Significance
Testing, contact tracing, and isolation programs have successfully mitigated the COVID-19 pandemic in some countries but have largely failed in the United States. We analyzed contact tracing data in a large US metropolitan area and used a stochastic model of COVID-19 transmission to project the epidemiological impacts of testing and tracing. We found that testing followed by case isolation would be expected to significantly reduce transmission but that the addition of contact tracing would have a relatively negligible impact, given the observed pace and success of follow-up with contacts in the community. Contact tracing programs can effectively mitigate local COVID-19 risks if testing and contact follow-up are significantly accelerated.
Keywords: COVID-19, pandemic, mathematical model, contact tracing, COVID-19 testing
Abstract
Although testing, contact tracing, and case isolation programs can mitigate COVID-19 transmission and allow the relaxation of social distancing measures, few countries worldwide have succeeded in scaling such efforts to levels that suppress spread. The efficacy of test-trace-isolate likely depends on the speed and extent of follow-up and the prevalence of SARS-CoV-2 in the community. Here, we use a granular model of COVID-19 transmission to estimate the public health impacts of test-trace-isolate programs across a range of programmatic and epidemiological scenarios, based on testing and contact tracing data collected on a university campus and surrounding community in Austin, TX, between October 1, 2020, and January 1, 2021. The median time between specimen collection from a symptomatic case and quarantine of a traced contact was 2 days (interquartile range [IQR]: 2 to 3) on campus and 5 days (IQR: 3 to 8) in the community. Assuming a reproduction number of 1.2, we found that detection of 40% of all symptomatic cases followed by isolation is expected to avert 39% (IQR: 30% to 45%) of COVID-19 cases. Contact tracing is expected to increase the cases averted to 53% (IQR: 42% to 58%) or 40% (32% to 47%), assuming the 2- and 5-day delays estimated on campus and in the community, respectively. In a tracing-accelerated scenario, in which 75% of contacts are notified the day after specimen collection, cases averted increase to 68% (IQR: 55% to 72%). An accelerated contact tracing program leveraging rapid testing and electronic reporting of test results can significantly curtail local COVID-19 transmission.
severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was declared a global pandemic on March 11, 2020 (1) and has claimed at least 5 million lives as of January 5, 2022 (2). Prior to the distribution of effective vaccines, countries relied on nonpharmaceutical interventions to mitigate the spread of the disease, such as school closures, travel restrictions, shelter-in-place orders, and closure of nonessential commercial activities (3). Although such measures have slowed transmission, their psychological, social, and economic costs have been substantial (4). Specifically, socially distanced populations are at increased risk of developing depression, anxiety, and loneliness (5, 6). Surveys from college students have shown that more than 70% of the participants had increased stress levels during the pandemic (7). Children experienced food insecurity, limited social and emotional development, and decreased academic achievement because of COVID-19–related school closure and remote education (8). The global economic growth was estimated to be an annualized rate of −4.5% to −6% (9) and the US gross domestic product decreased by 3.5% in 2020 (10), largely because of the pandemic-associated mortality, morbidity, and productivity loss caused by social distancing policies (11). In order to safely relax these costly measures, additional intervention strategies are required to prevent new waves of the COVID-19 pandemic, such as cocooning high-risk populations, wearing face masks, vaccination, and extensive testing, contact tracing, and isolation (henceforth, test-trace-isolate) programs (12, 13).
Tracing and testing close contacts of someone with COVID-19 can identify new cases earlier than self-identification of symptoms; immediate isolation can then limit transmission to others (14). Contact tracing has traditionally been used by public health departments to control a myriad of infectious diseases, including tuberculosis, HIV, Ebola, and SARS (15). Despite historic use, COVID-19 contact tracing efforts have been implemented with mixed success across the globe (16). Successful programs in South Korea, Singapore, and China (17) have relied heavily on the use of digital technology to enhance routine surveillance of cases. In South Korea, where testing is widespread, the number of cases remained under 30,000 until mid-November 2020, when a third pandemic wave led to a rapid rise in case counts (18). South Korea initially kept case counts low by integrating data from case interviews, medical records, Global Positioning System (GPS) from mobile phones, credit card transactions, and footage from video cameras to identify as many contacts as possible (19, 20). The first two waves were characterized by local clusters that were aggressively and effectively contained via these measures. However, during the third wave, delayed social distancing policies and widespread community transmission challenged these efforts. Contact tracing resources in South Korea were eventually diverted toward vaccination as the country’s case load surged in 2021 (21). In Singapore, traditional surveillance methods were initially extended to include contact tracing of suspected cases (20) and the use of Bluetooth-based technology on mobile phones to rapidly identify close contacts. A separate electronic visitor registration system was developed to track visitors to places providing essential services (20). Unlike South Korea, Singapore maintained its aggressive contact tracing efforts (22, 23) in addition to implementing strict social distancing measures, resulting in only 832 deaths as of January 5, 2022 (24).
