either analysis.
Fig. 3. Forest plot.
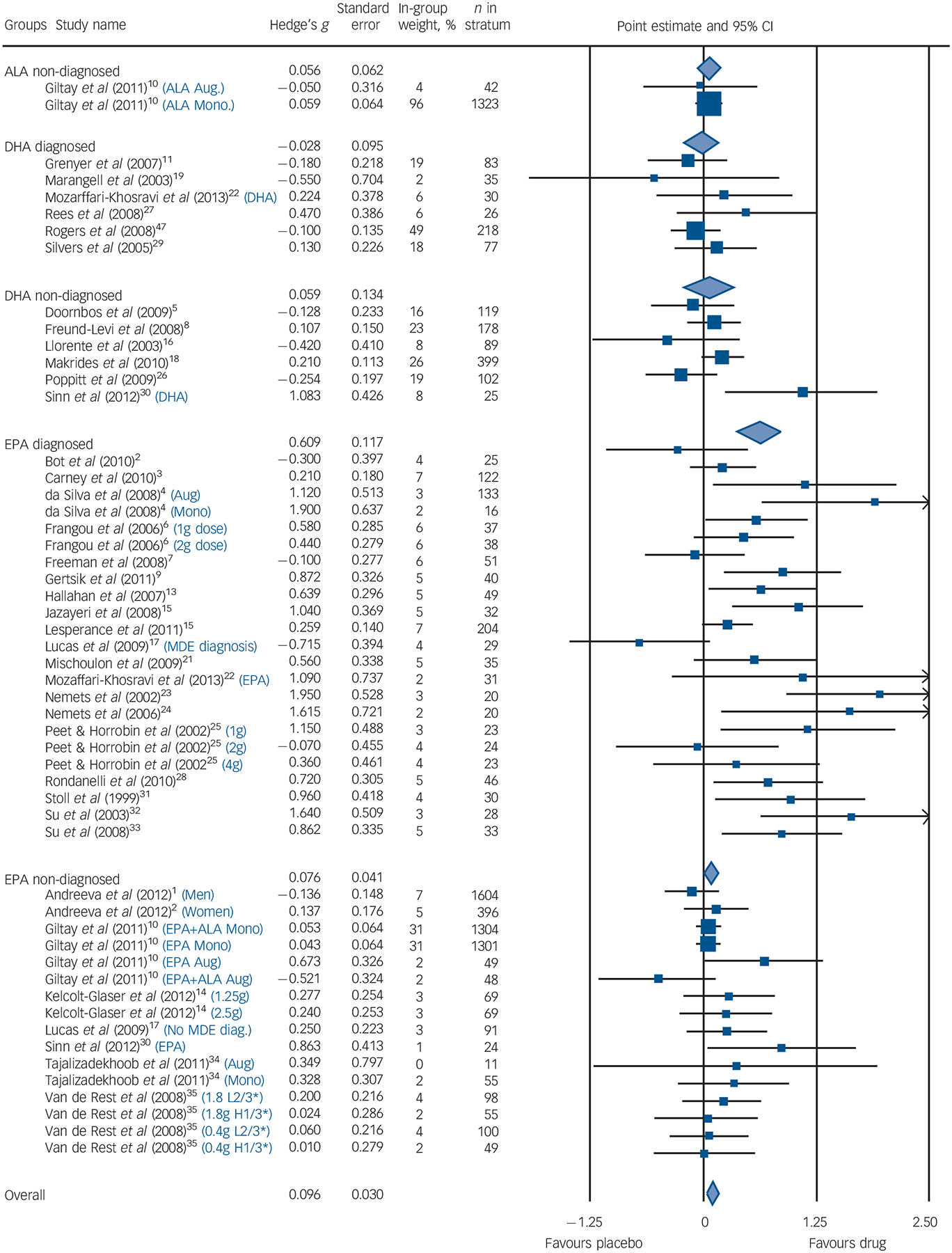
Size of blue squares proportional to weight in meta-analysis. Size of diamonds proportional to standard error of group. Black lines, show confidence intervals. The blue text indicates that a study was split into multiple strata and the criteria used to do so. H1/3* is the strata with the highest tertile of baseline depression scores; L2/3* is the strata with the lowest two teriles of baseline depression scores. ALA, α-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; MDE, major depressive episode; Aug, augmentation; Mono, monotherapy.
Hypothesis 3: composition of EPA, selectively enriched EPA v. mixed EPA
Both mixed EPA and selectively enriched (>80%) EPA formulations demonstrated an antidepressant effect. Mixed EPA formulations demonstrated an effect size of 0.62 (95% CI 0.31–0.94, P<0.001, I2 = 68%) and selectively enriched EPA formulations demonstrated an effect size of 0.61 (95% CI 0.26–0.96, P = 0.001, I2 = 49%), with the heterogeneity between groups not significant. Sensitivity analyses corroborated these findings.
