Abstract
Acute ST elevation myocardial infarction (STEMI) remains a leading cause of morbidity and mortality worldwide despite continuous advances in diagnostic, prognostic and therapeutic methods. Myocardial work (MW) indices and miRNAs have both emerged as potential prognostic markers in acute coronary syndromes in recent years. In this study we aim to assess the prognostic role of myocardial work indices and of a group of miRNAs in young patients with STEMI. We enrolled 50 young patients (<55 years) with STEMI who underwent primary PCI and 10 healthy age-matched controls. We performed standard 2D and 3D echocardiography; we also calculated left ventricular global longitudinal strain (GLS) and the derived myocardial work indices. Using RT-PCR we determined the plasmatic levels of six miRNAs: miR-223-3p, miR-142-3p, miR-146a-5p, miR-125a-5p, miR-486-5p and miR-155-5p. We assessed the occurrence of major adverse cardiac events (MACE) at up to one year after STEMI. Out of 50 patients, 18% experienced MACE at the one-year follow-up. In a Cox univariate logistic regression analysis, myocardial work indices were all significantly associated with MACE. The ROC analysis showed that GWI, GCW and GWE as a group have a better predictive value for MACE than each separately (AUC 0.951, p = 0.000). Patients with higher miRNAs values at baseline (miR-223-3p, miR-142-3p and miR-146a-5p) appear to have a higher probability of developing adverse events at 12 months of follow-up. ROC curves outlined for each variable confirmed their good predictive value (AUC = 0.832, p = 0.002 for miR-223-3p; AUC = 0.732, p = 0.031 for miR-142-3p and AUC = 0.848, p = 0.001 for miR-146a-5p); the group of three miRNAs also proved to have a better predictive value for MACE together than separately (AUC = 0.862). Moreover, adding each of the miRNAs (miR-233, miR-142-3p and miR-146a-5p) or all together over the myocardial work indices in the regression models improved their prognostic value. In conclusion, both myocardial work indices (GWI, GCW and GWE) and three miRNAs (miR-223-3p, miR-142-3p and miR-146a-5p) have the potential to be used as prognostic markers for adverse events after acute myocardial infarction. The combination of miRNAs and MW indices (measured at baseline) rather than each separately has very good predictive value for MACE in young STEMI patients (C-statistic 0.977).
Keywords: STEMI, young, MACE, myocardial work indices, miRNA
1. Introduction
Ischaemic heart disease remains to this day a leading cause of morbidity and mortality worldwide [1,2]. Although myocardial infarction is considered a disease occurring in older adults, in recent years its prevalence in the young population has increased [3,4]. So far, there has been no standard definition of “young” age in patients with STEMI. Previous studies used different age thresholds, varying from <30 years to <55 years [5,6,7]. We decided to use the broader cut off and included in our study adult patients, younger than 55 years old.
Patients with an acute coronary syndrome at a young age are at high risk for other future major cardiovascular events; therefore, an optimal follow-up and preventive strategies are of paramount importance in this population category [8] Considering the significant health and socioeconomic burden of AMI in younger patients, the discovery of new markers is essential to improve the diagnosis, risk stratification and prediction of adverse events in this group.
1.1. Myocardial Work Indices
Left ventricular systolic function is one of the most important and used prognostic marker after STEMI. It is traditionally expressed by left ventricular ejection fraction (LVEF), based on systolic and diastolic volume changes. However, LVEF is subjective, there are technical limitations and inconsistency, impaired reproducibility and a high interobserver variability [9]. Myocardial strain analysis has been developed as a more comprehensive technique for the evaluation of LV function enabling the assessment of global and regional myocardial deformation during the cardiac cycle [10]. Moreover, prior studies have advocated that LV GLS measured after STEMI has incremental prognostic value over LVEF [11,12]. Unfortunately, like LVEF, LV GLS is afterload-dependent, especially in patients with impaired left ventricular function, but to a lesser extent [13].
Echocardiographic assessment of myocardial work may further improve the evaluation of myocardial function. This non-invasive method is based on a standardised LV pressure curve adjusted to arterial pressure. Myocardial work parameters offer an estimation of LV systolic function taking into account loading conditions; therefore, this method is very promising for evaluating the failing heart and predicting prognosis [14,15]. There are four indices that can be assessed noninvasively with 2D speckle tracking imaging for myocardial work evaluation: global work index (GWI), global contraction work (GCW), global wasted work (GWW) and global work efficiency (GWE). Their prognostic potential has so far been assessed in various cardiovascular pathologies such as mitral regurgitation [16], aortic stenosis [17], advanced heart failure [18] and acute coronary syndromes [19]. In acute myocardial infarction, two studies have shown promising results: Lustosa et al., who tested the long-term prognostic value of GWE in STEMI patients, found that lower GWE values in the acute phase were associated with worse long-term survival [20] and Butcher et al. concluded that lower GWI values were independently associated with increased all-cause mortality at 6 months of follow-up [21]. However, the research regarding myocardial work indices in STEMI focused mainly on one parameter rather than testing the predictive power of all four indices; moreover, so far, no study has tested these echocardiographic markers in young patients. Therefore, we aim to evaluate the prognostic potential of all myocardial work indices as MACE predictors in young STEMI patients.
1.2. microRNAs
In recent years, microRNAs (miRNAs) have appeared as promising diagnostic and prognostic markers involved in the pathophysiology of cardiovascular diseases [22]. Many studies suggest that miRNAs play crucial roles in a variety of essential biological processes, including proliferation, development, differentiation and apoptosis [23]. Their small size, simple chemical composition, high stability, capability to withstand extreme conditions and a cost-effective quantification by RT-PCR make them excellent diagnostic and prognostic markers [22,24]. In addition, many miRNAs are remarkably stable and easily detectable in the peripheral blood [25,26]. The levels of circulating miRNAs are different in specific ways under specific pathological conditions [27,28,29]. This indicates that circulating miRNAs may be excellent candidate diagnostic and prognostic biomarkers of various diseases [30,31].
In this study, we tested six miRNAs (miR-233, miR-142-3p, miR-155-5p, miR-486-5p, miR-125a-5p and miR-146a-5p), known to be associated with coronary artery disease from previous research. MiR-233-3p is almost exclusively of platelet or megakaryocyte origin [32]; the biological activity of miR-223-3p is related to aggregation and granule secretion [32]. It has proved to be a marker of atherosclerotic plaque instability in patients with CAD [33] and also a predictor of thrombotic events that could be used for ischemic risk stratification after AMI [34]. MiR-142-3p plays a role in various inflammatory diseases, such as atherosclerosis [35]. Higher plasmatic levels of miR-142-3p were potential markers to predict MACE in CAD patients after PCI in a study form 2019 [36]. MiR-146a-5p exhibits a protective effect against cardiac ischaemia/hypoxia-induced apoptosis [37] and is also related to coronary artery disease (CAD) [38,39,40]. miR-146a-5p is also expressed in vascular endothelial cells, smooth muscle cells and monocytes/macrophages, and regulates the development of atherosclerosis by acting on different target genes [41,42,43]. It has proved to be a negative feedback regulator of inflammatory response; it is involved in regulating innate immune responses and its expression in myocardial tissue has been reported to increase with the onset of MI [44]. MiR-125a-5p is thought to regulate macrophage activation, lipid metabolism and the regulation of atherogenesis [45] critical processes in coronary artery disease [46] MiR-486-5p is a muscle-enriched miRNA, found to be upregulated in patients with acute coronary syndrome; Zhang et al. proved its diagnostic potential in AMI [47]. MiR-155-5p is an inflammatory-related miRNA, upregulated in activated inflammatory cells; it modulates immune responses via cell differentiation and function and inflammatory cytokine secretion [48,49]. In mice, the decreased expression of the miR-155-5p has been associated with enhanced atherosclerosis, decreased plaque stability and decreased T cell regulation [50].
Considering all the above, we hypothesised that both myocardial work parameters and the six miRNAs might play a role in predicting outcome patients with acute coronary syndromes; therefore, we propose to assess their value as prognostic markers in young patients (<55 years) with STEMI.
2. Materials and Methods
2.1. Study Population
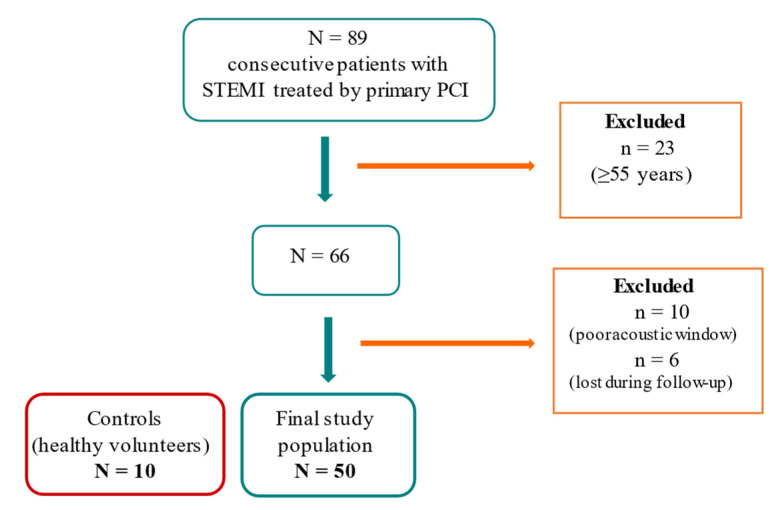
We enrolled in this prospective study young patients (<55 years old) with STEMI admitted to our hospital (in 2019–2020) and treated by primary PCI. We first selected 89 consecutive STEMI patients, excluded 23 of them for being >55 years, 10 were excluded due to poor acoustic window and 6 were lost during follow-up, leaving a final study group of 50 (Figure 1). We also chose 10 age-matched controls (healthy volunteers) for the miRNA results validation.
