Significance
The demand for electric vehicles with long driving range requires energy storage technology beyond Li-ion batteries. Aprotic Li-O2 batteries with high theoretical energy density have attracted significant interest as a potential alternative, but severe side reactions, especially singlet oxygen (1O2) and its related reactions, induce capacity decay and shorten the lifespan for Li-O2 batteries, which is also challenging for Li-rich materials. Although quenchers have been attempted to suppress 1O2, the reaction mechanism is ambiguous, which is important for rational design of quenchers. Here, an electrochemically stable and chemically reversible triphenylamine is demonstrated to be an efficient quencher to suppress 1O2 via nonradiative intersystem crossing and hence its associated side reactions, and then it significantly prolongs the lifetime for Li-O2 batteries.
Keywords: Li-O2 battery, singlet oxygen, triphenylamine, intersystem crossing mechanism, quencher
Abstract
Aprotic Li-O2 batteries are a promising energy storage technology, however severe side reactions during cycles lead to their poor rechargeability. Herein, highly reactive singlet oxygen (1O2) is revealed to generate in both the discharging and charging processes and is deterimental to battery stability. Electron-rich triphenylamine (TPA) is demonstrated as an effective quencher in the electrolyte to mitigate 1O2 and its associated parasitic reactions, which has the tertiary amine and phenyl groups to manifest excellent electrochemical stability and chemical reversibility. It reacts with electrophilic 1O2 to form a singlet complex during cycles, and it then quickly transforms to a triplet complex through nonradiative intersystem crossing (ISC). This efficiently accelerates the conversion of 1O2 to the ground-state triplet oxygen to eliminate its derived side reactions, and the regeneration of TPA. These enable the Li-O2 battery with obviously reduced overvoltages and prolonged lifetime for over 310 cycles when coupled with a RuO2 catalyst. This work highlights the ISC mechanism to quench 1O2 in Li-O2 battery.
Aprotic Li-O2 battery has gained extensive interests because of its high theoretical energy density of ∼3,600 Wh kg−1 (O2 + 2Li + 2e− ↔ Li2O2, E0 = 2.96 V vs. Li+/Li), however, it is hampered by the irreversible parasitic reactions at cathodes, leading to large discharge/charge overvoltages and poor rechargeability (1–4). This necessitates adoption of efficient cathode catalysts and suppression of inferior side reactions; the former has aroused a lot of efforts in recent years, while the latter is ambiguous and cannot be fully clarified for a long time (5–9). Recently, highly reactive singlet oxygen (1O2, the first excitedstate of oxygen) was demonstrated as a crucially important cause for parasitic reactions in Li-O2 batteries, which is mainly generated via the disproportionation of superoxide anion (O2−) and the oxidation of peroxide/superoxide species in the respective discharge and charge processes (10–14). The strong electrophilic 1O2 easily attacks most of cell components, like the organic solvents, electrolyte salts, and electrodes, to yield passivating by-products and induce capacity decay and even premature battery death (15–18). It is imperative to eliminate the reactive 1O2 and suppress the associated parasitic reactions for the development of reversible Li-O2 batteries.
The feasible solutions to 1O2 in aprotic Li-O2 batteries involve the reduction of charge voltages and the usage of 1O2 quenchers (10, 13, 15). The former is to lower the charge potentials below the equilibrium potential of 1O2 (∼3.55 V vs. Li+/Li) to partially mitigate the formation of 1O2, further improving the cycling stability of Li-O2 battery (5, 19–21). Alternatively, electron-rich quenchers are able to accelerate the decay of 1O2 to the ground-state triplet oxygen (3O2) and restrain the harmful 1O2-related side reactions (22). However, most adopted quenchers, such as organic amines, radical molecules, and redox mediators (RMs), are limited by their electrochemical irreversibility, insufficient chemical stability and low quenching efficiencies (23). For instance, 1,4-diazabicyclo[2.2.2]octane (DABCO) and RM of 5,10-dimethylphenazine (DMPZ) have manifested modest quenching efficiencies, but they are hindered by the electrochemical irreversibility and moderate chemical stability against 1O2, respectively (24–26). Exploration of effective and chemically/electrochemically stable quenchers to scavenge 1O2 remains quite challenging for Li-O2 batteries. Furthermore, the quenching mechanism for the decay of 1O2 has seldom been studied and urgently needs to be unveiled.
Herein, we report an electron-rich and robust triphenylamine (TPA) as the 1O2 quencher for Li-O2 batteries, which is generated in both the discharge and charge processes. The TPA quencher features as excellent electrochemical and chemical reversibility against 1O2. The elimination of 1O2 and suppression of its associated parasitic reactions are confirmed by in-situ UV-visible absorption spectroscopy, 1H NMR, and differential electrochemical mass spectrometry. During discharge and charge, TPA combines with electrophilic 1O2 to form a singlet complex, which rapidly converts to a triplet complex via radiationless intersystem crossing (ISC), accompanied with the transformation of 1O2 to 3O2 and regeneration of TPA. It effectively hinders the 1O2-driven parasitic reactions and rewards the Li-O2 battery with reduced discharge/charge voltage gaps and prolonged lifespan. This work provides insights into the design of efficient and stable 1O2 quenchers for long cycling Li-O2 batteries.
