Abstract
Background
Little is known about the role of early obesity or weight change during adulthood in the development of liver cancer and biliary tract cancer (BTC).
Methods
We investigated the associations of body mass index (BMI) and weight trajectories with the risk of liver cancer and BTC in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. BMI was self-reported at ages 20 years and 50 years and at enrollment. BMI trajectories were determined using latent class growth models. Cox regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs).
Results
During a median follow-up of 15.9 years among 138 922 participants, 170 liver cancer and 143 BTC cases were identified. Compared with those whose BMI did not exceed 25 kg/m2, participants with BMI exceeding 25 kg/m2 at age 20 years had increased risks of liver cancer (HR = 2.03, 95% CI = 1.26 to 3.28) and BTC (HR = 1.99, 95% CI = 1.16 to 3.39). Compared with participants maintaining normal BMI until enrollment, trajectory of normal weight at age 20 years to obesity at enrollment was associated with increased risk for liver cancer (HR = 2.50, 95% CI = 1.55 to 4.04) and BTC (HR = 1.83, 95% CI = 1.03 to 3.22). Compared with adults with stable weight (±5 kg) between age 20 years and 50 years, weight gain of 20 kg and greater between ages 20 years and 50 years had higher hazard ratios of 2.24 (95% CI = 1.40 to 3.58) for liver cancer and 1.86 (95% CI = 1.12 to 3.09) for BTC.
Conclusions
Being overweight and/or obese at age 20 years and BMI trajectories that result in being overweight and/or obese may increase risk for both liver cancer and BTC.
Hepatobiliary cancers include liver cancer, the most common type of which is hepatocellular carcinoma (HCC, approximately 80%), and biliary tract cancer (BTC), which encompasses cancers of the gallbladder, extrahepatic bile duct, and ampulla of Vater (1). All are highly lethal, with liver being the fifth-most common cause of cancer death among men worldwide in 2020 (2). In the United States, the incidence of liver plus intrahepatic bile duct cancer has been increasing recently, particularly for HCC, with incidence rates of 5.8 per 105 during 2008-2012 and 1.8 per 105 during 1978-1982, and the mortality of liver cancer has also increased during this period (3.2 per 105 during 2012-2017 vs 2.0 per 105 during 1985-1995) (2-4).
Coinciding with the increased rate of liver cancer and BTC, the prevalence of obesity has increased markedly in the United States (5,6). According to a recent report by the World Cancer Research Fund International/American Institute for Cancer Research, excess adult adiposity (measured by body mass index [BMI]) is a well-demonstrated cause of liver cancer (7,8). More than 30% of liver cancer cases in the United States have been attributed to obesity and other metabolic disorders (9). However, several questions are unanswered regarding the association between obesity and hepatobiliary cancers. First, little is known about the role of adiposity in early adulthood in the development of liver cancer and BTC. Only 3 studies (10-12) have investigated BMI at young adult ages in relation to HCC and 1 examined its association with BTC (13). Second, the relationship between weight change across the life course and liver cancer has not been widely studied (10). Because the influence of obesity on cancer development may vary at different ages (14), trajectory changes in BMI across one’s lifespan or a more integrated lifetime measure might better reflect the long-term effect of obesity on carcinogenesis compared with obesity measured at a single time point or during a short period. Third, the impact of adulthood weight changes on hepatobiliary cancer remains largely undefined. Weight gain during adulthood depends mostly on accumulation of fat rather than lean mass; thus, weight change is more likely to reflect adiposity than adult-attained weight itself, which tends to also reflect lean mass (7,15). However, few studies have investigated weight gain during adulthood (10,12,16,17). In addition, whether the influence of weight gain on cancer risk differs according to when it occurs in adulthood remains unclear.
Herein, we investigated early adulthood BMI, trajectories of adiposity change, and BMI over time in relation to the subsequent risk of developing liver cancer and BTC.
Methods
Study Population
The data were collected by the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Details of the study design, scientific rationale, and baseline characteristics of the participants have been described elsewhere (18). In brief, 154 887 eligible participants aged 42-78 years from 10 study sites (Washington, DC; Detroit, Michigan; Marshfield, Wisconsin; Honolulu, Hawaii; Birmingham, Alabama; Aurora, Colorado; Minneapolis, Minnesota; Pittsburgh, Pennsylvania; Boise, Idaho; and St Louis, Missouri) were enrolled between November 1993 and July 2001. Information on anthropometric measurements, demographics, cancer risk factors, and medical histories were collected using a self-administered baseline questionnaire in 1993-2001. Dietary information was collected using the Diet History Questionnaire, which collected frequency of intake and portion size of 124 food items in 1999 or 2000. The response rates for the dietary questionnaire and the baseline questionnaire were 84% and 89%, respectively.
