Abstract
X-linked hypophosphatemia (XLH) or vitamin D-resistant rickets (MIM#307800), is a monogenic disorder with X-linked inheritance. It is caused by mutations present in the Phosphate Regulating Endopeptidase Homolog X-Linked (PHEX) gene responsible for the degradation of the bone-derived hormone fibroblast growth factor 23 (FGF23) into inactive fragments, but the entire mechanism is currently unclear. The inactivation of the gene prevents the degradation of FGF23, causing increased levels of FGF23, which leads to decreased tubular reabsorbtion of phosphorus. Clinical aspects are growth delay, limb deformities, bone pain, osteomalacia, dental anomalies, and enthesopathy. Laboratory evaluation shows hypophosphatemia, elevated alkaline phosphatase (ALP), and normal serum calcium levels, whereas parathormone (PTH) may be normal or increased and FGF23 greatly increased. Conventional treatment consists of administration of oral phosphate and calcitriol. Treatment with Burosumab, a monoclonal antibody that binds to FGF23, reducing its activity, was approved in 2018. Methods. We describe a case of two siblings, a girl and a boy, diagnosed with XLH, monitored by the Genetic Department of the County Emergency Clinical Hospital since 2019. The clinical picture is suggestive for XLH, both siblings exhibiting short stature, lower limb curvature, bone pain, marked walking weakness, and fatigue. Radiological aspects showed marked deformity of the lower limbs: genu varum in the girl, genu varum and valgum in the boy. Laboratory investigations showed hypophosphathemia, hyperphosphaturia, elevated ALP, normal PTH, and highly increased FGF23 in both. DNA analysis performed on the two siblings revealed a nonsense mutation in exone 5 of the PHEX gene: NM_000444.6(PHEX):c.565C > T (p.Gln189Ter). Results. At the age of 13½ on 7 June 2021, the two children started treatment with Burosumab in therapeutic doses and were monitored clinically and biochemically at regular intervals according to the protocol established by the Endocrinology Commission of the Romanian Health Ministry. Conclusions. The first results of the Burosumab treatment in the two siblings are extremely encouraging and suggest a favorable long-term evolution under this treatment.
Keywords: X-linked hypophosphatemia, PHEX gene, FGF23, Burosumab
1. Introduction
X-linked hypophosphatemia is a monogenic disorder and the most common form of hereditary rickets with a prevalence of 1:20,000–1:60,000 in newborns [1,2,3,4]. The clinical picture includes hypophosphataemia, disharmonic dwarfism, bone deformities (curvature of the lower limbs), osteoalgia, dental abscesses, osteomalacia, and enthesopathies. From a physiopathological point of view, phosphate metabolism is affected by mutations in the PHEX gene, which cause elevated serum levels of FGF23. The latter is involved in the tubular reabsorbtion of phosphorus and the production of calcitriol [5,6]. Elevated FGF23 levels cause loss of phosphorus in urine by suppressing the activity of the Na-phosphate cotransporter and by suppressing 25(OH)D3 1-hydroxylase expression in the kidney [7,8]. The mechanism by which mutations in PHEX gene increase FGF23 levels is still unclear, although both PHEX and FGF23 are derived from osteocytes [9]. The PHEX gene has a wide variety of mutations, with over 615 recorded to date [10,11,12,13,14]. Reported mutations are diverse and include frameshift mutations, missense, nonsense mutations, intronic splice-site mutations, and deletions; these are distributed throughout the gene with no hot spot regions. Conventional treatment for XLH consists of administering calcitriol and phosphorus, but this treatment has a wide range of side effects, such as nephrocalcinosis, hyperparathyroidism, and gastrointestinal disorders. Biological therapy with monoclonal antibodies (Burosumab) was introduced in 2018. Recently published phase 2 and 3 studies demonstrated the extremely promising therapeutic effects of Burosumab, including improvement in growth curve and bone pain, increased capacity for effort, and in biological terms a normalization of phosphorus, FGF23 and PTH levels [15,16].
The purpose of this article is to present the clinical evolution of two siblings diagnosed with XLH one year after initiating treatment with Burosumab.
X-linked hypophosphatemia rickets (XLH) is a severe and rare disease
The authors describe a familial case of XLH
The clinical picture is different for the two siblings, especially in terms of bone changes
The Burosumab treatment has favorable effects, improving growth curve and bone pain, and normalizing phosphorus levels
2. Materials and Methods
2.1. Case Report
We describe a familial case of two siblings referred to the Genetics Department of the County Emergency Clinical Hospital Oradea, Romania, for short stature and severe lower limb deformities. Case ≠ 1, a girl, was the first child in the family, coming from a pregnancy with physiological evolution, vaginal delivery at 40 weeks, birth weight 3000 g, and birth length 48 cm. Case ≠ 2, a boy, was the second child in the family, coming from a pregnancy with physiological evolution, vaginal delivery at 38 weeks, birth weight 4200 g, and birth length 50 cm. Family history: young, unrelated parents; the mother’s maternal grandfather had disharmonic dwarfism, excruciating bone pain, waddling gait, and moderate genu varum deformity of the lower limbs.
2.2. Laboratory Investigations
Laboratory tests were focused on the assessment of phosphorus, alkaline phosphatase, PTH and 1.25 (OH)2 vitamin D at periodic intervals of 3, 6 and 12 months, according to the protocol established by the Endocrinology Commission of the Romanian Health Ministry.
2.3. Molecular Investigations
Written informed consent was obtained from the mother prior to participation in the study. DNA was extracted from peripheral blood lymphocytes of the participating family members using standard extraction procedures. Molecular tests were performed at Synlab Laboratories, Lausanne. The patients were tested using next-generation sequencing (NGS) with a multi-gene panel of 13 genes selected based on associations with hypophosphatemia, as reported in the medical literature: ALPL, CLCN5, CYP2R1, mCYP27B1, DMP1, ENPP1, FAH, FAM20C, FGF23, FGFR1, PHEX, SLC34A3, VDR. Each gene in the NGS panel was targeted with oligonucleotide baits (Agilent Technologies, Santa Clara, CA, USA; Roche, Pleasanton, CA, USA; IDT, Coralville, IA, USA). Genomic DNA was extracted from the biological sample using the bead-based method. DNA quality and quantity were assessed using electrophoretic methods. The sequencing library was prepared by ligating sequencing adapters to both ends of DNA fragments and were size-selected using the bead-based method. Regions of interest (exons and intronic targets) were targeted using a hybridization-based target capture method. The sequencing libraries that passed quality control were sequenced by Illumina’s sequencing-by-synthesis method, using paired-end sequencing. Sequence alterations were confirmed using bi-directional Sanger sequencing. The variant classification follows the modified Blueprint Genetics Variant Classification Schemes from the ACMG guideline 2015. A heterozygous variant of PHEX was identified c.565C > T, p.(Gln189 *) and classified as pathogenic. Written parental consent was obtained in order to include case details and accompanying images.
3. Results
3.1. Morphological Evaluation of the Patients
The siblings, sister (case#1) and brother (case#2), were referred to the Genetics Department in 2019, at the ages of 10 and 4, respectively, by their family doctor (Table 1). Treatment with Burosumab was initiated in June 2021, and the phenotypic characteristics of the patients prior to starting the treatment are shown in Table 1.
Table 1.
Morphological evaluation of the patients at the initiation of treatment with Burosumab.
