Abstract
No periplasmic binding protein has been demonstrated for the ATP-binding cassette (ABC)-type cobalamin transporter BtuCD. New mutations (btuF) are described that affect inner-membrane transport. The BtuF protein has a signal sequence and resembles the periplasmic binding proteins of several other ABC transporters.
Cobalamin is actively transported by Salmonella typhimurium and Escherichia coli (8, 24). The BtuB protein (in concert with the TonB protein) transports cobalamin across the outer membrane. The btuCED operon encodes two membrane proteins (BtuC and BtuD) that provide transport across the inner membrane (4, 5, 17, 22). The inner-membrane BtuCD system is an ATP-binding cassette (ABC) or “traffic ATPase” transporter (2, 7), a type that generally includes a periplasmic binding protein in addition to membrane-spanning components. None of the E. coli transport mutants is defective for a periplasmic binding protein. Direct assays of osmotic shock fluid revealed a protein able to bind cobalamin (8, 15, 27, 28), but no role in transport has been demonstrated (14, 22). It was suggested that the outer-membrane transport system (BtuB-TonB) concentrates cobalamin in the periplasm sufficiently to reduce the need for a binding protein (7, 8, 22). We describe a new class of Salmonella cobalamin transport mutations that may eliminate a periplasmic vitamin B12 binding protein.
New transport mutants.
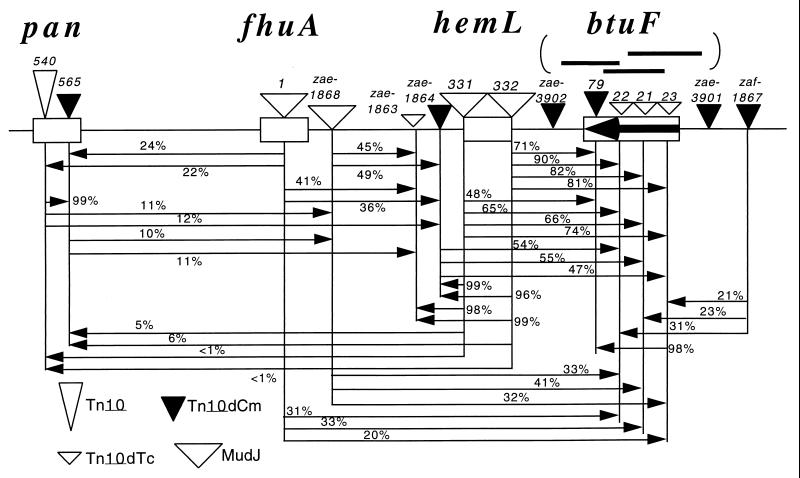
Under aerobic conditions, a metE mutant, which, under aerobic conditions, requires methionine unless exogenous vitamin B12 is provided (24) at a minimum concentration of about 0.1 nM. Known cobalamin transport mutants (btuB or btuCED mutants) require a higher level of exogenous cobalamin (Table 1). The btuF insertion mutants described here were isolated in the metE deletion strain, TT15696. Their phenotype resembled that of previously known btuCD mutants (Table 1). The btuF gene maps near 5 min of the S. typhimurium chromosome (Fig. 1), far from btuB and btuCED.
TABLE 1.
Effect of btu mutations on minimum level of CN–B12 required to support methionine synthesis
| Strain | Relevant genotypea | Ability to grow on minimal medium containing the indicated supplementb
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nothing | Met | CN–B12 (Concn) | |||||||||
| 0.1 nM | 1 nM | 10 nM | 100 nM | 1 μM | 10 μM | 100 μM | 500 mM | ||||
| LT2 | WT | + | + | + | + | + | + | + | + | + | + |
| TT15696 | metE | − | + | + | + | + | + | + | + | + | + |
| TT20702 | btuB12::Tn10dCm | − | + | − | − | − | − | ± | + | + | + |
| TT20703 | btuCED9::Tn10dCm | − | + | − | ± | + | + | + | + | + | + |
| TT20698 | btuF21::Tn10dTc | − | + | − | ± | + | + | + | + | + | + |
| TT20704 | btuF21::Tn10dTc butCED9::Tn10dCm | − | + | − | ± | + | + | + | + | + | + |
| TT20706 | btuF21::Tn10dTc btuB12::Tn10dCm | − | + | − | − | − | − | − | − | − | + |
| TT20705 | btuB20::Tn10dTc btuCED9::Tn10dCm | − | + | − | − | − | − | − | − | − | + |
All strains except LT2 carry a metE deletion mutation (Del1077) which renders methionine synthesis dependent on the B12-dependent MetH enzyme. Three other btuF mutations (TT20699 through TT20701) showed the same phenotype as strain TT20698. WT, wild type.