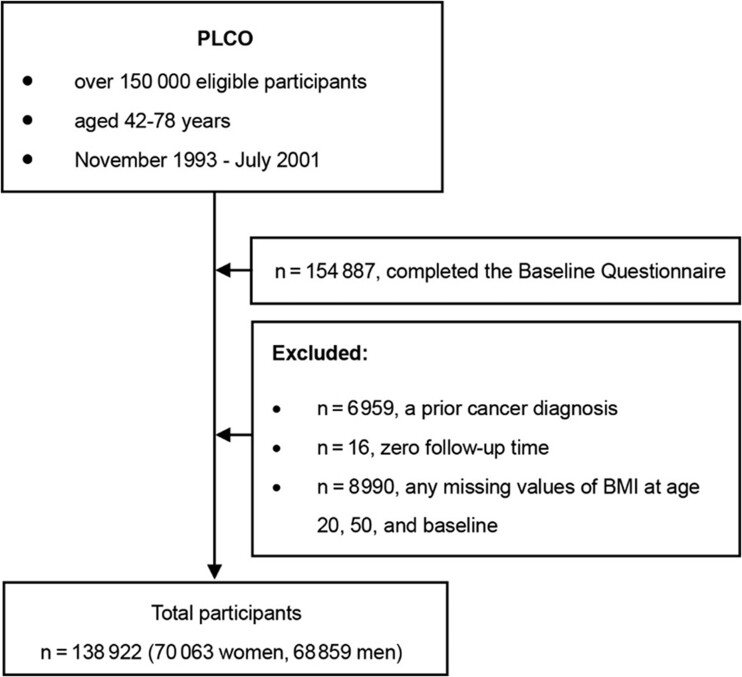
Among the 154 887 participants who completed the baseline questionnaire, we excluded individuals with a previous cancer diagnosis except nonmelanoma skin cancer (n = 6959, 4.49%), zero follow-up time (n = 16, 0.01%), or any missing values of BMI at age 20 years and 50 years and at baseline (n = 8990, 5.80%) to model trajectory of BMI evolution across adulthood. After exclusions, a total of 138 922 participants (89.69%, 70 063 women and 68 859 men) were included in the present analysis. The flow chart is shown in Figure 1. The usage of the database in the PLCO study was approved by the ethics committees of the data providers.
Figure 1.
Flowchart depicting the process of selecting participants. BMI = body mass index; PLCO = Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial.
Exposure Assessments
At the time of enrollment into PLCO, participants recorded their current height and body weight and recalled their weight at age 20 years and 50 years. BMI was calculated as the weight in kilograms divided by height in meters squared. Persons were classified by their BMI at each age according to the World Health Organization criteria: underweight (<18.5 kg/m2), normal weight (18.5-24.9 kg/m2, reference), overweight (25-29.9 kg/m2), and obese (≥30 kg/m2). Consistent with previous studies (19,20), changes in weight between ages 20 years and 50 years were classified in 5 categories: 1) weight loss of at least −5 kg; 2) stable weight, defined as either weight loss between 0 and less than −5 kg or weight gain between 0 and less than 5 kg (reference); 3) moderate weight gain between less than or equal to 5 and 10 kg; 4) statistically significant weight gain between more than 10.0 and 20.0 kg; and 5) extreme weight gain of 20.0 kg or more.
Assessments of Covariates
The PLCO study has collected data on age (years), sex (men, women), center (see Study Population section), race and ethnicity (Hispanic, non-Hispanic Black, non-Hispanic White, other [Asian, Pacific Islander, American Indian, and unknown]), randomization arm (intervention, control), education (less than high school, high school, post high school, some college, college graduate, postgraduate), job status (homemaker, working, unemployed, retired, extended sick leave, disabled, other), aspirin use longer than 12 months (yes, no), smoking status (current, former, never smokers), family history of liver cancer (yes, no), family history of biliary tract cancer (yes, no), and alcohol (never, current, former, unknown). Healthy Eating Index-2010 (HEI-2010) scores, histories of diabetes mellitus (yes, no), hypertension (yes, no), diverticulitis (yes, no), liver cirrhosis (yes, no), and gallstones stones or inflammation (yes, no) were derived from the diet history questionnaire.
Liver Cancer and BTC Ascertainment
Incident liver cancer and BTC cases were ascertained through annual self-report, state cancer registries and linkage to the National Death Index (for completeness), which were further confirmed by medical record reviews. Cancer cases were defined using International Classification of Diseases for Oncology, Third Edition (codes C22.0 and C22.1 for liver cancer, codes C23.9-C24.1 and C24.8-C24.9 for BTC).
Statistical Analysis
We calculated person-years from the date of return of the baseline questionnaire to the date of diagnosis of liver cancer or BTC, death from any cause, loss to follow-up, or the end of the follow-up (December 31, 2009), whichever came first. We used a group-based trajectory modeling approach implemented by SAS PROC TRAJ (21,22) to identify persons with similar patterns of BMI evolution across adulthood (ie, BMI at ages 20 years and 50 years and at study enrollment). We determined the optimal number of groups and model pattern according to the change in Bayesian Information Criterion and the mean posterior probability of each group, where each trajectory category needed to include at least 1% of participants. This approach was based on the latent class growth model and has been successfully applied in several cohort studies (23-25).
Age-adjusted and multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazards models. When examining liver cancer as the primary outcome, BTC was a censored event and vice versa. There was no evidence of violation of proportional hazard assumptions after testing an interaction term between exposures and follow-up time. The adjusted variables in the multivariable models included age, sex, race and ethnicity, randomization arm, education, study site, job status, smoking status, use of aspirin, family history of liver cancer, and family history of BTC. In analyses for weight change, we further adjusted for BMI at age 20 years.
We used several sensitivity analyses to test the robustness of our findings. First, we adjusted for HEI-2010 score and alcohol drinking in a subset, accounting for the possible confounding due to diet. Second, we adjusted for several comorbidities, including diabetes mellitus, hypertension, diverticulitis, liver cirrhosis, gallstones, and inflammation, some of which were considered as potential intermediates in the associations between obesity and hepatobiliary cancers. Third, participants diagnosed with liver cancer or BTC within 3 years after baseline survey were excluded, addressing for the potential reverse causality. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Study Enrollment Characteristics
Participants with a higher BMI at study enrollment were younger, less likely to be current smokers, and more likely to use aspirin and to have a family history of liver cancer (Table 1). The baseline characteristics according to weight change are summarized in Supplementary Table 1 (available online).
Table 1.