| Case #1 Sister | Case #2 Brother | |
|---|---|---|
| Age at diagnosis | 13½ years | 7 years |
| Age of onset | 3 years | 2 years |
| Weight | 39 kg | 19 kg |
| Stature | 131 cm (−3DS) | 107 cm (−3DS) |
| Craniofacial dysmorphism | macrocephaly: head circumference 53 cm frontal bossing flattened facies bilateral epicanthal folds, high arched palate |
|
| Chest deformity | pectus excavatum, thoracolumbar scoliosis, Harrison’s groove and rachitic rosary | pectus excavatum, Harrison’s groove and rachitic rosary |
| Musculoskeletal abnormalities |
thickened wrists and ankles, severe bilateral bowing of lower limbs (genu varum) | thickened wrists and ankles, genu valgum deformity (knees touching each other while the ankles remain spaced apart) |
| Walk | waddling gait | impaired |
| Walking fatigue | ++++++ | +++ |
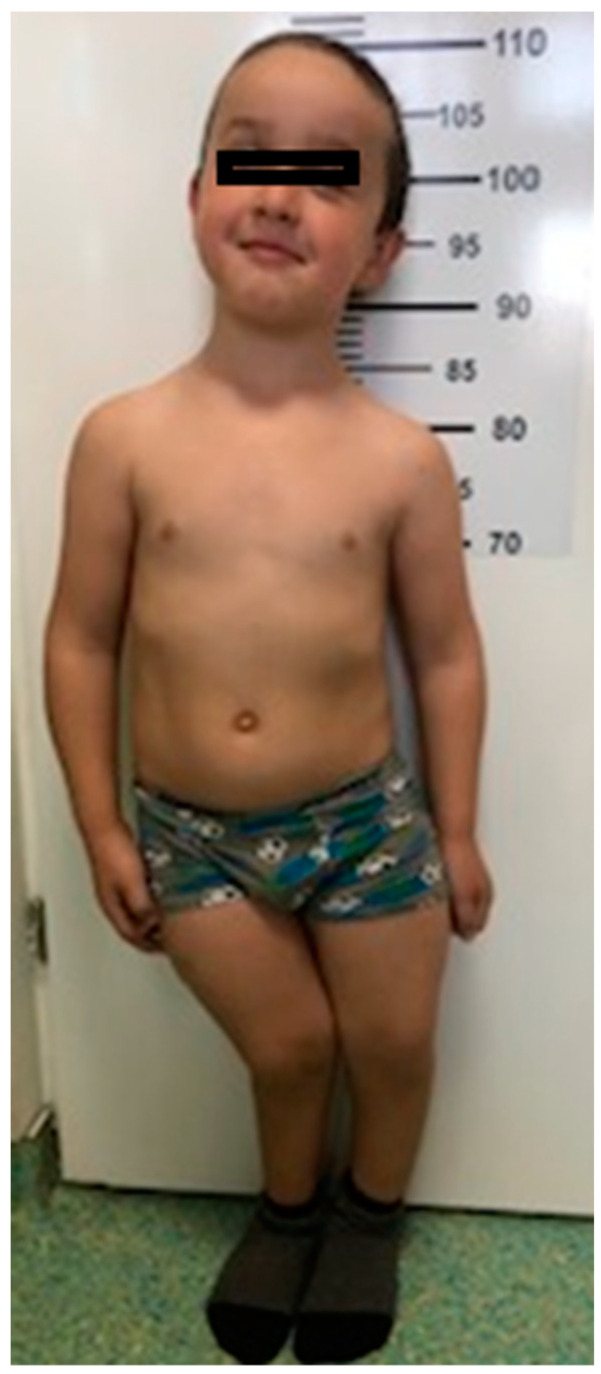
Case#1. The girl, 13½ years old, displayed very short stature: 131 cm (−3 SD) and normal weight: 39 kg. Chest examination revealed pectus excavatum, thoracolumbar scoliosis, Harrison’s groove and rachitic rosary. Musculoskeletal examination showed thickened wrists and ankles, severe bilateral bowing of lower limbs (genu varum). She had walking difficulties: slow walking, waddling gait, and marked fatigue after covering short distances (50 m), which is why she made frequent stops; intercondylar distance was 39 cm. Ophthalmological exam showed amblyopia (Figure 1).
Figure 1.

Case #1. Sister at 13½ years old: short stature, genu varum deformation of lower limbs.
Case#2. The boy, 7 years old, also displayed short stature: 107 cm (−3 SD) and normal weight: 19 kg. He also presented craniofacial dysmorphism: macrocephaly, with an ocipitofrontal diameter of 53 cm (above the 90th percentile), flattened facies, frontal bossing, bilateral epicanthal folds, and high-arched palate. Chest examination showed pectus excavatum, Harrison’s groove and rachitic rosary. Musculoskeletal examination revealed thickened wrists and ankles, bilateral bowing of lower limbs and impaired walk (Figure 2).
Figure 2.
Case #2. Brother at 7 years old: short stature, macrocephaly, genu valgum deformation of lower limbs, knees touching each other while ankles remain spaced apart.
3.2. Laboratory Investigations
Lab tests performed at the time of diagnosis and 1 year after the initiation of the Burosumab treatment are shown in Table 2. In the same table it can be seen that under the action of the Burosumab the values of phosphatemia and alkaline phosphatase changed in the direction of normalization.
Table 2.
Laboratory investigations.
| Investigations (Reference Values) |
Case#1 | Case#2 | ||
|---|---|---|---|---|
| Initiation of Treatment with Burosumab |
1 Year after Starting Treatment with Burosumab |
Initiation of Treatment with Burosumab |
1 Year after Starting Treatment with Burosumab |
|
| Phosphorus (2.4–4.4 mg/dL) |
1.85 | 2.6 | 2.41 | 3.0 |
| Alcaline phosphatase (3–10 years: 130–260 U/L, 10–14 years 130–340 U/L) |
488 | 159 | 788 | 379 |
| Total Calcium (8.8–10.8 mg/dL) |
10.2 | 9.5 | 9.9 | 9.40 |
| FGF23 (26–110 kRU/l) |
201 | 215 | ||
| Parathormone (PTH) (12–65 pg/mL) |
24 | 63 | 63.1 | 94 |
| 1,25(HO)2 dehydrogenase (25–86 pg/mL) |
59.5 | 83.7 | 62.7 | 89.10 |
| Glomerular filtration rate (over 90 mL/min) |
122.3 | 142.7 | 134.5 | 149 |
3.3. Radiological Investigations
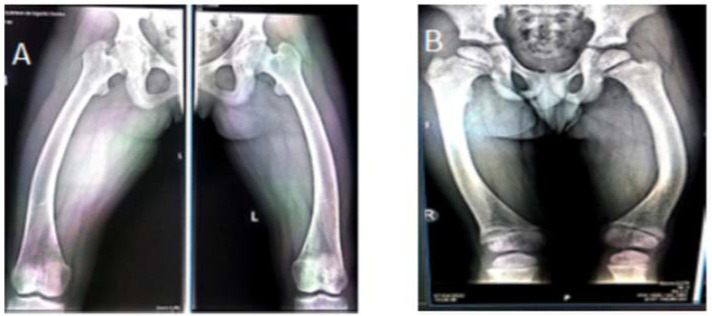
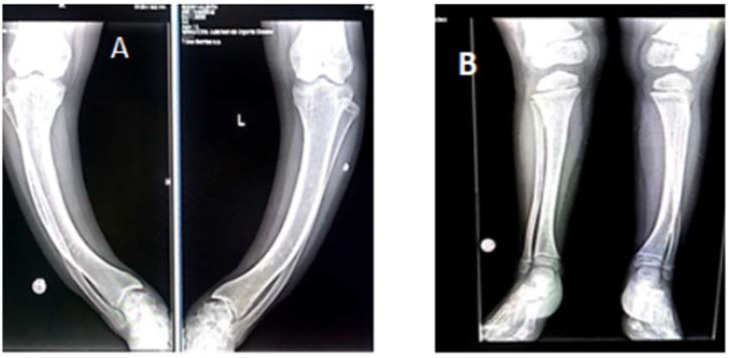
Radiological investigations show a genu varum deformity in both femural and tibial bones in Case#1. Case #2 presented genu varum deformity in femural bones; in tibial bones: left tibia genu varum, right tibia genu valgum (Figure 3 and Figure 4). These are summarized in Table 3.
Figure 3.
Femoral radiography (April 2022): femoral scoliosis, (A) case #1, (B) case #2.
Figure 4.
Tibial and fibular radiography (April 2022). (A): Case #1: tibial scoliostosis, mild bilateral fibular deformation, closed growth plates. (B): Case #2: left tibia: scoliostosis in varum, right tibia: scoliostosis invalgum.
Table 3.
Radiological investigations.
| Initiation of Treatment with Burosumab | 1 Year after Starting Treatment with Burosumab | |
|---|---|---|
| Case# 1 | Chest: discrete dextroconvex dorsolumbar scoliosis Bilateral femur: bilateral femural scoliostosis, bilateral enlargement of the distal metaphysis and epiphysis. Bilateral knee, leg, ankle: major bilateral tibial scoliostosis; mild bilateral fibular deformation. Bilateral enlargement of the proximal and distal tibial epiphysis and metaphysis Bone demineralization of the radiographed skeleton, with fine opaque lines Bone age corresponds to chronological age |
Bilateral femural scoliostosis (in varum); Marked bilateral tibial scoliostosis; mild bilateral fibular deformation. Closed growth plates. Bone age corresponds to chronological age |
| Case # 2 | Chest: Marked bilateral widening of the anterior ends of the ribs. Costal rosaries. Femur: Deformed, curved femoral diaphysis, widened distal metaphysis and irregular contour at growth plates level. Deformed, curved fibular and tibial diaphysis with widened metaphysis and irregular contours Bone age corresponds to chronological age |
Bilateral femoral scoliostosis (in varum); Widened femoral metaphysis In varum scoliostosis of the left tibia, in valgum scoliostosis of the right tibia. Bone age corresponds to chronological age |
3.4. Psychological Aspects: Case#1 Displays Low Self-Esteem Due to Motor Difficulties (Increased Emotional Sensitivity, Shyness in Unfamiliar Environments). Frequent Episodes of Sadness. Case#2 Periods of Anxiety and Anger
3.5. Molecular Investigations
NGS shows a pathogenic variant in PHEX gene c.565C > T, p.(Gln189 *). This variant generates a premature stop codon in exon and is predicted to lead to loss of normal protein function, either through protein truncation (188 out of 749 aa) or nonsense-mediated mRNA decay.
