Abstract
Acetate was shown to improve glucose fermentation in Lactococcus lactis deficient in lactate dehydrogenase. 13C and 1H nuclear magnetic resonance studies using [2-13C]glucose and [2-13C]acetate as substrates demonstrated that acetate was exclusively converted to ethanol. This novel pathway provides an alternative route for NAD+ regeneration in the absence of lactate dehydrogenase.
Lactococcus lactis is a lactic acid bacterium of major importance in the manufacture of dairy products. This bacterium displays an exclusive fermentative metabolism under dairy fermentation conditions: sugars are first converted by the Embden-Meyerhof-Parnas pathway to pyruvate, which is further reduced into l-lactate by an l-lactate dehydrogenase (l-LDH) (homolactic fermentation). The ATP is mainly produced (2 mol of ATP per mol of glucose) through glycolysis by substrate-level phosphorylation. A strict balance in the NADH/NAD+ ratio is maintained: the NAD+ cofactor reduced during glycolysis is regenerated during pyruvate reduction by the l-LDH (5).
A shift from the homolactic fermentation to a mixed-acid fermentation has been observed under certain conditions, such as carbohydrate limitation (21), galactose utilization (7, 22) or aerobic conditions (3). Besides l-LDH, three enzymes are able to dissipate pyruvate under different physiological conditions (reviewed in reference 10) (Fig. 1). The first is α-acetolactate synthase (ALS), which is active at a high pyruvate concentration and a low pH. Dissipation of pyruvate through this pathway can produce acetoin, diacetyl, and 2,3-butanediol. The second is the pyruvate dehydrogenase complex, which is active in aerobic conditions and at low pH and is inhibited at high NADH concentrations. The end products from this pathway are acetate and/or ethanol. The third is pyruvate formate-lyase, which is active under anaerobic conditions and at high pH. The end products are in this case a mixture of formate, acetate, and/or ethanol.
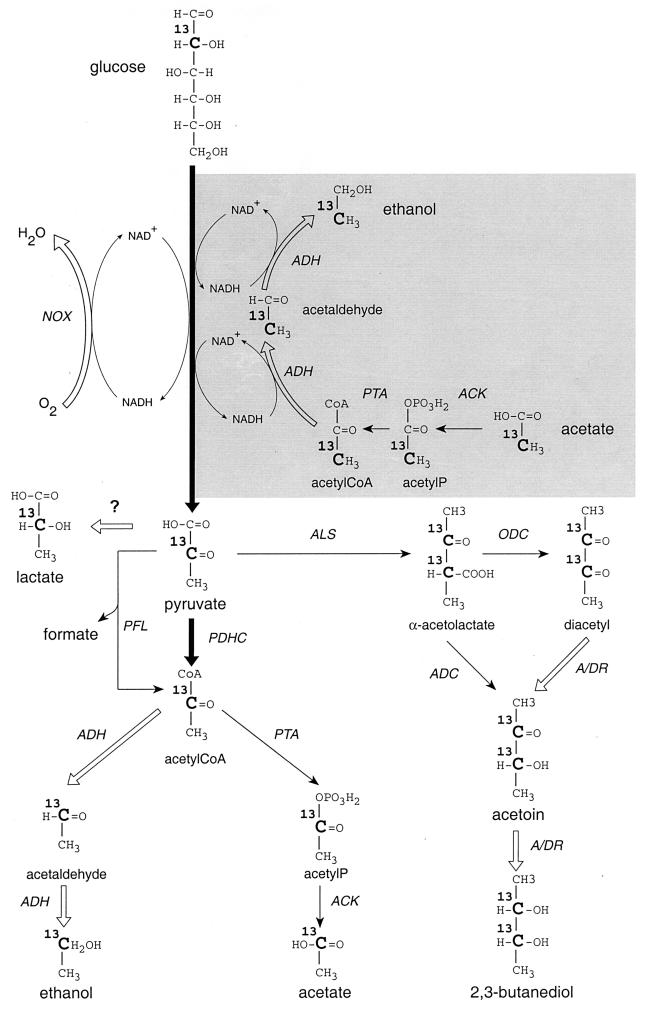
FIG. 1.