Results
Generation of 1O2 During Discharge and Charge.
The generation of 1O2 during discharge and charge is primarily quantified by monitoring the concentration change of 9,10-dimethylanthracene (DMA) via in situ UV-visible (UV-vis) measurement. DMA is chosen as the 1O2 probe because its absorbance of the characteristic peak at 379 nm is proportional to its concentration and decreases after selectively reacting with 1O2 to form endoperoxide (DMA-O2) (5), as confirmed by UV-vis and 1H-NMR measurements (Fig. 1A and SI Appendix, Figs. S1–S5). For the in situ UV-vis measurements, the Li-O2 battery is assembled inside a gas-tight quartz cuvette with an electrolyte of 50 μM DMA and 1.0 M lithium bis(trifluorosulfonyl)imide (LiTFSI) in diethylene glycol dimethyl ether (G2) solvent. Fig. 1B presents the discharge/charge profile and the corresponding DMA concentration changes of a Super P (SP)-based Li-O2 battery. During discharge, the DMA concentration maintains roughly constant at 50 μM within the detection accuracy of the UV-vis instrument. Alternatively, high-performance liquid chromatography coupled with mass spectrometry (HPLC-MS) is applied to detect the conversion of DMA to DMA-O2 after discharging a SP-based Li-O2 battery to 1 mAh in Fig. 1C, in which a high concentration of 30 mM DMA is added in the electrolyte to guarantee adequate capture of 1O2. The blue curve of initial electrolyte reveals the existence of DMA, and the red profile of discharged electrolyte presents a prominent peak of DMA and a small peak of DMA-O2. This suggests a tiny amount of 1O2 generated during discharge.
Fig. 1.
(A) UV-vis absorption spectrum of DMA in G2 electrolyte. The embedded equation shows the addition reaction of DMA and 1O2. (B) Discharge/charge profile of a SP-based Li-O2 battery and the corresponding DMA concentration changes. (C) HPLC-MS analyses of initial electrolyte and the electrolyte after discharged to 1 mAh. (D) DMA consumption profile of a SP-based Li-O2 battery charged at different voltage plateaus. (E) Schematic generation of 1O2 during discharging and charging.
When the SP-based Li-O2 battery is charged in Fig. 1B, the DMA concentration decreases constantly from the beginning to the end of charge, indicating that 1O2 is mainly generated in the charging process. The calculated 1O2 yield of 15.1 μM is corresponding to a 1O2/O2 ratio of ∼2.1%, in agreement with the previous report (5). The 1O2 generation rate profile is obtained by taking the derivative of DMA consumption (SI Appendix, Fig. S6), revealing that the 1O2 generation increases with the rising charge voltage. It is further verified by a voltage-step charging test in Fig. 1D, in which a predischarged Li-O2 battery is gradually charged at each varied voltage from 3.2 to 4.2 V for 20 min. It is found that DMA is slowly consumed from 50.0 to 48.4 μM when charged at low plateaus of 3.2 V and 3.4 V. However, the DMA concentration is promptly reduced to 40.0 μM when the charge plateau surpasses the equilibrium potential of 1O2 (∼3.55 V), from 3.6 to 4.2 V. When controlling the charge voltages below ∼3.6 V by employing a RuO2 catalyst (SI Appendix, Figs. S7 and S8), the 1O2 yield of Li-O2 battery is decreased to 9.5 μM (SI Appendix, Fig. S6). These imply that the generation of 1O2 is promoted by high charge voltages.
Fig. 1E illustrates the chemical and electrochemical generation routes of 1O2 in the discharging and charging processes of a Li-O2 battery. During discharge, 3O2 is firstly reduced to O2− anion via one-electron process, and then Li+ induces the disproportionation of O2− in the electrolyte for the formation of either 3O2 or 1O2, denoted as the chemical 1O2 generation route. This process is more thermodynamically favorable to form the ground-state 3O2 as product rather than the excited-state 1O2, leading to a tiny amount of 1O2 generated during discharge, as confirmed in Fig. 1 B and C. Upon charging, the discharge product of Li2O2 is initially oxidized to superoxide species, like O2− and Li-deficient Li2-xO2, and the soluble part is slightly converted to 1O2 via the chemical route in the whole charging process. The further electrochemical oxidation of peroxide and superoxide species mostly evolves 3O2 under low charge voltages, so only a small amount of 1O2 is generated at the beginning of charge. However, the formation of 1O2 becomes thermodynamically favorable when the charge voltage surpasses the equilibrium potential of 1O2 (∼3.55 V), resulting in the generation of massive 1O2 from the middle to the end of charge, denoted as the electrochemical 1O2 generation route.
