Abstract
Non-alcoholic fatty liver disease (NAFLD) is an important health concern during childhood; indeed, it is the most frequent cause of chronic liver diseases in obese children. No valid pharmacological therapies for children affected by this condition are available, and the recommended treatment is lifestyle modification, usually including nutrition and exercise interventions. In this narrative review, we summarized up-to-date information on the benefits of physical exercise on NAFLD in children and adolescents with obesity. The role of exercise as non-pharmacological treatment was emphasized in order to provide recent advances on this topic for clinicians not deeply involved in the field. Several studies on obese children and adults confirm the positive role of physical activity (PA) in the treatment of NAFLD, but to date, there are no pediatric randomized clinical trials on exercise versus usual care. Among the pathogenic mechanisms involved in the PA effects on NAFLD, the main players seem to be insulin resistance and related inflammation, oxidative stress, and gut dysbiosis, but further evaluations are necessary to deeply understand whether these factors are correlated and how they synergistically act. Thus, a deeper research on this theme is needed, and it would be extremely interesting.
Keywords: steatosis, obesity, pediatrics, exercise, physical activity, prevention
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is an important health concern during childhood; indeed, it is the most frequent cause of chronic liver diseases in obese children [1,2]. Further, it should be reported that having NAFLD in childhood could increase the risk to develop hepatocellular carcinoma in adulthood [3].
NAFLD represents a spectrum of pathological conditions ranging from steatosis to nonalcoholic steato-hepatitis (NASH) and fibrosis [4]. The accumulation of liver fat mainly depends on imbalances between the influx of fatty acids to the liver and the liver’s ability to export these fatty acids in the form of very-low-density lipoprotein (VLDL). Several factors, such as genetic susceptibility, epigenetic mechanisms, gut–liver axis, hormone levels, sedentary lifestyle, and hypercaloric diets [4], have been considered in pathogenesis of NAFLD.
NAFLD diagnosis is not straightforward, as this condition may be asymptomatic until the progression to end-stage liver disease [5]. The diagnosis of the disease was based on any diagnostic method, including liver enzymes, ultrasound, magnetic resonance, and liver biopsy.
Nowadays, no valid pharmacological therapies are available for children affected by this condition, and the recommended treatment is lifestyle modification, usually including nutrition and exercise interventions [3,6,7,8]. Physical activity has been included in the focus of research in children and adolescents with NAFLD either as the only intervention or in association with other lifestyle approaches [6,9].
In this narrative review, we summarized up-to-date information on the benefits of physical exercise on NAFLD in children and adolescents with obesity. The role of exercise as non-pharmacological treatment was emphasized in order to provide recent advances on this topic for clinicians not deeply involved in the field.
2. Methods
As a literature narrative review [10], we present a non-systematic summation and analysis of literature available on the topic of benefits of physical exercise as an approach to NAFLD in children and adolescents with obesity. Inclusion criteria to refine the narrative review were: articles in the English language; original scientific papers, clinical trials, meta-analyses, and reviews published on a specific topic in the last 15 years, up to June 2022; and case reports or series and letters were not considered. The authors assessed the abstracts of the available literature (n = 206) and reviewed the full texts of potentially relevant papers (n = 106) that were analyzed and critically discussed. The reference list of all articles was checked to consider relevant studies. The terms adopted for the research, alone and/or combined, are non-alcoholic fatty liver disease, obesity, adolescents, children, physical exercise, childhood obesity, complications, and physical activity. The databases PubMed, Scopus, Web of Science, and EMBASE were used from for research purposes.
3. Childhood Obesity and Non-Alcoholic Fatty Liver Disease
3.1. Childhood Obesity
Childhood obesity has become one of the major public health concerns not merely in the U.S. and in the Western countries but also in many other areas worldwide [11].
Before the outbreak of the COVID-19 pandemic, obesity prevalence in children and adolescents had plateaued in many wealthy countries, while its levels had risen in low- and middle-income countries [11]. However, during the pandemic, academics have reported a different trend: obesity and overweight in children has started to increase in the richest countries as well.
According to this, the U.S. data show the effects of the pandemic on the obesity trends. As reported by Centers for Disease Control and Prevention (CDC) [12], just before the COVID outbreak, the prevalence of obesity was equal to 19.7% for U.S. children and adolescents in the age group 2–19 years. Furthermore, obesity prevalence was 12.7% for age group 2–5, 20.7% for age group 6–11, and 22.2% for age group 12–19. Instead, only 1 year after the pandemic outbreak, the prevalence of childhood obesity in the U.S. increased to 22.4% in the age group 2–19 years based on a longitudinal cohort of 432,302 persons [13]. The total amount of obese children in the U.S. had a monthly increase of 0.07% a month before the pandemic. This coefficient rose to 0.37% (five times higher) just a month after the virus appeared [13,14].
Risk factors for obesity in the earlier stages of life have a twofold nature. Firstly, it is crucial to take into account the role played by biological risk factors, which include age, gender, and genetic predisposition. Secondly, one must consider the impact of environmental factors in early life, including as dietary intake, physical activity, and sedentary behavior throughout childhood [15].
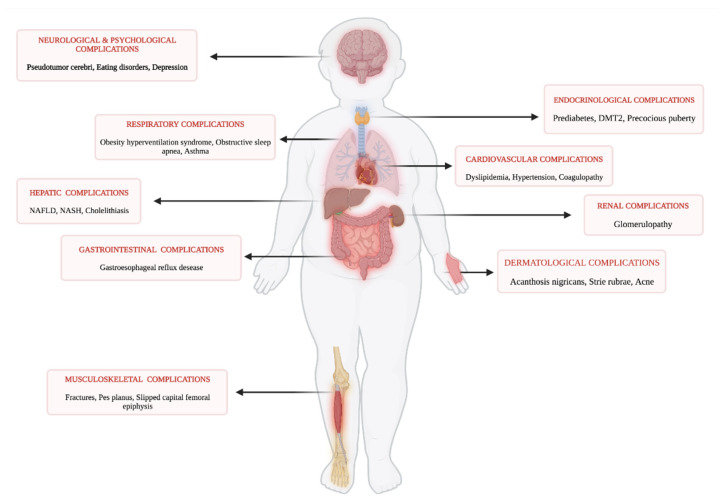
Childhood obesity is at the root of multiple systemic comorbidities. These include, among others, unbalances in the endo-metabolic system (e.g., type 2 diabetes mellitus, dyslipidemia), respiratory system (e.g., obstructive sleep apnea, asthma), and gastrointestinal system including NAFLD and its evolutions as well as at the cardiovascular and musculoskeletal systems [16,17,18,19,20] (Figure 1). The severity of these comorbidities increases proportionally to the degree of obesity [21].
Figure 1.
Multiorgan complications related to obesity in children and adolescents (created with BioRender.com, accessed on 1 July 2022).
Prevention remains the best approach to face the burden of this condition. Lifestyle interventions are feasible and effective in both the prevention and treatment of childhood obesity, and pharmacological and surgical treatment are available for a selected population of children and adolescents with obesity [17,18,22,23,24].
A multidisciplinary approach, including physical activity programs, nutrition education, and behavioral modification with highly qualified specialists, represents a key point for the approach to childhood obesity.
3.2. Epidemiology of NAFLD in General Pediatric Population and in Children with Obesity
NAFLD is defined as a chronic disease characterized by an accumulation of fat in the liver in the absence of excessive alcohol consumption or other known liver diseases [25], which initially manifests as steatosis in at least 5% of hepatocytes but which can progress to NASH, cirrhosis, and, in severe cases, to hepatocellular carcinoma [26]. It is mainly associated with an incorrect lifestyle and obesity [27] as well as other metabolic disorders such as dyslipidemia and DMT2 [28].
However, the presence of NAFLD can be detected in a minority of lean patients with impaired metabolic status [29]. In these subjects, it seems that genetics have a significant impact [30,31,32]; for example, it has been reported that the s738409 polymorphism of PNPLA3, which results in a loss of the hydrolytic function of the protein and leads to an accumulation of intrahepatic triglycerides and the development of insulin resistance (IR) [33], is higher in non-obese patients than in obese patients [34].
The growing diffusion of obesity in modern society has led to a parallel increase in the incidence of NAFLD, currently statistically widespread in about a quarter of adults worldwide [27]. NAFLD is an increasingly important health problem also among children and adolescents, where it represents one of the most common causes of chronic liver disease [35,36]. Specifically, according to the data collected, the global prevalence is 7.6% in the general pediatric population, with an increase of up to 34.2% in the pediatric population with obesity [37].
An analysis by the National Health and Examination Survey, conducted during the period 2007–2010, found a 6.9% prevalence of NAFLD in a large sample of American adolescents aging from 12 to 19 years old; considering the group of obese adolescents, the prevalence of NAFLD rose from 13% to 27% among obese females, and it rose from 29.5% to 48.3% among obese males from 1988–1994 to 2007–2010 [38].
The Child and Adolescent Trial for Cardiovascular Health (CATCH) reported a prevalence of NAFLD of 36% in Hispanic obese subjects, of 22% in Caucasian obese ones and of 14% in African-American obese ones [39].
Moreover, two Italian studies deployed ultrasound to assess the prevalence of suspected fatty liver among obese children. The results showed echogenic liver in 53% and in 42% of prepubertal and pubertal children, respectively [40].
A study conducted in Malaysia underlined a NAFLD prevalence of 63.3% within obese and overweight children’s groups, with a diagnosis made via ultrasonographic evidence of fatty liver [41]. The 62% of the NAFLD group showed metabolic syndrome based on the definition by the International Diabetes Federation (IDF) [41]. An interesting cross-sectional study was conducted on obese and overweight Iranian children: in these patients, abdominal ultrasound was used to evaluate liver echogenicity. In the research, NAFLD was diagnosed in 8.5% of children with overweight and 24.9% obesity. The NAFLD prevalence was 22.3% for patients aged 6–11 years and 35.5% for patients aged 12–19 years, without significant difference between girls and boys [42].
Differences in prevalence relative to gender have been reported, with a higher incidence rate in males [26] but also with a greater risk of developing fibrosis in females [43,44], as well as regarding age [45] and in relation to the geographical area; in Asia, the prevalence is 10.2% among children [46]; in Europe, it is about 2.5% [47], while in the Hispanic population, it rises up to 11.8% [48]. It seems that Mexican descendants are more predisposed, while young Black individuals seem to have genetic characteristics that protect them from the accumulation of liver fat [27,35,38].
However, it is necessary to underline that the literature lacks data on the pediatric population and that the studies on which those are based are not recent. Furthermore, the inability to make a precise and definitive diagnosis, caused by the limitations imposed by the type of population under consideration, could lead to an underestimation of the real incidence rate of NAFLD among children and adolescents [49]. Finally, the different diagnostic methods used in the studies conducted could influence the prevalence estimate [50].
