Significance
Subtropical East Asian caves are characterized by high levels of biodiversity and endemism but most do not fall within protected areas. Here, we investigate the timing and mode of cave colonization through a multi-taxon meta-analysis combined with investigation of paleoenvironmental dynamics. We show that most cave colonization events occurred after the Oligocene-Miocene boundary. Importantly, we document that biotic colonization of subtropical East Asian caves was not a random process, but was subject to periods of acceleration and decrease, in association with the climate and vegetational changes and the establishment of seasonal climate in subtropical East Asia. Our results provide insights into how subterranean biodiversity in subtropical East Asia has been shaped over time and also have implications for biodiversity conservation.
Keywords: climate change, East Asian monsoon, Miocene, multiple taxa, troglobites
Abstract
Caves are home to unique and fragile biotas with high levels of endemism. However, little is known about how the biotic colonization of caves has developed over time, especially in caves from middle and low latitudes. Subtropical East Asia holds the world's largest karst landform with numerous ancient caves, which harbor a high diversity of cave-dwelling organisms and are regarded as a biodiversity hotspot. Here, we assess the temporal dynamics of biotic colonization of subtropical East Asian caves through a multi-taxon analysis with representatives of green plants, animals, and fungi. We then investigate the consequences of paleonviromental changes on the colonization dynamics of these caves in combination with reconstructions of vegetation, temperature, and precipitation. We discover that 88% of cave colonization events occurred after the Oligocene-Miocene boundary, and organisms from the surrounding forest were a major source for subtropical East Asian cave biodiversity. Biotic colonization of subtropical East Asian caves during the Neogene was subject to periods of acceleration and decrease, in conjunction with large-scale, seasonal climatic changes and evolution of local forests. This study highlights the long-term evolutionary interaction between surface and cave biotas; our climate-vegetation-relict model proposed for the subtropical East Asian cave biota may help explain the evolutionary origins of other mid-latitude subterranean biotas.
Caves are formed by chemical reactions between circulating groundwater and surrounding rocks and have an isolated and strongly zonal environment, characterized by dim light, oligotrophy, and low diurnal and annual variation in temperature and humidity (1) (SI Appendix, Fig. S1). Based on the extent to which light and the external climate impact the cave environment, cave internal space can be divided into Entrance, Twilight, and Dark zones (2). The unique cave habitat hosts a large array of biodiversity with more than 50,000 obligate troglophilic species around the world (3) and has served as a natural laboratory for ecological, evolutionary, and biomedical studies (1, 4). Because of their low reproductive potential and small population sizes (5), endemic cave-dwelling species that are only known from caves are often at risk for extinction due to climate change and other anthropogenic activities (6). Globally, most caves (∼93%) do not fall within protected areas (7). To date, the evolutionary origins of cave biotas are still poorly explored (1).
Just as with insular or other island-like habitats (8–10), immigrants have contributed greatly to cave biodiversity (4, 11). Caves may serve as refugia for troglobites owing to relatively stable microclimates, whereas their surface counterparts have often gone extinct during periods of unfavorable climatic conditions (i.e., some endemic cave biotas are relicts). This climatic relict hypothesis was initially proposed based on studies of cave-dwelling species in temperate latitudes, which mainly evolved in association with Pleistocene glaciation (12). However, we know little about how ancient environmental changes affected colonization of caves in middle and low latitudes, in which numerous cave systems occur, especially in karst regions (13). A study of the spider genus Nesticella suggests that middle Miocene climate change promoted the origin of a subterranean lifestyle in Asian middle latitudes (14). To broadly understand the evolutionary origin of the endemic cave biota, molecular phylogenetic study of multiple clades across the tree of life is essential (9, 15).
In this study, we investigated the timing and mode of adaptation to subtropical East Asian caves by conducting a meta-analysis with multiple species of green plants, animals, and fungi, and tested whether cave colonization was a dynamic process over time. We find that most colonization events occurred after the Oligocene-Miocene (O-M) boundary and that colonization accelerated between 21–4 Ma, coinciding with the development of the East Asian subtropical evergreen broadleaf forests (EBLFs), as well as Neogene climate changes. We also document that species from subtropical EBLFs in East Asia are a major species “pool” for the origin of cave biodiversity in this region.