Suppression of 1O2 and Associated Parasitic Reactions.
1O2 with a fully occupied and an empty π∗ orbitals is highly electrophilic, and can be accepted by electron-rich molecules or groups (21, 22). Tertiary amine with three phenyl groups, namely TPA, is used as the quencher to suppress 1O2 and its derived side reactions for Li-O2 battery, as depicted in Fig. 2A. TPA has been widely used as electrode material because of its electrochemical reversibility and chemical stability against various reactive oxygen species (ROS) in the absence of active hydrogen atoms (17, 27, 28). The cyclic voltammetry (CV) curve in Fig. 2A shows the reversible redox behavior of TPA+/TPA, and indicates its excellent electrochemical stability compared with the unstable DABCO (SI Appendix, Figs. S9 and S10). The 1H-NMR spectra of TPA reveal its chemical stability against multiple ROS (including 1O2, O2−, Li2O2, and O2) and Li metal (Fig. 2B and SI Appendix, Figs. S11 and S12). The quenching effect of TPA is firstly verified in an external confirmatory test, where 1O2 is generated through the disproportionation of O2− driven by Li+. DMA is quickly consumed from 50.0 to 36.6 μM in the blank counterpart, while its concentration remains almost unchanged after addition of an optimized concentration of 10 mM TPA (SI Appendix, Figs. S13–S15). This results in its high 1O2 quenching efficiency of 99.3%, as compared with 83.2% of DABCO based on the equation (SI Appendix, Fig. S16). The quenching effect of TPA in Li-O2 battery is evaluated by monitoring the DMA concentration change during charge, since the tiny generation of 1O2 during discharge is below the detection limit, as revealed in Fig. 1B. When TPA is used as an electrolyte additive in the SP-based Li-O2 battery, the DMA concentration is close to 50 μM with tiny consumption at the end of charge in Fig. 2C, and it leads to low 1O2 yield of 2.2 μM and high 1O2 quenching efficiency of 85.4% (SI Appendix, Fig. S16). The quenching efficiency can be further improved to 94.8% by replacing SP with the RuO2 catalyst, which mitigates the generation of 1O2 via reducing charge voltages (SI Appendix, Fig. S6).
Fig. 2.
(A) CV profile of TPA under Ar atmosphere. (B) Stability of TPA against O2− and 1O2. (C) DMA concentration profiles in charging with SP or RuO2 cathode and TPA. (D–F) Gas evolution during charging with RuO2 + TPA, SP + TPA, and SP. Red and blue curves represent the respective evolution rates of O2 and CO2. (G) 1H-NMR spectra of the electrolytes at the initial state and after 10 cycles in DMSO-d6. (H) Quenching efficiencies of electron-rich TPA analogs.
The gas evolution of Li-O2 battery is detected by differential electrochemical mass spectrometry (DEMS) measurement in charging processes. In Fig. 2D, only O2 is evolved in the TPA-mediated Li-O2 battery with the RuO2 catalyst (denoted as RuO2 + TPA), and no other gases like CO2 and H2 are detected. The gas flux of O2 approaches to the theoretical value in the whole charging process, resulting in a remarkable charge-to-O2 ratio of 2.04 e−/O2. The high reversibility is originated from the effective prevention of 1O2, high voltages, and the related parasitic reactions with the combination of TPA and RuO2 catalyst. For the TPA-mediated Li-O2 battery with SP catalyst (denoted as SP + TPA) in Fig. 2E or the TPA-free Li-O2 battery with RuO2 catalyst (SI Appendix, Fig. S17), they present good reversibility with charge-to-O2 ratios of 2.61 and 2.41 e−/O2, respectively, in which TPA can suppress 1O2 and RuO2 contributes to reducing the charge voltages. The residual parasitic reactions lead to small amount of CO2 evolution at the end of charge. In Fig. 2F, the charge voltage easily reaches up to 4.2 V in the TPA-free Li-O2 battery with SP catalyst, which accelerates the generation of 1O2 as revealed by UV-vis analyses. The formation of 1O2 and high charge voltages induce severe side reactions, resulting in a high charge-to-O2 ratio of 3.39 e−/O2, massive CO2 release during charge, as well as the inferior stability of Li-O2 battery.