Hypothesis 4: augmentation and monotherapy
Compared with placebo, EPA was effective in both augmentation (G = 0.59, 95% CI 0.42–0.77, P= 0.004, I2 = 57%) and monotherapy (G = 0.33, 95% CI 0.13–0.52, P<0.003, I2 = 68%). This again was corroborated by sensitivity analysis. Augmentation studies demonstrated a trend towards greater antidepressant efficacy compared with monotherapy studies (P = 0.07) (which is consistent with the one direct study demonstrating greater efficacy in augmentation studies13).
Hypothesis 5: duration
The median duration for all trials was 12 weeks, which is also concordant with animal studies for brain omega-3 HUFA restoration. We failed to find any evidence of greater clinical efficacy among longer trial durations (i.e. >12 weeks). Trials of both <6 weeks (G = 0.55, 95% CI 0.30–0.81, P<0.001, I2 = 57%) and >6 weeks (G = 1.07, 95% CI 0.21–1.93, P = 0.02, I2 = 79%) demonstrated clinical efficacy. However, all trials assessing clinical depression with omega-3 HUFAs were relatively short in duration (⩽26 weeks, median 11 weeks). There was no evidence of a linear relationship when individual trial length was plotted against effect size (see online supplement DS1).
Hypothesis 6: EPA in bipolar depression
The effect size for bipolar depression was 0.59 (95% CI 0.24–0.94, P<0.001, I2 = 0%), however there were too few strata (n = 3) to make any definitive conclusions.
Publication bias
The Duval and Tweedie’s trim and fill test, using a random-effects model on diagnosed depression studies where EPA was the predominant formulation, estimated that several studies with negative effect sizes might never have been published (Fig. 4). The adjusted values with the imputed studies reduced the effect size from G = 0.61 to G = 0.42 (95% CI 0.18–0.65, P<0.001).
Fig. 4. (a) Effect size in diagnosed and non-diagnosed studies; (b) funnel plot of standard error by effect size in eicosapentaenoic acid (EPA), diagnosed group.
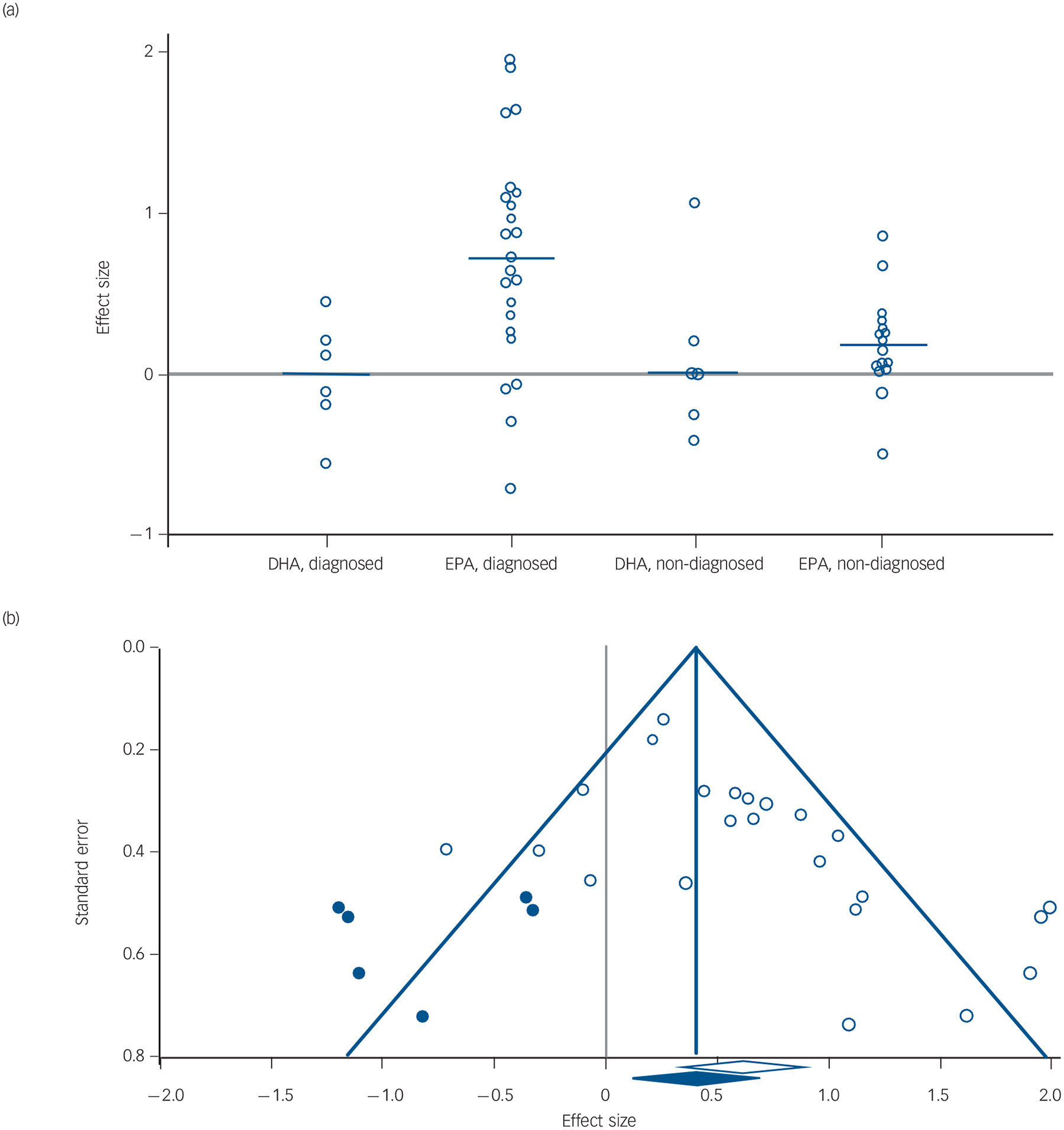
In (a) each white dot represents one study and its effect size; the blue line represents the median effect size for the group and the grey line goes across the zero point (no difference between drug and placebo). In (b) each white dot represents a real study; each blue dot represents an imputed study; the white diamond indicates the calculated effect size, extending left and right to show 95% confidence intervals; the blue diamond indicates the imputed effect size, extending left and right to show 95% confidence intervals; the blue triangle outlines the ‘funnel’, centred at the imputed effect size and the blue vertical line extends upward, centred at the imputed effect size.
