Abstract
The current levels of breast cancer in African women have contributed to the high mortality rates among them. In South Africa, the incidence of breast cancer is also on the rise due to changes in behavioural and biological risk factors. Such low survival rates can be attributed to the late diagnosis of the disease due to a lack of access and the high costs of the current diagnostic tools. Breast cancer is asymptomatic at early stages, which is the best time to detect it and intervene to prevent high mortality rates. Proper risk assessment, campaigns, and access to adequate healthcare need to be prioritised among patients at an early stage. Early detection of breast cancer can significantly improve the survival rate of breast cancer patients, since therapeutic strategies are more effective at this stage. Early detection of breast cancer can be achieved by developing devices that are simple, sensitive, low-cost, and employed at point-of-care (POC), especially in low-income countries (LICs). Nucleic-acid-based lateral flow assays (NABLFAs) that combine molecular detection with the immunochemical visualisation principles, have recently emerged as tools for disease diagnosis, even for low biomarker concentrations. Detection of circulating genetic biomarkers in non-invasively collected biological fluids with NABLFAs presents an appealing and suitable method for POC testing in resource-limited regions and/or LICs. Diagnosis of breast cancer at an early stage will improve the survival rates of the patients. This review covers the analysis of the current state of NABLFA technologies used in developing countries to reduce the scourge of breast cancer.
Keywords: breast cancer, NABLFA, circulating biomarkers, point-of-care, low income countries
1. Introduction
Globally, breast cancer is the second most prevalent cancer affecting women after cervical cancer. The number of diagnosed cases has increased rapidly, and 2.3 million cases are reported annually [1]. The majority (90–95%) of breast cancer cases are attributed to lifestyle, whereas 5–10% of these cases are heredity [2]. Strategies and campaigns have been developed to bring awareness and encourage women to screen and test for breast cancer. However, these are inaccessible to women in low and middle-income countries (LMCs), and as a result they are often diagnosed at an advanced stage when the disease can no longer be treated. Consequently, more than 685,000 mortalities are reported globally each year due to delayed diagnosis of breast cancer [3].
The breast cancer survival rate can be improved by early detection and therapeutic intervention. Currently, mammography, magnetic resonance imaging (MRI), X-ray imaging, ultrasound, CT scans, and tissue biopsies are the standard approved methods for breast cancer detection [4]. Despite their effectiveness, these methods have limitations in LICs, which include high cost, lack of trained personnel to operate and analyse data, and critically, a primary need for an invasive sampling procedure, such as a biopsy [5,6]. As such, countries with weak health infrastructure and limited screening or prevention programs continue to be affected by high mortality rates [7]. Therefore, there is a need to develop rapid and low-cost diagnostic tools that will be useful in LICs but still provide high sensitivity and specificity. Breast cancer diagnosis at the early stages is a crucial factor that will inform the disease treatment and management. Molecular changes during cancer development and progression are key to identifying individuals at risk, and it is critical to monitor these biomarkers, since this process could provide an effective way to follow the progress of breast cancer [8]. Cancer biomarkers are involved in various cellular processes that are essential for human life; any alteration in a biomarker’s level and expression could potentially serve as an indication for the development of cancer. Such biomarkers can be in the form of deoxyribonucleic acid (DNA), ribonucleic acid (RNA), proteins, metabolites [9], etc., and changes in their expression levels can be exploited to differentiate between healthy and infected individual [5].
Over the years, standard methods have been developed and used to detect or measure biomarker expression. These include enzyme-linked immunosorbent assays (ELISA) [10], radioimmunoassay (RIA) [11], and electrophoretic immunoassays [12]. These standard methods are known to give precise results, but their shortfall is that they require complicated equipment, multiple washing steps, and long turnaround times [13]. Therefore, the development of lateral flow biosensors (LFBs) for use at the POC has emerged as a feasible strategy for LMCs. Most importantly, LFB allows for the detection of circulating biomarkers in various biological fluids, such as saliva, blood, urine, serum, and plasma. Elevated levels of cell-free nucleic acids in the blood of cancer patients are amongst the biomarkers used to detect breast cancer [14] by ELISA and real-time polymerase chain reaction (RT or qPCR). However, these techniques are time-consuming and require costly instruments for detection, which becomes a limitation for use in LICs. The newly redesigned nucleic-acid based systems have shown potential for reducing the turnaround time. As a result, this review focuses on addressing the current trends in nucleic-acid-based lateral flow immunoassay systems for the detection of breast cancer.
2. Breast Cancer in Africa
Cancer has been reported to be the second major cause of death worldwide after heart-related diseases [15]. The most common cancers are breast, lung, prostate, cervix, brain, colon, thyroid, and pancreatic cancers [16]. Cervical cancer is the number one killer amongst women; 85% of cases and 88% of deaths occur in LMCs [17]. Even more concerning is that women in Sub-Saharan Africa (SSA) account for over a third of these cases, despite occupying only 14% of the female population in the world [18]. This region has also experienced a significant surge in incidence of breast cancer: from 19.7 per 100,000 to 36.9 per 100,000 in 2000 and 2015, respectively. In South Africa (SA) alone, breast cancer accounts for 22% of all malignancies [19], and it is predicted that 1 in 25 women are at risk of developing breast cancer in their lives [20].
Breast cancer survival rates vary globally, and high-income countries (HICs) have better survival than LMCs. For example, the United States of America (USA) has a survival rate of 89.9%, compared to 52.3% in SSA [21]. Most African countries have a five-year survival rate from the time of diagnosis to death/reoccurrence which is below 57%. For example, Kenya, Uganda, Malawi, and Nigeria had a 51.1% survival rate; compare that to New Zealand’s 90% survival rate [22]. The low survival rates in SSA are due to several factors, such as breast cancer diagnosis occurring at advanced clinical stages, co-morbid diseases, race, and aggressive pathological characteristics of breast cancer [23]. Global guidelines were developed to support program planning, implementation, monitoring, and early detection programs for breast cancer. These programs were found to be successful when tailored for specific communities and their needs, and by exploiting infrastructures and funding used for human immunodeficiency virus (HIV) programs [17]. Nonetheless, these programs are not accessible in LMCs. Furthermore, SSA countries are also burdened by other infectious diseases, further reducing the survival rates.
For instance, HIV/acquired immunodeficiency syndrome (AIDS) is one of the dominant infectious diseases in SSA; SA carries 20% of the global HIV burden, including 15% of new infections and 11% of AIDS-related deaths [23]. In 2018, about 7.52 million South Africans were reported to be living with HIV/AIDS, with 62% of them were on antiretroviral therapy [24]. Even though HIV is not an oncogenic disease, it can indirectly cause infected patients to develop other malignancies by suppressing T-cell function [25]. An estimated 30–40% of HIV patients are expected to have cancer in their lives [26]. HIV-positive patients usually present with advanced stages (stage III/IV) of breast cancer at diagnosis when compared to HIV-negative patients [20]. The survival rate of HIV/AIDS patients diagnosed with breast cancer is minimal [27]. This may be associated with the socioeconomic ills and inequalities that exist in SA, despite being rated by the World Bank as an upper-middle-income country in Africa [28]. Despite having multiple tertiary hospitals, state of the art oncology facilities [29], and national pathological laboratories with histopathological services, the survival rate in SA is still very low [30]. Private care patients are mostly funded by medical aid schemes, which only offer voluntary healthcare insurance to less than 15% of the population, whilst the majority of residents depend on public healthcare [31]. Women treated through private healthcare systems are likely to be treated by specialists with better oncological outcomes, whereas women cared by the public health system will only receive palliative attention instead of actual treatment. Additionally, the public healthcare system is burdened with high numbers [32], compounding issues related to late-stage diagnosis that are caused by inaccessibility to proper healthcare. The logistics and administration that must accompany diagnosis and treatment cause delays in therapeutic intervention and decrease the survival rate. The issues include (1) long-distance travel to hospitals and at times requiring visits to more than two healthcare facilities for diagnosis and treatment [33], (2) a patient needing a referral from a healthcare centre prior to attendance by a nearby hospital [34], and (3) the lengthy and costly diagnosis procedure. The procedure for diagnosis involves clinical examination and staging; imaging with mammography and ultrasonography; an image-guided core needle biopsy for histological diagnostic confirmation; tumour grading and receptor subtyping, which are performed by the national pathology laboratories [32]. The results take two to three weeks to obtain. This lengthy waiting period discourages the patients from returning for their results, as this will invariably involve a cost. The aforementioned reasons could be some of the reasons that women of African origin have the lowest registered cases of breast cancer [35]. Though contradictorily, the number of women diagnosed with breast cancer is now increasing due to the behavioural and biological risk factors [23]. Other reasons include illiteracy, health beliefs, policy constraints, and social-cultural factors [36]. Therefore, a screening device at a point-of-care for breast cancer has the potential to save lives and lessen the burden on the SA healthcare system [37] and the other LICs.
3. Breast Cancer Symptoms and Diagnosis
Breast cancer is a type of cancer that occurs mainly in women, though a small percentage of men are affected [38]. The cancerous cells form a tumour or a lump in the breast that can be felt or visualised through an X-ray. Like other cancers, breast cancer can invade and spread to other tissues surrounding the breast and other parts of the body [5], such as bones, the liver, lungs, or the brain [39]. It can be classified as invasive or non-invasive. In an invasive breast cancer, the cancerous cells spread to the ducts and possibly to the lymph nodes. Conversely, non-invasive cancer is confined to ducts or lobules where the cancer originated [40].