Figure 1.
Inclusion flow chart of the study.
Patients with previous myocardial infarction or cardiac surgery, recent stroke (within six months), recent surgery or trauma (within 6 months), active malignancy or autoimmune diseases, chronic renal failure (eGFR < 30 mL/min/1.73 m2), chronic liver failure (defined as a Child–Pugh score of 3), chronic respiratory failure (defined as PO2 < 50 mmHg and/or PCO2 > 50 mmHg), patients with addictions, poor compliance or those who refused to sign the informed consent were excluded.
This study was approved by the Ethics Committee from the Clinical Emergency Hospital of Bucharest and all patients signed an informed consent form at enrolment.
2.2. Echocardiography
We performed 2D standard echocardiography in all patients included in this study using a GE VIVID E9 ultrasound system, both at baseline and at follow-up. Baseline evaluation was made within 5 days after admission. At six months of follow-up, we assessed the evolution in time of the echocardiographic parameters.
The recordings and measurements were performed in accordance with the European [51] and American [52] echocardiographic guidelines. An offline data analysis was done by two independent operators experienced in echocardiography using EchoPAC software.
Besides the conventional parameters, we also measured the LV global longitudinal strain and LV mechanical dispersion using the speckle tracking technique and also 3D left ventricle echocardiography, for a better quantification of the ventricular function.
Myocardial Work Analysis
Myocardial work indices were calculated from LV pressure–strain loops by integrating the LV strain data and noninvasively estimating the LV pressure (considered to be equal to arterial blood pressure measured with a brachial cuff sphygmomanometer) [14,20].
The quantification of noninvasive myocardial work was performed using a commercially available software package (EchoPAC, GE Medical Systems).
LV strain data were acquired using 2D speckle-tracking echocardiography by manually tracing the LV endocardial border in the apical long-axis, 2 and 4-chamber views. The peak LV pressure was measured using the patient’s brachial cuff blood pressure recordings with the peak systolic LV pressure assumed to be equal to the peak arterial pressure. An LV pressure–strain curve was then automatically constructed using a reference curve provided by the software package and adjusted to the different cardiac cycle phases using valvular event timing (opening and closing timings of the aortic and mitral valves). LV myocardial work was calculated by integrating the product of the rate of segmental shortening and instantaneous LV pressure over time, thus obtaining myocardial work as a function of time during systole and isovolumic relaxation [21].
The following parameters were obtained from this analysis [14,53,54]:
Global work index (GWI)—the area within the global LV pressure–strain loop (calculated from mitral valve closure to mitral valve opening), representing the total LV work performed in a single cardiac cycle.
Global constructive work (GCW)—the myocardial work performed during the shortening of a myocardial segment in systole and during lengthening in isovolumic relaxation, representing the total work contributing to the pump function.
Global wasted work (GWW)—the negative myocardial work performed during the lengthening of a myocardial segment in systole or during shortening in isovolumic relaxation, and which therefore does not contribute to LV ejection.
Global work efficiency (GWE)—the sum of the constructive work in all LV segments, divided by the sum of the constructive and wasted work in all LV segments; it is expressed as a percentage: GCW/(GCW + GWW).
2.3. Blood Sample Collection and Storage
Whole blood samples harvested in EDTA tubes were obtained by peripheral venous puncture in the first 24–48 h after admission for STEMI. All blood samples were collected after primary PCI, in all patients. Plasma was separated from blood samples after centrifuging (1000× g) for 15 min at −4 °C within 30 min of collection and then aliquoted in Eppendorf tubes (300 µL each) and frozen at −80 °C immediately.
2.4. miRNA Isolation and Quantification
miRNAs were isolated from plasma using the miRNeasy Serum/Plasma kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. A quantity of 25 fmol of synthetic cel-miR-39 was added to each sample during miRNA purification as previously described [38].
The plasmatic levels of (hsa)-miR-223-3p (ID 002295), hsa-miR-146a-5p (ID 000468), hsa-miR-486-5p (ID 001278), hsa-miR-125a-5p (ID 002198), hsa-miR-142-3p (ID 000464) and hsa-miR-155-5p (ID 002623) were determined by TaqMan technology. Reverse-transcription was performed with a pool of TaqMan miRNA-specific stem-loop primers on a Veriti PCR system. Real-time quantitative PCR was performed using the hydrolysis probes of miRNA TaqMan assays on a ViiA7 real-time PCR system (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA) and for each sample, triplicate measurements were done on 384-well reaction plates.
The data were analysed using ViiA7 Software v1.2 with the automatic Cq setting. The expression level of each miRNA was determined relative to that of exogenously added cel-miR-39-2p (ID 000200) as previously reported [55].
2.5. Coronary Angiography
According to the European society of cardiology practice guidelines [56], invasive coronary angiography followed by primary PCI was performed at admission in all patients with STEMI from our study. None of the included patients had significant coronary lesions at hospital discharge.
2.6. Follow-Up and Outcomes
Patients were followed up for up to one year after STEMI. At 6 months, we performed a more detailed follow-up—clinical examination, standard echocardiography—and at one year, a telephone follow-up.
All included patients were followed up for one year after the acute ischemic event. The one-year follow-up consisted of a telephone questionnaire whereas at 6 months, a more detailed examination was performed. At 6 months of follow-up, we performed a clinical examination, electrocardiography, an echocardiographic evaluation and blood harvesting to assess specific biomarkers (miRNAs).
During this one year of follow-up, we assessed the occurrence of MACE. In this study, MACE was defined as death from cardiovascular causes, heart failure requiring hospital admission or repeat PCI/CABG due to ischaemia/infarction (in concordance with previous trials [57]).
It is important to mention that all patients received optimal medical therapy at hospital discharge, according to current clinical practice guidelines [56].
2.7. Statistical Analysis
We performed the statistical analysis using SPSS software (IBM SPSS Statistics v.22.0, IBM Corp., Armonk, NY, USA) and GraphPad software (GraphPad Prism 9.0.0, San Diego, CA, USA). We presented the categorical data as percentages and the continuous variables as means. A Kolmogorov–Smirnov test and Mann–Whitney U-test were used for normal and non-normal distribution of data, respectively. Student t and X2 tests were used to compare continuous and categorical variables. To determine the predictors of MACE, we performed a Cox univariate regression, further incorporating the statistically significant variables in a multivariate analysis. An ROC analysis (receiver operating curve) was used to determine the AUC (area under the curve) representing the predictive power of the tested parameters. We also determined cut-off values for the significant variables using the Youden index. To further test the added values of miRNAs over myocardial work indices, we constructed prediction models and compared their statistical power using the C statistic and Akaike information criterion (AIC). We considered p under 0.05 as statistically significant.
3. Results
3.1. Characteristics of the Study Population
We included 50 young patients (<55 years), mean age of 44.78, with STEMI treated by primary PCI and 10 healthy control subjects (for miRNA results validation). Patients were divided into MACE group (9 cases, accounting for 18%) and non-MACE group (41 cases, accounting for 82%). The baseline characteristics of the entire study group divided according to the presence or absence of MACE are detailed in Table 1.
Table 1.
Baseline characteristics of the entire study population divided in two subgroups according to the occurrence of MACE during follow up.
| Study Population (n = 50) |
MACE (n = 9) |
Without MACE (n = 41) |
p Value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (years) | 44.7 ± 5.62 | 44 ± 3.78 | 45 ± 5.98 | 0.99 |
| Systolic blood pressure (mmHg) | 119.54 ± 16.66 | 120.44 ± 20.35 | 119.34 ± 16.03 | 0.859 |
| Cardiovascular risk factors | ||||
| Smoking | 86% | 77.8% | 87% | 0.370 |
| Obesity | 22% | 0% | 24.2% | 0.109 |
| Hypertension | 46% | 33.3% | 48.8% | 0.321 |
| Dyslipidaemia | 75.6% | 77.8% | 82.9% | 0.517 |
| Diabetes | 17.1% | 11.1% | 12.2% | 0.707 |
| Metabolic syndrome | 12.2% | 40% | 17.6% | 0.248 |
| Clinical presentation | ||||
| Killip class ≥2 | 17% | 77.7% | 4.8% | <0.0001 |
| Angiographic characteristics | ||||
| LAD | 48% | 77.8% | 41.5% | 0.069 |
| RCA | 48% | 77.8% | 41.5% | 0.67 |
| LCX | 24% | 0% | 29.3% | 0.092 |
| Multivessel CAD | 34.6% | 22.2% | 77.8% | 0.459 |
| Occluded artery | 53.8% | 66.7% | 33.3% | 0.713 |
| Symptom to balloon time | 6.6 ± 5.31 | 7.5 ± 5.44 | 6.55 ± 7.26 | 0.692 |
| Laboratory characteristics | ||||
| WBC count, × 103/mm3 | 11,260 ± 3628 | 16,088.89 ± 3417.39 | 13,807 ± 1711.6 | 0.695 |
| Haemoglobin, g/dL | 14.06 ± 1.44 | 13.41 ± 1.24 | 14.02 ± 2.81 | 0.411 |
| Creatinine (mg/dL) | 0.83 ± 0.23 | 0.90 ± 0.40 | 0.82 ± 0.17 | 0.38 |
| Glycaemia (mg/dL) | 118.02 ± 38.62 | 136.22 ± 48.41 | 108.69 ± 33.36 | 0.047 |
| Cholesterol (mg/dL) | 217.21 ± 64.36 | 199.40 ± 67.15 | 224.08 ± 52.61 | 0.347 |
| Triglycerides (mg/dL) | 202.37 ± 181.288 | 125.47 ± 72.66 | 151.61 ± 71.65 | 0.321 |
| HDL-cholesterol | 28.08 ± 11.95 | 26.47 ± 12.30 | 28.47 ± 12.01 | 0.482 |
| LDL-cholesterol | 159.30 ± 53.95 | 147.84 ± 63.52 | 162.09 ± 51.69 | 0.658 |
| Peak CK-MB (U/L) | 251.58 ± 211.26 | 479.67 ± 296.824 | 198.00 ± 144.125 | 0.022 |
3.2. Echocardiographic Parameters
Echocardiographic parameters measured at baseline are reported in Table 2. Patients with MACE at follow-up had lower 2D LVEF (32.88 ± 5.79 vs. 43 ± 6.6 p = 0.000), more impaired LVGLS (−8.85 ± 1.58 vs. −13.8± 2.8, p < 0.0001) and higher 2D LVEDV (118.55 ± 29.43 vs. 99.26 ± 22.27, p = 0.031) and 2D LVESV (81.77 ± 25.36 vs. 54.87 ± 16.55, p = 0.013) at baseline.