Discussion
Main findings
Our purpose was to understand why some controlled studies found omega-3 HUFAs efficacious and other did not. Bloch42 and Bloch & Hannestad41 attributed heterogeneous results to publication bias, concluding that no further clinical trials should be undertaken and that state agencies should spend their research dollars elsewhere. Since evaluating the correct formulation in an inappropriate population or evaluating an inactive preparation in a sensitive population can confound results, we explored omega-3 HUFA formulations and grades of depression simultaneously, to explain the reasons for these discrepant results. This systematic review and exploratory hypothesis-testing meta-analysis, testing multiple, predefined ordered hypotheses demonstrated that EPA-predominant formulations are more efficacious than placebo for treatment of clinical depression. DHA-predominant formulations are consistently and homogenously ineffective. All formulae are also consistently and homogenously ineffective in non-depressed populations. We can confirm the findings of Bloch & Hannestad that publication bias exists, but disagree with their conclusion that a definitive confirmation study should not be undertaken; their verdict of ‘not yet proven’ is not definitive proof of absence of efficacy. Indeed, we insist that a positive finding with publication bias specifically suggests that multicentre RCTs of EPA-predominant formulations, with attention to the diagnostic confirmation and severity of depression, can now be appropriately powered and conducted.
Publication and other biases
The effect size for EPA-predominant formulations in clinical depression (G = 0.61) is comparable with that of conventional antidepressants but high or statistically significant effect sizes do not in themselves demonstrate a genuine effect. There is a widespread phenomenon for earlier small studies to have larger effect sizes than later larger studies. We can confirm Bloch & Hannestad’s41 finding of publication bias as we found, using Duval and Tweedie’s trim and fill test, using both random-(G = 0.42) and fixed-effect models (G = 0.47), a reduction in the effect size in relation to the antidepressant efficacy of EPA. We found evidence of potential publication bias with the magnitude of effect increased in strata of under 40 participants (see online supplement DS1). Whereas the overall mean stratum size was 207 participants, the mean stratum size for studies in diagnosed depression using EPA formulae was 42 with 74% of strata having less than 42 participants. We largely replicated, except in one instance, the Jadad ratings of study quality of Bloch & Hannestad41 (see online supplement DS1 for this and the Cochrane risk of bias tool).
Biological plausibility
The putative role of omega-3 HUFAs in the treatment of depression is biologically plausible, a hypothesis supported by epidemiological observations, tissue compositional comparisons, clinical and treatment studies in a large, complex and not entirely consistent literature.40 Humans are unable to synthesise EPA or DHA de novo and make limited amounts of DHA and EPA from the dietary precursor alpha-linolenic acid (ALA). Fish and shellfish are the primary dietary sources of pre-formed EPA and DHA, and epidemiological or tissue studies cannot disentangle the respective role of EPA from DHA.
Our analysis of differences comparing EPA or DHA v. placebo is indirect. There is only one study with direct comparison between EPA and DHA in patients with depression, demonstrating clear superiority for the EPA-predominant formulation over DHA or placebo.22 Mixed-EPA formulations where DHA was present in significant quantities proved to be clinically effective to at least the same degree as ‘selectively enriched’ EPA formulations. The RCT studies that have been published since Sublette et al’s38 theory of antidepressant efficacy relating to EPA being unopposed by DHA, support such a hypothesis, although there have been no recent studies assessing the antidepressant effects of ‘super-high’ doses of EPA.
