Abstract
Simple Summary
HPV-associated oropharyngeal squamous cell carcinoma (OPSCC) is unique amongst oropharyngeal cancers in its high responsiveness to treatment and its lower mortality rate. As a result, numerous clinical trials have been conducted to identify treatment modalities and protocols. In order for these trials to have meaningful impact on HPV-associated OPSCC patients, proper demographic representation by trial participants is essential. The aim of our systematic review and meta-analysis was to assess the demographics of trial participants for HPV-associated OPSCC clinical trials and compare them with those reported by national databases. We determined that clinical-trial participants were predominately non-smoking white men, with tonsils as the primary tumor site. These findings reflect the demographics reported by the National Cancer Database. Our results imply that HPV-associated OPSCC clinical trials appropriately represent the target population and offer immense benefit.
Abstract
The objective of our paper was to answer the following question: how do patients with HPV-related oropharyngeal squamous cell carcinoma OPSCC (Population) enrolled in clinical trials (Intervention), compared with national database reports of HPV-associated OPSCC patients (Comparison), present demographically (Outcome)? We conducted a systematic review and meta-analysis of studies pertaining to clinical trials of HPV-associated OPSCC and participant demographics in the United States. PubMed, Scopus, CINAHL, and the Cochrane Library were searched from inception to 2 February 2022. Studies of overlapping participant cohorts and/or studies conducted outside of the United States were excluded. Primary outcomes were patient age, sex, and race. Secondary outcomes were smoking history, alcohol history, history of prior cancer, and tumor origin site. Meta-analysis of single means (mean, N for each study, and standard deviation) for age, pack years, and smoking years was performed. Pooled prevalence rates of gender, race, alcohol history, tobacco history, and tumor origin site were expressed as a percentage, with 95% confidence intervals. Meta-analysis found patients to be predominately non-smoking white males, with tumors originating from the tonsil. Our findings reflected the demographics reported by the National Cancer Database (NCDB) for HPV-associated OPSCC. This indicates that HPV-associated OPSCC patients are appropriately represented in clinical trial demographics.
Keywords: clinical trial, oropharyngeal cancer, HPV-associated cancer
1. Introduction
In the last two decades, there has been an exponential rise of oropharyngeal squamous cell carcinoma (OPSCC), despite simultaneous decreases in both head and neck cancer mortality rates and rates of cigarette use in the United States [1,2]. This is largely due to elevated rates of oropharyngeal infection with oncogenic HPV strains. Regardless of origin, OPSCC poses notable potential for mortality, along with dramatic impairments in one’s daily functions and abilities [3]. Furthermore, treatment options, ranging from surgery to intensity-modulated radiotherapy and concurrent cisplatin, carry significant burden; surgeries include the risk of postoperative complications, potential rehospitalization, and significant postoperative disability, while chemo-radiotherapy (CRT) can result in treatment-induced toxicities, such as mucositis, dysphagia, xerostomia, and dysgeusia.
Amongst OPSCC’s variety of etiologies, human papilloma virus (HPV) holds particular interest and promise. HPV-associated OPSCC, especially of the p16/HPV-DNA subtype, demonstrates increased cellular chemo- and radio-sensitivity, corresponding to higher response rates and greater reductions in both disease progression and fatality. HPV-related OPSCC’s ubiquitous responsivity to surgery (especially compared to non-HPV-associated OPSCC), combined with the morbidities of current guidelines, have catalyzed numerous clinical trials in efforts to identify opportunities for CRT de-intensification and less-invasive surgeries. Recent clinical trials, including ORATOR, MC1273, and AVOID, have focused on HPV-related OPSCC management and have compared outcomes of chemoradiation with transoral robotic surgery and deintensification of adjuvant therapies, with progressively more trials in recruitment [4]. Although these trials have demonstrated significant promise, questions about population representation from trial to clinic remain. To best utilize clinical trial findings in treatment protocols for various patients, it is essential that the trial demographics are representative of said patients.
Instances of discrepancies between trial participants and patient populations have been well-documented throughout multiple specialties. Heiat et al. compared the demographics of patients in heart-failure-related randomized control trials with those of the general population and found that trial participants markedly differed from the general population, with an overrepresentation of white and male patients [5]. Johnston et al.’s systematic review of sex, age, race, and intervention type in clinical trials for HIV determined that females, older patients, and non-white patients were underrepresented in trial populations [6]. In reviewing randomized clinical trials for lipid-lowering therapies, Khan et al. noted consistent underrepresentation of female and older patients, limiting the evidence base for efficacy and safety in the treatment of these patient group [7]. Conversely, Strait et al.’s systematic review of participant demographics for rheumatoid arthritis randomized clinical trials found that males and nonwhite patients were significantly underrepresented in comparison to national statistics [8].
Currently, there are no equivalent reviews of demographics in clinical trials for HPV-associated OPSCC. The goal of this study was to ascertain the demographics of participants in HPV-OPSCC clinical trials in the United States and compare with those of national databases.
2. Materials and Methods
2.1. Systematic Literature Search
This systematic review/meta-analysis aimed to answer the following question: how do patients with HPV-related OPSCC (Population) enrolled in clinical trials, (Intervention), compared with national database reports of HPV-related OPSCC patients (Comparison), present demographically (Outcome)? A detailed search strategy (Appendix A) was developed in the following four databases: PubMed (National Library of Medicine, National Institutes of Health), Scopus (Elsevier), CINAHL (EBSCO), and Cochrane Library (Wiley). The search strategy used a combination of subject headings (e.g., Medical Subject Headings [Mesh] in PubMed). The PubMed search strategy was modified for the other three databases, replacing Mesh terms with appropriate subject headings, when available, and maintaining similar keywords. The databases were searched from inception through 7 February 2022, and results were limited to English language and clinical trials. References were uploaded to Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) and screened for relevance.
