Abstract
Physical inactivity is documented as a health risk factor for chronic diseases, accelerated aging, and cognitive impairment. Physical exercise, on the other hand, plays an important role in healthy aging by promoting positive muscular, cardiovascular, and central nervous system adaptions. Prior studies on the effects of exercise training on cerebral perfusion have focused largely on elderly cohorts or patient cohorts, while perfusion effects of exercise training in young sedentary adults have not yet been fully assessed. Therefore, the present study examined the physiological consequence of a 6-month endurance exercise training on brain perfusion in 28 young sedentary adults randomly assigned to an intervention group (IG; regular physical exercise) or a control group (CG; without physical exercise). The IG performed an extensive running interval training three times per week over 6 months. Performance diagnostics and MRI were performed every 2 months, and training intensity was adapted individually. Brain perfusion measurements with pseudo-continuous arterial spin labeling were analyzed using the standard Oxford ASL pipeline. A significant interaction effect between group and time was found for right superior temporal gyrus (STG) perfusion, driven by an increase in the IG and a decrease in the CG. Furthermore, a significant time effect was observed in the right middle occipital region in the IG only. Perfusion increases in the right STG, in the ventral striatum, and in primary motor areas were significantly associated with increases in maximum oxygen uptake (VO2max). Overall, this study identified region-specific increases in local perfusion in a cohort of young adults that partly correlated with individual performance increases, hence, suggesting exercise dose dependency. Respective adaptations in brain perfusion are discussed in the context of physical exercise-induced vascular plasticity.
Keywords: maximum oxygen uptake, endurance training, perfusion, pseudo-continuous arterial spin labeling, brain perfusion, CBF
Introduction
There is a broad consensus across disciplines that a sedentary lifestyle is an important risk factor for chronic diseases (Booth et al., 2012), accelerated aging (Mattson and Arumugam, 2018), and cognitive impairment (Steinberg et al., 2015). Physical exercise (PE) plays an important role in current concepts of “healthy aging” (Bull et al., 2020). Effects on the central nervous system are promoted by exercise-induced neurogenesis, synaptic plasticity, angiogenesis, and improved cerebral blood flow (CBF)/metabolism (Tarumi and Zhang, 2015; Vivar and Van Praag, 2017). Notably, these mechanisms impact the brain areas typically affected by aging, thereby maintaining or even improving cognitive function (Hillman et al., 2008; Erickson et al., 2011).
The human brain consumes ∼20% of total oxygen consumption at rest, and the brain function critically depends on continuous delivery of oxygen and metabolic nutrients (Smith and Ainslie, 2017). Regional brain perfusion is tightly coupled to regional cerebral metabolism (Chen et al., 2015). Brain perfusion and metabolism are inversely associated with age, and CBF is typically compromised in neurological diseases (Chen et al., 2015). On the other hand, intact cortical perfusion is considered a surrogate for an uncompromised tissue delivery of metabolites through cerebral microvessels that influence neuronal energy metabolism (Watts et al., 2018). PE is known to trigger angiogenesis via a complex interplay of growth factors, particularly the vascular endothelial growth factor (VEGF), and various receptors and signaling pathways that serve as stimuli for capillary growth (Egginton, 2009). Subsequent adaptations of CBF are necessary for meeting the changing metabolic demands associated with repeated exercise challenges (Ogoh and Ainslie, 2009), as well as non-acute or long-term neuroplastic changes induced by PE (Hillman et al., 2008; Erickson et al., 2011).
While structural and functional brain plasticity have been thoroughly investigated in numerous previous exercise imaging studies (for meta-analyses see Ji et al., 2021; Yu et al., 2021), the effects of PE on resting levels of global and regional brain perfusion have not yet been fully elucidated (Lehmann et al., 2020). Moreover, the existing PE interventions so far were exclusively conducted in older healthy cohorts (Burdette et al., 2010; Chapman et al., 2013; Flodin et al., 2017; Kleinloog et al., 2019) or patient populations (Robertson et al., 2017; Alfini et al., 2019; Thomas et al., 2020), but not in healthy young adults.
Given that recommendations on brain health and disease prevention are shifting to earlier ages (Ortega et al., 2008; Wen et al., 2011; Lin et al., 2015), our study aimed at determining whether exercise training for a 6-month duration significantly increases CBF in younger cohorts also. Optimal brain perfusion is considered to be an important component of “brain health” responsible for an adequate supply of nutrients and oxygen. In particular, we were interested where and when CBF changes would occur in the brain. For this purpose, we designed a randomized controlled intervention trial testing the effects of PE training over a 6-month-period on CBF in sedentary adults aged 20–35 years. We used whole-brain pseudo-continuous arterial spin labeling (pCASL) to measure PE-induced CBF increases and to relate changes in CBF to individual performance increases, as assessed in repeated graded exercise tests. Based on previous literature, we hypothesized that PE-induced perfusion increases in the following areas: (i) in the temporal lobe where PE interventions of 3 (Maass et al., 2015) or 4 (Burdette et al., 2010) months duration have highlighted hippocampal perfusion increases in healthy elderly cohorts (Pereira et al., 2007; Burdette et al., 2010; Maass et al., 2015); (ii) in the anterior cingulate cortex where other randomized PE studies of 12-weeks duration reported CBF increases in the elderly subjects (Chapman et al., 2013; Kleinloog et al., 2019); (iii) in areas of the frontal cortex which show exercise-induced plasticity (Zheng et al., 2019; Northey et al., 2020; Tari et al., 2020; Zhu et al., 2021); and finally (iv) in the primary motor cortex where structural plasticity was reported in endurance runners (Cao et al., 2021).
Materials and methods
Subjects
Men and women in the age group of 18–35 years were recruited via flyers distributed at the local university and on social media. Before the study inclusion, we verified that subjects had a sedentary lifestyle and a poor to fair fitness level. Subjects with prior history as competitive athletes (according to a custom questionnaire on different life spans, namely, up to 12 years of age, 13–18 years of age, after 18 years of age until inclusion) or regular physical exercise training in the last 2 years preceding this study (according to a custom questionnaire on frequency, time, and type of exercise) were excluded. As an add-on, the International Physical Activity Questionnaire (IPAQ) assessed participants’ general physical activity behavior, including leisure and work-associated activities, during the previous 7 days.
Eligible subjects did not meet the evidence-based recommendations for frequency, intensity, time, and type of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness regarding ACSM Position Stand (Garber et al., 2011). Physical activities like slow walking or other incidental activities did not lead to exclusion.
To determine that the participants were healthy and to exclude major physical health risks for subsequent exercise tests and trainings, a medical healthcare check was done before randomization into the study in all subjects. This included an anamnestic questionnaire, auscultation of lung and heart, and a 12-channel resting electrocardiogram, as well as questionnaires regarding neurologic and psychiatric diseases (current or in the past). Based on these tests, the sedentary participants were classified as being eligible for inclusion into a randomized exercise intervention. Screening questionnaires (Mini International Neuropsychiatric Interview (M.I.N.I, german version 5.0.0; Sheehan et al., 1998), trait anxiety of the State-Trait-Anxiety Inventory (STAI; Spielberger et al., 1983), and Beck Depression Inventory (BDI-I; Hautzinger et al., 1994) provided no indications for neurological or psychological diseases. MRI-related exclusion criteria were pregnancy, claustrophobia, non-removable metal, and tattoos exceeding a certain size (>20 cm or covering > 5% body surface or in head or neck area). All subjects were informed about the procedure and protocols and signed a declaration of agreement before inclusion in the study. The procedures were approved by the local ethics committee (Ethikkommission an der Medizinischen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn: Nr. 370/15) and were conducted according to the Declaration of Helsinki. The study was registered in the DRKS – German Clinical Trials Register (clinical trial registration number DRKS00021460).