4. Discussion
The diagnosis of familial hypophosphatemia was based on clinical aspect and laboratory changes (hypophosphatemia, increased ALP, decreased 1,25-dihydroxy vitamin D, increased FGF23), as well as on the radiological aspect of the bones. The diagnosis was confirmed by molecular investigations which highlight the mutation in the PHEX gene.
4.1. The Gene
The PHEX gene is located on the short arm of chromosome X (Xp22.11) and was identified in 1995. It contains 22 exons and encodes a protein (PHEX protein) made up of 749 amino acids, belonging to the M13 protease family (type II cell surface zinc-dependent proteases) involved in the degradation of the extracellular matrix. The protein consists of an extracellular domain, where the active site is located (positions 581, 646), three zinc coordination sites, but also other glycosylation links and Cys Cys disulfide bonds. PHEX also has other functions not related to FGF23 degradation, which is expressed in osteoblasts and osteocytes, but also in other tissues (ovary, testicles, lung, brain, muscle). In vitro studies showed that PHEX cleaves an extracellular matrix protein called osteopontin that inhibits bone mineralization and is found in bones and teeth [17,18].
The FGF23 gene is located on chromosome 12 and encodes a protein made up of 251 aminoacids. Although it is expressed by osteoblasts/osteocytes, its low levels are also expressed in other tissues in rodents (brain, teeth) [19].
The family of fibroblast growth factors (FGF) contains 22 de members involved in many metabolic processes such as angiogenesis, cell growth and differentiation, cell migration and tissue repair processes. FGF23 belongs to this family of proteins, and more specifically to the endocrine subfamily FGF19, along with FGF19 and FGF21 [20,21,22].
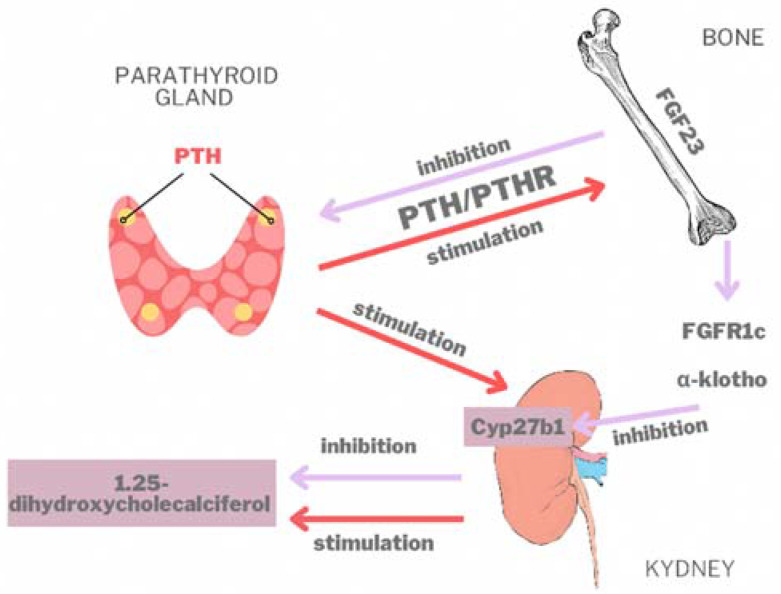
Although there is no direct link between PHEX and FGF23, the inactivation of the PHEX gene leads to increased FGF23 levels [23,24,25,26]. Phosphate and 1,25-dihydroxyvitamin D [1,25(OH)2D] levels are the main regulating factors of the FGF23 expression. In circulation, once FGF23 is activated by osteoblasts and osteocytes, it binds to a transmembrane protein, more precisely to the α-Klotho cofactor, and modifies phosphocalcic homeostasis. Specifically, it stimulates phosphate excretion and inhibits the formation of 1,25(OH)2D3, the active form of vitamin D (1,25 dihydroxycholecalciferol) ([27]). By activating the PTH/PTH receptor in osteoblasts and osteocytes, PTH stimulates FGF23 synthesis (Figure 5). FGF23 decreases PTH synthesis and secretion (downregulation) in the parathyroid [28]. FGF23 bound to the FGFR1 receptor, in the presence of α-Klotho, inhibits the action of 1-α-hydroxylase enzyme (encoded by Cyp27b1) in the kidneys and FGF23 stops the synthesis of 1,25-dihydroxyvitamin D. On the other hand, PTH stimulates Cyp27b1 expression in the kidneys (upregulation) and increases the serum concentration of 1,25(OH)2D3 [29,30].
Figure 5.
The role of FGF23 in circulation; PTH-parathormone; PTHR-receptor of parathormone.
Besides phosphate and 1,25(OH)2D3, other regulatory factors such as calcium, leptin, estrogen, glucocorticoids, iron metabolism and bone mineralization are involved in FGF23 expression [31].
Elevated levels of FGF23 are found in the autosomal dominant form of hypophosphatemic rickets caused by activating mutations of FGF23c, and also in the autosomal recessive form (due to mutations in dentin 1 matrix protein).
Sarafrazi Sodabben et al. have developed a new PHEX gene locus-specific database, (PHEX LSDB) for the purpose of centralizing and disseminating information about PHEX gene variants. Data was collected from several databases as follows. 1: The older 2000 version of PHEX LSDB which is currently inactive. 2: Genetic data obtained from clinical trials of Burosumab. 3: Recent data published in the literature. 4: A state-of-the-art free molecular testing program initiated by Ultragenyx Pharmaceutical Inc. and Invitae Corporation [31,32,33]. By April 2021, 870 unique variants of PHEX had been identified and collected in PHEX LSDB from the four databases listed above; more than 800 disease-causing PHEX variants have also been found.
In our patients, the nonsense mutation was identified in exon 5 of the PHEX gene: c.565C > T (p.Gln189Ter). The PHEX c.565C > T, p.(Gln189 *) variant had been previously reported in four unrelated individuals affected with X-linked hypophosphatemia, of which two were reported to be sporadic cases (Table 4) [34,35,36].
Table 4.
The c.565C > T (p.Gln189Ter) mutation in literature.
| Author | Title | References |
|---|---|---|
| Yamazaki Y et al. | Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia | [34] |
| Zhang C et al. | Clinical and genetic analysis in a large Chinese cohort of patients with X-linked hypophosphatemia. | [35] |
| Morey M et al. | Genetic diagnosis of X-linked dominant Hypophosphatemic Rickets in a cohort study: tubular reabsorption of phosphate and 1,25(OH)2D serum levels are associated with PHEX mutation type | [36] |
| Vila-Pérez D et al. | Four Cases of X-Linked Hypophosphatemic Rickets, Clinical Description and Genetic Testing | [37] |
4.2. Clinical Aspects
The disease is characterized by high morbidity and the appearance of bone deformities accompanied by severe bone pain, which affects the lives of patients [38]. These signs and symptoms affect the patients’ quality of life (QoL). In the case presented by the authors, the sister (case ≠ 1) who has reached the age of puberty, displays school adjustment issues, an inferiority complex, shyness, and reluctance in expressing her feelings.
The growth. Disharmonic dwarfism, with a predominant shortening of the lower body compared to the upper body, is the main feature in XLH, although it presents great variability [39,40]. The final stature in adults is −3 SD when compared to normal stature. The clinical picture is more severe in males than in females. The lower limbs are much more affected, with their marked curvature, because they are exposed to mechanical forces much more strongly than the upper limbs. It must be noted that growth plate activity is intense in the knee, causing the growth of the bone in length and the formation of the enchondral bone. Additionally, growth plate activity is often strongest around the knees, where it influences the rate of endochondral bone production and, consequently, bone length [41]. In adulthood, the clinical picture is dominated by the appearance of osteomalacia and enthesopathies associated with bone and joint pain due to secondary arthritis [42]. In our patients, Case#1 only registered an increase of 2.5 cm in height; at 1 year after the initiation of the treatment she measures 133.5 cm. Case #2 registered a 4 cm increase in height, and at 1 year after the initiation of the Burosumab treatment he measures 111 cm.