Contact tracing has been less effective in other countries, such as the United States and United Kingdom. Privacy concerns have impeded mass use of digital tracing technology (16). Instead, public health authorities have primarily relied on phone interviews with cases and contacts (25). This highly manual process is slower and may be less comprehensive than digital approaches, especially when relying on voluntary participation by cases (26), insufficient public health resources, and long delays in receiving test results (27). In the United States, 55% of survey respondents in early September 2020 reported a test turnaround time of more than 3 days (28); in Singapore, the average delay was 1 day (29). Data collected from 62 health departments across the United States between June 25 and July 24, 2020 (30) illustrate the delays and gaps that have plagued test-trace-isolate programs throughout the pandemic, with a median of 57% (interquartile range [IQR]: 27% to 82%) of cases interviewed within 1 day of receipt of a positive test result (which does not account for the initial test turnaround time) and a median of 55% (IQR: 32% to 79%) of contacts reached within 1 day of the interview. Higher caseloads were associated with even longer delays and lower proportions of cases immediately investigated. During this period, North Carolina counties reported a median delay of 6 days between specimen collection and contact notification, suggesting a diminished capacity to interrupt transmission during a pandemic surge (31).
In March 2020, when the pandemic emerged in the city of Austin, TX, Dell Medical School at the University of Texas at Austin (UT) began contact tracing through a partnership with Austin Public Health. By June 2020, 281 student and community volunteers were recruited and trained. Existing software applications were configured to capture data from COVID-19 cases and their contacts, and the onboarding and scheduling of volunteers were automated for efficiency. As cases surged in summer 2020, local laboratory and contact tracing operations that relied heavily on human resources became strained. Public health authorities rapidly procured additional isolation facilities and scrambled to determine an effective allocation of limited contact tracing resources. Specifically, they faced a trade-off between tracing coverage (i.e., the numbers of contacts traced per index case) and tracing speed (i.e., time between identification of index case and notification of infected contact). In this study, we use a COVID-19 transmission model informed by local COVID-19 hospital admissions and contact tracing data to evaluate the impact of test-trace-isolate efforts on mitigating the pandemic. We estimate the impacts of expanding test availability while accelerating contact tracing on the expected number of SARS-CoV-2 infections over an 7-mo period.
Results
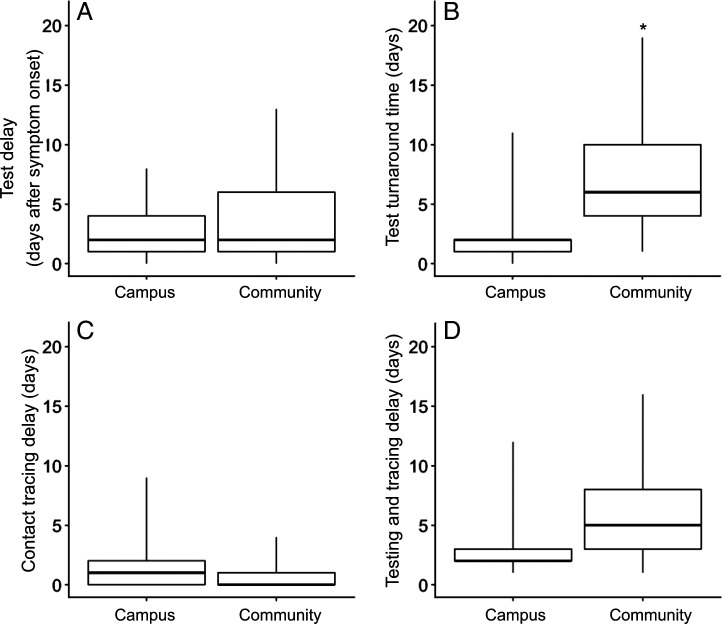
Between October 1, 2020, and January 1, 2021, symptomatic cases sought a COVID-19 test a median of 2 days (Fig. 1A) after symptom onset both at UT (campus) (IQR: 1 to 4) and in the surrounding community (community) (IQR: 1 to 6). Case reports (i.e., positive test confirmation) were acquired by the contact tracing program (Fig. 1B) a median of 2 days after specimen collection on campus (IQR: 1 to 2) and a median of 6 days (IQR: 4 to 10) in the community. Contacts of cases tested on campus were notified of their exposure (Fig. 1C) a median of 1 day following receipt of the index case report (IQR: 0 to 2 days). For cases tested in the community, contacts were typically notified on the day of case report receipt (median: 0, IQR: 0 to 1 days). Among the 620 cases reported from campus testing sites, 519 cases (83.7%) were successfully interviewed. Of the 133 cases that tested in the community, 96 cases (72.2%) were successfully interviewed (Table 1). Among all named contacts, 1,144 out of 1,506 contacts (76.0%) were notified of their exposure on campus and 133 out of 194 contacts (68.6%) were notified in the community. The total testing and tracing delay (Fig. 1D) was a median of 2 days (IQR: 2 to 3) and 5 days (IQR: 3 to 8) for cases on campus and in the community, respectively.
Fig. 1.
Delays associated with seeking test, test turnaround, and contact tracing of cases reported from community and campus testing sites from October 1, 2020, to January 1, 2021, in Austin, TX. (A) Delay from symptom onset to specimen collection from an index case. (B) Delay from specimen collection from an index case, to report of the index case to contact tracing. (C) Delay from report of an index case to notification of exposure to contacts. (D) Delay from specimen collection from an index case to notification of exposure to contacts. Boxplots display medians, IQRs, and ranges; the star in B indicates an outlier from community testing sites.
Table 1.