Study Area
Subtropical East Asia is extremely rich in ancient gorges and large cave systems (16). In particular, southern China holds the world's largest karst landform with approximately 500,000 terrestrial caves (13), among which some have been dated to the Eocene to Carboniferous (SI Appendix, Table S1). The India-Eurasia collision started during the Paleocene (∼61 Ma) (17) and subsequently resulted in the deformation and uplift of the Tibet-Himalaya-Hengduan (THH) region (18) and the Yunnan-Guizhou Plateau (YGP) (19), which drove regional and global environmental changes. Establishment of the East Asian monsoon system (20) and the rise of East Asian subtropical EBLFs (21–24) took place around the O-M boundary (∼23 Ma). The climate and vegetation of subtropical East Asia changed dramatically from the Neogene onward in association with the tectonic activities that occurred in response to the collision of the Indo-Eurasian plates (25, 26). Most subtropical East Asian caves are located in EBLFs, which are currently under the influence of a warm and moist monsoon climate (27). These caves encompass a high diversity of subterranean-adapted organisms and are considered a biodiversity hotspot (28, 29). More than 100 plant species endemic to China's karst caves have recently been described as new to science (6). Furthermore, many new species are likely still to be discovered (6, 29). However, these unique subtropical East Asian cave biotas are severely threatened at present because of climate change, anthropogenic deforestation, and absence of legislated protection (6, 11).
Results and Discussion
To unravel the colonization history of subtropical East Asian caves, we selected 28 clades with 1,437 species, belonging to 43 genera representing seven major clades of life: ferns, angiosperms, arachnids, amphibians, reptiles, fishes, and fungi (SI Appendix, Table S2). A total of 189 endemic cave-dwelling species were included (SI Appendix, Table S3). This sampling strategy maximized taxon representation in subtropical East Asian caves (Fig. 1). We generated a dated phylogeny for each of these 28 clades (Fig. 2 and SI Appendix, Figs. S2 and S3). We then compiled age estimates of immigration events into caves and calculated the maximum number of observed immigration events (MIEs) per million years (Fig. 3 and SI Appendix, Table S4). In particular, we characterized the relationship between immigration events and East Asian subtropical EBLFs by modeling the variation of suitable area of this biome over time (Fig. 3 and Movie S1). We also explored the possible correlation of cave colonizations with temperature and precipitation variation over time.
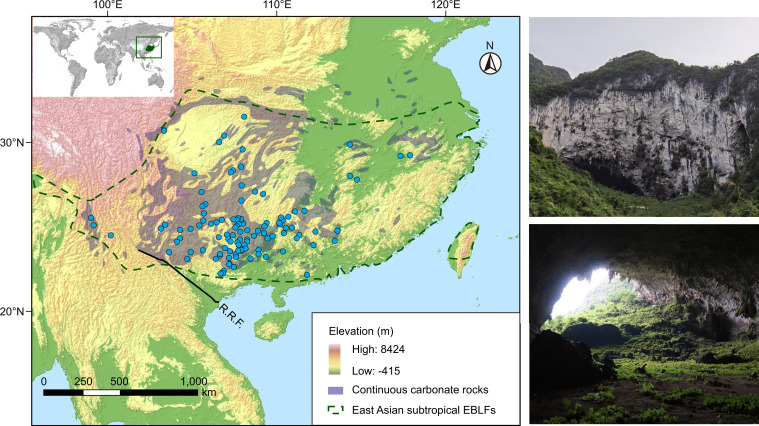
Fig. 1.
Distribution of study caves in subtropical East Asia. Blue circles denote cave localities, which are determined by the specimen records of the sampled endemic cave-dwelling species. The range of karsts throughout subtropical East Asia (in gray) is modified from https://www.un-igrac.org/resource/world-karst-aquifer-map-wokam. Inset Upper Left, the location of East Asian subtropical evergreen broadleaf forests in the world, modified from (27). An example of karst caves, the Yangzi Cave in Guangxi, is presented in the right (outside and inner view). R.R.F., Ailao Shan-Red River Fault zone.
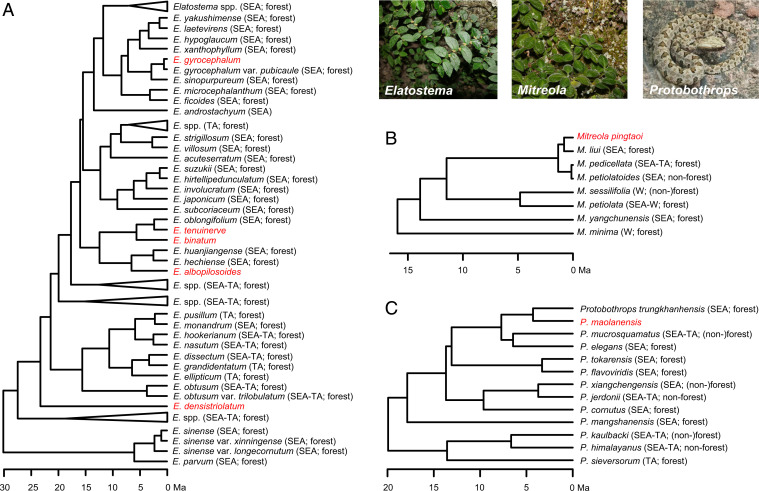
Fig. 2.