The stabilities of electrolytes are analyzed by monitoring the signals of byproducts via 1H-NMR measurements. The NMR spectrum of initial electrolyte in Fig. 2G displays prominent peaks of tetraethylene glycol dimethyl ether (G4), dimethyl sulfoxide (DMSO), and tetramethylsilane (TMS), which are originated from electrolyte solvent of distilled G4 and DMSO-d6 deuterated reagent, respectively. For the TPA-mediated Li-O2 battery with RuO2 catalyst, the NMR spectra display no impurity peaks after ten cycles and minimal byproducts after one hundred cycles (Fig. 2G and SI Appendix, Fig. S18). This proves the long-term stability of TPA and the successful suppression of electrolyte degradation by preventing 1O2, high voltages, and the associated parasitic reactions, as confirmed by DEMS results. In sharp contrast, a number of distinctive peaks associated with alkyls and hydroxyls of carboxylates appear in the NMR spectra of the TPA-free counterparts with SP and RuO2 catalysts after 10 cycles, and become pronounced after 100 cycles (Fig. 2G and SI Appendix, Fig. S18). These electrolyte counterparts are relatively stable after 1 cycle (SI Appendix, Figs. S18 and S19), however, the hazards of 1O2-induced parasitic reactions accumulate during cycling and gradually induce the instability of Li-O2 battery. The blue profile of TPA-mediated Li-O2 battery with SP catalyst represents minor signals of byproducts, suggesting inhibition of the 1O2-driven side reactions. These reveal that TPA can efficiently eliminate 1O2 and relevant side reactions.
Two electron-rich TPA analogs, such as Tris[4-(diethylamino)phenyl]amine (TDPA) and 4,4',4''-trimethyltriphenylamine (TPA-CH3), are also selected as potential quenchers for Li-O2 battery (SI Appendix, Fig. S20). The CV curves of TDPA and TPA-CH3 reveal their electrochemical reversibility in the operating voltage window of Li-O2 battery (SI Appendix, Figs. S21 and S22). The quenching effects of TDPA and TPA-CH3 for 1O2 in SP-based Li-O2 batteries are measured via in situ UV-vis and HPLC-MS analyses (SI Appendix, Figs. S23–S25) and compared with TPA as well as DABCO in Fig. 2H. TPA shows the highest quenching efficiency of 85.4%, against 82.8% and 82.4% of TDPA and TPA-CH3, respectively. Both TDPA and TPA-CH3 possess excellent quenching effect, mainly because of the robust and electron-rich structure of tertiary amine with three phenyl groups. Conversely, the unsatisfactory chemical stability of DABCO limits its quenching effect in battery systems (23, 24), resulting in low quenching efficiency of 65.6%. In general, robust and electron-rich chemicals own universal 1O2 quenching effect for Li-O2 battery.
Electrochemical Performance.
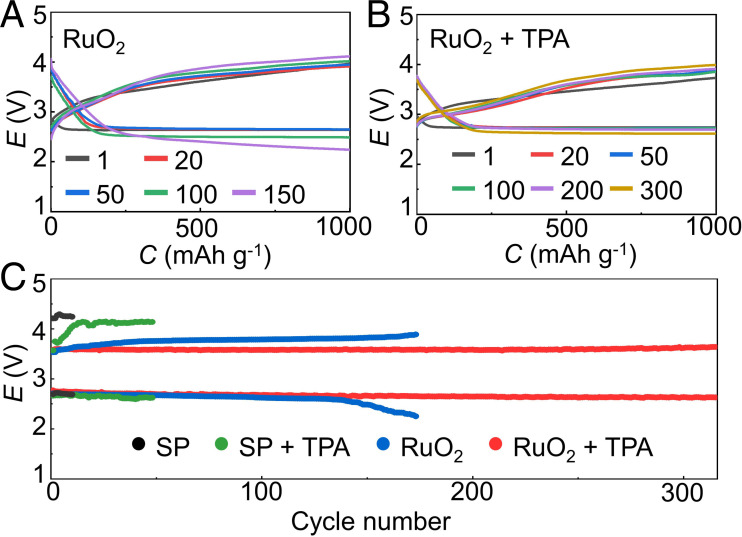
The charge voltage of SP-based battery surpasses 4.0 V with or without TPA (SI Appendix, Figs. S26 and S27), resulting in serious electrolyte degradation as demonstrated in Fig. 2G. RuO2 is introduced as cathode catalyst to accelerate the decomposition of Li2O2 and reduce the charge voltages for Li-O2 battery. The cycling performance of RuO2-based Li-O2 batteries with and without TPA is displayed in Fig. 3 A and B with a fixed capacity of 1000 mAh g−1 at 500 mA g−1. Both the Li-O2 batteries with and without TPA present small discharge/charge voltage gaps of ∼0.92 V for the first 5 cycles. In the TPA-mediated battery, it can be continuously discharged and charged for 310 cycles with little deterioration of discharge and charge polarization, indicating excellent cycling stability. The terminal charge voltage remains below 3.90 V after 300 cycles to exclude oxidation of TPA. Accordingly, the reversible formation and decomposition of toroidal Li2O2 are also verified by X-ray diffraction (XRD), Raman spectra and scanning electron microscopy (SEM) images (SI Appendix, Figs. S28–S30), respectively. In contrast, the discharge/charge plateaus of TPA-free battery present increasing voltage gaps of ∼1.14 V after 20 cycles, due to the accumulation of 1O2-driven parasitic reactions, as confirmed by DEMS and 1H-NMR analyses (SI Appendix, Figs. S17 and S18). It can only maintain for 150 cycles with the terminal discharge voltage quickly dropping to 2.2 V in Fig. 3C. The superior cycle stability of TPA-mediated battery is ascribed to the elimination of harmful 1O2 and its derived reactions at low charge voltages, however the degradation of Li anode also impairs the cycle stability and shortens the operation time to some extent (as discussed in SI Appendix, Fig. S31).