All tests were performed with cells grown overnight in E-glucose, methionine, and histidine. Cells were diluted in NaCl, and approximately 10 to 100 cells (in 5 μl) were dropped onto an E-glucose–histidine plate with the indicated concentration of CN–B12 or methionine (Met). Growth was scored as the appearance of colonies after 3 days.
FIG. 1.
Genetic map of the btuF region. This region is at min 5 of the latest Salmonella map; the homologous region of the E. coli at min 3.7 includes the yadT gene, which is homologous to the btuF gene of Salmonella. Linkages are presented as percent cotransduction in P22-mediated transduction crosses. The arrowheads point to the selective donor marker used in the cross. Transduction methods have been described previously (12).
Epistasis tests show that BtuF and BtuCD act together.
The transport defect of a btuF btuCED double mutant was indistinguishable from that of either single mutant, suggesting that the BtuF and BtuCD proteins contribute to the same function (Table 1). That is, the residual transport ability in each mutant type does not require the function encoded by the other gene. In contrast, the combination of a btuF and a btuB mutation causes a much more severe defect than either single mutation. This synergistic effect is also seen for a btuCD btuB double mutant. This suggests that BtuB acts in both btuCD and btuF single mutants to provide the residual transport seen. The BtuF protein does not seem to be needed for outer-membrane transport but appears to act with BtuCD to provide inner-membrane transport.
Repression of the cob operon in btuF insertion mutants.
Transcription of the cob operon is induced by propanediol (6, 23) and repressed by adenosylcobalamin (Ado–B12) (1). Repression by exogenous CN–B12 requires transport and internal adenosylation (13, 24). If the btuF mutation impairs cobalamin transport, it should also impair repression of the cob operon by exogenous CN–B12.
In wild-type strains, the cob operon is repressed by 0.1 μM CN–B12. Strains with a btuF (or btuCED) mutation required a 10-fold-higher CN–B12 concentration for repression. Strains with a btuB mutation were not fully repressed even by 1 mM CN–B12. These results were obtained with derivatives of strain TT20707, which carries a cobD24::MudJ insertion (forming a cob-lac operon fusion) and a deletion mutation (cobR4) which renders transcription independent of propanediol but still subject to repression by Ado–B12 (3, 10).
Transport assays.
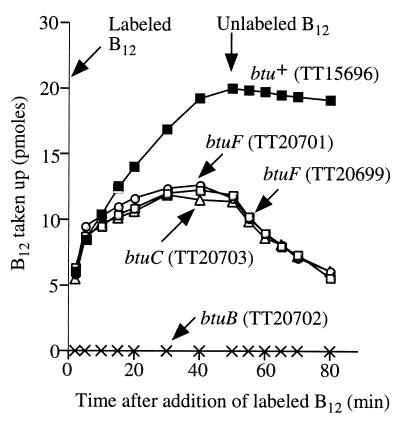
Assays of cyanocobalamin (CN-Cbl) transport were initiated by addition of 57Co-labeled CN–B12. After 50 min of incubation, an excess of unlabeled CN–B12 was added to stop uptake and allow observation of loss of previously assimilated cobalamin from cells. The btuF mutants were indistinguishable from btuC mutants (Fig. 2). Both mutant types initially took up vitamin B12 (12.7 nM) as well as wild-type cells, suggesting no defect in outer-membrane transport; this initial transport is eliminated by a btuB mutation. Both btuF and btuC mutants accumulated less CN-Cbl at steady state, suggesting that import is ultimately opposed by leakage of periplasmic CN-Cbl back out through the outer membrane. In wild-type cells, unlabeled CN–B12 stopped the accumulation of vitamin B12, but in btuF and btuC mutants, addition led to loss of labeled vitamin B12 by diffusion out of the periplasm. Four other btuF mutants behaved essentially like those in Fig. 2. In all cases, btuF mutations appear to block inner-membrane transport.
FIG. 2.
Transport of CN-Cbl by btu mutants. Cells were grown to mid-log phase (optical density at 660 nm [OD660], 0.4 to 0.8) in the Davis-Mingioli minimal medium (11). Harvested cells were suspended to a final OD660 of 6 in 0.1 M potassium phosphate buffer (pH 6.6) containing 20 μM calcium nitrate and 1% glucose. These suspensions were stored on ice and assayed within 3 h of harvest. Transport was assayed by the method of Bradbeer and Woodrow (9). Each 15-ml reaction mixture was 0.1 M potassium phosphate buffer (pH 6.6) containing final concentrations of 20 μM calcium nitrate, 1% glucose, and sufficient cells to give an OD660 of 0.2. After 5 min of incubation at 37°C, the reaction was started by adding 57Co-labeled CN-Cbl to a final concentration of 12.67 nM. Samples were removed at intervals and filtered (0.45-μm-pore-size Millipore filter HAWP) to collect cells. After 50 min of uptake, unlabeled CN-Cbl was added (final concentration, 5 μM) and sampling was continued. All filters were washed twice with 10 ml of 0.1 M lithium chloride, air dried, placed in Beckman Ready Safe scintillation fluid, and counted in a Beckman LS6500 liquid scintillation counter. Results are expressed as picomoles of CN-Cbl taken up per milliliter of cell suspension with an OD660 of 0.6.