Examples of dated phylogenetic trees. (A) Elatostema. (B) Mitreola. (C) Protobothrops. The cave-dwelling species endemic to subtropical East Asia (SEA) are indicated in red. For other trees, see SI Appendix, Fig. S2. TA, tropical Asia; W, the rest of the world.
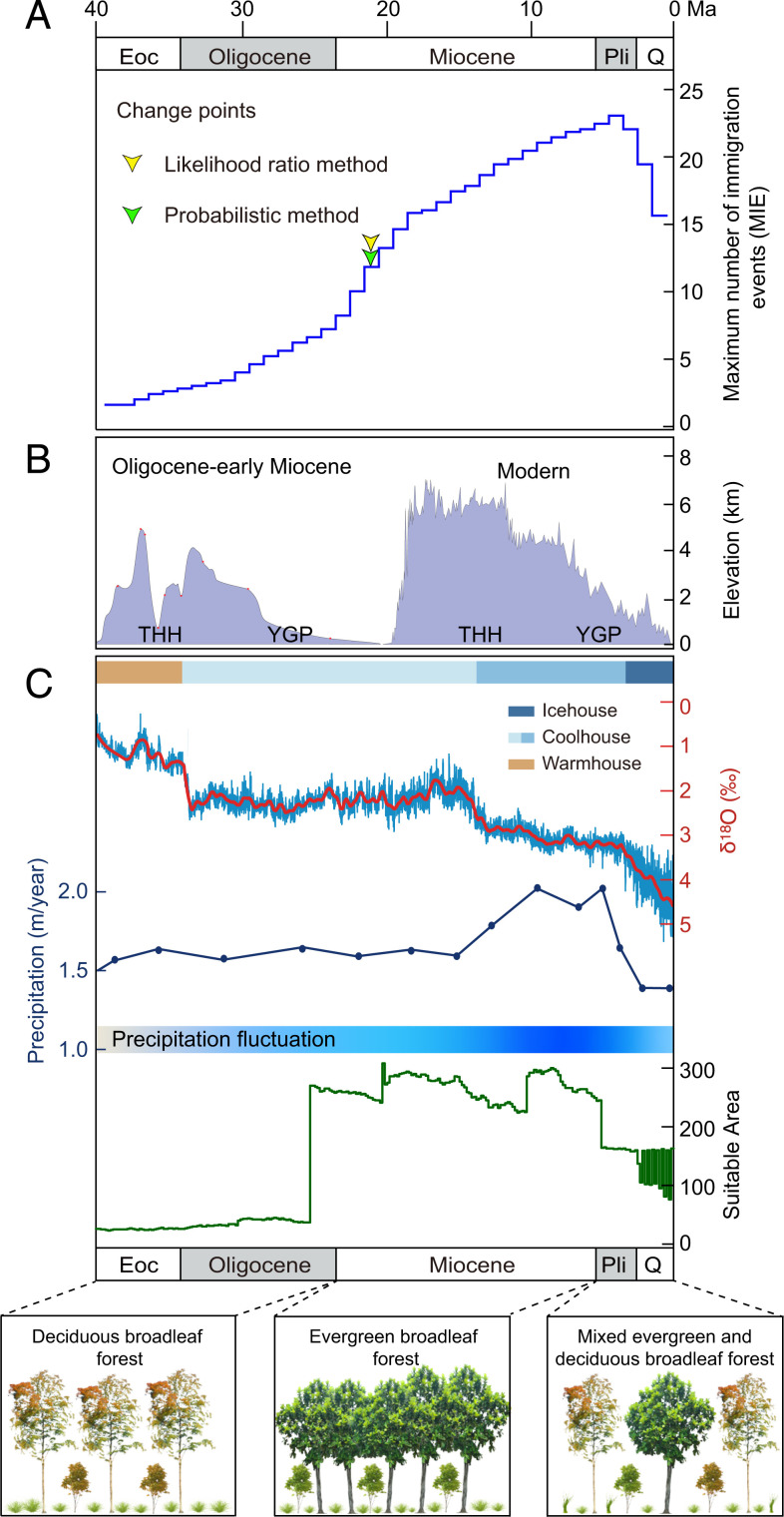
Fig. 3.
Colonization dynamics of subtropical East Asian caves in relation to paleogeoclimate and vegetation changes. (A) Immigration rates of subtropical East Asian caves based on the maximum number of observed immigration events (MIE) per Myr (modified from SI Appendix, Fig. S5). Arrowheads indicate estimated change points. (B) Schematic representation of the topography of the Tibet-Himalaya-Hengduan region and Yunnan-Guizhou Plateau in two phases, from the Oligocene to the present. Red circles show sites from which data were used to estimate the paleoelevations (see SI Appendix, Table S5 for details). (C) Global temperature inferred from δ18O levels in benthic foraminifera (25), modeled mean annual precipitation at idealized CO2 levels (32), suitable area (grid cell) dynamics of East Asian subtropical evergreen broadleaf forests, and the floristic turnover in subtropical East Asia since 40 Ma (23).