Fig. 3.
(A and B) Discharge and charge profiles of the RuO2-based Li-O2 batteries with and without TPA at 500 mA g−1. (C) Cycling performance of Li-O2 batteries (y axis represents average discharge/charge voltage).
Quenching Reaction Mechanism.
The Gibbs free energy diagram in Fig. 4A illustrates the quenching process, and the interaction region indicator (IRI) analysis in Fig. 4B visualizes the interaction between TPA and 1O2 during quenching. After the chemical or electrochemical generation of 1O2 during cycling, electrophilic 1O2 is firstly adsorbed at the phenyl group of TPA via weak attraction as shown in Fig. 4B and forms encounter complex (denoted as EC). It is prone to converting to singlet charge-transfer complex (denoted as 1CT), rather than to undergo endothermic addition reaction and form a byproduct of endoperoxide, in line with NMR spectra in Fig. 2B. Based on the Gibbs free energy diagram in Fig. 4A, 1CT with high Gibbs free energy of 0.99 eV spontaneously decays to triplet charge-transfer complex (3CT) with low Gibbs free energy of -0.78 eV through ISC mechanism, accompanied with the release of heat energy to the environment. The optimized structure of 3CT indicates that the oxygen in 3CT is changed from 1O2 to 3O2 after ISC. Because of the absence of electrophilicity for 3O2, it interacts with TPA through weak Van der Waals force in Fig. 4B, and then 3O2 easily dissociates from 3CT to the environment.
Fig. 4.
(A) Gibbs free energy diagram of quenching process. Intermediate complex EC, 1CT and 3CT are encounter complex, singlet charge-transfer complex, and triplet charge-transfer complex, respectively. Endoperoxide is a possible by-product of 1O2 and phenyl. (B) IRI analyses of intermediates during quenching. Red, green, and blue regions represent repulsion, Van der Waals interaction, and attraction, respectively. (C) Schematic diagram of conversion of spin state during ISC. Red and blue arrows represent spin-up and spin-down electrons, respectively. (D) Decay of 1O2 with or without TPA.
ISC is a nonradiative transition from singlet to triplet chemicals, accompanied with the release of energy and the transformation of multiplicity. The former has been revealed by the Gibbs free energy diagram in Fig. 4A, and the transformation from singlet to triplet state is illustrated in Fig. 4C. Both TPA and 1O2 are spin singlet since all the electrons are spin-paired with opposite directions, leading to total spin (S) of 0 and then spin multiplicity (M = 2S + 1) of 1. When EC converts to 1CT, a spin-up electron (↑) from the Pz orbital of N atom in TPA transfers to the empty π∗ orbital of 1O2, between which strong attraction is present, as proven by IRI analysis, molecular orbital component, and Mulliken analyses (Fig. 4B and SI Appendix, Figs. S32 and S33 and Table S2), respectively. 1CT is spin singlet because the single electrons in the π∗ orbital of 1O2 and in the Pz orbital of N atom of TPA have opposite spin directions, leading to a total spin of 0. Along with the release of energy during ISC, a spin-down electron (↓) in the fully occupied π∗ orbital of 1O2 in 1CT transfers back to the central N atom of TPA (SI Appendix, Fig. S32) and undergoes spin-flip and fills the spin-up vacancy in the Pz orbital of N atom, due to Pauli exclusion principle, with formation of triplet 3CT. The electronic structure of 3CT in Fig. 4C indicates that the oxygen is transformed from 1O2 to 3O2 after ISC, with two spin-up electrons occupying two different π∗ orbitals. Finally, 3O2 and TPA are dissociated from 3CT, and TPA participates another quenching process. Fig. 4D exhibits the decay of 1O2 to 3O2 without or with TPA quencher. Generally, 1O2 decays slowly to 3O2 via a radiative transition mechanism (denoted as RT) with light emission, since the direct ISC from 1O2 to 3O2 is inhibited (21, 22). After introduction of TPA as a 1O2 quencher, it interacts with 1O2 to form 1CT and relaxes to 3CT through quick nonradiative ISC, with release of 3O2. Therefore, TPA can greatly expedite the decay of 1O2 to 3O2 and mitigate the 1O2-driven side reactions for stable and prolonged Li-O2 battery.