Demographic and lifestyle characteristics of participants in the PLCO Cancer Screening Triala
| Variables | BMI at study enrollment, kg/m2 |
|||
|---|---|---|---|---|
| <18.5 | 18.5 to <25 | 25 to <30 | ≥30 | |
| No. of participants | 954 | 46 196 | 59 032 | 32 740 |
| Mean age, y | 63.7 (5.4) | 62.9 (5.5) | 62.7 (5.3) | 61.8 (5.1) |
| Sex | ||||
| Men | 23.2 | 40.1 | 59.7 | 48.7 |
| Women | 76.8 | 59.8 | 40.1 | 51.3 |
| Race and ethnicity | ||||
| Hispanic | 1.8 | 1.6 | 2.0 | 2.0 |
| Non-Hispanic Black | 4.2 | 3.3 | 4.7 | 8.1 |
| Non-Hispanic White | 82.0 | 88.4 | 89.4 | 87.7 |
| Otherb | 12.1 | 6.7 | 4.0 | 2.2 |
| Family history of liver cancer | 1.4 | 2.0 | 2.1 | 2.2 |
| Family history of biliary tract cancer | 0.2 | 0.3 | 0.3 | 0.3 |
| Alcohol drinking status | ||||
| Never | 14.9 | 10.2 | 9.0 | 10.7 |
| Former | 16.4 | 12.4 | 14.4 | 17.9 |
| Current | 64.2 | 74.0 | 73.0 | 67.0 |
| Unknown | 4.43 | 3.4 | 3.6 | 4.4 |
| Education | ||||
| Less than high school | 6.5 | 5.5 | 7.2 | 9.8 |
| High school | 23.2 | 21.1 | 22.9 | 25.5 |
| Post high school training other than college | 13.0 | 11.6 | 12.7 | 13.7 |
| Some college | 21.0 | 21.3 | 21.7 | 22.8 |
| College graduate | 17.5 | 19.4 | 16.9 | 14.1 |
| Postgraduate | 18.8 | 21.1 | 18.6 | 14.1 |
| Smoking status | ||||
| Never | 46.1 | 48.8 | 44.8 | 45.0 |
| Current | 27.2 | 38.0 | 45.5 | 47.0 |
| Former | 26.7 | 13.2 | 9.7 | 8.0 |
| Job status | ||||
| Homemaker | 17.1 | 13.8 | 9.1 | 11.0 |
| Working | 30.0 | 38.5 | 40.8 | 41.1 |
| Unemployed | 1.5 | 1.0 | 1.0 | 1.2 |
| Retired | 43.6 | 42.9 | 45.0 | 40.4 |
| Extended sick leave and disabled | 5.3 | 1.6 | 1.9 | 4.1 |
| Other | 2.5 | 2.2 | 2.2 | 2.2 |
| Mean HEI-2010 score | 66.6 (12.2) | 67.0 (11.1) | 64.7 (10.7) | 63.6 (10.4) |
| Aspirin usec | 41.4 | 44.0 | 48.7 | 48.9 |
| Mean BMI, kg/m2 | ||||
| At age 20 y | 19.3 (2.6) | 20.7 (2.2) | 22.2 (2.6) | 24.0 (3.7) |
| At age 50 y | 19.3 (3.0) | 22.6 (2.1) | 25.9 (2.4) | 30.6 (4.7) |
| At study enrollment | 17.5 (0.8) | 22.8 (1.5) | 27.2 (1.4) | 34.1 (4.1) |
All data reported as percentage (%) or mean (SD), unless noted otherwise. BMI = body mass index; HEI-2010 score = the Healthy Eating Index 2010 score; PLCO = Prostate, Lung, Colorectal and Ovarian.
“Other” race category includes Asian, Pacific Islander, American Indian, and unknown.
Aspirin use for longer than 12 months.
Associations of BMI With Liver Cancer and BTC
A total of 170 liver cancer cases and 143 BTC cases occurred during a median follow-up of 15.9 years among 138 922 participants. Compared with normal weight, obesity (BMI ≥30 kg/m2) at age 50 years or at study enrollment (mean age = 62.6 years) was statistically significantly associated with 91%-158% increased risk for liver cancer and 115%-120% increased risk for BTC. When modeled continuously, BMI at ages 20 years and 50 years and at study enrolment were associated with increased risks of both cancers, with multivariable-adjusted hazard ratios ranging from 1.32 to 1.45 per 5 kg/m2 for liver cancer and 1.24 to 1.35 for BTC (Table 2), although such positive associations were slightly attenuated when we additionally controlling for diabetes mellitus, hypertension, diverticulitis, liver cirrhosis, gallstones, inflammation, HEI-2010 score, and alcohol drinking. When we further adjusted for the average BMI during age 50 years to study enrollment, participants with BMI ≥30 vs 18.5–24.9 kg/m2 at age 20 years had hazard ratios of 1.60 (95% CI = 0.73 to 3.54) for liver cancer and 1.53 (95% CI = 0.61 to 3.89) for BTC (Supplementary Table 2, available online).
Table 2.