Our molecular dating and ancestral habitat reconstruction analyses identified 75 immigration events into subtropical East Asian caves, which occurred in a wide time range, from 0.81 million years to 106.35 million years (SI Appendix, Figs. S2–S4 and SI Appendix, Table S4). This time frame is prior to catastrophic deforestation in the 20th century (11), suggesting the origins of the endemic cave-dwelling species we sampled are irrelevant to modern human activities. Importantly, 88% of these took place after the O-M boundary, the time span during which the East Asian biotic realm approached that of the present day (23, 30). Only four arachnid, one fish, and four fungal lineages appeared prior to the O-M boundary. These nine exceptions may represent overestimates if our sampling did not contain the closest epigean sister lineage, or if these lineages are not strictly hypogean. Alternatively, these cave species may truly be very old endemics, as some ancient caves have existed in southern China since the Carboniferous (SI Appendix, Table S1).
Our MIE analyses suggest that colonization of subtropical East Asian caves significantly accelerated at approximately 21 Ma (Fig. 3A and SI Appendix, Figs. S5 and S6). This would be temporally congruent with the apparent tectonic activities in the THH region (Fig. 3B and SI Appendix, Table S5), which have greatly changed the Asian climate, drainage, and vegetation (23, 30, 31). Paleovegetational and paleoclimatic evidence shows the establishment of the East Asian summer monsoon at approximately 24.9 Ma (20), which would bring abundant summer rainfall and consequently promote the expansion of humid area in East Asia. Our paleoenvironmental reconstruction indicates that the suitable area of subtropical EBLFs in East Asia sharply increased during this period (Fig. 3C). Fossil and molecular phylogenetic data also support the rise of this biome around the O-M boundary (21–24). The MIE shows a virtually linear increase between 21 and 5 Ma (Fig. 3A). This coincides with the continual uplift of the THH region and the YGP and the intensification of the East Asian monsoon (Fig. 3B). In particular, the development of the “super monsoon” in East Asia at approximately 13 Ma increased the persistence of the wet season through the year (32). During the Miocene, the East Asian subtropical EBLFs covered a relatively large area (Fig. 3C), allowing for a potential expansion of this biome (10, 22, 33).
Our ancestral vegetation type and biogeographic analyses show that all cave-dwelling lineages (except one fungal species) originated from terrestrial species inhabiting the East Asian subtropical EBLFs (SI Appendix, Figs. S7 and S8 and SI Appendix, Table S4), and their closest relatives also inhabit this biome (Fig. 2 and SI Appendix, Figs. S2 and S3). These findings suggest that subtropical East Asian cave biotas are the extensions of the local surface biotas. During the Miocene, a warm and moist subtropical monsoon climate together with mountain building and river incision promoted the development of large caves in subtropical East Asia (13), which produced vacant niches. With the rise of East Asian subtropical EBLFs, organisms inhabiting those forests, especially in the extensive understory, appear to have colonized those caves during the Miocene. In addition, with the establishment of the monsoon climate from the O-M boundary onwards (20), seasonality with a lower winter mean temperature and humidity occurred in East Asia (SI Appendix, Fig. S1); this seasonality could have resulted in the extinction of the surface counterparts, whereas caves with relatively stable temperature and humidity could have offered ideal refugia for local species (14).
