Abstract
Periodically, new disease-associated variants of the human pathogen Neisseria meningitidis arise. These meningococci diversify during spread, and related isolates recovered from different parts of the world have different genetic and antigenic characteristics. An example is the ET-5 complex, members of which were isolated globally from the mid-1970s onwards. Isolates from a hyperendemic outbreak of meningococcal disease in Worcester, England, during the late 1980s were characterized by multilocus sequence typing and sequence determination of antigen genes. These data established that the Worcester outbreak was caused by ET-5 complex meningococci which were not closely related to the ET-5 complex bacteria responsible for a hyperendemic outbreak in the nearby town of Stroud during the years preceding the Worcester outbreak. A comparison with other ET-5 complex meningococci established that there were at least three distinct globally distributed subpopulations within the ET-5 complex, characterized by particular housekeeping and antigen gene alleles. The Worcester isolates belonged to one of these subpopulations, the Stroud isolates belonged to another, and at least one representative of the third subpopulation identified in this work was isolated elsewhere in the United Kingdom. The sequence data demonstrated that ET-5 variants have arisen by multiple complex pathways involving the recombination of antigen and housekeeping genes and de novo mutation of antigen genes. The data further suggest that either the ET-5 complex has been in existence for many years, evolving and spreading relatively slowly until its disease-causing potential was recognized, or it has evolved and spread rapidly since its first identification in the 1970s, with each of the subpopulations attaining a distribution spanning several continents.
The emergence and spread of bacterial pathogens are important phenomena that remain incompletely understood. Recent developments in the technology of nucleotide sequence determination, together with reductions in its cost, have made epidemiological studies by sequence analysis possible (19). Such studies have several advantages, including improvements in the precision, portability, and reproducibility of the data obtained. They also enable evolutionary and population studies to be performed on data collected for routine epidemiological purposes (30). As nucleotide sequence-based data collection techniques improve, the challenge is to develop appropriate analytical tools to extract the maximum population genetic and evolutionary inference from the data produced. These inferences can, in turn, be used to improve health care interventions.
The human pathogen Neisseria meningitidis, which represents a health hazard throughout the world (6), is an instructive model of the spread of bacterial pathogens as it is genetically, epidemiologically, and pathologically diverse (20). The great majority of meningococcal infections are harmless colonizations of the throat or nasopharynx (4), but some of these lead to life-threatening meningitis and septicemia, which may occur separately or in combination (26). Some meningococci are very much more likely to cause disease than others, with three of the 13 recognized capsular serogroups (serogroups A, B, and C) (37) causing approximately 95% of cases of meningococcal invasive disease (13). Within serogroups A, B, and C, most disease is caused by a limited number of groups of genetically related bacteria which have been referred to variously as complexes (39), clusters, subgroups (38) and recently as hyperinvasive lineages (19).
At intervals, hyperinvasive lineages arise and spread locally or globally (1, 8, 24). These may be novel but are sometimes related to lineages previously identified as causing disease outbreaks (2). For serogroup B and C meningococci, multilocus enzyme electrophoresis studies have shown that such lineages diversify during spread, resulting in complexes of genetically related but nonidentical isolates being recovered from diseased patients (7, 10, 25, 38, 39). The members of such complexes often change antigenically (40), while retaining distinctive epidemiologies and pathologies, creating problems for the development of vaccines and identification of hyperinvasive strains by serology (17). A good example of such spread is that of the ET-5 complex of mainly serogroup B meningococci, which was identified as spreading globally, causing elevated levels of meningococcal infection in a number of countries, from the mid-1970s onwards (9, 32, 40).
In England and Wales, ET-5 complex meningococci with the serological characteristics B:15:P1.7,16 (serogroup:serotype:serosubtype [14]) caused a protracted hyperendemic disease outbreak in the town of Stroud, Gloucestershire, England (5). This outbreak, which persisted for a number of years, was characterized by elevated levels of infection in a small geographical area, particularly in teenagers and young adults. ET-5 complex meningococci with the same serological and epidemiological characteristics were observed in Norway and have been reported in other countries (40). The present study uses recently developed sequence typing and analytical approaches to identify and characterize ET-5 variant strains from the United Kingdom (UK) with different antigenic characteristics. These meningococci were isolated during a hyperendemic disease outbreak which occurred in the town of Worcester, which is geographically close to Stroud, during the late 1980s and early 1990s. The advantages of sequence typing included the ability to characterize and compare variants rapidly and effectively with previously identified variants electronically and the ability to use the data directly in phylogenetic analyses.
MATERIALS AND METHODS
Bacterial isolates examined in this study.
