Abstract
The Escherichia coli chemoreceptors and their associated cytoplasmic proteins, CheA and CheW, cluster predominantly at the cell poles. The nature of the clustering remains a mystery. Recent studies suggest that CheR binding to and/or methylation of the chemoreceptors may play a role in chemoreceptor complex aggregation. In this study, we examined the intracellular distribution of the chemoreceptors by immunoelectron microscopy in strains lacking either the methyltransferase CheR or the methylesterase CheB. The localization data revealed that, in vivo, aggregation of the chemoreceptor complex was independent of either CheR or CheB.
Escherichia coli cells are able to respond to changes in environmental chemoeffector concentrations through reversal of their flagellar motors (for recent reviews, see references 6, 13, and 17). Cell behavior depends upon the nature of the stimulus. Attractants promote counterclockwise rotation of the flagella, resulting in smooth swimming, whereas repellents promote clockwise rotation, resulting in tumbling. These responses are mediated by membrane-bound methyl-accepting chemotaxis proteins (MCPs). Chemoeffectors bind to the amino-terminal periplasmic domain of the MCP. The ligand occupancy state is communicated to the carboxyl-terminal cytoplasmic signaling domain and its associated proteins, CheA and CheW. The MCP, CheA, and CheW proteins form ternary complexes that are clustered predominantly at the cell poles, and the polar clustering of each protein requires association with the other proteins (16). The role of chemoreceptor complex clustering is unknown. However, clustering may enhance communication between chemoreceptors or mediate signal amplification (3). The ability of ternary complexes to stimulate CheA in vitro is correlated with the formation of higher-order receptor structures (15) which might correspond to a receptor cluster in vivo.
The cytoplasmic signaling domain also contains several reversibly methylatable glutamate side chains, which are located in two segments, designated K1 and R1 (10, 11, 18, 22, 25). Methyl groups are transferred from S-adenosylmethionine to the chemoreceptors by CheR, a 31-kDa methyltransferase (23), and hydrolyzed to methanol by CheB, a 35-kDa methylesterase (24). Recently, it has been demonstrated that CheR binds directly to a 5-amino-acid sequence (NWETF) that is present at the carboxyl terminus of the high-abundance (Tar and Tsr) receptors but not the low-abundance (Trg and Tap) receptors (4, 26). In vitro, Tar is a better acceptor for methylation than Trg (2), and the difference in methylation is attributable to the presence or absence of the NWETF binding site for CheR (2). Methylation of the chemoreceptors can occur between dimers, further demonstrating a critical role for a close association between ternary complexes (12, 14).
Given the direct binding of CheR to the high-abundance chemoreceptors, the aggregation of the chemoreceptor complexes in vivo, and the close association between chemoreceptors that is necessary for interdimer methylation, we envision that either (i) CheR binding to the high-abundance transducers mediates the aggregation of the chemoreceptor complex or (ii) CheR is recruited to the aggregates, where it facilitates interdimer methylation. To test directly whether the CheR protein is necessary for chemoreceptor complex aggregation, we examined the intracellular location of the chemoreceptors in a strain lacking CheR. In addition, we performed a similar analysis of a strain lacking the CheB protein.
The spatial distribution of chemoreceptors in exponentially grown E. coli RP437 (F− thi thr leu his met eda rpsL) and derivatives RP1254 and RP4792 was analyzed by immunogold electron microscopy, using preadsorbed anti-Tsr antibody (1) as previously described (8, 16). RP1254 and RP4792 carry nonpolar deletions of cheR and cheB, respectively (20a). The strains were grown in parallel and embedded simultaneously. Cell sections were incubated in a 1:500 dilution of anti-Tsr in PBST+2%BSA (140 mM NaCl, 2 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, 0.05% Tween 20, 2% bovine serum albumin). The primary antibody was preadsorbed with acetone powders prepared from E. coli KO607 (19), which lacks the four major chemoreceptors, and visualized with a secondary antibody, goat anti-rabbit immunoglobulin G coupled with 12-nm-diameter colloidal gold particles (Jackson Immunoresearch), diluted 1:30 in PBST+2%BSA. The antibody reactions were performed simultaneously to reduce variability due to background noise from the antibody reactions.
We previously demonstrated that the MCPs are predominantly clustered at the cell poles (80% of membrane particles at the poles, and 81% of polar gold particles in clusters) in cells grown in minimal medium at 32°C. In addition, we showed a similar pattern of polar clustering for the cytoplasmic proteins CheA and CheW. The polar clustering of each protein in the chemoreceptor ternary complex required the presence of the other two proteins (16). Therefore, the location of the ternary complex can be effectively determined by the examination of one member of the complex. In this study, we chose to examine the distribution of the complex by determining the position of the MCPs, since high-affinity anti-MCP antibodies were readily available.