Cases investigated and contacts notified by UT contact tracing from October 1, 2020, to January 1, 2021, in Austin, TX
| % of cases interviewed (No. of interviewed/ total reported) |
% of contacts notified (No. of notified/ total reported) |
|
|---|---|---|
| Campus | 83.7% (519/620) | 76.0% (1,144/1,506) |
| Community | 72.2% (96/133) | 68.6% (133/194) |
Assuming symptomatic cases are tested and isolated an average of 2 days after symptom onset, we simulated two epidemic scenarios across all combinations of three symptomatic case detection ratios, four levels of contact tracing coverage, and two lags between specimen collection from the index case and contact notification.
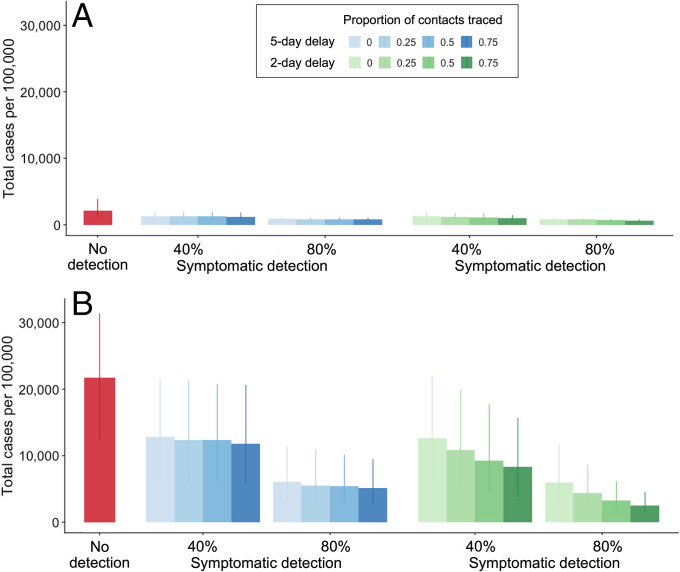
We projected that the total number of COVID-19 infections in the Austin–Round Rock Metropolitan Statistical Area (MSA) from November 8, 2020, to May 31, 2021, would total 45,992 (IQR: 26,506 to 84,578) and 471,143 (IQR: 269,684 to 680,501) assuming R0 = 0.95 and R0 = 1.2, respectively (Fig. 2). From November 8, 2020, to May 31, 2021, isolating 40% of symptomatic cases 2 days after their symptom onset (i.e., at the time of specimen collection) would be expected to reduce the total number of COVID-19 cases by 39.3% (IQR: 28.3% to 49.0%) and 39.4% (IQR: 29.5% to 44.8%) in the low-transmission (R0 = 0.95) and high-transmission (R0 = 1.2) scenarios, respectively (Fig. 2). These effect sizes account for the cumulative impact of isolation on averting multiple generations of transmission. A higher symptomatic detection ratio of 80% would be expected to further mitigate spread, with corresponding reductions in overall infections of 57.8% (IQR: 45.7% to 69.9%) and 69.1% (IQR: 58.4% to 72.7%) across the 7-mo study period. Under the 40% case detection scenario, if we also traced and isolated 25%, 50%, or 75% of contacts within 5 days of specimen collection, then the expected reductions in infections would increase only slightly to 39.5% (IQR: 29.7% to 45.5%), 40.4% (IQR: 31.8% to 46.3%), and 41.0% (IQR: 32.4% to 47.5%), respectively. The expected impacts would be significantly larger if we assumed testing and tracing delays were shortened to the levels in the campus setting (i.e., 2 days), with expected cases averted increasing to 46.2% (IQR: 35.3% to 52.0%), 53.2% (IQR: 41.9% to 58.4%), and 59.1% (IQR: 48.2% to 64.6%), respectively (Fig. 2B), during the 7th-mo period. If the delay is shortened to a single day, the expected cases averted would increase to 50.0% (IQR: 39.2% to 55.5%), 59.4% (IQR: 49.4% to 64.6%), 67.6% (IQR: 55.1% to 71.7%), respectively, within the study period. In the most optimistic scenario of an 80% symptomatic case detection rate combined with 75% contacts isolated or quarantined the next day of specimen collection, we estimated that contact tracing could avert 90.4% (IQR: 86.9% to 91.7%) of infections.
Fig. 2.
Expected COVID-19 attack rate from November 8, 2020, to May 31, 2021, across a range of scenarios for case detection and proportion of contacts traced, assuming reproduction numbers of (A) R0 = 0.95 and (B) R0 = 1.2. Red bars represent the projected number of COVID-19 cases per 100,000 in the absence of testing. The left and right sets of bars correspond to 40% and 80% symptomatic case detection rates, respectively, assuming that detected cases seek a test 2 days after symptom onset and isolate at the time of specimen collection for the duration of their infectious period. Blue and green shading ranges from no contact tracing (light) to 75% of contacts isolated (dark) either 5 days (blue) or 2 days (green) after specimen collection from the index case. Bars and whiskers are medians and IQRs from 200 stochastic simulations, respectively.