Several different mechanisms of antidepressant effects for both EPA and DHA can be postulated. DHA is highly enriched in brain synaptosomal membrane phospholipids, where it alters the biophysical properties of membranes, enhances neurotransmission,23 increases inflammation resolution56 and neurogenesis,57 all of which have plausible beneficial effects for mood disorders. These central actions are, however, dependent on the enrichment of DHA in brain tissue from circulating unesterified DHA. Low levels of DHA have been reported in post-mortem orbitofrontal cortex of patients with major depression.58 The complete restoration of DHA into the brain is about 12 weeks from deficiency states in animals.59 However, in the replete steady state, DHA turnover in human brain is slow, with a half-life of approximately 2.5 years.60 Omega-3 HUFAs to date have not been evaluated in participants with significant clinical depression beyond 16 weeks; our findings suggest that an emerging antidepressant effect may be evident at 4 weeks post-treatment.
A putative novel mechanism differentiating EPA from DHA
The late David Horrobin initially hypothesised that EPA had a superior beneficial antidepressant effect to DHA, suggesting that EPA probably works by modulating post-receptor signal transduction processes.61 Here we posit a novel mechanism for EPA potentially underlying the selective reduction of significant depressive symptoms as compared with DHA. Although EPA is not abundant in the brain at steady state, EPA rapidly enters the brain as a free fatty acid and is not reacylated into phospholipid membrane stores, being rapidly metabolised and beta oxidised.62 EPA is the natural ligand for the peroxisome proliferator-activated receptor gamma (PPARγ) nuclear transcription receptor that downregulates expression of nuclear factor-kappa B (Nf-κB) and inhibits neuronal parainflammatory cascades implicated in the pathophysiology of dysregulated stress responses and depression.63 Free fatty acids activate PPARs by stabilising the activation function 2 (AF-2) helix; carbon atom (C) lengths shorter than C16 fail to stabilise and greater than C20 (such as DHA 22:6n-3) fail to fit in the ligand binding pocket, with PPARγ having the greatest fatty acid specificity.64 Low concentrations EPA (20:5n-3) bind with very high affinity to all PPARs65 and 20:5n-3 co-crystals have been characterised,64 but in contrast, the binding affinity of DHA to PPARγ is so low it cannot be measured.65Although several studies have reported that adding DHA to diets or model systems activates PPARγ, this may be attributable to retro conversion of DHA to EPA.66 In addition the DNA binding-independent transactivity, that indicates the formation of the PPARα-associated coactivator-transcriptional complex PPARα–retinoid X receptor alpha (RXRα), is promoted by polyunsaturated fatty acids of 18 to 20 carbon groups with 3–5 double bonds, but not by DPA n-3 or DHA.67 EPA increases transactivation of PPARα–RXRα at low concentrations (1–10 μM) whereas in contrast DHA inhibited transactivation of PPARα–RXRα at concentrations higher than 50 μM.68 Since DHA is at high concentrations in the brain at steady state it may provide tonic inhibition of PPARs, whereas transient low concentrations of EPA may activate PPARγ. The selective discrimination of EPA from DHA in activating PPARγ–RXR nuclear transcription regulating the Nf-κB parainflammatory pathway is consistent with a proof-of-concept trial of the PPARγ agonist pioglitazone for depressive symptoms69 and may extend to other psychiatric illnesses including preventing alcohol relapse.70
In contrast to DHA, supplemental EPA is rapidly incorporated into membrane phospholipids of circulating mononuclear cells, resulting in attenuated production of proinflammatory cytokines interleukin-1 (IL-1) and tumour necrosis factor alpha (TNFα).43 Increased circulating IL-1 and TNFα occur in people with major depressive disorder; perhaps the therapeutic antidepressant effects of EPA may be related in some part to its anti-inflammatory effect. Indeed, EPA downregulates the release of inflammatory cytokines that can produce clinical symptoms of depression, especially IL-1β, TNFα and IL-6.71 Inflammatory biomarkers are associated with an antidepressant response to EPA monotherapy whereas DHA is not.72 Individuals with elevated interferon (IFN)-α as a result of chronic hepatitis C frequently experience fatigue, arthralgia and myalgia and many fulfil operational diagnostic criteria for depression. These patients respond to antidepressants.73 Similarly, in a recent, RCT, EPA but not DHA was also noted to ameliorate IFN-α-induced depression.74
In the rodent olfactory bulbectomy depression model, EPA treatment normalised depressive behaviours by attenuating prostaglandin E2-mediated activation of IL-6, decreasing mRNA expression for corticotrophin-releasing hormone (CRH) and inhibiting hyperactivation of the hypothalamic–pituitary–adrenal (HPA) axis.75 EPA may also exert a greater neurotrophic effect compared with DHA, with EPA supplementation shown to increase brain derived neurotrophic factor (BDNF) levels after traumatic brain injury.76,77 BDNF has been linked with the ‘neurotrophic hypothesis’ such as: (a) impairment of hippocampal BDNF signalling produces certain depression-related behaviours and impairs the actions of antidepressants;78 (b) experimental increases in hippocampal BDNF levels produce antidepressant-like effects;79 and (c) EPA’s antidepressant effect may be in some part related to its ability to enhance dopaminergic and serotonergic neurotransmission.57
Clinically diagnosed depression v. symptoms in non-clinical populations
Our primary distinction to separate episodes of diagnosed depressive illness from milder depressive symptoms among non-clinical populations at risk for depression was a dichotomous classification. It was supported by both a sensitivity analysis using alternative criteria and a meta-regression analysis based on the grade of depression present, from diagnosed clinical depression in individuals seeking treatment to mild symptoms of depression in a non-clinical population. Of note, one recent meta-analysis80 also demonstrated a benefit for depressive symptoms in non-clinical depression, however their inclusion criteria for this group were significantly different to ours, potentially explaining this difference in results. In augmentation studies, patients must be sufficiently depressed to require antidepressants, and may have treatment-resistant depression. In contrast, monotherapy studies sometimes explicitly exclude patients with moderate to severe depression and in any case such patients may not be referred if antidepressants were clearly needed. Other methodological problems include large placebo effects, regression to the mean and frequency of prior episodes in studies of prophylactic antidepressants to prevent relapse. Changes in mood in non-clinical populations may not be the same thing as in clinical depression. This dimension is important in clinical trial design and we discuss it in more detail in the online supplement. In contrast to a recent meta-analysis,80 EPA-predominant formulations demonstrated antidepressant efficacy both as augmentation agents and in monotherapy. Meta-analyses81 that do not appropriately evaluate the dimensions of both clinical severity and use of EPA-predominant formulations may underestimate clinical effect sizes and prematurely conclude that n-3 HUFA interventions have only limited therapeutic utility.