Dental abscesses. The appearance of dental abscesses is another main feature manifested from childhood, with devitalized teeth (especially mandibular incisor and canine teeth), moderate to severe periodontitis, or several asymptomatic periapical lesions on teeth that have already been treated endodontically or not can be seen in adults. The dental changes of patients appear as soon as the teeth erupt and persist throughout their lives, resulting in severe functional, aesthetic, and nutritional issues. The patients must be managed in specialized medical services, with constant attention paid to both functional and aesthetic rehabilitation. Our patients had not displayed any dental abscesses to date.
Craniofacial dysmorphism is discrete, and some patients may have cranial dysmorphism with macrocephaly and dolichocephaly [43]. Case #2 presents macrocrania, with a high forehead, frontal protuberances and flattened parietal bones.
4.3. Paraclinical Aspects
The major sign is the decrease of phosphatemia due to decreased renal tubular reabsorbtion. Alkaline phosphatase and FGF23 levels are highly elevated. Normal FGF23 levels do not rule out the presence of XLH, so in the presence of low hypophosphatemia, they should be interpreted as inadequately normal [44]. Increased levels of FGF23 are also present in other forms of hypophosphatemic rickets (autosomal dominant and recessive forms). PTH levels may be normal or at the upper limit, calcium level is low in most cases and 1,25(OH)2 vitamin D level is decreased. In addition to hypophosphatemia, our patients displayed elevated ALP and FGF23 levels, with normal levels of PTH.
4.4. Radiological Aspects
The changes that occur are those present in rickets, including enlarged, abnormally shaped metaphyses, and irregular diaphyses in the long bones; unlike in deficiency rickets, the cortical region of the bone is thickened, and bone resorption is absent. These changes are visible in areas of rapid growth, such as the distal part of the femoral and tibial bones or the distal part of the radius. An X-ray of the knee joint is sufficient to diagnose XLH, because the lower limbs are the first affected [45,46]. Our patients display characteristic changes of rickets. Case #1 displays genu varum deformation at femoral and tibial level and closed growth plates at femoral and tibial level. Case #2 displays femoral genu varum deformation, genu varum deformation in the left tibia, and genu valgum deformation in the right tibia.
4.5. Genotype-Phenotype Correlation
Although the disease manifests in all patients with PHEX gene mutations, severity may differ between members of the same family, irrespective of gender, as noted in the literature [47,48,49].
Mutations in the PHEX gene are extremely varied, including nonsense, missense, splicing, and frameshift mutations, so the pathogenic variants can be spread on the entire length of the gene [50,51,52]. There are currently more than 615 variants in this gene identified in the Human Genome Mutation Database (HGMD). According to Gaucher et al., the increased and diverse number of PHEX gene mutations makes the genotype-phenotype correlation less obvious [53]. Pathological variants lead to alteration of the protein, with alteration of protein function and truncated protein appearing in over 70% of the cases. This wide range of mutations disrupts the cell processing mechanism, alters the conformation of the protein, and also alters endopeptidase activity, as proven by numerous studies [54,55,56]. Some authors reported that women with certain variations in the PHEX gene have a milder clinical picture (moderately low levels of serum phosphate, milder bone deformations) than males with the same genetic variants. Other authors believe that pathogenic variants that lead to the formation of truncated proteins or variants in the C-terminal region of the gene increase the severity of the clinical and paraclinical picture [57,58].
Some authors, such as Cho et al., when comparing missense mutations and nonsense mutations, did not establish a genotype-phenotype correlation [59]. Although our patients have the same mutation, there are some differences: the bone deformities are more pronounced in the girl, with marked genu varum deformity and an intercondylar distance of 39 cm causing severe disharmonic dwarfism (with treatment, the intercondylar distance was reduced to 36 cm), whereas macrocephaly with a high forehead and frontal protuberances was present only in the boy.
4.6. Medical Treatment
There is currently no cure for XLH, so the goal of the current treatment is to improve bone deformities and other anomalies caused by severe hypophosphatemia [60].
The classical treatment of XLH consists of administering phosphate and calcitriol. Phosphate is administered in doses of 20–60 mg/kg/day, 4–6 times/day. The sooner the treatment is started, the more beneficial the effects are. A normalization of phosphorus and ALP levels is observed, and radiological alterations are improved; growth rate is also enhanced and bone pain is relieved. Calcitriol is associated with the phosphate treatment, in doses that vary from patient to patient. This treatment does not have the expected effects, both drugs having severe side effects, most commonly nephrocalcinosis, reported in 30–70% of patients, and secondary hyperparathyroidism, which further aggravates phosphaturia and bone resorption [61]. Our cases followed this treatment inconsistently for a year, after which they stopped it for unknown reasons.
Although growth hormone (GH) has beneficial effects on growth rate and PTH values (normalization of values), its administration is not recommended because its effects cause asymmetric growth in which some areas grow more than others, eventually leading to skeletal deformities and disproportion between body segments by stimulating chondrocyte proliferation, leading to increased growth rate without normalization of the growth plate in these patients [62,63,64].
Biological therapy with monoclonal antibodies that prevent the excessive activity of FGF23 is beneficial and is administered especially in patients with abnormal development of the growth plate. In 2018 Kaneko et al. demonstrated that blocking FGF23 in various ways leads to improvement in hypophosphatemia, bone integrity and also in enthesopathies [65,66].
Burosumab treatment for XLH was approved by the European Medicines Agency (EMA) in February 2018 in Europe, and in April 2018 by the US Food and Drug Administration (FDA) for patients older than 1 year. Treatment consists of subcutaneous injections with doses of 0.8 mg/kg/2 weeks, with the possibility of increasing the dose by 0.4 mg/kg if normal serum phosphorus levels are not reached, without exceeding the normal dose of 2 mg/kg. Burosumab bioavailability is almost 100% due to its good subcutaneous absorption [67,68]. The drug degrades into small molecules, and maximum levels are reached between 7.0–8.5 days, with a half-life of 16.4 days [69].
As a general rule, it is recommended that treatment with phosphate and calcitriol should be stopped one week before the start of treatment with Burosumab. The most common side effects are redness around the injection site, headache, and bone pain in the extremities.
Studies have shown that treatment over a period of 4 to 8 weeks increases femoral bone density, causes thinning in the growth plate, improves serum levels of phosphate and 1,25 (OH)2D3 [70]. If serum phosphorus levels do not reach normal levels after the first month of treatment, doses may be increased by 0.4 mg/kg to a maximum of 2 mg/kg. In our patients’ case, Burosumab doses were progressively increased until normal levels of phosphorus were reached: in the case of the girl the phosphorus values normalized after 3 months of treatment, while in the case of the brother after 4 months. With both patients a progressive increase of doses with 0.4 mg/kg was necessary. One year after the start of treatment, the doses administered were: 1.2 mg/kg/2 weeks (50 mg/2 weeks) for the girl and 1.4 mg/kg/2 weeks (40 mg/2 weeks) for the boy.
As it is a newly approved treatment, there are no studies to prove long-term efficiency and impact on the quality of life in adult patients undergoing this treatment [71]. It is certain that the Burosumab treatment improves the growth curve and anthropometric parameters without causing disharmonious growth, as it is the case with GH administration [72].
There are currently several uncontrolled open-label studies that have demonstrated the effectiveness of the Burosumab treatment. In an open-label phase 2 trial study, Carpenter et al. followed short term effects in 52 children, aged 1 to 12, diagnosed with XLH [73]. The aim of the study was to change the biochemical parameters to 40 and 64 weeks, respectively, after starting treatment, by following the Rickets Severity Score (RSS or Thacher score) [74]. RSS is a quantitative parameter measuring the severity of rickets according to radiological alterations in the wrists and knees. It measures the degree of wear in the mataphysis and the concavity and the percentage of growth plates damage. The results are interpreted on a scale from 1 to 10, 10 being the highest degree of severity, and 0 representing the absence of radiological changes specific to rickets. The authors of the study found that the effects of Burosumab treatment are beneficial: phosphorus levels reach the lower limit of reference values, and bone pain is decreased, while the capacity for effort is increased and children are able to walk longer distances until fatigue sets in.
In a randomized active controlled open-label phase 3 study (with data collected from 16 clinical sites), Imel et al. compared the effects of classical phosphate and calcitriol treatment and those of the Burosumab treatment in children with XLH. The study highlighted the favorable effects of Burosumab: a clinical improvement in rickets, in the growth curve and in biochemical parameters (phosphorus, PTH) [75,76]. In another phase 2 study, Whyte et al. showed the same beneficial effects of the treatment with this monoclonal antibody in children with XLH (Table 5) [77].