Table 2.
Echocardiographic parameters at baseline in the entire study population and divided in two subgroups according to the occurrence of MACE.
| Population | MACE (n = 9) |
Without MACE (n = 41) |
p Value | |
|---|---|---|---|---|
| 2D LVEDV (mL) | 102.74 ± 24.54 | 118.55 ± 29.43 | 99.26 ± 22.27 | 0.031 |
| 2D LVEDV (mL/mp) | 53.97 ± 12.6 | 64.18 ± 13.91 | 51.75 ± 11.28 | 0.06 |
| 2D LVESV (mL) | 59.72 ± 20.91 | 81.77 ± 25.36 | 54.87 ± 16.55 | 0.013 |
| 2D LVESV (mL/mp) | 59.72 ± 20.91 | 81.77 ± 25.36 | 54.87 ± 16.55 | 0.013 |
| 2D EF (%) | 41.94 ± 7.07 | 32.88 ± 5.79 | 43 ± 6.6 | <0.0001 |
| 3D LVEDV (mL) | 113.46 ± 24.46 | 127.66 ± 28.48 | 110.34 ± 22.7 | 0.053 |
| 3D LVEDV (ml/mp) | 59.77 ± 13.02 | 69.24 ± 13.45 | 57.69 ± 12.36 | 0.016 |
| 3D LVESV (mL) | 65.74 ± 21.15 | 87 ± 25.91 | 61.07 ± 17.02 | 0.001 |
| 3D LVESV (mL/mp) | 34.67 ± 11.34 | 47.13 ± 12.73 | 31.93 ± 9.08 | <0.0001 |
| 3D LVEF (%) | 40.02 ± 8.05 | 33 ± 6.55 | 45.24 ± 6.5 | <0.0001 |
| LV GLS | −12.93 ± 2.2 | −8.85 ± 1.58 | −13.8 ± 2.8 | <0.0001 |
| LV mechanical dispersion | 72.57 ± 26.49 | 93.11 ± 29.36 | 68.06 ± 23.9 | 0.009 |
| E/e’ (LV filling pressure) | 8.2 ± 2.92 | 10.68 ± 2.01 | 7.59 ± 2.03 | <0.0001 |
| Myocardial work indices | ||||
| LV GWI, mmHg% | 1089.66 ± 318.97 | 1167.07 ± 295.67 | 737 ± 124.24 | <0.0001 |
| LV GCW, mmHg% | 1430.54 ± 325.37 | 1499.68 ± 304.01 | 1115.55 ± 224.06 | 0.001 |
| LV GWW, mmHg% | 193.14 ± 105.84 | 172.75 ± 96.3 | 286 ± 102.07 | 0.003 |
| LV GWE, % | 86.12 ± 6.55 | 87.95 ± 5.53 | 77.77 ± 3.8 | <0.0001 |
Regarding medical treatment, there was no significant difference between MACE and no-MACE groups.
Echocardiography was performed both at baseline and at 6 months of follow-up. The echocardiographic parameters measured at follow-up are depicted in Table S1. Figure S1 (Supplementary Materials) represents the evolution of the myocardial work indices from baseline to follow-up. GWI, GCW and GWW appear to improve over time, but no significant change is observed in GWW.
3.3. miRNAs
Patients with STEMI had significantly higher levels of miRNA when compared to the control group (p < 0.005). All of the miRNAs associated with cardiovascular disease tested in this study were significantly upregulated in STEMI compared to the control group: miR-233-3p (p = 0.04), miR-142-3p (p = 0.009), miR-155-5p (p = 0.001), miR-486-5p (p = 0.001), miR-125a-5p (p = 0.013) and miR-146a-5p (p = 0.029). miRNA levels at baseline and at 6 months of follow-up are depicted in Figure S2.
We tested the correlations between miRNAs and cardiovascular risk factors (smoking, hypertension, dyslipidaemia and diabetes)—no significant correlations were observed. Interestingly, miR-125a-5p inversely correlated with age (Pearson −0.380, p = 0.006) and miR-142-3p with gender (Pearson −0.359, p = 0.01). miR-223-3p and miR-146a-5p both correlated with the Killip class (Pearson 0.291, p = 0.040 for miR-223-3p and Pearson 0.564, p = 0.000 for miR-146a-5p).
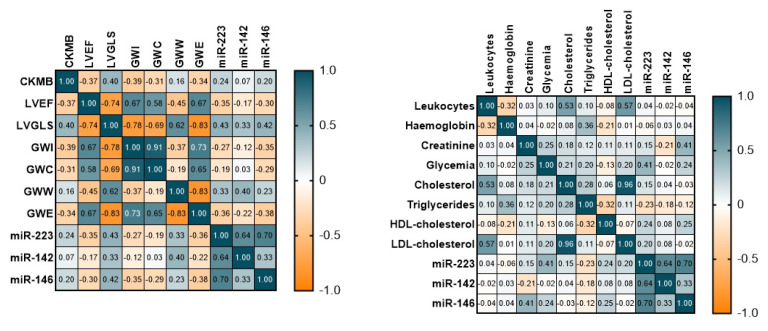
We also evaluated the correlation between echocardiographic parameters and miRNAs: the LV GLS values correlated with miR-223-3p (Pearson 0.429, p = 0.015); miR-142-3p (Pearson 0.335, p = 0.018); miR-4865p (Pearson 0.329, p = 0.02) and miR-146a-5p (Pearson 0.417, p = 0.003). The 2D LVEF values inversely correlated with miR-223-3p (Pearson −0.352, p = 0.012) and miR-146a-5p (Pearson −0.299. p = 0.035). miR-146a-5p inversely correlated with myocardial work indices—GWI (Pearson −0.347, p = 0.014), GCW (Pearson −0.288, p = 0.042) and GWE (Pearson = −0.378, p = 0.007). A few of the correlations are depicted below (Figure 2).
Figure 2.
Correlation matrix: correlation between echocardiographic, biochemical parameters and miRNAs.
3.4. Clinical End Points—MACE
We divided the study cohort in two groups considering the presence or absence of MACE at the one-year follow-up (see Table 1 and Table 2 for detailed information). MACE occurred in 18% of the studied patients—12% readmissions for heart failure, 5% requiring PCI and 2% cardiovascular deaths.
No significant differences between the two groups regarding cardiovascular risk factors, clinical or angiographic characteristics were observed. There were a few differences in laboratory data—patients with MACE had higher CK-MB levels (p = 0.002) and glycaemic levels (p = 0.047). As expected, patients with MACE had a higher Killip class.
Table S2 depicts the associations between the echocardiographic parameters and the occurrence of MACE at follow-up (determined by a Cox regression analysis). We concluded that patients with MACE at follow-up had lower 2D and 3D LVEF, higher LV volumes, higher left ventricular filling pressures and more impaired LV strain.
3.4.1. Myocardial Work Indices as Predictors of MACE
All four myocardial work indices (baseline values) were independent predictors for MACE at follow-up. They remained significant MACE predictors after adjustment for age, gender, 2D LVEF, LV dispersion and LV GLS. Moreover, after checking for collinearity, we obtained that GWI, GWE and GCW had a better predictive value as a group than separately. In the multivariate Cox regression analysis of the three variables, only GWI (p = 0.022, Wald 5.27) and GWE (p = 0.010, Wald 6.55) contributed significantly to the model—Table S3.