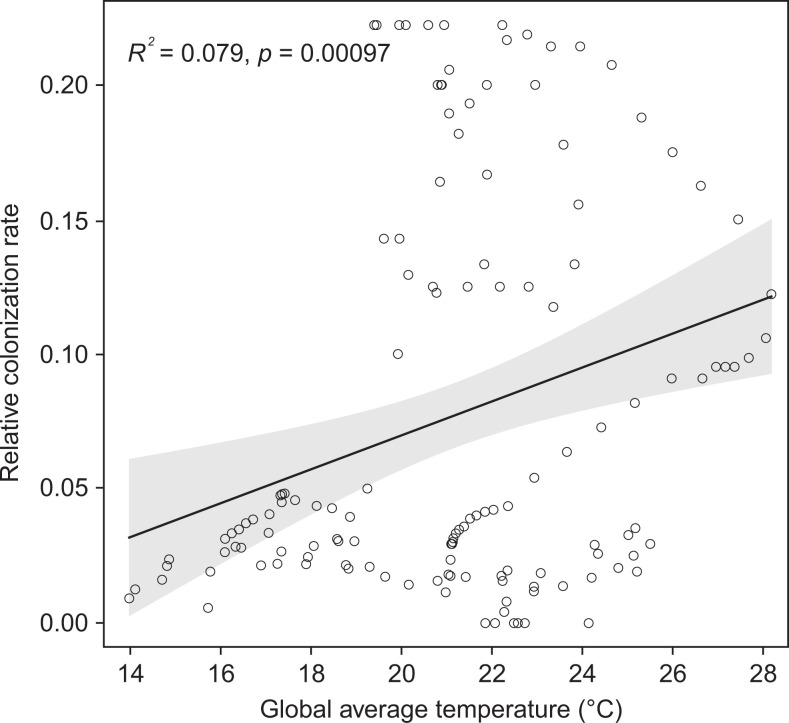
From 4 Ma onwards, MIE decreased, temporally in agreement with a shift to the Icehouse state (25) with a marked decrease of precipitation in East Asia (32). Our statistical analysis of paleoclimate data from the 19 fossil sites shows that both the minimum temperature of the coldest month and the mean annual precipitation, two key limiting factors for the distribution and species richness of East Asian subtropical EBLFs (27, 34), dropped in subtropical East Asia after the Miocene (SI Appendix, Fig. S9). These changes might have resulted in the increasing fragmentation of East Asian subtropical EBLFs since the Pliocene, supported by our estimation of the suitability area of this biome (Fig. 3C). Fossil evidence also indicates that East Asian subtropical EBLFs were replaced locally by mixed forests from the Pliocene onwards (23). Furthermore, we found that the colonization rate of subtropical East Asian caves is significantly positively correlated with global average temperature (R2 = 0.079, P = 10−4; Fig. 4), which experienced a marked decrease in the Pliocene (25). Thus, we suggest that the fragmentation of East Asian subtropical EBLFs, together with a temperature drop, might have reduced the colonization of subtropical East Asian caves after 4 Ma.
Fig. 4.
Correlation between the relative colonization rates of subtropical East Asian caves and global average temperature. Solid line represents the best-fit linear relationship, whereas the circles show the raw data. Gray shading indicates 95% credible intervals.
Similar to findings for East Asian terrestrial alpine (9, 35) and karst (10) organisms, our study shows that biotic colonization of subtropical East Asian caves has been a dynamic process. Recent work on terrestrial organisms of subtropical East Asia suggests that climatic and geological changes might have similarly impacted the historical assembly of the surface biota (36). Collectively, these studies indicate that geological and climate changes might have affected simultaneously the historical assembly of both surface and subterranean biotas from this region. Our analyses further reveal that the colonization dynamics of subtropical East Asian caves are associated with the evolution of local EBLFs. This climate-vegetation-relict model may be applicable to explain the evolutionary history of other subterranean biotas. We suggest that East Asian subtropical EBLFs are an essential source for cave biodiversity in subtropical East Asia, and when external unfavorable conditions occur, caves in turn serve as refugia. In addition, our results identify nine transitions from caves back to the surface environment: one in plants, five in animals, and three in fungi (Fig. 2 and SI Appendix, Figs. S2 and S3), suggesting that cave populations can sometimes represent a critical source of germplasm, not only for the survival of these lineages in caves, but also for the restoration of forest (11). These findings emphasize the importance of conservation of surface and corresponding subterranean biodiversity in geographically and ecologically associated regions.
Materials and Methods
Phylogenetic Analysis and Divergence Time Estimation.
We collected phylogenetic datasets representing 28 clades from GenBank or published articles (see details in SI Appendix, SI Text). For each clade, we first performed maximum-likelihood (ML) searches using RAxML v8.2.10 (37) for each locus and deleted those species that were a source of significant incongruence between different locus trees based on a threshold bootstrap value >70% (38). For the final concatenated datasets, we performed RAxML analyses with the GTR+Γ substitution model for each locus. All RAxML analyses were conducted using the fast bootstrap option and 1,000 replicates.
For each clade, divergence times were estimated using the penalized likelihood method in treePL (39) based on the optimal RAxML tree. A cross-validation with treePL was run in a test of seven values for the smoothing parameter (0.001, 0.01, 0.1, 1, 10, 100, and 1,000). Confidence intervals for each node were calculated from the 1,000 RAxML bootstrap trees in SumTrees (40). Time calibration was done using fossil constraints or secondary calibration points, depending on the information available for each clade (for details of calibrations and analyses, see the SI Appendix, SI Text).
Habitat, Vegetation Type, and Biogeographic Analyses.
