Abstract
The interaction between tumor cells and non-malignant hosts cells within the tumor microenvironment (TME) is critical to the pathophysiology of cancer. These non-malignant host cells, consisting of a variety of stromal, immune, and endothelial cells, engage in a complex bidirectional crosstalk with the malignant tumor cells. Mesenchymal stem/stromal cells (MSCs) are one of these host cells, and they play a critical role in directing the formation and function of the entire TME. These MSCs are epigenetically reprogrammed by cancer cells to assume a strongly pro-tumorigenic phenotype and are referred to as carcinoma-associated mesenchymal stem/stromal cells (CA-MSCs). Studies over the last decade demonstrate that CA-MSCs not only directly interact with cancer cells to promote tumor growth and metastasis but also orchestrate the formation of the TME. Carcinoma-associated mesenchymal stem/stromal cells can differentiate into virtually all stromal sub-lineages present in the TME, including pro-tumorigenic cancer-associated fibroblasts (CAF), myofibroblasts, and adipocytes. carcinoma-associated mesenchymal stem/stromal cells and the CAFs they produce, secrete much of the extracellular matrix in the TME. Furthermore, CA-MSC secreted factors promote angiogenesis, and recruit immunosuppressive myeloid cells effectively driving tumor immune exclusion. Thus CA-MSCs impact nearly every aspect of the TME. Despite their influence on cancer biology, as CA-MSCs represent a heterogenous population without a single definitive marker, significant confusion remains regarding the origin and proper identification CA-MSCs. This review will focus on the impact of CA-MSCs on cancer progression and metastasis and the ongoing work on CA-MSC identification, nomenclature and mechanism of action.
Keywords: mesenchymal stem cell, tumor microenvironment, metastatic niche, stroma
Grahical Abstract

Significance Statement.
This review focuses on the role of mesenchymal stem cells in the formation of the tumor microenvrionment. We highlight known tumor supportive functions as well as areas which require further research. Collectively, this provides a comprehensive state-of-the-science review of mesenchymal stem cells in cancer.
Introduction
The Tumor Microenvironment and Mesenchymal Stem Cells
Throughout the course of neoplasia, tumor cells interact extensively with the surrounding non-malignant cell populations within the tumor microenvironment (TME). Research from the past 2 plus decades clearly demonstrates that the TME is not a passive backdrop, but rather an active “organ” consisting of a complex network of stromal, immune, and endothelial cells that interact both directly and indirectly with the tumor to influence disease progression and clinical outcomes.1 The TME has been implicated in a wide variety of tumor supporting functions including mediating therapeutic resistance, escape from the host immune response and promotion of metastasis.1-3 In addition to the cellular populations, the TME contains non-cellular components such as the extracellular matrix (ECM) and soluble factors which are also critical to cancer biology.2
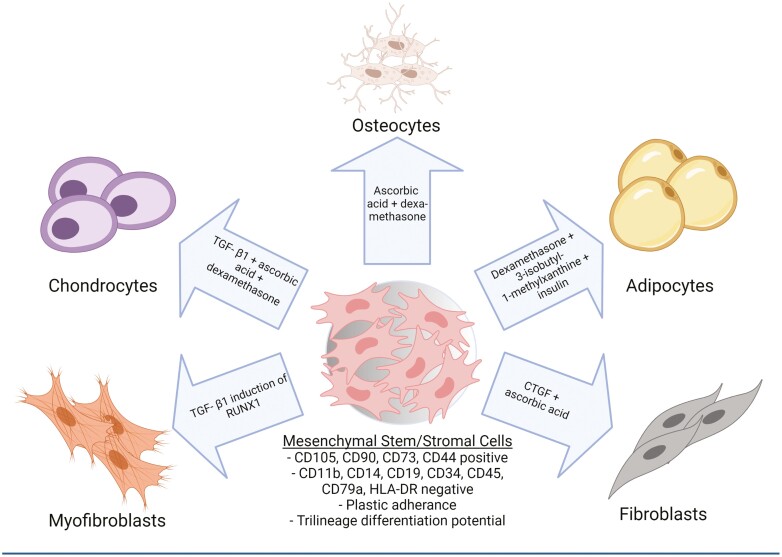
Mesenchymal stem/stromal cells (MSCs) are multipotent stromal progenitor cells that reside within the stromal TME and play an important role in formation and function of the tumor supportive TME. Mesenchymal stem/stromal cells were first identified in bone marrow but have since been shown to reside in almost all adult tissues including adipose, liver, kidney, lung, brain and ovary tissues, amongst others..4 These stromal progenitors possess the capacity to differentiate into most stromal lineages, including adipocytes, chondrocytes, osteoblasts, fibroblasts, and myofibroblasts.4,5 In healthy tissues, MSCs play a critical role in wound healing and immune modulation.4,6 Within the bone marrow niche, MSCs play an important role in hematopoietic stem cell maintenance and lineage determination via secreted factors as well as contributing to the architecture of the niche.7
In malignancy, MSCs make up a relatively small proportion of the TME with studies reporting they account for 0.01–5% of the total cellular population; however, this is highly disease and organ specific.8-10 Nonetheless, given the capacity for differentiation, MSCs can magnify their impact by directly altering the stromal composition of the TME and influencing the behavior of their progeny (Fig. 1). Indeed, MSCs play a fundamental role in tumor development and metastasis through direct interactions with tumor cells and through interactions with other TME components.11 Our group and others have shown that MSCs that reside within tumor tissue, compared with MSCs from healthy tissues, have a unique expression profile and are highly pro-tumorigenic.12,13 We have termed these tumor-associated MSCs as “carcinoma-associated mesenchymal stem cells” (CA-MSCs). Understanding the origin and functions of CA-MSCs is critical to understanding disease progression and represents a potential target for novel therapeutic interventions.
Figure 1.
Mesenchymal stem/stromal cells (MSCs) can differentiate into several lineages which make up the stromal tumor microenvironment (TME).
Origin of CA-MSCs
Functional Heterogeneity of MSC Populations Drive Contradictory Phenotypes
Much debate has occurred over the ability of MSCs to demonstrate both pro- and anti-tumorigenic phenotypes. The duality of MSCs in cancer is more thoroughly reviewed elsewhere.14,15 Mesenchymal stem/stromal cells represent a heterogenous population of cells with distinct expression profiles based on tissue of origin as well as sub-populations within tissues which show distinct differentiation potentials.16-18 Indeed, this heterogeneity likely contributes to the seemingly contradictory phenotypes observed. As shown below, critical to the development of a CA-MSC from naïve MSCs is both the MSC tissue source and tumor type.
Distinguishing CA-MSC from MSC
Understanding the origins of CA-MSCs and their mechanisms of action within the TME first requires the ability to properly identify and distinguish MSCs and CA-MSCs. Mesenchymal stem/stromal cells were originally identified as a second stem cell population (in addition to previously defined hematopoietic stem cells) within the bone marrow. Mesenchymal stem/stromal cells were defined by their ability to differentiate into osteocytes, adipocytes, and chondrocytes.19 Since their original isolation by Friedenstein in the 1970s, there has been much debate over the proper nomenclature, stemness, and identification of MSCs.20 The International Society for Cellular Therapy (ISCT) published minimal criteria for MSC identification in 2006 based on 3 tenants: (1) plastic adherence, (2) expression or lack of expression of multiple surface antigens, and (3) trilineage differentiation into adipocytes, osteoblasts, and chondroblasts.19 While the ISCT definition and its update published in 2019 has helped somewhat standardize the definition of MSCs; however, the lack of a single marker is one of the largest hinderances to elucidating the role of MSCs in biological processes, including cancer.21
Numerous studies have shown that CA-MSCs have a distinct RNA expression profile;13,22-24 however, using RNA-sequencing to distinguish CA-MSCs from MSCs or other stromal components is impractical and costly. Carcinoma-associated mesenchymal stem/stromal cells fit the same ISCT criteria for MSCs, making delineation based on these criteria impossible. Additionally, the CA-MSC phenotype does not arise through the acquisition of genetic mutations thus recurrent mutations cannot be used to distinguish CA-MSCs from MSCs.9 Further, MSCs demonstrate significant heterogeneity based on their tissue source.25 While several newly identified candidate markers have been proposed for better identification of various tissue-derived MSC subpopulations, there still remains a critical lack of markers for CA-MSC identification.26 Given the large degree of heterogeneity, the likelihood of identifying a single marker specific to CA-MSCs may be impossible.