3.3. Pathogenesis and Risk Factors of NAFLD
NAFLD is a complex and multifactorial disease [51,52,53]. At first, the hepatic fat accumulation and the oxidative stress were believed to be the two driving forces causing NAFLD, hence the name of the “two-hit” theory [46]. According to this model, NAFLD is characterized by fat deposition in liver parenchyma, caused when lipid uptake and disposal are not well-balanced: while de novo lipogenesis (DNL) is stimulated, β-oxidation and secretion of VLDLs are impaired [53,54,55]. Free fatty acid accumulation causes the liver to be more susceptible to the “second” hit, represented by oxidative stress, mitochondrial dysfunction, stellate cells activation, and pro-inflammatory cytokines imbalance. All these insults can worsen the damage, leading to the progression of non-alcoholic steatohepatitis and fibrosis. However, this “two-hit” hypothesis has been recently replaced by the “multi-hit” theory, which takes into account also the impact of nutritional elements, gut microbiota, and genetic and epigenetic factors [54]. It is well-known that children and adolescents ingest high quantity of sugars, especially fructose, which are responsible for lipid accumulation and DNL. Furthermore, the genetic predisposition could promote NAFLD pathogenesis as well as affect its severity [55]. In fact, genome-wide association studies identified genetic variants associated with NAFLD in several fatty acid genes [56,57,58,59], among which are PNPLA3, KLB, MBOAT7, TM6SF2, GCK, HSD17B13, PPP1R3B, IRGM, and LPIN1 [59,60,61,62]. Fundamental in nature are the genetic variants of the PNPLA3 (also known as adiponutrin) and, in particular, the amino acid substitution p.I148 M of the PNPLA3 gene. Studies show that this variant is associated with hepatic fat content, increased serum liver enzymes, and, moreover, an increased risk of NASH and the progression of fibrosis [59]. Although the biological mechanisms are not exactly defined, the common I148M variant impairs the function of the enzyme with a secondary compromise of lipid catabolism. The association between I148M variant and steatosis was confirmed in obese children in different studies [63,64,65,66].
Besides genetics, epigenetic mechanisms, such as DNA methylation, histone modification, and non-coding RNA regulation, have been gaining more and more interest in NAFLD understanding [56].
The role of gut microbiota (GM) in NAFLD pathogenesis has recently been taken into account. In NAFLD, a GM alteration and enhanced gut permeability determine a major liver exposure to gut-derived bacterial products such as lipopolysaccharides, activating the signaling pathways involved in liver inflammation and fibrogenesis [63,64].
Besides primary NAFLD, associated with insulin resistance, metabolic syndrome, obesity, and DMT2, it is important to also mention secondary causes of NAFLD, including medications, inborn errors of metabolism (IEM), and prenatal factors [65,66]. It has been reported that prolonged administration of aspirin as well as antibiotics can induce pediatric NAFLD [67,68]. In addition, the chronic use of antidepressant and antiepileptic drugs affects body weight and lipid metabolism, stimulating hepatic steatosis both in adults [69] and in children [70,71]. Several IEM, among which are glycogen and lysosomal storage diseases and fatty acid oxidation disorders, if diagnosed late or untreated, can lead to NAFLD [72]. Recent evidences have shown that prenatal factors may predispose to NAFLD. In fact, an association between maternal BMI and newborn hepatic fat accumulation has been established in both animal models [73] and human studies [74]. In addition, preterm birth, intrauterine growth retardation, and low and high birth weight are considered risk factors for severe steatosis in childhood [75,76,77].
3.4. Diagnosis of NAFLD
Many of the patients presenting NAFLD are asymptomatic, and therefore, it is difficult to promptly intervene to prevent the disease from progressing. Thus, NAFLD should be suspected in all children who are overweight and in adolescents with a waist circumference > 95th percentile for sex and age [78]. According to what was reported by the ESPGHAN Hepatology Committee, in children over 10 years with predisposing factors, NAFLD is probably attributable to obesity, while for those who are younger, a detailed diagnostic evaluation is required to exclude any other causes of hepatic steatosis [79]. In any case, according to the guidelines published in 2012, the panel of examinations for the differential diagnosis of NAFLD should contain: complete blood count, biochemical profile, coagulation test, glycosylated hemoglobin, glucose tolerance test, thyroid function, serum copper, ceruloplasmin, 24 h copper in urine, liver autoantibodies, A1 antitrypsin levels, viral hepatitis antibodies, sweat test, anti-transglutaminase A and total IgA, serum lactate, amino and organic acids, free fatty acids plasma, acylcarnitine profile, creatine phosphokinase (CPK), and history of drug treatment.
However, as reported in some studies, BMI does not reflect true body composition [80], while aminotransferases appear to have low sensitivity and specificity in NAFLD screening [81]. Indeed, it has been shown that the levels of ALT and aspartate aminotransferase (AST) fluctuate during the disease and do not correlate with fibrosis [82,83], and the serum ALT cutoff has not yet been precisely defined in the pediatric population [84]. Therefore, it is possible to consider the evaluation of these parameters as a support rather than as the main diagnostic reference [44]. Indeed, the presence of hepatic steatosis must be confirmed by ultrasound or by liver biopsy [43,85].
Hypertriglyceridemia is another biochemical marker frequently reported in obese children with NAFLD [86], with a positive correlation with an increased ALT [87,88]. In children’s atherogenic lipid profile, this correlates with the severity of liver injury [89]. Increased serum uric acid level has been also reported in the majority of subjects with the metabolic syndrome and has been proposed as an independent predictor of NAFLD both in adults [90] and children [91].
Liver biopsy represents the gold standard for the diagnosis of NAFLD and allows to determine traces of steatosis, fibrosis, or a local inflammatory state [92]. Furthermore, it allows to exclude the presence of any other liver diseases, such as autoimmune hepatitis [36,79]. However, it turns out to be quite invasive and not without risks [36,79], which are facts that make it a complicated examination to perform in the pediatric population and an unethically adoptable option in the field of research. However, histological analysis of liver biopsies allows to diagnose the extent of steatosis and subsequently to grade the pathology as mild, moderate, and severe.
Furthermore, some studies seem to associate it with a high rate of false negatives [44,93]. Therefore, the first-line test to date is represented by ultrasound [94]; available, non-invasive, and convenient, it has a sensitivity of 85% and a specificity of 95% [44] although, according to the systematic review by Awai HI et al., the positive predictive value of NAFLD varies between 47% and 62% [95].
Among other instrumental tests, computed tomography allows to observe the liver in its entirety and to evaluate moderate or severe states of steatosis. Considered superior to simple ultrasound, it has a sensitivity of 82% and a specificity of 100% [96].
Conventional magnetic resonance imaging (MRI) can also be used to determine minor steatosis states [97] but not to diagnose NASH or to evaluate fibrosis [98]. The magnetic resonance imaging-derived proton density fat fraction (MRI-PDFF) allows to overcome the problem and to identify any degree of steatosis [99] with high sensitivity and specificity, while the magnetic resonance spectroscopy (MRS) can detect steatosis with a percentage greater than 5% with good diagnostic reliability [100]. However, the validity of these techniques is still to be investigated [95,101].
The evaluation of specific markers such as IL-8, monocyte chemoattractant protein-1, resistin, soluble IL-1 receptor I, soluble IL-2 receptor α, and TNF-α [93] as well as serum panels related to liver fibrosis, such as FibroTest and FIB4 index, although the latter have been tested mainly in adults [102,103], has recently been suggested.
Finally, three scoring systems are currently in use to determine the severity of the disease: the Brunt system [104], the NASH CRN system [105], and the SAF system [106].
3.5. Treatments of NAFLD
Since NAFLD may progress to cirrhosis and end-stage liver disease [26], it is fundamental to treat patients as soon as possible. NASPGHAN and ESPGHAN guidelines recommend lifestyle modification (see below), which is considered the only effective treatment for NAFLD in both adults and children [107].
A diet low in carbohydrates, fructose, saturated fatty acids, and trans-fatty acids [92,108,109,110] and an active lifestyle are the key components of treatment of NAFLD. BMI reduction is associated to a significant improvement of ALT and histological feature [111,112].
Physical activity as the only intervention or in connection with other lifestyle proposal was recently reported in children with NAFLD, and it will be explained in detail in the next sections.
Unfortunately, pharmacological therapies for pediatric steatosis, such as the administration of metformin, an insulin sensitizer, or the supplementation with the antioxidant vitamin E, are limited and with contrasting results [111,113]. Other options that revealed conflicting effects include the treatment with docosahexaenoic and eicosapentaenoic acids as sources of omega-3 fatty acids, which might inhibit lipogenesis and promote fatty acid oxidation and reduce inflammation, and probiotics aimed to restore gut microbiome [114,115]. Although novel molecules are actually under investigation in adults (obeticholic acid, an analog of bile acids; elafibranor, an insulin sensitizer; liraglutide), no ongoing pediatric trials evaluates the efficacy of these drugs in children [113].
In selected morbidly obese adolescents in which bariatric surgery could be indicated after growth completion, and only as a part of multidisciplinary treatment, reported studies show that the weight loss after bariatric surgery results in NASH and fibrosis reversal [26,116,117].
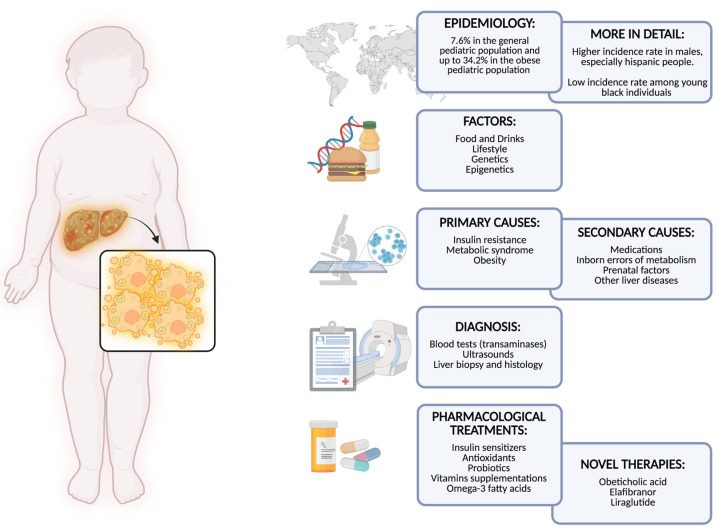
In Figure 2, epidemiology, pathogenicity and risk factors, and diagnostic and therapeutical approaches are explained.
Figure 2.
Nonalcoholic fatty liver disease in children and adolescents (created with BioRender.com, accessed on 1 July 2022).
4. Physical Activity Levels in Children and Adolescents with Obesity
Regular PA practice is crucial for correct development and growth of children [118,119], and it has a main role in the prevention of overweight and obesity during childhood and adolescence, decreasing the health risks related to weight gain [16]. The current guidelines by the World Health Organization (WHO) recommend to perform at least 60 min of moderate- to vigorous-intensity daily amount of PA for subjects from 5 to 17 years old in multiple short bouts during the day [120]. These activities include play, games, sports, active transportation, recreation, and physical education as well as planned exercise or training sessions [121]. The daily routine activities, both structured and unstructured, should be enjoyable and sustainable [122]. Moreover, the addition of resistance strength training is also recommended in order to ameliorate muscular strength and bone health in children and adolescents [120].
Despite established benefits of regular PA practice [18,123], children with obesity usually have lower levels of PA and physical fitness compared to peers [124,125] due to greater difficulties in performing different motor skills and negative feelings and self-esteem related to PA practice [126,127]. As demonstrated by Yu et al. [128], pediatric subjects with obesity spent 30% less time engaging in PA pursuits and 51% more time in sedentary behaviors during time dedicated to waking activities. Moreover, Babic et al. [129] reported that a higher physical self-esteem level in adolescents has been associated with more probability to engage in PA; conversely, a perception of low performance limits participation in PA/exercise programs and the consequent enjoyment [130,131,132]. Vandoni et al. [16] previously showed the crucial role of assessing the physical self-esteem and PA pleasure in children and adolescents before engaging in a regular exercise program. An accurate self-reported physical fitness evaluation could identify low-fitness (and possibly low-activity) children to enact surveillance and design dedicated PA interventions to increase long-time PA adherence. Moreover, across ages, several children and adolescents with obesity encountered barriers related to demographic, personal, social, and environmental factors that limit their exercise practice and PA goals. Indeed, the exercise habits of the youngsters and the amount of the exercise done by the parents influence preschool-aged children’s lifestyle [133]. Conversely, children who spend more time indoors are less active than those who spend more time outside [134]. Additionally, urban environments contribute to spending less time outdoors, and the perception of the lack of access to playgrounds, supervision, and equipment contribute to spending less time outside as well [135]. For these reasons, an active lifestyle should be promoted early both in children and in parents by intervening on family-based exercise habits that could improve children’s future fitness. Additionally, gender differences in PA level across age were found by Riddoch et al. [136], showing boys more active than girls; in particular, 21% of the children were more active at the age of 9 years old and 26% at the age of 15 years old, and gender differences in actively spent time in activities of moderate intensity were even more noticeable (20% and 36% of difference of less activity in girls). In a research by Garaulet et al. [137], with participants from 14 to 18 years old, girls with overweight had comparable levels of PA with their non-overweight counterparts, but overweight boys had a lower sport participation rate compared to their peers without overweight. In fact, sports clubs usually are too competition-oriented and hence do not include less-fit youth or particular subjects with obesity. Indeed, commonly, children and adolescents with obesity show low levels in sport participation, and the encouragement to engage in activities is crucial for the prevention and treatment of weight gain from an early age.