The bacterial isolates (Table 1) were from two sources: (i) meningococci isolated from patients with invasive meningococcal disease, submitted for characterization to the England and Wales Public Health Laboratory Service Meningococcal Reference Unit (MRU) and (ii) ET-5 isolates present in the collection of reference strains used in the development of the multilocus sequence typing (MLST) system (19). Each of the MRU isolates was serogrouped, serotyped, and serosubtyped on receipt by the MRU and was retested prior to this study. The serogroup was identified by coagglutination with polyclonal antisera specific to the capsular polysaccharide, and serotyping and serosubtyping were determined by using serotype-specific monoclonal antibodies in a dot blotting method as described previously (12). All isolates were resistant to sulfonamides (>50 μg of sulfadiazine per ml).
TABLE 1.
N. meningitidis strains
| Isolate | Location, yr | MLST allele
|
ST | porA allele | porA VR1,VR2 | porB allele | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| abcZ | adk | aroE | fumC | gdh | pdhC | pgm | ||||||
| H44/76 | Norway, 1976 | 4 | 10 | 5 | 4 | 6 | 3 | 8 | 32 | 2 | 7,16 | 3-24 |
| NG 080 | Norway, 1981 | 4 | 10 | 5 | 4 | 6 | 3 | 8 | 32 | 2 | 7,16 | 3-24 |
| NG144/82 | Norway, 1982 | 4 | 10 | 5 | 4 | 6 | 3 | 8 | 32 | 2 | 7,16 | 3-63 |
| BZ83 | Holland, 1984 | 8 | 10 | 5 | 4 | 5 | 3 | 8 | 34 | 16 | 5c,10 | 3-1 |
| E360 | Bury St. Edmunds, UK, 1984 | 4 | 10 | 5 | 4 | 6 | 3 | 8 | 32 | 2 | 7,16 | 3-24 |
| BZ 169 | Holland, 1985 | 4 | 10 | 5 | 4 | 6 | 3 | 8 | 32 | 14 | 7b,16 | 3-14 |
| EG 329 | Germany, 1985 | 4 | 10 | 5 | 4 | 6 | 3 | 8 | 32 | 60 | 7a,16 | 3-24 |
| NG PB24 | Norway, 1985 | 4 | 10 | 5 | 4 | 6 | 3 | 8 | 32 | 57 | 7b,16g | 3-24 |
| F126 | Stroud, UK, 1986 | 4 | 10 | 5 | 4 | 5 | 3 | 2 | 74 | 12 | 7,16b | 3-24 |
| G1960 | Stroud, UK, 1987 | 4 | 10 | 5 | 4 | 5 | 3 | 2 | 74 | 12 | 7,16b | 3-24 |
| 196/87 | Norway, 1987 | 4 | 10 | 5 | 4 | 6 | 3 | 8 | 32 | 61 | 7b,16l | 3-24 |
| 8680 | Chile, 1987 | 4 | 10 | 5 | 4 | 6 | 3 | 8 | 32 | 41 | 7b,3 | 3-24 |
| J129 | Hackney, UK, 1988 | 8 | 10 | 5 | 4 | 6 | 3 | 8 | 33 | 4 | 19,15 | 3-3 |
| K80-01508 | Worcester, UK, 1989 | 8 | 10 | 5 | 4 | 6 | 3 | 8 | 33 | 16 | 5c,10 | 3-1 |
| K89-01713 | Worcester, UK, 1989 | 8 | 10 | 5 | 4 | 6 | 3 | 8 | 33 | 16 | 5c,10 | 3-1 |
| K89-02151 | Worcester, UK, 1989 | 8 | 10 | 5 | 4 | 6 | 3 | 8 | 33 | 16 | 5c,10 | 3-1 |
| K89-02160 | Worcester, UK, 1989 | 8 | 10 | 5 | 4 | 6 | 3 | 8 | 33 | 16 | 5c,10 | 3-1 |
| K89-02224 | Worcester, UK, 1989 | 8 | 10 | 5 | 4 | 6 | 3 | 8 | 33 | 16 | 5c,10 | 3-1 |
| L90-00684 | Hereford, UK, 1990 | 8 | 10 | 5 | 4 | 6 | 3 | 8 | 33 | 16 | 5c,10 | 3-1 |
| L90-01144 | Birmingham, UK, 1990 | 8 | 10 | 5 | 4 | 6 | 3 | 8 | 33 | 16 | 5c,10 | 3-1 |
| L90-01047 | Kidderminster, UK, 1990 | 8 | 10 | 5 | 4 | 6 | 3 | 8 | 33 | 16 | 5c,10 | 3-1 |
| 204/92 | Cuba, 1992 | 8 | 10 | 5 | 4 | 6 | 3 | 8 | 33 | 4 | 19,15 | 3-8 |
DNA extraction and PCR.
DNA was extracted either as described previously (31) or by the Isoquick DNA extraction procedure (Orca Research Inc.). Amplification by PCR, purification of PCR products (11), and sequence extension reactions were carried out by using the primers and conditions previously described for MLST loci (19) with the addition of an additional locus, fumC (14a), porA (31), and porB (34, 35) by using Big Dye terminators (PE Applied Biosystems). Extension products were analyzed with a PE Applied Biosystems model 377 automated sequencer, and the data were assembled with the STADEN suite of computer programs (29). Each compiled sequence was determined at least once on each strand.
