Significance
Long-term memories are considered to be optimally formed during sleep, but they may also be formed during wakefulness. Using behavioral indicators dissociating object and context memory in a novel-object recognition (NOR) paradigm, in combination with pharmacological inhibition of hippocampal activity during postencoding consolidation, we show that, in contrast to sleep consolidation, wake consolidation does not comprise the spatial–contextual integration of the NOR memory and is impaired by ongoing hippocampal activity. Accordingly, remote NOR memory after wake consolidation was even superior to that after sleep consolidation when tested in a context different from encoding. Our study directly comparing sleep and wake-dependent consolidation demonstrates that memories consolidated during wake and sleep differ in quality, but not necessarily in strength.
Keywords: novel-object recognition memory, memory consolidation, sleep, wake
Abstract
Memory consolidation is promoted by sleep. However, there is also evidence for consolidation into long-term memory during wakefulness via processes that preferentially affect nonhippocampal representations. We compared, in rats, the effects of 2-h postencoding periods of sleep and wakefulness on the formation of long-term memory for objects and their associated environmental contexts. We employed a novel-object recognition (NOR) task, using object exploration and exploratory rearing as behavioral indicators of these memories. Remote recall testing (after 1 wk) confirmed significant long-term NOR memory under both conditions, with NOR memory after sleep predicted by the occurrence of EEG spindle–slow oscillation coupling. Rats in the sleep group decreased their exploratory rearing at recall testing, revealing successful recall of the environmental context. By contrast, rats that stayed awake after encoding showed equally high levels of rearing upon remote testing as during encoding, indicating that context memory was lost. Disruption of hippocampal function during the postencoding interval (by muscimol administration) suppressed long-term NOR memory together with context memory formation when animals slept, but enhanced NOR memory when they were awake during this interval. Testing remote recall in a context different from that during encoding impaired NOR memory in the sleep condition, while exploratory rearing was increased. By contrast, NOR memory in the wake rats was preserved and actually superior to that after sleep. Our findings indicate two distinct modes of long-term memory formation: Sleep consolidation is hippocampus dependent and implicates event-context binding, whereas wake consolidation is impaired by hippocampal activation and strengthens context-independent representations.
Sleep is known to promote memory consolidation, the process that transforms newly encoded information into a more stable form of memory (1, 2). The robust nature of the memory-enhancing effects of postencoding sleep in the context of a wide range of tasks has fueled speculation that this brain state may be a prerequisite for effective long-term memory formation generally. However, recent studies on fruit flies have provided evidence for sleep-independent consolidation processes that support long-term memory formation in the waking state (3). Studies on rodents have also suggested that long-term memory for objects can be formed even if the animal is deprived of sleep during the postencoding period (4–6). These findings raise the question of whether consolidation processes during sleep and wakefulness are of the same kind.
Sleep-dependent consolidation has been described as an active systems-level process of consolidation of experienced episodes, that is, events that are integrated into a spatial–temporal context with hippocampal networks representing the different contexts to which the events are bound (4, 7, 8). Replay of hippocampal neuronal ensembles involved in episodic context binding is considered to be the key to active systems consolidation that occurs during sleep (9, 10). On the other hand, the mechanisms governing consolidation during wakefulness are less well understood (11, 12). Notably, however, significant long-term memory formation under conditions of postencoding wakefulness has often been observed for memories that can also be encoded and retrieved without a functioning hippocampus—such as objects presented in a novel-object recognition (NOR) task—which indicates that they primarily reside in extra-hippocampal circuits, for example, in the perirhinal cortex (12–14).
Here we compared long-term memory formation following an NOR task in rats that had slept or remained awake during a 2-h postencoding interval. Remote NOR memory was assessed a week later, based on the standard procedure, that is, the rat’s preferential exploration of the novel, relative to the familiar, object. In addition, we used the rat’s rearing behavior during NOR testing to assess the extent to which remote recall of object memory simultaneously involves recall of the original spatial context. Rearing on hind legs is an exploratory behavior mediated by the hippocampus. Its purpose is to identify distal context cues, and declines once the rat becomes familiar with the context (15–17). To further examine hippocampal contributions, we inactivated the hippocampus during the 2-h postencoding wake and sleep periods by bilateral infusion of the GABA-A receptor agonist muscimol. We show that, unlike sleep-dependent consolidation, wake consolidation strengthens a context-independent representation of objects and is independent of hippocampal function.