To assess the evolutionary mode of adaptation to caves in subtropical East Asia, we reconstructed the ancestral state for habitat at the node subtending the split between each endemic cave-dwelling species and its sister clade using the Bayesian Binary MCMC (BBM) method in RASP v. 4.2 (41) and the optimal time-calibrated tree for each clade from treePL. Habitat was coded as two states: epigean (including entirely outside caves and most commonly outside) and hypogean (including caves and other comparable habitats, e.g., underground rivers, tunnels, sinkholes). To take into account phylogenetic uncertainty, we also sampled 1,000 ultrametric trees obtained using treePL. We applied 10 simultaneous chains optimized with the fixed JC + G model for 5 × 106 generations and sampled the posterior distribution every 1,000 generations.
In parallel with the habitat analysis, we inferred the ancestral vegetation type for each of 23 plant and animal clades. The five clades of fungi were not included in this analysis, as their vegetation types are unknown. We coded three vegetation types: forest, mountain shrub, and meadow. The probability of each vegetation type for the node subtending each endemic cave-dwelling species and its sister clade was estimated using the BBM method, as described in habitat reconstruction.
Ancestral ranges were inferred for each clade in BioGeoBEARS (42) under the Dispersal-Extinction-Cladogenesis (DEC) model (43). Based on the current distribution of extant species, known geological history of the areas, and modern climate, eleven biogeographical regions were defined: subtropical East Asia, Indochina, tropical Asian islands, India, Australia, Central Asia, West Asia, Europe, Africa, North America, and South America. For each clade, the analysis was conducted without constraining the availability of connections between areas, and the maximum number of areas was set as the maximum number of areas observed in the extant taxa. The inferred stem-node ages, ancestral vegetation types, and source areas of 75 lineages/species endemic to subtropical East Asian caves are listed in SI Appendix, Table S4.
Colonization Dynamic Meta-Analysis.
To investigate the colonization dynamics that led to the endemic cave-dwelling species, we first extracted 75 credibility intervals of divergence times at nodes for which we inferred immigration based on the results of the 28 habitat analyses (SI Appendix, Table S4). We then calculated the maximum number of observed immigration events (MIE) per million years by summing up potential immigration events over all datasets through time using time slices of one million years. The MIE indicates that immigration events are more likely to have happened when the corresponding divergence time intervals overlap, given that these immigration events have the same abiotic causes (44). The data were smoothed by calculating mean values using a sliding window approach with a time frame of 5 My. We also extracted immigration times for ferns (4 events), angiosperms (37 events), arachnids (12 events), reptiles (2 events), amphibians (2 events), fishes (4 events), and fungi (14 events). Their respective MIEs were calculated using the same approach.
We identified change points in the MIE curve using two methods. First, the Bayesian change point analysis was performed using the R package bcp (45). The analysis was run for 100,000 MCMC iterations and discarded the first 10% as burn-in. Based on Barnes et al. (46), we set p0 to 10−2 and allowed w0 to decrease iteratively from 10−25 to 10−10 until at least one change point was identified. Identified change points with posterior probabilities <0.80 were excluded. Second, significant change points were located using the cpt.mean function with default settings, implemented in the R package changepoint (47).
Macroecological Analyses.
To characterize the relationship between cave colonization and East Asian subtropical EBLFs, we estimated which grid cells from the paleoenvironmental reconstruction corresponded approximately to this biome through time by defining these cells as those with mean annual temperature between 13 °C to 20 °C and aridity index = 0. This equates to a rough approximation of a subtropical environment likely to be dominated by East Asia subtropical EBLFs (27) (Movie S1). We then recorded changes in suitable area by summing up the number of grid cells through time (km2). Given that nearly all immigration events occurred after 40 Ma (SI Appendix, Table S4), we compiled East Asia landscape data from 40 Ma to the present using the database of Hagen et al. (48).
Because low temperature in winter and annual rainfall are two major limiting factors for the distribution and species richness of East Asian subtropical EBLFs (27, 34), we plotted the changes of the minimum temperature of the coldest month (MTCM) and the mean annual precipitation (MAP) in subtropical East Asia. We collected these two indicators of paleoclimate data from 19 fossil sites, for which quantitative parameters have been provided (SI Appendix, Table S6). The corresponding present-day climate variables for each of these 19 fossil sites were calculated from WorldClim2 data (49).
To further assess the correlation between cave colonization and temperature variation over time, we first calculated immigration rates using the equation cXtoY(t1) = dXtoY(t1)/Br(t1), where dXtoY(t1) is the number of inferred immigration events from area X to Y in a 1-My bin, and Br(t1) is the total length of all branches in the 1-My bin (50). We then fitted a linear regression to relate colonization rates with global temperature changes (51) in R v.4.0.0 (52).