2.2. Selection Criteria
Abstracts were first independently reviewed by two reviewers (T.M.G. and R.G.) to identify all studies pertaining to clinical trials of HPV-related OPSCC and participant demographics. Non-English studies, review articles, nonhuman studies, non-journal articles (e.g., abstract only), and studies of clinical trials conducted outside of the United States were excluded. Studies that included overlapping participant cohorts with other trials were also excluded. Any conflicts were resolved by discussion. To identify additional articles, the reference lists of relevant articles were hand searched.
2.3. Data Collection
Data included in the analysis and discussion were extracted by two reviewers (T.M.G. and R.G.). Disagreements were resolved by discussion. Primary outcomes were patient age, sex, and race. Secondary outcomes included smoking history, alcohol history, history of prior cancer, and origin site of HPV-related OPSCC, classified as tonsil, base of tongue (BOT), both sites, or not otherwise specified (NOS). In instances of incomplete data, an attempt was made to contact the primary author via email for clarification or sharing of primary data.
2.4. Statistical Analysis
Meta-analysis of single means (mean, N for each study, and standard deviation) for age, pack years, and smoking years was performed by Comprehensive Meta-Analysis version 3 (Biostat Inc., Englewood, NJ, USA). Meta-analysis of proportions was performed using MedCalc 19.6 (MedCalc Software Ltd., Ostend, Belgium; https://www.medcalc.org; accessed on 2020). The pooled prevalence rate of gender, race, alcohol history, tobacco history, and tumor origin site were expressed as a percentage with 95% confidence intervals (CI). Each measure was weighted according to the number of patients affected. The weighted-summary proportion was calculated by the Freeman–Tukey transformation [9]. Heterogeneity among studies was assessed using χ2 and I2 statistics. I2 < 50% indicated acceptable heterogeneity, and, therefore, the fixed-effects model was used. Otherwise, the random-effects model was performed. Finally, Egger’s tests with funnel plots were performed to further assess the risk of publication bias [10,11]. In a funnel plot, the treatment effect is plotted on the horizontal axis, and the standard error is plotted on the vertical axis. The vertical line represents the summary estimated derived using a fixed-effect meta-analysis. Two diagonal lines represent (pseudo) 95% confidence limits (effect ± 1.96 SE) around the summary effect for each standard error on the vertical axis. These show the expected distribution of studies in the absence of heterogeneity or selection bias. In the absence of heterogeneity, 95% of the studies should lie within the funnel defined by these diagonal lines. Potential publication bias was evaluated by visual inspection of the funnel plot, as bias results in asymmetry of the funnel plot, and Egger’s test, which statistically examines this asymmetry. A p-value of <0.05 was considered to indicate a statistically significant difference for all statistical tests.
3. Results
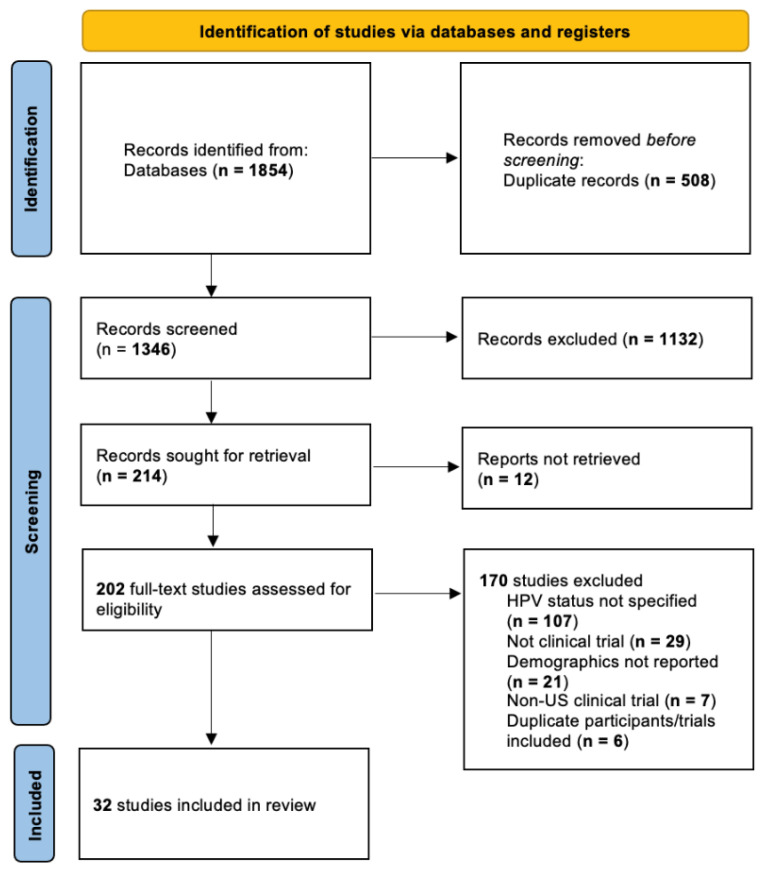
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRSIMA) guidelines (Figure 1). A total of 32 studies with 2995 patients were included in the review [2,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. Table 1 shows the year, location, trial identifier (NCT number, RTO, etc.) or study identifier, and trial phase for each included study.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flowchart for data search performed in PubMed (National Library of Medicine, National Institutes of Health), Scopus (Elsevier), CINAHL (EBSCO), and Cochrane Library (Wiley).
Table 1.