Table 5.
Open-label studies and multiple case reports about the effects of the Burosumab treatment.
| Study and Patients | Results | Observations | References |
|---|---|---|---|
| Open-label phase 2 trial No. of patients: 52 Age: 1–12 Tracking period: 64 weeks |
|
Positive effects of Burosumab treatment. | [73] |
| Open-label phase 3 trial at 16 clinical sites No. of patients: 61: 29 received Burosumab, 32 conventional therapies Age: 1–12 Tracking period: 64 weeks |
|
Burosumab offers a promising new treatment approach for children with XLH in comparison with conventional therapy | [75] |
| Open-label phase 2 trial at three hospitals in the US No. of patients: 13 Age: 1–4 Tracking period: 64 weeks |
|
Burosumab had a favorable safety profile | [77] |
The effects of the Burosumab treatment in adults were highlighted in a recent double-blind placebo-controlled phase 3 study by Insogna et al. The effects of the Burosumab treatment were monitored over a 24-week follow-up period. A total of 134 adult patients with XLH were included in the study. Burosumab proved to be effective both in terms of biochemical parameters, specifically phosphorus (increasing serum phosphate levels) and in terms of osteoarthritis development [78]. Similar effects were highlighted by Cheong et al. in Japanese and Korean adults with XLH, in a multicenter open-level single-dose study [79].
4.7. Surgical and Orthopedic Treatment
Surgical treatment aims at obtaining an equal length in the lower limbs, their correct alignment at the end of bone growth, and maintaining a joint as mobile and functional as possible.
The basic surgery technique is osteotomy performed in places where bone deformity is major. The result is an immediate correction of the deformity by internal fixation or gradual correction by external fixation. This technique is not often used anymore, due to the fact that it has been associated with frequent complications and a high risk of recurrence. In 2017 Gizard et al. showed that up to 57% of patients surgically treated by osteotomy suffered from postoperative complications, such as recurrent deformities present in 29% of patients [80]. However, if the bone deformity is severe and causes instability in the knee, surgical treatment is recommended before the end of the growth process.
Nowadays surgical techniques are more efficient and less invasive. Guided growth surgery is becoming more and more frequent and is performed in early childhood. It is recommended if there is no improvement in bone deformity after 1 year of medical treatment. The purpose is to correct the growth plate defect of the deformed bone before marked diaphyseal deformity occurs. The principle is to correct the mechanical axis. The forces acting on the diaphysis and implicitly on the joint are brought back to normal, leading to normal growth, and the procedure does not require immobilization. The technique involves setting a metal plate on the lateral or medial surface of the bone (for genu varum or genu valgum deformities) at the level of the growth plate; the metal plate is fixed with screws placed one proximal and one distal. In time, bone alignment is achieved; when the desired result is obtained, the metal plates are extracted, bone growth following its physiological course. This technique helps correct the bone only in coronal plane, without improving the median correction of the tibial bone [81].
4.8. Diagnosis and Monitoring
Diagnosis and follow-up monitoring of XLH patients represent a real challenge for practitioners. The process involves a multidisciplinary team that includes a geneticist, a pediatrician, an orthopedist, an ENT specialist, a dentist, a rheumatologist, an internist, a nephrologist, an endocrinologist, a physiotherapist and a psychologist. In 2011, Carpenter et al. published the first XLH Guide, “Clinician’s guide to XLH” [82]. More recently, in 2019, Hafner et al. published an article on practical clinical recommendations for diagnosing, treating and monitoring XLH patients, providing important and precise details on all aspects of this condition from childhood to adulthood.
Genetic counselling is extremely important. Being an X-linked dominant disorder, the affected father will have all daughters affected, and the affected mother runs a 50% risk of having affected children, be they boys or girls. Molecular diagnosis, which identifies pathogenic variants, provides correct genetic advice and allows for the screening of relatives at risk [83].
5. Conclusions
X-linked hypophosphatemia has a wide spectrum of clinical presentations. Burosumab treatment has beneficial effects on the growth curve, on bone changes and also mitigates fatigue. At the same time, it improves the patients’ quality of life, as it is a drug that revitalizes the lives of XLH patients. In Romania, the number of undiagnosed cases is still high; currently only 15 patients are being treated with Burosumab. Genetic counselling plays a key role, while early diagnosis and interdisciplinary cooperation are essential for proper management leading to improvement of the patients’ quality of life.
Acknowledgments
A medical review of the manuscript was provided by MedInteractiv Plus.
Author Contributions
Conceptualization, C.M.J. and A.D.J.; methodology, C.D.P.; software, K.K.; validation, C.P.; formal analysis, M.B.; investigation, K.K. and S.J.; resources, O.I.; data curation, O.I.; writing—original draft preparation, C.M.J. and S.J.; writing—review and editing, D.C.Z. and A.D.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Emergency Clinical County Hospital Oradea (protocol code 20357/23.06.2022).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Medical review and publication fees were funded by Kyowa Kyrin International.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beck-Nielsen S.S., Brock-Jacobsen B., Gram J., Brixen K., Jensen T.K. Incidence and Prevalence of Nutritional and Hereditary Rickets in Southern Denmark. Eur. J. Endocrinol. 2009;160:491–497. doi: 10.1530/EJE-08-0818. [DOI] [PubMed] [Google Scholar]
- 2.Holm I.A., Huang X., Kunkel L.M. Mutational Analysis of the PEX Gene in Patients with X-Linked Hypophosphatemic Rickets. Am. J. Hum. Genet. 1997;60:790–797. [PMC free article] [PubMed] [Google Scholar]
- 3.Fuente R., García-Bengoa M., Fernández-Iglesias Á., Gil-Peñ H., Santos F., López J.M. Cellular and Molecular Alterations Underlying Abnormal Bone Growth in X-Linked Hypophosphatemia. Int. J. Mol. Sci. 2022;23:934. doi: 10.3390/ijms23020934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuente R., Gil-Peña H., Claramunt-Taberner D., Hernández-Frías O., Fernández-Iglesias Á., Hermida-Prado F., Anes-González G., Rubio-Aliaga I., Lopez J.M., Santos F. Marked Alterations in the Structure, Dynamics and Maturation of Growth Plate Likely Explain Growth Retardation and Bone Deformities of Young Hyp Mice. Bone. 2018;116:187–195. doi: 10.1016/j.bone.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Reid I.R., Hardy D.C., Murphy W.A., Teitelbaum S.L., Bergfeld M.A., Whyte M.P. X-Linked Hypophosphatemia: A Clinical, Biochemical, and Histopathologic Assessment of Morbidity in Adults. Medicine. 1989;68:336–352. doi: 10.1097/00005792-198911000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Liu S., Quarles L.D. How Fibroblast Growth Factor 23 Works. J. Am. Soc. Nephrol. 2007;18:1637–1647. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- 7.Saito H., Kusano K., Kinosaki M., Ito H., Hirata M., Segawa H., Miyamoto K.-I., Fukushima N. Human Fibroblast Growth Factor-23 Mutants Suppress Na+-Dependent Phosphate Co-Transport Activity and 1alpha,25-Dihydroxyvitamin D3 Production. J. Biol. Chem. 2003;278:2206–2211. doi: 10.1074/jbc.M207872200. [DOI] [PubMed] [Google Scholar]