In summary, positive predictors of PA level include familiar context, peer, stimuli from community, an active and sportive self-identity and attitude, personal achievement [138], and perceived competence [139]. Public policies should enhance programs aimed to play outside, play at recess, and walk or bike to school. Finally, pediatricians and sport specialists should carefully evaluate barriers to the practice of exercise and implement PA and sports strategies to augment long-term PA adherence.
5. The Effects of Physical Exercise on NAFLD
5.1. Exercise Intervention in NAFLD Patients
Lifestyle intervention is considered the first line, as it has no side effects and confers multiple cardiometabolic benefits [6,107,140]. Diet- and exercise-intervention-based reduction of body weight has been shown to be effective in reducing hepatic fat deposition in children and adolescents with obesity and NAFLD [141].
Exercise is beneficial both in the prevention and in the treatment of NAFLD in adulthood, aside from dietary changes and independently from weight loss [142,143]. Indeed, it has been shown that hepatic steatosis prevalence is significantly lower in physically active men who regularly exercise (≥2–3 days per week) with respect to men with sedentary lifestyle [140]. A significant inverse association between physical activity (PA) and intrahepatic lipid content was also shown in both men and women independently of age and BMI [144]. In addition, regular exercise was significantly correlated to decreased AST, ALT, gamma-glutamyl transferase (GGT), total cholesterol (TC), triglycerides (TG), and low-density lipoproteins (LDL) in adults affected by NAFLD [143]. Although exercise is universally recommended for the treatment of NAFLD [26,50], and sedentary lifestyle is considered a risk factor for this condition during childhood [46,145], no randomized clinical trials on exercise alone versus usual care were found in children with NAFLD. Thus, for children affected by NAFLD, as for all children, WHO recommends 60 min of daily moderate- to vigorous-intensity PA and at least 3 days per week of muscle-strengthening [146]. Unfortunately, studies evaluating the effects of exercise alone as a therapy in NAFLD in children are extremely limited, and to date, no studies have investigated exercise as a treatment for children with biopsy-proven NAFLD. The most of available research evaluated the effect of exercise on obese children as the target population and not focusing only on obese children with a clear diagnosis of NAFLD [3,7].
Different clinical trials conducted on obese pediatric populations (without a proper diagnosis of NAFLD) demonstrated beneficial effects of physical exercise on liver characteristics and metabolic factors, both as preventive strategy (in children still not diagnosed with NAFLD) and as a therapeutic aid (in children affected by NAFLD) [147,148,149,150,151].
Interestingly, Gonzalez-Ruiz et al., in a meta-analysis of 14 randomized controlled trials (RCTs) in children with overweight and obesity, evaluated the effect of exercise (aerobic, resistance, and combined activities) on liver enzymes and intrahepatic triglycerides (IHTG) [152]. Exercise programs were associated with significant reductions in IHTG and GGT levels with respect to usual care. As the study was based on obese patients with obesity without a diagnosis of NAFLD per se, no specific recommendations for NAFLD pediatric patients can be driven, and the positive effects observed could be considered an efficacious preventive measure rather than a therapeutical effect for NAFLD [152]. De Piano et al. in their RCT compared the effects of aerobic training (AT) with aerobic plus resistance training (AT+RT) in adolescents with obesity and NAFLD [153]. The authors showed that 180 min of AT+RT was superior to 180 min AT on ALT and several other non-invasive NAFLD biomarkers associated with the high disease progression risk in the pediatric population [153]. In this case, PA has a therapeutical role. Lee et al. investigated the effect of AT and RT versus control on obese children with two studies: one involving boys and one girls [148,149]. Each of the study had 44 children [148,149]. The AT was structured in three sessions of 60 min per week, with treadmill, elliptical, or stationary bike. The RT consisted of three 60-minute sessions of ten whole-body exercises per week. Changes in hepatic fat fraction (HFF) for both groups were reported. However, very few children had sufficient liver fat to be considered as having NAFLD. Moreover, whether the changes reported in HFF are clinically relevant remained not fully elucidated [148,149].
Van der Heijden et al. also evaluated the effect of aerobic and resistance exercise on children [151]; specifically, in their work on AT, children with obesity (n = 15) and normal-weight children (n = 14) were provided with a 30 min aerobic intervention twice a week for 12 weeks [151]. These subjects overall showed a decrease in the mean HFF (from 9 to 6%) but no significant change in mean ALT [151]. In the study of resistance training, 12 adolescents with obesity performed a 60 min session twice a week for 12 weeks, without significant changes in HFF. Moreover, ALT levels were evaluated neither at baseline nor in response to the intervention [150].
In a recent semi-experimental study, Iraji et al. evaluated 34 obese male adolescents with clinically defined NAFLD in order to compare liver enzymes and metabolic profile changes upon selected school-based exercise (SBE) and high-intensity interval training (HIIT) [154]. The patients were divided into three groups: the HIIT group, the SBE group, and controls [154]. BMI, body fat percentage, and waist-to-hip ratio of the participants decreased after the exercise intervention. In both groups, significant reductions in the levels of IR, TG, TC, ALT, and AST were detected [154]. Moreover, the HIIT group showed a decrease in high-density lipoprotein levels that were significantly relevant with respect to the SEB group, and both the training groups showed a significant reduction in low-density lipoprotein level with respect to controls. Indeed, the authors concluded that HIIT and SBE are equally effective in improving health parameters in obese children and adolescents affected by NAFLD [154]. This is fundamental, as it highlights not only PA as a therapeutical tool for these patients but also the fact that the proper training should be tailored on patient’s abilities and choices in order to increase exercise enjoyability and improve adherence to training [154,155].
5.2. Exercise in NAFLD: Mechanisms of Action
The mechanisms by which exercise acts on liver fat are still greatly unknown, and pediatric studies are missing, but different works on adults showed a decrease in the hepatic fat content upon exercise even when overall weight loss was not observed [156,157,158], which is consistent with the idea that exercise has a direct effect on the liver [156,159].
Some mechanistic studies described different pathways linking increased PA to NAFLD improvement [160]. Importantly, in NAFLD, there is a complex interaction between adipose tissue, liver, and skeletal muscles, and exercise was shown to improve IR in these organs [160,161]. An improvement of IR in peripheral tissues was correlated to a lower glucose transport to the liver and a lower free fatty acid flux to the liver, with a consequent decreased liver fat content [161,162]. Moreover, PA reduces the risk of sarcopenia, which is an important risk factor for non-obese NAFLD [160,162,163]. Exercise also improves muscle function, which may further reduce systemic IR and improve hepatic inflammation [160,161] and increases cardiorespiratory fitness, which is fundamental to reduce cardiovascular risk in patients with NAFLD [161,162].
Moreover, NAFLD oxidative stress (OS), caused by reactive oxygen species (ROS) and inflammation, causes a significant damage on hepatic cells and leads to tissue injury [159]. The major sources of ROS in NAFLD are mitochondrial abnormalities, down-regulation of several antioxidant enzymes, accumulation of leukocytes, and hepatic inflammation [159]. In this context, exercise can be useful, as it was shown to suppress OS and ROS overproduction via up-regulation of several antioxidant enzymes and anti-inflammatory mediators [159]. Several experimental studies reported that exercise can decrease ROS production and pro-inflammatory cytokines, including TNF-α and IL-1β, through the antioxidant activity improvement and acting on NF-κB pathway [159,164,165,166]. Further studies are necessary to confirm these results and drive definitive conclusions about the mechanisms by which exercise can attenuate hepatic oxidative damage in NAFLD and improve hepatic status, especially in the pediatric field.
Finally, among the mechanisms by which PA can act on NAFLD, the link with GM plays a crucial role. Indeed, accumulated data clarified that dysbiosis or disruption of the GM is associated with NAFLD in both children and adolescents [167,168,169]. Despite the absence of a consistent signature of dysbiosis in NAFLD-associated gut microbiota in children, all of the pediatric studies have found decreased α-diversity (richness and evenness), an altered β-diversity, and a significant difference in the abundance of bacteria at the phylum and genus level with respect to the microbiota of control subjects [167,169,170,171,172]. Although the mechanism through which exercise can alter the gut microbiota and ameliorate NAFLD is unclear, and pediatric studies are missing, regular exercise and a balanced diet are unequivocally beneficial in the maintenance of a healthy gut microbiota [169]. In terms of exercise, Clarke et al. evaluated 40 young athletes and showed that gut microbiota diversity is greater in athletes than in non-athletic healthy persons [173]; in addition, Allen et al. demonstrated that aerobic exercise itself—independent of diet— alters gut microbiome composition in human adults by increasing fecal concentrations of short-chain fatty acids (SCFAs) [174]. Specifically, in their study, 32 lean and obese, previously sedentary, subjects participated in 6 weeks of supervised, endurance-based exercise training (three trainings per week) that progressed from low to vigorous intensity [174]. Subsequently, participants were allowed to return to sedentary lifestyle activity for a 6-week period. Fecal samples were collected before and after the 6-week period of regular exercise and after the sedentary washout period. The fecal samples were analyzed, and exercise resulted in increased fecal concentrations of SCFAs in lean but not obese participants [174]. The exercise-induced change in metabolic output of the microbiota was consistent with the parallel shift in bacterial genes and taxa capable of SCFAs production. Interestingly, exercise-induced microbiota changes were largely reversed once exercise training stopped [174]. Quiroga et al. demonstrated that 12 weeks of exercise training favorably altered the deleterious obesity-related microbiota profile and reduced microbial inflammatory signaling in children with obesity [175]. The authors enrolled 39 obese children that were randomly assigned to the control or training (combined strength and endurance exercise) group [175]. The exercise group not only had reduced blood glucose and GOT levels with respect to controls but also significantly decreased abundance of the obesity-related Proteobacteria phylum and Gammaproteobacteria class and increased levels of some beneficial bacterial genera, such as Blautia, Dialister, and SCFAs-producing Roseburia [175].
These findings suggest that exercise can positively modify the deleterious microbiota profile present in obese subjects affected by NAFLD. Nevertheless, additional research is needed to understand how exercise determines these changes and all their related effects both in terms of NAFLD prevention and therapy.