In order to address this major hurdle, Coffman et al recently developed a novel algorithm based on transcriptional differences between CA-MSCs and normal MSCs. Transcriptional profiling of MSCs isolated from ovarian cancer and normal ovarian and omental tissue was used to create a unique algorithm which can accurately distinguish CA-MSC from MSC, as well as CA-MSC/MSC from other TME components such as CAFs.13 This algorithm is able to correctly classify pancreatic and endometrial cancer CA-MSCs, suggesting potentially broader applicability.13 Classifier scores and the tumor supportive phenotype were observed in CA-MSCs after multiple passages (cells were used up to passage 8) removed from the influence of the tumor, suggesting that the CA-MSC phenotype may be stable. This is consistent with the subsequent finding that epigenetic reprogramming drives the formation of a CA-MSC, leading to a durable phenotype.9
Carcinoma-Associated Mesenchymal Stem/Stromal Cell Origins: Local reprogrammed or Recruited Populations?
While the influence of CA-MSCs on the tumor and TME has been established, the fundamental question regarding the origin of these tumor supporting cells is less clear. The first reports of CA-MSC populations focused on naïve MSCs recruited toward the tumor site.6,27 Mesenchymal stem/stromal cells are highly migratory and display a remarkable tropism towards sites of inflammation, representative of their traditionally ascribed role in wound healing.4,6 Tumors have been long thought of as “wounds that do not heal” and secrete numerous chemokines that can recruit MSCs, such as CC-chemokine ligand 2 (CCL2), CCL5, CXCL12, and CXCL16.27-30 Using both syngeneic and xenograft models of breast cancer, systemically delivered luciferase tagged MSCs were found to colocalize at tumor sites.6 Similar colocalization was observed in a xenograft mouse model of human glioma as well as melanoma.31,32
While the migratory MSC population cannot be discounted, emerging evidence suggests that resident MSCs are the more likely source of CA-MSCs (Fig. 2). Tissue resident MSCs are reprogrammed early on during tumorigenesis by interactions with both the tumor cells and the local environment, giving rise to CA-MSCs which help promote tumor growth as well as develop the mature TME (see below).13,22,33 Given that MSCs inhabit most of the tissues within the body and given their local proximity to the developing tumor, these MSC populations most likely represent the first populations of CA-MSCs to occupy the TME and support early tumor development.13 Later recruitment of migratory MSCs is likely, as the developing TME becomes more inflammatory.34
Figure 2.
Carcinoma-associated mesenchymal stem cells (CA-MSCs) are reprogrammed from naïve MSCs and have several tumor supporting functions.
The mechanisms that drive the development of the CA-MSC phenotype have largely remained elusive. Initial studies focused on bone marrow-derived MSCs identified several paracrine signaling mechanisms that promote the pro-tumorigenic CA-MSC phenotype. Naïve bone marrow mesenchymal stem cells (BM-MSCs) from a healthy donor cultured in tumor cell conditioned media or patient-derived ascites demonstrated increased expression of genes associated with tumor growth, metastasis and angiogenesis and functionally increased tumor cell resistance to carboplatin.24 The secretome from the invasive breast cancer cell line MDA-WT was shown to induce global changes in the transcriptome of naïve BM-MSC, resulting in changes to pathways related to immune modulation and angiogenesis and these tumor conditioned MSCs demonstrated enhance macrophage activation.35 In a lymphoma model, TNFα stimulation was also shown to induce a CA-MSC phenotype in naïve BM-MSCs.33
Interestingly, while paracrine signaling is important to MSC reprogramming, it may only partially drive the conversion to a stable CA-MSC phenotype. Using the CA-MSC classifier, we demonstrated that ovary and omental-derived MSCs indirectly cocultured with ovarian tumor cells only resulted in a partial conversion to a CA-MSC.13 The combination of direct tumor cell and MSC culture under hypoxic conditions was necessary for full CA-MSC conversion.13 Hypoxia-related pathways have also been implicated in studies of CA-MSC formation in hepatocellular carcinoma.36 Thus, indirect and direct tumor stimulation in the setting of critical environmental factors such as hypoxia appear to drive the conversion of MSCs to CA-MSCs.
There is also some evidence suggesting CA-MSCs themselves can convert naïve MSCs into tumor-promoting CA-MSCs. Local resident lymphoma CA-MSCs were found to reprogram naïve BM-MSCs to adopt a CA-MSC phenotype independent of tumor cells. This appears to be driven by direct contact, but soluble factors were shown to partially induce naïve MSCs to develop CCL-2 expression characteristic of CA-MSCs.22 However, contextually this work explored the ability of this mechanism to promote tumor growth via CCR2-mediated macrophage recruitment and further exploration of this mechanism is required.
It is important to note that the conversion of an MSC to a CA-MSC appears to be disease and tissue source dependent. Ovarian cancer is capable of converting normal MSCs derived from the omentum or ovary into cancer supportive CA-MSCs; however, BM-MSCs are resistant to this conversion while breast cancer cells readily convert BM-MSCs into breast cancer supportive MSCs.13 Consistent with this, ovarian cancer almost universally metastasizes to the omentum, but rarely metastasizes to the bone while breast cancer commonly spreads to the bone. Thus, the propensity of a given tumor type to metastasize to specific organs may be reflective of the ability of the cancer to reprogram the local tissue MSC. This also highlights the need to understand the tissue source and level of cancer education of the MSCs used in each experimental system to accurately interpret results and clinical applicability.