Early breast cancer usually does not cause pain or show any noticeable symptoms, and can go unnoticed for years. As the cancer progresses, early signs and symptoms can be physically observed: a lump or thickening in or near the breast; a change in the size or shape of the breast; nipple discharge, tenderness or retraction (turning inward); skin irritation, dimpling or scanlines [41]. These changes are not breast-cancer-specific and can occur as part of different conditions, such as fribrocystic [42] and mastitis cystic breast disease [43]. Nevertheless, having one or more of these symptoms can raise health concerns for breast cancer [39].
3.1. Breast Cancer Susceptibility Genes
Breast cancer occurs because of the genetic modifications or mutations in normal breast cells. Some mutations significantly increase the risks of certain cancers. DNA mutations linked to breast cancer are hereditary, whereas some are acquired [44]. Hereditary breast cancers usually occur earlier in life than the acquired (sporadic) cases, and are more likely to involve both breasts [45]. It is estimated that 5% to 10% of all hereditary breast cancers are due to known breast cancer susceptibility genes [46]. These genes are divided into low, moderate, and high-risk breast cancer susceptibility genes (Table 1). The high-risk breast cancer susceptibility genes include breast cancer 1 (BRCA1), BRCA2, phosphatase and tensin homolog (PTEN), tumour protein p53 (TP53), serine/threonine kinase 11 (STK), and CDHI. Checkpoint kinase 1 (CHEK1), transforming growth factor β1 (TGF-β1), caspase (CASP)8, and Ataxia telangiectasia mutated (ATM) genes belong to the low to moderate-risk breast cancer susceptibility genes [45].
Table 1.
List of known breast cancer genes.
| Low-Risk Genes | Moderate Risk Genes | High-Risk Genes |
|---|---|---|
| Fibroblast growth factor receptor 2 | CHEK2 | BRCA1 |
| TOX high mobility group box family member 3 | Partner and localizer of BRCA (PALB) 2 | BRCA2 |
| mitogen-activated protein kinase kinase kinase 1 | BRCA1 Interacting Protein 1 | PTEN |
| FAM84B/C-MYC | ATM | TP53 |
| lymphocyte-specific protein 1 | STK11 | |
| NIMA Related Kinase 10 | CDHI | |
| Cytochrome c oxidase 11 | ||
| CASP8(D302H) | ||
| TNP/IGFBP5/IGFBP2/TNSI | ||
| Neurogenic locus notch homolog protein 2/Fc gamma receptor Ib | ||
| RAD5ILI | ||
| mitochondrial ribosomal protein S30/FcgammaRI | ||
| Estrogen receptor I |
BRAC1 and BRAC2 are high-risk genes with 59–87% and 35–80% chances of developing cancer, respectively. BRAC1 and BRAC2 mutations are responsible for more deadly tumours and are located on chromosome 17 and chromosome 13, respectively. BRAC1 has 300 mutations that cause cancer, and BRAC2 has 1600 [47]. Some of the mutations include 185delAG; 6174delT; 5382ins; CS1832P; T2766I; N2781I; and K2860T, K3083E, or 9475A > G. These mutations are found to be more common among certain geographic or ethnic groups; for example, BRAC1 (3036del4) and BRAC2 mutations are high in Jewish women from Ashkenazi (Eastern Europe) [48]. Asian, Hispanic, and Native American women are at a lower risk of carrying breast cancer susceptibility genes [49], whereas in SA, BRCA1, PALB2 and RAD5IC genes are often responsible for breast cancer diagnosis [50]. Women who have inherited some of these genetic mutations have a high risk of developing breast cancer, ovarian cancer, colon cancer, and other types of cancer during their lives. Men with BRCA1 mutations (3232A>G) also have an increased risk of developing breast cancer [51]. BRCA1 mutations are also associated with increased risks of other cancers; for example, pancreatic cancer, prostate cancer, and ovarian cancer [52]. Similarly, mutations in the BRCA2 gene are also associated with increased chances of developing male breast cancer and cancers of the prostate and pancreas. An aggressive form of skin cancer (melanoma) is also more common amongst people who have BRCA2 mutations [53]. Identification of these mutations has been a crucial breakthrough in the research and development of more specific and selective diagnostic tools.
3.2. Diagnosis of Breast Cancer
Breast cancer screening and diagnosis starts with self-examination, which is performed lying down or standing, placing the right arm behind the head. The left hand is used to feel for lumps by using an over-lapping dime-sized circular motion of the finger to touch the breast for any lumps [54]. The screening can be followed by clinical validation. The current diagnostic methods used for breast cancer are shown in Table 2. These methods include biopsy, endoscopy, diagnostic imaging methods, and mammography. In biopsies, a small tissue sample is surgically removed from the suspicious growth area of the breast and examined under a microscope for the presence of cancer cells. This procedure can be performed by a surgeon or a radiologist [55]. Endoscopy involves the insertion of a flexible plastic tube with a tiny camera at the end into the nipple through the breast ducts, deep into the breast. This tube allows a physician to view the lining of the lactiferous ducts and look for abnormal tissue [54]. Diagnostic imaging methods such as X-ray imagine, computerized axial tomography (CAT), magnetic resonance imaging (MRI), and ultrasound are image-based methods for studying the anatomy of the breast and can identify any possible abnormalities [56,57]. Ultrasound evaluates whether the breast is filled with fluid (a cyst) or solid objects (tumours) [58]. The advantages and disadvantages of these diagnostic tests are summarized in Table 2. Most of these methods are invasive, time-consuming, require skilled personnel, use expensive equipment, have long turnaround times, and most importantly, are costly. The limitations of physical and image-based methods for breast cancer diagnosis [56] could be overcome by the use of molecular methods that detect disease biomarkers, such as immunoblotting, immunohistochemistry (IHC), enzyme-linked immunosorbent assay (ELISA), and in-situ fluorescence hybridization (FISH) [59]. Advancements in research and technology have enabled researchers to come up with improved molecular methods for diagnosis. The latest addition to breast cancer diagnostics is the Prosigna assay, which was approved by the FDA to determine the risk of recurrence in breast cancer patients after surgery. The assay studies changes in mRNA expression of a panel of 50 genes associated with various molecular subtypes of breast cancer, collectively known as the Prediction Analysis of Microarray 50 (PAM50) [60,61,62]. Other new and emerging preclinical assays that are being explored for diagnosis include electrochemical technologies such as electrochemical biosensors, nano-transistors, photonic crystals, and microfluidics-based technologies [63]. Although all these technologies provide improved diagnostic methods, they are not suitable for use in LMCs, as they require expensive equipment, sample pre-treatment, and trained personnel [60,63]. Thus, there is an urgent need for a quick breast cancer diagnostic method or technique that will be minimally invasive, rapid, and less expensive [54].
Table 2.
Advantages and disadvantages of different techniques for the diagnosis of breast cancer.
| Technique | Advantage | Disadvantage | * Cost Per Consultation (ZAR) |
|---|---|---|---|
| Biopsy | The results provide all the characteristics of the cancer cells. | Require surgery to get a sample women who may not have breast cancer will have the surgery just to clear them. | R11,000–R26,000 |
| Endoscopy | More details about the cancer cells (colour, texture). Short operation time Can see cancer in hidden areas. |
Requires surgery and may leave a scar. | R1000–R4000 |
| Diagnostic Imaging (CAT, X-rays, MRI) | Screening of high-risk woman gives more information about suspicious area. Detect the spreading of cancer to other parts of the body and Monitor reoccurrence after treatment. | A contrast solution (dye) is intravenously injected into your arm. This dye can affect your kidneys, a test for kidneys must be performed before it’s injected. The procedure is invasive and requires too many tests. |
R6000–R12,000 |
| Breast self-exam | Detect tumour at an early stage. | Validation must be followed-up with molecular tests. | Free |
* Note: The costs for consultation in South Africa were adapted from [64]. 2021, Mediclinic tariffs.
4. Nucleic Acids in Breast Cancer Diagnosis
Body fluids such as blood, urine, and cerebrospinal fluid contain the blueprint that can reflect the health status of an individual. Blood has been ubiquitously used over the years to diagnose various diseases. An average human adult has about five litres of continuously circulating blood that delivers nutrients and transports metabolic waste throughout the body [65]. Blood is made up of 54.3% plasma, 45% red blood cells, and 0.7% white blood cells by volume [66]. Plasma, the fluid part of blood, consists of proteins, nucleic acids, nutrients, and waste products. It also maintains the electrolyte balance and protects the body from infection and blood disorders [67]. Serum, obtained after blood clotting [54], tends to be used for detection of biological molecules present in the blood. Nucleic acids are some of the circulating biomarkers found in blood, and possibly other easily accessible biological fluids; thus, nucleic acids can be used to differentiate between healthy and disease states in diagnostics [68].