- 8.Shimada T., Mizutani S., Muto T., Yoneya T., Hino R., Takeda S., Takeuchi Y., Fujita T., Fukumoto S., Yamashita T. Cloning and Characterization of FGF23 as a Causative Factor of Tumor-Induced Osteomalacia. Proc. Natl. Acad. Sci. USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruchon A.F., Marcinkiewicz M., Siegfried G., Tenenhouse H.S., DesGroseillers L., Crine P., Boileau G. Pex MRNA Is Localized in Developing Mouse Osteoblasts and Odontoblasts. J. Histochem. Cytochem. 1998;46:459–468. doi: 10.1177/002215549804600405. [DOI] [PubMed] [Google Scholar]
- 10.Ishihara Y., Ohata Y., Takeyari S., Kitaoka T., Fujiwara M., Nakano Y., Yamamoto K., Yamada C., Yamamoto K., Michigami T., et al. Genotype-Phenotype Analysis, and Assessment of the Importance of the Zinc-Binding Site in PHEX in Japanese Patients with X-Linked Hypophosphatemic Rickets Using 3D Structure Modeling. Bone. 2021;153:116135. doi: 10.1016/j.bone.2021.116135. [DOI] [PubMed] [Google Scholar]
- 11.Santos F., Fuente R., Mejia N., Mantecon L., Gil-Peña H., Ordoñez F.A. Hypophosphatemia and Growth. Pediatr. Nephrol. 2013;28:595–603. doi: 10.1007/s00467-012-2364-9. [DOI] [PubMed] [Google Scholar]
- 12.Forero-Delgadillo J.M., Cleves D., Ochoa V., Londoño-Correa H., Restrepo J.M., Nastasi-Catanese J.A., Pachajoa H. PHEX Gene Mutation in a Patient with X-Linked Hypophosphatemic Rickets in a Developing Country. Appl. Clin. Genet. 2020;13:57–62. doi: 10.2147/TACG.S232448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razali N.N., Hwu T.T., Thilakavathy K. Phosphate Homeostasis and Genetic Mutations of Familial Hypophosphatemic Rickets. J. Pediatr. Endocrinol. Metab. 2015;28:1009–1017. doi: 10.1515/jpem-2014-0366. [DOI] [PubMed] [Google Scholar]
- 14.Francis F., Hennig S., Korn B., Reinhardt R., de Jong P., Poustka A., Lehrach H., Rowe P.S.N., Goulding J.N., Summerfield T., et al. A Gene (PEX) with Homologies to Endopeptidases Is Mutated in Patients with X-Linked Hypophosphatemic Rickets. The HYP Consortium. Nat. Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 15.Pavone V., Testa G., Gioitta Iachino S., Evola F.R., Avondo S., Sessa G. Hypophosphatemic Rickets: Etiology, Clinical Features and Treatment. Eur. J. Orthop. Surg. Traumatol. 2015;25:221–226. doi: 10.1007/s00590-014-1496-y. [DOI] [PubMed] [Google Scholar]
- 16.Filisetti D., Ostermann G., von Bredow M., Strom T., Filler G., Ehrich J., Pannetier S., Garnier J.M., Rowe P., Francis F., et al. Non-Random Distribution of Mutations in the PHEX Gene, and under-Detected Missense Mutations at Non-Conserved Residues. Eur. J. Hum. Genet. 1999;7:615–619. doi: 10.1038/sj.ejhg.5200341. [DOI] [PubMed] [Google Scholar]
- 17.Barros N.M.T., Hoac B., Neves R.L., Addison W.N., Assis D.M., Murshed M., Carmona A.K., McKee M.D. Proteolytic Processing of Osteopontin by PHEX and Accumulation of Osteopontin Fragments in Hyp Mouse Bone, the Murine Model of X-Linked Hypophosphatemia. J. Bone Miner. Res. 2013;28:688–699. doi: 10.1002/jbmr.1766. [DOI] [PubMed] [Google Scholar]
- 18.Qin C., Baba O., Butler W.T. Post-Translational Modifications of Sibling Proteins and Their Roles in Osteogenesis and Dentinogenesis. Crit. Rev. Oral Biol. Med. 2004;15:126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- 19.Yoshiko Y., Wang H., Minamizaki T., Ijuin C., Yamamoto R., Suemune S., Kozai K., Tanne K., Aubin J.E., Maeda N. Mineralized Tissue Cells Are a Principal Source of FGF23. Bone. 2007;40:1565–1573. doi: 10.1016/j.bone.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Yu X., Xia Y., Jia J., Yuan G. The Role of Fibroblast Growth Factor 19 Subfamily in Different Populations Suffering from Osteoporosis. Front. Endocrinol. (Lausanne) 2022;13:830022. doi: 10.3389/fendo.2022.830022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurpas A., Supeł K., Idzikowska K., Zielińska M. FGF23: A Review of Its Role in Mineral Metabolism and Renal and Cardiovascular Disease. Dis. Markers. 2021;2021:8821292. doi: 10.1155/2021/8821292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamashita T., Yoshioka M., Itoh N. Identification of a Novel Fibroblast Growth Factor, FGF-23, Preferentially Expressed in the Ventrolateral Thalamic Nucleus of the Brain. Biochem. Biophys. Res. Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 23.Quarles L.D. FGF23, PHEX, and MEPE Regulation of Phosphate Homeostasis and Skeletal Mineralization. Am. J. Physiol. Endocrinol. Metab. 2003;285:E1–E9. doi: 10.1152/ajpendo.00016.2003. [DOI] [PubMed] [Google Scholar]
- 24.Sabbagh Y., Carpenter T.O., Demay M.B. Hypophosphatemia Leads to Rickets by Impairing Caspase-Mediated Apoptosis of Hypertrophic Chondrocytes. Proc. Natl. Acad. Sci. USA. 2005;102:9637–9642. doi: 10.1073/pnas.0502249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mäkitie O., Doria A., Kooh S.W., Cole W.G., Daneman A., Sochett E. Early Treatment Improves Growth and Biochemical and Radiographic Outcome in X-Linked Hypophosphatemic Rickets. J. Clin. Endocrinol. Metab. 2003;88:3591–3597. doi: 10.1210/jc.2003-030036. [DOI] [PubMed] [Google Scholar]
- 26.Murali S.K., Andrukhova O., Clinkenbeard E.L., White K.E., Erben R.G. Excessive Osteocytic Fgf23 Secretion Contributes to Pyrophosphate Accumulation and Mineralization Defect in Hyp Mice. PLoS Biol. 2016;14:e1002427. doi: 10.1371/journal.pbio.1002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Degirolamo C., Sabbà C., Moschetta A. Therapeutic Potential of the Endocrine Fibroblast Growth Factors FGF19, FGF21 and FGF23. Nat. Rev. Drug Discov. 2016;15:51–69. doi: 10.1038/nrd.2015.9. [DOI] [PubMed] [Google Scholar]
- 28.Mace M.L., Olgaard K., Lewin E. New Aspects of the Kidney in the Regulation of Fibroblast Growth Factor 23 (FGF23) and Mineral Homeostasis. Int. J. Mol. Sci. 2020;21:8810. doi: 10.3390/ijms21228810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ewendt F., Feger M., Föller M. Role of Fibroblast Growth Factor 23 (FGF23) and AKlotho in Cancer. Front. Cell Dev. Biol. 2020;8:601006. doi: 10.3389/fcell.2020.601006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saki F., Kassaee S.R., Salehifar A., Omrani G.H.R. Interaction between Serum FGF-23 and PTH in Renal Phosphate Excretion, a Case-Control Study in Hypoparathyroid Patients. BMC Nephrol. 2020;21:176. doi: 10.1186/s12882-020-01826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang F., Leibrock C., Pandyra A.A., Stournaras C., Wagner C.A., Föller M. Phosphate Homeostasis, Inflammation and the Regulation of FGF-23. Kidney Blood Press. Res. 2018;43:1742–1748. doi: 10.1159/000495393. [DOI] [PubMed] [Google Scholar]