Schematic pathways of glucose and acetate cometabolism in l-LDH-deficient L. lactis. The proposed pathway of acetate conversion to ethanol and its coupling to glycolysis for cofactor regeneration is highlighted (grey area). Reactions or pathways producing NADH are denoted by large black arrows, those that are NADH independent are denoted by thin black arrows, and those producing NAD+ are denoted by large white arrows. The fate of the label derived from [2-13C]glucose and [2-13C]acetate (shaded region) is shown. ACK, acetate kinase; ADC, α-acetolactate decarboxylase; ADH, acetaldehyde/alcohol dehydrogenase; A/DR, α-acetolactate/diacetyl reductase; NOX, NADH oxidase; ODC, oxidative decarboxylation (chemical reaction); PDHC, pyruvate dehydrogenase complex; PFL, pyruvate/formate lyase; PTA, phosphotransacetylase; ?, unknown enzyme(s) involved in residual lactate production.
Under aerobic conditions, mixed-acid fermentation was shown to mainly result from the activation of an H2O-forming NADH oxidase (13) which strongly lowered the pool of NADH available for l-LDH, leading to a rerouting of pyruvate through the other aerobic catabolic pathways. The importance of NADH oxidase in controlling the NADH/NAD+ balance was recently exploited as a novel metabolic engineering strategy (cofactor engineering) in L. lactis (14). Overproduction of NADH oxidase resulted in the complete replacement of lactate by a mixture of acetate and acetoin (14). In this situation, all NADH-dependent enzymes were inhibited and the pyruvate pool was rerouted toward oxidative or NADH-independent pathways.
Platteeuw et al. (16) reported the various end products of sugar fermentation obtained with growing cells of an l-LDH-deficient strain. Under anaerobic conditions (initial pH 6.0), these are mainly a mixture of formate (34% of lactose utilization), ethanol (28%), acetoin (11%), and 2,3-butanediol (22%). Under aerobic conditions (initial pH 6.0), a mixture of acetoin (52%), 2,3-butanediol (31.5%), and ethanol (17%) was observed. Surprisingly, the production of acetate in both conditions was less than 2%. Therefore, the l-LDH-deficient strain was suspected to use acetate as an exogenous electron acceptor, as was reported for the metabolism of sorbitol and mannitol by lactobacilli (15, 20).
This paper reports the cometabolism of acetate and glucose in an l-LDH-deficient strain of L. lactis in which acetate is exclusively converted to ethanol, providing an alternative pathway for NAD+ regeneration.
End product formation from glucose-acetate cometabolism by an l-LDH-deficient strain.
Metabolic studies were performed with buffered cell suspensions, which greatly facilitate end product analysis. The L. lactis cells grown in GM17 (M17 medium [Merck] supplemented with 0.5% [wt/vol] glucose) were collected at an optical density at 600 nm of 2.0, washed, and resuspended at a concentration of approximately 3.3 g (dry weight) per liter in 100 mM KH2PO4–100 mM Na2HPO4 (pH 7.0) buffer supplemented with glucose (50 mM) and/or ammonium acetate (100 mM). The cell suspensions were incubated under low- or high-aeration conditions by shaking the cells either in 10-ml tubes at 50 rpm (Table 1, low aeration) or in 30-ml tubes at 200 rpm (Table 1, high aeration) in an upright position in a G76 waterbath (New Brunswick Scientific, Edison, N.J.). The supernatant (4 ml) was recovered after 1 h of incubation at 30°C, and end products were analyzed by high-pressure liquid chromatography (HPLC) (19) except α-acetolactate and acetoin, which were determined by using chemical assays (24).
TABLE 1.