First, we examined the distribution of the MCPs in chemotactically wild-type cells (RP437) grown in tryptone broth (TB) (1% tryptone, 0.5% NaCl) at 32°C, conditions often used in the characterization of chemotaxis mutants. The positions of colloidal gold particles in 80 longitudinal sections of nonseptating cells were examined and recorded as defined previously (8, 16). Using these growth conditions, we observed a pattern of MCP localization similar to that seen in cells grown in minimal medium (16). The majority (87%) of the membrane-associated gold particles were at the cell poles, and 92% of these particles were in clusters (Table 1). The few MCPs along the lateral edges of the cell also tended to be clustered (52%). As had been previously observed, the polar clusters were significantly larger than the lateral clusters (10 and 7 gold particles, respectively) (Table 2). Thus, the distributions of chemoreceptor complexes in exponential-phase cells grown in minimal medium and TB are comparable.
TABLE 1.
Spatial distribution of chemoreceptorsa
| Strain | Total no. of particles | % of total particles in:
|
Polar membrane particles
|
Lateral membrane particles
|
|||
|---|---|---|---|---|---|---|---|
| Cyto-plasm | Mem-brane | % | % in clusters | % | % in clusters | ||
| RP437 (wild type) | 634 | 2 | 98 | 87 | 92 | 13 | 52 |
| RP4792 (ΔcheB) | 1,658 | 3 | 97 | 87 | 87 | 13 | 57 |
| RP1254 (ΔcheR) | 1,275 | 3 | 97 | 88 | 88 | 12 | 61 |
Cells were prepared for immunoelectron microscopy as described in the text. Eighty longitudinal sections of RP437 and 160 longitudinal sections each of RP4792 and RP1254 were examined.
TABLE 2.
Size and distribution of clusters in chemotactically wild-type and nonchemotactic E. coli strainsa
| Strain | Polar clusters
|
Lateral clusters
|
||
|---|---|---|---|---|
| No. | Size | No. | Size | |
| RP437 (wild type) | 50 | 9.9 ± 0.6 | 6 | 7.0 ± 1.3 |
| RP4792 (ΔcheB) | 125 | 9.8 ± 0.5 | 15 | 7.7 ± 0.9 |
| RP1254 (ΔcheR) | 109 | 8.8 ± 0.4 | 12 | 8.8 ± 1.6 |
Cells were prepared for immunoelectron microscopy as described in the text. Eighty longitudinal sections of RP437 and 160 longitudinal sections each of RP4792 and RP1254 were examined. The number of gold particle clusters located either in the polar or lateral membrane was determined. The mean size (number of gold particles) and standard deviation of the clusters are presented for each strain.
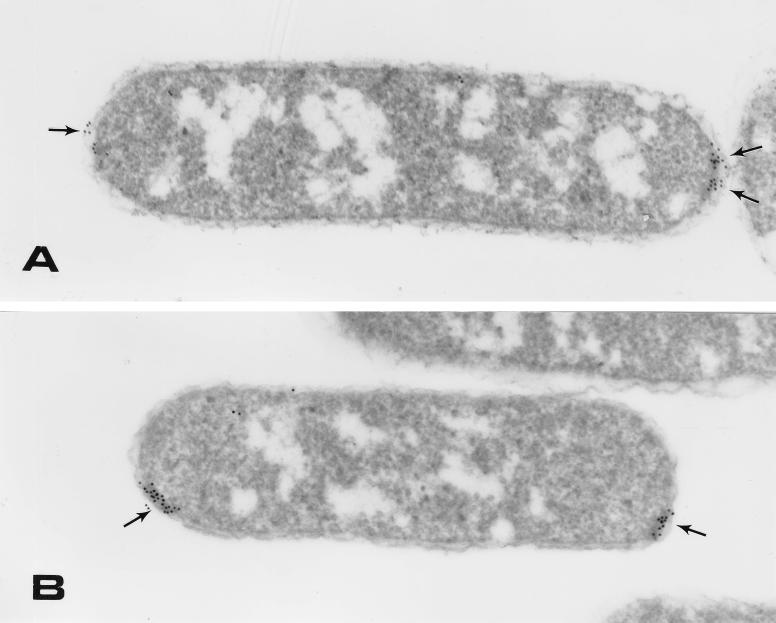
Next, we examined the spatial distribution of MCPs in nonchemotactic cells lacking either CheR or CheB. Remarkably, the distribution of MCPs was similar to that observed in chemotactically wild-type cells (Fig. 1 and Tables 1 and 2). In RP4792 (ΔcheB), the majority (87%) of the MCPs were at the cell poles, and 87% of these MCPs were in clusters of approximately 10 gold particles. Smaller lateral clusters (eight gold particles) were observed as well. In RP1254 (ΔcheR), 88% of the membrane-associated gold particles were at the cell poles and 88% of these particles were in clusters. The size and abundance of the lateral clusters were similar to those of the other strains (61% of lateral spots were in clusters with a size of nine gold particles).