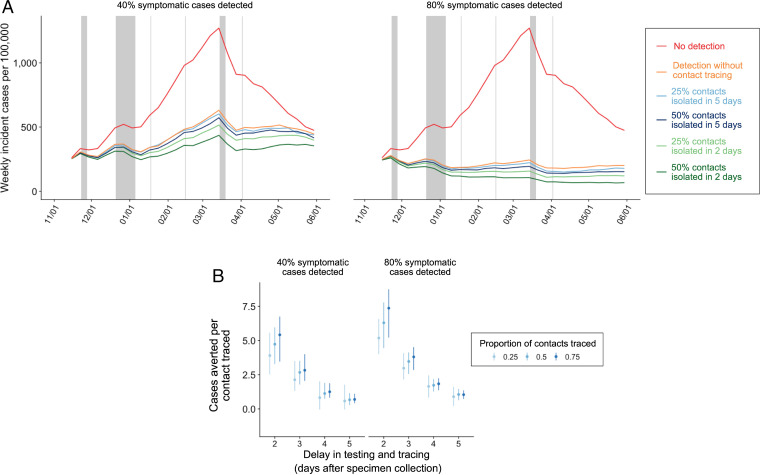
Assuming R0 = 1.2, a 40% symptomatic case detection rate would be expected to flatten the epidemic curve and an 80% detection rate would be expected to effectively contain spread (Fig. 3). Contact tracing could further suppress transmission, especially when accelerated. If test turnaround time and contact tracing takes as long as 5 days, it would be expected to have only a modest impact on population-level disease burden. However, if contacts are traced within 2 days, a pessimistic scenario of a 40% symptomatic case detection rate and 25% tracing success rate would avert an expected 3.9 (IQR: 2.5 to 5.6) infections per index case traced; a more optimistic scenario of an 80% detection rate and 75% success rate would be expected to increase the impact of each traced index case to 7.4 (IQR: 5.2 to 8.7) infections averted (Fig. 3).
Fig. 3.
Expected COVID-19 incidence and cases averted per contact traced from November 8, 2020, to May 31, 2021, across a range of case detection and contact tracing scenarios, assuming R0 = 1.2. (A) Median estimated weekly incident COVID-19 cases across 200 stochastic simulations. Red curves correspond to the no testing or tracing scenario. Left and Right assume 40% and 80% of all symptomatics are detected and isolated, respectively. Orange curves correspond to testing without tracing, blue curves assume 25% or 50% of the contacts of the confirmed cases are traced and isolated 5 days after the isolation of the confirmed case, and green curves assume 25% or 50% of the contacts of the confirmed cases are traced and isolated after 2 days. The gray vertical shading represents Thanksgiving break, winter break, and spring break for the local school district, and the other vertical lines represent other school holidays including Martin Luther King Day, Presidents’ Day, and Easter (32). (B) Number of COVID-19 cases averted per contact successfully traced as a function of lag from specimen collection from the index case to isolation of the contact. Left and Right assume 40% and 80% of symptomatic cases are detected, respectively. The blue shading indicates contact tracing success rates of 25%, 50%, or 75%. The points are medians from 200 paired stochastic simulations, and the error bars are IQRs.
We estimated the impact of isolation on secondary transmission from successfully traced contacts, depending on the delay between index case specimen collection and isolation of the traced contacts, assuming a 40% symptomatic case detection rate with a 2-day lag between symptom onset and testing and a 50% contact tracing success rate. If a contact is isolated within 1 day of the index case’s specimen collection, then isolation occurs after an estimated 20.6% of the case’s total infectivity has transpired, effectively blocking 79.4% of overall infectivity. For the observed 2- and 5-day delays in tracing, contact isolation is expected to block 55.1% and 15.6% of infectivity, respectively (Table 2). At a reproduction number of R0 = 1.2, these estimates imply that a 1-, 2-, or 5-day lag between index case specimen collection and contact isolation will prevent 65.1% (IQR: 53% to 70%), 53% (IQR: 43% to 58%), or 40.4% (IQR: 32% to 47%) of secondary infections from the isolated contacts, respectively.
Table 2.
Cumulative infectivity of a traced contact prior to isolation and percentage of infections averted by isolation under two transmission scenarios
| Days from index case specimen collection to contact isolation | Cumulative infectivity prior to isolation (%) | % of infections averted (R0 = 0.95) | % of infections averted (R0 = 1.2) |
|---|---|---|---|
| 0 | 20.6 | 54 (42–66) | 65 (53–70) |
| 1 | 31.8 | 51 (39–63) | 59 (48–65) |
| 2 | 44.9 | 47 (35–59) | 53 (42–58) |
| 3 | 58.9 | 43 (31–54) | 47 (35–53) |
| 4 | 72.5 | 41 (29–52) | 43 (33–49) |
| 5 | 84.4 | 39 (28–51) | 40 (32–47) |
Two transmission scenarios: R0 = 0.95 and R0 = 1.2. Estimates assume a 40% detection rate for symptomatic cases, a 2-day lag between symptom onset and specimen collection from the index case, and a 50% contact tracing success rate. Values are medians and IQRs from 200 simulations.
Discussion
Using a mathematical model of COVID-19 transmission, we estimated the epidemiological impacts of contact tracing and isolation based on data from a large contact tracing program in Austin, TX. The symptomatic case detection rate and speed of contact tracing are positively correlated with reduced transmission. Given the observed pace of contact tracing in the Austin community, our model projects that contact tracing in similar contexts would only modestly reduce transmission beyond reductions achieved through the isolation of index cases. The more rapid contact tracing on UT’s campus may have had a greater impact on preventing spread, which could be amplified by increasing the proportion of contacts successfully traced. We estimate that reducing the delay between specimen collection of the index case and contact isolation from 5 to 2 days would reduce the number of secondary infections by 40%. The importance of case detection and rapid isolation is also noted in a modeling study by Grantz et al. (33). The authors found that in most scenarios, the largest expected reductions in transmission result from increasing the proportion of cases detected and isolated. Only when a sufficient proportion of cases are detected do other factors, such as speed and contact coverage, begin to matter.