Concentrations of glucose and acetate fermentation products determined by HPLC analysis from cell suspensions of L. lactis NZ3900 (wild type) and NZ3950 (l-LDH deficient) at an initial pH of 7.0a
| Fermentation conditions | Strain | Carbon source(s) | Substrate consumption (mM)
|
Product formation (mM)b
|
Carbon balance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Acetate | Lactate | Acetoin or α-acetolactate | Ethanol | Pyruvate | Acetate | Formate | 2,3-Butanediol | ||||
| Low aeration | NZ3900 | Glucose | 17.8 | 39.6 (111.2) | ND | ND | ND | ND | ND | ND | 1.11 | |
| NZ3950 | Glucose | 9.4 | 2.8 (14.9) | 3.9 (41.5) | ND | 2.4 (12.8) | ND | 0.9 (4.8) | ND | 0.74 | ||
| NZ3900 | Glucose + acetate | 24.8 | ND | 54.7 (110.3) | ND | ND | ND | ND | ND | ND | 1.10 | |
| NZ3950 | Glucose + acetate | 25.5 | 21.5 | 2.4 (4.7) | 13.9 (54.5) | 22.5 (2.0) | 17.4 (34.1) | ND | ND | 0.95 | ||
| High aeration | NZ3950 | Glucose | 44.5 | 4.1 (4.6) | 22.0 (49.4) | 11.2 (12.6) | 6.5 (7.3) | 8.1 (9.1) | 1.4 (1.6) | ND | 0.83 | |
| NZ3950 | Glucose + acetate | 44.8 | 15.6 | 3.7 (4.1) | 23.9 (53.3) | 22.4 (7.6) | 17.8 (19.9) | 0.7 (0.8) | ND | 0.85 | ||
Data are representative of at least two experiments.
Values in parentheses are percentages of glucose converted to the product. ND, not detected.
Glucose catabolism by resting cells of the l-LDH-deficient strain (NZ3950 [ΔlacF ldh pepN::nisRK]; MG1363 derivative [9]) resulted in a mixed-acid fermentation (Table 1) as observed previously by Platteeuw et al. for growing cells (16). However, at a low aeration rate, glucose consumption was threefold lower than in the wild-type strain (NZ3900 [ΔlacF pepN::nisRK]; MG1363 derivative [4]); pyruvate was mainly rerouted toward the ALS pathway (41.5% of glucose utilization) as expected, but acetate and ethanol were not detected, and a significant amount (12.8%) of pyruvate was excreted. At a high aeration rate, glucose consumption was significantly higher and the expected distribution of end products, including acetate (9.1%) and ethanol (12.6%), was observed as previously reported for growing cells (16). Excretion of pyruvate by the l-LDH-deficient strain could easily be explained by a low efficiency of the alternative pyruvate-dissipating enzymes at a low aeration rate and a high initial pH (7.0), as indicated above. The low level of glucose consumption probably resulted from an inefficient regeneration of NAD+ since the acetoin pathway (via ALS) is NADH independent and NAD+ regeneration by NADH oxidase is not fully operative. At a higher aeration rate, the NADH oxidase activity is enhanced, resulting in an increased regeneration of NAD+ (favoring glycolysis) and a decreased inhibition of the (pyruvate dehydrogenase complex by a high NADH concentration (allowing the production of acetate and ethanol).
The addition of acetate to cell suspensions of the l-LDH-deficient strain at low aeration rates resulted in a nearly equimolar consumption of glucose and acetate (acetate/glucose ratio of 0.84), while no acetate was consumed by cell suspensions of the wild-type strain under the same conditions (Table 1). Furthermore, glucose was consumed to an extent similar to that for the wild-type strain, and ethanol was produced at a level nearly equimolar to that of acetate utilization (acetate/ethanol ratio of 0.95). These observations suggested that the most probable pathway of conversion of acetate to ethanol is as presented in Fig. 1. In this scheme, acetate is first converted to acetyl coenzyme A (acetyl-CoA) by acetate kinase and phosphotransacetylase, and acetyl-CoA is next reduced to ethanol by alcohol dehydrogenase with a concomitant oxidation of 2 mol of NADH to NAD+. This pathway enables a coupling of acetate utilization (1 acetate + 2 NADH + 1 ATP → 1 ethanol + 2 NAD+ + 1 ADP + 1 Pi) to glycolysis (glucose + 2 NAD+ + 2 ADP + 2 Pi → 2 pyruvate + 2 NADH + 2 ATP) and cofactor regeneration. The acetate pathway also consumes 1 mol of ATP per mol of acetate, which could eventually improve the ATP turnover by stimulating glycolytic enzymes (pyruvate kinase and phosphofructokinase) and thus favoring glucose utilization. However, acetate metabolism at a high aeration rate does not improve glucose utilization in contrast to low-aeration conditions. At a high aeration rate, high glucose consumption correlates with activation of the NADH oxidase, which plays a crucial role in maintaining an equilibrated redox balance without any direct impact on ATP turnover. Table 1 shows that glucose-acetate cometabolism in the l-LDH-deficient strain at a high aeration rate results in a lower utilization of acetate (acetate/glucose ratio of 0.35) than in low-aeration conditions (acetate/glucose ratio of 0.84). These observations could be explained by a different level of competition for NADH between the NADH oxidase and the acetate utilization pathway (Fig. 1), which plays a predominant role in NAD+ regeneration rather than in ATP turnover.