Experimental design
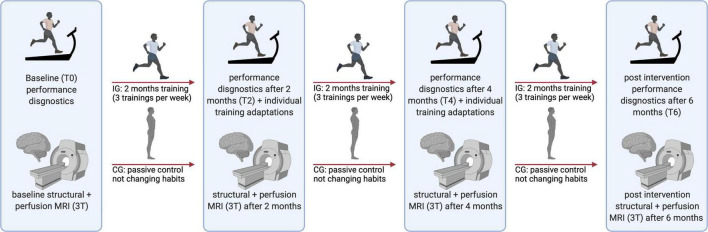
This study is a sub-analysis within the “RUNSTUD” study (Maurer et al., 2020). To test the influence of an extensive interval training over 6 months on brain structure and function, all subjects completed an extensive battery of neuropsychological, 3T and 7T MRI, and pain assessments before (timepoint at baseline: T0) and every 2 months (2, 4, and 6 months; timepoints T2, T4, and T6) after being randomized into an intervention group (IG) or control group (CG) (Figure 1).
FIGURE 1.
Flow diagram of the study showing examinations at each time point (T0, T2, T4, and T6). 3T, 3 Tesla; MRI, magnetic resonance imaging; CG, control group; IG, intervention group. Figure was created with BioRender.com.
To exclude major physical health risks for subsequent exercise tests and trainings, a medical healthcare check was done before inclusion in the study in all subjects. This included an anamnestic questionnaire, auscultation of the lungs and heart, and a 12-channel resting electrocardiogram. To test the influence of an extensive interval training over 6 months on brain perfusion, all subjects (CG and IG) performed a maximal graded exercise test on the treadmill to determine physical fitness. Subjects were not allowed to perform strenuous exercise or drink alcohol 24 h before the examination day and were instructed to refrain from caffeine (and for exercise testing: food) at least 2 h before the testing sessions.
Incremental exercise test
The incremental exercise test was performed on a treadmill (PPS S70, Woodway GmBH, Weil am Rhein, Germany) with an initial running speed of 6 km h–1 and an incline set to 1%. Running speed increased in 1 km h–1 increments every 3 min until voluntary exhaustion to determine maximal oxygen uptake (VO2max) and maximal heart rate (HRmax). Oxygen uptake (VO2), metabolic respiratory quotient (respiratory exchange ratio, RER), and heart rate (HR) were continuously measured during the test (Cortex meta-analyzer 3B, Leipzig, Germany, and Polar Electro A360, Kempele, Finland). Capillary blood lactate samples were taken from the fingertip in the last 15 s of each step. To determine lactate concentration, 20 μl of capillary blood was directly mixed with 1 mL of the EBIO plus system hemolysis solution and analyzed amperometric-enzymatically using EBIOplus (EKF Diagnostic Sales, Magdeburg, Germany). At the same time points, the rating of perceived exertion (RPE) was assessed using the 6–20 points Borg scale (Borg, 1970). Exhaustion was considered with the attainment of at least two of the following criteria: leveling off in VO2, RER ≥ 1.10, high levels of blood lactate (BLa) (≥8 mmol L–1), a perceived rate of exertion of ≥18, and/or a HR of ±10 bpm of age-predicted maximum (220 – age) (Midgley et al., 2007). The relative VO2max was defined as the highest 30 s moving average of VO2 divided by body mass (mL min–1 kg–1).
Endurance exercise intervention
Throughout the 6-month running intervention phase, subjects of the intervention group completed three running sessions per week lasting 25–45 min, consisting of two supervised exercise sessions on the treadmill, one in the laboratory and one at home on flat terrain. This resulted in a total of 78 training sessions of which 60.0 ± 11.1 (77 ± 14%) were performed on average. An activity tracker was used to track subjects’ training data of the home trainings in the IG and to rule out deliberate physical activity in the CG.
The endurance exercise training of the intervention group was designed as an extensive interval training with 3–5-min intervals at 75–80% of HRmax and 3–5 min of active recovery, with six to eight repetitions. To ensure a steady progression of physical adaptations, exercise intensity was adapted individually according to the results of each performance test (2 and 4 months after baseline) (Figure 1).
Subjects in the CG were instructed to stick to their usual lifestyle, refrain from any kind of exercise, and continue their normal dietary and physical activity practices throughout the study.
Magnetic resonance imaging acquisition
The magnetic resonance imaging (MRI) data were acquired using a 3T Siemens Magnetom Skyra scanner. Perfusion data was acquired using an in-house developed 3D pCASL sequence (Boland et al., 2018) with TR = 4,130 ms; TE = 21.50 ms; FOV = 224 mm; slice thickness = 3.5 mm; bolus length = 1,800 ms, post-labeling duration = 1,800 ms, and total duration = 5 min:48 s.
We also acquired structural data sets using a custom T1-weighted MPRAGE sequence (Brenner et al., 2014), which employs threefold parallel imaging acceleration with CAIPIRINHA (Breuer et al., 2006) and elliptical sampling (Bernstein et al., 2001). The sequence parameters were as follows: TR = 2,500 ms, TE = 5 ms, TI = 1,100 ms, flip angle = 7°; FOV = 256 mm; voxel size = 1 mm3 × 1 mm3 × 1 mm3, 192 slices, scan duration 2 min:53 s.
Data analysis
Physiological data
A linear mixed effect (LME) model was performed to investigate the fitness measure VO2max among the groups (IG and CG) at the different time points (T0, T2, T4, and T6), as well as their interaction (group × time). LME models allow to overcome the issue of missing data values across time points (Ibrahim and Molenberghs, 2009). In this model, we included age and sex as fixed effects and a random intercept to account for random individual-level effects. Post hoc comparisons were performed using an LME model with time as the categorical variable using the “emmeans” package (Searle et al., 1980). The above models were obtained from the package lme4 (Bates et al., 2015) in the open access platform R, version 4.1.0 (R Core Team, 2013).
Perfusion data
Pseudo-continuous arterial spin labeling data were analyzed using the standard Oxford ASL pipeline available in the FSL toolbox1 (Chappell et al., 2009). Voxel-wise CBF maps were created after correcting for motion and registering to the native gray matter and white matter segmentation maps. Additionally, partial volume corrections were performed to avoid the effect of overlapping CBF values between different tissues. Images were quantified using the default parameters recommended in ASL white paper (Alsop et al., 2015) with the following parameters: longitudinal relaxation time of blood (T1b) = 1,650 ms, longitudinal relaxation time of tissue (T1tissue) = 1,300 ms, gray matter (GM) threshold = 0.8. The resulting GM CBF maps were further normalized to the global mean GM perfusion to create relative GM CBF maps correcting for any time-point-related bias (Yang et al., 2020).
The statistical analysis of these relative GM CBF maps was performed on a voxel by voxel level, including all time points, using the linear mixed-effects model (3dLMEr) (Chen et al., 2013) implemented in the AFNI toolbox (AFNI version: AFNI_21.3.02; 3dLMEr version 0.1.4). Age and sex were included as fixed effects and a random intercept to account for random individual-level effects. Significance was considered at a cluster-defining threshold of p < 0.001 (uncorrected) and an FWE-corrected threshold of p < 0.05. Later, we performed post hoc tests using emmeans package (Searle et al., 2012) implemented in R (version 4.1.0, R Core Team, 2013) and the multivariate t-distribution method to adjust p-values. The degrees-of-freedom method was Kenward–Roger (Kenward and Roger, 1997). Results of post hoc tests are reported with Cohen’s d as the effect size (Cohen, 1992).