Discussion
In summary, electron-rich TPA is demonstrated to be an efficient quencher to eliminate 1O2 and the relevant side reactions in Li-O2 battery. The robust tertiary amine and phenyl groups in TPA ensures its electrochemical reversibility and chemical stability against diverse ROS, as verified by CV and 1H-NMR measurements. The electron-rich TPA can interact with electrophilic 1O2 to form 1CT by transferring a spin-up electron (↑) from N atom of TPA to 1O2. Then, 1CT quickly decays to 3CT through ISC mechanism, and simultaneously heat energy is released to the environment and a spin-down electron (↓) in 1O2 undergoes spin-flip and transfers back to the central N atom of TPA. This accelerates the relaxing of 1O2 and efficiently restrains O2-driven side reactions. Coupled with RuO2 to reduce charge voltages and mitigate the generation of 1O2, the TPA-mediated Li-O2 battery exhibits superior reversibility of 2.04 e−/O2 during charge, reduced discharge/charge overvoltages and prolonged lifespan of 310 cycles. In addition, two electron-rich and robust TPA analogs of TDPA and TPA-CH3 also demonstrate their universal 1O2 quenching effect for Li-O2 battery. This work highlights the vital role of ISC to quench 1O2 and provides insights into the qualified quenchers for durable Li-O2 battery.
Materials and Methods
Materials.
All chemicals were stored in an argon-filled glovebox. G2 and G4 were distilled and kept with activated molecular sieves. LiTFSI was dried under vacuum for 12 h at 80 °C. DMA and KO2 were directly used without further treatment. A series of 1O2 quenchers such as TPA, DABCO, TPA-CH3, and TDPA were dried under vacuum before use. RuO2 catalyst was synthesized via precipitation reaction and calcination process (29). Carbon nanotube (denoted as CNT) was pretreated with nitric acid at 110 °C for 4 h, and then washed with ultrapure water and ethanol. One hundred milligrams of CNT and 200 mg RuCl3 were dispersed in 30 mL ultrapure water by stirring for 30 min, into which a 0.2 M NaHCO3 aqueous solution was added drop by drop until the pH reached 7. After stirring for 10 h, the sediment was washed with ultrapure water and ethanol, dried at 80 °C for 6 h and calcined at 150 °C for 4 h. The black powder was collected and denoted as RuO2.
Material Characterization.
SEM (JEOL-JSM7500F) was utilized to study the morphology of discharged products. XRD (Rigaku MiniFlex600) and Raman spectroscopy (LabRAM HR800 with a 532 nm laser) were applied to confirm the formation and decomposition of discharged products. The electrodes for the tests were washed with distilled DMA, dried under vacuum to remove residual solvents, and characterized under argon protection. UV-vis absorption spectra (Agilent Cary 60) were conducted to record the generation of 1O2. 1H-NMR (Bruker, 400 MHz) was operated to check electrolyte decomposition. Transmission electron microscopy (TEM; FEI Talos F200X G2) was utilized to characterize the RuO2 catalyst. EPR spectrometer (Bruker, E580) is utilized to optimize the concentration of TPA.
Electrochemical Measurements.
RuO2 catalyst or SP was dispersed in ethanol solution by sonication with 10 w% sodium carboxymethylcellulose as the binder. The black suspension was sprayed on carbon cloth with a mass loading of ∼0.3 mg cm−2. The prepared carbon cloth was cut into discs (Ф10 mm) and dried in vacuum. The electrolyte was prepared by dissolving 1.0 M LiTFSI in G4. The electrolyte with 1O2 quencher was prepared by adding extra 10 mM TPA or other quenchers. The Li-O2 batteries were assembled in top-holed CR2032 coin cells in an argon-filled glovebox. Each cell contained a piece of Li foil (Ф10 mm), a glassy fiber separator (Ф16 mm) with 60 μL of electrolyte, and a catalyst-loaded carbon cathode (Ф10 mm). Galvanostatic cycling tests were run on Land battery instrument (CT2001A). CV measurement was performed on a Solartron 1470E electrochemical workstation.
In-situ DEMS measurement.
DEMS measurement was conducted on a customized QMG250M1 commercial instrument from Linglu Instruments (Shanghai) Co., Ltd. Modified Swagelok-type Li-O2 cells were used for DEMS measurement. Each cell contained a piece of Li foil (Ф14 mm), a glassy fiber separator (Ф22 mm) with 40 μL of G4 electrolyte, and a catalyst-loaded carbon cathode (Ф14 mm) with loading density of 0.5 mg cm−2. The cell was discharged to 200 µAh before DEMS testing, then linked to a mass spectrometer by a gas-purging system and charged to 200 µAh at 100 µA. The flow rate of carrier gas (Ar) was set at 1 mL min−1. The evolution of O2 and CO2 was monitored by the mass spectrometer during charge.
In situ and Ex situ 1O2 Detection.