Hazard ratios and 95% confidence intervals for incident liver and biliary tract cancers according to BMI in the PLCO Cancer Screening Trial
| Model | BMI (kg/m2) |
BMI (per 5 kg/m2)c | |||
|---|---|---|---|---|---|
| <18.5 | 18.5 to <25 | 25 to <30 | ≥30 | ||
| Liver cancer | |||||
| BMI, age 20 y, kg/m2 | |||||
| No. of cases/person-years | 11/183 461 | 118/1 701 222 | 34/288 085 | 7/37 537 | — |
| Age-adjusted HR (95% CI) | 0.86 (0.46 to 1.60) | 1 (Referent) | 1.79 (1.22 to 2.63) | 2.95 (1.37 to 6.34) | 1.59 (1.31 to 1.93) |
| Multivariable adjusted HR (95% CI)a | 1.04 (0.56 to 1.94) | 1 (Referent) | 1.35 (0.92 to 1.99) | 2.54 (1.18 to 5.46) | 1.37 (1.10 to 1.72) |
| BMI, age 50 y, kg/m2 | |||||
| No. of cases/person-years | 0/16 314 | 55/1 043 112 | 76/860 936 | 39/289 942 | — |
| Age-adjusted HR (95% CI) | — | 1 (Referent) | 1.77 (1.25 to 2.51) | 3.02 (1.99 to 4.58) | 1.47 (1.28 to 1.68) |
| Multivariable adjusted HR (95% CI)a | — | 1 (Referent) | 1.40 (0.98 to 2.01) | 2.58 (1.69 to 3.96) | 1.45 (1.24 to 1.70) |
| BMI, age at study enrollment | |||||
| No. of cases/person-years | 1/13 942 | 44/746 192 | 72/944 652 | 53/505 518 | — |
| Age-adjusted HR (95% CI) | 1.15 (0.15 to 8.37) | 1 (Referent) | 1.31 (0.90 to 1.91) | 1.90 (1.27 to 2.85) | 1.26 (1.10 to 1.45) |
| Multivariable adjusted HR (95% CI)a | 1.18 (0.16 to 8.64) | 1 (Referent) | 1.14 (0.78 to 1.68) | 1.91 (1.26 to 2.89) | 1.32 (1.14 to 1.54) |
| Average BMI across the adult life course (age 20 y to study enrollment) | |||||
| No. of cases/person-years | 0/16 905 | 80/1 317 070 | 75/730 876 | 15/145 454 | — |
| Age-adjusted HR (95% CI) | — | 1 (Referent) | 1.75 (1.28 to 2.40) | 1.90 (1.09 to 3.31) | 1.54 (1.27 to 1.85) |
| Multivariable adjusted HR (95% CI)a | — | 1 (Referent) | 1.56 (1.13 to 2.16) | 1.86 (1.06 to 3.26) | 1.50 (1.22 to 1.85) |
| Biliary tract cancer | |||||
| BMI, age 20 y, kg/m2 | |||||
| No. of cases/person-years | 10/183 461 | 104/1 701 222 | 24/288 085 | 5/37 537 | — |
| Age-adjusted HR (95% CI) | 0.89 (0.46 to 1.70) | 1 (Referent) | 1.44 (0.92 to 2.24) | 2.37 (0.96 to 5.84) | 1.34 (1.05 to 1.71) |
| Multivariable adjusted HR (95% CI)b | 0.90 (0.47 to 1.72) | 1 (Referent) | 1.32 (0.83 to 2.08) | 2.15 (0.87 to 5.31) | 1.28 (0.99 to 1.64) |
| BMI, age 50 y, kg/m2 | |||||
| No. of cases/person-years | 1/6314 | 52/1 043 112 | 62/860 936 | 28/289 942 | — |
| Age-adjusted HR (95% CI) | 1.21 (0.16 to 8.80) | 1 (Referent) | 1.53 (1.06 to 2.22) | 2.28 (1.43 to 3.64) | 1.37 (1.16 to 1.60) |
| Multivariable adjusted HR (95% CI)b | 1.13 (0.16 to 8.18) | 1 (Referent) | 1.46 (0.99 to 2.14) | 2.15 (1.33 to 3.47) | 1.35 (1.14 to 1.59) |
| BMI, age at study enrollment | |||||
| No. of cases/person-years | 0/13 942 | 36/746 192 | 57/944 652 | 50/505 518 | — |
| Age-adjusted HR (95% CI) | — | 1 (Referent) | 1.27 (0.83 to 1.92) | 2.19 (1.42 to 3.37) | 1.24 (1.06 to 1.44) |
| Multivariable adjusted HR (95% CI)b | — | 1 (Referent) | 1.24 (0.81 to 1.90) | 2.20 (1.41 to 3.43) | 1.24 (1.06 to 1.45) |
| Average BMI across the adult life course (age 20 y to study enrollment) | |||||
| No. of cases/person-years | 1/16 905 | 72/1 317 070 | 55/730 876 | 15/145 454 | — |
| Age-adjusted HR (95% CI) | 1.06 (0.14 to 7.62) | 1 (Referent) | 1.42 (1.00 to 2.03) | 2.10 (1.20 to 3.69) | 1.40 (1.13 to 1.73) |
| Multivariable adjusted HR (95% CI)b | 1.01 (0.14 to 7.31) | 1 (Referent) | 1.38 (0.96 to 1.98) | 2.01 (1.14 to 3.54) | 1.37 (1.09 to 1.71) |
Models were adjusted for age, sex, center, race and ethnicity, randomization arm, education, job status, aspirin use longer than 12 months, smoking status, and family history of liver cancer. BMI = body mass index; CI = confidence interval; HR = hazard ratio; PLCO = Prostate, Lung, Colorectal and Ovarian.
Models were adjusted for age, sex, center, race and ethnicity, randomization arm, education, job status, aspirin use longer than 12 months, smoking status, and family history of biliary tract cancer.
The tests were conducted using BMI as a continuous variable.
We observed an increased risk of BTC among participants who were obese at age 20 years (HR = 1.99, 95% CI = 1.16 to 3.39) or at age 50 years (HR = 1.67, 95% CI = 1.06 to 2.61) compared with those who were never overweight. For liver cancer, we observed an increased risk among participants who were overweight at age 20 years (HR = 2.03, 95% CI = 1.26 to 3.28) and age 50 years (HR = 1.63, 95% CI = 1.06 to 2.49) (Table 3). These associations were in the same direction, albeit not statistically significant after further adjusting for diabetes mellitus, hypertension, diverticulitis, liver cirrhosis, gallstones, inflammation, HEI-2010 score, and alcohol drinking. The hazard ratios of liver cancer for BMI first exceeded 25 kg/m2 were 1.30 (95% CI = 0.69 to 2.46) at age 20 years and 1.01 (95% CI = 0.58 to 1.76) at age 50 years. The hazard ratios of BTC for BMI first exceeded 25 kg/m2 were 1.96 (95% CI = 0.98 to 3.94) at age 20 years and 1.68 (95% CI = 0.95 to 3.00) at age 50 years (Supplementary Table 3, available online). Likewise, results did not essentially change after further exclusion of participants diagnosed with liver cancer or BTC within 3 years after baseline survey (data not shown).