4.1. Circulating Biomarkers for Breast Cancer Diagnosis
In infectious diseases, nucleic acids offer additional advantages as a type of biomarker over antigens, antibodies, and metabolites. Specificity is one such advantage, as bacterial and viral DNA that are shed in the host during infection can be distinguished from the hosts’ DNA and microbial strains. These discriminating features are not always expressed at the phenotypic level; for instance, severe acute respiratory syndrome corona virus (SARS-CoV-1) and SARS-CoV-2 spike proteins can be differentiated via DNA rather than protein. Additionally, DNA, unlike antibodies, is detected almost immediately after infection, which means that a disease can be timeously diagnosed without requiring further incubation [69].
Nucleic acids are carriers of genetic information and can be secreted into the bloodstream in the form of circulating nucleic acids. They are detectable in small amounts in the sera of healthy individuals [70], which means elevated levels would suggest epigenetic alterations of a primary tumour [71]. It has been reported that the circulating nucleic acids are released into the bloodstream by proliferating or dying (both necrotic and apoptotic) cells [72], and also carried by exosomes shed in body fluids. Thus, exosomes can also be targeted as biomarker reservoirs, as they contain cellular components derived from their parental cells [73]. The circulating tumour DNA (ctDNA) and microRNA (miRNA) found in serum are known to contain tumour-specific mutations [65]. These molecular biomarkers are released in the blood during cancer growth and progression [70], and their expression repertoire can be used to detect and classify cancer stages or prognoses. From this, better therapeutic and diagnostic strategies can therefore be derived and implemented [74].
DNA in the human bloodstream was first reported in 1948 [75]. Attention was drawn to these findings in 1966 when the presence of DNA was reported in serum from patients suffering from systemic lupus erythematosus. Since then, DNA has also been detected in patients with other diseases, such as hepatitis, metastatic carcinoma, and miliary tuberculosis; and it was proposed that these DNA molecules originate from endogenous tissue breakdown [76]. Several years later, the presence of DNA in the sera of cancer patients with various cancers, such as breast, lung, cervical, ovary, and lymphosarcoma cancers, was detected using RIA. The amounts of DNA in these samples were quantified based on their affinity for DNA antibodies produced from patients with lupus erythematosus. High levels of DNA were detected in 50% of sera from cancer patients, of whom the majority were metastatic cancer patients [77]. Stroun et al. reported that the increased ctDNA content exhibited genetically identical characteristics to tumour DNA [65]. Two groups also confirmed the presence of tumour-associated oncogenes, namely, BCR ABL [78] and CA19.9 [79], for leukaemia and pancreatic cancer, respectively.
The discovery of DNA in the blood led researchers to find other types of blood circulating nucleic acids. RNA was also found to be one of the nucleic acids secreted in plasma in the form of microRNAs (miRNA) [68]. Elevated levels of microRNAs were also found in the blood samples from breast cancer patients, and were confirmed to be associated with tumour development and progression. The circulating concentrations of miRNAs, particularly miR-10b, miR-34a, miR-141, and miR-155, were higher in patients with primary breast cancer than in patients with no cancer [80]. Elevated levels of miR-885-5p, miR-1, miR-95, and miR-929, were also reported in blood from patients with breast cancer [81]. Iorio et al. also identified 13 miRNAs, which included miR-21, miR-125a, miR-205, miR-335, and miR-126, which were secreted into the bloodstream of breast cancer patients, and this revealed valuable biological information about the tumour [82]. These reports provided evidence that breast cancer cells do secrete cancer-related nucleic acids (DNA and miRNAs) which can be detected in the bloodstream.
4.2. PCR-Based Diagnostic Methods for Detection of Nucleic Acids
Molecular techniques such as PCR, ELISA, IHC, FISH, and mass spectrometry (MS) [83] have been instrumental in the detection of genetic mutations caused by pathogens. These tests are used for molecular diagnoses of diseases from various type of samples, including blood [74]. PCR-based techniques are by far the most reliable and most sensitive tests. The various methods are summarised in Table 3 below, including their disadvantages, which signify a need for cost-effective, easy to use, and rapid test devices.
Table 3.
Clinical and pre-clinical molecular methods for detection of nucleic acids.
| Method | Advantage | Disadvantage | Ref. |
|---|---|---|---|
| Microarrays | Analysis of thousands of genes in a single test to create molecular tumour profiles | Require long hybridisation times Prolonged wash steps that can take up to 24 h |
[84] |
| RT-PCR | DNA amplification increases sensitivity Test multiple samples simultaneously |
Requires a series of temperature changes Tedious sample preparation Equipment |
[85] |
| Nano pore sensor | Label-free. Small sample size Amplification free Distinguish single-nucleotide differences |
No reproducibility or adaptability of biological system | [86] |
| Micro-fluid devices | Rapid purification of nucleic acids | Challenging to integrate blood pre-treatment steps | [87] |
| A three-mode electrochemical sensor (HPD-SENS) |
Detect low concentrations of miRNA 10 aM to 1 mM range Multiple miRNAs on a single electrode. Exhibits high selectivity and specificity. |
Detection of low of copy number of sample of DNA/RNA in samples for early onset of a disease | [88] |
5. NABLFA for Rapid Diagnostics
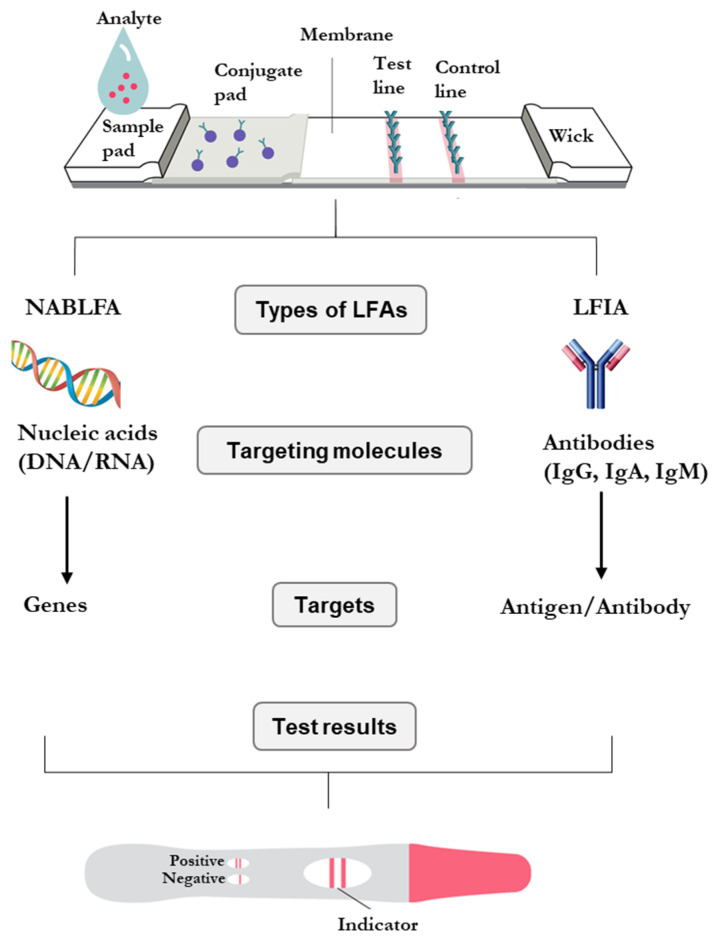
LFAs are based on the detection of analyte/antigens in body fluids and have emerged as reliable techniques for the diagnosis of several diseases [89]. There are two types of lateral flow formats, namely, the antibody and nucleic acid-based LFAs, as shown in Figure 1 [90]. The sandwich LFAs are used to detect antigens with multiple epitopes, such as those used to detect infectious diseases. The competitive LFAs test for antigens with single epitopes; examples are drug abuse tests [91]. LFAs are cost-effective and rapid compared to the molecular tests, with turnaround times of 10–15 min. Although the immuno-based assays (lateral flow immunoassay, LFIA) have been successful in diagnostics for decades, they have some limitations: their sensitivity relies on the concentration of the test specimen, and the test samples must be in solution [92]. The sensitivity of LFAs was shown to be improved from the μM level in LFIA to the aM level in systems that incorporate a pre-amplification step for test samples in NABLFAs. The sensitivity of an NABLFA is comparable to those of molecular tests and immunoassays [93].
Figure 1.
Types of clinically used LFAs for detection of nucleic acids (NABLFA) and antibodies (LFIA) found in biological fluids [90].
5.1. NABLFA
NABLFAs are under development for the detection of various genetic markers (DNA, RNA, or miRNA) that are specific for infectious and chronic diseases [94]. The NABLFAs have the added advantage of amplifying the nucleic acid targets that are specific to the analyte, as such the concentration can be enriched and detected [95]. NABLFA gives high sensitivity and specificity, similar to that of molecular tests, compared to the antibody-based LFAs [96].
Nucleic acid-based tests are essential in the diagnosis of genetic diseases. Several NABLFAs have been successfully developed to detect DNA, mRNA, proteins, and other biological agents [97]. Developing these nucleic-acid-based tests for fundamental research and clinical applications has become widely attractive because they offer simplicity, and are less time-consuming and labour intensive compared to conventional PCR methods [98,99]. Most NABLFAs are based on binding of hapten to target molecules, such as antibody or protein, in the test sample. The target molecules are first amplified using hapten-labelled primers [99]. The NABFLA follows the same principles as LFAs, except that the detection procedure starts with the amplification of genes of interest using PCR and use of the PCR product (amplicons) as a test sample [100]. Colorimetric detection is also an integral part of NABLFA which offers a more straightforward option to detect or identify PCR products by the naked eye, without additional equipment or the need for skilled personnel. NABLFAs are favourable diagnostic devices due to their ease of use [94]. The NABLFA follows in the success of nucleic acid amplification technologies (NAATs) [101], and unlike NAATs, the end-point test uses a rapid and POC system that can be used in scarcely resourced settings. The NABLFAs have been used to detect nucleic acids in food pathogens [91], infectious diseases [102], and cancer biomarkers [89].