Analysis of glucose-acetate cometabolism by 13C and 1H NMR.
Nuclear magnetic resonance spectroscopy (NMR) was used to test the hypothesis that acetate was converted to ethanol in the l-LDH-negative strain of L. lactis. NMR is a powerful technique for studying metabolic pathways since the use of 13C-labeled substrates allows evaluation of the fate of individual carbon atoms in a noninvasive way (12, 23). Furthermore, when a mixture of selectively enriched substrates is used (mixotrophic conditions), the origin of the carbon atoms in the end products formed can be determined by analysis of their labeling patterns (17, 18). In our experiments, a mixture of glucose labeled on C-2 and acetate labeled on the methyl group was supplied to the cells. The fate of each substrate can be determined easily from analysis of the isotopic enrichment in the two carbon atoms of ethanol. In fact, the label would end on the methylene group if ethanol were derived from glucose and on the methyl group if it were derived from acetate. This approach provided a definite proof for the operation of the pathway converting acetate to ethanol.
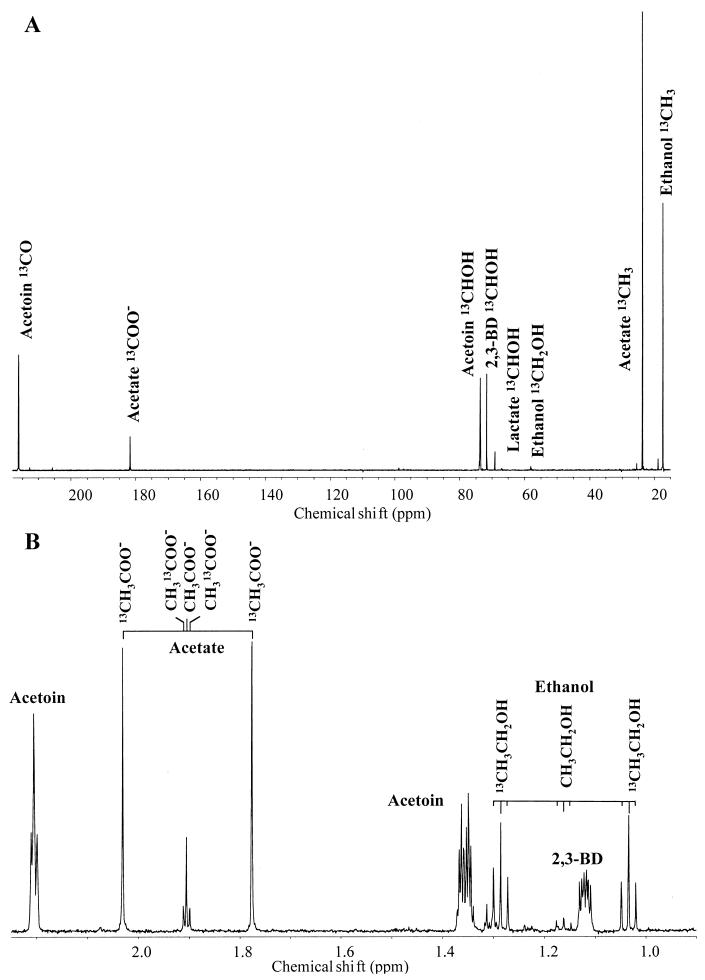
As for the previous studies, fermentation was performed with resting cells. L. lactis cells were harvested in the mid-exponential growth phase, washed, and resuspended at a concentration of approximately 20 g (dry weight) per liter in potassium phosphate buffer (50 mM, pH 5.5) supplemented with [2-13C]glucose (40 mM) and [2-13C]acetate (sodium salt, 40 mM). The supernatant was recovered for end product analysis by NMR following 1 h of incubation at 30°C. Figure 2A shows the 13C NMR spectrum of the supernatant containing the end products of [2-13C]acetate and [2-13C]glucose cometabolism by the l-LDH-deficient strain at pH 5.5. Catabolism of [2-13C]glucose produced acetoin labeled on the CO and CHOH groups, 2,3-butanediol labeled on both CHOH groups, acetate labeled on the COOH group, ethanol labeled on the CH2OH group, and a small amount of lactate enriched on the CHOH group (Fig. 1 and 2A). These end products were as expected from a fermentation performed in the presence of oxygen at low pH. The production of 2,3-butanediol in these conditions was reported previously by Platteeuw et al. (16). On the other hand, no pyruvate was detected, which suggested a better efficiency of pyruvate-dissipating enzymes at low pH, as previously reported for the ALS enzyme (10, 16).