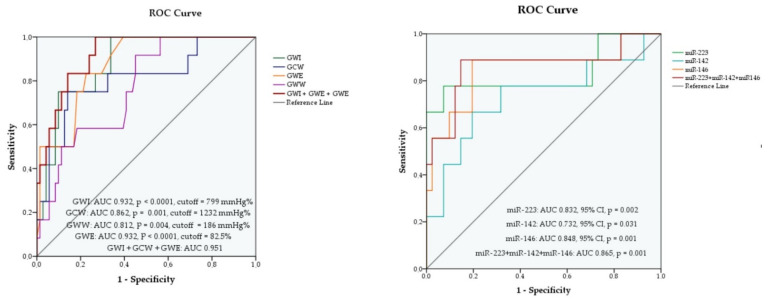
The four myocardial work indices had good prediction potential for MACE, with an AUC greater than 0.7 in the ROC curve analysis as follows: AUC 0.932 (95% CI), p < 0.0001 for GWI; AUC 0.862 (95% CI), p = 0.001 for GCW; AUC 0.812 (95% CI), p = 0.004 for GWW and AUC 0.932 (95% CI), p < 0.0001 for GWE, as shown in Figure 3. Out of the three variables, GWI and GWE proved to have the best predictive value for MACE, both with AUC 0.932, p < 0.0001. The highest AUC was obtained for the combination of the three parameters GWI, GCW and GWE, with AUC 0.951, p < 0.0001, proving a better prediction ability than each variable separately (Figure 3 and Figure S3).
Figure 3.
ROC curve analysis for baseline values of myocardial work indices (left) and miRNAs (right) as predictors of MACE at follow up.
For each variable we determined a cut-off value, based on the maximum value of the Youden index as follows: 799 mmHg% (sensitivity 88.9%, specificity 92.7%) for GWI, 1232 mmHg% (sensitivity 88.9%, specificity 82.9%) for GCW, 186 mmHg% (sensitivity 77.8%, specificity 63.4%) for GWW and 82.5% (sensitivity 88.9%, specificity 70.7%) for GWE as shown in Table S4.
For comparison, we performed an ROC analysis for classical echocardiographic MACE predictors: LV GLS (AUC 0.924, p < 0.0001), LV mechanical dispersion (AUC 0.764, p = 0.014) and 3D LVEF (AUC 0.888, p < 0.0001). Considering this, GWI and GWE are better at predicting an outcome in this patient group than the standard parameters.
3.4.2. Association of miRNA Levels with Cardiovascular Outcome
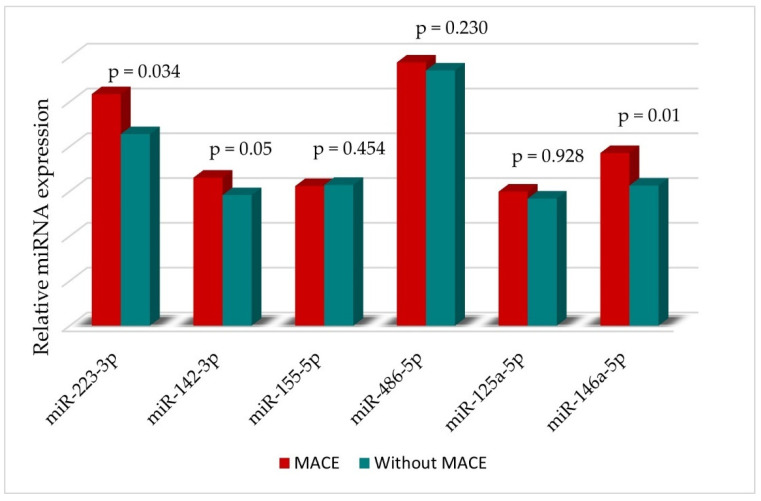
We observed that the expression levels of the three miRNAs were higher in patients in the MACE group compared with the non-MACE group. STEMI patients with MACE had a higher expression of miR-223-3p and miR-146a-5p plasma levels at baseline than those without MACE (Figure 4).
Figure 4.
miRNA expression (log 10) at baseline in the entire population and divided according to the occurrence of MACE.
Using a Cox binary univariate regression analysis, we tested the predictive potential of these markers and found that only three of the circulating miRNAs were significantly associated with the primary endpoint (MACE) in young STEMI patients: miR-223-3p (p = 0.000), miR-142-3p (p = 0.022) and miR-146a-5p (p = 0.000). Introducing the variables in a multivariable Cox regression model (chi-square = 13.792, p of model = 0.003), only miR-146a-5p remained independently associated with MACE (p = 0.012, Wald 6.37).
We then estimated the prediction potential of the three plasma miRNAs for MACE in patients with STEMI, using a receiver operator curve analysis (ROC). According to this analysis, the three miRNAs had good prediction abilities for MACE with an AUC greater than 0.7: AUC 0.832 (95% CI), p = 0.002 for miR-223-3p; AUC 0.732 (95% CI), p = 0.031 for miR-142-3p and AUC 0.848 (95% CI), p = 0.001 for miR-146a-5p (Figure 3). For each variable we determined a cut-off value, based on the maximum value of the Youden index as follows: 146,133 (sensitivity 77.8%, specificity 87.8%) for miR-233-3p, 1115 (sensitivity 77.8%, specificity 68.3%) for miR-142-3p and 4155 (sensitivity 88.9%, specificity 80.5%) for miR-146a-5p, as shown in Table S5. Out of the three miRNAs, miR-146a-5p proved to have the best predictive value for MACE. We also assessed their predictive value as a group and obtained an AUC of 0.865, 95% CI, p < 0.0001 Table S5. It is worth mentioning that in the Cox multivariate logistic regression analysis with the combination of the three miRNAs, miR-146a-5p had a significant contribution to the model with p = 0.012.
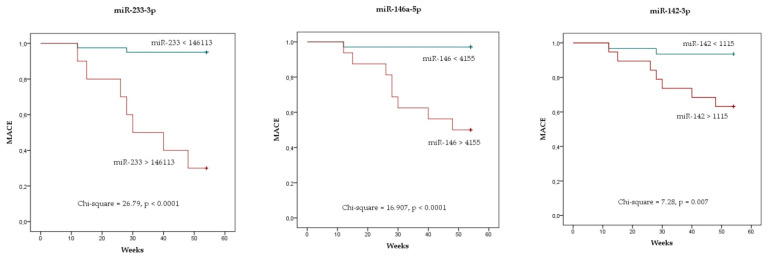
A Kaplan–Meier analysis showed the survival curves for the risk of MACE with respect to miR-223-3p, miR-142-3p and miR-146a-5p expression. Patients with higher miRNA levels at baseline had a higher probability of MACE at follow-up (details below in Figure 5).
Figure 5.
Kaplan–Meyer analysis curves showing the risk of MACE stratified by miR-223 (left), miR-146 (middle) and miR-142 (right). Cut-off value for each variable was calculated by ROC analysis.
3.4.3. Comparison between the Prognostic Power of Myocardial Work Indices and miRNAs
Myocardial work indices (GWI, GWC and GWE) proved to have a better prognostic potential than the three miRNAs (AUC = 0.951 for myocardial work indices and AUC = 0.862 for miRNAs) (Figure S3). However, their combination had better prognostic power than each separately as shown in the next paragraph.
3.4.4. Incremental Prognostic Value of Circulating miRNAs over Myocardial Work Indices
To further test the added value of miRNAs as prognostic markers, we built two logistic models: model 1 included myocardial work indices (GWI, GCW, GWE) and model 2 included sex, age and myocardial work indices. We determined the ability of each miRNA and of the combination of miRNAs to improve the predictive value of the three models.
Each of the three miRNAs and their associations added value to all the proposed predictive models as depicted below (Table 3 and Table S6).
Table 3.
C-statistics, AIC and likelihood ratio test for incremental predictive values of MACE obtained for model 1 by addition of miRNAs.
| p Value | Statistic log Likelihood Ratio | AIC | C-Statistic | Likelihood Ratio Test | |
|---|---|---|---|---|---|
| Model 1 (GWI + GCW + GWE) | p < 0.0001 | 27.577 | 44.07 | 0.938 (0.884–0.991) | |
| +miR 223-3p | p < 0.0001 | 33.064 | 40.53 | 0.9504 (0.909–0.991) | 0.0186 |
| +miR 142-3p | p = 0.0024 | 35.027 | 38.11 | 0.9504 (0.905–0.995) | 0.0048 |
| +miR 146a-5p | p < 0.0001 | 34.674 | 38.58 | 0.9603 (0.932–0.988) | 0.0062 |
| +miR 223-3p + miR 142-3p | p < 0.0001 | 37.049 | 38 | 0.9553 (0.9165–0.9942) | 0.0067 |
| +miR 142-3p + miR 146a-5p | p < 0.0001 | 42.719 | 31.19 | 0.975 (0.949–1.001)) | 0.0002 |
| +miR 223-3p + miR 146a-5p | p < 0.0001 | 34.934 | 40 | 0.960 (0.9329–0.9877) | 0.0216 |
| +miR 223 + miR-142 + miR-146 | p < 0.0001 | 44.068 | 31 | 0.9777 (0.952–1.003) | 0.0003 |
To support our findings, we compared the prediction potential of the combination of the three miRNAs with myocardial indices and the combination of the three miRNAs with LVGLS and 2D LVEF and in each case, the combination of miRNAs with myocardial work parameters yielded the best AIC and C-statistic.
4. Discussion
This was the first study to assess the potential prognostic value of a group of miRNAs together with new echocardiographic parameters (myocardial work indices) in predicting adverse events/MACE in a group of young patients with STEMI.
We focused our attention on young adults with STEMI considering its increasing prevalence in this group. The diagnosis of AMI at a younger age exerts a significant health, socioeconomic and psychological burden not only upon the patient but also upon the entire community. Finding a good prognostic marker might help improve the stratification risk and prognosis in these patients.