Pro-tumorigenic Functions of CA-MSCs
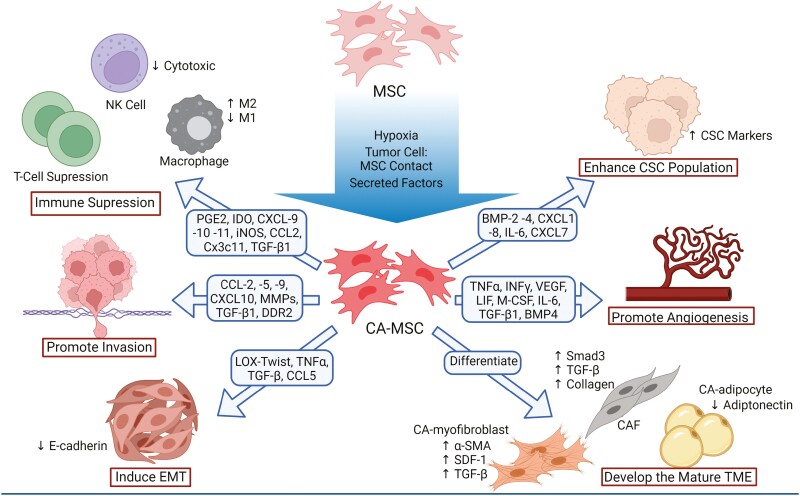
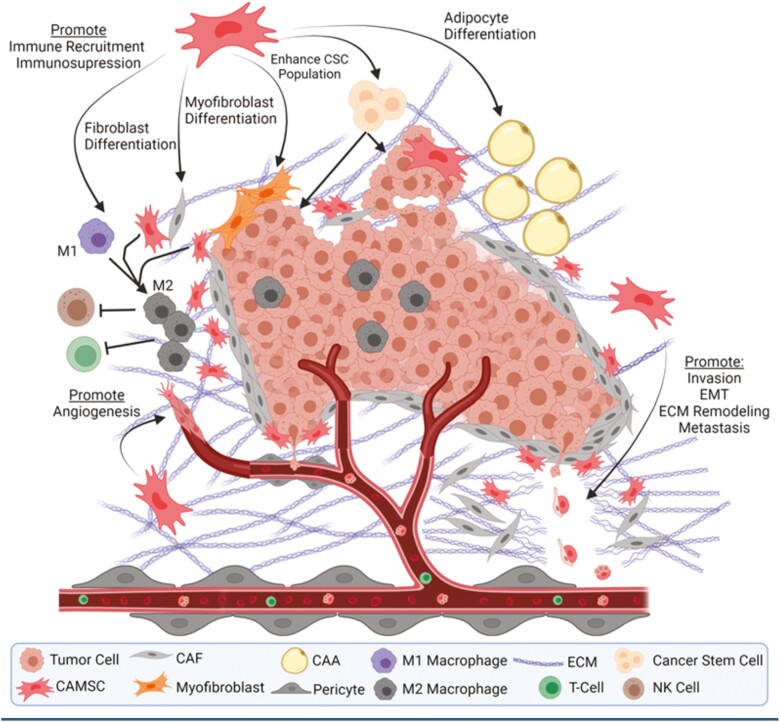
Carcinoma-associated mesenchymal stem/stromal cells have been implicated in a variety of tumor supporting functions across numerous cancer types including breast, ovarian, gastric, colon, prostate, pancreatic, and head and neck cancers as well as lymphoma and glioma.37 Once reprogrammed, CA-MSCs can support tumor progression through several mechanisms, such as aiding in the formation of the TME through differentiation into other tumor supporting stroma, immunosuppression, promotion of angiogenesis, and enhancing invasion and metastasis (Fig. 3).33,38-46
Figure 3.
CA-MSCs function within and form the mature TME through differentiation and direct interaction.
Carcinoma-Associated Mesenchymal Stem/Stromal Cells Help Form the Mature TME Through Differentiation
The field is continuing to improve methods of tracking MSC differentiation (during benign and malignant processes) including lineage tracing, fluorescent labeling, and single-cell sequencing thus providing addition evidence for in vivo multilineage stromal differentiation.47 Using these techniques, CA-MSCs have been shown to differentiate into multiple stromal lineages which can help form the mature stromal TME.9 Carcinoma-associated mesenchymal stem/stromal cells can differentiate into other tumor supporting cells, such as carcinoma-associated fibroblasts (CAFs), myofibroblasts and adipocytes (cancer associated adipocytes [CAAs]).38-41,48 Using single-cell sequencing, Miyazaki et al. demonstrate adipose MSCs differentiate into CAFs in a pancreatic cancer xenograft model.40
Carcinoma-associated fibroblasts are the most abundant cell type found in the stromal TME and promote tumor progression thus a CA-MSC source of CAFs within the TME amplifies the tumor-promoting impact of CA-MSCs. Carcinoma-associated mesenchymal stem/stromal cells derived from lung tumor tissue were shown to have higher expression of the CAF markers α-SMA, HI-1α, MMP11, VEGF, CXCL12, TGF-β1, TGF-βRII, IL6, and TNFα when compared with paired healthy lung tissue MSCs.49 Co-transplantation of human adipose-derived MSCs and the human pancreatic cancer cell line Capan-1 into mice recapitulated the functional stromal TME, and subsequent single-cell RNA sequencing and histological analysis demonstrated the CAF population derived through differentiation of transplanted adipose mesenchymal stem cells (AD-MSCs).35 Additionally, exosomes secreted by ovarian and breast tumor cells trigger MSC differentiation into pro-angiogenic and pro-invasive myofibroblasts.38,39 BM-MSCs treated with breast cancer cell conditioned media differentiate into a myofibroblast-like CAF phenotype including SDF-1, α-SMA, and fibroblast surface protein (FSP) expression and functionally enhance tumor growth both in vitro and in vivo.41
Carcinoma-associated mesenchymal stem/stromal cells differentiation into other tumor-supporting lineages has also been shown. Treatment of BM-MSCs with conditioned media from PC-3 prostate cancer cells was shown to increase osteoblastic lineage commitment through upregulation of the pro-osteoblastic factors α5/β1 integrins, fibronectin, and osteoprotegerin when compared with control media or media from non-metastatic 22RV1 cells.50 Secretion of FGF-9 by PC-3 cells was identified as critical to driving this differentiation.50 CAAs function to support metabolic demands of tumor cells and can be derived from CA-MSCs. Exosomes isolated from MSC-derived adipocytes enhanced the proliferation and chemoresistance of breast cancer MCF7 cells, and the depletion of exosomes in vivo reduced tumor growth, possibly through activation of the Hippo signaling pathway.51
Immunosuppressive Properties of CA-MSCs
As a canonical function of their role in wound healing, MSCs engage in direct and indirect crosstalk with immune cells at the wound and suppress both the innate and adaptive immune responses.4 Several cytokines commonly secreted by tumors or the TME, such as tumor necrosis factor alpha (TNFα), IFNγ, and interleukin-1 (IL-1), induce CA-MSCs to secrete molecules involved in regulation of the innate and adaptive immune systems including prostaglandin E2 and indoleamine 2,3-dioxygenase chemokines CXCL-9, -10, and -11, as well as nitric oxide synthase, which leads to suppression of recruited T cells.42 Carcinoma-associated mesenchymal stem/stromal cells have been shown to affect the phenotype of and decrease the cytotoxic activity of recruited NK cells as well as inducing differentiation of myeloid-derived suppressor cells (MDSCs) into immunosuppressive M2-polarized macrophages.43,44 Carcinoma-associated mesenchymal stem/stromal cells can likewise directly induce M2 macrophage polarization in pancreatic cancer.52 Suppression of the active immune system can occur through induction of Tregs via release of TGFβ1, which was shown to protect breast cancer cells from immune clearance.53 Our recent work also demonstrates CA-MSCs modulate the immune response in ovarian cancer. We found that addition of CA-MSCs to an “immune hot” model of ovarian cancer completely eliminated the response to immune checkpoint inhibitor therapy. Mechanistically, this was related to CA-MSC expression of CCL2, CX3CL1, and TGFβ1, and recruitment of CCR2+ monocytes and M2 TAMs into the TME.54 This is consistent with previous findings that CA-MSC expression of CCL2 in lymphoma, melanoma, and breast cancer models recruit CCR2+ tumor-associated macrophages to the TME to promote tumor growth.33
Promotion of Angiogenesis
The formation of new vasculature, or angiogenesis, is a hallmark of cancer progression and is necessary to provide nutrients to the expanding tumor. Carcinoma-associated mesenchymal stem/stromal cells help govern this process through direct interactions and secretion of pro-angiogenic factors such as TNFα, VEGF, INFγ, LIF, and M-CSF.55,56 Secretion of IL-6 from CA-MSCs increases the secretion of endothelin-1 in tumor cells, resulting in the activation and recruitment of endothelial cells via Akt/ERK and promotion of angiogenesis.57 Additionally, TGFβ1 appears to be critical to CA-MSC-driven angiogenesis.45,58 Carcinoma-associated mesenchymal stem/stromal cells also induce angiogenesis via a paracrine signaling loop involving BMP4 and Hedgehog, as demonstrated in an ovarian cancer model.59 Carcinoma-associated mesenchymal stem/stromal cells, in response to tumor secreted lysophosphatidic acid, stimulate angiogenesis vis the expression of VEGF-A and SDF-1.60 While the ability of MSCs to undergo mesenchymal-to-endothelial transition and acquire an endothelial phenotype is controversial, a potential mechanism driven by TGFβ/JNK signaling and negatively regulated by p38α has been proposed .58]. Finally, as noted above, CA-MSCs recruit “M2” macrophages to the TME. As M2 macrophages are highly angiogenic, this also provides an indirect mechanism whereby CA-MSCs can promote angiogenesis.61