Other limitations
General limitations of meta-analyses include issues such as limitations of available studies of varying quality, non-uniformity of formulations, duration of trials and that this meta-analysis itself is not a randomised selection of trials, but rather an observational analysis of existing trials. Many of the studies did not have the assessor guess the participants’ consumption (active omega-3 HUFA formulation or placebo). Treatment adherence and/or the alteration in lipid compositions secondary to omega-3 HUFA intake were similarly not measured in most studies. We did not explore issues relating to tolerability of omega-3 HUFAs in this study.
Implications
Omega-3 HUFAs with EPA-predominant formulations demonstrated evidence for an antidepressant effect both when used alone and as augmentation agents for operationally diagnosed episodes of depression in contrast to DHA-predominant compounds where no antidepressant efficacy was demonstrated. This suggests that larger multisite, RCTs testing the clinical antidepressant efficacy and safety of EPA-predominant formulations both in monotherapy and as an augmentation agent in populations with clinical depression are warranted. We recommend that such studies should be accompanied by monitoring of treatment adherence including monitoring of biochemical levels of omega-3 HUFAs. Furthermore, studies should aim to determine optimal dose, enrol patients with major depression who have moderate to severe symptoms, with the methodological protocols used in clinical trials and ascertain how their putative therapeutic actions add to, and interact with, conventional antidepressant agents.
Supplementary Material
Acknowledgements
We acknowledge J.P.S. as originating the idea that selective binding at PPARγ may explain the differential efficacy of EPA, and Rachel Gow for her work in proofreading this article.
Funding
The intramural programme of the National Institute of Alcohol Abuse and Alcoholism provided support for this work.
Footnotes
Declaration of interest
None.
This study, including predefined hypotheses and meta-analysis design, were previously presented at the 49th American College of Neuropsychopharmacology 2010 meeting in Florida, USA.
References
- 1.Andreeva VA, Galan P, Torres M, Julia C, Hercberg S, Kesse-Guyot E. Supplementation with B vitamins or n-3 fatty acids and depressive symptoms in cardiovascular disease survivors: ancillary findings from the SUpplementation with FOLate, vitamins B-6 and B-12 and/or OMega-3 fatty acids (SU.FOL.OM3) randomised trial. Am J Clin Nutr 2012; 96: 208–14. [DOI] [PubMed] [Google Scholar]
- 2.Bot M, Pouwer F, Assies J, Jansen EH, Diamant M, Snoek FJ, et al. Eicosapentaenoic acid as an add-on to antidepressant medication for comorbid major depression in patients with diabetes mellitus: a randomized, double-blind placebo-controlled study. J Affect Disord 2010; 126: 282–6. [DOI] [PubMed] [Google Scholar]
- 3.Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, Harris WS. Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: a randomized controlled trial. JAMA 2009; 302: 1651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.da Silva TM, Munhoz RP, Alvarez C, Naliwaiko K, Kiss A, Andreatini R, et al. Depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord 2008; 111: 351–9. [DOI] [PubMed] [Google Scholar]
- 5.Doornbos B, van Goor SA, Dijck-Brouwer DA, Schaafsma A, Korf J, Muskiet FA. Supplementation of a low dose of DHA or DHA+AA does not prevent peripartum depressive symptoms in a small population based sample. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33: 49–52. [DOI] [PubMed] [Google Scholar]
- 6.Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry 2006; 188: 46–50. [DOI] [PubMed] [Google Scholar]
- 7.Freeman MP, Davis M, Sinha P, Wisner KL, Hibbeln JR, Gelenberg AJ. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. J Affect Disord 2008; 110: 142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freund-Levi Y, Basun H, Cederholm T, Faxen-Irving G, Garlind A, Grut M, et al. Omega-3 supplementation in mild to moderate Alzheimer’s disease: effects on neuropsychiatric symptoms. Int J Geriatr Psychiatry 2008; 23: 161–9. [DOI] [PubMed] [Google Scholar]
- 9.Gertsik L, Poland RE, Bresee C, Rapaport MH. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J Clin Psychopharmacol 2012; 32: 61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giltay EJ, Geleijnse JM, Kromhout D. Effects of n-3 fatty acids on depressive symptoms and dispositional optimism after myocardial infarction. Am J Clin Nutr 2011; 94: 1442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grenyer BF, Crowe T, Meyer B, Owen AJ, Grigonis-Deane EM, Caputi P, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31: 1393–6. [DOI] [PubMed] [Google Scholar]