5.2. NABLFA in Cancer Diagnosis
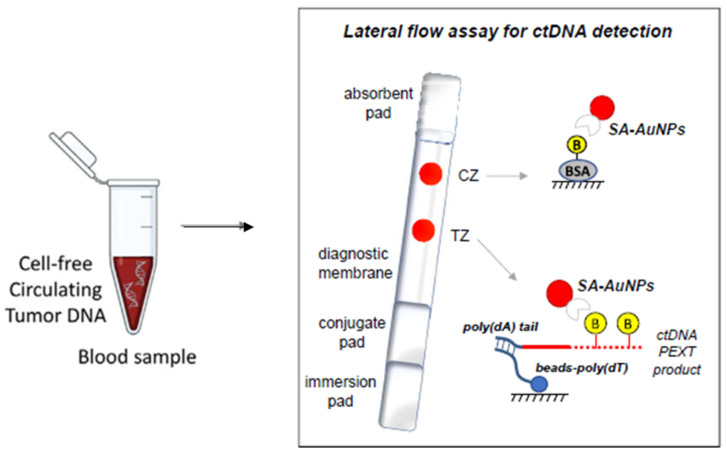
The feasibility of NABLFA has been demonstrated in the detection of various cancers [89]. Notably, a group in Greece developed an NABLFA for the detection of Kirsten rat sarcoma viral oncogene homologue (KRAS) mutations in DNA samples extracted from colorectal cancer (CRC) cells and blood [103]. The NABLFA was developed for the analysis of blood samples from CRC patients, wherein four single nucleotide polymorphisms (SNPs) that matched the normal KRAS gene and three of the most common mutations in the KRAS gene correlating to CRC in synthetic DNA samples, cancer cells, and ctDNA were detected [103]. KRAS is commonly used for cancer prognosis, response to chemotherapy, and resistance to anti-EGFR therapy [104], and its presence in blood samples could serve as a theragnostic biomarker. The gold nanoparticles (AuNPs)-based NABLFA (Figure 2) had high specificity and was able to differentiate single KRAS mutations in ctDNA extracted from cells and blood. This system demonstrated that the existing biomarkers present in low amounts can be detected in various diseases, including breast cancer [103]. Although the amplification step is crucial in cases where the biomarker is present in undetectable or low levels, it becomes a limitation for low resource settings and is unsuited for a POC testing. Therefore, there is still a need to develop less expensive diagnostic methods that can lead to rapid detection of breast cancer [54] at a POC to accommodate the LMCs.
Figure 2.
AuNPs-based NABLFA for rapid detection of ctDNA in CRC blood samples. Adapted with permission from [103]. 2021, Elsevier.
Next-generation PCR technologies that involve rapid amplification and detection of DNA and have a potential use in LMCs were devised, such as continuous flow PCR, droplet PCR, digital PCR, ultrafast photonic PCR, and insulated isothermal PCR [105]. However, temperature control in these devices still proves to be a limiting factor; hence, isothermal amplification methods such as loop-mediated isothermal amplification (LAMP) are better constructed for in-field use [106]. Despite the existence of other isothermal methods, LAMP has stood out amongst the other amplification assays, which include the strand displacement amplification, helicase-dependent amplification, rolling circle amplification, recombinase polymerase amplification, and nicking enzyme amplification reaction (NEAR) [107]. LAMP is considered superior due to several reasons: (1) LAMP is inhibitor-tolerant—that is, amplification is not suppressed by biologically-borne inhibitors found in blood, urine, and saliva; (2) amplification can be readily achieved from unprocessed raw samples, such as swabs and whole blood; (3) the prolonged storage time of reagents that have been preserved by lyophilisation; and lastly, (4) the protocols for this method are readily accessible, and its patent is not as restrictive as the NEAR and recombinase polymerase amplification assays [106,107].
In the year 2000, Notomi et al., discovered a PCR-inspired LAMP method. Amongst the many features of this isothermal method that make it favourable, is the use of the high strand displacement Bst polymerase, which operates under isothermal conditions. This transcends the limitations of using complex and expensive heating instruments that are needed for denaturing DNA [107,108]. As this method gained popularity, different and improved versions of it evolved—namely, the reverse transcription LAMP (RT-LAMP), which is the most widely used, the multiplex LAMP, electric LAMP, and in-disc LAMP [108]. The relatively high specificity of this technique has been attributed to the four (or six) primers that recognise and bind to six (or eight) different sites of the target DNA [109]. Once amplification of the targeted DNA is completed, the amplicon needs to be detected and/or quantified. LAMP is a highly flexible assay that accommodates different detection methods, such as turbidimetric, fluorescent, chemiluminescent, electrochemical, and colorimetric detection [107]. LAMP was then integrated with other technologies for rapid diagnosis of disease biomarkers. A LAMP-based microfluidic device that incorporates the nucleic acid extraction, amplification, and LFA steps in a single device demonstrated the feasibility of these systems for use at POC [110].
Clustered regularly interspaced short palindromic repeats (CRISPR), which are found in bacteria and archaea, confer acquired immunity against foreign genetic material, such as bacteriophages and plasmids. These hypervariable CRISPR are able to take up and store the foreign genetic material to form short DNA sequences. Ultimately, these sequences are recognised and hydrolysed by caspases, forming part of the basic concept of the CRISP–Cas based diagnostic assays [111]. Efforts to detect circulating nucleic acids using amplification-free assays were reported. This assay used oligonucleotide-templated LFAs to detect circulating microRNAs in blood samples [112].
5.3. NABLFA in Breast Cancer Diagnosis
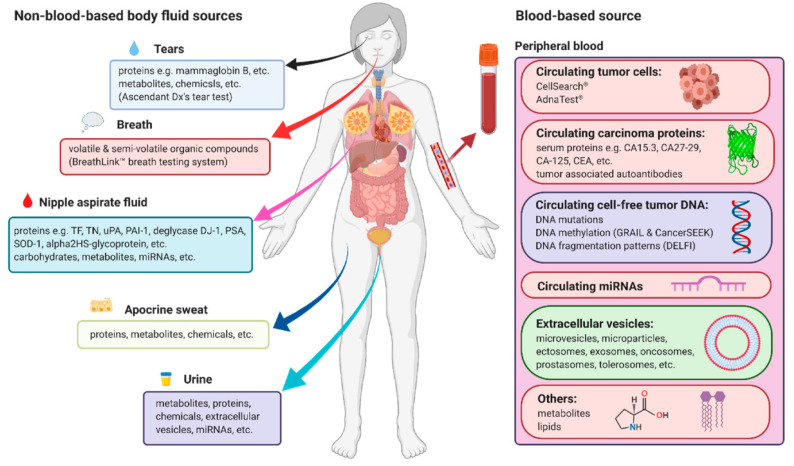
NAATs have played a huge role in the development of molecular tests that are highly sensitive and capable of studying genes associated with various diseases and their diagnosis, including breast cancer. PCR-based assays and FISH are still used to evaluate the expression of BRCA and HER2/neu genes in breast tissues, among others. Using a one-step nucleic acid amplification assay, forkhead box P3 and cluster of differentiation 4 genes were detected in sentinel lymph nodes samples from metastatic breast cancer patients [113]. However, tissue and liquid biopsy sampling are highly invasive, which led to the use of less invasive sampling methods. Circulating biomarkers associated with breast cancer were found in various body fluids, such as blood [80,81,82], urine [114], nipple aspirate fluid, tears, and sweat [68] (Figure 3); it is pending validation for clinical application. The non-invasive biomarkers, their sources, and tests used in their detection have been reviewed elsewhere [68]. Most of these biomarkers are measured in the body fluids using molecular tests, and in recent years, these techniques have been evolving into systems that can be used at the POC and in low-resource settings. The proposed NABLFA diagnostic test is user friendly and offers rapid detection of target biomarkers in solution. The NABLFA is compatible with the current screening or molecular diagnostic tests for disease biomarkers and presents a cost-effective system for LMCs. Thus, the NABLFA is of clinical value and displays features that are desirable for POC testing even in resource-limited settings. The lack of clinical NABLFAs or LFAs for diagnosis of cancer does not discredit their value; in fact, they could tap into the same success and market as the LFAs for infectious diseases [115], and their convenience can encourage patients to do regular check-ups. The LFAs are currently being integrated for cancer diagnostics; this was brought to light by the collaborative effort between SCIENION (Berlin, Germany) and the Institute for Prevention and Occupational Medicine of the German Social Accident Insurance (North Rhine-Westphalia, Germany) to develop a bladder cancer LFA. The multiplex LFA will be used to detect ten bladder cancer biomarkers in urine samples at a POC [116], further validating the usefulness of these systems in LICs. Although NABLFAs have the potential for improving testing accessibility and clinical outcomes, the technology is novel and has limitations. Less than 20% of healthcare facilities in seven Sub-Saharan countries have the capacity for deploying this technology, compared to 80% coverage in HICs. The limitations have been attributed to minimal operational education, high cost, and limited technological options [117]. Additionally, the POC device has to be functional under various environmental conditions. Similarly to TB testing, only a third of newly infected patients were diagnosed using LFA, and these are some of factors that cause resistance toward new technologies. When the LFIA-based TB LAM Alere Determine™ was initially introduced in South Africa, the uptake was very slow, owing to lack of proper establishment of procurement processes, low confidence in use of the device, and inaccessibility in some areas. Since its rollout, the detection of TB cases more than quadrupled between 2017 and 2020 [118]. Thus, with much persistence through education, acquiring resources, and increasing capacity, the prospects for NABLFAs in LICs are encouraging.