Promotion of Therapeutic Resistance
Resistance to chemotherapy remains a major barrier to the effective treatment of cancer. Carcinoma-associated mesenchymal stem/stromal cells have been shown to promote chemoresistance through several different mechanisms, including secretion/induction of anti-apoptotic and resistance promoting factors, promotion of quiescence in tumor cells, metabolic support of tumor cells and enrichment of the cancer stem cell (CSC) population.62-72
Both resistance driving juxtacrine and paracrine signaling mechanisms between CA-MSCs and tumor cells have been identified. Physical contact between breast cancer cells and adipose-derived MSCs was shown to modulate trastuzumab resistance by activation of nonreceptor tyrosine kinase c-Src, downregulation of PTEN, and activation of the PI3K/AKT pathway in vitro.63 Expression of the tetraspanins CD9 and CD81 by bone-marrow MSCs was found to promote resistance to doxorubicin and 5-fluorouracil in HCC1806 breast cancer cells, with MSC CD9 knockdown leading to decreased expression of drug resistance protein BCRP and serum cytokines CCL5, CCR5, and CXCR12.71 Ovarian tumor cells were shown to induce the secretion of the CXC chemokine receptors 1/2 ligands CXCL1, CXCL2, and IL-8 by CA-MSCs, which induce resistance to carboplatin.24 Carcinoma-associated mesenchymal stem/stromal cells also secrete BMP4 creating a BMP4/hedgehog positive feedback loop with ovarian cancer cells which increases cancer cell cisplatin resistance.59 Cisplatin pretreatment of adipose-derived MSCs was shown to induce IL-6 and IL-8 secretion and subsequent coculture with breast cancer cells led to increased chemoresistance in vivo, demonstrating therapy may have a direct effect on CA-MSC function.70 Carcinoma-associated mesenchymal stem/stromal cells/stromal IL-6 secretion has been shown in a variety of other neoplasias, including lung, liver, colorectal. and pancreatic cancers.73-77 Additionally, CA-MSC secretion of platelet-derived growth factor was also shown to induce chemoresistance and promote stemness.68
As traditional chemotherapy targets rapidly dividing cells, quiescence—induction of a reversible G0-G1 arrest—is an effective mechanism for chemotherapeutic resistance.72 Tumor cells escaping the primary site can be disseminated throughout the body through the blood or lymphatic circulatory system in a quiescent state.72 These quiescent disseminated tumor cells (DTCs) can lay dormant for decades, eventually leading to disease recurrence.72 Carcinoma-associated mesenchymal stem/stromal cells are capable of inducing quiescence in tumor cells, which has been best characterized in models of breast cancer.62,66,69,72 Periarteriolar NG2+/Nestin+ MSCs were depleted in a C57BL/6 NG2-CreER inducible diphtheria toxin receptor mouse model and subsequent intracardiac injection of E0771 tumor cells led to an accumulation of DTCs in depleted mice vs wild type, with accumulated tumor cells expressing significantly more Ki67 and decreased p27+, indicating reactivation of dormant DTCs.66 NG2+/Nestin+ MSCs were identified as the source of pro-dormancy factors TGF-β2 and BMP7, and conditional knockout of TGF-β2 in vivo resulted in awakening of dormant DTCs in the bone marrow.66 Dormancy may also be induced by MSC exosomes in a subset of tumor cells.62,69 BM-MSCs primed by breast cancer cells release exosomes containing the microRNAs miR-222/223 which were shown to promote quiescence and chemoresistance.62
Cancer cells undergo metabolic reprogramming which supports their growth and survival.67 Fatty acid oxidation (FAO) is required for cancer cell growth and survival, and recently CA-MSCs have been shown to promote chemoresistance and stemness in tumor cells through FAO in vitro and in vivo.65,67 TGF-β1 secretion by MSCs activated SMAD2/3 in gastric cancer cells and led to long non-coding RNA (lncRNA) MACC1-AS1 expression, which promoted FAO-dependent chemoresistance through antagonizing miR-145-5p.65 Another recent publication showed CA-MSCs enhance chemoresistance by supporting metabolic requirements of tumor cells and acting as an antioxidant defense to prevent apoptosis. Nestin+ BM-MSCs were found to increase oxidative phosphorylation, ATP production, and TCA activity in acute myeloid leukemia (AML) cells.64 Interestingly, the transfer of mitochondria from BM-MSC to tumor cell was observed, possibly identifying the source of this increased energy production. Chemotherapy generates reactive oxygen species (ROS) which lead to cell damage and death; BM-MSCs were observed to protect AML cells by reducing ROS and lipid peroxidation through glutathione-mediated antioxidant defense.64
Carcinoma-associated mesenchymal stem/stromal cell enhancement of the CSC population is also a mechanism by which CA-MSCs can promote chemoresistance. Carcinoma-associated mesenchymal stem/stromal cell:CSC niche interactions are discussed later in this review.
Carcinoma-Associated Mesenchymal Stem/Stromal Cells and Metastasis
Carcinoma-Associated Mesenchymal Stem/Stromal Cells Enhance Tumor Cell Invasion
Metastasis is a highly complex cascade of events that is responsible for the majority of cancer-associated mortalities. Metastasis can be simplified into 2 major phases: (1) detachment and translocation of tumor cells from the primary site into the vasculature and (2) invasion and colonization at a distant site.78 Carcinoma-associated mesenchymal stem/stromal cells play a role in both initiating and promoting the metastatic cascade at the primary tumor, as well as aiding tumor cell colonization at the site of metastasis.