Analysis of nucleotide sequences.
Allele designation for MLST loci was carried out by using the MLST website (19, 24a), with new allele numbers assigned as appropriate. For each strain the allele present at each locus was identified and used to define the sequence type (ST) for that strain. For the porin genes, alignments were performed manually, so as to maintain the reading frame, and with regard to the proposed structural models for these proteins (36), and allele assignments were made in accordance with our in-house database (26a).
Phylogenetic analysis and manipulation of nucleotide sequences were done with MEGA (16) and SPLITSTREE, version 2.4 (15). SPLITSTREE was used to visualize the data as split graphs, generated by split decomposition analysis (3), which draws networks between sequences if there are potentially multiple evolutionary pathways linking them. This was a more appropriate way of representing these data than a conventional bifurcating phylogenetic tree, as evolutionary relationships in N. meningitidis can be obscured by inter- and intraspecific recombination events. Sequences were analyzed individually and as concatenated sequences comprising all the MLST and antigen gene data. As the overall divergence of these sequences was small, uncorrected (“p” or “Hamming”) distances were used throughout.
RESULTS
Epidemiology of meningococcal disease in the Worcester area in the late 1980s.
From 1987 onwards, an elevated level of meningococcal disease was observed in the town of Worcester, England. The epidemiology of the cluster of cases was similar to that observed in the nearby town of Stroud, with a high proportion of isolates coming from teenagers, and was consistent with a meningococcal strain belonging to the ET-5 complex being present in the area. However, the meningococci associated with the Worcester disease outbreak were serologically B:NT:P1.10, a rare combination of serogroup, serotype, and serosubtype antigens in England and Wales at that time and not previously associated with ET-5 meningococci. Of 7,000 meningococcal strains typed from England and Wales, with a combined population of approximately 49 million, over the period from 1987 to 1990 only 38 had the B:NT:P1.10 phenotype and of these 18 (47%) were from Worcester, which has a population of approximately 81,000. Analysis of the strains from Worcester by pulsed-field gel electrophoresis fingerprinting with restriction endonuclease SfiI and restriction fragment length polymorphism analyses with several probes showed that they were similar to those isolated during the Stroud outbreak and to the Norwegian ET-5 complex strain, H44/76 (data not shown).
STs of ET-5 isolates.
The isolates related to the Worcester outbreak all belonged to ST-33, one of three STs shown to form part of the ET-5 complex, which was originally defined and named by multilocus enzyme electrophoresis analyses (9). Most members of the ET-5 complex examined by MLST to date belonged to ST-32 (19), which differed from ST-33 at the abcZ locus by having allele 4 rather than allele 8 (Table 1). A further ST, ST-34, also belongs to the ET-5 complex: this ST also differed from ST-32 at the abcZ locus in having allele 4 but had the additional difference of allele 5, rather than 6, at the gdh locus (Table 1). Of the other UK isolates included in this study for comparative purposes, one, which originated in Hackney, was ST-33. Two isolates obtained from the Stroud hyperendemic outbreak in successive years had a previously unreported ST, ST-74, differing from ST-32 at the gdh locus (allele 5 rather than allele 6) and at the pgm locus (allele 2 in place of allele 8). A further UK isolate, obtained in geographically remote Bury St. Edmunds, was ST-32 (Table 1).
Nucleotide sequence analysis of porB antigen genes.
The nucleotide sequences of the porB genes, encoding the serotyping antigens, were determined and assigned allele numbers in accordance with reference 33 (Table 1). Six porB alleles were identified, all encoding class 3 porB proteins: porB3-1, porB3-3, porB3-8, porB3-14, porB3-24, and porB3-63. These belonged to three distinct groups of related sequences. Alleles porB3-1, porB3-3, and porB3-8 encoded antigens recognized by the serotype 4 monoclonal antibody (34), alleles porB3-24 and porB3-63 were related to alleles encoding serotype 15, and porB3-14 encoded a PorB protein known to react with serotype 1. These results explained why the Worcester isolates were originally designated nontypeable, as the serotype 4 monoclonal antibody was not in routine use at the MRU before 1995 (34).
Nucleotide sequence analysis of porA antigen genes.