FIG. 1.
Immunolocalization of chemoreceptors. Immunoelectron micrographs depicting the intracellular position of MCPs in strains lacking either CheR (strain RP1254) (A) or CheB (strain RP4792) (B) are shown. Antibody reactions were performed as described in the text. Sections were poststained with 1% uranyl acetate and examined on a Philips CM10 electron microscope at 60 kV.
These data demonstrate that neither CheR nor CheB are necessary for the aggregation of the chemoreceptor complex in vivo. Receptor clustering may still play a crucial role in allowing communication between receptors; for example, oligomerization of the receptors could facilitate interdimer methylation by CheR.
These data imply that the methylation state, and possibly the signaling state, of the chemoreceptors does not affect in vivo clustering of the chemoreceptor complex. E. coli cells require the CheR and CheB proteins in order to adapt appropriately, and yet we observed optimal chemoreceptor clustering in strains that should display very different methylation states. In cells lacking CheR, MCP methylation is reduced (7), and there is an extreme counterclockwise bias resulting in constant smooth swimming behavior (21). In the absence of CheB, MCP methylation levels are very high (9) and there is an extreme clockwise bias resulting in constant tumbling behavior (20). Here we show that chemoreceptor clustering, as monitored by immunoelectron microscopy, is not affected by the signaling state of the receptors.
Finally, in vitro, large chemoreceptor bundles composed of seven MCP dimers, four CheW monomers, and one CheA dimer have been observed (15). The relationship between the chemotaxis bundles observed in vitro and the receptor clustering observed in vivo is not known. Methylation plays a critical role in the stability of the in vitro bundles (15), yet chemoreceptor aggregation in vivo is not affected in mutants lacking the methyltransferase or methylesterase. Clearly, further studies are warranted to determine the relationship between bundles and clusters, to characterize the mechanism by which receptor clustering occurs, and to elucidate the function of the in vivo aggregations.
Acknowledgments
We thank Sandy Parkinson for continued gifts of strains and antibodies and David Parker for technical assistance. We also thank Sandy Parkinson, Mike Manson, and Sue Sullivan for critical evaluation of the manuscript and Ken Balazovich for unwavering patience and retrieval of critical tools.
This research was supported in part by National Institute of Health grant GM-55133.
REFERENCES
- 1.Ames P, Parkinson J S. Constitutively signaling fragments of Tsr, the Escherichia coli serine chemoreceptor. J Bacteriol. 1994;176:6340–6348. doi: 10.1128/jb.176.20.6340-6348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnakov A N, Barnakova L A, Hazelbauer G L. Comparison in vitro of a high- and a low-abundance chemoreceptor of Escherichia coli: similar kinase activation but different methyl-accepting activities. J Bacteriol. 1998;180:6713–6718. doi: 10.1128/jb.180.24.6713-6718.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray D, Levin M D, Morton-Firth C J. Receptor clustering as a cellular mechanism to control sensitivity. Nature. 1998;393:85–88. doi: 10.1038/30018. [DOI] [PubMed] [Google Scholar]
- 4.Djordjevic S, Stock A M. Chemotaxis receptor recognition by protein methyltransferase CheR. Nat Struct Biol. 1998;5:446–450. doi: 10.1038/nsb0698-446. [DOI] [PubMed] [Google Scholar]
- 5.Falke J J, Bass R B, Butler S L, Chervitz S A, Danielson M A. The two-component signaling pathway of bacterial chemotaxis: a molecular view of signal transduction by receptors, kinases, and adaptation enzymes. Annu Rev Cell Dev Biol. 1997;13:457–512. doi: 10.1146/annurev.cellbio.13.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goudreau P N, Stock A M. Signal transduction in bacteria: molecular mechanisms of stimulus-response coupling. Curr Opin Microbiol. 1998;1:160–169. doi: 10.1016/s1369-5274(98)80006-4. [DOI] [PubMed] [Google Scholar]
- 7.Goy M F, Springer M S, Adler J. Failure of sensory adaptation in bacterial mutants that are defective in a protein methylation reaction. Cell. 1978;15:1231–1240. doi: 10.1016/0092-8674(78)90049-1. [DOI] [PubMed] [Google Scholar]