- 12.Hallahan B, Hibbeln JR, Davis JM, Garland MR. Omega-3 fatty acid supplementation in patients with recurrent self-harm. Single-centre double-blind randomised controlled trial. Br J Psychiatry 2007; 190: 118–22. [DOI] [PubMed] [Google Scholar]
- 13.Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust NZ J Psychiatry 2008; 42: 192–8. [DOI] [PubMed] [Google Scholar]
- 14.Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Hwang BS, Glaser R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav Immun 2012; 26: 988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lesperance F, Frasure-Smith N, St-Andre E, Turecki G, Lesperance P, Wisniewski SR. The efficacy of omega-3 supplementation for major depression: a randomized controlled trial. J Clin Psychiatry 2011; 72: 1054–62. [DOI] [PubMed] [Google Scholar]
- 16.Llorente AM, Jensen CL, Voigt RG, Fraley JK, Berretta MC, Heird WC. Effect of maternal docosahexaenoic acid supplementation on postpartum depression and information processing. Am J Obstet Gynecol 2003; 188: 1348–53. [DOI] [PubMed] [Google Scholar]
- 17.Lucas M, Asselin G, Merette C, Poulin MJ, Dodin S. Ethyl-eicosapentaenoic acid for the treatment of psychological distress and depressive symptoms in middle-aged women: a double-blind, placebo-controlled, randomized clinical trial. Am J Clin Nutr 2009; 89: 641–51. [DOI] [PubMed] [Google Scholar]
- 18.Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P, et al. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA 2010; 304: 1675–83. [DOI] [PubMed] [Google Scholar]
- 19.Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry 2003; 160: 996–8. [DOI] [PubMed] [Google Scholar]
- 20.Mattes E, McCarthy S, Gong G, van Eekelen JA, Dunstan J, Foster J, et al. Maternal mood scores in mid-pregnancy are related to aspects of neonatal immune function. Brain Behav Immun 2009; 23: 380–8. [DOI] [PubMed] [Google Scholar]
- 21.Mischoulon D, Papakostas GI, Dording CM, Farabaugh AH, Sonawalla SB, Agoston AM, et al. A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder. J Clin Psychiatry 2009; 70: 1636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mozaffari-Khosravi H, Yassini-Ardakani M, Karamati M, Shariati-Bafghi SE. Eicosapentaenoic acid versus docosahexaenoic acid in mild-to-moderate depression: a randomized, double-blind, placebo-controlled trial. Eur Neuropsychopharmacol 2013; 23: 636–44. [DOI] [PubMed] [Google Scholar]
- 23.Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002; 159: 477–9. [DOI] [PubMed] [Google Scholar]
- 24.Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry 2006; 163: 1098–100. [DOI] [PubMed] [Google Scholar]
- 25.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry 2002; 59: 913–9. [DOI] [PubMed] [Google Scholar]
- 26.Poppitt SD, Howe CA, Lithander FE, Silvers KM, Lin RB, Croft J, et al. Effects of moderate-dose omega-3 fish oil on cardiovascular risk factors and mood after ischemic stroke: a randomized, controlled trial. Stroke 2009; 40: 3485–92. [DOI] [PubMed] [Google Scholar]
- 27.Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust NZ J Psychiatry 2008; 42: 199–205. [DOI] [PubMed] [Google Scholar]
- 28.Rondanelli M, Giacosa A, Opizzi A, Pelucchi C, La Vecchia C, Montorfano G, et al. Effect of omega-3 fatty acids supplementation on depressive symptoms and on health-related quality of life in the treatment of elderly women with depression: a double-blind, placebo-controlled, randomized clinical trial. J Am Coll Nutr 2010; 29: 55–64. [DOI] [PubMed] [Google Scholar]
- 29.Silvers KM, Woolley CC, Hamilton FC, Watts PM, Watson RA. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids 2005; 72: 211–8. [DOI] [PubMed] [Google Scholar]
- 30.Sinn N, Milte CM, Street SJ, Buckley JD, Coates AM, Petkov J, et al. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. Br J Nutr 2012; 107: 1682–93. [DOI] [PubMed] [Google Scholar]
- 31.Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999; 56: 407–12. [DOI] [PubMed] [Google Scholar]
- 32.Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol 2003; 13: 267–71. [DOI] [PubMed] [Google Scholar]
- 33.Su KP, Huang SY, Chiu TH, Huang KC, Huang CL, Chang HC, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. Clin Psychiatry 2008; 69: 644–51. [DOI] [PubMed] [Google Scholar]
- 34.Tajalizadekhoob Y, Sharifi F, Fakhrzadeh H, Mirarefin M, Ghaderpanahi M, Badamchizade Z, et al. The effect of low-dose omega 3 fatty acids on the treatment of mild to moderate depression in the elderly: a double-blind, randomized, placebo-controlled study. Eur Arch Psychiatry Clin Neurosci 2011; 261: 539–49. [DOI] [PubMed] [Google Scholar]