Sequence determination of the genes encoding the serosubtyping antigen, porA, identified nine alleles in the ET-5 complex strains examined (Table 1) which were assigned allele numbers. These fell into three groups, expressing subtypes P1.7,16, P1.19,15, or P1.5c,10. The P1.5c,10-encoding gene (porA-16) was found in the Worcester isolates and one isolate from The Netherlands (BZ83). As the P1.5c variant is not recognized by the P1.5 monoclonal antibody (31), this observation explained the P1.10 serosubtype determined for the Worcester isolates. All of these genes were identical, with no minor variants. One of the UK isolates (J129) had a porA-4 allele, encoding the subtypes P1.19,15, which was identical to that found in the Cuban isolate 204/92. The remaining isolates contained alleles related to porA-2, which encoded the P1.7,16 subtypes (Table 1). One of these (allele porA-60) had a variant of the P1.7 epitope (P1.7a) which appeared to have evolved by a duplication event plus a point mutation in VR1 (Table 2). However, it is likely that these events occurred outside the ET-5 complex, as the DNA encoding this variant was surrounded by four synonymous mutations outside the antigenically variable region which are found in meningococcal porA genes not included in this analysis (22).
TABLE 2.
Changes in PorA protein variable regions with variants of P1.7,16
| Allele | VR combination | Amino acid sequence
|
|
|---|---|---|---|
| VR1 | VR2 | ||
| porA-2 | 7,16 | AQAANGG--ASGQVKVTKVTKA | YYTKDTN-----NNLTLVP |
| porA-60 | 7a,16 | AQAANGGAGASGQVKVTKVTKA | YYTKDTN-----NNLTLVP |
| porA-14 | 7b,16 | AQAANGG--ASGQV---KVTKA | YYTKDTN-----NNLTLVP |
| porA-57 | 7b,16g | AQAANGG--ASGQV---KVTKA | YYTKDTNTKDTNNNLTLVP |
| porA-12 | 7,16b | AQAANGG--ASGQVKVTKVTKA | YYTKNTN-----NNLTLVP |
| porA-61 | 7b,16l | AQAANGG--ASGQV---KVTKA | YYTKDTN-----DNLTLVP |
| porA-41 | 7b,3 | AQAANGG--ASGQV---KVTKA | TLANGAN-----NTIIRVP |
Combination of ST and antigen type data.
Each genetic difference shown in Table 1 was assessed for the likelihood of its being a result of recombination or de novo mutation. Changes were regarded as recombination if entire genes, or substantial portions of them, had contiguous segments of sequence changes, while those events that could have been introduced by a single mutational event, such as a single base substitution, deletion, or duplication, were regarded as likely to be the result of mutation. In this data set, all of the housekeeping genes appeared to have varied by recombination. Both porB (Fig. 1) and porA (Fig. 2), however, appeared to have changed within the ET-5 complex by gene replacement, horizontal genetical exchange of gene fragments resulting in mosaic genes, and the accumulation of new mutations.
FIG. 1.
The six porB alleles found in ET-5 complex meningococci, compared to the sequence of allele porB3-1. The sequences have been edited such that invariant bases have been removed, leaving only the variable sites. The positions in the sequence alignments of the variable bases are indicated by the vertical numbers at the top of the figure. Where a sequence varies from the porB3-1 sequence in a given allele this is shown by the appropriate letter. A period indicates that the sequence is identical to that of allele 3-1 at that position. Single changes, which are likely to be the result of de novo point mutational events, are shown as white text on black.
FIG. 2.
The variable nucleotide sites of the nine porA alleles of ET-5 complex meningococci, compared with allele porA-2, are shown with the same conventions as used for porB in Fig. 1. The bases in those regions in loops I, IV, and V, encoding the variable regions of the gene, are indicated with arrowheads (<>). Variations likely to be the result of single de novo genetic events (point mutations, duplications, or deletions) are indicated as white text on a black background.
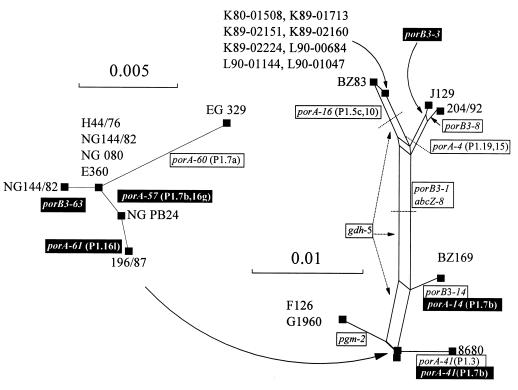
Split decomposition was used to visualize the relationships of concatenated nucleotide sequence data of all genes analyzed (Fig. 3). This was performed as a convenient way of visualizing isolate relatedness, but as there were differences in the diversity of the alleles present at each locus the scale bars in Fig. 3 are not quantitative indicators of interisolate relatedness. The split graph was annotated by adding the gene changes, relative to strain H44/76, contributing each of the edges present in the graph with each change assigned as likely to have resulted from either recombination (boxed) or mutation (white text on a black background). From the annotations the number of identified genetic events separating the isolates are apparent. For example, isolate BZ83 was separated from isolate H44/76 by four recombination events replacing gdh-6 with gdh-5, abcZ-4 with abcZ-8, porB3-24 with porB3-1, and porA-2 (P1.7,16) with porA-16 (P1.5c,10).