Furthermore, for regression analysis, the relationship between relative CBF and VO2max was assessed using the above-mentioned 3dLMEr model, with age and sex as covariates, and also using a cluster-defining threshold of p < 0.001 (uncorrected) and an FWE-corrected threshold of p < 0.05. The Pearson correlation coefficient was calculated by extracting the beta values from the corresponding clusters and implementing the correlation with VO2max in the open access platform R, version 4.1.0 (R Core Team, 2013). The Talairach atlas available in AFNI (Lancaster et al., 2000) was used for labeling the significant results. For reporting and visualization, the statistical maps were transformed into MNI space and overlaid onto the MNI template using MRIcroGL.2 Finally, we conducted a targeted analysis for a hippocampal region of interest (ROI) created using the Harvard-Oxford-subcortical structural atlas (Frazier et al., 2005). Following this, we applied the abovementioned LME model to assess changes over time within and between groups.
Results
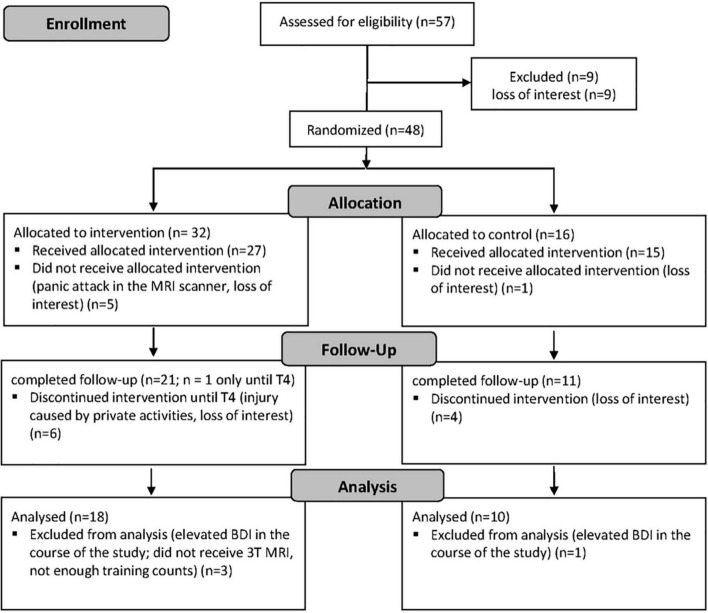
In total, 57 subjects with a sedentary lifestyle were assessed for eligibility after giving informed consent (Figure 2). Of these, nine were excluded due to loss of interest. Of the remaining 48 subjects, 32 were randomized to the intervention group (IG) and 16 to the control group (CG), respectively (randomization 2:1 IG and CG). Five of the subjects allocated to the IG did not receive the intervention due to panic attacks in the MRI or loss of interest, and another six subjects discontinued the intervention due to injury and loss of interest, leaving a total of 21 subjects in the IG who completed the follow-up. Of the 16 subjects allocated to the CG, one subject did not receive the intervention due to loss of interest, and four dropped out during the intervention due to loss of interest, leaving a total of 11 control subjects who completed the follow-up. After the final exclusion of three further subjects from the IG and one subject from the CG for reasons that became apparent during the intervention, the final dataset consisted of 28 subjects, including 18 in the IG and 10 in the CG. Subject characteristics are given in Table 1. According to the ACSM fitness categories VO2max values below 35.2 mL min–1 kg–1 in women and below 44.6 mL min–1 kg–1 in men are defined as poor and, respectively, fair physical fitness. Mean VO2max at T0 in all male subjects was 43.5 ± 3.7 mL min–1 kg–1 and 32.7 mL min–1 kg–1 in the female subjects.
FIGURE 2.
Flow chart diagram. T4, examination timepoint after 4 months.
TABLE 1.
Subject characteristics.
| Intervention group (n = 18) mean ± SD | Control group (n = 10) mean ± SD | P-value | |
| Age (years) | 23.9 ± 3.9 | 23.7 ± 4.2 | 0.80 |
| Gender (M/F) | 7/11 | 6/4 | 0.50 |
| Height (cm) | 173.6 ± 12.1 | 176.9 ± 7.9 | 0.45 |
| Weight (kg) | 69.9 ± 15.1 | 71.2 ± 14.1 | 0.82 |
| EHI | 74.2 ± 16.2 | 79.5 ± 13.3 | 0.39 |
| WST | 107.0 ± 9.9 | 107.3 ± 8.8 | 0.94 |
| VO2max (ml⋅min–1 kg–1) | |||
| T0 | 38.5 ± 3.4 | 41.7 ± 7.5 | 0.663 |
| T2 | 41.0 ± 4.1 | 39.1 ± 7.2 | 0.585 |
| T4 | 42.9 ± 5.4 | 41.1 ± 8.4 | 0.164 |
| T6 | 42.4 ± 5.0 | 40.3 ± 7.4 | 0.086 |
| HRrest (bpm) | |||
| T0 | 80.5 ± 14.2 | 75.6 ± 10.2 | 0.741 |
| T2 | 76.9 ± 8.4 | 71.4 ± 11.4 | 0.768 |
| T4 | 72.7 ± 10.9 | 80.8 ± 9.2 | 0.239 |
| T6 | 75.5 ± 11.9 | 72.1 ± 11.6 | 0.964 |
| BPDiastolic (mmHg) | |||
| T0 | 74.3 ± 7.3 | 76.0 ± 11.5 | 0.956 |
| T2 | 76.0 ± 6.4 | 68.3 ± 6.1 | 0.325 |
| T4 | 77.5 ± 7.1 | 71.6 ± 9.0 | 0.435 |
| T6 | 78.4 ± 9.6 | 74.0 ± 6.2 | 0.649 |
| BPsystolic (mmHg) | |||
| T0 | 118.8 ± 10.5 | 119.0 ± 13.6 | 0.999 |
| T2 | 118.0 ± 10.1 | 118.6 ± 12.5 | 0.999 |
| T4 | 115.8 ± 12.4 | 116.9 ± 8.4 | 0.966 |
| T6 | 117.0 ± 9.38 | 116.6 ± 11.8 | 0.999 |
| BDI | 2.6 ± 3.4a | 1.4 ± 1.5 | 0.300 |
| STAI trait | 33.9 ± 9.3 | 31.1 ± 5.8 | 0.400 |
aTwo missing values. bpm, beats per minute; BDI, Beck Depression Inventory 1 (score ≤9: no depression); cm, centimeter; EHI, Edinburgh Handedness Inventory; F, female; HRmax, maximum heart rate; kg, kilogram; M, male; min, minute; ml, milliliter; SD, standard deviation; STAI, State Trait Anxiety Inventory (range: 20 = not being afraid up to 80 = maximum intensity of anxiety); VO2max, maximum oxygen uptake; WST, Wortschatztest. p-value shows differences between groups.
Relative maximum oxygen uptake (VO2max)
The LME model with time as the random slope and random intercept revealed an increase in VO2max in the IG over time. The VO2max showed a significant time effect [F(1, 22.89) = 9.39, p = 0.006] and a significant group × time interaction [F(1, 22.85) = 29.67, p < 0.001]. There was no significant group effect [F(1, 22.47) = 0.42, p = 0.523]. Additionally, a significant effect of sex [F(1, 22.38) = 14.93, p < 0.001], driven by higher VO2max values in men than in women was found. Moreover, the covariate age showed no significant impact on the VO2max [F(1, 41.39) = 0.07, p = 0.787]. Results of post hoc tests within and between IG and CG at each time point are summarized in Table 2.