With sufficient testing and isolation in place (i.e., 40% of symptomatic cases), we found that the addition of rapid and effective contact tracing (i.e., 1-day delay achieving 75% coverage) is expected to prevent roughly as many infections as doubling the symptomatic isolation rate from 40% to 80%. However, the addition of slow contact tracing (i.e., a 5-day delay) does little to prevent transmission. Whether delayed or not, contact tracing brings other unmeasured benefits. The case and contact information gathered through interviews provides critical epidemiological insight into disease transmission in various communities (34). During these interviews, contact tracers can provide valuable health education, including information about mask wearing, other precautionary behavior, and vaccination. Contacts are encouraged to seek a test even if asymptomatic, which can improve case detection. These interviews also provide an opportunity to link cases and contacts to community resources to address any unmet health, social, and economic needs during their time in isolation or quarantine. Furthermore, the education and emotional support provided by contact tracers can lead to higher compliance with isolation and quarantine guidelines.
Given the many benefits of contact tracing, we believe these results should inform strategic efforts to increase the effectiveness rather than disband existing programs. Specifically, we find that accelerating tracing efforts and increasing the fraction of contacts successfully traced can reduce the COVID-19 burden. The contact tracing success rate is largely dependent on case and contact participation. While our estimates of case participation among those that test in the community are similar to other settings (26), we reported higher participation among cases that tested on campus. Institutional settings, such as universities and workplaces, may be able to take advantage of a shared sense of social responsibility and institutional identity to encourage higher participation in the test-trace-isolate strategy.
The shorter test turnaround times estimated on UT campus in comparison to the surrounding community reflect the efficiency provided by same-day reporting alongside contact tracing at a single institution. During epidemic surges, the increased demand on laboratories and healthcare providers can significantly slow result notification. For example, during the 2020 summer wave of COVID-19 in Texas, a 7-day turnaround from specimen collection was common (35). Expanding laboratory capacity, as well as interoperable public health surveillance and electronic health record systems, can reduce such delays.
In addition, rapid antigen tests have the potential to significantly improve contact tracing by accelerating that isolation of cases and, through nearly instant in-person notification, encouraging compliance. Test kits such as the Abbott BinaxNOW have been demonstrated to have high sensitivity and specificity in community settings among cases with high viral load (36). Increased investment in these point-of-care tests should be considered in high-risk settings such as daycares, schools, university residences, jails, and other congregate settings.
Finally, test-trace-isolate programs could be improved through backward contact tracing, which involves identifying individuals who may have infected the index case and tracing their contacts. Theoretical studies suggest that backward tracing is especially effective when the index case is infected by a superspreader (37, 38). Our results indicate that the impact of test-trace-isolate programs depends on tracing delays, which may be more extreme for backward tracing.
The study period included a large winter COVID-19 surge that peaked in January 2021. While other US counties reported long lags between case reports and contact notification during such peaks (31), UT managed to avoid delays using a large network of on-call volunteers. In general, a flexible workforce of public health professionals and volunteers that can pivot among multiple activities may be key to managing infectious disease surges. Reliable surveillance and predictive models can support the adaptive management of essential personnel. Gardner and Kilpatrick (39) even suggest that shifting personnel from high- or low-burden areas to areas with intermediate burden of COVID-19 could maximize impact on the epidemic. Similar to the conclusions made by Gardner and Kilpatrick (39) and Grantz et al. (33), our results highlight that tracing speed should be prioritized over the proportion of contacts traced. On a given day, if contact tracing programs are not able to call all reported cases and contacts, they could prioritize and possibly reset their priority list. The Centers for Disease Control and Prevention (CDC) recommends prioritizing cases with recent symptom onset or a recent positive test result for tracing (40). Priority might also be given to highly infectious cases, such as those with high viral loads or highly infectious variants. Alternatively, contacts may be prioritized according to how recent or high risk their exposures were (39, 41) and whether they live in high-risk environments (e.g., long-term care facilities).
Our model is stratified by age and underlying conditions. While some age groups engage in more frequent contacts than others, we do not explicitly model individuals with anomalously large numbers of contacts who could become superspreaders. Early identification and isolation of superspreaders could lead to greater reductions in transmission than those estimated. We also make the simplifying assumption that symptomatic cases comply with testing and isolation within 2 days of symptom onset and possibly before receiving test results. However, data from a LatinX community in the United States demonstrate that while adherence to self-isolation guidelines may be moderate to high, the median time from symptom onset to isolation can be as long as 7 days (42). Lower or delayed compliance would lead to lower expected efficacy of index case testing and isolation (SI Appendix, Section 3). We further assume that only symptomatic cases seek testing, which was typical early in the pandemic when testing was limited across the United States (43). With expanded and rapid testing of presymptomatic and asymptomatic cases, test-trace-isolate programs could further mitigate transmission of SARS-CoV-2.