FIG. 3.
Split graph of the relationships among members of the ET-5 complex. The split graphs were generated from concatenated sequences of the seven housekeeping loci with the porB and porA gene sequences. The smaller graph shows the detailed relationships within those isolates most closely related to the Norwegian ET-5 strain, H44/76. These isolates occupy the location marked by the arrow on the larger graph. Each of the edges in the graph has been annotated with the genetic change that it represents. Changes likely to be the result of single de novo genetic events (point mutations, deletions, or duplications) are shown as white text on a black background, and changes likely to be the result of recombination events are shown as boxed black text.
Several isolates were identical to strain H44/76 at all loci, with a number of further isolates that varied only in their porA or porB genes, shown in the enlarged portion of the split graph. Two ST-32 isolates were distinct from H44/76 as a result of mutational and recombinational changes in their porA or porB genes. The Chilean isolate 8680 had a mosaic porA gene (porA-41) relative to H44/76 (porA-2), with the distal portion of its genes encoding the P1.3 subtype (Fig. 2 and Table 2).
Other isolates had different STs, a result of variations in their housekeeping genes, in addition to changes in their porA and porB genes. The two isolates from the Stroud outbreak in Gloucestershire were identical to each other and had different pgm (pgm-2) and gdh (gdh-5) alleles relative to H44/76 in addition to a mutational change in porA described previously (23). The isolates from the Worcester outbreak were more distantly related to H44/76, sharing a variant abcZ allele (abcZ-8) and distinct porB allele (porB-1) with a number of other ET-5 variants. Among these meningococci, UK isolate J129 was similar to isolate 204/92 from Cuba, both possessing an identical porA allele, with the two strains differing in these data only by two single base changes in the porB gene of isolate J129 and an apparent replacement in the porB gene of isolate 204/92 (alleles porB3-3 and porB3-8; Fig. 1). These two isolates were distinct from the 1984 isolate from The Netherlands, BZ83, and the Worcester isolates, which had the subtype P1.5c,10 porA-16 allele and porB3-1. Isolate BZ83 was distinct from the Worcester isolates in having the same pgm allele, pgm-5, present in the outbreak strains from Stroud, although otherwise these isolates were not closely related.
DISCUSSION
The meningococcus has a complex population biology, which is in part a consequence of its natural competence for DNA uptake (18, 28). Rates of horizontal genetical exchange by transformation in this species are sufficiently high that the clonal population structure is disrupted and different members of the species can exhibit different population structures (20). This poses a number of problems for public health interventions, for example, making the identification of disease-associated meningococci difficult (19) and complicating the design and use of vaccines based on variable antigens such as PorA (12).
The nucleotide sequence-based typing procedures outlined here enable some of the evolutionary events that have occurred during the spread of ET-5 meningococci to be described. Although the genotype of the meningococcus ancestral to the members of this complex of related organisms cannot be identified with certainty, it is possible to make comparisons within the data set. The annotations in Fig. 3 illustrate the genetic changes in ET-5 variants relative to the Norwegian isolate H44/76, which was one of the earliest ET-5 organisms isolated and which occupied a central position in the split graph (Fig. 3). A comparison of the variants revealed a number of unexpected findings. First, despite the temporal and geographical proximity of the Worcester and Stroud outbreaks, the Worcester-related isolates were more closely related to meningococci isolated outside the UK than to the isolates from Stroud. Indeed, it appears that the Worcester-related isolates belong to a distinct subset of ET-5 meningococci, characterized by abcZ allele 8 and porB genes expressing proteins associated with the serotype 4 phenotype (porB alleles 3-1, 3-3, and 3-8) and not possessing porA alleles related to porA-2 (P1.7,16 and variants). Despite these genetic changes, these variants apparently retained their ability to cause disease outbreaks.
The various combinations of the porA-16 (P1.5c,10) and the gdh-5 alleles introduced a number of complications into the analysis. As the parallel edges in Fig. 3 imply, there is no parsimonious explanation for the relationships of isolates H44/76 and BZ83 and the Worcester and Stroud isolates that does not involve one recombinational event occurring twice independently. These data suggest that gdh-5 has been independently acquired by meningococci belonging to the ET-5 complex at least twice. Given the number of meningococcal gdh alleles known to exist (19), this appears to be a very unlikely event. While selection at this locus is a possible explanation, there is no a priori reason to invoke it, and this observation highlights the complexity of the events involved in the emergence of variants of meningococcal clones.