Canonically, invasion of the basement membrane and cell migration represents the first step of metastasis. The CA-MSC secretome can direct tumor cell invasion through expression of multiple chemokines. In a xenograft mouse model of melanoma and breast cancer, CA-MSCs secrete CCL-2, -5, -9, and CXCL10 and activate tumor cell migration and invasion, promoting metastasis to bone, lung, and lymph nodes.4,46,79-81 Additionally, chemokine activation of matrix metalloproteinases (MMPs) may direct remodeling the ECM, promoting escape of tumor cells from the primary TME.81 Paracrine signaling via TGFβ1 has also been shown to enhance invasion and metastasis in human Glioblastoma.82 Carcinoma-associated mesenchymal stem/stromal cell-derived exosomes were shown to promote migration of MCF7 breast cancer cells through upregulation of Wnt and β-catenin signaling pathways.83 Carcinoma-associated mesenchymal stem/stromal cell conditioned media was also shown to increase growth and metastasis of hepatocellular carcinoma via induction of the lncRNA DNM3OS within tumor cells.84
CA-MSCs Alter the ECM
There is also emerging evidence that CA-MSCs can enhance metastasis through alterations of the ECM. Changes in collagen deposition and resultant alterations in tumor cell and MSC interactions with deposited matrix has been shown to promote metastasis. For example, CA-MSCs are able to both increase discoidin domain receptor 2 (DDR2) expression and the deposition of its ligand, collagen I, to enhance breast cancer metastasis.85 In ovarian cancer, CA-MSCs shift the balance of basement membrane ECM from collagen 15 to collagen 4 which is also correlated with increased metastasis.9 As mentioned previously, CA-MSCs can differentiate into CAFs, which also alter the ECM to promote invasion and metastasis.86
CA-MSCs Induced Epithelial-to-Mesenchymal Transition
In addition, CA-MSCs play a critical role to enhance invasion and metastasis by promoting epithelial-to-mesenchymal transition (EMT) in tumor cells. Epithelial-to-mesenchymal transition is a dynamic process by which epithelial cells lose their cell-cell adhesion and polarity, gain a more mesenchymal phenotype and generally become more migratory and invasive. Carcinoma-associated mesenchymal stem/stromal cells were shown to induce EMT of metastatically weak breast cancer cells via LOX-Twist signaling, leading to increased metastasis to bone and lung in a breast cancer xenograft model.87 Stromal-derived TNFα and TGFβ1 increase tumor cell EMT in colon carcinoma cells via p38 MAPK activity and ERK activation88 and both factors are secreted by CA-MSCs.45,53,57,58 Tumor cell induction of CA-MSC CCL5 secretion was shown to enhance EMT of breast carcinoma cells via paracrine signaling through the chemokine receptor CCR5.89 Direct interaction between CA-MSCs and tumor cells has also been shown to increase expression of EMT-related genes, particularly at the stroma-epithelial invasive edge of the tumor.90,91
Carcinoma-Associated Mesenchymal Stem/Stromal Cells Directly Bind to and Travel with Cancer Cells
Finally, CA-MSCs can enhance metastasis by physically binding to tumor cells forming a heterocellular metastatic unit. In this multicellular complex, CA-MSCs and tumor cells escape the primary tumor as a unit and CA-MSCs serve as a chaperone to the tumor cells as they traverse to the metastatic site. Indeed, CA-MSCs have been identified in complex with tumor cells in the ascites of patients with ovarian cancer.9 Mechanistically, as tumor cells undergo CA-MSC-induced EMT, a bidirectional partial mesenchymal-to-epithelial transition (MET) occurs in CA-MSCs. This MET increases the adhesive properties of CA-MSCs allowing them to tightly bind to tumor cells.9 Carcinoma-associated mesenchymal stem/stromal cells which travel with tumor cells as a metastatic unit may aid in construction of the metastatic TME through proliferation and differentiation into supportive stroma. Further, CA-MSC interactions with other non-tumor components may help drive metastatic TME formation. For example, CA-MSCs were shown to promote the survival of lung cancer cells via expansion of myeloid-derived suppressor cells (MDSCs) at the distant metastatic site.92
Carcinoma-Associated Mesenchymal Stem/Stromal Cells and the Pre-metastatic Niche
Carcinoma-associated mesenchymal stem/stromal cells may also help influence the formation of the pre-metastatic niche (PMN), “priming” future sites of metastasis allowing for successful engraftment and survival of tumor cells in an otherwise hostile environment. Several factors identified as promoting PMN formation are either directly secreted by CA-MSCs or induced in tumor cells by CA-MSCs.93 TGFβ1 is significantly upregulated in CA-MSCs and secreted within the primary TME.13,45,53,88 Secreted TGFβ1 attracts myeloid cells to the pre-metastatic lung, inducing an inflammatory state and enhancing subsequent metastasis.93 TGFβ1 secretion has also been shown to lead to PMN formation via Treg accumulation in breast cancer lung metastasis, concurrent with observed immune cell recruitment in PMN formation.94 Carcinoma-associated mesenchymal stem/stromal cells can induce tumor cell secretion of ECM remodeling factors such as LOX which accumulate at PMNs and enhance the recruitment and growth of metastasizing tumor cells to this niche.87,95 Additionally, other ECM remodeling factors such as MMPs important to PMN formation are secreted by CA-MSCs.96,97 While these studies suggest an important function for CA-MSCs, further research is required to fully elucidate their role in PMN formation.
Carcinoma-Associated Mesenchymal Stem/Stromal Cells and the Cancer Stem Cell Niche
Cancer stem-like cells (CSCs) represent a subset of tumor cells characterized by the ability to self-renew, differentiate, and initiate tumors. These cell populations, while rare, can potentially recapitulate the entire tumor cell population and may be responsible for cancer initiation, therapeutic resistance and metastasis.11,30 This CSC population is maintained in part through the crosstalk with the TME and stroma.30 Specially, several mechanisms of CA-MSC:CSC niche interactions have been identified.
Carcinoma-associated mesenchymal stem/stromal cells isolated from human ovarian cancer ascites were shown to increase the CD133+/ALDH+ CSC population via BMP2 and BMP4.12,13 Carcinoma-associated mesenchymal stem/stromal cells also induce the expression of the microRNAs mir-199 and mir-214 in breast tumor cells, resulting in decreases to FoxP2 expression and increases in both metastasis and cancer cell stemness.98 Attachment of BM-MSCs to gastric cancer cells in coculture led to upregulation of the WNT and TGFβ signaling pathways and subsequent increases in the CSC population.99 The CA-MSC secretome plays a role in modulating the CSC niche through production of CXCL1, CXCL8, IL-6, and CXCL7.74,100,101 Direct coculture with MSCs resulted in upregulation of IL-6 and CXCL7 pathways and both increased sphere formation and self-renewal capacity in breast cancer cells.74 Additionally, CA-MSCs may indirectly modulate the CSC population by differentiation into CAFs.102,103 Taken together, these studies identify a crucial role for CA-MSCs in the maintenance of the CSC niche and disease progression through CA-MSC:CSC interactions.
Therapeutic Implications of CA-MSCs
Given the role of CA-MSCs in promoting tumor growth, progression and therapeutic resistance, the development of therapeutics targeting CA-MSC-tumor cell interactions or tumor education of naïve MSCs present a novel approach to treating cancer. Therapeutics targeting CA-MSCs may arise from novel or repurposed drugs which can be used in conjunction with other established treatments.104 Pharmacological disruption of the crosstalk between CA-MSCs and tumor cells may lead to improve response to treatment.54,59,73,105 Targeting a HH/BMP4 signaling loop between CA-MSCs and ovarian tumor cells with HHi IPI-926 was able to sensitize tumor cells to cisplatin.59 IPI-926 treatment was also able to sensitize desmoplastic ovarian tumors in mice to anti-PD-L1 immune checkpoint inhibitor treatment.54 HHi treatment was also able to sensitize pancreatic carcinoma cells to paclitaxel.105 Additionally, treatment using the lipid-lowering drug simvastatin was able to disrupt crosstalk between CA-MSCs and squamous cell lung carcinoma cells, leading to decreased CA-MSC secretion of several tumor-supporting cytokines.73
As reported previously, CA-MSCs potentially promote the recurrence of disease.72 Identification and targeting of CA-MSCs in patients after successful treatment may present an additional treatment strategy. As CA-MSCs are virtually indistinguishable from naïve MSCs, the development of other modalities to test for remaining tumor-supportive stroma may help identify patients at-risk for recurrence and could benefit from treatment. For example, use of the CA-MSC predictive algorithm developed by Coffman et al could be used to stratify patients based on the likelihood of tumor supportive stroma remaining post-chemotherapy/debulking.13 These patients could potentially be treated to reduce or eliminate the remaining tumor supporting stroma, while patients with non-supportive stroma could be treated with therapeutics that inhibit conversion of naïve MSCs to CA-MSCs.9
Of additional import is the emerging use of MSC-based therapies both in benign and malignant conditions. Indeed, MSC-based therapies are already FDA approved for the treatment of graft vs host disease. Given this, it is extremely important to better understand the pro- vs anti-tumor properties of MSCs (CA-MSCs vs MSCs) and to understand the nuances of the heterogenous MSC population in order to guarantee the clinical safety of MSC-based treatments.13,106,107 While full exploration of this topic is outside the scope of this review, there are numerous comprehensive articles which cover this topic in greater detail.106-109
Conclusion
Carcinoma-associated mesenchymal stem/stromal cells are key contributors to the formation of the tumor supporting microenvironment and may play a pivotal role in the establishment of the pre-metastatic and metastatic niche. Carcinoma-associated mesenchymal stem/stromal cells are most likely converted from native MSC populations at the primary site early on in cancer initiation through epigenetic reprogramming to acquire a tumor supporting phenotype. Once converted, CA-MSCs support tumor progression through several mechanisms and can differentiate into other tumor supporting stromal cells which give rise to the mature TME. Carcinoma-associated mesenchymal stem/stromal cells aid tumor cells in escaping the primary site and chaperone them to secondary metastatic sites, both increasing their chances of survival and potentially acting as architects of the new metastatic niche. Additionally, CA-MSCs may assist in forming the pre-metastatic niche prior to the arrival of DTCs, increasing the likelihood of successful metastatic spread. Given the multifaceted roles CA-MSCs play in cancer progression, they represent an untapped yet potentially powerful target for improved cancer therapeutics. In addition, a more nuanced understanding of MSC-CA-MSC dynamics can be used to ensure safe use of MSCs in clinical applications.