4.1. Myocardial Work Indices as MACE Predictors in STEMI
Recently, the LV myocardial work analysis was proposed as a new method for evaluation of LV systolic function. Combining LV pressure–strain loops derived from GLS and blood pressure measurements, this new method takes into account the loading conditions [14]. Even though 2D LVEF and GLS are currently the most used echocardiographic parameters for LV systolic function assessment, myocardial work parameters have proved to be less load-dependent, therefore more reliable [14].
Previous studies evaluated the clinical applications of this new method of assessing systolic function in various cardiovascular diseases. In ischaemic heart disease, myocardial work indices have proved to have good diagnostic value in a few studies. Guo et al. demonstrated that regional myocardial work measured by echocardiography exhibited a good diagnostic value in detecting significant myocardial ischaemia compared to the standard fractional flow reserve approach (measured invasively in the catheterisation laboratory) [58]. The use of regional GWE was able to identify at baseline CAD patients with critical coronary artery stenosis before invasive angiography with excellent performance (AUC = 0.92) in a trial from 2021 [59].
In our study cohort of young STEMI patients treated by primary PCI, we found that myocardial work parameters measured noninvasively were independent predictors of MACE at one-year follow-up.
We first assessed the evolution of MW in time in the entire cohort. At follow-up, we observed that GWI, GCW and GWE values were higher than baseline (1180.29 at baseline vs. 1138.4 at follow-up for GWI, p = 0.007; 1493 at baseline vs. 1663.29 at follow-up for GCW p = 0.089, 87 at baseline vs. 90 at follow-up, for GWE p = 0.004) but with little to no changes in GWW (182.88 at baseline vs. 181.51 at follow-up, p = 0.943) results, consistent with a recent study [60]. We then compared the evolution in time of myocardial work parameters between the MACE and non-MACE groups. While in the non-MACE group the myocardial work indices improved significantly at follow-up, in the MACE group they did not. This emphasises the potential prognostic potential of GWI, GCW and GWE.
Butcher et al. found GWI as independently associated with all-cause mortality at 6 months of follow-up after STEMI, providing an incremental prognostic value over LVEF and a minor incremental prognostic value over LV GLS in a study of 179 patients with reduced left ventricular ejection fraction [21]. While Lustosa et al. demonstrated the prognostic power of GWE at 80 months after STEMI in 507 patients [20], in another study GCW proved to be an independent predictor of segmental and global LV remodelling in patients with anterior MI treated by primary PCI [61]. Taking these trials into consideration, an evaluation of all myocardial work indices as MACE predictors in young patients with STEMI is currently lacking.
In our study on young STEMI patients, GWI, GWE and GCW had significantly lower values in the MACE group compared to the group without MACE. In an ROC analysis, the GWI, GWE but also the GCW baseline values proved to have good prediction abilities for MACE at one-year follow-up after STEMI with: AUC 0.932, p < 0.0001 for GWI; AUC 0.862, p = 0.001 for GCW; AUC 0.812, p = 0.004 for GWW and AUC 0.932, p < 0.0001 for GWE. GWI and GWE proved to be the best predictors for MACE among the myocardial indices. Moreover, we demonstrated that GWI, GWE and GCW had a better predictive value as a group than separately with AUC = 0.951, p < 0.0001 (Figure 3). It is worth mentioning that GWI and GWE proved to be better predictors compared with other standard echocardiographic parameters (LVEF, LVGLS and mechanical dispersion).
STEMI patients with lower values of GWI, GCW and GWE at baseline proved to have a higher probability of developing adverse events in time.
4.2. MIRNAs as MACE Predictors in STEMI
MiRNAs have been proved to participate in many cardiovascular disorders and pathological processes of cardiovascular diseases, such as atherosclerosis, coronary artery disease, heart failure, cardiac remodelling, arrhythmias and myocardial ischaemia [62,63].
Recent research emphasises the rising potential of miRNAs as novel biomarkers in ischemic heart disease, and its extreme manifestation, acute coronary syndrome [64].
In our study, higher circulating levels of miR-233-3p, miR-142-3p and miR-146a-5p measured within 48 h from symptom onset in a group of young patients were identified as independent predictors of future adverse cardiac events one year after STEMI.
miR-223-3p levels at baseline were higher in STEMI patients compared to controls. This could be explained by the fact that this is a cardiac-specific miRNA, moderately expressed in cardiomyocytes [65]. Previous studies have found that miR-223-3p is strongly upregulated during the early stages of myocardial infarction before the elevation of troponin I and CK-MB [66] with a higher expression in the ischemic compared to the normal myocardium. Our findings are in agreement with previous trials that demonstrated its connection to multiple pathological processes including atherosclerosis (miR-233-3p levels are significantly elevated in patients with atherosclerosis) [67]; it also has a role in platelet activation [68], the modulation of cholesterol homeostasis [69] and the transport function of lipoproteins [70]. Previous trials have stated its role as a marker of atherosclerotic plaque instability in patients with CAD [33] and a predictor of thrombotic events that could be used for ischemic risk stratification after AMI [34]. All these data support its potential role in STEMI patients.
We obtained that a value of miR-233-3p expression over the cut-off value (AUC 0.832, 95% CI, p = 0.002) at baseline was an independent predictor of MACE. Furthermore, in the Kaplan–Meier analysis, patients with higher levels of miR-223-3p in the acute setting had a higher probability of developing MACE at follow-up (Log rank chi-square 26.46, p < 0.0001). These results are consistent with Schulte et al. [71], who reported that increased circulating miR-223-3p in coronary artery disease could be used to predict cardiovascular death risk for patients, in particular for patients with ACS, in a study on 873 patients. Moreover, elevated levels of miR-223-3p positively associated with the severity of coronary atherosclerotic lesions evaluated by Gensini scores in a study from 2018, supporting its role as prognostic marker [72].
miR-146a-5p has proved to be upregulated in atherosclerotic plaques [73] and also in patients with coronary artery disease [38,39]. Takahashi et al. discovered that circulating levels of miR-146a and miR-146b (related to inflammation) were elevated in patients with CAD (compared with patients without CAD) [74]. Supporting these data, higher miR-146a-5p levels were encountered in patients with ACS compared with unstable angina [75,76]. Moreover, Xiao et al. found that miR-146a might serve as a marker for MACE in STEMI patients [77].
We also tested its predictive power in STEMI patients and observed a correlation between high levels of miR-146a-5p and MACE. In Cox univariate regression analysis, it proved to be a predictor of MACE at one year follow up. ROC curve analysis confirmed that-AUC 0.848 (95% CI), p = 0.001. miR-146a-5p had the highest AUC between the three miRNAs, therefore the greatest value as potential predictor of adverse events.
miR-142-3p, known to be involved in atherosclerosis [55] and ischemic heart disease [35] proved to be an independent prognostic marker of adverse outcome in our group of young patients with STEMI. Its prognostic value was tested using an ROC curve analysis, where we obtained an AUC of 0.732 (95% CI), p = 0.031, and determined an optimal cut-off value of 1115 (sensitivity 77.8%, specificity 68.3%) for MACE prediction. In good agreement with these data, a strong predictive potential for subsequent cardiovascular events was proved in our previous study on multiple vascular atherosclerotic patients with peripheral artery disease [55]. It is worth mentioning that among the three tested miRNAs, miR-142-3p had the lowest predictive power for MACE in a Cox univariate regression and in an ROC curve analysis (AUC).
STEMI patients with miRNAs values higher than the cut-off points (previously obtained by ROC analysis) appeared to have a higher probability of developing MACE in a Kaplan–Meier analysis. The increased plasma levels of the three miRNAs could be used to predict unfavourable outcomes in STEMI patients.
4.3. miRNAs and Myocardial Work Parameters
As far as we know, this is the first study to assess the power of a group of miRNA and myocardial work indices to predict the occurrence of MACE after STEMI in young patients. In this study, we found that both miRNAs and myocardial work parameters had good prognostic power in the studied population.
miR-223-3p, miR-142-3p and miR-146a-5p had incremental prognostic value over myocardial work indices and together, they could better predict unfavourable outcomes than each separately. The addition of each of the three miRNAs over myocardial work indices (GWI, GCW and GWE) in a Cox multivariate regression analysis, yielded a higher AIC and C-statistic than those of the myocardial indices alone (the best model between two was chosen based on the likelihood ratio test).
Our findings hold great potential for future treatment monitoring and personalised patient management according to risk stratification.
4.4. Limitations
Our study had several limitations. The first limitation is the small sample population (due to the age threshold of the study group and COVID 19 pandemic)—the performance and precision of predictions may have been affected. Second, another limitation is related to the lack of a comparison group of older STEMI patients. Third, at one year, we only had a telephonic follow-up available due to COVID-19 pandemic restrictions.
Larger further studies are required to validate our findings.
Despite these limitations, our study holds great potential, considering the obtained results and their further possible utility as MACE predictors in a young population.
5. Conclusions
Myocardial work indices and the three miRNAs tested in this study (miR-223-3p, miR-142-3p and miR-146a-5p) have a promising prognostic potential, as independent markers and also as a group, in young STEMI patients. The complementary use of miRNAs has incremental prognostic value over the tested echocardiographic parameters (GWI, GCW and GWE).
miRNAs together with myocardial work indices are better at predicting MACE than each separately and have the potential to be used as prognostic biomarkers; this might further facilitate risk stratification and the guidance of clinical care, improve secondary prevention and even lower cardiovascular mortality in young patients after STEMI.