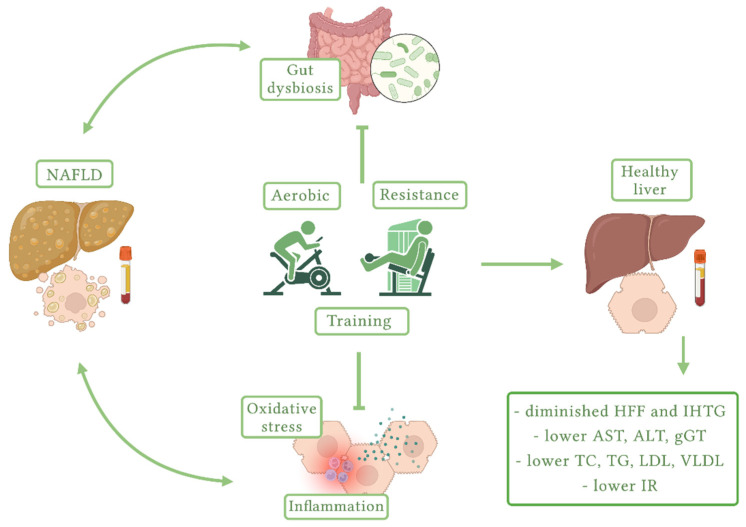
Benefits of exercise on NAFLD are schematized in Figure 3.
Figure 3.
Benefits of exercise on NAFLD (created with BioRender.com, accessed on 1 July 2022). NAFLD, non-alcoholic fatty liver disease; HFF, hepatic fat fraction; IHTG, intrahepatic triglycerides; AST, aspartate aminotransferase; ALT, alanine aminotransferase; gGT, gamma-glutamyl transferase; TC, total cholesterol; TG, triglycerides; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein; IR, insulin resistance.
6. Conclusions
As sedentary behavior is a risk factor for NAFLD development independent from weight gain, tailored exercise has been highlighted as effective strategy to prevent and treat NAFLD. Several studies on obese children and adults confirm the positive role of PA in the treatment of NAFLD, but to date, there are no pediatric RCTs on exercise versus usual care. Moreover, literature concerning the prognostic value of exercise in the treatment of pediatric NAFLD is missing. Among the mechanisms involved in the effects of PA on NAFLD, the main players seem to be IR and related inflammation, oxidative stress, and gut dysbiosis, but further evaluations are necessary to deeply understand whether these factors are correlated and how they synergistically act. Thus, deeper research on this theme is needed, and it would be extremely interesting.
Author Contributions
Conceptualization, V.C. and M.V.; methodology V.C., V.C.M., M.V., C.B., L.M., A.B., E.C., G.S.S., C.C. and G.Z.; investigation, V.C.M., C.B., A.B., E.C., G.S.S. and V.C.P.; data curation, V.C., M.V. and L.M.; writing—original draft preparation, V.C., V.C.M., M.V., C.B., L.M., A.B., E.C., G.S.S., D.S., V.C.P., C.C. and G.Z.; writing—review and editing, V.C., M.V., L.M. and G.Z.; supervision, V.C., M.V. and G.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nobili V., Alisi A., Valenti L., Miele L., Feldstein A.E., Alkhouri N. NAFLD in Children: New Genes, New Diagnostic Modalities and New Drugs. Nat. Rev. Gastroenterol. Hepatol. 2019;16:517–530. doi: 10.1038/s41575-019-0169-z. [DOI] [PubMed] [Google Scholar]
- 2.Shaunak M., Byrne C.D., Davis N., Afolabi P., Faust S.N., Davies J.H. Non-Alcoholic Fatty Liver Disease and Childhood Obesity. Arch. Dis. Child. 2021;106:3–8. doi: 10.1136/archdischild-2019-318063. [DOI] [PubMed] [Google Scholar]
- 3.Africa J.A., Newton K.P., Schwimmer J.B. Lifestyle Interventions Including Nutrition, Exercise, and Supplements for Nonalcoholic Fatty Liver Disease in Children. Dig. Dis. Sci. 2016;61:1375–1386. doi: 10.1007/s10620-016-4126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nobili V., Svegliati-Baroni G., Alisi A., Miele L., Valenti L., Vajro P. A 360-Degree Overview of Paediatric NAFLD: Recent Insights. J. Hepatol. 2013;58:1218–1229. doi: 10.1016/j.jhep.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Cananzi M., Vajro P., Rela M., Dhawan A. NAFLD and Liver Transplantation in Children—Working Group Report From the ILTS Single Topic Conference on NAFLD. Transplantation. 2019;103:68–70. doi: 10.1097/TP.0000000000002490. [DOI] [PubMed] [Google Scholar]
- 6.Mann J.P., Tang G.Y., Nobili V., Armstrong M.J. Evaluations of Lifestyle, Dietary, and Pharmacologic Treatments for Pediatric Nonalcoholic Fatty Liver Disease: A Systematic Review. Clin. Gastroenterol. Hepatol. 2019;17:1457–1476. doi: 10.1016/j.cgh.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Kwak M.-S., Kim D. Non-Alcoholic Fatty Liver Disease and Lifestyle Modifications, Focusing on Physical Activity. Korean J. Intern. Med. 2018;33:64–74. doi: 10.3904/kjim.2017.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin G., Xinhe Z., Haoyu T., Xing J., Dan L., Ningning W., Jing S., Xue W., Zilu Z., Yiling L. Epidemiology and Lifestyle Survey of Non-Alcoholic Fatty Liver Disease in School-Age Children and Adolescents in Shenyang, Liaoning. BMC Pediatr. 2022;22:286. doi: 10.1186/s12887-022-03351-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 10.Gregory A.T., Denniss A.R. An Introduction to Writing Narrative and Systematic Reviews—Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018;27:893–898. doi: 10.1016/j.hlc.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Jebeile H., Kelly A.S., O’Malley G., Baur L.A. Obesity in Children and Adolescents: Epidemiology, Causes, Assessment, and Management. Lancet Diabetes Endocrinol. 2022;10:351–365. doi: 10.1016/S2213-8587(22)00047-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Childhood Obesity Facts|Overweight & Obesity|CDC. [(accessed on 29 June 2022)]; Available online: https://www.cdc.gov/obesity/data/childhood.html.
- 13.Lange S.J., Kompaniyets L., Freedman D.S., Kraus E.M., Porter R., Blanck H.M., Goodman A.B. Longitudinal Trends in Body Mass Index Before and During the COVID-19 Pandemic Among Persons Aged 2–19 Years—United States, 2018–2020. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1278–1283. doi: 10.15585/mmwr.mm7037a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyer O. Obesity in US Children Increased at an Unprecedented Rate during the Pandemic. BMJ. 2021;374:n2332. doi: 10.1136/bmj.n2332. [DOI] [PubMed] [Google Scholar]
- 15.Sahoo K., Sahoo B., Choudhury A., Sofi N., Kumar R., Bhadoria A. Childhood Obesity: Causes and Consequences. J. Fam. Med. Prim. Care. 2015;4:187. doi: 10.4103/2249-4863.154628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandoni M., Calcaterra V., Pellino V.C., De Silvestri A., Marin L., Zuccotti G.V., Tranfaglia V., Giuriato M., Codella R., Lovecchio N. “Fitness and Fatness” in Children and Adolescents: An Italian Cross-Sectional Study. Children. 2021;8:762. doi: 10.3390/children8090762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calcaterra V., Zuccotti G. Prevention and Treatment of Cardiometabolic Diseases in Children with Overweight and Obesity: The Future of Healthcare. Children. 2022;9:176. doi: 10.3390/children9020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calcaterra V., Larizza D., Codrons E., De Silvestri A., Brambilla P., Abela S., Arpesella M., Vandoni M. Improved Metabolic and Cardiorespiratory Fitness during a Recreational Training Program in Obese Children. J. Pediatr. Endocrinol. Metab. 2013;26:271–276. doi: 10.1515/jpem-2012-0157. [DOI] [PubMed] [Google Scholar]
- 19.Calcaterra V., Verduci E., Ghezzi M., Cena H., Pascuzzi M.C., Regalbuto C., Lamberti R., Rossi V., Manuelli M., Bosetti A., et al. Pediatric Obesity-Related Asthma: The Role of Nutrition and Nutrients in Prevention and Treatment. Nutrients. 2021;13:3708. doi: 10.3390/nu13113708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calcaterra V., Verduci E., Cena H., Magenes V.C., Todisco C.F., Tenuta E., Gregorio C., De Giuseppe R., Bosetti A., Di Profio E., et al. Polycystic Ovary Syndrome in Insulin-Resistant Adolescents with Obesity: The Role of Nutrition Therapy and Food Supplements as a Strategy to Protect Fertility. Nutrients. 2021;13:1848. doi: 10.3390/nu13061848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S., Kelly A.S. Review of Childhood Obesity. Mayo Clin. Proc. 2017;92:251–265. doi: 10.1016/j.mayocp.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Valerio G., Maffeis C., Saggese G., Ambruzzi M.A., Balsamo A., Bellone S., Bergamini M., Bernasconi S., Bona G., Calcaterra V., et al. Diagnosis, Treatment and Prevention of Pediatric Obesity: Consensus Position Statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital. J. Pediatr. 2018;44:88. doi: 10.1186/s13052-018-0525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calcaterra V., Vandoni M., Rossi V., Berardo C., Grazi R., Cordaro E., Tranfaglia V., Carnevale Pellino V., Cereda C., Zuccotti G. Use of Physical Activity and Exercise to Reduce Inflammation in Children and Adolescents with Obesity. Int. J. Environ. Res. Public. Health. 2022;19:6908. doi: 10.3390/ijerph19116908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calcaterra V., Cena H., Pelizzo G., Porri D., Regalbuto C., Vinci F., Destro F., Vestri E., Verduci E., Bosetti A., et al. Bariatric Surgery in Adolescents: To Do or Not to Do? Children. 2021;8:453. doi: 10.3390/children8060453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellentani S., Marino M. Epidemiology and Natural History of Non-Alcoholic Fatty Liver Disease (NAFLD) Ann. Hepatol. 2009;8((Suppl. S1)):S4–S8. doi: 10.1016/S1665-2681(19)31820-4. [DOI] [PubMed] [Google Scholar]
- 26.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 27.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 28.Lu F.-B., Hu E.-D., Xu L.-M., Chen L., Wu J.-L., Li H., Chen D.-Z., Chen Y.-P. The Relationship between Obesity and the Severity of Non-Alcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Expert Rev. Gastroenterol. Hepatol. 2018;12:491–502. doi: 10.1080/17474124.2018.1460202. [DOI] [PubMed] [Google Scholar]
- 29.Golabi P., Paik J.M., Arshad T., Younossi Y., Mishra A., Younossi Z.M. Mortality of NAFLD According to the Body Composition and Presence of Metabolic Abnormalities. Hepatol. Commun. 2020;4:1136–1148. doi: 10.1002/hep4.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwimmer J.B., Celedon M.A., Lavine J.E., Salem R., Campbell N., Schork N.J., Shiehmorteza M., Yokoo T., Chavez A., Middleton M.S., et al. Heritability of Nonalcoholic Fatty Liver Disease. Gastroenterology. 2009;136:1585–1592. doi: 10.1053/j.gastro.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wattacheril J., Lavine J.E., Chalasani N.P., Guo X., Kwon S., Schwimmer J., Molleston J.P., Loomba R., Brunt E.M., Chen Y.-D.I., et al. Genome-Wide Associations Related to Hepatic Histology in Nonalcoholic Fatty Liver Disease in Hispanic Boys. J. Pediatr. 2017;190:100–107.e2. doi: 10.1016/j.jpeds.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santoro N., Caprio S., Pierpont B., Van Name M., Savoye M., Parks E.J. Hepatic De Novo Lipogenesis in Obese Youth Is Modulated by a Common Variant in the GCKR Gene. J. Clin. Endocrinol. Metab. 2015;100:E1125–E1132. doi: 10.1210/jc.2015-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oniki K., Saruwatari J., Izuka T., Kajiwara A., Morita K., Sakata M., Otake K., Ogata Y., Nakagawa K. Influence of the PNPLA3 Rs738409 Polymorphism on Non-Alcoholic Fatty Liver Disease and Renal Function among Normal Weight Subjects. PLoS ONE. 2015;10:e0132640. doi: 10.1371/journal.pone.0132640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou Z.Y., Wong V.W., Fan J.G. Epidemiology of Nonalcoholic Fatty Liver Disease in Non-obese Populations: Meta-analytic Assessment of Its Prevalence, Genetic, Metabolic, and Histological Profiles. J. Dig. Dis. 2020;21:372–384. doi: 10.1111/1751-2980.12871. [DOI] [PubMed] [Google Scholar]