Table 3.
Hazard ratios and 95% confidence intervals for incident liver and biliary tract cancers according to timing of being first overweight in the PLCO Cancer Screening Trial
| Time when BMI first exceeded 25 kg/m2 | Liver cancer |
Biliary tract cancer |
||
|---|---|---|---|---|
| No. of cases/person-years | HR (95% CI)a | No. of cases/person-years | HR (95% CI)b | |
| Never | 32/545 121 | 1 (Referent) | 30/545 121 | 1 (Referent) |
| By age 20 y | 41/262 785 | 2.03 (1.26 to 3.28) | 29/262 785 | 1.99 (1.16 to 3.39) |
| By age 50 y | 74/679 922 | 1.63 (1.06 to 2.49) | 62/679 922 | 1.67 (1.06 to 2.61) |
| By age at study enrollment | 23/290 756 | 1.34 (0.78 to 2.29) | 22/290 756 | 1.30 (0.74 to 2.26) |
Models were adjusted for age, sex, center, race and ethnicity, randomization arm, education, job status, aspirin use longer than 12 months, smoking status, and family history of liver cancer. BMI = body mass index; CI = confidence interval; HR = hazard ratio; PLCO = Prostate, Lung, Colorectal and Ovarian.
Models were adjusted for age, sex, center, race and ethnicity, randomization arm, education, job status, aspirin use longer than 12 months, smoking status, and family history of biliary tract cancer.
Associations of BMI Trajectories With Liver Cancer and BTC
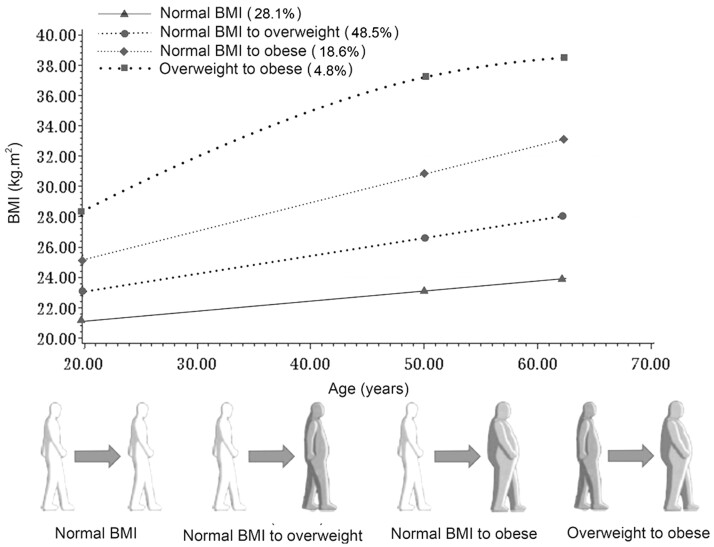
We identified 4 distinct trajectories of body adiposity from age 20 years up to study enrollment: 28.1% participants had the “stable normal” trajectory, where they maintained normal BMI throughout; 48.5% individuals had the “normal to overweight” trajectory, where they progressed from a normal BMI to overweight; 18.6% participants had the “normal to obese” trajectory, where they started lean and became obese; the remaining (4.8%) had the “overweight to obese” trajectory, where they started overweight and became obese (Figure 2).
Figure 2.
Trajectories of adulthood body mass index among participants in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Each trajectory was modeled using quadratic polynomials. BMI = body mass index.
Compared with the “stable normal” group, the participants in the other groups showed an elevated risk of developing liver cancer, with the hazard ratio of 2.50 (95% CI = 1.55 to 4.04) in the normal to obese group. A similar pattern was observed for BTC (Table 4). The adjusted hazard ratios of incident BTC were 1.75 (95% CI = 1.09 to 2.79) for the normal to overweight group, 4.26 (95% CI = 2.16 to 8.41) for the overweight to obese group, and 1.83 (95% CI = 1.03 to 3.22) for the normal to obese group. Overall, the BMI trajectories that resulted in obesity increased the risk of both liver cancer and BTC.
Table 4.
Hazard ratios and 95% confidence intervals for incident liver and biliary tract cancers according to BMI trajectories across adulthood in the PLCO Cancer Screening Trial
| BMI trajectories | Liver cancer |
Biliary tract cancer |
||
|---|---|---|---|---|
| No. of cases/person-years | HR (95% CI)a | No. of cases/person-years | HR (95% CI)b | |
| Stable normal | 28/600 919 | 1 (Referent) | 24/600 919 | 1 (Referent) |
| Normal to overweight | 83/1 116 850 | 1.33 (0.86 to 2.05) | 78/1 116 850 | 1.75 (1.09 to 2.79) |
| Overweight to obese | 8/95 321 | 1.99 (0.89 to 4.44) | 14/95 321 | 4.26 (2.16 to 8.41) |
| Normal to obese | 51/397 214 | 2.50 (1.55 to 4.04) | 27/397 214 | 1.83 (1.03 to 3.22) |
Models were adjusted for age, sex, center, race and ethnicity, randomization arm, education, job status, aspirin use longer than 12 months, smoking status, and family history of liver cancer. BMI = body mass index; CI = confidence interval; HR = hazard ratio; PLCO = Prostate, Lung, Colorectal and Ovarian. Note: underweight, BMI < 18.5 kg/m2; normal weight, BMI = 18.5 to <25.0 kg/m2; overweight, BMI = 25 to <30 kg/m2; obese BMI ≥ 30 kg/m2.
Models were adjusted for age, sex, center, race and ethnicity, randomization arm, education, job status, aspirin use longer than 12 months, smoking status, and family history of biliary tract cancer.