- 35.van de Rest O, Geleijnse JM, Kok FJ, van Staveren WA, Hoefnagels WH, Beekman AT, et al. Effect of fish-oil supplementation on mental well-being in older subjects: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2008; 88: 706–13. [DOI] [PubMed] [Google Scholar]
- 36.Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis 2007; 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry 2012; 17: 1144–9; discussion 63-7. [DOI] [PubMed] [Google Scholar]
- 38.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry 2011; 72: 1577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Appleton KM, Hayward RC, Gunnell D, Peters TJ, Rogers PJ, Kessler D, et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials. Am J Clin Nutr 2006; 84: 1308–16. [DOI] [PubMed] [Google Scholar]
- 40.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr 2010; 91: 757–70. [DOI] [PubMed] [Google Scholar]
- 41.Bloch MH, Hannestad J. Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol Psychiatry 2012; 17: 1272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bloch MHH J Response to critiques on ‘Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis’. Mol Psychiatry 2012; 17: 1163–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr 1996; 63: 116–22. [DOI] [PubMed] [Google Scholar]
- 44.Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med 2008; 5: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 2010; 303: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American College of Neuropsychopharmacology. ACNP 49th Annual Meeting Final Program (available at www.acnp.org/annualmeeting/programbooks.aspx). ACNP, 2010. [Google Scholar]
- 47.Rogers PJ, Appleton KM, Kessler D, Peters TJ, Gunnell D, Hayward RC, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr 2008; 99: 421–31. [DOI] [PubMed] [Google Scholar]
- 48.Lucas M, Asselin G, Merette C, Poulin MJ, Dodin S. Effects of ethyl-eicosapentaenoic acid omega-3 fatty acid supplementation on hot flashes and quality of life among middle-aged women: a double-blind, placebo-controlled, randomized clinical trial. Menopause 2009; 16: 357–66. [DOI] [PubMed] [Google Scholar]
- 49.Corbett MS H J, Woolacott NF. Assessing baseline imbalance in randomised controlled trials: implications for the Cochrane risk of bias tool. Res Syn Meth 2014; 5: 79–85. [DOI] [PubMed] [Google Scholar]
- 50.Chiu CC, Huang SY, Chen CC, Su KP. Omega-3 fatty acids are more beneficial in the depressive phase than in the manic phase in patients with bipolar I disorder. J Clin Psychiatry 2005; 66: 1613–4. [DOI] [PubMed] [Google Scholar]
- 51.Marangell LB, Suppes T, Ketter TA, Dennehy EB, Zboyan H, Kertz B, et al. Omega-3 fatty acids in bipolar disorder: clinical and research considerations. Prostaglandins Leukot Essent Fatty Acids 2006; 75: 315–21. [DOI] [PubMed] [Google Scholar]
- 52.Murphy BL, Stoll AL, Harris PQ, Ravichandran C, Babb SM, Carlezon WA Jr, et al. Omega-3 fatty acid treatment, with or without cytidine, fails to show therapeutic properties in bipolar disorder: a double-blind, randomized add-on clinical trial. J Clin Psychopharmacol 2012; 32: 699–703. [DOI] [PubMed] [Google Scholar]
- 53.Gracious BL, Chirieac MC, Costescu S, Finucane TL, Youngstrom EA, Hibbeln JR. Randomized, placebo-controlled trial of flax oil in pediatric bipolar disorder. Bipolar Disord 2010; 12: 142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krauss-Etschmann S, Shadid R, Campoy C, Hoster E, Demmelmair H, Jimenez M, et al. Effects of fish-oil and folate supplementation of pregnant women on maternal and fetal plasma concentrations of docosahexaenoic acid and eicosapentaenoic acid: a European randomized multicenter trial. Am J Clin Nutr 2007; 85: 1392–400. [DOI] [PubMed] [Google Scholar]
- 55.Keck PE Jr, Mintz J, McElroy SL, Freeman MP, Suppes T, Frye MA, et al. Double-blind, randomized, placebo-controlled trials of ethyleicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry 2006; 60: 1020–2. [DOI] [PubMed] [Google Scholar]
- 56.Kim HY. Novel metabolism of docosahexaenoic acid in neural cells. J Biol Chem 2007; 282: 18661–5. [DOI] [PubMed] [Google Scholar]
- 57.McNamara RK, Able J, Liu Y, Jandacek R, Rider T, Tso P, et al. Omega-3 fatty acid deficiency during perinatal development increases serotonin turnover in the prefrontal cortex and decreases midbrain tryptophan hydroxylase-2 expression in adult female rats: dissociation from estrogenic effects. J Psychiatr Res 2009; 43: 656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moriguchi T, Loewke J, Garrison M, Catalan JN, Salem N Jr. Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, and serum. J Lipid Res 2001; 42: 419–27. [PubMed] [Google Scholar]