- 35.Schwimmer J.B., Deutsch R., Kahen T., Lavine J.E., Stanley C., Behling C. Prevalence of Fatty Liver in Children and Adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 36.Wieckowska A., Feldstein A. Diagnosis of Nonalcoholic Fatty Liver Disease: Invasive versus Noninvasive. Semin. Liver Dis. 2008;28:386–395. doi: 10.1055/s-0028-1091983. [DOI] [PubMed] [Google Scholar]
- 37.Anderson E.L., Howe L.D., Jones H.E., Higgins J.P.T., Lawlor D.A., Fraser A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS ONE. 2015;10:e0140908. doi: 10.1371/journal.pone.0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welsh J.A., Karpen S., Vos M.B. Increasing Prevalence of Nonalcoholic Fatty Liver Disease Among United States Adolescents, 1988–1994 to 2007–2010. J. Pediatr. 2013;162:496–500.e1. doi: 10.1016/j.jpeds.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patton H.M., Sirlin C., Behling C., Middleton M., Schwimmer J.B., Lavine J.E. Pediatric Nonalcoholic Fatty Liver Disease: A Critical Appraisal of Current Data and Implications for Future Research. J. Pediatr. Gastroenterol. Nutr. 2006;43:413–427. doi: 10.1097/01.mpg.0000239995.58388.56. [DOI] [PubMed] [Google Scholar]
- 40.Guzzaloni G., Grugni G., Minocci A., Moro D., Morabito F. Liver Steatosis in Juvenile Obesity: Correlations with Lipid Profile, Hepatic Biochemical Parameters and Glycemic and Insulinemic Responses to an Oral Glucose Tolerance Test. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2000;24:772–776. doi: 10.1038/sj.ijo.0801224. [DOI] [PubMed] [Google Scholar]
- 41.Mohamed R.Z., Jalaludin M.Y., Anuar Zaini A. Predictors of Non-Alcoholic Fatty Liver Disease (NAFLD) among Children with Obesity. J. Pediatr. Endocrinol. Metab. JPEM. 2020;33:247–253. doi: 10.1515/jpem-2019-0403. [DOI] [PubMed] [Google Scholar]
- 42.Maleki F., Hosseinpour M., Motlagh B.M., Esmaeili Gouvarchin Ghaleh H., Gheibi S. OC49 Non-Alcoholic Fatty Liver Disease in Obese and Overweight Iranian Children: A Cross Sectional Study. Arch. Dis. Child. 2019;104:A20–A21. [Google Scholar]
- 43.Dyson J.K., Anstee Q.M., McPherson S. Non-Alcoholic Fatty Liver Disease: A Practical Approach to Diagnosis and Staging. Frontline Gastroenterol. 2014;5:211–218. doi: 10.1136/flgastro-2013-100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jennison E., Patel J., Scorletti E., Byrne C.D. Diagnosis and Management of Non-Alcoholic Fatty Liver Disease. Postgrad. Med. J. 2019;95:314–322. doi: 10.1136/postgradmedj-2018-136316. [DOI] [PubMed] [Google Scholar]
- 45.Li B., Selmi C., Tang R., Gershwin M.E., Ma X. The Microbiome and Autoimmunity: A Paradigm from the Gut–Liver Axis. Cell. Mol. Immunol. 2018;15:595–609. doi: 10.1038/cmi.2018.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang Y.-L., Chen H., Wang C.-L., Liang L. Pathogenesis of Non-Alcoholic Fatty Liver Disease in Children and Adolescence: From “Two Hit Theory” to “Multiple Hit Model”. World J. Gastroenterol. 2018;24:2974–2983. doi: 10.3748/wjg.v24.i27.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawlor D.A., Callaway M., Macdonald-Wallis C., Anderson E., Fraser A., Howe L.D., Day C., Sattar N. Nonalcoholic Fatty Liver Disease, Liver Fibrosis, and Cardiometabolic Risk Factors in Adolescence: A Cross-Sectional Study of 1874 General Population Adolescents. J. Clin. Endocrinol. Metab. 2014;99:E410–E417. doi: 10.1210/jc.2013-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jain A. Pediatric Fatty Liver Disease. Mo. Med. 2019;116:123. [PMC free article] [PubMed] [Google Scholar]
- 49.Chiesa C., Andreoli G.M., Pacifico L. Pediatric Nonalcoholic Fatty Liver Disease. J. Pediatr. Rio J. 2019;95:4–6. doi: 10.1016/j.jped.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Vos M.B., Abrams S.H., Barlow S.E., Caprio S., Daniels S.R., Kohli R., Mouzaki M., Sathya P., Schwimmer J.B., Sundaram S.S., et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) J. Pediatr. Gastroenterol. Nutr. 2017;64:319–334. doi: 10.1097/MPG.0000000000001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Sessa A., Cirillo G., Guarino S., Marzuillo P., Miraglia del Giudice E. Pediatric Non-Alcoholic Fatty Liver Disease: Current Perspectives on Diagnosis and Management. Pediatr. Health Med. Ther. 2019;10:89–97. doi: 10.2147/PHMT.S188989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nassir F., Rector R.S., Hammoud G.M., Ibdah J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015;11:167–175. [PMC free article] [PubMed] [Google Scholar]
- 53.Berardo C., Di Pasqua L.G., Cagna M., Richelmi P., Vairetti M., Ferrigno A. Nonalcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis: Current Issues and Future Perspectives in Preclinical and Clinical Research. Int. J. Mol. Sci. 2020;21:9646. doi: 10.3390/ijms21249646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buzzetti E., Pinzani M., Tsochatzis E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 55.Lin Y.-C., Wu C.-C., Ni Y.-H. New Perspectives on Genetic Prediction for Pediatric Metabolic Associated Fatty Liver Disease. Front. Pediatr. 2020;8:603654. doi: 10.3389/fped.2020.603654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jonas W., Schürmann A. Genetic and Epigenetic Factors Determining NAFLD Risk. Mol. Metab. 2021;50:101111. doi: 10.1016/j.molmet.2020.101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dongiovanni P., Crudele A., Panera N., Romito I., Meroni M., De Stefanis C., Palma A., Comparcola D., Fracanzani A.L., Miele L., et al. β-Klotho Gene Variation Is Associated with Liver Damage in Children with NAFLD. J. Hepatol. 2020;72:411–419. doi: 10.1016/j.jhep.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 58.Goyal N.P., Schwimmer J.B. The Genetics of Pediatric Nonalcoholic Fatty Liver Disease. Clin. Liver Dis. 2018;22:59–71. doi: 10.1016/j.cld.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valenti L., Al-Serri A., Daly A.K., Galmozzi E., Rametta R., Dongiovanni P., Nobili V., Mozzi E., Roviaro G., Vanni E., et al. Homozygosity for the Patatin-like Phospholipase-3/Adiponutrin I148M Polymorphism Influences Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Hepatology. 2010;51:1209–1217. doi: 10.1002/hep.23622. [DOI] [PubMed] [Google Scholar]
- 60.Romeo S., Sentinlli F., Cambuli V.M., Incani M., Congiu T., Matta V., Pilia S., Huang-Doran I., Cossu E., Loche S., et al. The 148M Allele of the PNPLA3 Gene Is Associated with Indices of Liver Damage Early in Life. J. Hepatol. 2010;53:335–338. doi: 10.1016/j.jhep.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 61.Lin Y.-C., Chang P.-F., Hu F.-C., Yang W.-S., Chang M.-H., Ni Y.-H. A Common Variant in the PNPLA3 Gene Is a Risk Factor for Non-Alcoholic Fatty Liver Disease in Obese Taiwanese Children. J. Pediatr. 2011;158:740–744. doi: 10.1016/j.jpeds.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 62.Goran M.I., Walker R., Le K.-A., Mahurkar S., Vikman S., Davis J.N., Spruijt-Metz D., Weigensberg M.J., Allayee H. Effects of PNPLA3 on Liver Fat and Metabolic Profile in Hispanic Children and Adolescents. Diabetes. 2010;59:3127–3130. doi: 10.2337/db10-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frasinariu O.E., Ceccarelli S., Alisi A., Moraru E., Nobili V. Gut-Liver Axis and Fibrosis in Nonalcoholic Fatty Liver Disease: An Input for Novel Therapies. Dig. Liver Dis. 2013;45:543–551. doi: 10.1016/j.dld.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 64.Berardis S., Sokal E. Pediatric Non-Alcoholic Fatty Liver Disease: An Increasing Public Health Issue. Eur. J. Pediatr. 2014;173:131–139. doi: 10.1007/s00431-013-2157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kneeman J.M., Misdraji J., Corey K.E. Secondary Causes of Nonalcoholic Fatty Liver Disease. Ther. Adv. Gastroenterol. 2012;5:199–207. doi: 10.1177/1756283X11430859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mann J., Valenti L., Scorletti E., Byrne C., Nobili V. Nonalcoholic Fatty Liver Disease in Children. Semin. Liver Dis. 2018;38:1–13. doi: 10.1055/s-0038-1627456. [DOI] [PubMed] [Google Scholar]
- 67.Faa G., Ekstrom J., Castagnola M., Gibo Y., Ottonello G., Fanos V. A Developmental Approach to Drug-Induced Liver Injury in Newborns and Children. Curr. Med. Chem. 2012;19:4581–4594. doi: 10.2174/092986712803306385. [DOI] [PubMed] [Google Scholar]
- 68.Chornomydz I., Boyarchuk O., Chornomydz A. Reye (Ray’s) syndrome: A problem everyone should remember. Georgian Med. News. 2017;272:110–118. [PubMed] [Google Scholar]
- 69.Di Pasqua L.G., Cagna M., Berardo C., Vairetti M., Ferrigno A. Detailed Molecular Mechanisms Involved in Drug-Induced Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis: An Update. Biomedicines. 2022;10:194. doi: 10.3390/biomedicines10010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mouzaki M., Yodoshi T., Arce-Clachar A.C., Bramlage K., Fei L., Ley S.L., Xanthakos S.A. Psychotropic Medications Are Associated with Increased Liver Disease Severity in Pediatric Nonalcoholic Fatty Liver Disease. J. Pediatr. Gastroenterol. Nutr. 2019;69:339–343. doi: 10.1097/MPG.0000000000002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmed Amin D., Ahmed Isma M., Mohamed Ib A. Non Alcoholic Fatty Liver Disease, Insulin Resistance, Dyslipidemia and Atherogenic Ratios in Epileptic Children and Adolescents on Long Term Antiepileptic Drug Therapy. Pak. J. Biol. Sci. 2012;15:68–77. doi: 10.3923/pjbs.2012.68.77. [DOI] [PubMed] [Google Scholar]