Clinical trial characteristics *.
| Author, Year | State(s) | Trial ID | Phase(s) | Total n |
|---|---|---|---|---|
| Aggarwal 2019 | PA | NCT02163057 | I/IIa | 22 |
| Anderson 2015 | MD, NY, OR | NCT01342978 | NR | 116 |
| Ang 2010 | TX | NCT00047008 | III | 206 |
| Chen 2017 | CA | NCT02048020/NCT01716195 | II | 44 |
| Chera 2015 | NC, FL | NCT01530997 | II | 44 |
| Chera 2019 | NC | NCT02281955 | II | 114 |
| Chera 2020 | NC, FL | NCT03077243 | II | 115 |
| Ding 2015 | TX | NCT01893307 | II/III | 31 |
| Dunn 2018 | NY | NCT01721525 | Ib | 10 |
| Fakhry 2008 | MD | ECOG protocol 2399 | II | 38 |
| Fakhry 2014 | MD | RTOG0129/RTOG0522 | III | 105 |
| Foster 2020 | IL | NCT02258659 | II | 62 |
| Gillison 2019 | TX | NCT01302834 | NR | 805 |
| Kumar 2008 | MI | UMCC9921 | NR | 50 |
| Ma 2019 | MN, AZ, FL | NCT01932697 | II | 79 |
| Marur 2016 | CT | NCT01084083 | II | 80 |
| Massarelli 2019 | TX | NCT02426892 | II | 22 |
| Miles 2021 | NY | NCT02072148 | II | 54 |
| Misiukiewicz 2019 | NY | NCT01706939 | III | 23 |
| Mowery 2020 | NC | NCT01908504 | NR | 62 |
| Oppelt 2021 | MO | NCT02101034 | II | 24 |
| Rosenberg 2021 | IL | NCT02258659 | II | 90 |
| Rosenthal 2016 | TX | NCT00004227 | III | 75 |
| Samuels 2016 | MI | PO1CA59827 | II | 53 |
| Seiwert 2019 | IL | NCT01816984 | II | 62 |
| Settle 2009 | MD | TAX 324 | III | 68 |
| Shaverdian 2019 | CA | NCT01716195 | II | 24 |
| Spector 2012 | MI | UCMCC02-021 | II | 78 |
| Swiecicki 2020 | MI | NCT01663259/NCT00904345 | II | 42 |
| Swisher-McClure 2020 | PA | NCT02159703 | II | 60 |
| Voskens 2012 | MD | NCT00257738 | I | 31 |
| Yom 2021 | CA | NCT02254278 | II | 306 |
NR = Not reported. * All trials were classified as Level 1 according to the OCEBM LOE.
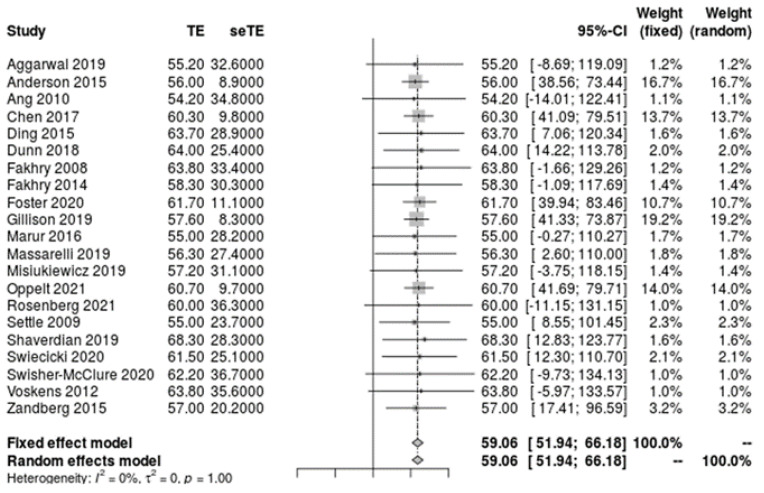
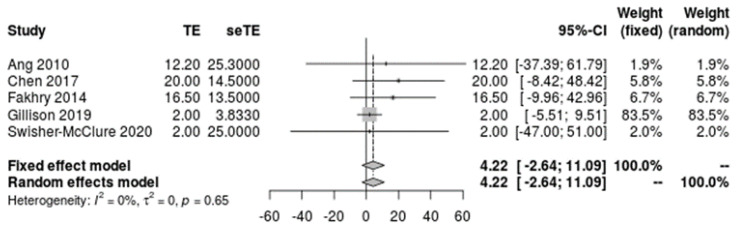
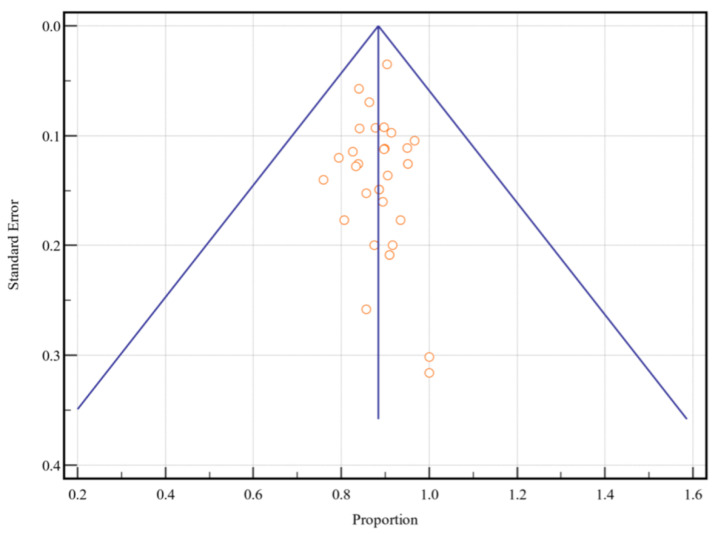
Of the 2995 included patients, 2918 patients were reported by sex, 2457 were reported by age, 1993 were reported by race, 2668 were reported by tobacco and alcohol history, and 2162 were reported by primary tumor site. The mean patient age was 59.1 [51.9, 66.2] years (Figure 2) and the mean pack years for smoking history was 4.2 [−2.6, 11.1] years (Figure 3). Overall, patients were found to be significantly predominately white and male, with no history of smoking tobacco, a current drinking status, and tumors originating from the tonsil. A funnel plot with Egger’s test (−0.11, 95% CI −1.10 to 0.88, p = 0.82) demonstrated all studies were within the funnel, suggesting little publication bias (Figure 4). Table 2 shows the meta-analysis of sex and race, Table 3 shows the meta-analysis of tobacco and alcohol history, and Table 4 shows the meta-analysis of tumor origin site. I2 values indicated high levels of heterogeneity amongst patients in regard to racial classification as African American, Hispanic, and other/NOS, smoking and alcohol history, and tumor origin site.