TABLE 2.
Results of post hoc test of the VO2max within and between IG and CG.
| Contrast | t | P-value | Cohen’s d |
| IG | |||
| T0 vs. T2 | −4.13 | <0.001 | −1.46 |
| T0 vs. T4 | −7.37 | <0.001 | −2.61 |
| T0 vs. T6 | −6.75 | <0.001 | −2.50 |
| CG | |||
| T0 vs. T2 | 1.09 | 0.697 | −0.55 |
| T0 vs. T4 | 0.84 | 0.834 | −0.39 |
| T0 vs. T6 | 1.94 | 0.219 | −0.87 |
| IG vs. CG | |||
| T0 | −0.78 | 0.682 | −0.90 |
| T2 | 0.94 | 0.570 | 1.11 |
| T4 | 1.80 | 0.158 | 2.09 |
| T6 | 2.11 | 0.089 | 2.46 |
Post hoc tests comparing different timepoints within group (intervention or control) and comparisons between group for each timepoint (intervention vs. control). All results were controlled for age and sex. CG, control group (n = 10); IG, intervention group [(n = 18) n = 16 at T6]; t, t-statistic; T0, T2, T4, and T6, examination timepoint after 0, 2, 4, and 6 months; VO2max, maximum oxygen uptake.
Comparison of the relative gray matter perfusion
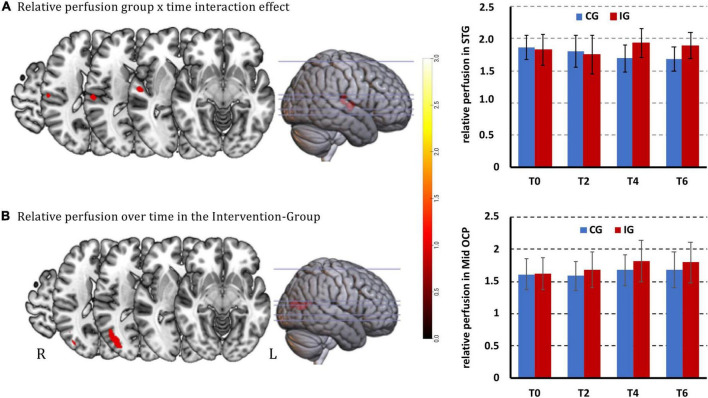
Analysis using the 3dLMEr model revealed a significant interaction effect (group × time; cluster size > 41 voxels) for relative GM perfusion in the right superior temporal gyrus (STG) [peak voxel (MNI) at x, y, z = 56.2, −14.9, 7.9]. This interaction was driven by a relative perfusion increase in the IG and a relative perfusion decrease in the CG over time (Figure 3A). Furthermore, a significant time effect was observed in the right middle occipital region (x, y, z = 38.5, −73.9, 13.8) in the IG group, namely an increase in relative perfusion (Figure 3B). The CG did not show significant changes over time in the 3dLMEr model.
FIGURE 3.
Significant relative perfusion changes [p < 0.001 (uncorrected), cluster level corrected at alpha = 0.05 with cluster size > 41 voxel for cluster-wise FWE]. (A) Increased group × time interaction effect in the right superior temporal gyrus and bar plots of beta values with standard deviations; (B) increased relative perfusion over time in the IG in the right middle occipital region. Clusters are overlaid on the structural MNI template of the brain and bar plots of beta values with standard deviations. The color bar from dark red to light yellow indicates the increasing relative GM CBF values.
Post hoc paired t-tests of the perfusion changes in right STG over time within the IG revealed a strong trend (after correction for multiple comparisons) for perfusion increase from T0 to T4, but no significance between T0 and T2 and T0 to T6. Post hoc paired t-tests in CG showed a significant perfusion decrease from T0 to T4 and from T0 to T6, but no significance between T0 and T2. Further, group comparisons showed significantly higher perfusion in the IG group at T4 and T6, but no significant difference at T0 and T2 when compared to the CG.
Post hoc paired t-test of the perfusion changes in the right middle occipital region showed significant increases from T0 to T4 and from T0 to T6 in the IG.
All detailed statistics are summarized in Table 3.
TABLE 3.
Results of post hoc tests of the relative gray matter (GM) perfusion analysis.
| Contrast | t | P-value | Cohen’s d |
| Right superior temporal gyrus | |||
| IG | |||
| T0 vs. T2 | 1.648 | 0.358 | 0.549 |
| T0 vs. T4 | −2.551 | 0.06 | −0.85 |
| T0 vs. T6 | 1.807 | 0.278 | −0.626 |
| CG | |||
| T0 vs. T2 | 1.06 | 0.715 | 0.474 |
| T0 vs. T4 | 2.939 | 0.022 | 1.314 |
| T0 vs. T6 | 3.048 | 0.016 | 1.363 |
| IG vs. CG | |||
| T0 | −0.159 | 0.999 | 0.11 |
| T2 | −0.268 | 0.994 | 0.185 |
| T4 | 2.972 | 0.015 | 2.054 |
| T6 | 2.692 | 0.03 | 1.879 |
| Right_middle occipital | |||
| IG | |||
| T0 vs. T2 | −1.129 | 0.673 | −0.376 |
| T0 vs. T4 | −4.343 | <0.001 | −1.448 |
| T0 vs. T6 | −4.134 | <0.001 | −1.434 |
| CG | |||
| T0 vs. T2 | 0.456 | 0.968 | 0.204 |
| T0 vs. T4 | –1.095 | 0.693 | –0.490 |
| T0 vs. T6 | –1.126 | 0.674 | -0.504 |
| IG vs. CG | |||
| T0 | –0.336 | 0.976 | –0.295 |
| T2 | –0.998 | 0.580 | –0.874 |
| T4 | –1.429 | 0.322 | –1.252 |
| T6 | –1.389 | 0.343 | –1.224 |
Post hoc tests comparing different timepoints within group (intervention or control) and comparisons between group for each timepoint (intervention vs. control). All results were controlled for age and sex. CG, control group (n = 10); IG, intervention group [(n = 18) n = 16 at T6]; t, t-statistic; T0, T2, T4, and T6, examination timepoint after 0, 2, 4, and 6 months.
Regression analysis
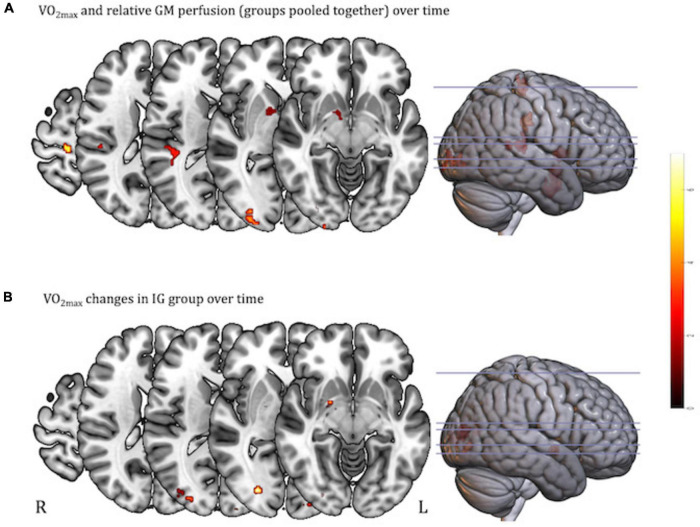
After pooling all subjects and time-points, regression analysis revealed that higher VO2max was associated with higher relative GM CBF in the right STG (x, y, z = 43, −31.9, 13.0; Supplementary Figure 1), the right middle occipital region (x, y, z = 30.4, −95.3, −1.0; Supplementary Figure 1), the right precentral gyrus (x, y, z = 15.7, −28.9, 66.9; Supplementary Figure 1), and the right ventral striatum (x, y, z = 12.7, 5.0, 4.2; Supplementary Figure 1), including the right lentiform nucleus (cluster size > 42 voxels; Figure 4A). To assess the contribution of each group separately, post hoc analysis assessed the relationship between VO2max and relative CBF in the IG and CG separately. Therefore, the regression was restricted to those areas showing significance in the above regression analysis (at an inclusive masking threshold of p < 0.01, uncorrected) of both groups pooled. Only the IG showed a significant relation between VO2max and relative GM CBF located in the right middle occipital region (x, y, z = 29, −87.9, 12.3; Supplementary Figure 1) and the left middle occipital region (x, y, z = −43.3, −73.9, 14.5; Supplementary Figure 1), as well as a trend in the right ventral striatum (x, y, z = 20.1, −2.4, −4.7; r = 0.19; p < 0.005 uncorrected, >10 voxel; Figure 4B and Supplementary Figure 1).