Materials and Methods
We built a city-level susceptible-exposed-infected-recovered model of SARS-CoV-2 transmission (SI Appendix, Fig. S1). The stochastic compartmental model (SI Appendix, Section 1) incorporates city-specific age and risk structures, contact patterns, and various testing and contact tracing efforts to measure the effectiveness of testing and contact tracing. Stochasticity is introduced by sampling multiple parameters from distributions for each simulation, as well as using the τ-leap method (44, 45) to model stochastic transitions between the compartments. We analyzed the model with a focus on the Austin–Round Rock MSA, but the results could be applied to all US cities.
Local Testing and Contact Tracing.
Data were collected through contact tracing conducted by Dell Medical School, UT, working in partnership with Austin Public Health from October 1, 2020, to January 1, 2021. The contact tracing program received notifications of university-related COVID-19 cases from campus testing sites. University-related cases testing at other sites in the city were identified in collaboration with Austin Public Health. Campus testing sites were open on weekdays to both asymptomatic and symptomatic UT staff, faculty, and students, as well as to patients of UT Health Austin at Dell Medical School. Only cases with an indication of a recent infection with SARS-CoV-2 detected through nucleic acid amplification tests or an antigen test were investigated. Contact tracing was carried out through phone interviews to cases and contacts. Attempts were made to call cases and their contacts up to three times. In this analysis, we examined the delay from 1) index case symptom onset to specimen collection among symptomatic cases (test delay); 2) specimen collection, to confirmation and reporting of the test result, to contact tracing (test turnaround time); 3) reporting of the test result to first attempt to notify a contact of an exposure (contact tracing delay); and 4) specimen collection to first attempt to notify a contact of an exposure (testing and tracing delay). We used data reported to Austin Public Health by off-campus providers as a proxy for the test delay, test turnaround time, and tracing delay in the community. Data from campus testing sites were used to provide estimates of analogous delays at the university. We evaluated the percentage of cases investigated (the number of cases investigated divided by the number of cases reported to contact tracing) and the percentage of contacts successfully notified of their exposure (the number of contacts successfully notified of their exposure and interviewed among all contacts named during case investigations). The University of Texas Institutional Review Board (IRB) determined that the proposed activity is not research involving human subjects.
Transmission Model.
We divided the population into five age groups: 0 to 4, 5 to 17, 18 to 49, 50 to 64, and 65+ y based on the 2017 American Community Survey (46). Each age group was further separated into two risk groups based on the prevalence of chronic health conditions (SI Appendix, Section 2). Individuals who were not previously infected were considered susceptible to the disease. Exposed people moved into a latent period, in which they were slightly infectious but free of symptoms upon infection, and then proceeded into either asymptomatic or symptomatic compartments, both of which were infectious. Symptomatic individuals with severe disease moved into a hospitalized compartment, and some went on to become deceased. Recovered individuals were considered fully immune to the disease.
To model the effects of testing, contact tracing, and isolation, we split the exposed, asymptomatic, and symptomatic compartments into three subcompartments: undetectable, detectable, and detected and isolated. The ratio of detectable to undetectable depended on the detection ratio for each compartment. Individuals who were detectable moved into the isolated status after a period of time. A full description of the model is provided in the SI Appendix. We assumed that only symptomatic individuals seek COVID-19 testing voluntarily (referred to as symptomatic case detection), it takes a symptomatic individual 2 days to seek healthcare after symptom onset, and they are isolated at the time of the test. Following national contact tracing guidelines (25), contacts with symptoms are in isolation and contacts without symptoms are in quarantine.
All model parameters were based on published literature about COVID-19, as well as from discussion with Austin Public Health officials. We assumed the reproduction number to be 0.95 and 1.2, based on estimates of Rt from hospitalization admission and discharge data on November 7, 2020 (47). The transmission rate was calculated using the next-generation matrix method based on the specified reproduction number (48). Age-specific contact patterns were obtained using the published contact matrices (49) and were adjusted based on the local school calendar (32). We assumed 44% of infections happened before symptom onset and sampled the incubation period parameter from a triangular distribution from 4.2 to 6.2 days, with a mean of 5.2 days (50). The infectiousness of asymptomatic individuals was assumed to be 67% of that of symptomatic individuals (50), and the recovery periods of the symptomatic and asymptomatic individuals were assumed to be the same. If a symptomatic case was severe and required hospital care, hospital admission was estimated to happen 5.9 days after symptom onset (51). We sampled the hospitalization duration from a triangular distribution from 9.4 to 12.8 days, with an average of 10.7 days, based on Austin admissions and discharge data (52, 53). Deaths after hospitalization are estimated to follow a triangular distribution from 5.2 to 10.1 days, with an average of 8.1 days (52, 53). Following the CDC’s planning scenarios, we assumed that the high-risk population was 10 times more likely to be hospitalized and deceased after hospitalization compared to the low-risk individuals in the same age group.
Stochastic simulations began with projected compartment occupancies (SI Appendix, Table S1.8) based on estimates from a healthcare forecasting model (54) fitted to local hospital admission and discharge data in the Austin–Round Rock MSA (47) on November 8, 2020, and were updated in 2.4-h intervals. For each epidemic scenario, we ran 200 simulations and reported the median and IQR of weekly quantities. To quantify the effect of specific testing and tracing delays, we calculated the cumulative infectivity of a contact at the time they were notified of their exposure. This can be interpreted as the proportion of all secondary infections from the index case that are expected to occur by the time of notification in a scenario where the case is not isolated. Cumulative infectivity was calculated based on published estimates for the daily infectiousness of a case following symptom onset (55), with formulas provided in the SI Appendix.