In situ UV-vis tests were conducted using a UV-vis spectrophotometer. The generation of 1O2 and quenching efficiency of different additives were detected by measuring the decay of DMA concentration. A 1 cm high-precision quartz cell with a custom-made gasproof polytetrafluoroethylene (PTFE) lid was used for the in situ UV-vis test. The cell contained a catalyst-loaded carbon cathode, a piece of Li foil as an anode, and a 1.2 mL G2 electrolyte with or without 10 mM of 1O2 quenchers. Before testing, O2 was bubbled into the electrolyte for 20 min. The DMA concentration of 50 μM was chosen to balance effective trapping concentration of DMA and excellent detecting precision of the instrument. For in situ UV-vis tests, the cells were discharged and charged under 50 μA cm−2 for 2 h, respectively.
HPLC-MS was utilized to analyze the transformation of DMA to DMA-O2, with 30 mM DMA in the electrolyte to guarantee enhanced sensitivity. The electrolyte was extracted from the separator and cathode of each cell using 200 µL DME. Then the DME solution was evaporated under vacuum. After dissolving the residue in 10 µL DME, a volume of 2 µL was injected into the HPLC-MS equipment. The DMA to DMA-O2 conversion was measured from the absorbance at 210 nm.
DFT Calculations.
Dispersion-corrected density functional theory calculations (DFT-D3) was performed by using Gaussian 16 software package (30, 31). Molecule structures were relaxed with Becky three parameter exchange function and Lee-Yang-Parr correlation functional (B3LYP) with 6-31+G (d,p) basis set (32–34). Vibrational frequency analyses were applied to ensure that the geometry optimization was at local minima and to determine the zero-point energies. The electronic ground state energy of complex was obtained at M06-2X level with 6-311+G (d,p) basis set (35). Thermal correction to Gibbs free energy was acquired from vibrational frequency calculations using B3LYP with 6-31+G (d,p) basis set. In this case of DFT energy calculation, the correction of 1O2 was 0.98 eV (16). The implicit solvation model based on density models was utilized to model solvent effects (eps = 7.68, epsinf = 1.432). IRI analysis revealing chemical bonding and weak interaction region, and natural atomic orbital analysis showing the contribution of each atom in orbitals based on DFT results were conducted by Multiwfn package (36, 37).
Supplementary Material
Acknowledgments
This work was supported by the Ministry of Science and Technology of China (2017YFA0206700), National Natural Science Foundation of China (51671107), Tianjin Natural Science Foundation (grant No. 19JCJQJC62400), the 111 project of B12015, and the Haihe Laboratory of Sustainable Chemical Transformations.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2202835119/-/DCSupplemental.
Data, Materials, and Software Availability
All study data are included in the article and/or supporting information.
References
- 1.Liu T., et al. , Current challenges and routes forward for nonaqueous lithium-air batteries. Chem. Rev. 120, 6558–6625 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Kwak W. J., et al. , Lithium-oxygen batteries and related systems: Potential, status, and future. Chem. Rev. 120, 6626–6683 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Tan C., et al. , True reaction sites on discharge in Li-O2 batteries. J. Am. Chem. Soc. 144, 807–815 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Zhang J., et al. , A versatile functionalized ionic liquid to boost the solution-mediated performances of lithium-oxygen batteries. Nat. Commun. 10, 602 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahne N., et al. , Singlet oxygen generation as a significant cause for parasitic reactions during cycling of aprotic lithium-oxygen batteries. Nat. Energy 2, 17036 (2017). [Google Scholar]
- 6.Lv Q., Zhu Z., Ni Y., Geng J., Li F., Spin-state manipulation of two-dimensional metal-organic framework with enhanced metal-oxygen covalency for lithium-oxygen batteries. Angew. Chem. Int. Ed. Engl. 61, e202114293 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Zhang J., et al. , A long-life lithium-oxygen battery via a molecular qenching/mediating mechanism. Sci. Adv. 8, eabm1899 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J., Sun B., Zhao Y., Kretschmer K., Wang G., Modified tetrathiafulvalene as an organic conductor for improving performances of Li-O2 batteries. Angew. Chem. Int. Ed. Engl. 56, 8505–8509 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Li X., Zhao R. X., Fu Y. Z., Manthiram A., Nitrate additives for lithium batteries: Mechanisms, applications, and prospects. eScience 1, 108–123 (2021). [Google Scholar]
- 10.Wandt J., Jakes P., Granwehr J., Gasteiger H. A., Eichel R. A., Singlet oxygen formation during the charging process of an aprotic lithium-oxygen battery. Angew. Chem. Int. Ed. Engl. 55, 6892–6895 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Córdoba D., Rodríguez H. B., Calvo E. J., Singlet oxygen formation during the oxygen reduction reaction in DMSO LiTFSI on lithium air battery carbon electrodes. ChemistrySelect 4, 12304–12307 (2019). [Google Scholar]