Ten distinct porA alleles were present in the ET-5 meningococci described here, requiring a minimum of two gene replacements and one, perhaps two, intragenic recombinations plus mutations to have occurred irrespective of the porA allele present in the common ancestor of the ET-5 complex meningococci. The Worcester isolates and the Dutch isolate BZ83 possessed the porA-16 allele, encoding subtype P1.5c,10, which was identical to a porA allele found frequently in the serogroup A meningococci (referred to as gene type α by Suker et al. [31]). This allele, which was associated with a number of serogroup A hyperinvasive lineages but particularly with subgroup I, was identical in serogroup A isolates obtained at various geographical locations over the last 50 years (31). The finding that allele porA-60 differed from the other alleles encoding variants of the P1.7,16 PorA proteins, probably as a result of a recombination event that exchanged one P1.7 variant with another, further underlines the complexity of events in the evolution of these organisms. The porB allele data for the ET-5 isolates investigated here are similar, requiring at least one gene replacement event and probably two intragenic events, represented by alleles porB-60 and porB-41, to explain them.
Although it is tempting to identify potential donors for each of the exchange events observed, particularly in the case of the porA-16 allele, it is not possible from these data to reconstruct reliably the series of events in the ET-5 complex, and the data are more appropriately explained by the concept of a meningococcal global gene pool (21, 31). In this model, rates of horizontal exchange are sufficiently high among N. meningitidis strains to ensure that a given allele, or part of an allele, is potentially available to all meningococci. For some genes, particularly those involved in conserved housekeeping functions, such a global gene pool may extend across species boundaries.
These results have implications for both public health monitoring and vaccination strategies against this important pathogen. The ET-5 meningococci, while retaining their epidemiological characteristic of causing hyperendemic disease in localized foci, have undergone an antigenic change involving gene replacement of two of the major surface antigens used in their characterization and in novel vaccines on several occasions. This has included nucleotide sequence changes within the parts of the porA gene that encode the variable regions of the PorA protein and gene replacement events that have resulted in the acquisition of entirely new PorA and PorB proteins. Such exchanges have also been reported for the capsular operon (32). This variation is most easily explained by a selective pressure on the structure of the surface antigens of the meningococcus and is most probably the result of selection imposed by human immune responses (27). These data further illustrate the potential shortcomings of both serosubtyping in characterization of meningococci and antimeningococcal vaccine design strategies that rely on variable antigens. It is essential that multilocus approaches are used in the identification and characterization of hypervirulent lineages of meningococci.
In conclusion, there are at least three distinct subpopulations within the ET-5 complex of meningococci, which can be defined by the presence of different housekeeping and antigen alleles. All three of these populations have caused disease in the UK and across several continents. Given the relatively short period in evolutionary time since the first isolation of meningococci belonging to the ET-5 complex, there are two explanations for these data. Either this complex of strains has evolved rapidly in the period of a decade or so with at least three distinct variants spreading to attain a global distribution, or the ET-5 complex arose some time before its association with elevated levels of disease, recognized only recently in its evolutionary history.
ACKNOWLEDGMENTS
M.C.J.M. is a Wellcome Trust Senior Fellow in Biodiversity and thanks the Wellcome Trust for financial support. J.E.R. is supported by the Meningitis Research Foundation.
REFERENCES
- 1.Achtman M. Clonal properties of meningococci from epidemic meningitis. Trans R Soc Trop Med Hyg. 1991;85(Suppl. 1):24–31. doi: 10.1016/0035-9203(91)90337-x. [DOI] [PubMed] [Google Scholar]
- 2.Achtman M. Global epidemiology of meningococcal disease. In: Cartwright K A, editor. Meningococcal disease. Chichester, England: John Wiley and Sons; 1995. pp. 159–175. [Google Scholar]
- 3.Bandelt H J, Dress A W. Split decomposition: a new and useful approach to phylogenetic analysis of distance data. Mol Phylogenet Evol. 1992;1:242–252. doi: 10.1016/1055-7903(92)90021-8. [DOI] [PubMed] [Google Scholar]
- 4.Broome C V. The carrier state: Neisseria meningitidis. J Antimicrob Chemother. 1986;18(Suppl. A):25–34. doi: 10.1093/jac/18.supplement_a.25. [DOI] [PubMed] [Google Scholar]
- 5.Cartwright K A, Stuart J M, Noah N D. An outbreak of meningococcal disease in Gloucestershire. Lancet. 1986;ii:558–561. doi: 10.1016/s0140-6736(86)90124-8. [DOI] [PubMed] [Google Scholar]