FIG. 2.
NMR spectra of end products formed from the cometabolism of [2-13C]glucose and [2-13C]acetate (40 mM each) by cell suspensions of l-LDH-deficient L. lactis at pH 5.5. (A) 13C NMR spectrum, acquired on a Bruker DRX500 spectrometer at 125.77 MHz with a quadruple nucleus probe head (spectral width, 30 kHz; pulse width, 13 μs; data points, 32K; repetition delay, 61.5 s; number of transients, 1,000). (B) 1H NMR spectrum of the same sample analyzed after a 30-fold dilution in 2H2O. Isotopomers of acetate and ethanol are indicated. The spectrum was acquired at 500.13 MHz with a 5-mm inverse detection probe head (spectral width, 8 kHz; pulse width, 6 μs; data points, 32K; repetition delay, 45 s). 2,3-BD, 2,3-butanediol.
Catabolism of [2-13C]acetate produced ethanol labeled on the methyl group (strong resonance at 17.3 ppm). The other intense resonance signal at a high field in this spectrum (δ = 23.6 ppm) was due to the methyl group of residual [2-13C]acetate. Ethanol was the only metabolite produced from the supplied acetate, since no other resonances with a labeling pattern consistent with derivation from 13CH3 acetate were observed in the spectrum. This observation was confirmed by analysis of the proton spectrum (Fig. 2B). The resonance patterns due to the methyl groups of acetate and ethanol are highlighted in Fig. 2B. The central resonance at 1.9 ppm in the acetate pattern is assigned to unlabeled acetate, the low-intensity inner doublet is due to CH313COO− (derived from glucose), and the high-intensity lateral resonance split by a coupling constant of 127 Hz is due to the added 13CH3COO− that remained in the supernatant. The ethanol derived from [2-13C]acetate, labeled on the CH3 group, originates the doublet of triplets clearly observed at a higher field in the ethanol pattern, while ethanol derived from glucose metabolism was not clearly detected on this spectrum. Integration of the areas of these resonances allowed us to determine that 12.5 mM ethanol was produced from [2-13C]acetate metabolism. The resonances due to other products of glucose metabolism (acetoin and 2,3-butanediol) are also identified in Fig. 2B.
13C and 1H NMR analysis of the supernatant resulting from the metabolism of [2-13C]glucose and [2-13C]acetate by the wild-type strain at pH 5.5 showed that acetate was not metabolized and that glucose was completely converted to lactate (data not shown).
Concluding remarks.