FIGURE 4.
Regression analysis between VO2max and relative gray matter (GM) perfusion using the 3dlmer model in AFNI [p < 0.001 (uncorrected), cluster corrected at alpha = 0.05 with cluster size (>42 voxel) for FWE]. (A) Higher VO2max associated with higher relative GM CBF is shown in right STG, right middle occipital region, right precentral gyrus, and right ventral striatum (groups pooled together); (B) regression analysis for IG only showing higher VO2max associated with higher relative GM CBF in the bilateral middle occipital region, as well as a trend in the right ventral striatum (displayed at the more liberal threshold of p < 0.005, uncorrected, >10 voxel). Clusters are overlaid on the structural MNI template of the brain. The color bar from dark red to light yellow indicates the increasing strength of the correlation between VO2max and relative GM perfusion values.
No significant changes were observed in relative CBF in the left or right hippocampus ROI. Neither group × time interaction nor the group and time main effects were significant.
Discussion
This randomized exercise intervention study provides novel evidence that 6 months of extensive interval training (three weekly training sessions of 25–45 min each) over a 6-month period leads to significant increases in regional cortical perfusion in young and previously sedentary adults. Specifically, an interaction effect between group and time was observed in the right STG, which was driven by an increase in perfusion over time in the IG and a significant decrease within the CG. These effects were significantly different between groups after 4 and 6 months of PE training. In addition, the perfusion increase in the right STG was significantly associated with the extent of improvement in physical performance (individual changes in VO2max), suggesting dose-dependent training effects on cerebral perfusion. Furthermore, positive associations between fitness changes and local perfusion enhancements were observed in the bilateral occipital lobe, the right precentral gyrus, and the ventral striatum.
To our knowledge, this is the first randomized intervention investigating long-term PE-induced perfusion changes as a marker of vascular plasticity in healthy young adults. As yet, the existing intervention studies reporting perfusion changes related to long-term exercise had been conducted exclusively in older healthy cohorts (Burdette et al., 2010; Chapman et al., 2013; Flodin et al., 2017; Kleinloog et al., 2019) or in patient populations (Robertson et al., 2017; Alfini et al., 2019; Thomas et al., 2020).
The temporal lobe is typically affected by age-related atrophy (Jack et al., 1998) at the levels of the cortex, the hippocampus, the temporal horn, and the white matter (Bigler et al., 2002). Temporal lobe atrophy is thought to contribute to the cognitive impairments in memory that occur in older age (Burke and Barnes, 2006). Results of enhanced perfusion due to PE point to a recent cross-sectional study in which VO2max was positively associated with cortical thickness in the left STG (Olivo et al., 2021). On a meta-analytic level, findings on structural plasticity in the STG associated with physical activity or exercise have been reported using the activation likelihood estimation (ALE) method, where 412 healthy older subjects pooled from nine randomized controlled trials showed significant structural increases in this area (Zheng et al., 2019). Notably, our study showed an association between temporal lobe perfusion and VO2max.
We would like to speculate that the interaction effect in the right STG is indeed related to the exercise intervention and the induction of vascular plasticity in the IG only. A cross-sectional MR angiography study found elevated small-caliber vessels in the cortex of aerobically active compared to low-activity healthy subjects (Bullitt et al., 2009). The same study also reported reduced brain-vessel tortuosity in the aerobically active subjects. This is in line with our interpretation that the observed interaction effect could stem from such fundamental effects on the cerebral perfusion driven by presence or absence of regular physical activity as brain-vessel tortuosity decreases cerebral flow.
Given the short duration of the study phase (6 months) and the young age of our participants, we consider it rather unlikely that the decrease in perfusion observed in the CG is caused by a veritable age-related decline. A possible factor to be considered could be lipid metabolism. It is known that a sedentary life-style correlates positively with increased LDL–cholesterol levels (Crichton and Alkerwi, 2015). In turn, higher levels of cholesterol were shown to be associated with decreased regional perfusion in the superior temporal lobe (Claus et al., 1998). Unfortunately, we have no measures to verify these putative mechanisms and this will need to be assessed in future randomized exercise interventions.
Outside the temporal lobe, we observed that higher perfusion in the precentral gyrus correlated positively with individual VO2max. The results suggest vascular plasticity of the motor system in an anatomically restricted area that is precisely confined to the relevant leg representation of the primary motor cortex. The results are consistent with the concepts of promoting motor function through movement, as proposed by Perrey (2013). More recently, somatotopically restricted changes in the leg representation of the primary motor cortex after exercise have been described using quantitative longitudinal relaxation rate (R1) MRI, where R1 reflects cortical myelin density (Rowley et al., 2020). These data from healthy older adults (age range of 65–90 years) who exercised three times per week or maintained their usual physical routine over a 12-week period suggest plasticity at the microstructural level. Similar to our results, the effects correlated with a marker of improvement in submaximal aerobic performance (Rowley et al., 2020). Our results are also consistent with work in adult rats trained with the running wheel for 30 days, indicating capillary growth in motor areas of the cerebral cortex as an adaptation to sustained motor activity (Swain et al., 2003). In addition to capillary growth, the vasculature showed increased flow under conditions of activation (Swain et al., 2003). In endurance runners, greater gray matter volume and cortical surface area were reported in the left precentral gyrus compared to controls (Cao et al., 2021). Thus, bringing these non-human and human data together, they imply PE-induced locoregional plasticity in the primary motor cortex and suggest that perfusion is increased to meet increased energy demand as a long-term effect of PE.
Furthermore, bilateral occipital lobe perfusion correlated positively with VO2max. The occipital lobe was described in previous cross-sectional work comparing sedentary (10 men and 16 women, 54 ± 1 years) and endurance-trained (11 men and 21 women, 52 ± 1 years) middle-aged adults. Less carotid artery stiffness was associated with better neuropsychological outcomes, independent of age, sex, and education, and correlated with greater occipitoparietal blood flow (Tarumi et al., 2013).