- 59.McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford KE, et al. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry 2007; 62: 17–24. [DOI] [PubMed] [Google Scholar]
- 60.Umhau JC, Zhou W, Carson RE, Rapoport SI, Polozova A, Demar J, et al. Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J Lipid Res 2009; 50: 1259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horrobin DF. A new category of psychotropic drugs: neuroactive lipids as exemplified by ethyl eicosapentaenoate (E-E). Prog Drug Res 2002; 59: 171–99. [DOI] [PubMed] [Google Scholar]
- 62.Chen CT, Domenichiello AF, Trepanier MO, Liu Z, Masoodi M, Bazinet RP. The low levels of eicosapentaenoic acid in rat brain phospholipids are maintained via multiple redundant mechanisms. J Lipid Res 2013; 54: 2410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gold PW, Licinio J, Pavlatou MG. Pathological parainflammation and endoplasmic reticulum stress in depression: potential translational targets through the CNS insulin, klotho and PPAR-gamma systems. Mol Psychiatry 2013; 18: 154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell 1999; 3: 397–403. [DOI] [PubMed] [Google Scholar]
- 65.Lin Q, Ruuska SE, Shaw NS, Dong D, Noy N. Ligand selectivity of the peroxisome proliferator-activated receptor alpha. Biochemistry 1999; 38: 185–90. [DOI] [PubMed] [Google Scholar]
- 66.Jump DB, Tripathy S, Depner CM. Fatty acid-regulated transcription factors in the liver. Annu Rev Nutr 2013; 33: 249–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mochizuki K, Suruga K, Fukami H, Kiso Y, Takase S, Goda T. Selectivity of fatty acid ligands for PPARalpha which correlates both with binding to cis-element and DNA binding-independent transactivity in Caco-2 cells. Life Sci 2006; 80: 140–5. [DOI] [PubMed] [Google Scholar]
- 68.Popeijus HE, van Otterdijk SD, van der Krieken SE, Konings M, Serbonij K, Plat J, et al. Fatty acid chain length and saturation influences PPARalpha transcriptional activation and repression in HepG2 cells. Mol Nutr Food Res 2014; 58: 2342–9. [DOI] [PubMed] [Google Scholar]
- 69.Kemp DE, Schinagle M, Gao K, Conroy C, Ganocy SJ, Ismail-Beigi F, et al. PPAR-gamma agonism as a modulator of mood: proof-of-concept for pioglitazone in bipolar depression. CNS Drugs 2014; 28: 571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, et al. Activation of nuclear PPARgamma receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol Psychiatry 2011; 69: 642–9. [DOI] [PubMed] [Google Scholar]
- 71.Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis 2009; 24: 27–53. [DOI] [PubMed] [Google Scholar]
- 72.Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R, et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry 2016; 21: 71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sarkar S, Schaefer M. Antidepressant pretreatment for the prevention of interferon alfa-associated depression: a systematic review and meta-analysis. Psychosomatics 2014; 55: 221–34. [DOI] [PubMed] [Google Scholar]
- 74.Su KP, Lai HC, Yang HT, Su WP, Peng CY, Chang JP, et al. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: results from a randomized, controlled trial. Biol Psychiatry 2014; 76: 559–66. [DOI] [PubMed] [Google Scholar]
- 75.Song C, Zhang XY, Manku M. Increased phospholipase A2 activity and inflammatory response but decreased nerve growth factor expression in the olfactory bulbectomized rat model of depression: effects of chronic ethyl-eicosapentaenoate treatment. J Neurosci 2009; 29: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma 2004; 21: 1457–67. [DOI] [PubMed] [Google Scholar]
- 77.Rao MS, Hattiangady B, Shetty AK. Fetal hippocampal CA3 cell grafts enriched with FGF-2 and BDNF exhibit robust long-term survival and integration and suppress aberrant mossy fiber sprouting in the injured middle-aged hippocampus. Neurobiol Dis 2006; 21: 276–90. [DOI] [PubMed] [Google Scholar]
- 78.Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry 2010; 15: 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res 2005; 1037: 204–8. [DOI] [PubMed] [Google Scholar]
- 80.Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C, et al. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PloS One 2014; 9: e96905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Appleton KM, Sallis HM, Perry R, Ness AR, Churchill R. Omega-3 fatty acids for depression in adults. Cochrane Database Syst Rev 2015; 11: CD004692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