Contributor Information
Len Frisbie, Department of Integrative Systems Biology, University of Pittsburgh, Pittsburgh, PA, USA.
Ronald J Buckanovich, Division of Hematology/Oncology, Department of Medicine, Hillman Cancer Center, University of Pittsburgh, Pittsburgh, PA, USA; Division of Gynecologic Oncology, Department of Obstetrics, Gynecology, and Reproductive Sciences, Magee Women’s Research Institute, University of Pittsburgh, Pittsburgh, PA, USA.
Lan Coffman, Division of Hematology/Oncology, Department of Medicine, Hillman Cancer Center, University of Pittsburgh, Pittsburgh, PA, USA; Division of Gynecologic Oncology, Department of Obstetrics, Gynecology, and Reproductive Sciences, Magee Women’s Research Institute, University of Pittsburgh, Pittsburgh, PA, USA.
Conflict of Interest
The authors indicated no financial relationships.
Authors Contributions
L.F.: conception and design, writing (original draft), writing (review and editing); R.J.B.: conception and design, writing (review and editing); L.C.: conception and design, writing (original draft), writing (review and editing).
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Wu T, Dai Y.. Tumor microenvironment and therapeutic response. Cancer Lett. 2017; 387:61-68. [DOI] [PubMed] [Google Scholar]
- 2. Wang M, Zhao J, Zhang L, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017; 8(5):761-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008; 27(45):5904-5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li P, Gong Z, Shultz LD, Ren G.. Mesenchymal stem cells: from regeneration to cancer. Pharmacol Therap. 2019; 200:42-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Almalki SG, Agrawal DK.. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation. 2016; 92(1-2):41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kidd S, Spaeth E, Dembinski JL, et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells (Dayton, Ohio). 2009; 27(10):2614-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crippa S, Bernardo ME.. Mesenchymal stromal cells: role in the BM Niche and in the support of hematopoietic stem cell transplantation. HemaSphere. 2018; 2(6):e151-e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Denton AE, Roberts EW, Fearon DT.. Stromal cells in the tumor microenvironment. In: Owens L.M. B., ed. Stromal Immunology. Springer; 2018: 99-114. Advances in Experimental Medicine and Biology. [DOI] [PubMed] [Google Scholar]
- 9. Fan H, Atiya HI, Wang Y, et al. Epigenomic reprogramming toward mesenchymal-epithelial transition in ovarian-cancer-associated mesenchymal stem cells drives metastasis. Cell Rep. 2020; 33(10):108473108473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brennen WN, Chen S, Denmeade SR, Isaacs JT.. Quantification of mesenchymal stem cells (MSCs) at sites of human prostate cancer. Oncotarget. 2013; 4(1):106-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atiya H, Frisbie L, Pressimone C, Coffman L.. Mesenchymal stem cells in the tumor microenvironment. Adv Exp Med Biol. 2020; 1234:31-42. [DOI] [PubMed] [Google Scholar]
- 12. McLean K, Gong Y, Choi Y, et al. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 2011; 121(8):3206-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coffman LG, Pearson AT, Frisbie LG, et al. Ovarian carcinoma-associated mesenchymal stem cells arise from tissue-specific normal stroma. Stem Cells. 2019; 37(2):257-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eiro N, Fraile M, Fernández-Francos S, Sánchez R, Costa LA, Vizoso FJ.. Importance of the origin of mesenchymal (stem) stromal cells in cancer biology: “alliance” or “war” in intercellular signals. Cell Biosci. 2021; 11(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liang W, Chen X, Zhang S, et al. Mesenchymal stem cells as a double-edged sword in tumor growth: focusing on MSC-derived cytokines. Cell Mol Biol Lett. 2021; 26(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cho K-A, Park M, Kim Y-H, Woo S-Y, Ryu K-H.. RNA sequencing reveals a transcriptomic portrait of human mesenchymal stem cells from bone marrow, adipose tissue, and palatine tonsils. Sci Rep. 2017; 7(1):17114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hou W, Duan L, Huang C, et al. Cross-tissue characterization of heterogeneities of mesenchymal stem cells and their differentiation potentials. Front Cell Dev Biol. 2021. 9:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang C, Han X, Liu J, et al. Single-cell transcriptomic analysis reveals the cellular heterogeneity of mesenchymal stem cells. Genom Proteom Bioinform. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8(4):315-317. [DOI] [PubMed] [Google Scholar]
- 20. Andrzejewska A, Lukomska B, Janowski M.. Concise review: mesenchymal stem cells: from roots to boost. Stem Cells (Dayton, Ohio). 2019; 37(7):855-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Viswanathan S, Shi Y, Galipeau J, et al. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019; 21(10):1019-1024. [DOI] [PubMed] [Google Scholar]
- 22. Ren G, Liu Y, Zhao X, et al. Tumor resident mesenchymal stromal cells endow naïve stromal cells with tumor-promoting properties. Oncogene. 2014; 33(30):4016-4020. [DOI] [PubMed] [Google Scholar]
- 23. Ren G, Zhao X, Wang Y, et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα. Cell Stem Cell. 2012; 11(6):812-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Le Naour A, Prat M, Thibault B, et al. Tumor cells educate mesenchymal stromal cells to release chemoprotective and immunomodulatory factors. J Mol Cell Biol. 2019; 12(3):202-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Phinney DG, Sensebé L.. Mesenchymal stromal cells: misconceptions and evolving concepts. Cytotherapy. 2013; 15(2):140-145. [DOI] [PubMed] [Google Scholar]
- 26. Galland S, Stamenkovic I.. Mesenchymal stromal cells in cancer: a review of their immunomodulatory functions and dual effects on tumor progression. J Pathol. 2020; 250(5):555-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jung Y, Kim JK, Shiozawa Y, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. 2013; 4:1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao H, Priebe W, Glod J, Banerjee D.. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells. 2009; 27(4):857-865. [DOI] [PubMed] [Google Scholar]
- 29. Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA.. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014; 2014:149185-149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Papaccio F, Paino F, Regad T, Papaccio G, Desiderio V, Tirino V.. Concise review: cancer cells, cancer stem cells, and mesenchymal stem cells: influence in cancer development. Stem Cells Transl Med. 2017; 6(12):2115-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang Y, Yu P, Li W, et al. p53 regulates mesenchymal stem cell-mediated tumor suppression in a tumor microenvironment through immune modulation. Oncogene. 2014; 33(29):3830-3838. [DOI] [PubMed] [Google Scholar]
- 32. Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005; 65(8):3307-3318. [DOI] [PubMed] [Google Scholar]