Results
Postencoding Sleep and Wakefulness Promote Long-Term NOR Memory, but Context Memory Is Evidenced Only after Postencoding Sleep.
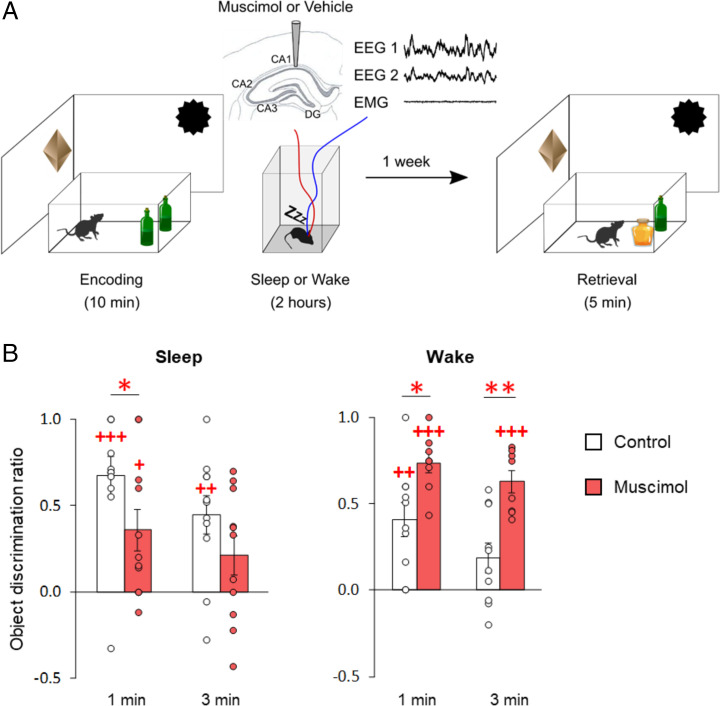
After encoding the NOR task, rats either slept or remained awake during a 2-h postencoding period, and retrieval was tested 1 wk later (see Fig. 1A for experimental design). Behavioral analyses focused on cumulative scores at 1 and 3 min into the test phase, which covers the period most sensitive for the novelty preference–based assessment of memory (9, 18). Exploration discrimination ratios indicated that, during the remote retrieval test 1 wk after exposure to either of the postencoding conditions, the rats displayed NOR memory at levels significantly above chance during the first minute (Fig. 1B, empty bars; mean ± SEM discrimination score: sleep, 0.67 ± 0.11, t(10) = 6.02, P = 0.0001; wake, 0.41 ± 0.10, t(9) = 4.21, P = 0.002) and first 3 min of the retrieval phase (sleep: 0.45 ± 0.11, t(10) = 4.11, P = 0.002; wake, 0.19 ± 0.09, t(9) = 2.19, P = 0.056). These findings confirm previous studies, which indicated that significant NOR long-term memory is also formed in animals that are kept awake after encoding (5, 9). However, our analyses also revealed that discrimination scores were, on average, slightly lower in the wake than the sleep condition (χ2[1] = 3.93, P = 0.048 for the sleep/wake main effect), suggesting that wake consolidation is less effective overall than sleep consolidation. Total object exploration and total distance traveled during encoding and retrieval were comparable for both conditions (all P > 0.7; SI Appendix, Fig. S1), excluding possible confounding factors associated with nonspecific changes in motivation or locomotion.
Fig. 1.
Remote NOR memory is present after postencoding sleep and wakefulness, but is oppositely influenced by postencoding inactivation of the hippocampus. (A) Experimental design. Rats explored two identical objects in an arena for 10 min (encoding), and then either slept or remained awake for 2 h. Retrieval of NOR memory was tested a week later. For retrieval testing, one of the two objects used in the encoding phase was replaced by a novel object. Recognition memory was assessed based on the increased exploration time the rat devoted to the novel object in comparison to that spent exploring the old object during the first 3 min of the retrieval phase (object discrimination ratio). To inactivate the hippocampus during the 2-h postencoding interval, muscimol was bilaterally infused into the dorsal hippocampus, either upon the first occurrence of continuous SWS or at a comparable time point during postencoding wakefulness. Controls included rats infused with vehicle and untreated rats. (B) Mean (±SEM) object discrimination ratios at 1 and 3 min into the remote retrieval phase, for animals of the sleep (Left) and wake (Right) groups with functioning hippocampus (empty bars), and following infusion with muscimol (red bars) during the 2-h postencoding interval n = 11, 11, 10, and 8 rats for sleep control, sleep muscimol, wake control, and wake muscimol groups, respectively. +++P < 0.001, ++P < 0.01, +P < 0.05 for one-sample t tests against chance level; **P < 0.01, *P < 0.05 for pairwise t tests (two-sided) between muscimol and control groups.