Figure 3.
Non-invasive sampling of biomarkers that can be used for early detection of breast cancer in human samples [68].
6. Conclusions
Early diagnosis is a vital part of treating and managing breast cancer and ensuring the higher rates of patient survival. Although NAATs have maintained a significant role in disease diagnosis, the systems used are not yet suitable for a POC testing, since nucleic extraction and the amplification steps are crucial for successfully developing a NABLFA with robust sensitivity that is suitable for POC testing. This challenge can be overcome by using technologies that incorporate the extraction and amplification processes as a single step, which ultimately requires limited use of instrumentation. This is true of isothermal techniques such as the RT-LAMP, which require no thermo-cycler. Thus, RT-LAMP can be combined with an LFA [119]. In the last few years, LFAs have been adopted for the detection of genetic markers in solution, and advanced into systems that can detect circulating molecules in biological fluids without the need for an instrument. Colorimetric LFAs are user friendly, and any person can use and interpret the results with ease. The presence of an analyte is reflected in a coloured line that can be visually detected with the naked eye. The popularity of LFAs is based on their simplicity and the enabled rapid diagnoses of diseases at POC [120]. As such, NABLFAs could overcome limitations that are associated with breast cancer diagnosis in LICs/LMCS. Circulating Breast cancer biomarkers have been identified, and they could be instrumental in the development of LFAs that do not require skilled personnel or a controlled environment. Moreover, these biomarkers can be detected in easy-to-access samples, such as saliva, urine, and sweat [68]. The LFA market is predicted to reach 12.6 billion USD in 2026, demonstrating the importance and uptake of these technologies by clinical practitioners [115]. Various companies have introduced various NABLFA for POC testing for cancer biomarkers [89]. Although these tests are not yet available for breast cancer, the studies under review serve as proof of the notion that they can be a reality for all cancers, including breast cancer.
Acknowledgments
A.S. is now working at the Council for Scientific and Industrial Research (CSIR), Next Generation Health Cluster, Advanced Chemistry and Life Sciences, Meiring Naude Road, Brummeria, Pretoria, 0001, South Africa.
Author Contributions
Writing—original draft preparation, project administration, B.D., P.P.S. and N.R.S.S. Conceptualization, supervision, resources, A.S., P.S.M., S.M. and N.R.S.S.; writing—review and editing, B.D., P.P.S., A.S., P.S.M., S.M. and N.R.S.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Busiswa Dyan‘s PhD project was funded by Mintek.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Senel M., Dervisevic M., Kokkokoğlu F. Electrochemical DNA biosensors for label-free breast cancer gene marker detection. Anal. Bioanal. Chem. 2019;411:2925–2935. doi: 10.1007/s00216-019-01739-9. [DOI] [PubMed] [Google Scholar]
- 2.Anand P., Kunnumakara A.B., Sundaram C., Harikumar K.B., Tharakan S.T., Lai O.S., Sung B., Aggarwal B.B. Cancer is a Preventable Disease that Requires Major Lifestyle Changes. Pharm. Res. 2008;25:2097. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jedy-Agba E., McCormack V., Adebamowo C., dos-Santos-Silva I. Stage at diagnosis of breast cancer in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob. Health. 2016;4:e923–e935. doi: 10.1016/S2214-109X(16)30259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karellas A., Vedantham S. Breast cancer imaging: A perspective for the next decade. Med. Phys. 2008;35:4878–4897. doi: 10.1118/1.2986144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranjan P., Parihar A., Jain S., Kumar N., Dhand C., Murali S., Mishra D., Sanghi S.K., Chaurasia J.P., Srivastava A.K., et al. Biosensor-based diagnostic approaches for various cellular biomarkers of breast cancer: A comprehensive review. Anal. Biochem. 2020;610:113996. doi: 10.1016/j.ab.2020.113996. [DOI] [PubMed] [Google Scholar]
- 6.Harbeck N., Penault-Llorca F., Cortes J., Gnant M., Houssami N., Poortmans P., Ruddy K., Tsang J., Cardoso F. Breast cancer. Nat. Rev. Dis. Prim. 2019;5:66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 7.Espina C., McKenzie F., dos-Santos-Silva I. Delayed presentation and diagnosis of breast cancer in African women: A systematic review. Ann. Epidemiol. 2017;27:659–671.e7. doi: 10.1016/j.annepidem.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van’t Veer L.J., Dai H., Van de Vijver M.J., He Y.D., Hart A.A.M., Mao M., Peterse H.L., Van Der Kooy K., Marton M.J., Witteveen A.T., et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 9.Quezada H., Guzmán-Ortiz A.L., Díaz-Sánchez H., Valle-Rios R., Aguirre-Hernández J. Omics-based biomarkers: Current status and potential use in the clinic. Boletín Médico Hosp. Infant. México (Engl. Ed.) 2017;74:219–226. doi: 10.1016/J.BMHIME.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Dong J., Ueda H. ELISA-type assays of trace biomarkers using microfluidic methods. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017;9:e1457. doi: 10.1002/wnan.1457. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y., Li H., Li G., Kang Y., Shi J., Kong T., Yang X., Xu J., Li C., Su K.P., et al. Active smoking, sleep quality and cerebrospinal fluid biomarkers of neuroinflammation. Brain. Behav. Immun. 2020;89:623–627. doi: 10.1016/j.bbi.2020.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Cheng S.B., Skinner C.D., Taylor J., Attiya S., Lee W.E., Picelli G., Harrison D.J. Development of a Multichannel Microfluidic Analysis System Employing Affinity Capillary Electrophoresis for Immunoassay. Anal. Chem. 2001;73:1472–1479. doi: 10.1021/ac0007938. [DOI] [PubMed] [Google Scholar]
- 13.Shawky A.M., El-Tohamy M. Signal amplification strategy of label-free ultrasenstive electrochemical immunosensor based ternary Ag/TiO2/rGO nanocomposites for detecting breast cancer biomarker CA 15-3. Mater. Chem. Phys. 2021;272:124983. doi: 10.1016/j.matchemphys.2021.124983. [DOI] [Google Scholar]
- 14.Li J., Peng Y., Duan Y. Diagnosis of breast cancer based on breath analysis: An emerging method. Crit. Rev. Oncol. Hematol. 2013;87:28–40. doi: 10.1016/j.critrevonc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Nagai H., Kim Y.H. Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis. 2017;9:448. doi: 10.21037/jtd.2017.02.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta S. Data mining classification techniques applied for breast cancer diagnosis and prognosis. Indian J. Comput. Sci. Eng. 2011;2:188–195. [Google Scholar]
- 17.Pierz A.J., Randall T.C., Castle P.E., Adedimeji A., Ingabire C., Kubwimana G., Uwinkindi F., Hagenimana M., Businge L., Musabyimana F., et al. A scoping review: Facilitators and barriers of cervical cancer screening and early diagnosis of breast cancer in Sub-Saharan African health settings. Gynecol. Oncol. Rep. 2020;33:100605. doi: 10.1016/j.gore.2020.100605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morhason-Bello I.O., Odedina F., Rebbeck T.R., Harford J., Dangou J.M., Denny L., Adewole I.F. Challenges and opportunities in cancer control in Africa: A perspective from the African organisation for research and training in cancer. Lancet Oncol. 2013;14:e142–e151. doi: 10.1016/S1470-2045(12)70482-5. [DOI] [PubMed] [Google Scholar]
- 19.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 20.Phakathi B., Nietz S., Cubasch H., Dickens C., Dix-Peek T., Joffe M., Neugut A.I., Jacobson J., Duarte R., Ruff P. Survival of South African women with breast cancer receiving anti-retroviral therapy for HIV. Breast. 2021;59:27–36. doi: 10.1016/j.breast.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galukande M., Wabinga H., Mirembe F. Breast cancer survival experiences at a tertiary hospital in sub-Saharan Africa: A cohort study. World J. Surg. Oncol. 2015;13:220. doi: 10.1186/s12957-015-0632-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sankaranarayanan R., Swaminathan R., Brenner H., Chen K., Chia K.S., Chen J.G., Law S.C., Ahn Y.O., Xiang Y.B., Yeole B.B., et al. Cancer survival in Africa, Asia, and Central America: A population-based study. Lancet. Oncol. 2010;11:165–173. doi: 10.1016/S1470-2045(09)70335-3. [DOI] [PubMed] [Google Scholar]