- 33. Ren G, Zhao X, Wang Y, et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα. Cell Stem Cell. 2012; 11(6):812-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang L, Lin PC.. Mechanisms that drive inflammatory tumor microenvironment, tumor heterogeneity, and metastatic progression. Semin Cancer Biol. 2017; 47:185-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blache U, Horton ER, Xia T, et al. Mesenchymal stromal cell activation by breast cancer secretomes in bioengineered 3D microenvironments. Life Sci Alliance. 2019; 2(3):e201900304e201900304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang C, Hu J, Chen Z, et al. Reversibility of hAT-MSCs phenotypic and metabolic changes after exposure to and withdrawal from HCC-conditioned medium through regulation of the ROS/MAPK/HIF-1α signaling pathway. Stem Cell Res Ther. 2020; 11(1):506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ridge SM, Sullivan FJ, Glynn SA.. Mesenchymal stem cells: key players in cancer progression. Mol Cancer. 2017; 16(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cho JA, Park H, Lim EH, et al. Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecol Oncol. 2011; 123(2):379-386. [DOI] [PubMed] [Google Scholar]
- 39. Cho JA, Park H, Lim EH, Lee KW.. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol. 2012; 40(1):130-138. [DOI] [PubMed] [Google Scholar]
- 40. Miyazaki Y, Oda T, Inagaki Y, et al. Adipose-derived mesenchymal stem cells differentiate into heterogeneous cancer-associated fibroblasts in a stroma-rich xenograft model. Sci Rep. 2021; 11(1):4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mishra PJ, Mishra PJ, Humeniuk R, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008; 68(11):4331-4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008; 2(2):141-150. [DOI] [PubMed] [Google Scholar]
- 43. Biswas S, Mandal G, Roy Chowdhury S, et al. Exosomes produced by mesenchymal stem cells drive differentiation of myeloid cells into immunosuppressive M2-polarized macrophages in breast cancer. J Immunol. 2019; 203(12):3447-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Galland S, Vuille J, Martin P, et al. Tumor-derived mesenchymal stem cells use distinct mechanisms to block the activity of natural killer cell subsets. Cell Rep. 2017; 20(12):2891-2905. [DOI] [PubMed] [Google Scholar]
- 45. Li G-C, Zhang H-W, Zhao Q-C, et al. Mesenchymal stem cells promote tumor angiogenesis via the action of transforming growth factor β1. Oncol Lett. 2016; 11(2):1089-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chaturvedi P, Gilkes DM, Wong CCL, et al. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Invest. 2013; 123(1):189-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shen F, Shi Y.. Recent advances in single-cell view of mesenchymal stem cell in osteogenesis. Front Cell Dev Biol. 2021; 9:809918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu Q, Li B, Li Z, Li J, Sun S, Sun S.. Cancer-associated adipocytes: key players in breast cancer progression. J Hematol Oncol. 2019; 12(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arena S, Salati M, Sorgentoni G, Barbisan F, Orciani M.. Characterization of tumor-derived mesenchymal stem cells potentially differentiating into cancer-associated fibroblasts in lung cancer. Clin Transl Oncol. 2018; 20(12):1582-1591. [DOI] [PubMed] [Google Scholar]
- 50. Fritz V, Brondello JM, Gordeladze JO, et al. Bone-metastatic prostate carcinoma favors mesenchymal stem cell differentiation toward osteoblasts and reduces their osteoclastogenic potential. J Cell Biochem. 2011; 112(11):3234-3245. [DOI] [PubMed] [Google Scholar]
- 51. Wang S, Su X, Xu M, et al. Exosomes secreted by mesenchymal stromal/stem cell-derived adipocytes promote breast cancer cell growth via activation of Hippo signaling pathway. Stem Cell Res Ther. 2019; 10(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mathew E, Brannon AL, Del Vecchio A, et al. Mesenchymal stem cells promote pancreatic tumor growth by inducing alternative polarization of macrophages. Neoplasia. 2016; 18(3):142-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P.. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol. 2010; 184(10):5885-5894. [DOI] [PubMed] [Google Scholar]
- 54. Cascio S, Chandler C, Zhang L, et al. Cancer associated MSC drive tumor immune exclusion and resistance to immunotherapy which can be overcome by hedgehog inhibition. Science Advances. 2021; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhu W, Huang L, Li Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012; 315(1):28-37. [DOI] [PubMed] [Google Scholar]
- 56. Beckermann BM, Kallifatidis G, Groth A, et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. 2008; 99(4):622-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huang WH, Chang MC, Tsai KS, Hung MC, Chen HL, Hung SC.. Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene. 2013; 32(37):4343-4354. [DOI] [PubMed] [Google Scholar]
- 58. Batlle R, Andrés E, Gonzalez L, et al. Regulation of tumor angiogenesis and mesenchymal–endothelial transition by p38α through TGF-β and JNK signaling. Nat Commun. 2019; 10(1):3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Coffman LG, Choi Y-J, McLean K, Allen BL, di Magliano MP, Buckanovich RJ.. Human carcinoma-associated mesenchymal stem cells promote ovarian cancer chemotherapy resistance via a BMP4/HH signaling loop. Oncotarget. 2016; 7(6):6916-6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jeon ES, Heo SC, Lee IH, et al. Ovarian cancer-derived lysophosphatidic acid stimulates secretion of VEGF and stromal cell-derived factor-1 alpha from human mesenchymal stem cells. Exp Mol Med. 2010; 42(4):280-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM.. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014; 17(1):109-118. [DOI] [PubMed] [Google Scholar]
- 62. Bliss SA, Sinha G, Sandiford OA, et al. Mesenchymal stem cell–derived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrow. Cancer Res. 2016; 76(19):5832-5844. [DOI] [PubMed] [Google Scholar]
- 63. Daverey A, Drain AP, Kidambi S.. Physical intimacy of breast cancer cells with mesenchymal stem cells elicits trastuzumab resistance through Src activation. Sci Rep. 2015; 5(1):13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Forte D, García-Fernández M, Sánchez-Aguilera A, et al. Bone marrow mesenchymal stem cells support acute myeloid leukemia bioenergetics and enhance antioxidant defense and escape from chemotherapy. Cell Metab. 2020; 32(5):829-843.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. He W, Liang B, Wang C, et al. MSC-regulated lncRNA MACC1-AS1 promotes stemness and chemoresistance through fatty acid oxidation in gastric cancer. Oncogene. 2019; 38(23):4637-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nobre AR, Risson E, Singh DK, et al. Bone marrow NG2+/Nestin+ mesenchymal stem cells drive DTC dormancy via TGF-β2. Nat Cancer. 2021; 2(3):327-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ohshima K, Morii E.. Metabolic reprogramming of cancer cells during tumor progression and metastasis. Metabolites. 2021; 11(1):2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Raghavan S, Snyder CS, Wang A, et al. Carcinoma-associated mesenchymal stem cells promote chemoresistance in ovarian cancer stem cells via PDGF signaling. Cancers (Basel). 2020; 12(8):2063-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sandiford OA, Donnelly RJ, El-Far MH, et al. Mesenchymal stem cell–secreted extracellular vesicles instruct stepwise dedifferentiation of breast cancer cells into dormancy at the bone marrow perivascular region. Cancer Res. 2021; 81(6):1567-1582. [DOI] [PubMed] [Google Scholar]