Supplementary Material
Acknowledgments
This research was supported by NIH grant R01 AI151176 (to X.W., Z.D., S.J.F., and L.A.M.), CDC grant U01 IP001136 (to X.W., Z.D., S.J.F., and L.A.M.), and a donation from Love, Tito’s (the philanthropic arm of Tito’s Homemade Vodka, Austin, TX) to the University of Texas to support the modeling of COVID-19 mitigation strategies (to X.W., Z.D., M.L., L.A.M., and D.B.). D.B.’s effort on this project was also supported by core funds of the Dell Medical School at UT.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2200652119/-/DCSupplemental.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Wan W., WHO declares a pandemic of coronavirus disease covid-19. The Washington Post, 11 March 2020. https://www.washingtonpost.com/health/2020/03/11/who-declares-pandemic-coronavirus-disease-covid-19/. Accessed 25 March 2020.
- 2.World Health Organization, WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Accessed 30 August 2021.
- 3.MITRE Corporation, COVID-19 Healthcare Coalition. https://c19hcc.org/. Accessed 9 December 2020.
- 4.Nicola M., et al. , The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 78, 185–193 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galea S., Merchant R. M., Lurie N., The mental health consequences of COVID-19 and physical distancing: The need for prevention and early intervention. JAMA Intern. Med. 180, 817–818 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Aknin L. B., et al. , Mental health during the first year of the COVID-19 pandemic: A review and recommendations for moving forward. Perspect. Psychol. Sci. 17, 915–936 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X., et al. , Investigating mental health of US college students during the COVID-19 pandemic: Cross-sectional survey study. J. Med. Internet Res. 22, e22817 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boutzoukas A. E., Akinboyo I. C., Wong C. A., D. K. Benjamin, Jr., Zimmerman K. O., Impact of COVID-19-related school closures on the drivers of child health. N. C. Med. J. 82, 50–56 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Congressional Research Service, Global Economic Effects of COVID-19. https://crsreports.congress.gov/product/pdf/R/R46270. Accessed 15 February 2021.
- 10.Bureau of Economic Analysis, Gross Domestic Product, 4th Quarter and Year 2020 (Advance Estimate) (2021). https://www.bea.gov/news/2021/gross-domestic-product-4th-quarter-and-year-2020-advance-estimate. Accessed 8 February 2021.
- 11.Thunström L., Newbold S. C., Finnoff D., Ashworth M., Shogren J. F., The benefits and costs of using social distancing to flatten the curve for COVID-19. J. Benefit Cost Anal. 11, 179–195 (2020). [Google Scholar]
- 12.Wang X., et al. , Effects of cocooning on coronavirus disease rates after relaxing social distancing. Emerg. Infect. Dis. 26, 3066–3068 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The White House, Opening Up America Again. https://www.whitehouse.gov/priorities/covid-19. Accessed 9 December 2020.
- 14.Larremore D. B., et al. , Test sensitivity is secondary to frequency and turnaround time for COVID-19 surveillance. medRxiv [Preprint] (2020). 10.1101/2020.06.22.20136309. Accessed 8 February 2021. [DOI] [PMC free article] [PubMed]
- 15.Watson C., Cicero A., Blumenstock J. S., Fraser M. R., A National Plan to Enable Comprehensive COVID-19 Case Finding and Contact Tracing in the US (Johns Hopkins Bloomberg School of Public Health, Center for Health Security, 2020). [Google Scholar]
- 16.Lewis D., Why many countries failed at COVID contact-tracing—but some got it right. Nature 588, 384–387 (2020). [DOI] [PubMed] [Google Scholar]
- 17.The Lancet Digital Health, Contact tracing: Digital health on the frontline. Lancet Digit. Health 2, e561 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Statista, South Korea: COVID-19 daily new cases 2020. Statista. https://www.statista.com/statistics/1102777/south-korea-covid-19-daily-new-cases. Accessed 15 February 2021.
- 19.COVID-19 National Emergency Response Center, Epidemiology & Case Management Team, Korea Centers for Disease Control & Prevention, Contact transmission of COVID-19 in South Korea: Novel investigation techniques for tracing contacts. Osong Public Health Res. Perspect. 11, 60–63 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai S. H. S., Tang C. Q. Y., Kurup A., Thevendran G., The experience of contact tracing in Singapore in the control of COVID-19: Highlighting the use of digital technology. Int. Orthop. 45, 65–69 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seong H., et al. , Comparison of the second and third waves of the COVID-19 pandemic in South Korea: Importance of early public health intervention. Int. J. Infect. Dis. 104, 742–745 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guest S. A. P., Singapore Implements New Level Of Contact Tracing To Fight Spread Of COVID-19. Forbes Magazine, 9 June 2021. https://www.forbes.com/sites/sap/2021/06/09/singapore-implements-new-level-of-contact-tracing-to-fight-spread-of-covid-19/. Accessed 7 September 2021.
- 23.Willis S., Singapore is trying to do what no other country has done: Pivot away from COVID-zero. Will it work? Fortune, 24 August 2021. https://fortune.com/2021/08/24/singapore-pivot-covid-zero-reopening-international-travels/. Accessed 7 September 2021.