Figure 2.
Meta-analysis of single means for participant age.
Figure 3.
Meta-analysis of single means for participant pack years.
Figure 4.
Funnel plot of all included studies.
Table 2.
Meta-analyses of patient sex and race and I2 values amongst all studies and patients.
| Identifier | Proportion % [95% CI] | I2 (%) |
|---|---|---|
| Male | 88.2 [86.4, 89.9] | 41.7 |
| Female | 11.8 [10.1, 13.6] | 41.7 |
| White | 91.1 [88.9, 93.0] | 44.1 |
| African American | 4.8 [2.7, 7.3] | 76.9 |
| Hispanic | 1.8 [0.7, 3.3] | 70.5 |
| Asian | 0.3 [0.1, 0.6] | 0.0 |
| Other/NOS | 2.5 [1.4, 3.9] | 58.7 |
Table 3.
Meta-analysis of tobacco and alcohol history and I2 values amongst 28 studies (n = 2691).
| History | Proportion % [95% CI] | I2 (%) |
|---|---|---|
| Current smoker | 13.0 [5.9, 22.2] | 87.8 |
| Former smoker | 30.6 [18.7, 44.0] | 90.6 |
| Never smoker | 50.0 [43.0, 57.1] | 90.2 |
| Unknown smoking history | 6.5 [3.8, 9.9] | 0.0 |
| History of <20 pack years | 16.5 [6.2, 30.4] | 83.8 |
| History of ≥20 pack years | 22.6 [12.7, 34.3] | 80.3 |
| History of ≤10 pack years | 24.4 [17.6, 31.8] | 87.1 |
| History of >10 pack years | 26.9 [19.2, 35.4] | 87.8 |
| Current drinker | 46.2 [26.3, 66.8] | 89.2 |
| Former drinker | 10.1 [1.5, 48.5] | 95.2 |
| Never drinker | 28.7 [6.6, 58.4] | 95.4 |
Table 4.
Meta-analysis of tumor origin site and I2 values amongst 21 studies (n = 2162).
| Tumor Origin Site | Proportion % [95% CI] | I2 (%) |
|---|---|---|
| Tonsil | 44.4 [39.2, 49.7] | 79.3 |
| Base of tongue | 41.4 [34.5, 48.5] | 89.0 |
| Tonsil and base of tongue | 2.6 [0.5, 6.3] | 93.4 |
| Other/not otherwise specified | 4.3 [2.3, 6.8] | 81.6 |
A review of the HPV-associated OPSCC population within the oropharyngeal cancer cohort in the National Cancer Database (NCDB) reported the demographics of 14,805 patients: patients were a mean age of 58.4 ± 9.5 years, 67.9% (n = 12,600) male, 67.7% (n = 13,479) white, and 69.7% (n = 8068) of high socioeconomic status, with 65.2% (n = 5598) having tumors originating from the tongue base [41,42].
4. Discussion
The objective of this study was to answer the following question: how do patients with HPV-related OPSCC (Population) enrolled in clinical trials (Intervention), compared with national database reports of HPV-related OPSCC patients (Comparison), present demographically (Outcome)? With the determination of HPV status for OPSCC rapidly becoming the standard of care, given HPV-positive patients’ approximate 60% reduction in risk of death and absolute survival difference of nearly 30% at five years, the importance of reviewing the demographics reflected in current advances for treatment cannot be understated [43]. Furthermore, even though overall recurrence rates are lower for HPV-positive OPSCC patients, they have a higher proportion of recurrences at distant sites than HPV-negative patients and are more likely to experience disseminated metastasis in non-traditional/non-pulmonary sites [44,45,46]. Thus, demographically-appropriate treatment modalities are essential.
According to Pytynia et al., the prototypical patient with HPV-positive OPSCC is a nonsmoker with a history of multiple sexual (oral and/or genital) partners [44]. Furthermore, associated tumors are most likely to originate in the tonsil or base of tongue [47]. This is reflected in the demographics of our included trials, as patients were predominantly Caucasian, male, and non-smokers, with tumors originating in nearly equal proportions from the tonsil or base of tongue. Our findings, therefore, suggest that HPV-associated OPSCC patients are, in fact, properly represented in clinical trials.
We were unable to perform analysis on sexual partner histories or socioeconomic status, as too few studies reported these variables. HPV-associated OPSCC differs drastically from other cancers in its incidence amongst Caucasians relative to non-Caucasians; whereas Camidge et al. reported that African American men are more likely to have malignant tumors and lower rates of survival than the general population, Pytynia et al. found that the stark preponderance of HPV-associated OPSCC in Caucasian males over the general population actually contributes to the diminishment in cancer-related disparities between Caucasian and African American males [44,48]. Although Kennedy-Martin et al. found that RCT participant populations are highly selective and have lower risk profiles than that of the general population, they did so in the context of cardiology, mental health, and general oncology [49]. Heiat et al. and Varma et al. demonstrated disparities in representation for clinical trials regarding cardiology and oncology, respectively, with trial demographics that were predominately Caucasian, despite significant incidence in African American and other non-Caucasian patients [5,50]. Conversely, HPV-associated OPSCC has been described with a more skewed incidence amongst Caucasian males.