- 23.Akarolo-Anthony S.N., Ogundiran T.O., Adebamowo C.A. Emerging breast cancer epidemic: Evidence from Africa. Breast Cancer Res. 2010;12:S8. doi: 10.1186/bcr2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phakathi B., Cubasch H., Nietz S., Dickens C., Dix-Peek T., Joffe M., Neugut A.I., Jacobson J., Duarte R., Ruff P. Clinico-pathological characteristics among South African women with breast cancer receiving anti-retroviral therapy for HIV. Breast. 2019;43:123–129. doi: 10.1016/j.breast.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hessol N.A., Pipkin S., Schwarcz S., Cress R.D., Bacchetti P., Scheer S. The impact of highly active antiretroviral therapy on Non-AIDS-Defining cancers among adults with AIDS. Am. J. Epidemiol. 2007;165:1143–1153. doi: 10.1093/aje/kwm017. [DOI] [PubMed] [Google Scholar]
- 26.Mounier N., Katlama C., Costagliola D., Chichmanian R.M., Spano J.P. Drug interactions between antineoplastic and antiretroviral therapies: Implications and management for clinical practice. Crit. Rev. Oncol. Hematol. 2009;72:10–20. doi: 10.1016/j.critrevonc.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Brandão M., Bruzzone M., Franzoi M.A., De Angelis C., Eiger D., Caparica R., Piccart-Gebhart M., Buisseret L., Ceppi M., Dauby N., et al. Impact of HIV infection on baseline characteristics and survival of women with breast cancer. AIDS. 2021;35:605–618. doi: 10.1097/QAD.0000000000002810. [DOI] [PubMed] [Google Scholar]
- 28.Coovadia H., Jewkes R., Barron P., Sanders D., McIntyre D. The health and health system of South Africa: Historical roots of current public health challenges. Lancet. 2009;374:817–834. doi: 10.1016/S0140-6736(09)60951-X. [DOI] [PubMed] [Google Scholar]
- 29.Abdel-Wahab M., Bourque J.M., Pynda Y., Izewska J., Van der Merwe D., Zubizarreta E., Rosenblatt E. Status of radiotherapy resources in Africa: An international atomic energy agency analysis. Lancet Oncol. 2013;14:e168–e175. doi: 10.1016/S1470-2045(12)70532-6. [DOI] [PubMed] [Google Scholar]
- 30.Dickens C., Duarte R., Zietsman A., Cubasch H., Kellett P., Schüuz J., Kielkowski D., McCormack V. Racial comparison of receptor-defined breast cancer in Southern African women: Subtype prevalence and age—Incidence analysis of nationwide cancer registry data. Cancer Epidemiol. Biomark. Prev. 2014;23:2311–2321. doi: 10.1158/1055-9965.EPI-14-0603. [DOI] [PubMed] [Google Scholar]
- 31.Conmy A. South African health care system analysis. Public Health Rev. 2018;1:1–8. [Google Scholar]
- 32.Cubasch H., Dickens C., Joffe M., Duarte R., Murugan N., Tsai Chih M., Moodley K., Sharma V., Ayeni O., Jacobson J.S., et al. Breast cancer survival in Soweto, Johannesburg, South Africa: A receptor-defined cohort of women diagnosed from 2009 to 11. Cancer Epidemiol. 2018;52:120–127. doi: 10.1016/j.canep.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nietz S., Ruff P., Chen W.C., O’Neil D.S., Norris S.A. Quality indicators for the diagnosis and surgical management of breast cancer in South Africa. Breast. 2020;54:187–196. doi: 10.1016/j.breast.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCormack V.A., Joffe M., van den Berg E., Broeze N., dos Santos Silva I., Romieu I., Jacobson J.S., Neugut A.I., Schüz J., Cubasch H. Breast cancer receptor status and stage at diagnosis in over 1,200 consecutive public hospital patients in Soweto, South Africa: A case series. Breast Cancer Res. 2013;15:R84. doi: 10.1186/bcr3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forouzanfar M.H., Foreman K.J., Delossantos A.M., Lozano R., Lopez A.D., Murray C.J.L., Naghavi M. Breast and cervical cancer in 187 countries between 1980 and 2010: A systematic analysis. Lancet. 2011;378:1461–1484. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 36.Dickens C., Joffe M., Jacobson J., Venter F., Schüz J., Cubasch H., McCormack V. Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: A South African public hospital case series of over 1000 women. Int. J. Cancer. 2014;135:2173–2182. doi: 10.1002/ijc.28861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeman E., Semeere A., Wenger M., Bwana M., Asirwa F.C., Busakhala N., Oga E., Jedy-Agba E., Kwaghe V., Iregbu K., et al. Pitfalls of practicing cancer epidemiology in resource-limited settings: The case of survival and loss to follow-up after a diagnosis of Kaposi’s sarcoma in five countries across sub-Saharan Africa. BMC Cancer. 2016;16:65. doi: 10.1186/s12885-016-2080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le E.P.V., Wang Y., Huang Y., Hickman S., Gilbert F.J. Artificial intelligence in breast imaging. Clin. Radiol. 2019;74:357–366. doi: 10.1016/j.crad.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Lee S.W., Hyun K.A., Kim S.I., Kang J.Y., Jung H.I. Continuous enrichment of circulating tumor cells using a microfluidic lateral flow filtration chip. J. Chromatogr. A. 2015;1377:100–105. doi: 10.1016/j.chroma.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 40.Zhang M., Lin Q., Su X.H., Cui C.X., Bian T.T., Wang C.Q., Zhao J., Li L.L., Ma J.Z., Huang J.L. Breast ductal carcinoma in situ with micro-invasion versus ductal carcinoma in situ: A comparative analysis of clinicopathological and mammographic findings. Clin. Radiol. 2021;76:787.e1–787.e7. doi: 10.1016/j.crad.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 41.CDC What Are the Symptoms of Breast Cancer? [(accessed on 8 June 2022)]; Available online: https://www.cdc.gov/cancer/breast/basic_info/risk_factors.htm.
- 42.Malherbe K., Khan M., Fatima S. Fibrocystic Breast Disease. [(accessed on 13 June 2022)]; Up to Date 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551609/
- 43.Stachs A., Stubert J., Reimer T., Hartmann S. Benign breast disease in women. Dtsch. Arztebl. Int. 2019;116:565. doi: 10.3238/arztebl.2019.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheikh A., Hussain S.A., Ghori Q., Naeem N., Fazil A., Giri S., Sathian B., Mainali P., Al Tamimi D.M. The spectrum of genetic mutations in breast cancer. Asian Pac. J. Cancer Prev. 2015;16:2177–2185. doi: 10.7314/APJCP.2015.16.6.2177. [DOI] [PubMed] [Google Scholar]
- 45.Oldenburg R.A., Meijers-Heijboer H., Cornelisse C.J., Devilee P. Genetic susceptibility for breast cancer: How many more genes to be found? Crit. Rev. Oncol. Hematol. 2007;63:125–149. doi: 10.1016/j.critrevonc.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Holland M.L., Huston A., Noyes K. Cost-effectiveness of testing for breast cancer susceptibility genes. Value Health. 2009;12:207–216. doi: 10.1111/j.1524-4733.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 47.Casey M., Bewtra C. Peritoneal carcinoma in women with genetic susceptibility: Implications for Jewish populations. Fam. Cancer. 2004;3:265–281. doi: 10.1007/s10689-004-9554-y. [DOI] [PubMed] [Google Scholar]
- 48.Friebel T.M., Andrulis I.L., Balmaña J., Blanco A.M., Couch F.J., Daly M.B., Domchek S.M., Easton D.F., Foulkes W.D., Ganz P.A., et al. BRCA1 and BRCA2 pathogenic sequence variants in women of African origin or ancestry. Hum. Mutat. 2019;40:1781–1796. doi: 10.1002/humu.23804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Struewing J.P., Hartge P., Wacholder S., Baker S.M., Berlin M., McAdams M., Timmerman M.M., Brody L.C., Tucker M.A. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N. Engl. J. Med. 1997;336:1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 50.PDQ Cancer Genetics Editorial Board . Genetics of Breast and Gynecologic Cancers (PDQ®): Health Professional Version. National Cancer Institute; Bethasda, MD, USA: 2002. PDQ Cancer Information Summaries. [PubMed] [Google Scholar]
- 51.Ibrahim M., Yadav S., Ogunleye F., Zakalik D. Male BRCA mutation carriers: Clinical characteristics and cancer spectrum. BMC Cancer. 2018;18:179. doi: 10.1186/s12885-018-4098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karami F., Mehdipour P. A comprehensive focus on global spectrum of BRCA1 and BRCA2 mutations in breast cancer. Biomed Res. Int. 2013;2013:928562. doi: 10.1155/2013/928562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang G., Sau C., Lai W., Cichon J., Li W. BRAC1 and BRAC2 mutation and treatment strategies for breast cancer. HHS Pulic Access. 2015;344:1173–1178. [Google Scholar]
- 54.Song Y., Huang Y.Y., Liu X., Zhang X., Ferrari M., Qin L. Point-of-care technologies for molecular diagnostics using a drop of blood. Trends Biotechnol. 2014;32:132–139. doi: 10.1016/j.tibtech.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roche Foundation Medicine|Cancer testing. [(accessed on 1 August 2022)]. Available online: https://www.rochefoundationmedicine.com/cancertesting.html.