- 24.World Health Organization, Singapore: WHO Coronavirus Disease (COVID-19) Dashboard With Vaccination Data. https://covid19.who.int/region/wpro/country/sg. Accessed 7 September 2021.
- 25.CDC, Contact Tracing for COVID-19 (2020). https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracing-plan/contact-tracing.html. Accessed 23 September 2020.
- 26.Miller J. S., et al. , COVID-19 case investigation and contact tracing in central Washington state, June-July 2020. J. Community Health 46, 918–921 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark E., Chiao E. Y., Amirian E. S., Why contact tracing efforts have failed to curb COVID-19 transmission in much of the U.S. Clin. Infect. Dis. 72, e415–e419 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGarry B. E., SteelFisher G. K., Grabowski D. C., Barnett M. L., COVID-19 test result turnaround time for residents and staff in US nursing homes. JAMA Intern. Med. 181, 556–559 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Health Sciences Authority, Testing and Diagnosis in Singapore (2020). https://covid19.healthserve.org.sg/en/info/testing-and-diagnosis-in-singapore/. Accessed 22 February 2021.
- 30.Spencer K. D., et al. , COVID-19 case investigation and contact tracing efforts from Health Departments—United States, June 25-July 24, 2020. MMWR Morb. Mortal. Wkly. Rep. 70, 83–87 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lash R. R., et al. ; Contact Tracing Assessment Team, COVID-19 contact tracing in two counties—North Carolina, June-July 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 1360–1363 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin ISD, Calendar of Events. https://www.austinisd.org/calendar. Accessed 26 March 2020.
- 33.Grantz K. H., et al. , Maximizing and evaluating the impact of test-trace-isolate programs: A modeling study. PLoS Med. 18, e1003585 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q., et al. , Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 382, 1199–1207 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dellinger H., As demand for COVID-19 testing surges, results lag in labs. Houston Chronicle, 2 July 2020. https://www.houstonchronicle.com/news/houston-texas/health/article/As-demand-for-COVID-19-testing-surges-results-15383430.php. Accessed 23 February 2021.
- 36.Pilarowski G., et al. , Field performance and public health response using the BinaxNOW TM Rapid SARS-CoV-2 antigen detection assay during community-based testing. Clin. Infect. Dis. 73, e3098-e3101. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kojaku S., Hébert-Dufresne L., Mones E., Lehmann S., Ahn Y.-Y., The effectiveness of backward contact tracing in networks. Nat. Phys. 17, 652–658 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Endo A., et al. ; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group, Implication of backward contact tracing in the presence of overdispersed transmission in COVID-19 outbreaks. Wellcome Open Res. 5, 239 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardner B. J., Kilpatrick A. M., Contact tracing efficiency, transmission heterogeneity, and accelerating COVID-19 epidemics. PLOS Comput. Biol. 17, e1009122 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.CDC, Prioritizing Case Investigations and Contact Tracing for COVID-19 in High Burden Jurisdictions (2020). https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracing-plan/prioritization.html. Accessed 2 December 2020.
- 41.Mack C. D., et al. ; NFL COVID-19 Advisory and Operational Team, Implementation and evolution of mitigation measures, testing, and contact tracing in the national football league, August 9-November 21, 2020. MMWR Morb. Mortal. Wkly. Rep. 70, 130–135 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubio L. A., et al. , The COVID-19 symptom to isolation cascade in a latinx community: A call to action. Open Forum Infect. Dis. 8, ofab023 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer R., America Isn’t Testing for the Most Alarming Coronavirus Cases. The Atlantic, 13 March 2020. https://www.theatlantic.com/science/archive/2020/03/who-gets-tested-coronavirus/607999/. Accessed 24 February 2021.
- 44.Keeling M. J., Rohani P., Modeling Infectious Diseases in Humans and Animals (Princeton University Press, 2011). [Google Scholar]
- 45.Gillespie D. T., Approximate accelerated stochastic simulation of chemically reacting systems. J. Chem. Phys. 115, 1716–1733 (2001). [Google Scholar]
- 46.US Census Bureau, American Community Survey (ACS). https://www.census.gov/programs-surveys/acs. Accessed 19 November 2019.
- 47.Austin Dashboard. https://covid-19.tacc.utexas.edu/dashboards/austin. Accessed 8 December 2020.
- 48.Diekmann O., Heesterbeek J. A. P., Roberts M. G., The construction of next-generation matrices for compartmental epidemic models. J. R. Soc. Interface 7, 873–885 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prem K., Cook A. R., Jit M., Projecting social contact matrices in 152 countries using contact surveys and demographic data. PLOS Comput. Biol. 13, e1005697 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He X., et al. , Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 26, 672–675 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Tindale L., et al. , Transmission interval estimates suggest pre-symptomatic spread of COVID-19. eLife 9, e57149 (2020).32568070 [Google Scholar]
- 52.Richardson S., et al. ; the Northwell COVID-19 Research Consortium, Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 323, 2052–2059 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewnard J. A., et al. , Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: Prospective cohort study. BMJ 369, m1923 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tec M., et al. , Austin COVID-19 transmission estimates and healthcare projections. https://sites.cns.utexas.edu/sites/default/files/cid/files/austin_dashboard_report_071520.pdf. Accessed 5 February 2021.
- 55.Ashcroft P., et al. , COVID-19 infectivity profile correction. Swiss Med. Wkly. 150, w20336 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.