While our findings indicate that the HPV-associated OPSCC population is adequately represented in clinical trials, it is also worth considering areas of potential underreporting in the general population, such that certain demographics are going unnoticed. Dunlop et al. and Dovido et al. showed that non-Caucasian patients have fewer physician contacts, utilize fewer hospital and outpatient surgery services, and are more likely to suffer from unequal geographic distribution of medical services [51,52]. Barriers that present before an initial clinical encounter could result in an underreporting of HPV-associated OPSCC cases in select patient groups, translating into underreporting of clinical trial participation and poor representation in treatment evaluations.
The largest limitation of this study was the lack of a proper bias assessment tool. While this was a meta-analysis of clinical trials, models such as ROBINS-I or Cochrane Collaboration’s tool were not appropriate, as they utilize pre-intervention, peri-intervention, and post-intervention metrics [53]. Since we focused solely on the demographics of each trial and not the specific potential deviations from intended interventions or protocol, we were unable to utilize the standard risk of bias assessments. A uniform assessment method that allows for analysis without the inclusion of specific interventions would allow for risk of bias to be determined in this context.
Disparity in patient representation for clinical trials is, unfortunately, a relatively familiar dilemma; Clark et al. reported that African Americans and Hispanics comprised 13% and 16% of the US population in 2016, respectively, yet made up only 5% and 1% of clinical-trial participants, respectively. In reviewing the barriers faced by patients in clinical trial representation, Clark et al., along with Ford et al., found that the five primary obstacles were physician mistrust, discomfort with the clinical trial process, lack of information, time and resource constraints, and lack of awareness of resources [54,55]. A review of Phase I–III trials for drugs targeting breast, colorectal, lung, and prostate cancer by Ramamoorthy et al. found that 79.7% of the trial participants were Caucasian, 12.4% Asian, 3.8% African American, and 3.6% Hispanic [56]. Similarly, Loree et al.’s review of clinical trials for FDA approvals for cancer drugs reported that Caucasians represented 76.3% of the trial participants, followed by 18.3% Asians, 6.1% Hispanics, and 3.1% African Americans [57].
This study was also potentially limited by the heterogeneity in reporting demographics and histories. The majority of included studies reported sex, age, and tobacco use; however, race was less frequently reported and variables such as socioeconomic status and sexual partner history were even more rarely documented. Since the latter two variables have also demonstrated key patterns in the general population, the addition of more studies that further specify these characteristics would enable more detailed analysis of trial participant demographics, in comparison to the overall patient population. In addition to heterogeneity in frequency of reporting, there was also heterogeneity in the specifics of describing certain features. For example, some studies provided mean pack years, some reported quantities of patients with pack years greater than, equal to, or less than 10 years, and others reported the equivalent for 20 pack years. Since the data was presented differently, fewer analyses were done, as each sub-group had a smaller sample size. Future analyses would be improved by standardizing the format of reporting patient demographics and history.
5. Conclusions
Having been described as an epidemic, HPV-associated OPSCC is both increasingly common in incidence as well as promising in treatment responses, warranting various clinical trials for treatment evaluation [45]. The demographics of participants for said trials were reviewed and meta-analyzed to answer the following question: how do patients with HPV-related OPSCC (Population) enrolled in clinical trials (Intervention), compared with national database reports of HPV-related OPSCC patients (Comparison), present demographically (Outcome)? Overall, our findings revealed a predominance of middle-aged Caucasian males without a history of smoking, reflective of the demographics for HPV-associated OPSCC in the United States, as reported by the NCDB. Further studies could increase both the sample size and power of these trial demographics and allow for even better reflection of the general population in participant groups for clinical trials.
Acknowledgments
Rachana Gudipudi, assisted in study selection.
Appendix A. Search Strategy
PubMed:
(“Human papillomavirus 16” [Mesh] OR “Oropharyngeal Neoplasms” [Mesh]) AND (“Clinical Trials as Topic” [Mesh] OR “Clinical Trial, Phase II” [Publication Type] OR “Clinical Trial, Phase III” [Publication Type] OR “Clinical Trial, Phase IV” [Publication Type] OR “Clinical Trial” [Publication Type])
Scopus:
TITLE-ABS-KEY (“Human papillomavirus 16” OR “Oropharyngeal Neoplasms”) AND (“Clinical Trials as Topic” OR “Clinical Trial, Phase II” OR “Clinical Trial, Phase III” OR “Clinical Trial, Phase IV” OR “Clinical Trial”)
CINAHL:
(MH “Human papillomavirus 16” OR “Oropharyngeal Neoplasms”) AND (MH “Clinical Trials as Topic” OR “Clinical Trial, Phase II” OR “Clinical Trial, Phase III” OR “Clinical Trial, Phase IV” OR “Clinical Trial”)
Cochrane:
(“Human papillomavirus 16” OR “Oropharyngeal Neoplasms”) AND (“Clinical Trials as Topic” OR “Clinical Trial, Phase II” OR “Clinical Trial, Phase III” OR “Clinical Trial, Phase IV” OR “Clinical Trial”)
Author Contributions