Supplementary Material
Acknowledgments
We thank the two anonymous reviewers for constructive comments, which greatly improved this manuscript. This research was partially funded by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB31030000), the National Natural Science Foundation of China (31770231, 31770233, and 32170210), and the K.C. Wong Education Foundation (GJTD-2020-05).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2207199119/-/DCSupplemental.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Romero R., Cave Biodiversity. Life in Darkness (Cambridge University Press, Cambridge, 2009). [Google Scholar]
- 2.Humphreys W. F., “Background and glossary” in Ecosystems of the World, 30: Subterranean Ecosystems, Wilkens H., Culver D. C., Humphries W. F., Eds. (Elsevier, Amsterdam, 2000), pp. 3–14. [Google Scholar]
- 3.Culver D. C., Pipan T., “Survey of subterranean life” in The Biology of Caves and Other Subterranean Habitats, Culver D. C., Pipan T., Eds. (Oxford University Press, Oxford, 2009), pp. 40–73. [Google Scholar]
- 4.Recknagel H., Trontelj P., From cave dragons to genomics: Advancements in the study of subterranean tetrapods. Bioscience 72, 254–266 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bichuette M. E., Trajano E., “Conservation of subterranean fishes” in Biology of Subterranean Fishes, Trajano E., Bichuette M. E., Kapoor B. G., Eds. (Science Publishers, Enfield, 2010), pp. 65–80. [Google Scholar]
- 6.Duan Y. F., Li M., Xu K. W., Zhang L., Zhang L. B., Protect China’s karst cave habitats. Science 374, 699 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Sánchez-Fernández D., Galassi D. M. P., Wynne J. J., Cardoso P., Mammola S., Don’t forget subterranean ecosystems in climate change agendas. Nat. Clim. Chang. 11, 458–459 (2021). [Google Scholar]
- 8.Warren B. H., et al. , Islands as model systems in ecology and evolution: Prospects fifty years after MacArthur-Wilson. Ecol. Lett. 18, 200–217 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Ding W. N., Ree R. H., Spicer R. A., Xing Y. W., Ancient orogenic and monsoon-driven assembly of the world’s richest temperate alpine flora. Science 369, 578–581 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Li X. Q., et al. , Immigration dynamics of tropical and subtropical Southeast Asian limestone karst floras. Proc. Biol. Sci. 289, 20211308 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monro A. K., Bystriakova N., Fu L., Wen F., Wei Y., Discovery of a diverse cave flora in China. PLoS One 13, e0190801 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holsinger J. R., “Ecological derivation, colonization, and speciation” in Subterranean Ecosystems, Wilkens H., Culver D. C., Humphries W. F., Eds. (Elsevier, Amsterdam, 2000), pp. 399–415. [Google Scholar]
- 13.Zhang Y. H., Zhu D. H., Large karst caves distribution and development in China. J. Guilin. Univer. Tech. 32, 20–28 (2012). [Google Scholar]
- 14.Ballarin F., Li S., Diversification in tropics and subtropics following the mid-Miocene climate change: A case study of the spider genus Nesticella. Glob. Change Biol. 24, e577–e591 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Merckx V. S. F. T., et al. , Evolution of endemism on a young tropical mountain. Nature 524, 347–350 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Schindler J. S., Karst of China. Ground Water 20, 226–230 (1982). [Google Scholar]
- 17.Yuan J., et al. , Rapid drift of the Tethyan Himalaya terrane before two-stage India-Asia collision. Natl. Sci. Rev. 8, nwaa173 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spicer R., et al. , Why ‘the uplift of the Tibetan Plateau’ is a myth? Natl. Sci. Rev. 8, nwaa091 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G. Z., Chu F. Y., Wang C. S., Paleoelevation reconstruction of Red River drainage areas in Western Yunnan Plateau since Miocene. J. Chengdu Univer. Tech. 31, 118–124 (2004). [Google Scholar]
- 20.Ding W. J., et al. , Palaeovegetation variation in response to the late Oligocene-early Miocene East Asian summer monsoon in the Ying-Qiong Basin, South China Sea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 567, 110205 (2021). [Google Scholar]
- 21.Yu X. Q., et al. , Insights into the historical assembly of East Asian subtropical evergreen broadleaved forests revealed by the temporal history of the tea family. New Phytol. 215, 1235–1248 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Chen X. H., et al. , Biogeographic diversification of Mahonia (Berberidaceae): Implications for the origin and evolution of East Asian subtropical evergreen broadleaved forests. Mol. Phylogenet. Evol. 151, 106910 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Li S. F., et al. , Orographic evolution of northern Tibet shaped vegetation and plant diversity in eastern Asia. Sci. Adv. 7, eabc7741 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y. S., Deng T., Zhou Z., Sun H., Is the East Asian flora ancient or not? Natl. Sci. Rev. 5, 920–932 (2018). [Google Scholar]