- 32.Sarafrazi S., Daugherty S.C., Miller N., Boada P., Carpenter T.O., Chunn L., Dill K., Econs M.J., Eisenbeis S., Imel E.A., et al. Novel PHEX Gene Locus-Specific Database: Comprehensive Characterization of Vast Number of Variants Associated with X-Linked Hypophosphatemia (XLH) Hum. Mutat. 2022;43:143–157. doi: 10.1002/humu.24296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rush E.T., Johnson B., Aradhya S., Beltran D., Bristow S.L., Eisenbeis S., Guerra N.E., Krolczyk S., Miller N., Morales A., et al. Molecular Diagnoses of X-Linked and Other Genetic Hypophosphatemias: Results from a Sponsored Genetic Testing Program. J. Bone Miner. Res. 2022;37:202–214. doi: 10.1002/jbmr.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamazaki Y., Okazaki R., Shibata M., Hasegawa Y., Satoh K., Tajima T., Takeuchi Y., Fujita T., Nakahara K., Yamashita T., et al. Increased Circulatory Level of Biologically Active Full-Length FGF-23 in Patients with Hypophosphatemic Rickets/Osteomalacia. J. Clin. Endocrinol. Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C., Zhao Z., Sun Y., Xu L., JiaJue R., Cui L., Pang Q., Jiang Y., Li M., Wang O., et al. Clinical and Genetic Analysis in a Large Chinese Cohort of Patients with X-Linked Hypophosphatemia. Bone. 2019;121:212–220. doi: 10.1016/j.bone.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Morey M., Castro-Feijóo L., Barreiro J., Cabanas P., Pombo M., Gil M., Bernabeu I., Díaz-Grande J.M., Rey-Cordo L., Ariceta G., et al. Genetic Diagnosis of X-Linked Dominant Hypophosphatemic Rickets in a Cohort Study: Tubular Reabsorption of Phosphate and 1,25(OH)2D Serum Levels Are Associated with PHEX Mutation Type. BMC Med. Genet. 2011;12:116. doi: 10.1186/1471-2350-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vila-Pérez D., Marín-del-Barrio S., Vila-Cots J., Camacho-Díaz J.A., Morey M., Loidi L. Four Cases of X-Linked Hypophosphatemic Rickets, Clinical Description and Genetic Testing. Open J. Genet. 2014;4:40–45. doi: 10.4236/ojgen.2014.41006. [DOI] [Google Scholar]
- 38.Rodríguez-Rubio E., Gil-Peña H., Chocron S., Madariaga L., de la Cerda-Ojeda F., Fernández-Fernández M., de Lucas-Collantes C., Gil M., Luis-Yanes M.I., Vergara I., et al. Phenotypic Characterization of X-Linked Hypophosphatemia in Pediatric Spanish Population. Orphanet J. Rare Dis. 2021;16:104. doi: 10.1186/s13023-021-01729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makras P., Hamdy N.A.T., Kant S.G., Papapoulos S.E. Normal Growth and Muscle Dysfunction in X-Linked Hypophosphatemic Rickets Associated with a Novel Mutation in the PHEX Gene. J. Clin. Endocrinol. Metab. 2008;93:1386–1389. doi: 10.1210/jc.2007-1296. [DOI] [PubMed] [Google Scholar]
- 40.Cauliez A., Zhukouskaya V.V., Hilliquin S., Sadoine J., Slimani L., Miceli-Richard C., Briot K., Linglart A., Chaussain C., Bardet C. Impact of Early Conventional Treatment on Adult Bone and Joints in a Murine Model of X-Linked Hypophosphatemia. Front. Cell Dev. Biol. 2020;8:591417. doi: 10.3389/fcell.2020.591417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zivičnjak M., Schnabel D., Billing H., Staude H., Filler G., Querfeld U., Schumacher M., Pyper A., Schröder C., Brämswig J., et al. Age-Related Stature and Linear Body Segments in Children with X-Linked Hypophosphatemic Rickets. Pediatr. Nephrol. 2011;26:223–231. doi: 10.1007/s00467-010-1705-9. [DOI] [PubMed] [Google Scholar]
- 42.Chanchlani R., Nemer P., Sinha R., Nemer L., Krishnappa V., Sochett E., Safadi F., Raina R. An Overview of Rickets in Children. Kidney Int. Rep. 2020;5:980–990. doi: 10.1016/j.ekir.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haffner D., Emma F., Eastwood D.M., Duplan M.B., Bacchetta J., Schnabel D., Wicart P., Bockenhauer D., Santos F., Levtchenko E., et al. Clinical Practice Recommendations for the Diagnosis and Management of X-Linked Hypophosphataemia. Nat. Rev. Nephrol. 2019;15:435–455. doi: 10.1038/s41581-019-0152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharkey M.S., Grunseich K., Carpenter T.O. Contemporary Medical and Surgical Management of X-Linked Hypophosphatemic Rickets. J. Am. Acad. Orthop. Surg. 2015;23:433–442. doi: 10.5435/JAAOS-D-14-00082. [DOI] [PubMed] [Google Scholar]
- 45.Sochett E., Doria A.S., Henriques F., Kooh S.W., Daneman A., Mäkitie O. Growth and Metabolic Control during Puberty in Girls with X-Linked Hypophosphataemic Rickets. Horm. Res. 2004;61:252–256. doi: 10.1159/000077401. [DOI] [PubMed] [Google Scholar]
- 46.Carpenter T.O., Shaw N.J., Portale A.A., Ward L.M., Abrams S.A., Pettifor J.M. Rickets. Nat. Rev. Dis. Primers. 2017;3:17101. doi: 10.1038/nrdp.2017.101. [DOI] [PubMed] [Google Scholar]
- 47.Holm I.A., Nelson A.E., Robinson B.G., Mason R.S., Marsh D.J., Cowell C.T., Carpenter T.O. Mutational Analysis and Genotype-Phenotype Correlation of the PHEX Gene in X-Linked Hypophosphatemic Rickets. J. Clin. Endocrinol. Metab. 2001;86:3889–3899. doi: 10.1210/jcem.86.8.7761. [DOI] [PubMed] [Google Scholar]
- 48.Rafaelsen S., Johansson S., Ræder H., Bjerknes R. Hereditary Hypophosphatemia in Norway: A Retrospective Population-Based Study of Genotypes, Phenotypes, and Treatment Complications. Eur. J. Endocrinol. 2016;174:125–136. doi: 10.1530/EJE-15-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chesher D., Oddy M., Darbar U., Sayal P., Casey A., Ryan A., Sechi A., Simister C., Waters A., Wedatilake Y., et al. Outcome of Adult Patients with X-Linked Hypophosphatemia Caused by PHEX Gene Mutations. J. Inherit. Metab. Dis. 2018;41:865–876. doi: 10.1007/s10545-018-0147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whyte M.P., Schranck F.W., Armamento-Villareal R. X-Linked Hypophosphatemia: A Search for Gender, Race, Anticipation, or Parent of Origin Effects on Disease Expression in Children. J. Clin. Endocrinol. Metab. 1996;81:4075–4080. doi: 10.1210/jcem.81.11.8923863. [DOI] [PubMed] [Google Scholar]
- 51.Beck-Nielsen S.S., Mughal Z., Haffner D., Nilsson O., Levtchenko E., Ariceta G., de Lucas Collantes C., Schnabel D., Jandhyala R., Mäkitie O. FGF23 and Its Role in X-Linked Hypophosphatemia-Related Morbidity. Orphanet J. Rare Dis. 2019;14:58. doi: 10.1186/s13023-019-1014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabbagh Y., Jones A.O., Tenenhouse H.S. PHEXdb, a Locus-Specific Database for Mutations Causing X-Linked Hypophosphatemia. Hum. Mutat. 2000;16:1–6. doi: 10.1002/1098-1004(200007)16:1<1::AID-HUMU1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 53.Gaucher C., Walrant-Debray O., Nguyen T.-M., Esterle L., Garabédian M., Jehan F. PHEX Analysis in 118 Pedigrees Reveals New Genetic Clues in Hypophosphatemic Rickets. Hum. Genet. 2009;125:401–411. doi: 10.1007/s00439-009-0631-z. [DOI] [PubMed] [Google Scholar]
- 54.Li B., Wang X., Hao X., Liu Y., Wang Y., Shan C., Ao X., Liu Y., Bao H., Li P. A Novel c.2179T>C Mutation Blocked the Intracellular Transport of PHEX Protein and Caused X-Linked Hypophosphatemic Rickets in a Chinese Family. Mol. Genet. Genomic Med. 2020;8:e1262. doi: 10.1002/mgg3.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sabbagh Y., Boileau G., Campos M., Carmona A.K., Tenenhouse H.S. Structure and Function of Disease-Causing Missense Mutations in the PHEX Gene. J. Clin. Endocrinol. Metab. 2003;88:2213–2222. doi: 10.1210/jc.2002-021809. [DOI] [PubMed] [Google Scholar]
- 56.Zheng B., Wang C., Chen Q., Che R., Sha Y., Zhao F., Ding G., Zhou W., Jia Z., Huang S., et al. Functional Characterization of PHEX Gene Variants in Children with X-Linked Hypophosphatemic Rickets Shows No Evidence of Genotype-Phenotype Correlation. J. Bone Miner. Res. 2020;35:1718–1725. doi: 10.1002/jbmr.4035. [DOI] [PubMed] [Google Scholar]
- 57.Alenazi B., Molla M.A.M., Alshaya A., Saleh M. X-Linked Hypophosphatemic Rickets (PHEX Mutation): A Case Report and Literature Review. Sudan. J. Paediatr. 2017;17:61–65. [PMC free article] [PubMed] [Google Scholar]