Conceptualization and data curation, T.M.G. and J.L.C.; methodology, software and formal analysis, S.A.N.; writing—original draft preparation, T.M.G.; writing—review and editing, T.M.G., J.L.C., S.A.N. and J.G.N. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iorio G.C., Arcadipane F., Martini S., Ricardi U., Franco P. Decreasing treatment burden in HPV-related OPSCC: A systematic review of clinical trials. Crit. Rev. Oncol. Hematol. 2021;160:103243. doi: 10.1016/j.critrevonc.2021.103243. [DOI] [PubMed] [Google Scholar]
- 2.Tota J.E., Best A.F., Zumsteg Z.S., Gillison M.L., Rosenberg P.S., Chaturvedi A.K. Evolution of the oropharynx cancer epidemic in the United States: Moderation of increasing incidence in younger individuals and shift in the burden to older individuals. J. Clin. Oncol. 2019;37:1538. doi: 10.1200/JCO.19.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung C.H., Schwartz D.L. Impact of HPV-related head and neck cancer in clinical trials: Opportunity to translate scientific insight into personalized care. Otolaryngol. Clin. N. Am. 2012;45:795–806. doi: 10.1016/j.otc.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Margalit D.N., Karam S.D., Chua M.L.K., Anderson C., Kimple R.J. Four influential clinical trials in human papilloma virus-associated oropharynx cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020;106:893–899. doi: 10.1016/j.ijrobp.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Heiat A., Gross C.P., Krumholz H.M. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern. Med. 2002;162:1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 6.Johnston R.E., Heitzeg M.M. Sex, age, race and intervention type in clinical studies of HIV cure: A systematic review. AIDS Res. Hum. Retrovir. 2015;31:85–97. doi: 10.1089/aid.2014.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan S.U., Khan M.Z., Subramanian C.R., Riaz H., Khan M.U., Lone A.N., Khan M.S., Benson E.-M., Alkhouli M., Blaha M.J., et al. Participation of women and older participants in randomized clinical trials of lipid-lowering therapies: A systematic review. JAMA Netw. Open. 2020;3:e205202. doi: 10.1001/jamanetworkopen.2020.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strait A., Castillo F., Choden S., Li J., Whitaker E., Falasinnu T., Schmajuk G., Yazdany J. Demographic characteristics of participants in rheumatoid arthritis randomized clinical trials: A systematic review. JAMA Netw. Open. 2019;2:e1914745. doi: 10.1001/jamanetworkopen.2019.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman M.F., Turkey J.W. Transformations related to the angular and the square root. Ann. Math. Statist. 1950;21:607–611. doi: 10.1214/aoms/1177729756. [DOI] [Google Scholar]
- 10.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterne J.A., Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001;54:1046–1055. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal C., Cohen R.B., Morrow M.P., Kraynyak K.A., Sylvester A.J., Knoblock D.M., Bauml J.M., Weinstein G.S., Lin A., Boyer J. Immunotherapy Targeting HPV16/18 Generates Potent Immune Responses in HPV-Associated Head and Neck CancerDNA Immunotherapy in HPV-Associated Head and Neck Cancer. Clin. Cancer Res. 2019;25:110–124. doi: 10.1158/1078-0432.CCR-18-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson K.S., Gerber J.E., D’Souza G., Pai S.I., Cheng J.N., Alam R., Kesiraju S., Chowell D., Gross N.D., Haddad R., et al. Biologic predictors of serologic responses to HPV in oropharyngeal cancer: The HOTSPOT study. Oral Oncol. 2015;51:751–758. doi: 10.1016/j.oraloncology.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ang K.K., Harris J., Wheeler R., Weber R., Rosenthal D.I., Nguyen-Tân P.F., Westra W.H., Chung C.H., Jordan R.C., Lu C., et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen A.M., Felix C., Wang P.C., Hsu S., Basehart V., Garst J., Beron P., Wong D., Rosove M.H., Rao S., et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: A single-arm, phase 2 study. Lancet Oncol. 2017;18:803–811. doi: 10.1016/S1470-2045(17)30246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chera B.S., Amdur R.J., Tepper J., Qaqish B., Green R., Aumer S.L., Hayes N., Weiss J., Grilley-Olson J., Zanation A., et al. Phase 2 trial of de-intensified chemoradiation therapy for favorable-risk human papillomavirus–associated oropharyngeal squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2015;93:976–985. doi: 10.1016/j.ijrobp.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 17.Ding Y., Hazle J.D., Mohamed A.S.R., Frank S.J., Hobbs B.P., Colen R.R., Gunn G.B., Wang J., Kalpathy-Cramer J., Garden A., et al. Intravoxel incoherent motion imaging kinetics during chemoradiotherapy for human papillomavirus-associated squamous cell carcinoma of the oropharynx: Preliminary results from a prospective pilot study. NMR Biomed. 2015;28:1645–1654. doi: 10.1002/nbm.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn L.A., Fury M.G., Sherman E.J., Ho A.A., Katabi N., Haque S.S., Pfister D.G. Phase I study of induction chemotherapy with afatinib, ribavirin, and weekly carboplatin and paclitaxel for stage IVA/IVB human papillomavirus-associated oropharyngeal squamous cell cancer. Head Neck. 2018;40:233–241. doi: 10.1002/hed.24938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fakhry C., Westra W.H., Li S., Cmelak A., Ridge J.A., Pinto H., Forastiere A., Gillison M.L. Improved Survival of Patients with Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. JNCI J. Natl. Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 20.Fakhry C., Zhang Q., Nguyen-Tan P.F., Rosenthal D., El-Naggar A., Garden A., Soulieres D., Trotti A., Avizonis V., Ridge J.A., et al. Human Papillomavirus and Overall Survival After Progression of Oropharyngeal Squamous Cell Carcinoma. J. Clin. Oncol. 