- 72.Yıldız Y., Sivri H.S. Inborn Errors of Metabolism in the Differential Diagnosis of Fatty Liver Disease. Turk. J. Gastroenterol. 2020;31:3–16. doi: 10.5152/tjg.2019.19367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han S., Zhu F., Huang X., Yan P., Xu K., Shen F., Sun J., Yang Z., Jin G., Teng Y. Maternal Obesity Accelerated Non-alcoholic Fatty Liver Disease in Offspring Mice by Reducing Autophagy. Exp. Ther. Med. 2021;22:716. doi: 10.3892/etm.2021.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brumbaugh D.E., Tearse P., Cree-Green M., Fenton L.Z., Brown M., Scherzinger A., Reynolds R., Alston M., Hoffman C., Pan Z., et al. Intrahepatic Fat Is Increased in the Neonatal Offspring of Obese Women with Gestational Diabetes. J. Pediatr. 2013;162:930–936.e1. doi: 10.1016/j.jpeds.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bugianesi E., Bizzarri C., Rosso C., Mosca A., Panera N., Veraldi S., Dotta A., Giannone G., Raponi M., Cappa M., et al. Low Birthweight Increases the Likelihood of Severe Steatosis in Pediatric Non-Alcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2017;112:1277–1286. doi: 10.1038/ajg.2017.140. [DOI] [PubMed] [Google Scholar]
- 76.Amadou C., Nabi O., Serfaty L., Lacombe K., Boursier J., Mathurin P., Ribet C., de Ledinghen V., Zins M., Charles M. Association between Birth Weight, Preterm Birth, and Nonalcoholic Fatty Liver Disease in a Community-based Cohort. Hepatology. 2022. in press . [DOI] [PMC free article] [PubMed]
- 77.Newton K.P., Feldman H.S., Chambers C.D., Wilson L., Behling C., Clark J.M., Molleston J.P., Chalasani N., Sanyal A.J., Fishbein M.H., et al. Low and High Birth Weights Are Risk Factors for Nonalcoholic Fatty Liver Disease in Children. J. Pediatr. 2017;187:141–146.e1. doi: 10.1016/j.jpeds.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tock L., Dâmaso A.R., de Piano A., Carnier J., Sanches P.L., Lederman H.M., Ernandes R.M.Y., de Mello M.T., Tufik S. Long-Term Effects of Metformin and Lifestyle Modification on Nonalcoholic Fatty Liver Disease Obese Adolescents. J. Obes. 2010;2010:831901. doi: 10.1155/2010/831901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vajro P., Lenta S., Socha P., Dhawan A., McKiernan P., Baumann U., Durmaz O., Lacaille F., McLin V., Nobili V. Diagnosis of Nonalcoholic Fatty Liver Disease in Children and Adolescents: Position Paper of the ESPGHAN Hepatology Committee. J. Pediatr. Gastroenterol. Nutr. 2012;54:700–713. doi: 10.1097/MPG.0b013e318252a13f. [DOI] [PubMed] [Google Scholar]
- 80.Brambilla P., Bedogni G., Heo M., Pietrobelli A. Waist Circumference-to-Height Ratio Predicts Adiposity Better than Body Mass Index in Children and Adolescents. Int. J. Obes. 2013;37:943–946. doi: 10.1038/ijo.2013.32. [DOI] [PubMed] [Google Scholar]
- 81.Schwimmer J.B., Newton K.P., Awai H.I., Choi L.J., Garcia M.A., Ellis L.L., Vanderwall K., Fontanesi J. Paediatric Gastroenterology Evaluation of Overweight and Obese Children Referred from Primary Care for Suspected Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2013;38:1267–1277. doi: 10.1111/apt.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Molleston J.P., Schwimmer J.B., Yates K.P., Murray K.F., Cummings O.W., Lavine J.E., Brunt E.M., Scheimann A.O., Unalp-Arida A. Histological Abnormalities in Children with Nonalcoholic Fatty Liver Disease and Normal or Mildly Elevated Alanine Aminotransferase Levels. J. Pediatr. 2014;164:707–713.e3. doi: 10.1016/j.jpeds.2013.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kwok R., Tse Y.-K., Wong G.L.-H., Ha Y., Lee A.U., Ngu M.C., Chan H.L.-Y., Wong V.W.-S. Systematic Review with Meta-Analysis: Non-Invasive Assessment of Non-Alcoholic Fatty Liver Disease—The Role of Transient Elastography and Plasma Cytokeratin-18 Fragments. Aliment. Pharmacol. Ther. 2014;39:254–269. doi: 10.1111/apt.12569. [DOI] [PubMed] [Google Scholar]
- 84.Schwimmer J.B., Dunn W., Norman G.J., Pardee P.E., Middleton M.S., Kerkar N., Sirlin C.B. SAFETY Study: Alanine Aminotransferase Cutoff Values Are Set Too High for Reliable Detection of Pediatric Chronic Liver Disease. Gastroenterology. 2010;138:1357–1364.e2. doi: 10.1053/j.gastro.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kumar A., Singh A.K., Panda P.K., Nischal N., Soneja M. Non-Alcoholic Fatty Liver Disease Diagnosis, Grading and Staging; a Simplified Tool for Clinicians. J. Adv. Med. 2017;6:15. doi: 10.5958/2319-4324.2017.00003.7. [DOI] [Google Scholar]
- 86.Schwimmer J.B., Deutsch R., Rauch J.B., Behling C., Newbury R., Lavine J.E. Obesity, Insulin Resistance, and Other Clinicopathological Correlates of Pediatric Nonalcoholic Fatty Liver Disease. J. Pediatr. 2003;143:500–505. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 87.Oliveira A.M., Oliveira N., Reis J.C., Santos M.V., Silva A.M., Adan L. Triglycerides and Alanine Aminotransferase as Screening Markers for Suspected Fatty Liver Disease in Obese Children and Adolescents. Horm. Res. Paediatr. 2009;71:83–88. doi: 10.1159/000183896. [DOI] [PubMed] [Google Scholar]
- 88.Engiz Ö., Berberoglu M., Siklar Z., Öçal G. Risk Factors for Non-Alcoholic Fatty Liver Disease in Obese Children. Horm. Res. 2009;72:63–64. doi: 10.1159/000224343. [DOI] [PubMed] [Google Scholar]
- 89.Nobili V., Alkhouri N., Bartuli A., Manco M., Lopez R., Alisi A., Feldstein A.E. Severity of Liver Injury and Atherogenic Lipid Profile in Children with Nonalcoholic Fatty Liver Disease. Pediatr. Res. 2010;67:665–670. doi: 10.1203/PDR.0b013e3181da4798. [DOI] [PubMed] [Google Scholar]
- 90.Lonardo A., Loria P., Leonardi F., Borsatti A., Neri P., Pulvirenti M., Verrone A.M., Bagni A., Bertolotti M., Ganazzi D., et al. Fasting Insulin and Uric Acid Levels but Not Indices of Iron Metabolism Are Independent Predictors of Non-Alcoholic Fatty Liver Disease. A Case-Control Study. Dig. Liver Dis. 2002;34:204–211. doi: 10.1016/S1590-8658(02)80194-3. [DOI] [PubMed] [Google Scholar]
- 91.Sartorio A., Del Col A., Agosti F., Mazzilli G., Bellentani S., Tiribelli C., Bedogni G. Predictors of Non-Alcoholic Fatty Liver Disease in Obese Children. Eur. J. Clin. Nutr. 2007;61:877–883. doi: 10.1038/sj.ejcn.1602588. [DOI] [PubMed] [Google Scholar]
- 92.Della Corte C., Alisi A., Saccari A., De Vito R., Vania A., Nobili V. Nonalcoholic Fatty Liver in Children and Adolescents: An Overview. J. Adolesc. Health. 2012;51:305–312. doi: 10.1016/j.jadohealth.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 93.Ajmera V., Perito E.R., Bass N.M., Terrault N.A., Yates K.P., Gill R., Loomba R., Diehl A.M., Aouizerat B.E., NASH Clinical Research Network Novel Plasma Biomarkers Associated with Liver Disease Severity in Adults with Nonalcoholic Fatty Liver Disease. Hepatology. 2017;65:65–77. doi: 10.1002/hep.28776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petäjä E., Yki-Järvinen H. Definitions of Normal Liver Fat and the Association of Insulin Sensitivity with Acquired and Genetic NAFLD—A Systematic Review. Int. J. Mol. Sci. 2016;17:633. doi: 10.3390/ijms17050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Awai H.I., Newton K.P., Sirlin C.B., Behling C., Schwimmer J.B. Evidence and Recommendations for Imaging Liver Fat in Children, Based on Systematic Review. Clin. Gastroenterol. Hepatol. 2014;12:765–773. doi: 10.1016/j.cgh.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park S.H., Kim P.N., Kim K.W., Lee S.W., Yoon S.E., Park S.W., Ha H.K., Lee M.-G., Hwang S., Lee S.-G., et al. Macrovesicular Hepatic Steatosis in Living Liver Donors: Use of CT for Quantitative and Qualitative Assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 97.Fishbein M., Castro F., Cheruku S., Jain S., Webb B., Gleason T., Stevens W.R. Hepatic MRI for Fat Quantitation: Its Relationship to Fat Morphology, Diagnosis, and Ultrasound. J. Clin. Gastroenterol. 2005;39:619–625. doi: 10.1097/00004836-200508000-00012. [DOI] [PubMed] [Google Scholar]
- 98.Saadeh S., Younossi Z.M., Remer E.M., Gramlich T., Ong J.P., Hurley M., Mullen K.D., Cooper J.N., Sheridan M.J. The Utility of Radiological Imaging in Nonalcoholic Fatty Liver Disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 99.Xiao G., Zhu S., Xiao X., Yan L., Yang J., Wu G. Comparison of Laboratory Tests, Ultrasound, or Magnetic Resonance Elastography to Detect Fibrosis in Patients with Nonalcoholic Fatty Liver Disease: A Meta-analysis. Hepatology. 2017;66:1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 100.Chan W., Nik Mustapha N.R., Wong G.L., Wong V.W., Mahadeva S. Controlled Attenuation Parameter Using the FibroScan® XL Probe for Quantification of Hepatic Steatosis for Non-alcoholic Fatty Liver Disease in an Asian Population. United Eur. Gastroenterol. J. 2017;5:76–85. doi: 10.1177/2050640616646528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nadeau K.J., Ehlers L.B., Zeitler P.S., Love-Osborne K. Treatment of Non-Alcoholic Fatty Liver Disease with Metformin versus Lifestyle Intervention in Insulin-Resistant Adolescents. Pediatr. Diabetes. 2009;10:5–13. doi: 10.1111/j.1399-5448.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- 102.Shah A.G., Lydecker A., Murray K., Tetri B.N., Contos M.J., Sanyal A.J. Comparison of Noninvasive Markers of Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ratziu V., Massard J., Charlotte F., Messous D., Imbert-Bismut F., Bonyhay L., Tahiri M., Munteanu M., Thabut D., Cadranel J.F., et al. Diagnostic Value of Biochemical Markers (FibroTest-FibroSURE) for the Prediction of Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. BMC Gastroenterol. 2006;6:6. doi: 10.1186/1471-230X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brunt E.M., Janney C.G., Di Bisceglie A.M., Neuschwander-Tetri B.A., Bacon B.R. Nonalcoholic Steatohepatitis: A Proposal for Grading and Staging the Histological Lesions. Am. J. Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 105.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., Ferrell L.D., Liu Y.-C., Torbenson M.S., Unalp-Arida A., et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 106.Bedossa P., Poitou C., Veyrie N., Bouillot J.-L., Basdevant A., Paradis V., Tordjman J., Clement K. Histopathological Algorithm and Scoring System for Evaluation of Liver Lesions in Morbidly Obese Patients. Hepatology. 2012;56:1751–1759. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