Acetate is cometabolized with glucose under various conditions of aeration and initial pH only when the l-LDH is inactivated in L. lactis. Acetate is exclusively converted into ethanol, as demonstrated by NMR analysis. A hypothetical acetate-ethanol pathway is presented in Fig. 1. This pathway suggests acetyl-CoA, not pyruvate, as an intermediate, since no product other than ethanol was detected from acetate metabolism. Acetate is proposed to be converted to acetyl-CoA by the acetate kinase-phosphotransacetylase pathway via acetyl-P, since both enzymatic activities have been found in L. lactis (10). However, we cannot exclude the possibility that acetate can be partially or totally converted to acetyl-CoA by an acetyl-CoA synthase enzyme (acetate + ATP + CoA → acetyl-CoA + AMP + PPi) as reported for other bacteria (2, 6, 8), although this activity has not been reported for L. lactis. The conversion of acetyl-CoA to ethanol is known to take place in L. lactis via a bifunctional alcohol dehydrogenase enzyme whose genetic inactivation is responsible for a strong reduction in ethanol production (1). This last reaction is responsible for NAD+ regeneration, thus allowing glucose metabolism through glycolysis in an l-LDH-negative strain in the absence of efficient reductive pathways. The role of the NADH oxidase in improving and/or modifying the redox balance is well known (13, 14). This newly described pathway performs a similar function and reinforces the predominant role of the NADH/NAD+ ratio in governing the glycolytic flux and the distribution of fermentation end products in L. lactis. Furthermore, one of the consequences of glucose-acetate cometabolism is the unexpected excretion of a large quantity of pyruvate (up to 34%), which is a key intermediate metabolite, now available to be rerouted by metabolic engineering toward new pathways unable to regenerate NAD+.
Acknowledgments
This research was carried out in the framework of the European Community Research Programme BIOTECH (contract BIO4-CT96-0498). P.H. holds an EC BIOTECH postdoctoral training grant (contract BIO4-CT96-5093). A.R. is grateful to Fundação de Ciência e Tecnologia, Portugal, for a postdoctoral fellowship.
We thank R. Holleman for technical help in HPLC analyses.
REFERENCES
- 1.Arnau J, Jørgensen F, Madsen S M, Vrang A, Israelsen H. Cloning of the Lactococcus lactis adhE gene, encoding a multifunctional alcohol dehydrogenase, by complementation of a fermentative mutant of Escherichia coli. J Bacteriol. 1998;180:3049–3055. doi: 10.1128/jb.180.12.3049-3055.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown T D K, Jones-Mortimer M C, Kornberg H L. The enzymatic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J Gen Microbiol. 1977;102:327–336. doi: 10.1099/00221287-102-2-327. [DOI] [PubMed] [Google Scholar]
- 3.Condon S. Responses of lactic acid bacteria to oxygen. FEMS Microbiol Rev. 1987;46:269–280. [Google Scholar]
- 4.de Ruyter P G G A, Kuipers O P, de Vos W M. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol. 1996;62:3662–3667. doi: 10.1128/aem.62.10.3662-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vos W M. Metabolic engineering of sugar catabolism in lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:223–242. doi: 10.1007/BF00395934. [DOI] [PubMed] [Google Scholar]
- 6.Eggen R I L, Geerling A C M, Boshoven A B P, de Vos W M. Cloning, sequence analysis, and functional expression of the acetyl coenzyme A synthetase gene from Methanothrix soehngenii in Escherichia coli. J Bacteriol. 1991;173:6383–6389. doi: 10.1128/jb.173.20.6383-6389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrigues C, Loubiere P, Lindley N D, Cocaign-Bousquet M. Control of the shift from homolactic acid to mixed-acid fermentation in Lactococcus lactis: predominant role of the NADH/NAD+ ratio. J Bacteriol. 1997;179:5282–5287. doi: 10.1128/jb.179.17.5282-5287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grundy F J, Waters D A, Takova T Y, Henkin T M. Identification of genes involved in utilization of acetate and acetoin in Bacillus subtilis. Mol Microbiol. 1993;10:259–271. doi: 10.1111/j.1365-2958.1993.tb01952.x. [DOI] [PubMed] [Google Scholar]