2014;32:3365–3373. doi: 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster C.C., Seiwert T.Y., MacCracken E., Blair E.A., Agrawal N., Melotek J.M., Portugal L., Brisson R.J., Gooi Z., Spiotto M.T., et al. Dose and Volume De-Escalation for Human Papillomavirus–Positive Oropharyngeal Cancer is Associated with Favorable Posttreatment Functional Outcomes. Int. J. Radiat. Oncol. 2020;107:662–671. doi: 10.1016/j.ijrobp.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Gillison M.L., Trotti A.M., Harris J., Eisbruch A., Harari P.M., Adelstein D.J., Jordan R.C.K., Zhao W., Sturgis E.M., Burtness B., et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet. 2019;393:40–50. doi: 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar B., Cordell K.G., Lee J.S., Worden F.P., Prince M.E., Tran H.H., Wolf G.T., Urba S.G., Chepeha D.B., Teknos T.N., et al. EGFR, p16, HPV Titer, Bcl-xL and p53, Sex, and Smoking as Indicators of Response to Therapy and Survival in Oropharyngeal Cancer. J. Clin. Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marur S., Li S., Cmelak A.J., Gillison M.L., Zhao W.J., Ferris R.L., Westra W.H., Gilbert J., Bauman J.E., Wagner L.I., et al. E1308: Phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx—ECOG-ACRIN Cancer Research Group. J. Clin. Oncol. 2017;35:490. doi: 10.1200/JCO.2016.68.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massarelli E., William W., Johnson F., Kies M., Ferrarotto R., Guo M., Feng L., Lee J.J., Tran H., Kim Y.U., et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16–related cancer: A phase 2 clinical trial. JAMA Oncol. 2019;5:67–73. doi: 10.1001/jamaoncol.2018.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miles B.A., Posner M.R., Gupta V., Teng M.S., Bakst R.L., Yao M., Misiukiewicz K.J., Chai R.L., Sharma S., Westra W.H., et al. De-escalated adjuvant therapy after transoral robotic surgery for human papillomavirus-related oropharyngeal carcinoma: The Sinai robotic surgery (SIRS) trial. Oncologist. 2021;26:504–513. doi: 10.1002/onco.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misiukiewicz K., Gupta V., Miles B., Bakst R., Genden E., Selkridge I., Surgeon J., Rainey H., Camille N., Roy E., et al. Standard of care vs reduced-dose chemoradiation after induction chemotherapy in HPV+ oropharyngeal carcinoma patients: The Quarterback trial. Oral Oncol. 2019;95:170–177. doi: 10.1016/j.oraloncology.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Mowery Y.M., Vergalasova I., Rushing C.N., Choudhury K.R., Niedzwiecki D., Wu Q.J., Yoo D.S., Das S., Wong T.Z., Brizel D. Early 18F-FDG-PET Response During Radiation Therapy for HPV-Related Oropharyngeal Cancer May Predict Disease Recurrence. Int. J. Radiat. Oncol. 2020;108:969–976. doi: 10.1016/j.ijrobp.2020.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Oppelt P., Ley J.C., Worden F., Palka K., Maggiore R., Liu J., Adkins D. Palbociclib and cetuximab in cetuximab-resistant human papillomavirus-related oropharynx squamous-cell carcinoma: A multicenter phase 2 trial. Oral Oncol. 2021;114:105164. doi: 10.1016/j.oraloncology.2020.105164. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg A.J., Agrawal N., Pearson A., Gooi Z., Blair E., Cursio J., Juloori A., Ginat D., Howard A., Chin J., et al. Risk and response adapted de-intensified treatment for HPV-associated oropharyngeal cancer: Optima paradigm expanded experience. Oral Oncol. 2021;122:105566. doi: 10.1016/j.oraloncology.2021.105566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenthal D., Harari P.M., Giralt J., Bell D., Raben D., Liu J., Schulten J., Ang K.K., Bonner J.A. Association of Human Papillomavirus and p16 Status with Outcomes in the IMCL-9815 Phase III Registration Trial for Patients with Locoregionally Advanced Oropharyngeal Squamous Cell Carcinoma of the Head and Neck Treated with Radiotherapy with or without Cetuximab. J. Clin. Oncol. 2016;34:1300–1308. doi: 10.1200/jco.2015.62.5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuels S.E., Tao Y., Lyden T., Haxer M., Spector M., Malloy K.M., Prince M.E., Bradford C.R., Worden F.P., Schipper M., et al. Comparisons of dysphagia and quality of life (QOL) in comparable patients with HPV-positive oropharyngeal cancer receiving chemo-irradiation or cetuximab-irradiation. Oral Oncol. 2016;54:68–74. doi: 10.1016/j.oraloncology.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seiwert T.Y., Foster C.C., Blair E.A., Karrison T.G., Agrawal N., Melotek J.M., Portugal L., Brisson R.J., Dekker A., Kochanny S., et al. OPTIMA: A phase II dose and volume de-escalation trial for human papillomavirus-positive oropharyngeal cancer. Ann. Oncol. 2019;30:297–302. doi: 10.1093/annonc/mdy522. [DOI] [PubMed] [Google Scholar]
- 34.Settle K., Posner M.R., Schumaker L.M., Tan M., Suntharalingam M., Goloubeva O., Strome S.E., Haddad R.I., Patel S.S., Cambell E.V., et al. Racial Survival Disparity in Head and Neck Cancer Results from Low Prevalence of Human Papillomavirus Infection in Black Oropharyngeal Cancer Patients. Cancer Prev. Res. 2009;2:776–781. doi: 10.1158/1940-6207.CAPR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaverdian N., Hegde J.V., Felix C., Hsu S., Basehart V., Steinberg M.L., Chen A.M. Patient perspectives and treatment regret after de-escalated chemoradiation for human papillomavirus-positive oropharyngeal cancer: Findings from a phase II trial. Head Neck. 2019;41:2768–2776. doi: 10.1002/hed.25760. [DOI] [PubMed] [Google Scholar]