- 8.Harrison D M, Skidmore J, Armitage J P, Maddock J R. Localization and environmental regulation of MCP-like proteins in Rhodobacter sphaeroides. Mol Microbiol. 1999;31:885–892. doi: 10.1046/j.1365-2958.1999.01226.x. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi H, Koiwai O, Kozuka M. Studies on bacterial chemotaxis. II. Effect of cheB and cheZ mutations on the methylation of methyl-accepting chemotaxis protein of Escherichia coli. J Biochem. 1979;85:1213–1223. [PubMed] [Google Scholar]
- 10.Kehry M R, Dahlquist F W. The methyl-accepting chemotaxis proteins of Escherichia coli. Identification of the multiple methylation sites on methyl-accepting chemotaxis protein I. J Biol Chem. 1982;257:10378–10386. [PubMed] [Google Scholar]
- 11.Kehry M R, Engstrom P, Dahlquist F W, Hazelbauer G L. Multiple covalent modifications of Trg, a sensory transducer of Escherichia coli. J Biol Chem. 1983;258:5050–5055. [PubMed] [Google Scholar]
- 12.Le Moual H, Quang T, Koshland D E J. Methylation of the Escherichia coli chemotaxis receptors: intra- and interdimer mechanisms. Biochemistry. 1997;36:13441–13448. doi: 10.1021/bi9713207. [DOI] [PubMed] [Google Scholar]
- 13.Levit M N, Liu Y, Stock J B. Stimulus response coupling in bacterial chemotaxis: receptor dimers in signalling arrays. Mol Microbiol. 1998;30:459–466. doi: 10.1046/j.1365-2958.1998.01066.x. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Li G, Weis R M. The serine chemoreceptor from Escherichia coli is methylated through an inter-dimer process. Biochemistry. 1997;36:11851–11857. doi: 10.1021/bi971510h. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Levit M, Lurz R, Surette M G, Stock J B. Receptor-mediated protein kinase activation and the mechanism of transmembrane signaling in bacterial chemotaxis. EMBO J. 1997;16:7231–7240. doi: 10.1093/emboj/16.24.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maddock J R, Shapiro L. Polar location of the chemoreceptor complex in the Escherichia coli cell. Science. 1993;259:1717–1723. doi: 10.1126/science.8456299. [DOI] [PubMed] [Google Scholar]
- 17.Manson M D, Armitage J P, Hoch J A, Macnab R M. Bacterial locomotion and signal transduction. J Bacteriol. 1998;180:1009–1022. doi: 10.1128/jb.180.5.1009-1022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowlin D M, Bollinger J, Hazelbauer G L. Sites of covalent modification in Trg, a sensory transducer of Escherichia coli. J Biol Chem. 1987;258:6039–6045. [PubMed] [Google Scholar]
- 19.Oosawa K, Mutoh N, Simon M I. Cloning of the C-terminal cytoplasmic fragment of the Tar protein and effects of the fragment on chemotaxis of Escherichia coli. J Bacteriol. 1988;170:2521–2526. doi: 10.1128/jb.170.6.2521-2526.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parkinson J S. cheA, cheB, and cheC genes of Escherichia coli and their role in chemotaxis. J Bacteriol. 1976;126:758–770. doi: 10.1128/jb.126.2.758-770.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Parkinson, J. S. Personal communication.
- 21.Parkinson J S, Revello P T. Sensory adaptation mutants of E. coli. Cell. 1978;15:1221–1230. doi: 10.1016/0092-8674(78)90048-x. [DOI] [PubMed] [Google Scholar]
- 22.Rice M S, Dahlquist F W. Sites of deamidation and methylation in Tsr, a bacterial chemotaxis sensory transducer. J Biol Chem. 1991;266:9746–9753. [PubMed] [Google Scholar]
- 23.Springer W, Koshland D E J. Identification of a protein methyltransferase as the cheR gene product in the bacterial sensing system. Proc Natl Acad Sci USA. 1977;74:533–537. doi: 10.1073/pnas.74.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stock J B, Koshland D E J. A protein methylesterase involved in bacterial sensing. Proc Natl Acad Sci USA. 1978;75:3659–3663. doi: 10.1073/pnas.75.8.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terwilliger T C, Koshland D E J. Sites of methyl esterification and deamination on the aspartate receptor involved in chemotaxis. J Biol Chem. 1984;259:7719–7725. [PubMed] [Google Scholar]
- 26.Wu J, Li J, Li G, Long D G, Weis R M. The receptor binding for the methyltransferase of bacterial chemotaxis is distinct from the sites of methylation. Biochemistry. 1996;35:4984–4993. doi: 10.1021/bi9530189. [DOI] [PubMed] [Google Scholar]