Abbreviations
| ACS | Acute Coronary Syndrome |
| AIC | Akaike information criterion |
| AMI | acute myocardial infarction |
| AUC | area under the curve |
| CAD | coronary artery disease |
| CI | confidence intervals |
| CK_MB | creatine kinase-MB |
| EDTA | ethylenediaminetetraacetic acid |
| GCW | global contraction work |
| GLS | global longitudinal strain |
| GWE | global work efficiency |
| GWI | global work index |
| GWW | global wasted work |
| HDL | high-density lipoprotein |
| LAD | left anterior descending artery |
| LCX | left circumflex artery |
| LDL | low-density lipoprotein |
| LV | left ventricular |
| LVEDV | LV end diastolic volume |
| LVEF | left ventricular ejection fraction |
| LVESV | LV end-systolic volume |
| MACE | major adverse cardiac events |
| MW | myocardial work |
| OR | odds ratio |
| PCI | percutaneous coronary intervention |
| RCA | right coronary artery |
| ROC | receiver operating characteristic |
| RT-PCR | reverse transcription polymerase chain reaction |
| STEMI | acute ST elevation myocardial infarction |
| WBC | white blood cells |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/diagnostics12081946/s1, Table S1: Echocardiographic parameters at baseline and at follow-up in the entire population, Figure S1: Changes in myocardial work indices after STEMI (baseline and 6-month follow-up): GWI (upper left), GCW (upper right), GWW (lower left), GWE (lower right), Figure S2: miRNA levels at baseline in the study population and in the control group, Table S2: Cox univariate regression for the occurrence of MACE, Table S3: Univariate and multivariate regression analysis of myocardial work indices (at baseline) as predictors of MACE, Table S4: ROC analysis for myocardial work indices at baseline as predictors for MACE at follow-up, Table S5: ROC analysis performance of MACE prediction using miRNAs baseline values, Figure S3: ROC analysis for Cox regression models of myocardial work indices and miRNAs (baseline values) as predictors for MACE at follow-up in young STEMI patients. Table S6: C-statistics, AIC and likelihood ratio test for incremental predictive values of MACE obtained for model 2 by adding miRNAs.
Author Contributions
Conceptualisation, A.I.S. and M.M.M.; formal analysis, A.I.S. and M.M.M.; methodology, A.I.S., M.M.M., L.Ș.N., T.B. and C.S.; validation, A.I.S., M.M.M., T.B. and L.Ș.N.; clinical investigation, A.I.S. and M.M.M.; visualization, A.I.S.; writing—original draft preparation, A.I.S.; writing—review and editing, M.M.M., A.I.S. and L.Ș.N.; supervision, M.M.M. and A.V.S.; project administration, M.M.M. and L.Ș.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Ethics Committees from Clinical Emergency Hospital of Bucharest (no. 8575/24.09.2019) and from the Institute of Cellular Biology and Pathology “N. Simionescu”, Bucharest (no. 03/08 October2019).
Informed Consent Statement
All patients gave a signed informed consent at enrolment.
Data Availability Statement
The data will be available based on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by a grant from the Romanian Ministry of Education and Research, CCCDI-UEFISCDI, project number PN-III-P2-2.1-PED-2019-1897, within PNCDI III.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nowbar A.N., Gitto M., Howard J.P., Francis D.P., Al-Lamee R. Mortality from Ischemic Heart Disease. Circ. Cardiovasc. Qual. Outcomes. 2019;12:e005375. doi: 10.1161/CIRCOUTCOMES.118.005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Townsend N., Nichols M., Scarborough P., Rayner M. Cardiovascular disease in Europe--epidemiological update 2015. Eur. Heart. J. 2015;36:2696–2705. doi: 10.1093/eurheartj/ehv428. [DOI] [PubMed] [Google Scholar]
- 3.Arora S., Stouffer G.A., Kucharska-Newton A.M., Qamar A., Vaduganathan M., Pandey A., Porterfield D., Blankstein R., Rosamond W.D., Bhatt D.L., et al. Twenty Year Trends and Sex Differences in Young Adults Hospitalized with Acute Myocardial Infarction. Circulation. 2019;139:1047–1056. doi: 10.1161/CIRCULATIONAHA.118.037137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang J., Biery D.W., Singh A., Divakaran S., DeFilippis E.M., Wu W.Y., Klein J., Hainer J., Ramsis M., Natarajan P., et al. Risk Factors and Outcomes of Very Young Adults Who Experience Myocardial Infarction: The Partners YOUNG-MI Registry. Am. J. Med. 2020;133:605–612 e1. doi: 10.1016/j.amjmed.2019.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Divakaran S., Singh A., Biery D., Yang J., DeFilippis E.M., Collins B.L., Ramsis M., Qamar A., Hainer J., Klein J., et al. Diabetes Is Associated with Worse Long-term Outcomes in Young Adults After Myocardial Infarction: The Partners YOUNG-MI Registry. Diabetes Care. 2020;43:1843–1850. doi: 10.2337/dc19-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yandrapalli S., Nabors C., Goyal A., Aronow W.S., Frishman W.H. Modifiable Risk Factors in Young Adults with First Myocardial Infarction. J. Am. Coll. Cardiol. 2019;73:573–584. doi: 10.1016/j.jacc.2018.10.084. [DOI] [PubMed] [Google Scholar]
- 7.Guo X., Li Z., Vittinghoff E., Sun Y., Pletcher M.J. Trends in rate of acute myocardial infarction among patients aged <30 years. Nat. Rev. Cardiol. 2018;15:119. doi: 10.1038/nrcardio.2017.191. [DOI] [PubMed] [Google Scholar]
- 8.Lv J., Ni L., Liu K., Gao X., Yang J., Zhang X., Ye Y., Dong Q., Fu R., Sun H., et al. Clinical Characteristics, Prognosis, and Gender Disparities in Young Patients with Acute Myocardial Infarction. Front. Cardiovasc. Med. 2021;8:720378. doi: 10.3389/fcvm.2021.720378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuster A., Backhaus S.J., Stiermaier T., Eitel I. Prognostic utility of global longitudinal strain in myocardial infarction. World J. Cardiol. 2018;10:35–37. doi: 10.4330/wjc.v10.i5.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smiseth O.A., Torp H., Opdahl A., Haugaa K.H., Urheim S. Myocardial strain imaging: How useful is it in clinical decision making? Eur. Heart J. 2016;37:1196–1207. doi: 10.1093/eurheartj/ehv529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ersboll M., Valeur N., Mogensen U.M., Andersen M.J., Moller J.E., Hassager C., Sogaard P., Kober L. Relationship between left ventricular longitudinal deformation and clinical heart failure during admission for acute myocardial infarction: A two-dimensional speckle-tracking study. J. Am. Soc. Echocardiogr. 2012;25:1280–1289. doi: 10.1016/j.echo.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Woo J.S., Kim W.S., Yu T.K., Ha S.J., Kim S.Y., Bae J.H., Kim K.S. Prognostic value of serial global longitudinal strain measured by two-dimensional speckle tracking echocardiography in patients with ST-segment elevation myocardial infarction. Am. J. Cardiol. 2011;108:340–347. doi: 10.1016/j.amjcard.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 13.Dahle G.O., Stangeland L., Moen C.A., Salminen P.R., Haaverstad R., Matre K., Grong K. The influence of acute unloading on left ventricular strain and strain rate by speckle tracking echocardiography in a porcine model. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H1330–H1339. doi: 10.1152/ajpheart.00947.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell K., Eriksen M., Aaberge L., Wilhelmsen N., Skulstad H., Remme E.W., Haugaa K.H., Opdahl A., Fjeld J.G., Gjesdal O., et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012;33:724–733. doi: 10.1093/eurheartj/ehs016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubert A., Le Rolle V., Leclercq C., Galli E., Samset E., Casset C., Mabo P., Hernandez A., Donal E. Estimation of myocardial work from pressure-strain loops analysis: An experimental evaluation. Eur. Heart J. Cardiovasc. Imaging. 2018;19:1372–1379. doi: 10.1093/ehjci/jey024. [DOI] [PubMed] [Google Scholar]
- 16.Verbeke J., Calle S., Kamoen V., De Buyzere M., Timmermans F. Prognostic value of myocardial work and global longitudinal strain in patients with heart failure and functional mitral regurgitation. Int. J. Cardiovasc. Imaging. 2022;38:803–812. doi: 10.1007/s10554-021-02474-y. [DOI] [PubMed] [Google Scholar]
- 17.Jain R., Bajwa T., Roemer S., Huisheree H., Allaqaband S.Q., Kroboth S., Perez Moreno A.C., Tajik A.J., Khandheria B.K. Myocardial work assessment in severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur. Heart J. Cardiovasc. Imaging. 2021;22:715–721. doi: 10.1093/ehjci/jeaa257. [DOI] [PubMed] [Google Scholar]