Sequencing.
The DNA sequence of the btuF gene was determined following PCR amplification of sequences between genetically characterized insertion mutations as indicated in the genetic map (Fig. 1). Methods and primers used are described elsewhere (18). All sequenced btuF insertion mutations lie within a 801-nucleotide open reading frame (ORF) near the hemL gene. Their positions are btuF23::Tn10dTc (bp 97), btuF22::Tn10dTc (bp 400), and btuF79::Tn10dCm (bp 477). The inferred BtuF sequence includes a highly probable signal sequence with a cleavage site at amino acid 22 (score, 0.97; maximum, 1), a feature expected of a periplasmic binding protein. The BtuF amino acid sequence shows a very strong similarity to the yadT ORF of E. coli. The sequence resembles those of three known ABC-type, periplasmic binding proteins. One transports hemin across in the inner membrane of Yersinia enterocolitica and Yersinia pestis (16, 26). Another (FecB of E. coli) transports citrate-iron chelates (25), and the third transports ferrisiderophores (CbrA of Erwinia chrysanthemi) (21).
Regulation of expression.
Expression of btuF is not regulated, based on tests using a MudJ insertion mutation (btuF80::MudJ), which fuses transcription of the inserted lac operon to the btuF gene. A strain with this fusion (TT20711) was grown on glucose, on glycerol, and on ethanolamine; the fused lacZ gene produced about 45 U of β-galactosidase under all conditions. Addition of CN–B12 (5 nM or 5 μM) had no effect on transcription during growth on any of these three carbon sources. This is interesting in light of the fact that the btuA gene is controlled (by Ado-B12) (19, 20), while the btuCED operon is not (22).
In summary, we infer that the BtuF protein acts as a periplasmic binding protein with BtuCD to transport cobalamins across the inner membrane. Such a protein is expected for a transporter of this type but was not found among previously characterized E. coli cobalamin transport mutants.
Nucleotide sequence accession number.
The Salmonella btuF sequence is GenBank accession no. AF096877.
Acknowledgments
This work was supported in part by NIH grant GM34804.
REFERENCES
- 1.Ailion M, Roth J R. Repression of the cob operon of Salmonella typhimurium by adenosylcobalamin is influenced by mutations in the pdu operon. J Bacteriol. 1997;179:6084–6091. doi: 10.1128/jb.179.19.6084-6091.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ames G F, Mimura C S, Holbrook S R, Shyamala V. Traffic ATPases: a superfamily of transport proteins operating from Escherichia coli to humans. Adv Enzymol. 1992;65:1–47. doi: 10.1002/9780470123119.ch1. [DOI] [PubMed] [Google Scholar]
- 3.Andersson D I, Roth J R. Mutations affecting regulation of cobinamide biosynthesis in Salmonella typhimurium. J Bacteriol. 1989;171:6726–6733. doi: 10.1128/jb.171.12.6726-6733.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassford P J, Jr, Bradbeer C, Kadner R J, Schaitman C A. Transport of vitamin B12 in tonB mutants of Escherichia coli. J Bacteriol. 1976;128:242–247. doi: 10.1128/jb.128.1.242-247.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassford P J, Jr, Kadner R J. Genetic analysis of components involved in vitamin B12 uptake in Escherichia coli. J Bacteriol. 1977;132:96–805. doi: 10.1128/jb.132.3.796-805.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobik T A, Ailion M, Roth J R. A single regulatory gene integrates control of vitamin B12 synthesis and propanediol degradation. J Bacteriol. 1992;174:2253–2266. doi: 10.1128/jb.174.7.2253-2266.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boos W, Lucht J. Periplasmic binding protein-dependent ABC transporters. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C: American Society for Microbiology; 1996. pp. 1175–1209. [Google Scholar]
- 8.Bradbeer C. Cobalamin transport in Escherichia coli. Biofactors. 1991;3:11–19. [PubMed] [Google Scholar]