- 58.Song H.R., Park J.W., Cho D.Y., Yang J.H., Yoon H.R., Jung S.C. PHEX Gene Mutations and Genotype-Phenotype Analysis of Korean Patients with Hypophosphatemic Rickets. J. Korean Med. Sci. 2007;22:981–986. doi: 10.3346/jkms.2007.22.6.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho H.Y., Lee B.H., Kang J.H., Ha I.S., Cheong H.I., Choi Y. A Clinical and Molecular Genetic Study of Hypophosphatemic Rickets in Children. Pediatr. Res. 2005;58:329–333. doi: 10.1203/01.PDR.0000169983.40758.7B. [DOI] [PubMed] [Google Scholar]
- 60.Hamdy N.A.T., Harvengt P., Usardi A. X-Linked Hypophosphatemia: The Medical Expert’s Challenges and the Patient’s Concerns on Their Journey with the Disease. Arch. Pediatr. 2021;28:612–618. doi: 10.1016/j.arcped.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Laurent M.R., De Schepper J., Trouet D., Godefroid N., Boros E., Heinrichs C., Bravenboer B., Velkeniers B., Lammens J., Harvengt P., et al. Consensus Recommendations for the Diagnosis and Management of X-Linked Hypophosphatemia in Belgium. Front. Endocrinol. 2021;12:641543. doi: 10.3389/fendo.2021.641543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borghi M.M.S., Coates V., Omar H.A. Evaluation of Stature Development during Childhood and Adolescence in Individuals with Familial Hypophosphatemic Rickets. ScientificWorldJournal. 2005;5:868–873. doi: 10.1100/tsw.2005.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santos Rodríguez F. X-Linked Hypophosphataemic Rickets and Growth. Adv. Ther. 2020;37((Suppl. 2)):55–61. doi: 10.1007/s12325-019-01178-z. [DOI] [PubMed] [Google Scholar]
- 64.Jurcă M.C., Bembea M., Kozma K., Şandor M.I., Negrean R.A., Dobjanschi L., Cuc E.A., Petcheşi C.D., Jurcă A.D. Empty Sella Associated with Growth Hormone Deficiency and Polydactyly. Rom. J. Morphol. Embryol. 2018;59:381–384. [PubMed] [Google Scholar]
- 65.Kaneko I., Segawa H., Ikuta K., Hanazaki A., Fujii T., Tatsumi S., Kido S., Hasegawa T., Amizuka N., Saito H., et al. Eldecalcitol Causes FGF23 Resistance for Pi Reabsorption and Improves Rachitic Bone Phenotypes in the Male Hyp Mouse. Endocrinology. 2018;159:2741–2758. doi: 10.1210/en.2018-00109. [DOI] [PubMed] [Google Scholar]
- 66.Beck-Nielsen S.S., Brusgaard K., Rasmussen L.M., Brixen K., Brock-Jacobsen B., Poulsen M.R., Vestergaard P., Ralston S.H., Albagha O.M.E., Poulsen S., et al. Phenotype Presentation of Hypophosphatemic Rickets in Adults. Calcif. Tissue Int. 2010;87:108–119. doi: 10.1007/s00223-010-9373-0. [DOI] [PubMed] [Google Scholar]
- 67.Drug Approval Package: CRYSVITA (Burosumab–Twza) [(accessed on 15 July 2022)]; Available online: https://medlineplus.gov/druginfo/meds/a618034.html.
- 68.EMA. [(accessed on 19 May 2022)]. Available online: https://www.ema.europa.eu/en/documents/product-information/crysvita-epar-product-information_en.p.
- 69.Baradhi K. Dramatic Transformation after Burosumab in a Young Boy with X-Linked Hypophosphatemia: A Life-Changing Saga. Cureus. 2022;14:e22340. doi: 10.7759/cureus.22340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kondo S., Takano T., Ono Y., Saito H., Matsumoto T. Eldecalcitol Reduces Osteoporotic Fractures by Unique Mechanisms. J. Steroid Biochem. Mol. Biol. 2015;148:232–238. doi: 10.1016/j.jsbmb.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 71.Raimann A., Mindler G.T., Kocijan R., Bekes K., Zwerina J., Haeusler G., Ganger R. Multidisciplinary Patient Care in X-Linked Hypophosphatemic Rickets: One Challenge, Many Perspectives. Wien. Med. Wochenschr. 2020;170:116–123. doi: 10.1007/s10354-019-00732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Insogna K.L., Rauch F., Kamenický P., Ito N., Kubota T., Nakamura A., Zhang L., Mealiffe M., San Martin J., Portale A.A. Burosumab Improved Histomorphometric Measures of Osteomalacia in Adults with X-Linked Hypophosphatemia: A Phase 3, Single-Arm, International Trial. J. Bone Miner. Res. 2019;34:2183–2191. doi: 10.1002/jbmr.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carpenter T.O., Whyte M.P., Imel E.A., Boot A.M., Högler W., Linglart A., Padidela R., van’t Hoff W., Mao M., Chen C.-Y., et al. Burosumab Therapy in Children with X-Linked Hypophosphatemia. N. Engl. J. Med. 2018;378:1987–1998. doi: 10.1056/NEJMoa1714641. [DOI] [PubMed] [Google Scholar]
- 74.Thacher T.D., Pettifor J.M., Tebben P.J., Creo A.L., Skrinar A., Mao M., Chen C.-Y., Chang T., San Martin J., Carpenter T.O. Rickets Severity Predicts Clinical Outcomes in Children with X-Linked Hypophosphatemia: Utility of the Radiographic Rickets Severity Score. Bone. 2019;122:76–81. doi: 10.1016/j.bone.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 75.Imel E.A., Glorieux F.H., Whyte M.P., Munns C.F., Ward L.M., Nilsson O., Simmons J.H., Padidela R., Namba N., Cheong H.I., et al. Burosumab versus Conventional Therapy in Children with X-Linked Hypophosphataemia: A Randomised, Active-Controlled, Open-Label, Phase 3 Trial. Lancet. 2019;393:2416–2427. doi: 10.1016/S0140-6736(19)30654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Padidela R., Whyte M.P., Glorieux F.H., Munns C.F., Ward L.M., Nilsson O., Portale A.A., Simmons J.H., Namba N., Cheong H.I., et al. Patient-Reported Outcomes from a Randomized, Active-Controlled, Open-Label, Phase 3 Trial of Burosumab versus Conventional Therapy in Children with X-Linked Hypophosphatemia. Calcif. Tissue Int. 2021;108:622–633. doi: 10.1007/s00223-020-00797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whyte M.P., Carpenter T.O., Gottesman G.S., Mao M., Skrinar A., San Martin J., Imel E.A. Efficacy and Safety of Burosumab in Children Aged 1-4 Years with X-Linked Hypophosphataemia: A Multicentre, Open-Label, Phase 2 Trial. Lancet Diabetes Endocrinol. 2019;7:189–199. doi: 10.1016/S2213-8587(18)30338-3. [DOI] [PubMed] [Google Scholar]
- 78.Insogna K.L., Briot K., Imel E.A., Kamenický P., Ruppe M.D., Portale A.A., Weber T., Pitukcheewanont P., Cheong H.I., Jan de Beur S., et al. A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial Evaluating the Efficacy of Burosumab, an Anti-FGF23 Antibody, in Adults with X-Linked Hypophosphatemia: Week 24 Primary Analysis. J. Bone Miner. Res. 2018;33:1383–1393. doi: 10.1002/jbmr.3475. [DOI] [PubMed] [Google Scholar]
- 79.Cheong H.I., Yoo H.-W., Adachi M., Tanaka H., Fujiwara I., Hasegawa Y., Harada D., Sugimoto M., Okada Y., Kato M., et al. First-in-Asian Phase I Study of the Anti-Fibroblast Growth Factor 23 Monoclonal Antibody, Burosumab: Safety and Pharmacodynamics in Adults with X-Linked Hypophosphatemia: First-in-Asian Phase i Study of Burosumab, Anti-Fgf23 Antibody. JBMR Plus. 2019;3:e10074. doi: 10.1002/jbm4.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gizard A., Rothenbuhler A., Pejin Z., Finidori G., Glorion C., de Billy B., Linglart A., Wicart P. Outcomes of Orthopedic Surgery in a Cohort of 49 Patients with X-Linked Hypophosphatemic Rickets (XLHR) Endocr. Connect. 2017;6:566–573. doi: 10.1530/EC-17-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kinoshita Y., Fukumoto S. X-Linked Hypophosphatemia and FGF23-Related Hypophosphatemic Diseases: Prospect for New Treatment. Endocr. Rev. 2018;39:274–291. doi: 10.1210/er.2017-00220. [DOI] [PubMed] [Google Scholar]
- 82.Carpenter T.O., Imel E.A., Holm I.A., Jan de Beur S.M., Insogna K.L. A Clinician’s Guide to X-Linked Hypophosphatemia. J. Bone Miner. Res. 2011;26:1381–1388. doi: 10.1002/jbmr.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baroncelli G.I., Mora S. X-Linked Hypophosphatemic Rickets: Multisystemic Disorder in Children Requiring Multidisciplinary Management. Front. Endocrinol. 2021;12:688309. doi: 10.3389/fendo.2021.688309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.