- 6.Cartwright K A, editor. Meningococcal disease. Chichester, England: John Wiley and Sons; 1995. [Google Scholar]
- 7.Caugant D A, Bol P, Hoiby E A, Zanen H C, Froholm L O. Clones of serogroup B Neisseria meningitidis causing systemic disease in the Netherlands, 1958–1986. J Infect Dis. 1990;162:867–874. doi: 10.1093/infdis/162.4.867. [DOI] [PubMed] [Google Scholar]
- 8.Caugant D A, Froholm L O, Bovre K, Holten E, Frasch C E, Mocca L F, Zollinger W D, Selander R K. Intercontinental spread of a genetically distinctive complex of clones of Neisseria meningitidis causing epidemic disease. Proc Natl Acad Sci USA. 1986;83:4927–4931. doi: 10.1073/pnas.83.13.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caugant D A, Froholm L O, Bovre K, Holten E, Frasch C E, Mocca L F, Zollinger W D, Selander R K. Intercontinental spread of Neisseria meningitidis clones of the ET-5 complex. Antonie Leeuwenhoek. 1987;53:389–394. doi: 10.1007/BF00415492. [DOI] [PubMed] [Google Scholar]
- 10.Caugant D A, Mocca L F, Frasch C E, Froholm L O, Zollinger W D, Selander R K. Genetic structure of Neisseria meningitidis populations in relation to serogroup, serotype, and outer membrane protein pattern. J Bacteriol. 1987;169:2781–2792. doi: 10.1128/jb.169.6.2781-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Embley T M. The linear PCR reaction: a simple and robust method for sequencing amplified rRNA genes. Lett Appl Microbiol. 1991;13:171–174. doi: 10.1111/j.1472-765x.1991.tb00600.x. [DOI] [PubMed] [Google Scholar]
- 12.Feavers I M, Fox A J, Gray S, Jones D M, Maiden M C J. Antigenic diversity of meningococcal outer membrane protein PorA has implications for epidemiological analysis and vaccine design. Clin Diagn Lab Immunol. 1996;3:444–450. doi: 10.1128/cdli.3.4.444-450.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frasch C E. Vaccines for prevention of meningococcal disease. Clin Microbiol Rev. 1989;2:S134–S138. doi: 10.1128/cmr.2.suppl.s134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frasch C E, Zollinger W D, Poolman J T. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev Infect Dis. 1985;7:504–510. doi: 10.1093/clinids/7.4.504. [DOI] [PubMed] [Google Scholar]
- 14a.Holmes E C, Urwin R, Maiden M C. The influence of recombination on the population structure of the human pathogen Neisseria meningitidis. Mol Biol Evol. 1999;16:741–749. doi: 10.1093/oxfordjournals.molbev.a026159. [DOI] [PubMed] [Google Scholar]
- 15.Huson D H. SPLITSTREE: a program for analysing and visualising evolutionary data. Bioinformatics. 1998;14:68–73. doi: 10.1093/bioinformatics/14.1.68. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, Tamura K, Nei M. MEGA: Molecular Evolutionary Genetics Analysis software for microcomputers. Comput Appl Biosci. 1994;10:189–191. doi: 10.1093/bioinformatics/10.2.189. [DOI] [PubMed] [Google Scholar]
- 17.Maiden M C J. The impact of molecular techniques on the study of meningococcal disease. In: Woodford N, Johnson A P, editors. Molecular bacteriology: protocols and clinical applications. Totowa, N.J: Humana Press; 1998. pp. 265–291. [DOI] [PubMed] [Google Scholar]
- 18.Maiden M C J. Population genetics of a transformable bacterium: the influence of horizontal genetical exchange on the biology of Neisseria meningitidis. FEMS Microbiol Lett. 1993;112:243–250. doi: 10.1111/j.1574-6968.1993.tb06457.x. [DOI] [PubMed] [Google Scholar]
- 19.Maiden M C J, Bygraves J A, Feil E, Morelli G, Russell J E, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant D A, Feavers I M, Achtman M, Spratt B G. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci USA. 1998;95:3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiden M C J, Feavers I M. Population genetics and global epidemiology of the human pathogen Neisseria meningitidis. In: Baumberg S, Young J P W, Wellington E M H, Saunders J R, editors. Population genetics of bacteria. Cambridge, England: Cambridge University Press; 1995. pp. 269–293. [Google Scholar]
- 21.Maiden M C J, Malorny B, Achtman M. A global gene pool in the neisseriae. Mol Microbiol. 1996;21:1297–1298. doi: 10.1046/j.1365-2958.1996.981457.x. [DOI] [PubMed] [Google Scholar]
- 22.Maiden M C J, Suker J, McKenna A J, Bygraves J A, Feavers I M. Comparison of the class 1 outer membrane proteins of eight serological reference strains of Neisseria meningitidis. Mol Microbiol. 1991;5:727–736. doi: 10.1111/j.1365-2958.1991.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 23.McGuinness B T, Clarke I N, Lambden P R, Barlow A K, Poolman J T, Jones D M, Heckels J E. Point mutation in meningococcal porA gene associated with increased endemic disease. Lancet. 1991;337:514–517. doi: 10.1016/0140-6736(91)91297-8. [DOI] [PubMed] [Google Scholar]
- 24.Morelli G, Malorny B, Muller K, Seiler A, Wang J F, del Valle J, Achtman M. Clonal descent and microevolution of Neisseria meningitidis during 30 years of epidemic spread. Mol Microbiol. 1997;25:1047–1064. doi: 10.1046/j.1365-2958.1997.5211882.x. [DOI] [PubMed] [Google Scholar]
- 24a.Multilocus sequence typing. 26 July 1999, revison date. [Online.] http://mlst.zoo.ox.ac.uk. [28 July 1999, last date accessed.]