- 36.Spector M.E., Gallagher K.K., Light E., Ibrahim M., Bs E.J.C., Moyer J.S., Prince M.E., Wolf G.T., Bradford C.R., Cordell K., et al. Matted nodes: Poor prognostic marker in oropharyngeal squamous cell carcinoma independent of HPV and EGFR status. Head Neck. 2012;34:1727–1733. doi: 10.1002/hed.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swiecicki P.L., Li P., Bellile E., Stucken C., Malloy K., Shuman A., Spector M.E., Chinn S., Casper K., McLean S., et al. Paired phase II trials evaluating cetuximab and radiotherapy for low risk HPV associated oropharyngeal cancer and locoregionally advanced squamous cell carcinoma of the head and neck in patients not eligible for cisplatin. Head Neck. 2020;42:1728–1737. doi: 10.1002/hed.26085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosch-Voskens C., Sewell D., Hertzano R., Ms J.D., Ms S.R., Lee M., Taylor R., Wolf J., Suntharalingam M., Gastman B., et al. inducTION of mage-A3 and HPV-16 immunity by Trojan vaccines in patients with head and neck carcinoma. Head Neck. 2012;34:1734–1746. doi: 10.1002/hed.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yom S.S., Torres-Saavedra P., Caudell J.J., Waldron J.N., Gillison M.L., Xia P., Truong M.T., Kong C., Jordan R., Subramaniam R.M., et al. Reduced-Dose Radiation Therapy for HPV-Associated Oropharyngeal Carcinoma (NRG Oncology HN002) J. Clin. Oncol. 2021;39:956–965. doi: 10.1200/JCO.20.03128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swisher-McClure S., Lukens J.N., Aggarwal C., Ahn P., Basu D., Bauml J.M., Brody R., Chalian A., Cohen R.B., Fotouhi-Ghiam A., et al. A Phase 2 Trial of Alternative Volumes of Oropharyngeal Irradiation for De-intensification (AVOID): Omission of the Resected Primary Tumor Bed After Transoral Robotic Surgery for Human Papilloma Virus–Related Squamous Cell Carcinoma of the Oropharynx. Int. J. Radiat. Oncol. 2020;106:725–732. doi: 10.1016/j.ijrobp.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 41.Liederbach E., Kyrillos A., Wang C.H., Liu J.C., Sturgis E.M., Bhayani M.K. The national landscape of human papillomavirus-associated oropharynx squamous cell carcinoma. Int. J. Cancer. 2017;140:504–512. doi: 10.1002/ijc.30442. [DOI] [PubMed] [Google Scholar]
- 42.Lechner M., Liu J., Masterson L., Fenton T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022;19:306–327. doi: 10.1038/s41571-022-00603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jordan R.C., Lingen M.W., Perez-Ordonez B., He X., Pickard R., Koluder M., Jiang B., Wakely P., Xiao W., Gillison M.L. Validation of Methods for Oropharyngeal Cancer HPV Status Determination in US Cooperative Group Trials. Am. J. Surg. Pathol. 2012;36:945–954. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pytynia K.B., Dahlstrom K.R., Sturgis E.M. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50:380–386. doi: 10.1016/j.oraloncology.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaturvedi A.K., Engels E.A., Pfeiffer R.M., Hernandez B.Y., Xiao W., Kim E., Jiang B., Goodman M.T., Sibug-Saber M., Cozen W., et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang M.B., Liu I.Y., Gornbein J.A., Nguyen C.T. HPV-positive oropharyngeal carcinoma: A systematic review of treatment and prognosis. Otolaryngol. Head Neck Surg. 2015;153:758–769. doi: 10.1177/0194599815592157. [DOI] [PubMed] [Google Scholar]
- 47.Sturgis E.M., Ang K.K. The Epidemic of HPV-associated oropharyngeal cancer is here: Is it time to change our treatment paradigms? J. Natl. Compr. Cancer Netw. 2011;9:665–673. doi: 10.6004/jnccn.2011.0055. [DOI] [PubMed] [Google Scholar]
- 48.Camidge D.R., Park H., E Smoyer K., Jacobs I., Lee L.J., Askerova Z., McGinnis J., Zakharia Y. Race and ethnicity representation in clinical trials: Findings from a literature review of Phase I oncology trials. Futur. Oncol. 2021;17:3271–3280. doi: 10.2217/fon-2020-1262. [DOI] [PubMed] [Google Scholar]
- 49.Kennedy-Martin T., Curtis S., Faries D., Robinson S., Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495. doi: 10.1186/s13063-015-1023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varma T., Wallach J.D., Miller J.E., Schnabel D., Skydel J.J., Zhang A.D., Dinan M.A., Ross J.S., Gross C.P. Reporting of study participant demographic characteristics and demographic representation in Premarketing and postmarketing studies of novel cancer therapeutics. JAMA Netw. Open. 2021;4:e217063. doi: 10.1001/jamanetworkopen.2021.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunlop D.D., Manheim L.M., Song J., Chang R.W. Gender and Ethnic/Racial Disparities in Health Care Utilization Among Older Adults. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2002;57:S221–S233. doi: 10.1093/geronb/57.4.S221. [DOI] [PubMed] [Google Scholar]
- 52.Dovido J.F., Penner L.A., Albrecht T.L., Norton W.E., Gaertner S.L., Shelton J.N. Disparities and distrust: The implications of psychological processes for understanding racial disparities in health and health care. Soc. Sci. Med. 2008;67:478–486. doi: 10.1016/j.socscimed.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 53.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark L.T., Watkins L., Piña I.L., Elmer M., Akinboboye O., Gorham M., Jamerson B., McCullough C., Pierre C., Polis A.B., et al. Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Curr. Probl. Cardiol. 2018;44:148–172. doi: 10.1016/j.cpcardiol.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 55.Ford J.G., Howerton M.W., Lai G.Y., Gary-Webb T., Bolen S., Gibbons M.C., Tilburt J., Baffi C., Tanpitukpongse T.P., Wilson R.F., et al. Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer. 2008;112:228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 56.Ramamoorthy A., Knepper T.C., Merenda C., Mendoza M., McLeod H.L., Bull J., Zhang L., Pacanowski M. Demographic composition of select oncologic new molecular entities approved by the FDA between 2008 and 2017. Clin. Pharmacol. Ther. 2018;104:940–948. doi: 10.1002/cpt.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loree J., Anand S., Dasari A., Unger J.M., Gothwal A., Ellis L.M., Varadhachary G., Kopetz S., Overman M.J., Raghav K. Disparity of Race Reporting and Representation in Clinical Trials Leading to Cancer Drug Approvals From 2008 to 2018. JAMA Oncol. 2019;5:e191870. doi: 10.1001/jamaoncol.2019.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]