- 9.Bradbeer C, Woodrow M L. Transport of vitamin B12 in Escherichia coli: energy dependence. J Bacteriol. 1976;128:99–104. doi: 10.1128/jb.128.1.99-104.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P, Andersson D I, Roth J R. The control region of the pdu/cob regulon in Salmonella typhimurium. J Bacteriol. 1994;176:5474–5482. doi: 10.1128/jb.176.17.5474-5482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis B D, Mingioli E. Mutants of Escherichia coli requiring methionine or B12. J Bacteriol. 1950;60:17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis R W, Botstein D, Roth J. Advanced bacterial genetics laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 13.Escalante-Semerena J C, Suh S-J, Roth J R. cobA function is required for both de novo cobalamin biosynthesis and assimilation of exogenous corrinoids in Salmonella typhimurium. J Bacteriol. 1990;172:273–280. doi: 10.1128/jb.172.1.273-280.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedrich M J, DeVeaux L C, Kadner R J. Nucleotide sequence of the btuCED genes involved in vitamin B12 transport in Escherichia coli and homology with components of periplasmic-binding-protein-dependent transport systems. J Bacteriol. 1986;167:928–934. doi: 10.1128/jb.167.3.928-934.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holroyd C, Bradbeer C. Cobalamin transport in Escherichia coli. In: Leive L, Schlessinger D, editors. Microbiology—1984. Washington, D.C: American Society for Microbiology; 1984. pp. 21–23. [Google Scholar]
- 16.Hornung J, Jones H, Perry R. The hmu locus of Yersinia pestis is essential for utilization of free haemin and haem–protein complexes as iron sources. Mol Microbiol. 1996;20:725–739. doi: 10.1111/j.1365-2958.1996.tb02512.x. [DOI] [PubMed] [Google Scholar]
- 17.Kadner R J, McElhaney G. Outer membrane-dependent transport systems in Escherichia coli: turnover of TonB function. J Bacteriol. 1978;134:1020–1029. doi: 10.1128/jb.134.3.1020-1029.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kofoid E, Rappleye C, Stojiljkovic I, Roth J. The 17-gene ethanolamine (eut) operon of Salmonella typhimurium encodes five homologues of carboxysome shell proteins. J Bacteriol. 1999;181:5317–5329. doi: 10.1128/jb.181.17.5317-5329.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundrigan M D, Kadner R J. Altered cobalamin metabolism in Escherichia coli btuR mutants affects btuB regulation. J Bacteriol. 1989;171:154–161. doi: 10.1128/jb.171.1.154-161.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundrigan M D, Koster W, Kadner R J. Transcribed sequences of the Escherichia coli btuB gene control its expression and regulation by vitamin B12. Proc Natl Acad Sci USA. 1991;88:1479–1483. doi: 10.1073/pnas.88.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahe B, Masclaux C, Rauscher L, Enard C, Expert D. Differential expression of two siderophore-dependent iron-acquisition pathways in Erwinia chrysanthemi 3937: characterization of a novel ferrisiderophore permease of the ABC transporter family. Mol Microbiol. 1995;18:33–43. doi: 10.1111/j.1365-2958.1995.mmi_18010033.x. [DOI] [PubMed] [Google Scholar]
- 22.Rioux C R, Kadner R J. Vitamin B12 transport in Escherichia coli K12 does not require the btuE gene of the btuCED operon. Mol Gen Genet. 1989;217:301–308. doi: 10.1007/BF02464897. [DOI] [PubMed] [Google Scholar]
- 23.Rondon M R, Escalante-Semerena J C. The poc locus is required for 1,2-propanediol-dependent transcription of the cobalamin biosynthetic (cob) and propanediol utilization (pdu) genes of Salmonella typhimurium. J Bacteriol. 1992;174:2267–2272. doi: 10.1128/jb.174.7.2267-2272.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roth J R, Lawrence J G, Bobik T A. Cobalamin (coenzyme B12): synthesis and biological significance. Annu Rev Microbiol. 1996;50:137–181. doi: 10.1146/annurev.micro.50.1.137. [DOI] [PubMed] [Google Scholar]
- 25.Staudenmaier H, Van Hove B, Yaraghi Z, Braun V. Nucleotide sequences of the fecBCDE genes and locations of the proteins suggest a periplasmic-binding-protein-dependent transport mechanism for iron(III) dicitrate in Escherichia coli. J Bacteriol. 1989;171:2626–2633. doi: 10.1128/jb.171.5.2626-2633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stojiljkovic I, Hantke K. Transport of haemin across the cytoplasmic membrane through a haemin-specific periplasmic binding-protein-dependent transport system in Yersinia enterocolitica. Mol Microbiol. 1994;13:719–732. doi: 10.1111/j.1365-2958.1994.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 27.Taylor R T, Norrell S A, Hanna M L. Uptake of cyanocobalamin by Escherichia coli B: some characteristics and evidence for a binding protein. Arch Biochem Biophys. 1972;148:366–381. doi: 10.1016/0003-9861(72)90154-3. [DOI] [PubMed] [Google Scholar]
- 28.White J C, di Girolamo P M, Fu M L, Preston Y A, Bradbeer C. Transport of vitamin B12 in Escherichia coli. Location and properties of the initial B12-binding site. J Biol Chem. 1973;248:3976–3986. [PubMed] [Google Scholar]