- 12.Mourad E., et al. , Singlet oxygen from cation driven superoxide disproportionation and consequences for aprotic Metal-O2 batteries. Energy Environ. Sci. 12, 2559–2568 (2019). [Google Scholar]
- 13.Petit Y. K., et al. , Mechanism of mediated alkali peroxide oxidation and triplet versus singlet oxygen formation. Nat. Chem. 13, 465–471 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Su Y., Zhao Z., Huang J., Wang E., Peng Z., Hunting the culprits: Reactive oxygen species in aprotic lithium-oxygen batteries. J. Phys. Chem. C 126, 1243–1255 (2022). [Google Scholar]
- 15.Kwak J. W., et al. , Mutual conservation of redox mediator and singlet oxygen quencher in lithium-oxygen batteries. ACS Catal. 9, 9914–9922 (2019). [Google Scholar]
- 16.Mullinax J. W., C. W. Bauschlicher, Jr, Lawson J. W., Reaction of singlet oxygen with the ethylene group: Implications for electrolyte stability in li-ion and Li-O2 batteries. J. Phys. Chem. A 125, 2876–2884 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Huang Z., et al. , A stable lithium-oxygen battery electrolyte based on fully methylated cyclic ether. Angew. Chem. Int. Ed. Engl. 58, 2345–2349 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Ruiz de Larramendi I., Ortiz-Vitoriano N., Unraveling the effect of singlet oxygen on metal-O2 batteries: Strategies toward deactivation. Front Chem. 8, 605 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv Q., et al. , Semiconducting metal-organic polymer nanosheets for a photoinvolved Li-O2 battery under visible light. J. Am. Chem. Soc. 143, 1941–1947 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Zhu Z., et al. , Internal electric field and interfacial bonding engineered step-scheme junction for a visible-light-involved lithium-oxygen battery. Angew. Chem. Int. Ed. Engl. 61, e202116699 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Schürmann A., Luerßen B., Mollenhauer D., Janek J., Schröder D., Singlet oxygen in electrochemical cells: A critical review of literature and theory. Chem. Rev. 121, 12445–12464 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Schweitzer C., Schmidt R., Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 103, 1685–1757 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Lee H. W., Kim H., Jung H. G., Sun Y. K., Kwak W. J., Ambilaterality of RM towards 1O2 in Li-O2 batteries: Trap and quencher. Adv. Funct. Mater. 31, 2102442 (2021). [Google Scholar]
- 24.Petit Y. K., et al. , DABCOnium: An efficient and high-voltage stable singlet oxygen quencher for metal-O2 cells. Angew. Chem. Int. Ed. Engl. 58, 6535–6539 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwak W. J., et al. , Deactivation of redox mediators in lithium-oxygen batteries by singlet oxygen. Nat. Commun. 10, 1380 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng J. K., Cao Y. L., Ai X. P., Yang H. X., Polytriphenylamine: A high power and high capacity cathode material for rechargeable lithium batteries. J. Power Sources 177, 99–204 (2008). [Google Scholar]
- 27.Su C., Ye Y. P., Bu X. D., Xu L. H., Zhang C., Preparation and properties of polytriphenylamine/multi-walled carbon nanotube composite as a cathode material for li-ion batteries. Adv. Mat. Res. 335, 1512–1515 (2011). [Google Scholar]
- 28.Zhang M., et al. , Suppressing singlet oxygen generation in lithium-oxygen batteries with redox mediators. Energy Environ. Sci. 13, 2870–2877 (2020). [Google Scholar]
- 29.Jian Z., Liu P., Li F., He P., Zhou H., Ru-RuO2/CNT hybrids as high-activity pH-universal electrocatalysts for water splitting within 0.73 V in an asymmetric-electrolyte electrolyzer. Nano Energy 61, 576–583 (2019). [Google Scholar]
- 30.Ehrlich S., Moellmann J., Reckien W., Bredow T., Grimme S., System-dependent dispersion coefficients for the DFT-D3 treatment of adsorption processes on ionic surfaces. ChemPhysChem 12, 3414–3420 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Frisch M. J., et al. , Gaussian 16, Revision A.03 (Gaussian, Inc., Wallingford, CT, 2016). [Google Scholar]
- 32.Lee C., Yang W., Parr R. G., Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 37, 785–789 (1988). [DOI] [PubMed] [Google Scholar]
- 33.Becke A. D., A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 98, 1372–1377 (1993). [Google Scholar]
- 34.Becke A. D., Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A Gen. Phys. 38, 3098–3100 (1988). [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y., Truhlar D. G., Theor. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2007). [Google Scholar]
- 36.Lu T., Chen Q., Interaction region indicator: A simple real space function clearly revealing both chemical bonds and weak interactions. Chemistry-Methods 1, 231–239 (2021). [Google Scholar]
- 37.Lu T., Chen F., Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.