- 25.Olyhoek T, Crowe B A, Achtman M. Clonal population structure of Neisseria meningitidis serogroup A isolated from epidemics and pandemics between 1915 and 1983. Rev Infect Dis. 1987;9:665–682. doi: 10.1093/clinids/9.4.665. [DOI] [PubMed] [Google Scholar]
- 26.Peltola H. Meningococcal disease: still with us. Rev Infect Dis. 1983;5:71–91. doi: 10.1093/clinids/5.1.71. [DOI] [PubMed] [Google Scholar]
- 26a.Russell, J. R. 26 July 1999, revision date. Allele database. [Online.] http://www.mlst.zoo.ox.ac.uk/Meningococcus. [28 July 1999, last date accessed.]
- 27.Smith N H, Maynard Smith J, Spratt B G. Sequence evolution of the porB gene of Neisseria gonorrhoeae and Neisseria meningitidis: evidence of positive Darwinian selection. Mol Biol Evol. 1995;12:363–370. doi: 10.1093/oxfordjournals.molbev.a040212. [DOI] [PubMed] [Google Scholar]
- 28.Spratt B G, Smith N H, Zhou J, O’Rourke M, Feil E. The population genetics of the pathogenic Neisseria. In: Baumberg S, Young J P W, Wellington E M H, Saunders J R, editors. Population genetics of bacteria. Cambridge, England: Cambridge University Press; 1995. pp. 143–160. [Google Scholar]
- 29.Staden R. The STADEN sequence analysis package. Mol Biotechnol. 1996;5:233–241. doi: 10.1007/BF02900361. [DOI] [PubMed] [Google Scholar]
- 30.Suerbaum S, Maynard Smith J, Bapumia K, Morelli G, Smith N H, Kunstmann E, Dyrek I, Achtman M. Free recombination within Helicobacter pylori. Proc Natl Acad Sci USA. 1998;95:12619–12624. doi: 10.1073/pnas.95.21.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suker J, Feavers I M, Achtman M, Morelli G, Wang J-F, Maiden M C J. The porA gene in serogroup A meningococci: evolutionary stability and mechanism of genetic variation. Mol Microbiol. 1994;12:253–265. doi: 10.1111/j.1365-2958.1994.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 32.Swartley J S, Marfin A A, Edupuganti S, Liu L J, Cieslak P, Perkins B, Wenger J D, Stephens D S. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci USA. 1997;94:271–276. doi: 10.1073/pnas.94.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urwin R. Ph.D. thesis. Stafford, United Kingdom: University of Stafford; 1998. [Google Scholar]
- 34.Urwin R, Feavers I M, Jones D M, Maiden M C J, Fox A J. Molecular variation of meningococcal serotype 4 antigen genes. Epidemiol Infect. 1998;121:95–101. doi: 10.1017/s0950268898008942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urwin R, Fox A J, Musilek M, Kriz P, Maiden M C J. Heterogeneity of the PorB protein in serotype 22 Neisseria meningitidis. J Clin Microbiol. 1998;36:3680–3682. doi: 10.1128/jcm.36.12.3680-3682.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Ley P, Heckels J E, Virji M, Hoogerhout P, Poolman J T. Topology of outer membrane porins in pathogenic Neisseria spp. Infect Immun. 1991;59:2963–2971. doi: 10.1128/iai.59.9.2963-2971.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vedros N A. Development of meningococcal serogroups. In: Vedros N A, editor. Evolution of meningococcal disease. II. Boca Raton, Fla: CRC Press Inc.; 1987. pp. 33–37. [Google Scholar]
- 38.Wang J-F, Caugant D A, Li X, Hu X, Poolman J T, Crowe B A, Achtman M. Clonal and antigenic analysis of serogroup A Neisseria meningitidis with particular reference to epidemiological features of epidemic meningitis in China. Infect Immun. 1992;60:5267–5282. doi: 10.1128/iai.60.12.5267-5282.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J-F, Caugant D A, Morelli G, Koumaré B, Achtman M. Antigenic and epidemiological properties of the ET-37 complex of Neisseria meningitidis. J Infect Dis. 1993;167:1320–1329. doi: 10.1093/infdis/167.6.1320. [DOI] [PubMed] [Google Scholar]
- 40.Wedege E, Kolberg J, Delvig A, Høiby E A, Holten E, Rosenqvist E, Caugant D A. Emergence of a new virulent clone within the electrophoretic type 5 complex of serogroup B meningococci in Norway. Clin Diagn Lab Immunol. 1995;2:314–321. doi: 10.1128/cdli.2.3.314-321.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]