Finally, we observed vascular plasticity in areas related to exercise-induced reward mechanisms. The association between VO2max and ventral striatal perfusion in the IG is a novel finding, as this region has not typically been related to fitness-related outcomes. The ventral striatum, and especially dopaminergic signaling within the nucleus accumbens, has traditionally been associated with reward-related functioning, for example, motivational energization of behavior and reinforcement learning processes (Haber and Knutson, 2010; Salamone and Correa, 2012). In line with this reasoning, clinical studies observed that reductions in ventral striatal blood flow were correlated with apathy in psychiatric patients (Schneider et al., 2019). In this context, it is interesting that a recent observational study in N = 111 young adult women (Gorrell et al., 2022) found positive correlations between self-reported exercise engagement energization and brain responses during BOLD fMRI reward paradigms in the medial orbitofrontal cortex and, slightly weaker, in the ventral striatum. It is tempting to speculate whether regular engagement in vigorous physical exercise training that effectively increases VO2max is also linked with upregulations in the motivational circuits of the brain, which would be consistent with findings from animal literature (Ruiz-Tejada et al., 2022), but needs more systematic research in humans. Consistent with our temporal and ventral striatal findings, Robinson et al. (2018) reported elevated resting brain glucose uptake in young and old healthy adults measured using 18F-fluorodeoxyglucose positron emission tomography after 12 weeks of high-intensity interval training (HIIT) training in parietal–temporal and caudate regions.
The present study convincingly shows that cortical perfusion increases can also be achieved by training interventions in young sedentary adults. We did not observe training-induced changes in hippocampal perfusion or associations between fitness and hippocampal CBF, as reported by previous studies in children (Chaddock-Heyman et al., 2016) and elderly populations (Burdette et al., 2010; Maass et al., 2015). This may have methodological reasons, but could also reflect developmental differences (Aghjayan et al., 2021), i.e., training effects on resting-state perfusion may be stronger during more dynamic phases of brain development and aging (or neurodegeneration). The available evidence from randomized controlled trials does suggest age-related differences regarding mechanisms and effects of exercise on brain health and cognition (reviewed in Stillman et al., 2020), although there are substantial gaps, especially in the research with young populations that preclude firm conclusions.
This study is not without limitations. We must acknowledge that the small sample size, particularly of the control cohort, may have limited the power of this study. Future studies should attempt to recruit larger samples of control subjects. Furthermore, the inactive control group, receiving the recommendation to maintain their usual (e.g., inactive) habits, might be a limitation of the study, as the missing attention, social contact, or light (physical) activities could have beneficial effects by themselves and may improve compliance. Moreover, we have to acknowledge that we did not quantify the entire daily physical activity in the CG. Rather, the activity tracker was used to rule out any deliberate physical activity violating the volunteer’s assignment to a passive control condition over a 6-month period. Ideally, future exercise studies should perform pCASL examinations at the same time of the day to rule out potential confounds due to diurnal variations of cerebral perfusion. For an in-depth review of additional external factors which may induce variation in cerebral blood flow measurements, empirical evidence for or against their significance and practical measures to minimize their influence, see Clement et al. (2018).
In summary, our randomized exercise intervention in sedentary young adults yielded region-specific increases in local perfusion that extend previous data from cross-sectional studies and provide a new database for younger cohorts. Interestingly, vascular plasticity in temporal, ventral striatal, and motor areas correlated with individual performance increases, suggesting a dependence on the exercise dose. These robust associations found with perfusion metrics are consistent with the literature showing positive effects of a physically active lifestyle on vascular health. The presented data support further efforts in initiating and maintaining physical exercise training in sedentary young individuals, as this might potentiate long-term beneficial health outcomes.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethikkommission an der Medizinischen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn. The patients/participants provided their written informed consent to participate in this study.
Author contributions
NU and TSc performed statistical analysis, interpreted the data, and drafted and edited the manuscript. AM was involved in all aspects of project administration, investigation, and supervision, as well as reviewed and edited the manuscript. JC performed statistical analysis. LS and MD were involved in the conception and investigation of the project, reviewed, and edited the manuscript. JM was involved in the conception and investigation of the project, supervised the physical training and exercise tests, reviewed, and edited the manuscript. RS and TSt developed and supervised the in-house pCASL sequence, reviewed, and edited the manuscript. AR and UA contributed to provision of the infrastructure and critically revised the manuscript. HB was involved in the conception of the study and all aspects of the investigation, drafted, edited, and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Footnotes
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.951022/full#supplementary-material
Regression analyses between VO2max and relative perfusion of (A) right superior temporal gyrus (rSTG), (B) right middle occipital region (rMOR), (C) right precentral gyrus (rPG), (D) right ventral striatum including the right lentiform nucleus (rVS), (E) rMOR, (F) left middle occipital region (lMOR), and (G) rVS. Part (A–D) show regressions pooled over all subjects (intervention group and control group) and all timepoints (T0, T2, T4, and T6), while (E–G) show regressions of all timepoints within the intervention group only.
References
- Aghjayan S. L., Lesnovskaya A., Esteban-Cornejo I., Peven J. C., Stillman C. M., Erickson K. I. (2021). Aerobic exercise, cardiorespiratory fitness, and the human hippocampus. Hippocampus 31 817–844. 10.1002/hipo.23337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfini A. J., Weiss L. R., Nielson K. A., Verber M. D., Smith J. C. (2019). Resting cerebral blood flow after exercise training in mild cognitive impairment. J. Alzheimers Dis. 67 671–684. 10.3233/JAD-180728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop D. C., Detre J. A., Golay X., Gunther M., Hendrikse J., Hernandez-Garcia L., et al. (2015). Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 73 102–116. 10.1002/mrm.25197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Machler M., Bolker B. M., Walker S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bernstein M. A., Fain S. B., Riederer S. J. (2001). Effect of windowing and zero-filled reconstruction of MRI data on spatial resolution and acquisition strategy. J. Magn. Reson. Imaging 14 270–280. 10.1002/jmri.1183 [DOI] [PubMed] [Google Scholar]
- Bigler E. D., Anderson C. V., Blatter D. D. (2002). Temporal lobe morphology in normal aging and traumatic brain injury. AJNR Am. J. Neuroradiol. 23 255–266. [PMC free article] [PubMed] [Google Scholar]
- Boland M., Stirnberg R., Pracht E. D., Kramme J., Viviani R., Stingl J., et al. (2018). Accelerated 3D-GRASE imaging improves quantitative multiple post labeling delay arterial spin labeling. Magn. Reson. Med. 80 2475–2484. 10.1002/mrm.27226 [DOI] [PubMed] [Google Scholar]
- Booth F. W., Roberts C. K., Laye M. J. (2012). Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2 1143–1211. 10.1002/cphy.c110025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G. (1970). Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 2, 92-98. [PubMed] [Google Scholar]