- 107.Kohut T., Panganiban J. Lifestyle Intervention as the Primary Treatment for Pediatric Nonalcoholic Fatty Liver Disease. Clin. Liver Dis. 2021;17:185–190. doi: 10.1002/cld.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Anania C., Perla F.M., Olivero F., Pacifico L., Chiesa C. Mediterranean Diet and Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2018;24:2083–2094. doi: 10.3748/wjg.v24.i19.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Della Corte C., Mosca A., Vania A., Alterio A., Iasevoli S., Nobili V. Good Adherence to the Mediterranean Diet Reduces the Risk for NASH and Diabetes in Pediatric Patients with Obesity: The Results of an Italian Study. Nutrition. 2017;39–40:8–14. doi: 10.1016/j.nut.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 110.Schwimmer J.B., Ugalde-Nicalo P., Welsh J.A., Angeles J.E., Cordero M., Harlow K.E., Alazraki A., Durelle J., Knight-Scott J., Newton K.P., et al. Effect of a Low Free Sugar Diet vs Usual Diet on Nonalcoholic Fatty Liver Disease in Adolescent Boys: A Randomized Clinical Trial. JAMA. 2019;321:256. doi: 10.1001/jama.2018.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nobili V., Manco M., Devito R., Ciampalini P., Piemonte F., Marcellini M. Effect of Vitamin E on Aminotransferase Levels and Insulin Resistance in Children with Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2006;24:1553–1561. doi: 10.1111/j.1365-2036.2006.03161.x. [DOI] [PubMed] [Google Scholar]
- 112.Nobili V., Manco M., Devito R., Di Ciommo V., Comparcola D., Sartorelli M.R., Piemonte F., Marcellini M., Angulo P. Lifestyle Intervention and Antioxidant Therapy in Children with Nonalcoholic Fatty Liver Disease: A Randomized, Controlled Trial. Hepatology. 2008;48:119–128. doi: 10.1002/hep.22336. [DOI] [PubMed] [Google Scholar]
- 113.Conjeevaram Selvakumar P.K., Kabbany M.N., Alkhouri N. Nonalcoholic Fatty Liver Disease in Children: Not a Small Matter. Pediatr. Drugs. 2018;20:315–329. doi: 10.1007/s40272-018-0292-2. [DOI] [PubMed] [Google Scholar]
- 114.Clerc P., Mouzaki M., Goldman R.D. Omega-3 for Nonalcoholic Fatty Liver Disease in Children. Can. Fam. Physician Med. Fam. Can. 2019;65:34–38. [PMC free article] [PubMed] [Google Scholar]
- 115.Gao X., Zhu Y., Wen Y., Liu G., Wan C. Efficacy of Probiotics in Non-Alcoholic Fatty Liver Disease in Adult and Children: A Meta-Analysis of Randomized Controlled Trials: Meta-Analysis. Hepatol. Res. 2016;46:1226–1233. doi: 10.1111/hepr.12671. [DOI] [PubMed] [Google Scholar]
- 116.Furthner D., Weghuber D., Dalus C., Lukas A., Stundner-Ladenhauf H.N., Mangge H., Pixner T. Nonalcoholic Fatty Liver Disease in Children with Obesity: Narrative Review and Research Gaps. Horm. Res. Paediatr. 2022;95:167–176. doi: 10.1159/000518595. [DOI] [PubMed] [Google Scholar]
- 117.Leoni S., Tovoli F., Napoli L., Serio I., Ferri S., Bolondi L. Current Guidelines for the Management of Non-Alcoholic Fatty Liver Disease: A Systematic Review with Comparative Analysis. World J. Gastroenterol. 2018;24:3361–3373. doi: 10.3748/wjg.v24.i30.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alves J.G.B., Alves G.V. Effects of Physical Activity on Children’s Growth. J. Pediatr. Rio J. 2019;95((Suppl. S1)):72–78. doi: 10.1016/j.jped.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 119.Veldman S.L.C., Paw C.A., Mai J.M., Altenburg T.M. Physical Activity and Prospective Associations with Indicators of Health and Development in Children Aged <5 Years: A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2021;18:6. doi: 10.1186/s12966-020-01072-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bull F.C., Al-Ansari S.S., Biddle S., Borodulin K., Buman M.P., Cardon G., Carty C., Chaput J.-P., Chastin S., Chou R., et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020;54:1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McMurray R.G., Berry D.C., Schwartz T.A., Hall E.G., Neal M.N., Li S., Lam D. Relationships of Physical Activity and Sedentary Time in Obese Parent-Child Dyads: A Cross-Sectional Study. BMC Public Health. 2016;16:124. doi: 10.1186/s12889-016-2795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Whiting S., Buoncristiano M., Gelius P., Abu-Omar K., Pattison M., Hyska J., Duleva V., Musić Milanović S., Zamrazilová H., Hejgaard T., et al. Physical Activity, Screen Time, and Sleep Duration of Children Aged 6–9 Years in 25 Countries: An Analysis within the WHO European Childhood Obesity Surveillance Initiative (COSI) 2015–2017. Obes. Facts. 2021;14:32–44. doi: 10.1159/000511263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ward D., Evans R. Physical Activity, Aerobic Fitness and Obesity in Children. Med. Exerc. Nutr. Health. 1995;4:3–16. [Google Scholar]
- 124.Guthold R., Stevens G.A., Riley L.M., Bull F.C. Worldwide Trends in Insufficient Physical Activity from 2001 to 2016: A Pooled Analysis of 358 Population-Based Surveys with 1·9 million Participants. Lancet Glob. Health. 2018;6:e1077–e1086. doi: 10.1016/S2214-109X(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 125.Vandoni M., Carnevale Pellino V., De Silvestri A., Lovecchio N., Rovida A., Gatti A., Biagioli V., Zuccotti G., Calcaterra V. The Temporal Association between Body Characteristics and Speed Performance over Twenty-Five Years in Italian Adolescents. Child. Basel Switz. 2022;9:521. doi: 10.3390/children9040521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schwarzfischer P., Gruszfeld D., Stolarczyk A., Ferre N., Escribano J., Rousseaux D., Moretti M., Mariani B., Verduci E., Koletzko B., et al. Physical Activity and Sedentary Behavior From 6 to 11 Years. Pediatrics. 2019;143:e20180994. doi: 10.1542/peds.2018-0994. [DOI] [PubMed] [Google Scholar]
- 127.Vandoni M., Lovecchio N., Carnevale Pellino V., Codella R., Fabiano V., Rossi V., Zuccotti G.V., Calcaterra V. Self-Reported Physical Fitness in Children and Adolescents with Obesity: A Cross-Sectional Analysis on the Level of Alignment with Multiple Adiposity Indexes. Children. 2021;8:476. doi: 10.3390/children8060476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu J.J., Capio C.M., Abernethy B., Sit C.H.P. Moderate-to-Vigorous Physical Activity and Sedentary Behavior in Children with and without Developmental Coordination Disorder: Associations with Fundamental Movement Skills. Res. Dev. Disabil. 2021;118:104070. doi: 10.1016/j.ridd.2021.104070. [DOI] [PubMed] [Google Scholar]
- 129.Babic M.J., Morgan P.J., Plotnikoff R.C., Lonsdale C., White R.L., Lubans D.R. Physical Activity and Physical Self-Concept in Youth: Systematic Review and Meta-Analysis. Sports Med. 2014;44:1589–1601. doi: 10.1007/s40279-014-0229-z. [DOI] [PubMed] [Google Scholar]
- 130.Lovecchio N., Zago M. Fitness Differences According to BMI Categories: A New Point of View. J. Sports Med. Phys. Fit. 2019;59:298–303. doi: 10.23736/S0022-4707.18.08271-3. [DOI] [PubMed] [Google Scholar]
- 131.Stodden D., Goodway J., Langendorfer S., Roberton M.A., Rudisill M., Garcia C., Garcia L. A Developmental Perspective on the Role of Motor Skill Competence in Physical Activity: An Emergent Relationship. Quest. 2008;60:290–306. doi: 10.1080/00336297.2008.10483582. [DOI] [Google Scholar]
- 132.Gatti A., Pugliese L., Carnevale Pellino V., Del Bianco M., Vandoni M., Lovecchio N. Self-Declared Physical Activity Levels and Self-Reported Physical Fitness in a Sample of Italian Adolescents during the COVID-19 Pandemic. Eur. J. Investig. Health Psychol. Educ. 2022;12:655–665. doi: 10.3390/ejihpe12060049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fuemmeler B.F., Anderson C.B., Mâsse L.C. Parent-Child Relationship of Directly Measured Physical Activity. Int. J. Behav. Nutr. Phys. Act. 2011;8:17. doi: 10.1186/1479-5868-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hinkley T., Crawford D., Salmon J., Okely A.D., Hesketh K. Preschool Children and Physical Activity: A Review of Correlates. Am. J. Prev. Med. 2008;34:435–441. doi: 10.1016/j.amepre.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 135.Nettle H., Sprogis E. Pediatric Exercise: Truth and/or Consequences. Sports Med. Arthrosc. Rev. 2011;19:75–80. doi: 10.1097/JSA.0b013e318209cf2b. [DOI] [PubMed] [Google Scholar]
- 136.Riddoch C.J., Bo Andersen L., Wedderkopp N., Harro M., Klasson-Heggebø L., Sardinha L.B., Cooper A.R., Ekelund U. Physical Activity Levels and Patterns of 9- and 15-Yr-Old European Children. Med. Sci. Sports Exerc. 2004;36:86–92. doi: 10.1249/01.MSS.0000106174.43932.92. [DOI] [PubMed] [Google Scholar]
- 137.Garaulet M., Martínez A., Victoria F., Pérez–Llamas F., Ortega R.M., Zamora S. Differences in Dietary Intake and Activity Level between Normal-Weight and Overweight or Obese Adolescents. J. Pediatr. Gastroenterol. Nutr. 2000;30:253–258. doi: 10.1097/00005176-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 138.Haverly K., Davison K.K. Personal Fulfillment Motivates Adolescents to Be Physically Active. Arch. Pediatr. Adolesc. Med. 2005;159:1115–1120. doi: 10.1001/archpedi.159.12.1115. [DOI] [PubMed] [Google Scholar]
- 139.Trost S.G., Pate R.R., Ward D.S., Saunders R., Riner W. Correlates of Objectively Measured Physical Activity in Preadolescent Youth. Am. J. Prev. Med. 1999;17:120–126. doi: 10.1016/S0749-3797(99)00056-2. [DOI] [PubMed] [Google Scholar]
- 140.Deldin A.R., Lee S. Role of Physical Activity in the Treatment of Nonalcoholic Fatty Liver Disease in Children and Adolescents. Appl. Physiol. Nutr. Metab. 2013;38:805–812. doi: 10.1139/apnm-2012-0503. [DOI] [PubMed] [Google Scholar]
- 141.Isoura Y., Cho Y., Fujimoto H., Hamazaki T., Tokuhara D. Effects of Obesity Reduction on Transient Elastography–Based Parameters in Pediatric Non-Alcoholic Fatty Liver Disease. Obes. Res. Clin. Pract. 2020;14:473–478. doi: 10.1016/j.orcp.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 142.Babu A.F., Csader S., Lok J., Gómez-Gallego C., Hanhineva K., El-Nezami H., Schwab U. Positive Effects of Exercise Intervention without Weight Loss and Dietary Changes in NAFLD-Related Clinical Parameters: A Systematic Review and Meta-Analysis. Nutrients. 2021;13:3135. doi: 10.3390/nu13093135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cho K., Park S., Koyanagi A., Jacob L., Yon D.K., Lee S.W., Kim M.S., Kim S.U., Kim B.K., Shin J.I., et al. The Effect of Pharmacological Treatment and Lifestyle Modification in Patients with Nonalcoholic Fatty Liver Disease: An Umbrella Review of Meta-analyses of Randomized Controlled Trials. Obes. Rev. 2022:e13464. doi: 10.1111/obr.13464. [DOI] [PubMed] [Google Scholar]