Associations of Weight Changes With Liver Cancer and BTC
After adjusting for BMI at age 20 years and other confounding factors, both liver cancer and BTC risk statistically significantly increased progressively with weight gain from 20 years to 50 years of age (both Ptrend < .05; Table 5). Compared with adults with stable weight (±5 kg), participants with weight gain of 20 kg and more between ages 20 years and 50 years had elevated hazard ratios of 2.24 (95% CI = 1.40 to 3.58) for liver cancer and 1.86 (95% CI = 1.12 to 3.09) for BTC. We did not observe any statistically significant associations of weight gain from age 50 years to the study enrollment with either liver cancer or BTC (data not shown). Results were slightly attenuated when we additionally adjusted for HEI-2010 score, diabetes mellitus, hypertension, diverticulitis, liver cirrhosis, gallstones, inflammation, and alcohol (Supplementary Table 4, available online) or excluded participants diagnosed with liver cancer or BTC within 3 years after baseline survey (Supplementary Table 5, available online).
Table 5.
Hazard ratios and 95% confidence intervals for incident liver and biliary tract cancers according to weight change during early to mid-adulthood (20-50 years of age) in the PLCO Cancer Screening Trial
| Model | Loss | Stable | Gain | Gain | Gain | P trend d |
|---|---|---|---|---|---|---|
| ≥ −5 kg | −5 to <5 kg | 5 to <10 kg | 10 to <20 kg | ≥ 20 kg | ||
| Liver cancer | ||||||
| No. of cases/person-years | 2/51 988 | 36/390 233 | 31/400 507 | 63/715 883 | 38/651 693 | — |
| Median (IQR) | −9.0 (−13.6 to −6.8) | 2.2 (0.0 to 4.5) | 7.7 (6.8 to 9.1) | 13.6 (11.3 to 15.8) | 24.9 (22.7 to 31.7) | — |
| Age-adjusted HR (95% CI) | 0.75 (0.18 to 3.13) | 1 (Referent) | 0.95 (0.59 to 1.55) | 1.71 (1.13 to 2.58) | 2.23 (1.40 to 3.54) | <.001 |
| MV, HR (95% CI)a | 0.60 (0.15 to 2.51) | 1 (Referent) | 0.96 (0.59 to 1.55) | 1.60 (1.06 to 2.43) | 1.99 (1.25 to 3.17) | .002 |
| MV+BMI at age 20 y, HR (95% CI)b | 0.39 (0.09 to 1.65) | 1 (Referent) | 1.02 (0.63 to 1.66) | 1.80 (1.19 to 2.73) | 2.24 (1.40 to 3.58) | <.001 |
| Biliary tract cancer | ||||||
| No. of cases/person-years | 1/51 988 | 34/390 233 | 38/400 507 | 40/715 883 | 30/651 693 | — |
| Median (IQR) | −9.0 (−13.6 to −6.8) | 2.2 (0.0 to 4.5) | 7.7 (6.8–9.1) | 13.6 (11.3 to 15.8) | 24.9 (22.7 to 31.7) | — |
| Age-adjusted HR (95% CI) | 0.39 (0.05 to 2.90) | 1 (Referent) | 1.24 (0.78 to 1.97) | 1.15 (0.72 to 1.81) | 1.86 (1.13 to 3.06) | .005 |
| MV, HR (95% CI)c | 0.37 (0.05 to 2.67) | 1 (Referent) | 1.23 (0.78 to 1.96) | 1.12 (0.71 to 1.78) | 1.74 (1.05 to 2.89) | .01 |
| MV+BMI at age 20 y, HR (95% CI)b | 0.26 (0.03 to 1.92) | 1 (Referent) | 1.29 (0.81 to 2.05) | 1.21 (0.76 to 1.92) | 1.86 (1.12 to 3.09) | .004 |
Models were adjusted for age, sex, center, race and ethnicity, randomization arm, education, job status, aspirin use longer than 12 months, smoking status, and family history of liver cancer. BMI = body mass index; CI = confidence interval; HR = hazard ratio; IQR = interquartile range; MV = multivariable model; PLCO = Prostate, Lung, Colorectal and Ovarian.
Models were further adjusted for the BMI at age 20 years.
Models were adjusted for age, sex, center, race and ethnicity, randomization arm, education, job status, aspirin use longer than 12 months, smoking status, and family history of biliary tract cancer.
The trend tests were conducted using BMI as a continuous variable.
Discussion
In this prospective cohort study of 138 922 US adults, we found that higher BMI at various ages across adulthood and BMI trajectories that resulted in being overweight and/or obese were associated with an increased risk of both liver cancer and BTC. Participants with weight gain during adulthood had higher risks of subsequently developing liver cancer and BTC. In addition, individuals who reported being overweight at age 20 years had a doubled risk of both diseases. Our findings on liver cancer and BTC were consistent with the prospective cohort studies (10,11,13) that examined adiposity at an earlier age and across the life course.
Excess adult adiposity, which is measured by BMI, is a well-established risk factor for various cancers, including liver cancer (7). Our findings regarding BMI at age 20 years were consistent with previous studies (10,11). In a hospital-based case-control study with 622 HCC cases and 660 sex- and age-matched cancer-free and genetically unrelated family members, obesity (BMI > 30 kg/m2) before the mid-20s and during the mid-20s to mid-40s was statistically significantly associated with 150% and 190% increased risk of HCC, respectively (11). In the National Institutes of Health‐American Association of Retired Persons (NIH-AARP) cohort study, in which 372 HCCs developed after a mean follow-up of 11.9 years, BMI greater than 30 kg/m2 at ages 18 years and 30 years showed 90% and 100% increased risk of developing HCC, respectively (10).