- Brenner D., Stirnberg R., Pracht E. D., Stocker T. (2014). Two-dimensional accelerated MP-RAGE imaging with flexible linear reordering. MAGMA 27 455–462. 10.1007/s10334-014-0430-y [DOI] [PubMed] [Google Scholar]
- Breuer F. A., Blaimer M., Mueller M. F., Seiberlich N., Heidemann R. M., Griswold M. A., et al. (2006). Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn. Reson. Med. 55 549–556. 10.1002/mrm.20787 [DOI] [PubMed] [Google Scholar]
- Bull F. C., Al-Ansari S. S., Biddle S., Borodulin K., Buman M. P., Cardon G., et al. (2020). World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 54 1451–1462. 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullitt E., Rahman F. N., Smith J. K., Kim E., Zeng D., Katz L. M., et al. (2009). The effect of exercise on the cerebral vasculature of healthy aged subjects as visualized by MR angiography. AJNR Am. J. Neuroradiol. 30 1857–1863. 10.3174/ajnr.A1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette J. H., Laurienti P. J., Espeland M. A., Morgan A., Telesford Q., Vechlekar C. D., et al. (2010). Using network science to evaluate exercise-associated brain changes in older adults. Front. Aging Neurosci. 2:23. 10.3389/fnagi.2010.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke S. N., Barnes C. A. (2006). Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 7 30–40. 10.1038/nrn1809 [DOI] [PubMed] [Google Scholar]
- Cao L., Zhang Y., Huang R., Li L., Xia F., Zou L., et al. (2021). Structural and functional brain signatures of endurance runners. Brain Struct. Funct. 226 93–103. 10.1007/s00429-020-02170-y [DOI] [PubMed] [Google Scholar]
- Chaddock-Heyman L., Erickson K. I., Chappell M. A., Johnson C. L., Kienzler C., Knecht A., et al. (2016). Aerobic fitness is associated with greater hippocampal cerebral blood flow in children. Dev. Cogn. Neurosci. 20 52–58. 10.1016/j.dcn.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S. B., Aslan S., Spence J. S., Defina L. F., Keebler M. W., Didehbani N., et al. (2013). Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front. Aging Neurosci. 5:75. 10.3389/fnagi.2013.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell M. A., Groves A. R., Whitcher B., Woolrich M. W. (2009). Variational bayesian inference for a nonlinear forward model. IEEE Trans. Signal Process. 57 223–236. 10.1109/TSP.2008.2005752 [DOI] [Google Scholar]
- Chen G., Saad Z. S., Britton J. C., Pine D. S., Cox R. W. (2013). Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage 73 176–190. 10.1016/j.neuroimage.2013.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. J., Jann K., Wang D. J. (2015). Characterizing resting-state brain function using arterial spin labeling. Brain Connect 5 527–542. 10.1089/brain.2015.0344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus J. J., Breteler M. M., Hasan D., Krenning E. P., Bots M. L., Grobbee D. E., et al. (1998). Regional cerebral blood flow and cerebrovascular risk factors in the elderly population. Neurobiol. Aging 19 57–64. 10.1016/S0197-4580(98)00004-9 [DOI] [PubMed] [Google Scholar]
- Clement P., Mutsaerts H. J., Vaclavu L., Ghariq E., Pizzini F. B., Smits M., et al. (2018). Variability of physiological brain perfusion in healthy subjects – A systematic review of modifiers. Considerations for multi-center ASL studies. J. Cereb. Blood Flow Metab. 38 1418–1437. 10.1177/0271678X17702156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1992). A power primer. Psychol. Bull. 112 155–159. 10.1037//0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- Crichton G. E., Alkerwi A. (2015). Physical activity, sedentary behavior time and lipid levels in the observation of cardiovascular risk factors in luxembourg study. Lipids Health Dis. 14:87. 10.1186/s12944-015-0085-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egginton S. (2009). Invited review: Activity-induced angiogenesis. Pflugers Arch. 457 963–977. 10.1007/s00424-008-0563-9 [DOI] [PubMed] [Google Scholar]
- Erickson K. I., Voss M. W., Prakash R. S., Basak C., Szabo A., Chaddock L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U.S.A. 108 3017–3022. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flodin P., Jonasson L. S., Riklund K., Nyberg L., Boraxbekk C. J. (2017). Does aerobic exercise influence intrinsic brain activity? An aerobic exercise intervention among healthy old adults. Front. Aging Neurosci. 9:267. 10.3389/fnagi.2017.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier J. A., Chiu S., Breeze J. L., Makris N., Lange N., Kennedy D. N., et al. (2005). Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am. J. Psychiatry 162 1256–1265. 10.1176/appi.ajp.162.7.1256 [DOI] [PubMed] [Google Scholar]
- Garber C. E., Blissmer B., Deschenes M. R., Franklin B. A., Lamonte M. J., Lee I. M., et al. (2011). American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 43 1334–1359. 10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- Gorrell S., Shott M. E., Frank G. K. W. (2022). Associations between aerobic exercise and dopamine-related reward-processing: Informing a model of human exercise engagement. Biol. Psychol. 171:108350. 10.1016/j.biopsycho.2022.108350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S. N., Knutson B. (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35 4–26. 10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautzinger M., Bailer M., Worall H., Keller F. (1994). Beck Depressionsinventar [Beck Depression Inventory]. Bern: Huber. [Google Scholar]
- Hillman C. H., Erickson K. I., Kramer A. F. (2008). Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 9 58–65. 10.1038/nrn2298 [DOI] [PubMed] [Google Scholar]
- Ibrahim J. G., Molenberghs G. (2009). Missing data methods in longitudinal studies: A review. Test 18 1–43. 10.1007/s11749-009-0138-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C. R., Jr., Petersen R. C., Xu Y., O’brien P. C., Smith G. E., Ivnik R. J., et al. (1998). Rate of medial temporal lobe atrophy in typical aging and Alzheimer’s disease. Neurology 51 993–999. 10.1212/wnl.51.4.993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L., Steffens D. C., Wang L. (2021). Effects of physical exercise on the aging brain across imaging modalities: A meta-analysis of neuroimaging studies in randomized controlled trials. Int. J. Geriatr. Psychiatry 36 1148–1157. 10.1002/gps.5510 [DOI] [PubMed] [Google Scholar]
- Kenward M. G., Roger J. H. (1997). Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53 983–997. 10.2307/2533558 [DOI] [PubMed] [Google Scholar]
- Kleinloog J. P. D., Mensink R. P., Ivanov D., Adam J. J., Uludag K., Joris P. J. (2019). Aerobic exercise training improves cerebral blood flow and executive function: A randomized, controlled cross-over trial in sedentary older men. Front. Aging Neurosci. 11:333. 10.3389/fnagi.2019.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J. L., Woldorff M. G., Parsons L. M., Liotti M., Freitas C. S., Rainey L., et al. (2000). Automated talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 10 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann N., Villringer A., Taubert M. (2020). Colocalized white matter plasticity and increased cerebral blood flow mediate the beneficial effect of cardiovascular exercise on long-term motor learning. J. Neurosci. 40 2416–2429. 10.1523/JNEUROSCI.2310-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Zhang X., Guo J., Roberts C. K., Mckenzie S., Wu W. C., et al. (2015). Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 4:e002014. 10.1161/JAHA.115.002014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A., Duzel S., Goerke M., Becke A., Sobieray U., Neumann K., et al. (2015). Vascular hippocampal plasticity after aerobic exercise in older adults. Mol. Psychiatry 20 585–593. 10.1038/mp.2014.114 [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Arumugam T. V. (2018). Hallmarks of brain aging: Adaptive and pathological modification by metabolic states. Cell Metab. 27 1176–1199. 10.1016/j.cmet.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer A., Deckert S., Levenig C., Schorkmaier T., Stangier C., Attenberger U., et al. (2020). Body image relates to exercise-induced antinociception and mood changes in young adults: A randomized longitudinal exercise intervention. Int. J. Environ. Res. Public Health 17:6801. 10.3390/ijerph17186801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley A. W., Mcnaughton L. R., Polman R., Marchant D. (2007). Criteria for determination of maximal oxygen uptake: A brief critique and recommendations for future research. Sports Med. 37 1019–1028. 10.2165/00007256-200737120-00002 [DOI] [PubMed] [Google Scholar]