- 18.Hedwig F., Nemchyna O., Stein J., Knosalla C., Merke N., Knebel F., Hagendorff A., Schoenrath F., Falk V., Knierim J. Myocardial Work Assessment for the Prediction of Prognosis in Advanced Heart Failure. Front. Cardiovasc. Med. 2021;8:691611. doi: 10.3389/fcvm.2021.691611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boe E., Russell K., Eek C., Eriksen M., Remme E.W., Smiseth O.A., Skulstad H. Non-invasive myocardial work index identifies acute coronary occlusion in patients with non-ST-segment elevation-acute coronary syndrome. Eur. Heart J. Cardiovasc. Imaging. 2015;16:1247–1255. doi: 10.1093/ehjci/jev078. [DOI] [PubMed] [Google Scholar]
- 20.Lustosa R.P., Butcher S.C., van der Bijl P., El Mahdiui M., Montero-Cabezas J.M., Kostyukevich M.V., Rocha De Lorenzo A., Knuuti J., Ajmone Marsan N., Bax J.J., et al. Global Left Ventricular Myocardial Work Efficiency and Long-Term Prognosis in Patients After ST-Segment-Elevation Myocardial Infarction. Circ. Cardiovasc. Imaging. 2021;14:e012072. doi: 10.1161/CIRCIMAGING.120.012072. [DOI] [PubMed] [Google Scholar]
- 21.Butcher S.C., Lustosa R.P., Abou R., Marsan N.A., Bax J.J., Delgado V. Prognostic implications of left ventricular myocardial work index in patients with ST-segment elevation myocardial infarction and reduced left ventricular ejection fraction. Eur. Heart J. Cardiovasc. Imaging. 2022;23:699–707. doi: 10.1093/ehjci/jeab096. [DOI] [PubMed] [Google Scholar]
- 22.Scarlatescu A.I., Micheu M.M., Popa-Fotea N.M., Dorobantu M. MicroRNAs in Acute ST Elevation Myocardial Infarction-A New Tool for Diagnosis and Prognosis: Therapeutic Implications. Int. J. Mol. Sci. 2021;22:4799. doi: 10.3390/ijms22094799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plasterk R.H. Micro RNAs in animal development. Cell. 2006;124:877–881. doi: 10.1016/j.cell.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 24.Cavarretta E., Frati G. MicroRNAs in Coronary Heart Disease: Ready to Enter the Clinical Arena? Biomed. Res. Int. 2016;2016:2150763. doi: 10.1155/2016/2150763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang G.K., Zhu J.Q., Zhang J.T., Li Q., Li Y., He J., Qin Y.W., Jing Q. Circulating microRNA: A novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010;31:659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 26.Hsu A., Chen S.J., Chang Y.S., Chen H.C., Chu P.H. Systemic approach to identify serum microRNAs as potential biomarkers for acute myocardial infarction. Biomed. Res. Int. 2014;2014:418628. doi: 10.1155/2014/418628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Empel V.P., De Windt L.J., da Costa Martins P.A. Circulating miRNAs: Reflecting or affecting cardiovascular disease? Curr. Hypertens. Rep. 2012;14:498–509. doi: 10.1007/s11906-012-0310-7. [DOI] [PubMed] [Google Scholar]
- 28.Kloosterman W.P., Plasterk R.H. The diverse functions of microRNAs in animal development and disease. Dev. Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell P.S., Parkin R.K., Kroh E.M., Fritz B.R., Wyman S.K., Pogosova-Agadjanyan E.L., Peterson A., Noteboom J., O’Briant K.C., Allen A., et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilad S., Meiri E., Yogev Y., Benjamin S., Lebanony D., Yerushalmi N., Benjamin H., Kushnir M., Cholakh H., Melamed N., et al. Serum microRNAs are promising novel biomarkers. PLoS ONE. 2008;3:e3148. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X., Ba Y., Ma L., Cai X., Yin Y., Wang K., Guo J., Zhang Y., Chen J., Guo X., et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 32.Landry P., Plante I., Ouellet D.L., Perron M.P., Rousseau G., Provost P. Existence of a microRNA pathway in anucleate platelets. Nat. Struct. Mol. Biol. 2009;16:961–966. doi: 10.1038/nsmb.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh S., de Ronde M.W.J., Kok M.G.M., Beijk M.A., De Winter R.J., van der Wal A.C., Sondermeijer B.M., Meijers J.C.M., Creemers E.E., Pinto-Sietsma S.J. MiR-223-3p and miR-122-5p as circulating biomarkers for plaque instability. Open Heart. 2020;7:e001223. doi: 10.1136/openhrt-2019-001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hromadka M., Motovska Z., Hlinomaz O., Kala P., Tousek F., Jarkovsky J., Beranova M., Jansky P., Svoboda M., Krepelkova I., et al. MiR-126-3p and MiR-223-3p as Biomarkers for Prediction of Thrombotic Risk in Patients with Acute Myocardial Infarction and Primary Angioplasty. J. Pers. Med. 2021;11:508. doi: 10.3390/jpm11060508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin B., Shu Y., Long L., Li H., Men X., Feng L., Yang H., Lu Z. MicroRNA-142-3p Induces Atherosclerosis-Associated Endothelial Cell Apoptosis by Directly Targeting Rictor. Cell. Physiol. Biochem. 2018;47:1589–1603. doi: 10.1159/000490932. [DOI] [PubMed] [Google Scholar]
- 36.Tang Q.J., Lei H.P., Wu H., Chen J.Y., Deng C.Y., Sheng W.S., Fu Y.H., Li X.H., Lin Y.B., Han Y.L., et al. Plasma miR-142 predicts major adverse cardiovascular events as an intermediate biomarker of dual antiplatelet therapy. Acta. Pharmacol. Sin. 2019;40:208–215. doi: 10.1038/s41401-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su Q., Lv X., Ye Z., Sun Y., Kong B., Qin Z., Li L. The mechanism of miR-142-3p in coronary microembolization-induced myocardiac injury via regulating target gene IRAK-1. Cell Death Dis. 2019;10:61. doi: 10.1038/s41419-019-1341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bao M.H., Xiao Y., Zhang Q.S., Luo H.Q., Luo J., Zhao J., Li G.Y., Zeng J., Li J.M. Meta-Analysis of miR-146a Polymorphisms Association with Coronary Artery Diseases and Ischemic Stroke. Int. J. Mol. Sci. 2015;16:14305–14317. doi: 10.3390/ijms160714305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roldan V., Arroyo A.B., Salloum-Asfar S., Manzano-Fernandez S., Garcia-Barbera N., Marin F., Vicente V., Gonzalez-Conejero R., Martinez C. Prognostic role of MIR146A polymorphisms for cardiovascular events in atrial fibrillation. Thromb. Haemost. 2014;112:781–788. doi: 10.1160/TH14-01-0092. [DOI] [PubMed] [Google Scholar]
- 40.Niculescu L.S., Simionescu N., Sanda G.M., Carnuta M.G., Stancu C.S., Popescu A.C., Popescu M.R., Vlad A., Dimulescu D.R., Simionescu M., et al. MiR-486 and miR-92a Identified in Circulating HDL Discriminate between Stable and Vulnerable Coronary Artery Disease Patients. PLoS ONE. 2015;10:e0140958. doi: 10.1371/journal.pone.0140958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang K., He Y.S., Wang X.Q., Lu L., Chen Q.J., Liu J., Sun Z., Shen W.F. MiR-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS Lett. 2011;585:854–860. doi: 10.1016/j.febslet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Cheng H.S., Sivachandran N., Lau A., Boudreau E., Zhao J.L., Baltimore D., Delgado-Olguin P., Cybulsky M.I., Fish J.E. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol. Med. 2013;5:1017–1034. doi: 10.1002/emmm.201202318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong S., Xiong W., Yuan J., Li J., Liu J., Xu X. MiRNA-146a regulates the maturation and differentiation of vascular smooth muscle cells by targeting NF-kappaB expression. Mol. Med. Rep. 2013;8:407–412. doi: 10.3892/mmr.2013.1538. [DOI] [PubMed] [Google Scholar]
- 44.Bukauskas T., Mickus R., Cereskevicius D., Macas A. Value of Serum miR-23a, miR-30d, and miR-146a Biomarkers in ST-Elevation Myocardial Infarction. Med. Sci. Monit. 2019;25:3925–3932. doi: 10.12659/MSM.913743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J., Wu Q., Yu J., Cao X., Xu Z. miR-125a-5p inhibits the expression of NLRP3 by targeting CCL4 in human vascular smooth muscle cells treated with ox-LDL. Exp. Ther. Med. 2019;18:1645–1652. doi: 10.3892/etm.2019.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen T., Huang Z., Wang L., Wang Y., Wu F., Meng S., Wang C. MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovasc. Res. 2009;83:131–139. doi: 10.1093/cvr/cvp121. [DOI] [PubMed] [Google Scholar]
- 47.Zhang R., Lan C., Pei H., Duan G., Huang L., Li L. Expression of circulating miR-486 and miR-150 in patients with acute myocardial infarction. BMC Cardiovasc. Disord. 2015;15:51. doi: 10.1186/s12872-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du F., Yu F., Wang Y., Hui Y., Carnevale K., Fu M., Lu H., Fan D. MicroRNA-155 deficiency results in decreased macrophage inflammation and attenuated atherogenesis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2014;34:759–767. doi: 10.1161/ATVBAHA.113.302701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eisenhardt S.U., Schmidt Y., Karaxha G., Iblher N., Penna V., Torio-Padron N., Stark G.B., Bannasch H. Monitoring molecular changes induced by ischemia/reperfusion in human free muscle flap tissue samples. Ann. Plast. Surg. 2012;68:202–208. doi: 10.1097/SAP.0b013e3181f77ba5. [DOI] [PubMed] [Google Scholar]