- 9.Hols P, Kleerebezem M, Schanck A N, Ferain T, Hugenholtz J, Delcour J, de Vos W M. Conversion of Lactococcus lactis from homolactic to homoalanine fermentation through metabolic engineering. Nat Biotechnol. 1999;17:588–592. doi: 10.1038/9902. [DOI] [PubMed] [Google Scholar]
- 10.Hugenholtz J. Citrate metabolism in lactic acid bacteria. FEMS Microbiol Rev. 1993;12:165–178. [Google Scholar]
- 11.Lees G J. Acetaldehyde: an intermediate in the formation of ethanol from glucose by lactic acid bacteria. J Dairy Res. 1976;43:63–73. doi: 10.1017/s0022029900015600. [DOI] [PubMed] [Google Scholar]
- 12.London R E. 13C labelling studies of metabolic regulation. Prog Nucl Magn Reson Spectrosc. 1988;20:337–383. [Google Scholar]
- 13.Lopez de Felipe F, Starrenburg M J C, Hugenholtz J. The role of NADH-oxidation in acetoin and diacetyl production from glucose in Lactococcus lactis. FEMS Microbiol Lett. 1997;156:15–19. [Google Scholar]
- 14.Lopez de Felipe F, Kleerebezem M, de Vos W M, Hugenholtz J. Cofactor engineering: a novel approach to metabolic engineering in Lactococcus lactis by controlled expression of NADH oxidase. J Bacteriol. 1998;180:3804–3808. doi: 10.1128/jb.180.15.3804-3808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McFeeters R F, Chen K-H. Utilization of electron acceptors for anaerobic mannitol metabolism by Lactobacillus plantarum. Compounds which serve as electron acceptors. Food Microbiol. 1986;3:73–81. [Google Scholar]
- 16.Platteeuw C, Hugenholtz J, Starrenburg M, van Alen-Boerrigter I, de Vos W M. Metabolic engineering of Lactococcus lactis: influence of the overproduction of α-acetolactate synthase in strains deficient in lactate dehydrogenase as a function of culture conditions. Appl Environ Microbiol. 1995;61:3967–3971. doi: 10.1128/aem.61.11.3967-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos A, Jordan K N, Cogan T M, Santos H. 13C nuclear magnetic resonance studies of citrate and glucose cometabolism by Lactococcus lactis. Appl Environ Microbiol. 1994;60:1739–1748. doi: 10.1128/aem.60.6.1739-1748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos A, Santos H. Citrate and sugar cofermentation in Leuconostoc oenos, a 13C nuclear magnetic resonance study. Appl Environ Microbiol. 1996;62:2577–2585. doi: 10.1128/aem.62.7.2577-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starrenburg M J C, Hugenholtz J. Citrate fermentation by Lactococcus and Leuconostoc spp. Appl Environ Microbiol. 1991;57:3535–3540. doi: 10.1128/aem.57.12.3535-3540.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi N, Kalfas S, Yamada T. Effect of acetate on sorbitol fermentation by oral lactobacilli. Oral Microbiol Immunol. 1995;10:349–354. doi: 10.1111/j.1399-302x.1995.tb00165.x. [DOI] [PubMed] [Google Scholar]
- 21.Thomas T D, Ellwood D C, Longyear V M C. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol. 1979;138:109–117. doi: 10.1128/jb.138.1.109-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas T D, Turner K W, Crow V L. Galactose fermentation by Streptococcus lactis and Streptococcus cremoris: pathways, products, and regulation. J Bacteriol. 1980;144:672–682. doi: 10.1128/jb.144.2.672-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veiga-da-Cunha M, Firme P, San Romão M V, Santos H. Application of 13C nuclear magnetic resonance to elucidate the unexpected biosynthesis of erythritol by Leuconostoc oenos. Appl Environ Microbiol. 1992;58:2271–2279. doi: 10.1128/aem.58.7.2271-2279.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veringa H A, Verburg E H, Stadhouders J. Determination of diacetyl in dairy products containing α-acetolactic acid. Neth Milk Dairy J. 1984;38:251–263. [Google Scholar]