We found that being overweight at ages 20 years or 50 years was associated with higher risks of both liver cancer and BTC, which was in line with the results of the NIH-AARP study (10). Although we do not know the exact age at which adiposity begins to influence cancer development, these findings indicate that maintaining a healthy weight, especially in young adulthood, could protect against development of these cancers. We identified 4 distinct trajectory changes in BMI and found that BMI trajectories that result in obesity were associated with approximately 90% increased liver cancer risk. Similarly, findings from the NIH-AARP study identified 5 distinct trajectories using latent-class group-based trajectory modeling and showed that BMI trajectories that resulted in obesity were associated with 70%-80% higher HCC risk (10).
In most observational studies, BMI was based on a single measurement, with the assumption that a single “one-off” measure is an approximation for long-term exposure; a group-based trajectory modeling approach has its advantages. First, it improves the assessment of etiological associations by phenotyping particular high-risk subpopulations. Second, BMI trajectory modeling offers a public health strategy to identify early divergent adverse trajectories as potential intervention targets. Third, the trajectory approach allows a better understanding of the causes of between-individual variation in certain features (eg, weight variation over age) by analyzing the trajectory as an exposure rather than an outcome (26,27).
Compared with BMI at one time point, adult weight gain is more likely to reflect the accumulation of adiposity (15). We found that weight gain during early to mid-20s to 50s of age was associated with higher risk of both liver cancer and BTC, independent of BMI at age 20 years. However, we did not find statistically significant associations with weight gain from 50 years to age at study enrollment (mean age = 62.6 years). These results, if confirmed by future studies, underscore the importance of maintaining a stable adult weight, especially during early-to-mid adulthood.
In this study, we also examined the risk of BTC, which has not, to our knowledge, been previously reported. Previous studies, however, have investigated adult BMI and other anthropometric parameters (eg, waist circumference and waist-to-hip ratio) at a single time point in relation to total BTC (13,16) and gallbladder cancer (28). In general, BTC showed patterns similar to those of liver cancer when examining weight changes and BMI trajectory changes across adulthood. Our analyses showed that BMI trajectories that resulted in overweight and obesity were both associated with higher risk of BTC. This finding contrasted slightly from the finding regarding liver cancer.
The exact mechanisms linking obesity and liver cancer or BTC development remain unclear. However, adiposity greatly increases the risk of nonalcoholic fatty liver disease (15,29), which can cause inflammation and hepatic damage and predispose to liver cancer (30). In addition, adiposity is associated with low-grade chronic inflammation and insulin resistance, which play an important role in the occurrence and progression of many types of cancer, including liver cancer and BTC (31-33).
Strengths of this study include a large prospective cohort design, relatively long-term follow-up, validated cancer outcomes, and adjustments for a wide range of risk factors. In addition, using a group-based trajectory approach may better characterize the association between evolution of adiposity across time. Our study has several limitations. First, BMI was based on self-report and recalled height and weight; we thus cannot rule out measurement error. Second, our study lacked other body size indicators, including waist and hip circumferences. Anthropometric indices based on these measurements may better reflect abdominal obesity, which could be a risk factor for liver cancer independent of BMI (16). In addition, we did not account for chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections status, given the lack of this variable. Third, this study had limited cancer cases, which also precluded subgroup or interaction analyses according to other risk factors, such as alcohol, coffee, HBV, and HCV.
In conclusion, our results suggest that being overweight or obese at age 20 years and BMI trajectories after age 20 years that result in obesity and/or being overweight may increase the risk of both liver cancer and BTC. Future studies with continuously collected body size indicators (eg, weight, height, and waist and hip circumferences via electronic medical record) are needed to comprehensively characterize the role of adiposity at various ages for hepatobiliary cancer development.
Funding
PLCO was funded by NIH/NCI. Dr Sudenga is supported by NIH K07 CA225404 and Dr Zhang is supported by NIH R37CA262299, the Dana-Farber Harvard Cancer Center, as well as the Zhu Family Center at Harvard Chan School.
Notes
Role of the funder: The study sponsor approved submission of the manuscript but did not have a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: The authors have no potential conflicts of interest to disclose.
Author contributions: WYS, XFZ: Data Curation, Formal Analysis, Writing—Original Draft Preparation, Writing—Review and Editing. JLP, CJD, AAF, BL, HMN, JTM, LW, HMZ, PTC, EG, KAM: Writing—Review and Editing. XHZ: Conceptualization, Methodology, Data curation, Formal Analysis, Supervision, Writing—Review and Editing.
Acknowledgements: The authors thank the National Cancer Institute for access to NCI’s data collected by the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial.
Disclaimers: The interpretation and reporting of these data are the sole responsibility of the authors. The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by NCI.
Supplementary Material
Contributor Information
Wanshui Yang, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; Department of Nutrition, School of Public Health, Anhui Medical University, Hefei, Anhui, P.R. China.
Xufen Zeng, Department of Nutrition, School of Public Health, Anhui Medical University, Hefei, Anhui, P.R. China.
Jessica L Petrick, Slone Epidemiology Center, Boston University, Boston, MA, USA.
Christopher J Danford, Division of Gastroenterology and Hepatology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Andrea A Florio, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Bing Lu, Division of Rheumatology, Inflammation and Immunity, Department of Medicine, Brigham and Women’s Hospital, Boston, MA, USA.
Hongmei Nan, Department of Epidemiology, Richard M Fairbanks School of Public Health, Indiana University, and Indiana University Melvin and Bren Simon Cancer Center, Indianapolis, IN, USA.
Jiantao Ma, Framingham Heart Study, Framingham, MA, USA; Division of Nutrition Data Science, Tufts University Friedman School of Nutrition Science and Policy, Boston, MA, USA.
Liang Wang, Department of Public Health, Robbins College of Health and Human Sciences, Baylor University, TX, USA.
Hongmei Zeng, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Staci L Sudenga, Division of Epidemiology, Vanderbilt University Medical Center, Nashville, TN, USA.
Peter T Campbell, Department of Epidemiology and Population Science, Albert Einstein College of Medicine, Bronx, NY, USA.