- 50.Donners M.M., Wolfs I.M., Stoger L.J., van der Vorst E.P., Pottgens C.C., Heymans S., Schroen B., Gijbels M.J., de Winther M.P. Hematopoietic miR155 deficiency enhances atherosclerosis and decreases plaque stability in hyperlipidemic mice. PLoS ONE. 2012;7:e35877. doi: 10.1371/journal.pone.0035877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of, Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2016;17:412. doi: 10.1093/ehjci/jew041. [DOI] [PubMed] [Google Scholar]
- 52.Mitchell C., Rahko P.S., Blauwet L.A., Canaday B., Finstuen J.A., Foster M.C., Horton K., Ogunyankin K.O., Palma R.A., Velazquez E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019;32:1–64. doi: 10.1016/j.echo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Van der Bijl P., Kostyukevich M., El Mahdiui M., Hansen G., Samset E., Ajmone Marsan N., Bax J.J., Delgado V. A Roadmap to Assess Myocardial Work: From Theory to Clinical Practice. JACC Cardiovasc. Imaging. 2019;12:2549–2554. doi: 10.1016/j.jcmg.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 54.Mahdiui M.E., van der Bijl P., Abou R., de Paula Lustosa R., van der Geest R., Ajmone Marsan N., Delgado V., Bax J.J. Myocardial Work, an Echocardiographic Measure of Post Myocardial Infarct Scar on Contrast-Enhanced Cardiac Magnetic Resonance. Am. J. Cardiol. 2021;151:1–9. doi: 10.1016/j.amjcard.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Barbalata T., Moraru O.E., Stancu C.S., Devaux Y., Simionescu M., Sima A.V., Niculescu L.S. Increased miR-142 Levels in Plasma and Atherosclerotic Plaques from Peripheral Artery Disease Patients with Post-Surgery Cardiovascular Events. Int. J. Mol. Sci. 2020;21:9600. doi: 10.3390/ijms21249600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., Caforio A.L.P., Crea F., Goudevenos J.A., Halvorsen S., et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 57.Poudel I., Tejpal C., Rashid H., Jahan N. Major Adverse Cardiovascular Events: An Inevitable Outcome of ST-elevation myocardial infarction? A Literature Review. Cureus. 2019;11:e5280. doi: 10.7759/cureus.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo Y., Yang C., Wang X., Pei Z., Zhu H., Meng X., Zhou Z., Lang X., Ning S., Zhang R., et al. Regional Myocardial Work Measured by Echocardiography for the Detection of Myocardial Ischemic Segments: A Comparative Study with Invasive Fractional Flow Reserve. Front. Cardiovasc. Med. 2022;9:813710. doi: 10.3389/fcvm.2022.813710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sabatino J., De Rosa S., Leo I., Strangio A., Spaccarotella C., Polimeni A., Sorrentino S., Di Salvo G., Indolfi C. Prediction of Significant Coronary Artery Disease Through Advanced Echocardiography: Role of Non-invasive Myocardial Work. Front. Cardiovasc. Med. 2021;8:719603. doi: 10.3389/fcvm.2021.719603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lustosa R.P., Fortuni F., van der Bijl P., Mahdiui M.E., Montero-Cabezas J.M., Kostyukevich M.V., Knuuti J., Marsan N.A., Delgado V., Bax J.J. Changes in Global Left Ventricular Myocardial Work Indices and Stunning Detection 3 Months After ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2021;157:15–21. doi: 10.1016/j.amjcard.2021.07.012. [DOI] [PubMed] [Google Scholar]
- 61.Meimoun P., Abdani S., Stracchi V., Elmkies F., Boulanger J., Botoro T., Zemir H., Clerc J. Usefulness of Noninvasive Myocardial Work to Predict Left Ventricular Recovery and Acute Complications after Acute Anterior Myocardial Infarction Treated by Percutaneous Coronary Intervention. J. Am. Soc. Echocardiogr. 2020;33:1180–1190. doi: 10.1016/j.echo.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 62.Navickas R., Gal D., Laucevicius A., Taparauskaite A., Zdanyte M., Holvoet P. Identifying circulating microRNAs as biomarkers of cardiovascular disease: A systematic review. Cardiovasc. Res. 2016;111:322–337. doi: 10.1093/cvr/cvw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solly E.L., Dimasi C.G., Bursill C.A., Psaltis P.J., Tan J.T.M. MicroRNAs as Therapeutic Targets and Clinical Biomarkers in Atherosclerosis. J. Clin. Med. 2019;8:2199. doi: 10.3390/jcm8122199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barraclough J.Y., Joan M., Joglekar M.V., Hardikar A.A., Patel S. MicroRNAs as Prognostic Markers in Acute Coronary Syndrome Patients-A Systematic Review. Cells. 2019;8:1572. doi: 10.3390/cells8121572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schirle N.T., Sheu-Gruttadauria J., MacRae I.J. Structural basis for microRNA targeting. Science. 2014;346:608–613. doi: 10.1126/science.1258040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodriguez A.E., Hernandez J.A., Benito R., Gutierrez N.C., Garcia J.L., Hernandez-Sanchez M., Risueno A., Sarasquete M.E., Ferminan E., Fisac R., et al. Molecular characterization of chronic lymphocytic leukemia patients with a high number of losses in 13q14. PLoS ONE. 2012;7:e48485. doi: 10.1371/journal.pone.0048485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shan Z., Qin S., Li W., Wu W., Yang J., Chu M., Li X., Huo Y., Schaer G.L., Wang S., et al. An Endocrine Genetic Signal Between Blood Cells and Vascular Smooth Muscle Cells: Role of MicroRNA-223 in Smooth Muscle Function and Atherogenesis. J. Am. Coll. Cardiol. 2015;65:2526–2537. doi: 10.1016/j.jacc.2015.03.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Willeit P., Zampetaki A., Dudek K., Kaudewitz D., King A., Kirkby N.S., Crosby-Nwaobi R., Prokopi M., Drozdov I., Langley S.R., et al. Circulating microRNAs as novel biomarkers for platelet activation. Circ. Res. 2013;112:595–600. doi: 10.1161/CIRCRESAHA.111.300539. [DOI] [PubMed] [Google Scholar]
- 69.Barbalata T., Zhang L., Dulceanu M.D., Stancu C.S., Devaux Y., Sima A.V., Niculescu L.S. Regulation of microRNAs in high-fat diet induced hyperlipidemic hamsters. Sci. Rep. 2020;10:20549. doi: 10.1038/s41598-020-77539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simionescu N., Niculescu L.S., Carnuta M.G., Sanda G.M., Stancu C.S., Popescu A.C., Popescu M.R., Vlad A., Dimulescu D.R., Simionescu M., et al. Hyperglycemia Determines Increased Specific MicroRNAs Levels in Sera and HDL of Acute Coronary Syndrome Patients and Stimulates MicroRNAs Production in Human Macrophages. PLoS ONE. 2016;11:e0161201. doi: 10.1371/journal.pone.0161201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schulte C., Molz S., Appelbaum S., Karakas M., Ojeda F., Lau D.M., Hartmann T., Lackner K.J., Westermann D., Schnabel R.B., et al. miRNA-197 and miRNA-223 Predict Cardiovascular Death in a Cohort of Patients with Symptomatic Coronary Artery Disease. PLoS ONE. 2015;10:e0145930. doi: 10.1371/journal.pone.0145930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo J.F., Zhang Y., Zheng Q.X., Zhang Y., Zhou H.H., Cui L.M. Association between elevated plasma microRNA-223 content and severity of coronary heart disease. Scand. J. Clin. Lab. Investig. 2018;78:373–378. doi: 10.1080/00365513.2018.1480059. [DOI] [PubMed] [Google Scholar]
- 73.Raitoharju E., Lyytikainen L.P., Levula M., Oksala N., Mennander A., Tarkka M., Klopp N., Illig T., Kahonen M., Karhunen P.J., et al. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis. 2011;219:211–217. doi: 10.1016/j.atherosclerosis.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 74.Takahashi Y., Satoh M., Minami Y., Tabuchi T., Itoh T., Nakamura M. Expression of miR-146a/b is associated with the Toll-like receptor 4 signal in coronary artery disease: Effect of renin-angiotensin system blockade and statins on miRNA-146a/b and Toll-like receptor 4 levels. Clin. Sci. 2010;119:395–405. doi: 10.1042/CS20100003. [DOI] [PubMed] [Google Scholar]
- 75.Oerlemans M.I., Mosterd A., Dekker M.S., de Vrey E.A., van Mil A., Pasterkamp G., Doevendans P.A., Hoes A.W., Sluijter J.P. Early assessment of acute coronary syndromes in the emergency department: The potential diagnostic value of circulating microRNAs. EMBO Mol. Med. 2012;4:1176–1185. doi: 10.1002/emmm.201201749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diehl P., Fricke A., Sander L., Stamm J., Bassler N., Htun N., Ziemann M., Helbing T., El-Osta A., Jowett J.B., et al. Microparticles: Major transport vehicles for distinct microRNAs in circulation. Cardiovasc. Res. 2012;93:633–644. doi: 10.1093/cvr/cvs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao S., Xue T., Pan Q., Hu Y., Wu Q., Liu Q., Wang X., Liu A., Liu J., Zhu H., et al. MicroRNA-146a Serves as a Biomarker for Adverse Prognosis of ST-Segment Elevation Myocardial Infarction. Cardiovasc. Ther. 2021;2021:2923441. doi: 10.1155/2021/2923441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be available based on reasonable request.