- 56.Ali M.A., Mondal K., Jiao Y., Oren S., Xu Z., Sharma A., Dong L. Microfluidic immuno-biochip for detection of breast cancer biomarkers using hierarchical composite of porous graphene and titanium dioxide nanofibers. ACS Appl. Mater. Interfaces. 2016;8:20570–20582. doi: 10.1021/acsami.6b05648. [DOI] [PubMed] [Google Scholar]
- 57.Allemani C., Weir H.K., Carreira H., Harewood R., Spika D., Wang X.S., Bannon F., Ahn J.V., Johnson C.J., Bonaventure A. Global surveillance of cancer survival 1995-2009: Analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee C.H., Dershaw D.D., Kopans D., Evans P., Monsees B., Monticciolo D., Brenner R.J., Bassett L., Berg W., Feig S., et al. Breast cancer screening with imaging: Recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J. Am. Coll. Radiol. 2010;7:18–27. doi: 10.1016/j.jacr.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 59.Dervisevic M., Alba M., Adams T.E., Prieto-Simon B., Voelcker N.H. Electrochemical immunosensor for breast cancer biomarker detection using high-density silicon microneedle array. Biosens. Bioelectron. 2021;192:113496. doi: 10.1016/j.bios.2021.113496. [DOI] [PubMed] [Google Scholar]
- 60.Grimaldi A.M., Conte F., Pane K., Fiscon G., Mirabelli P., Baselice S., Giannatiempo R., Messina F., Franzese M., Salvatore M., et al. The new paradigm of network medicine to analyze breast cancer phenotypes. Int. J. Mol. Sci. 2020;21:6690. doi: 10.3390/ijms21186690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang C.-Y., Chiao C.-C., Phan N.N., Li C.-Y., Sun Z.-D., Jiang J.-Z., Hung J.-H., Chen Y.-L., Yen M.-C., Weng T.-Y., et al. Gene signatures and potential therapeutic targets of amino acid metabolism in estrogen receptor-positive breast cancer. Am. J. Cancer Res. 2020;10:95. [PMC free article] [PubMed] [Google Scholar]
- 62.Lien T.G., Ohnstad H.O., Lingjærde O.C., Vallon-Christersson J., Aaserud M., Sveli M.A.T., Borg Å., Garred Ø., Borgen E., Naume B., et al. Sample Preparation Approach Influences PAM50 Risk of Recurrence Score in Early Breast Cancer. Cancers. 2021;13:6118. doi: 10.3390/cancers13236118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li F., You M., Li S., Hu J., Liu C., Gong Y., Yang H., Xu F. Paper-based point-of-care immunoassays: Recent advances and emerging trends. Biotechnol. Adv. 2020;39:107442. doi: 10.1016/j.biotechadv.2019.107442. [DOI] [PubMed] [Google Scholar]
- 64.Mediclinic The Mediclinic Southern Africa Private Tariff Schedule. 2021. [(accessed on 2 July 2022)]. Available online: https://www.mediclinic.co.za/content/dam/mc-sa-corporate/downloads/stay-and-visit/Mediclinic%20Southern%20Africa%20Private%20Tariff%20Schedule%202021%20(South%20Africa%20only).pdf.
- 65.Stroun M., Anker P., Maurice P., Lyautey J., Lederrey C., Beljanski M. Neoplastic characteristics of the dna found in the plasma of cancer patients. Oncology. 1989;46:318–322. doi: 10.1159/000226740. [DOI] [PubMed] [Google Scholar]
- 66.Lenshof A., Ahmad-Tajudin A., Järås K., Swärd-Nilsson A.M., Åberg L., Marko-Varga G., Malm J., Lilja H., Laurell T. Acoustic whole blood plasmapheresis chip for prostate specific antigen microarray diagnostics. Anal. Chem. 2009;81:6030–6037. doi: 10.1021/ac9013572. [DOI] [PubMed] [Google Scholar]
- 67.Marieb E. Essentials of Human Anatomy and Physiology. 6th ed. Benjamin Cummings; San Francisco, CA, USA: 2000. [Google Scholar]
- 68.Li J., Guan X., Fan Z., Ching L.M., Li Y., Wang X., Cao W.M., Liu D.X. Non-invasive biomarkers for early detection of breast cancer. Cancers. 2020;12:2767. doi: 10.3390/cancers12102767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suea-Ngam A., Bezinge L., Mateescu B., Howes P.D., Demello A.J., Richards D.A. Enzyme-assisted nucleic acid detection for infectious disease diagnostics: Moving toward the point-of-care. ACS Sens. 2020;5:2701–2723. doi: 10.1021/acssensors.0c01488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dennis Lo Y.M. Circulating nucleic acids in plasma and serum: An overview. Ann. N.Y. Acad. Sci. 2001;945:1–7. doi: 10.1111/j.1749-6632.2001.tb03858.x. [DOI] [PubMed] [Google Scholar]
- 71.Fleischhacker M., Schmidt B. Free circulating nucleic acids in plasma and serum (CNAPS)—Useful for the detection of lung cancer patients? Cancer Biomark. 2009;6:211–219. doi: 10.3233/CBM-2009-0131. [DOI] [PubMed] [Google Scholar]
- 72.Manoharan A., Sambandam R., Bhat V. Recent technologies enhancing the clinical utility of circulating tumor DNA. Clin. Chim. Acta. 2020;510:498–506. doi: 10.1016/j.cca.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 73.Kalishwaralal K., Kwon W.Y., Park K.S. Exosomes for non-invasive cancer monitoring. Biotechnol. J. 2019;14:1800430. doi: 10.1002/biot.201800430. [DOI] [PubMed] [Google Scholar]
- 74.Phan J.H., Moffitt R.A., Stokes T.H., Liu J., Young A.N., Nie S., Wang M.D. Convergence of biomarkers, bioinformatics and nanotechnology for individualized cancer treatment. Trends Biotechnol. 2009;27:350–358. doi: 10.1016/j.tibtech.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmidt B., Weickmann S., Witt C., Fleischhacker M. Improved method for isolating cell-free DNA. Clin. Chem. 2005;51:1561–1563. doi: 10.1373/clinchem.2005.051003. [DOI] [PubMed] [Google Scholar]
- 76.Tan E.M., Schur P.H., Carr R.I., Kunkel H.G. Deoxyribonucleic Acid (DNA) and antibodies to DNA in the serum of patients with systemic lupus erythematosus. J. Clin. Investig. 1966;45:1732–1740. doi: 10.1172/JCI105479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leon S.A., Shapiro B., Sklaroff D.M., Yaros M.J. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37:646–650. [PubMed] [Google Scholar]
- 78.Scherer F., Kurtz D.M., Diehn M., Alizadeh A.A. High-throughput sequencing for noninvasive disease detection in hematologic malignancies. Blood. 2017;130:440. doi: 10.1182/blood-2017-03-735639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sefrioui D., Blanchard F., Toure E., Basile P., Beaussire L., Dolfus C., Perdrix A., Paresy M., Antonietti M., Iwanicki-Caron I., et al. Diagnostic value of CA19.9, circulating tumour DNA and circulating tumour cells in patients with solid pancreatic tumours. Br. J. Cancer. 2017;117:1017. doi: 10.1038/bjc.2017.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kodahl A.R., Lyng M.B., Binder H., Cold S., Gravgaard K., Knoop A.S., Ditzel H.J. Novel circulating microRNA signature as a potential non-invasive multi-marker test in ER-positive early-stage breast cancer: A case control study. Mol. Oncol. 2014;8:874–883. doi: 10.1016/j.molonc.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heneghan H.M., Miller N., Lowery A.J., Sweeney K.J., Newell J., Kerin M.J. Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann. Surg. 2010;251:499–505. doi: 10.1097/SLA.0b013e3181cc939f. [DOI] [PubMed] [Google Scholar]
- 82.Iorio M.V., Casalini P., Piovan C., Braccioli L., Tagliabue E. Breast cancer and microRNAs: Therapeutic impact. Breast. 2011;20:S63–S70. doi: 10.1016/S0960-9776(11)70297-1. [DOI] [PubMed] [Google Scholar]
- 83.Sapountzi E.A., Tragoulias S.S., Kalogianni D.P., Ioannou P.C., Christopoulos T.K. Lateral flow devices for nucleic acid analysis exploiting quantum dots as reporters. Anal. Chim. Acta. 2015;864:48–54. doi: 10.1016/j.aca.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 84.Trevino V., Falciani F., Barrera-Saldaña H.A. DNA microarrays: A powerful genomic tool for biomedical and clinical research. Mol. Med. 2007;13:527–541. doi: 10.2119/2006-00107.Trevino. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chikkaveeraiah B.V., Bhirde A.A., Morgan N.Y., Eden H.S., Chen X. Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano. 2012;6:6546–6561. doi: 10.1021/nn3023969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi W., Friedman A.K., Baker L.A. Nanopore sensing. Anal. Chem. 2017;89:157. doi: 10.1021/acs.analchem.6b04260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu G., Mao X., Phillips J.A., Xu H., Tan W., Zeng L. Aptamer-nanoparticle strip biosensor for sensitive detection of cancer cells. Anal. Chem. 2009;81:10013–10018. doi: 10.1021/ac901889s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Labib M., Khan N., Ghobadloo S.M., Cheng J., Pezacki J.P., Berezovski M.V. Three-mode electrochemical sensing of ultralow MicroRNA levels. J. Am. Chem. Soc. 2013;135:3027–3038. doi: 10.1021/ja308216z. [DOI] [PubMed] [Google Scholar]