- 144.Perseghin G., Lattuada G., De Cobelli F., Ragogna F., Ntali G., Esposito A., Belloni E., Canu T., Terruzzi I., Scifo P., et al. Habitual Physical Activity Is Associated with Intrahepatic Fat Content in Humans. Diabetes Care. 2007;30:683–688. doi: 10.2337/dc06-2032. [DOI] [PubMed] [Google Scholar]
- 145.Wang S., Song J., Shang X., Chawla N., Yang Y., Meng X., Wang H., Ma J. Physical Activity and Sedentary Behavior Can Modulate the Effect of the PNPLA3 Variant on Childhood NAFLD: A Case-Control Study in a Chinese Population. BMC Med. Genet. 2016;17:90. doi: 10.1186/s12881-016-0352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.WHO . WHO Global Recommendations on Physical Activity for Health. World Health Organization; Geneva, Switzerland: 2011. [PubMed] [Google Scholar]
- 147.Katsagoni C.N., Papachristou E., Sidossis A., Sidossis L. Effects of Dietary and Lifestyle Interventions on Liver, Clinical and Metabolic Parameters in Children and Adolescents with Non-Alcoholic Fatty Liver Disease: A Systematic Review. Nutrients. 2020;12:2864. doi: 10.3390/nu12092864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee S., Deldin A.R., White D., Kim Y., Libman I., Rivera-Vega M., Kuk J.L., Sandoval S., Boesch C., Arslanian S. Aerobic Exercise but Not Resistance Exercise Reduces Intrahepatic Lipid Content and Visceral Fat and Improves Insulin Sensitivity in Obese Adolescent Girls: A Randomized Controlled Trial. Am. J. Physiol.-Endocrinol. Metab. 2013;305:E1222–E1229. doi: 10.1152/ajpendo.00285.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee S., Bacha F., Hannon T., Kuk J.L., Boesch C., Arslanian S. Effects of Aerobic Versus Resistance Exercise Without Caloric Restriction on Abdominal Fat, Intrahepatic Lipid, and Insulin Sensitivity in Obese Adolescent Boys. Diabetes. 2012;61:2787–2795. doi: 10.2337/db12-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Van Der Heijden G.-J., Wang Z.J., Chu Z., Toffolo G., Manesso E., Sauer P.J.J., Sunehag A.L. Strength Exercise Improves Muscle Mass and Hepatic Insulin Sensitivity in Obese Youth. Med. Sci. Sports Exerc. 2010;42:1973–1980. doi: 10.1249/MSS.0b013e3181df16d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.van der Heijden G.-J., Wang Z.J., Chu Z.D., Sauer P.J.J., Haymond M.W., Rodriguez L.M., Sunehag A.L. A 12-Week Aerobic Exercise Program Reduces Hepatic Fat Accumulation and Insulin Resistance in Obese, Hispanic Adolescents. Obesity. 2010;18:384–390. doi: 10.1038/oby.2009.274. [DOI] [PubMed] [Google Scholar]
- 152.González-Ruiz K., Ramírez-Vélez R., Correa-Bautista J.E., Peterson M.D., García-Hermoso A. The Effects of Exercise on Abdominal Fat and Liver Enzymes in Pediatric Obesity: A Systematic Review and Meta-Analysis. Child. Obes. 2017;13:272–282. doi: 10.1089/chi.2017.0027. [DOI] [PubMed] [Google Scholar]
- 153.De Piano A., de Mello M.T., Sanches P. de L.; da Silva, P.L.; Campos, R.M.S.; Carnier, J.; Corgosinho, F.; Foschini, D.; Masquio, D.L.; Tock, L.; et al. Long-Term Effects of Aerobic plus Resistance Training on the Adipokines and Neuropeptides in Nonalcoholic Fatty Liver Disease Obese Adolescents. Eur. J. Gastroenterol. Hepatol. 2012;24:1313–1324. doi: 10.1097/MEG.0b013e32835793ac. [DOI] [PubMed] [Google Scholar]
- 154.Iraji H., Minasian V., Kelishadi R. Changes in Liver Enzymes and Metabolic Profile in Adolescents with Fatty Liver Following Exercise Interventions. Pediatr. Gastroenterol. Hepatol. Nutr. 2021;24:54. doi: 10.5223/pghn.2021.24.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Logan G.R.M., Harris N., Duncan S., Schofield G. A Review of Adolescent High-Intensity Interval Training. Sports Med. 2014;44:1071–1085. doi: 10.1007/s40279-014-0187-5. [DOI] [PubMed] [Google Scholar]
- 156.Van der Windt D.J., Sud V., Zhang H., Tsung A., Huang H. The Effects of Physical Exercise on Fatty Liver Disease. Gene Expr. 2018;18:89–101. doi: 10.3727/105221617X15124844266408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jin Y.-J., Kim K.M., Hwang S., Lee S.G., Ha T.-Y., Song G.-W., Jung D.-H., Kim K.-H., Yu E., Shim J.H., et al. Exercise and Diet Modification in Non-Obese Non-Alcoholic Fatty Liver Disease: Analysis of Biopsies of Living Liver Donors: Lifestyle Modification in Non-Obese NAFLD. J. Gastroenterol. Hepatol. 2012;27:1341–1347. doi: 10.1111/j.1440-1746.2012.07165.x. [DOI] [PubMed] [Google Scholar]
- 158.Eckard C., Cole R., Lockwood J., Torres D.M., Williams C.D., Shaw J.C., Harrison S.A. Prospective Histopathologic Evaluation of Lifestyle Modification in Nonalcoholic Fatty Liver Disease: A Randomized Trial. Ther. Adv. Gastroenterol. 2013;6:249–259. doi: 10.1177/1756283X13484078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Farzanegi P., Dana A., Ebrahimpoor Z., Asadi M., Azarbayjani M.A. Mechanisms of Beneficial Effects of Exercise Training on Non-Alcoholic Fatty Liver Disease (NAFLD): Roles of Oxidative Stress and Inflammation. Eur. J. Sport Sci. 2019;19:994–1003. doi: 10.1080/17461391.2019.1571114. [DOI] [PubMed] [Google Scholar]
- 160.Zhang X.-L., Wang T.-Y., Targher G., Byrne C.D., Zheng M.-H. Lifestyle Interventions for Non-Obese Patients Both with, and at Risk, of Non-Alcoholic Fatty Liver Disease. Diabetes Metab. J. 2022;46:391–401. doi: 10.4093/dmj.2022.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Berzigotti A., Saran U., Dufour J.-F. Physical Activity and Liver Diseases. Hepatology. 2016;63:1026–1040. doi: 10.1002/hep.28132. [DOI] [PubMed] [Google Scholar]
- 162.Cigrovski Berkovic M., Bilic-Curcic I., Mrzljak A., Cigrovski V. NAFLD and Physical Exercise: Ready, Steady, Go! Front. Nutr. 2021;8:734859. doi: 10.3389/fnut.2021.734859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pan X., Han Y., Zou T., Zhu G., Xu K., Zheng J., Zheng M., Cheng X. Sarcopenia Contributes to the Progression of Nonalcoholic Fatty Liver Disease- Related Fibrosis: A Meta-Analysis. Dig. Dis. 2018;36:427–436. doi: 10.1159/000491015. [DOI] [PubMed] [Google Scholar]
- 164.Farzanegi P., Abbaszadeh H., Abbassi Daloii A., Kazemi M., Sabbaghian M., Shoeibi A., Nabipour R., Abuhosseini Z., Azarbayjani M.A. Effects of Aerobic Exercise on Histopathology and Toxicology of ZnO and Nano ZnO in Male Rats. Toxicol. Environ. Chem. 2018;100:103–114. doi: 10.1080/02772248.2018.1430233. [DOI] [Google Scholar]
- 165.Cui J., Guo Y., Yang W., Zhao X., Yu F., Tang W., Pang B., Su X. Effects of Exercise on Learning and Memory, Oxidative Stress and NNOS Expression in Marginal Division of Striatum of Ovariectomized Rats. J. Sports Med. Phys. Fit. 2018;58:356–365. doi: 10.23736/S0022-4707.17.06957-2. [DOI] [PubMed] [Google Scholar]
- 166.Kawanishi N., Yano H., Mizokami T., Takahashi M., Oyanagi E., Suzuki K. Exercise Training Attenuates Hepatic Inflammation, Fibrosis and Macrophage Infiltration during Diet Induced-Obesity in Mice. Brain. Behav. Immun. 2012;26:931–941. doi: 10.1016/j.bbi.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 167.Del Chierico F., Nobili V., Vernocchi P., Russo A., De Stefanis C., Gnani D., Furlanello C., Zandonà A., Paci P., Capuani G., et al. Gut Microbiota Profiling of Pediatric Nonalcoholic Fatty Liver Disease and Obese Patients Unveiled by an Integrated Meta-omics-based Approach. Hepatology. 2017;65:451–464. doi: 10.1002/hep.28572. [DOI] [PubMed] [Google Scholar]
- 168.Moran-Ramos S., Lopez-Contreras B.E., Villarruel-Vazquez R., Ocampo-Medina E., Macias-Kauffer L., Martinez-Medina J.N., Villamil-Ramirez H., León-Mimila P., Del Rio-Navarro B.E., Ibarra-Gonzalez I., et al. Environmental and Intrinsic Factors Shaping Gut Microbiota Composition and Diversity and Its Relation to Metabolic Health in Children and Early Adolescents: A Population-Based Study. Gut Microbes. 2020;11:900–917. doi: 10.1080/19490976.2020.1712985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tokuhara D. Role of the Gut Microbiota in Regulating Non-Alcoholic Fatty Liver Disease in Children and Adolescents. Front. Nutr. 2021;8:700058. doi: 10.3389/fnut.2021.700058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Monga Kravetz A., Testerman T., Galuppo B., Graf J., Pierpont B., Siebel S., Feinn R., Santoro N. Effect of Gut Microbiota and PNPLA3 Rs738409 Variant on Nonalcoholic Fatty Liver Disease (NAFLD) in Obese Youth. J. Clin. Endocrinol. Metab. 2020;105:e3575–e3585. doi: 10.1210/clinem/dgaa382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stanislawski M.A., Lozupone C.A., Wagner B.D., Eggesbø M., Sontag M.K., Nusbacher N.M., Martinez M., Dabelea D. Gut Microbiota in Adolescents and the Association with Fatty Liver: The EPOCH Study. Pediatr. Res. 2018;84:219–227. doi: 10.1038/pr.2018.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schwimmer J.B., Johnson J.S., Angeles J.E., Behling C., Belt P.H., Borecki I., Bross C., Durelle J., Goyal N.P., Hamilton G., et al. Microbiome Signatures Associated with Steatohepatitis and Moderate to Severe Fibrosis in Children With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;157:1109–1122. doi: 10.1053/j.gastro.2019.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Clarke S.F., Murphy E.F., O’Sullivan O., Lucey A.J., Humphreys M., Hogan A., Hayes P., O’Reilly M., Jeffery I.B., Wood-Martin R., et al. Exercise and Associated Dietary Extremes Impact on Gut Microbial Diversity. Gut. 2014;63:1913–1920. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- 174.Allen J.M., Mailing L.J., Niemiro G.M., Moore R., Cook M.D., White B.A., Holscher H.D., Woods J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018;50:747–757. doi: 10.1249/MSS.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 175.Quiroga R., Nistal E., Estébanez B., Porras D., Juárez-Fernández M., Martínez-Flórez S., García-Mediavilla M.V., de Paz J.A., González-Gallego J., Sánchez-Campos S., et al. Exercise Training Modulates the Gut Microbiota Profile and Impairs Inflammatory Signaling Pathways in Obese Children. Exp. Mol. Med. 2020;52:1048–1061. doi: 10.1038/s12276-020-0459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.