- 70. Skolekova S, Matuskova M, Bohac M, et al. Cisplatin-induced mesenchymal stromal cells-mediated mechanism contributing to decreased antitumor effect in breast cancer cells. Cell Commun Signal. 2016; 14(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ullah M, Akbar A, Ng NN, Concepcion W, Thakor AS.. Mesenchymal stem cells confer chemoresistance in breast cancer via a CD9 dependent mechanism. Oncotarget. 2019; 10(37):3435-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhao L, Zhang K, He H, et al. The relationship between mesenchymal stem cells and tumor dormancy. Front Cell Dev Biol. 2021; 9:731393-731393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Galland S, Martin P, Fregni G, Letovanec I, Stamenkovic I.. Attenuation of the pro-inflammatory signature of lung cancer-derived mesenchymal stromal cells by statins. Cancer Lett. 2020; 484:50-64. [DOI] [PubMed] [Google Scholar]
- 74. Hsu HS, Lin JH, Hsu TW, et al. Mesenchymal stem cells enhance lung cancer initiation through activation of IL-6/JAK2/STAT3 pathway. Lung Cancer. 2012; 75(2):167-177. [DOI] [PubMed] [Google Scholar]
- 75. Kesh K, Garrido VT, Dosch A, et al. Stroma secreted IL6 selects for “stem-like” population and alters pancreatic tumor microenvironment by reprogramming metabolic pathways. Cell Death Dis. 2020; 11(11):967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mi F, Gong L.. Secretion of interleukin-6 by bone marrow mesenchymal stem cells promotes metastasis in hepatocellular carcinoma. Biosci Rep. 2017; 37(4):BSR20170181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang X, Hu F, Li G, et al. Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling. Cell Death Dis. 2018; 9(2):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chaffer CL, Weinberg RA.. A perspective on cancer cell metastasis. Science. 2011; 331(6024):1559. [DOI] [PubMed] [Google Scholar]
- 79. Kudo-Saito C, Fuwa T, Murakami K, Kawakami Y.. Targeting FSTL1 prevents tumor bone metastasis and consequent immune dysfunction. Cancer Res. 2013; 73(20):6185-6193. [DOI] [PubMed] [Google Scholar]
- 80. Luo J, Ok Lee S, Liang L, et al. Infiltrating bone marrow mesenchymal stem cells increase prostate cancer stem cell population and metastatic ability via secreting cytokines to suppress androgen receptor signaling. Oncogene. 2014; 33(21):2768-2778. [DOI] [PubMed] [Google Scholar]
- 81. Swamydas M, Ricci K, Rego SL, Dréau D.. Mesenchymal stem cell-derived CCL-9 and CCL-5 promote mammary tumor cell invasion and the activation of matrix metalloproteinases. Cell Adh Migr. 2013; 7(3):315-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rodini CO, Gonçalves da Silva PB, Assoni AF, Carvalho VM, Okamoto OK.. Mesenchymal stem cells enhance tumorigenic properties of human glioblastoma through independent cell-cell communication mechanisms. Oncotarget. 2018; 9(37):24766-24777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lin R, Wang S, Zhao RC.. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013; 383(1-2):13-20. [DOI] [PubMed] [Google Scholar]
- 84. Wang W, Wang Q, Huang D-B, et al. Tumor-associated mesenchymal stem cells promote hepatocellular carcinoma metastasis via a DNM3OS/KDM6B/TIAM1 axis. Cancer Lett. 2021; 503:19-31. [DOI] [PubMed] [Google Scholar]
- 85. Gonzalez ME, Martin EE, Anwar T, et al. Mesenchymal stem cell-induced DDR2 mediates stromal-breast cancer interactions and metastasis growth. Cell Rep. 2017; 18(5):1215-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zeltz C, Primac I, Erusappan P, Alam J, Noel A, Gullberg D.. Cancer-associated fibroblasts in desmoplastic tumors: emerging role of integrins. Semin Cancer Biol. 2020; 62:166-181. [DOI] [PubMed] [Google Scholar]
- 87. El-Haibi CP, Bell GW, Zhang J, et al. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc Natl Acad Sci USA. 2012; 109(43):17460-17465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bates RC, Mercurio AM.. Tumor necrosis factor-alpha stimulates the epithelial-to-mesenchymal transition of human colonic organoids. Mol Biol Cell. 2003; 14(5):1790-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007; 449(7162):557-563. [DOI] [PubMed] [Google Scholar]
- 90. Martin FT, Dwyer RM, Kelly J, et al. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res Treat. 2010; 124(2):317-326. [DOI] [PubMed] [Google Scholar]
- 91. Takigawa H, Kitadai Y, Shinagawa K, et al. Mesenchymal stem cells induce epithelial to mesenchymal transition in colon cancer cells through direct cell-to-cell contact. Neoplasia. 2017; 19(5):429-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sai B, Dai Y, Fan S, et al. Cancer-educated mesenchymal stem cells promote the survival of cancer cells at primary and distant metastatic sites via the expansion of bone marrow-derived-PMN-MDSCs. Cell Death Dis. 2019; 10(12):941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hiratsuka S, Watanabe A, Sakurai Y, et al. The S100A8–serum amyloid A3–TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008; 10(11):1349-1355. [DOI] [PubMed] [Google Scholar]
- 94. Olkhanud PB, Damdinsuren B, Bodogai M, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. 2011; 71(10):3505-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Erler JT, Bennewith KL, Cox TR, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009; 15(1):35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Peinado H, Zhang H, Matei IR, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017; 17(5):302-317. [DOI] [PubMed] [Google Scholar]
- 97. Yang Y, Bucan V, Baehre H, von der Ohe J, Otte A, Hass R.. Acquisition of new tumor cell properties by MSC-derived exosomes. Int J Oncol. 2015; 47(1):244-252. [DOI] [PubMed] [Google Scholar]
- 98. Cuiffo BG, Campagne A, Bell GW, et al. MSC-regulated microRNAs converge on the transcription factor FOXP2 and promote breast cancer metastasis. Cell Stem Cell. 2014; 15(6):762-774. [DOI] [PubMed] [Google Scholar]
- 99. Nishimura K, Semba S, Aoyagi K, Sasaki H, Yokozaki H.. Mesenchymal stem cells provide an advantageous tumor microenvironment for the restoration of cancer stem cells. Pathobiology. 2012; 79(6):290-306. [DOI] [PubMed] [Google Scholar]
- 100. Li HJ, Reinhardt F, Herschman HR, Weinberg RA.. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discov. 2012; 2(9):840-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Liu S, Ginestier C, Ou SJ, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011; 71(2):614-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chen WJ, Ho CC, Chang YL, et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat Commun. 2014; 5:3472. [DOI] [PubMed] [Google Scholar]
- 103. Huang T-X, Guan X-Y, Fu L.. Therapeutic targeting of the crosstalk between cancer-associated fibroblasts and cancer stem cells. Am J Cancer Res. 2019; 9(9):1889-1904. [PMC free article] [PubMed] [Google Scholar]
- 104. Jin M-Z, Jin W-L.. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020; 5(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhao J, Wang H, Hsiao C-H, et al. Simultaneous inhibition of hedgehog signaling and tumor proliferation remodels stroma and enhances pancreatic cancer therapy. Biomaterials. 2018; 159:215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hmadcha A, Martin-Montalvo A, Gauthier BR, Soria B, Capilla-Gonzalez V.. Therapeutic potential of mesenchymal stem cells for cancer therapy. Front Bioeng Biotechnol. 2020; 8:43-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lan T, Luo M, Wei X.. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021; 14(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sohrabi B, Dayeri B, Zahedi E, et al. Mesenchymal stem cell (MSC)-derived exosomes as novel vehicles for delivery of miRNAs in cancer therapy. Cancer Gene Ther. 2022. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 109. Mosallaei M, Simonian M, Ehtesham N, et al. Genetically engineered mesenchymal stem cells: targeted delivery of immunomodulatory agents for tumor eradication. Cancer Gene Ther. 2020; 27(12):854-868. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.