Edward Giovannucci, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Katherine A McGlynn, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Xuehong Zhang, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Data Availability
The datasets presented in this article are not readily available because the data that support the findings of this study are available from NIH PLCO study group. Restrictions apply to the availability of these data, which were used under license for this study. Requests to access the datasets should be directed to https://biometry.nci.nih.gov/cdas/datasets/plco/.
References
- 1. De Groen PC, Gores GJ, LaRusso NF, et al. Biliary tract cancers. N Engl J Med. 1999;341(18):1368-1378. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7-30. [DOI] [PubMed] [Google Scholar]
- 3. Petrick JL, Florio AA, Znaor A, et al. International trends in hepatocellular carcinoma incidence, 1978-2012. Int J Cancer. 2020;147(2):317-330. doi: 10.1002/ijc.32723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Dyke AL, Shiels MS, Jones GS, et al. Biliary tract cancer incidence and trends in the United States by demographic group, 1999-2013. Cancer. 2019;125(9):1489-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307(5):483-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491-497. [DOI] [PubMed] [Google Scholar]
- 7. World Cancer Research Fund International/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and liver cancer. 2018. http://wcrf.org/sites/default/files/Liver-Cancer-2015-Report.pdf.
- 8. Campbell PT, Newton CC, Freedman ND, et al. Body mass index, waist circumference, diabetes, and risk of liver cancer for U.S. adults. Cancer Res. 2016;76(20):6076-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122(11):1757-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang B, Petrick JL, Kelly SP, et al. Adiposity across the adult life course and incidence of primary liver cancer: the NIH-AARP cohort. Int J Cancer. 2017;141(2):271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hassan MM, Abdel-Wahab R, Kaseb A, et al. Obesity early in adulthood increases risk but does not affect outcomes of hepatocellular carcinoma. Gastroenterology. 2015;149(1):119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simon TG, Kim MN, Luo X, et al. Adiposity, adulthood weight change, and risk of incident hepatocellular carcinoma. Cancer Prev Res (Phila). 2021;14(10):945-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jackson SS, Van Dyke AL, Zhu B, et al. Anthropometric risk factors for cancers of the biliary tract in the biliary tract cancers pooling project. Cancer Res. 2019;79(15):3973-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Preston SH, Mehta NK, Stokes A.. Modeling obesity histories in cohort analyses of health and mortality. Epidemiology. 2013;24(1):158-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Cancer Research Fund International/American Institute for Cancer Research. Body fatness and weight gain and the risk of cancer. 2018. https://www.wcrf.org/sites/default/files/Body-fatness-and-weight-gain_0.pdf. https://www.wcrf.org/sites/default/files/Body-fatness-and-weight-gain_0.pdf. Accessed March 11, 2021.
- 16. Schlesinger S, Aleksandrova K, Pischon T, et al. Abdominal obesity, weight gain during adulthood and risk of liver and biliary tract cancer in a European cohort. Int J Cancer. 2013;132(3):645-657. [DOI] [PubMed] [Google Scholar]
- 17. Luo J, Hendryx M, Manson JE, et al. Intentional weight loss and obesity-related cancer risk. JNCI Cancer Spectr. 2019;3(4):pkz054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prorok PC, Andriole GL, Bresalier RS, et al. ; Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Project Team. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(suppl 6):273S-309S. [DOI] [PubMed] [Google Scholar]
- 19. Zheng Y, Manson JE, Yuan C, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA. 2017;318(3):255-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eliassen AH, Colditz GA, Rosner B, et al. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296(2):193-201. [DOI] [PubMed] [Google Scholar]
- 21. Jones BL, Nagin DS, Roeder K.. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29(3):374-393. [Google Scholar]
- 22. Jones BL, Nagin DS.. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res. 2007;35(4):542-571. [Google Scholar]
- 23. Kelly SP, Graubard BI, Andreotti G, et al. Prediagnostic body mass index trajectories in relation to prostate cancer incidence and mortality in the PLCO Cancer Screening Trial. J Natl Cancer Inst. 2017;109(3):djw225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zheng R, Du M, Zhang B, et al. Body mass index (BMI) trajectories and risk of colorectal cancer in the PLCO cohort. Br J Cancer. 2018;119(1):130-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Song M, Willett WC, Hu FB, et al. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer. 2016;138(10):2383-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lennon H, Kelly S, Sperrin M, et al. Framework to construct and interpret latent class trajectory modelling. BMJ Open. 2018;8(7):e020683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qian T, Masino AJ.. Latent patient cluster discovery for robust future forecasting and new-patient generalization. PLoS One. 2016;11(9):e0162812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Campbell PT, Newton CC, Kitahara CM, et al. Body size indicators and risk of gallbladder cancer: pooled analysis of individual-level data from 19 prospective cohort studies. Cancer Epidemiol Biomarkers Prev. 2017;26(4):597-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khan FZ, Perumpail RB, Wong RJ, et al. Advances in hepatocellular carcinoma: nonalcoholic steatohepatitis-related hepatocellular carcinoma. World J Hepatol. 2015;7(18):2155-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Starley BQ, Calcagno CJ, Harrison SA.. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51(5):1820-1832. [DOI] [PubMed] [Google Scholar]
- 31. Van de Velde F, Bekaert M, Geerts A, et al. Insulin resistance associates with hepatic lobular inflammation in subjects with obesity. Endocr Connect. 2019;8(9):1294-1301. doi: 10.1530/EC-19-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dietrich P, Hellerbrand C.. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28(4):637-653. [DOI] [PubMed] [Google Scholar]
- 33. Sun B, Karin M.. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56(3):704-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this article are not readily available because the data that support the findings of this study are available from NIH PLCO study group. Restrictions apply to the availability of these data, which were used under license for this study. Requests to access the datasets should be directed to https://biometry.nci.nih.gov/cdas/datasets/plco/.