- Northey J. M., Rattray B., Pumpa K. L., Pryor D. J., Fraser M. A., Shaw M. E., et al. (2020). Objectively measured physical activity is associated with dorsolateral prefrontal cortex volume in older adults. Neuroimage 221 117150. 10.1016/j.neuroimage.2020.117150 [DOI] [PubMed] [Google Scholar]
- Ogoh S., Ainslie P. N. (2009). Regulatory mechanisms of cerebral blood flow during exercise: New concepts. Exerc. Sport Sci. Rev. 37, 123–129. 10.1097/JES.0b013e3181aa64d7 [DOI] [PubMed] [Google Scholar]
- Olivo G., Nilsson J., Garzon B., Lebedev A., Wahlin A., Tarassova O., et al. (2021). Higher VO2max is associated with thicker cortex and lower grey matter blood flow in older adults. Sci. Rep. 11:16724. 10.1038/s41598-021-96138-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega F. B., Ruiz J. R., Castillo M. J., Sjostrom M. (2008). Physical fitness in childhood and adolescence: A powerful marker of health. Int. J. Obes. 32 1–11. [DOI] [PubMed] [Google Scholar]
- Pereira A. C., Huddleston D. E., Brickman A. M., Sosunov A. A., Hen R., Mckhann G. M., et al. (2007). An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. U.S.A. 104 5638–5643. 10.1073/pnas.0611721104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrey S. (2013). Promoting motor function by exercising the brain. Brain Sci. 3 101–122. 10.3390/brainsci3010101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2013). R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Robertson A. D., Marzolini S., Middleton L. E., Basile V. S., Oh P. I., Macintosh B. J. (2017). Exercise training increases parietal lobe cerebral blood flow in chronic stroke: An observational study. Front. Aging Neurosci. 9:318. 10.3389/fnagi.2017.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. M., Lowe V. J., Nair K. S. (2018). Increased brain glucose uptake after 12 weeks of aerobic high-intensity interval training in young and older adults. J. Clin. Endocrinol. Metab. 103 221–227. 10.1210/jc.2017-01571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley C. D., Bock N. A., Deichmann R., Engeroff T., Hattingen E., Hellweg R., et al. (2020). Exercise and microstructural changes in the motor cortex of older adults. Eur. J. Neurosci. 51 1711–1722. [DOI] [PubMed] [Google Scholar]
- Ruiz-Tejada A., Neisewander J., Katsanos C. S. (2022). Regulation of voluntary physical activity behavior: A review of evidence involving dopaminergic pathways in the brain. Brain Sci 12:333. 10.3390/brainsci12030333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone J. D., Correa M. (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron 76 470–485. 10.1016/j.neuron.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K., Michels L., Hartmann-Riemer M. N., Burrer A., Tobler P. N., Stampfli P., et al. (2019). Cerebral blood flow in striatal regions is associated with apathy in patients with schizophrenia. J. Psychiatry Neurosci. 44 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle S. R., Speed F. M., Milliken G. A. (1980). Population marginal means in the linear-model – an alternative to least-squares means. Am. Stat. 34 216–221. [Google Scholar]
- Searle S. R., Speed F. M., Milliken G. A. (2012). Population marginal means in the linear model: An alternative to least squares means. Am. Stat. 34 216–221. [Google Scholar]
- Sheehan D. V., Lecrubier Y., Sheehan K. H., Amorim P., Janavs J., Weiller E., et al. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59(Suppl. 20), 22–33;quiz34–57. [PubMed] [Google Scholar]
- Smith K. J., Ainslie P. N. (2017). Regulation of cerebral blood flow and metabolism during exercise. Exp. Physiol. 102 1356–1371. [DOI] [PubMed] [Google Scholar]
- Spielberger C., Goruch R., Lushene R., Vagg P., Jacobs G. (1983). Manual for the state-trait inventory STAI (form Y). Palo Alto, CA: Mind Garden. [Google Scholar]
- Steinberg S. I., Sammel M. D., Harel B. T., Schembri A., Policastro C., Bogner H. R., et al. (2015). Exercise, sedentary pastimes, and cognitive performance in healthy older adults. Am. J. Alzheimers Dis. Other Demen. 30 290–298. 10.1177/1533317514545615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman C. M., Esteban-Cornejo I., Brown B., Bender C. M., Erickson K. I. (2020). Effects of exercise on brain and cognition across age groups and health states. Trends Neurosci. 43 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain R. A., Harris A. B., Wiener E. C., Dutka M. V., Morris H. D., Theien B. E., et al. (2003). Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience 117 1037–1046. 10.1016/s0306-4522(02)00664-4 [DOI] [PubMed] [Google Scholar]
- Tari B., Vanhie J. J., Belfry G. R., Shoemaker J. K., Heath M. (2020). Increased cerebral blood flow supports a single-bout postexercise benefit to executive function: Evidence from hypercapnia. J. Neurophysiol. 124 930–940. 10.1152/jn.00240.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarumi T., Zhang R. (2015). The role of exercise-induced cardiovascular adaptation in brain health. Exerc. Sport Sci. Rev. 43 181–189. 10.1249/JES.0000000000000063 [DOI] [PubMed] [Google Scholar]
- Tarumi T., Gonzales M. M., Fallow B., Nualnim N., Pyron M., Tanaka H., et al. (2013). Central artery stiffness, neuropsychological function, and cerebral perfusion in sedentary and endurance-trained middle-aged adults. J. Hypertens. 31 2400–2409. 10.1097/HJH.0b013e328364decc [DOI] [PubMed] [Google Scholar]
- Thomas B. P., Tarumi T., Sheng M., Tseng B., Womack K. B., Cullum C. M., et al. (2020). Brain perfusion change in patients with mild cognitive impairment after 12 months of aerobic exercise training. J. Alzheimers Dis. 75 617–631. 10.3233/JAD-190977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C., Van Praag H. (2017). Running changes the brain: The long and the short of it. Physiology 32 410–424. 10.1152/physiol.00017.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts M. E., Pocock R., Claudianos C. (2018). Brain energy and oxygen metabolism: Emerging role in normal function and disease. Front. Mol. Neurosci. 11:216. 10.3389/fnmol.2018.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C. P., Wai J. P., Tsai M. K., Yang Y. C., Cheng T. Y., Lee M. C., et al. (2011). Minimum amount of physical activity for reduced mortality and extended life expectancy: A prospective cohort study. Lancet 378 1244–1253. 10.1016/S0140-6736(11)60749-6 [DOI] [PubMed] [Google Scholar]
- Yang W., Yang R., Tang F., Luo J., Zhang J., Chen C., et al. (2020). Decreased relative cerebral blood flow in unmedicated heroin-dependent individuals. Front. Psychiatry 11:643. 10.3389/fpsyt.2020.00643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Herold F., Becker B., Klugah-Brown B., Zhang Y., Perrey S., et al. (2021). Cognitive benefits of exercise interventions: An fMRI activation likelihood estimation meta-analysis. Brain Struct. Funct. 226 601–619. 10.1007/s00429-021-02247-2 [DOI] [PubMed] [Google Scholar]
- Zheng G., Ye B., Zheng Y., Xiong Z., Xia R., Qiu P., et al. (2019). The effects of exercise on the structure of cognitive related brain regions: A meta-analysis of functional neuroimaging data. Int. J. Neurosci. 129 406–415. 10.1080/00207454.2018.1508135 [DOI] [PubMed] [Google Scholar]
- Zhu L., Yu Q., Herold F., Cheval B., Dong X., Cui L., et al. (2021). Brain structure, cardiorespiratory fitness, and executive control changes after a 9-week exercise intervention in young adults: A randomized controlled trial. Life 11:292. 10.3390/life11040292 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Regression analyses between VO2max and relative perfusion of (A) right superior temporal gyrus (rSTG), (B) right middle occipital region (rMOR), (C) right precentral gyrus (rPG), (D) right ventral striatum including the right lentiform nucleus (rVS), (E) rMOR, (F) left middle occipital region (lMOR), and (G) rVS. Part (A–D) show regressions pooled over all subjects (intervention group and control group) and all timepoints (T0, T2, T4, and T6), while (E–G) show regressions of all timepoints within the intervention group only.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.