- 25.Westerhold T., et al. , An astronomically dated record of Earth’s climate and its predictability over the last 66 million years. Science 369, 1383–1387 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Liu X. D., et al. , Where were the monsoon regions and arid zones in Asia prior to the Tibetan Plateau uplift? Natl. Sci. Rev. 2, 403–416 (2015). [Google Scholar]
- 27.Song Y. C., Da L. J., “Evergreen broad-leaved forest of East Asia” in Vegetation Structure and Function at Multiple Spatial, Temporal and Conceptual Scales, Box E. O., Ed. (Springer, 2016), pp. 101–128. [Google Scholar]
- 28.Wynne J., et al. , A conservation roadmap for the subterranean biome. Conserv. Lett. 14, e12834 (2021). [Google Scholar]
- 29.Zhang Z. F., et al. , Culturable mycobiota from karst caves in China II, with descriptions of 33 new species. Fungal Divers. 106, 29–136 (2021). [Google Scholar]
- 30.Deng T., Wu F. X., Wang S. Q., Su T., Zhou Z. K., Major turnover of biotas across the Oligocene/Miocene boundary on the Tibetan Plateau. Palaeogeogr. Palaeoclimatol. Palaeoecol. 567, 110241 (2021). [Google Scholar]
- 31.Zheng H., Birth of the Yangtze River: Age and tectonic-geomorphic implications. Natl. Sci. Rev. 2, 438–453 (2015). [Google Scholar]
- 32.Farnsworth A., et al. , Past East Asian monsoon evolution controlled by paleogeography, not CO2. Sci. Adv. 5, eaax1697 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W., et al. , A dated phylogeny of Lardizabalaceae reveals an unusual long-distance dispersal across the Pacific Ocean and the rapid rise of East Asian subtropical evergreen broadleaved forests in the late Miocene. Cladistics 36, 447–457 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Wang Z., Fang J., Tang Z., Lin X., Patterns, determinants and models of woody plant diversity in China. Proc. Biol. Sci. 278, 2122–2132 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu W., et al. , Herpetological phylogeographic analyses support a Miocene focal point of Himalayan uplift and biological diversification. Natl. Sci. Rev. 8, nwaa263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang C. Q., et al. , Identifying long-term stable refugia for relict plant species in East Asia. Nat. Commun. 9, 4488 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamatakis A., RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang W., Li H., Chen Z., Analysis of plastid and nuclear DNA data in plant phylogenetics-evaluation and improvement. Sci. China Life Sci. 57, 280–286 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Smith S. A., O’Meara B. C., treePL: Divergence time estimation using penalized likelihood for large phylogenies. Bioinformatics 28, 2689–2690 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Sukumaran J., Holder M. T., DendroPy: A Python library for phylogenetic computing. Bioinformatics 26, 1569–1571 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Yu Y., Blair C., He X., RASP 4: ancestral state reconstruction tool for multiple genes and characters. Mol. Biol. Evol. 37, 604–606 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Matzke N. J., Probabilistic historical biogeography: New models for founder-event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Front. Biogeogr. 5, 242–248 (2013). [Google Scholar]
- 43.Ree R. H., Smith S. A., Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Syst. Biol. 57, 4–14 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Klaus S., Morley R. J., Plath M., Zhang Y. P., Li J. T., Biotic interchange between the Indian subcontinent and mainland Asia through time. Nat. Commun. 7, 12132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erdman C., Emerson J. W., Bcp: An R package for performing a Bayesian analysis of change point problems. J. Stat. Softw. 23, 1–13 (2007). [Google Scholar]
- 46.Barnes B. D., Sclafani J. A., Zaffos A., Dead clades walking are a pervasive macroevolutionary pattern. Proc. Natl. Acad. Sci. U.S.A. 118, e2019208118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Killick R., Eckley I., Changepoint: An R package for changepoint analysis. J. Stat. Softw. 58, 1–19 (2014). [Google Scholar]
- 48.Hagen O., et al. , gen3sis: A general engine for eco-evolutionary simulations of the processes that shape Earth’s biodiversity. PLoS Biol. 19, e3001340 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fick S. E., Hijmans R. J., WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017). [Google Scholar]
- 50.Antonelli A., et al. , Amazonia is the primary source of Neotropical biodiversity. Proc. Natl. Acad. Sci. U.S.A. 115, 6034–6039 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scotese R., Song H. J., Mills B. J., van der Meer W. D. G., Phanerozoic paleotemperatures: The earth’s changing climate during the last 540 million years. Earth Sci. Rev. 215, 103503 (2021). [Google Scholar]
- 52.R Core Team. R: A Language and Environment for Statistical Computing. Version 4.0.0. R Foundation for Statistical Computing (2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.