- 89.Mahmoudi T., de la Guardia M., Baradaran B. Lateral flow assays towards point-of-care cancer detection: A review of current progress and future trends. TrAC Trends Anal. Chem. 2020;125:115842. doi: 10.1016/j.trac.2020.115842. [DOI] [Google Scholar]
- 90.Martin D.R., Sibuyi N.R., Dube P., Fadaka A.O., Cloete R., Onani M., Madiehe A.M., Meyer M. Aptamer-based diagnostic systems for the rapid screening of TB at the point-of-care. Diagnostics. 2021;11:1352. doi: 10.3390/diagnostics11081352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blažková M., Koets M., Rauch P., van Amerongen A. Development of a nucleic acid lateral flow immunoassay for simultaneous detection of listeria spp. and listeria monocytogenes in food. Eur. Food Res. Technol. 2009;229:867–874. doi: 10.1007/s00217-009-1115-z. [DOI] [Google Scholar]
- 92.Andryukov B.G. Six decades of lateral flow immunoassay: From determining metabolic markers to diagnosing COVID-19. AIMS Microbiol. 2020;6:280–304. doi: 10.3934/microbiol.2020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Y., Zhan L., Qin Z., Sackrison J., Bischof J.C. Ultrasensitive and highly specific lateral flow assays for point-of-care. Diagnosis. 2021;15:3593–3611. doi: 10.1021/acsnano.0c10035. [DOI] [PubMed] [Google Scholar]
- 94.Sajid M., Kawde A.N., Daud M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015;19:689–705. doi: 10.1016/j.jscs.2014.09.001. [DOI] [Google Scholar]
- 95.Jauset-Rubio M., Svobodová M., Mairal T., McNeil C., Keegan N., Saeed A., Abbas M.N., El-Shahawi M.S., Bashammakh A.S., Alyoubi A.O., et al. Ultrasensitive, rapid and inexpensive detection of DNA using paper based lateral flow assay. Sci. Rep. 2016;6:37732. doi: 10.1038/srep37732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen A., Yang S. Replacing antibodies with aptamers in lateral flow immunoassay. Biosens. Bioelectron. 2015;71:230–242. doi: 10.1016/j.bios.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 97.Quesada-González D., Merkoçi A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015;73:47–63. doi: 10.1016/j.bios.2015.05.050. [DOI] [PubMed] [Google Scholar]
- 98.Zhang M., Wang X., Han L., Niu S., Shi C., Ma C. Rapid detection of foodborne pathogen Listeria monocytogenes by strand exchange amplification. Anal. Biochem. 2018;545:38–42. doi: 10.1016/j.ab.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 99.Mao X., Wang W., Du T.E. Dry-reagent nucleic acid biosensor based on blue dye doped latex beads and lateral flow strip. Talanta. 2013;114:248–253. doi: 10.1016/j.talanta.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 100.Shen G., Zhang S., Hu X. Signal enhancement in a lateral flow immunoassay based on dual gold nanoparticle conjugates. Clin. Biochem. 2013;46:1734–1738. doi: 10.1016/j.clinbiochem.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 101.Cantera J.L., White H., Diaz M.H., Beall S.G., Winchell J.M., Lillis L., Kalnoky M., Gallarda J., Boyle D.S. Assessment of eight nucleic acid amplification technologies for potential use to detect infectious agents in low-resource settings. PLoS ONE. 2019;14:e0215756. doi: 10.1371/journal.pone.0215756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang D., Ma J., Zhang Q., Li N., Yang J., Raju P.A., Peng M., Luo Y., Hui W., Chen C., et al. Polyelectrolyte-coated gold magnetic nanoparticles for immunoassay development: Toward point of care diagnostics for syphilis screening. Anal. Chem. 2013;85:6688–6695. doi: 10.1021/ac400517e. [DOI] [PubMed] [Google Scholar]
- 103.Kalligosfyri P., Nikou S., Bravou V., Kalogianni D.P. Liquid biopsy genotyping by a simple lateral flow strip assay with visual detection. Anal. Chim. Acta. 2021;1163:338470. doi: 10.1016/j.aca.2021.338470. [DOI] [PubMed] [Google Scholar]
- 104.Tan C., Du X. KRAS mutation testing in metastatic colorectal cancer. World J. Gastroenterol. 2012;18:5171. doi: 10.3748/WJG.V18.I37.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhu H., Zhang H., Xu Y., Laššáková S., Korabečná M., Neužil P. PCR past, present and future. Biotechniques. 2020;69:317–325. doi: 10.2144/btn-2020-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Paul R., Ostermann E., Wei Q. Advances in point-of-care nucleic acid extraction technologies for rapid diagnosis of human and plant diseases. Biosens. Bioelectron. 2020;169:112592. doi: 10.1016/j.bios.2020.112592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moehling T.J., Choi G., Dugan L.C., Salit M., Meagher R.J. LAMP diagnostics at the point-of-care: Emerging trends and perspectives for the developer community. Expert Rev. Mol. Diagn. 2021;21:43–61. doi: 10.1080/14737159.2021.1873769. [DOI] [PubMed] [Google Scholar]
- 108.Warkiani M.E., Guan G., Luan K.B., Lee W.C., Bhagat A.A.S., Kant Chaudhuri P., Tan D.S.W., Lim W.T., Lee S.C., Chen P.C.Y., et al. Slanted spiral microfluidics for the ultra-fast, label-free isolation of circulating tumor cells. Lab Chip. 2014;14:128–137. doi: 10.1039/C3LC50617G. [DOI] [PubMed] [Google Scholar]
- 109.Wong Y., Othman S., Lau Y., Radu S., Chee H. Loop-mediated isothermal amplification (LAMP): A versatile technique for detection of micro-organisms. J. Appl. Microbiol. 2018;124:626–643. doi: 10.1111/jam.13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen D., Mauk M., Qiu X., Liu C., Kim J., Ramprasad S., Ongagna S., Abrams W.R., Malamud D., Corstjens P.L.A.M., et al. An integrated, self-contained microfluidic cassette for isolation, amplification, and detection of nucleic acids. Biomed. Microdevices. 2010;12:705–719. doi: 10.1007/s10544-010-9423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Horvath P., Barrangou R. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010;327:167–170. doi: 10.1126/science.1179555. [DOI] [PubMed] [Google Scholar]
- 112.Pavagada S., Channon R.B., Chang J.Y.H., Kim S.H., MacIntyre D., Bennett P.R., Terzidou V., Ladame S. Oligonucleotide-templated lateral flow assays for amplification-free sensing of circulating microRNAs. Chem. Commun. 2019;55:12451–12454. doi: 10.1039/C9CC05607F. [DOI] [PubMed] [Google Scholar]
- 113.Ribeiro C., Gante I., Dias M.F., Gomes A., Silva H.C. A new application to one-step nucleic acid amplification-discarded sample in breast cancer: Preliminary results. Mol. Clin. Oncol. 2021;15:216. doi: 10.3892/mco.2021.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beretov J., Wasinger V.C., Millar E.K.A., Schwartz P., Graham P.H., Li Y. Proteomic analysis of urine to identify breast cancer biomarker candidates using a label-free LC-MS/MS approach. PLoS ONE. 2015;10:e0141876. doi: 10.1371/journal.pone.0141876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.MarketsandMarketsTM INC Lateral Flow Assays Market worth $12.6 Billion by 2026—Report by MarketsandMarketsTM. [(accessed on 13 July 2022)]. Available online: https://www.marketsandmarkets.com/PressReleases/lateral-flow-assay.asp.
- 116.Labmedica Point-of-Care Lateral Flow Test Detects Bladder Cancer Using Urine Sample within Minutes—Molecular Diagnostics. [(accessed on 7 July 2022)]. Available online: https://www.labmedica.com/molecular-diagnostics/articles/294791303/point-of-care-lateral-flow-test-detects-bladder-cancer-using-urine-sample-within-minutes.html.
- 117.Jani I.V., Peter T.F. Nucleic acid point-of-care testing to improve diagnostic preparedness. Clin. Infect. Dis. 2022:ciac013. doi: 10.1093/cid/ciac013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.University Research, Co Rapid Test Improves TB Diagnosis in People with HIV in South Africa—URC. [(accessed on 11 July 2022)]. Available online: https://www.urc-chs.com/news/rapid-test-improves-tb-diagnosis-in-people-with-hiv-in-south-africa/
- 119.Sun Y., Qin P., He J., Li W., Shi Y., Xu J., Wu Q., Chen Q., Li W., Wang X., et al. Rapid and simultaneous visual screening of SARS-CoV-2 and influenza virufses with customized isothermal amplification integrated lateral flow strip. Biosens. Bioelectron. 2022;197:113771. doi: 10.1016/j.bios.2021.113771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aveyard J., Mehrabi M., Cossins A., Braven H., Wilson R. One step visual detection of PCR products with gold nanoparticles and a nucleic acid lateral flow (NALF) device. Chem. Commun. 2007;41:4251–4253. doi: 10.1039/b708859k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.