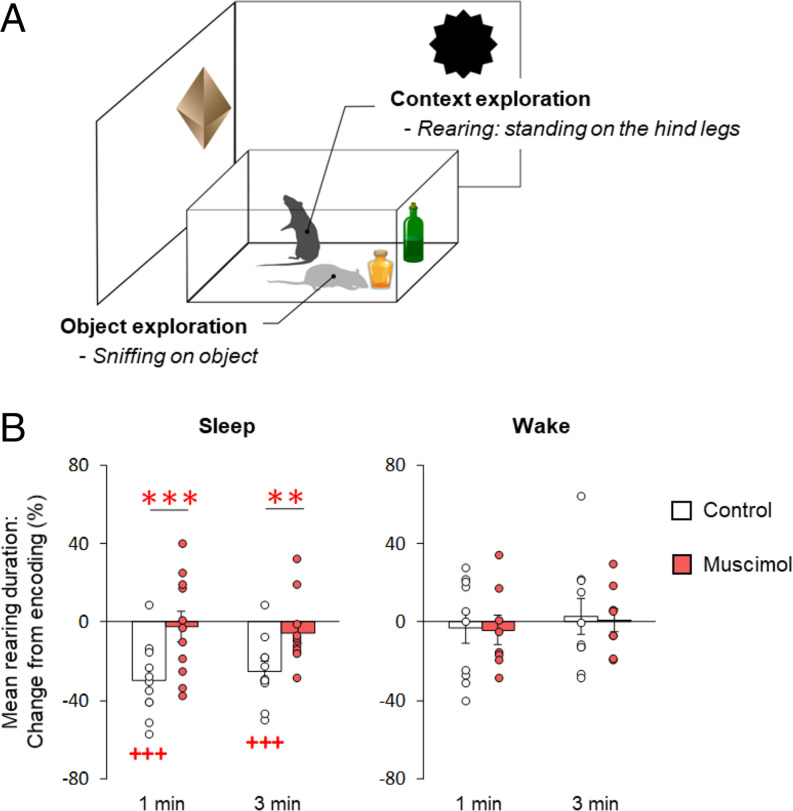
In order to assess the extent to which remote retrieval involved recall of environmental context, that is, distal spatial cues (17), we analyzed how much time the rats spent on their hind legs in an exploratory rearing posture (Fig. 2A). In rats that slept during the postencoding interval, mean rearing duration decreased from encoding to remote retrieval testing (Fig. 2B, gray bars; first minute: −29.67 ± 5.61%, t(10) = −5.29, P = 0.0004; third minute: −29.67 ± 5.61%, t(10) = −4.99, P = 0.0005). This decrease reflects habituation, as the rat has become familiar with the spatial context (16, 17). In contrast, rats that stayed awake in the postencoding interval displayed the same high level of exploratory rearing upon remote retrieval testing as they showed during encoding (Fig. 2B, empty bars; first minute: −2.90 ± 8.04%, t(9) = −0.36, P = 0.73; third minute: 2.93 ± 8.94%, t(9) = 0.33, P = 0.75), indicating that these rats had no recollection of the spatial context (χ2[1] = 9.01, P = 0.003, for the difference in rearing change between sleep and wake conditions). The number of rearing events during the retrieval phase did not differ between conditions (χ2[1] = 0.22, P = 0.64). Control analyses revealed no differences in mean rearing duration or number during the encoding phase between sleep and wake conditions (mean ± SEM; mean rearing duration: sleep, 1.17 ± 0.06 s; wake, 1.18 ± 0.08 s, t(17.4) = −0.07, P = 0.95; number of rearing events: sleep, 98.18 ± 9.06; wake, 120.10 ± 17.09, t(13.8) = −1.13, P = 0.28; SI Appendix, Fig. S2), excluding the possibility that the changes observed in the remote retrieval test derived from baseline differences in exploration patterns between the groups. NOR performance was not correlated with exploratory rearing on hind legs at remote retrieval (all P > 0.26).
Fig. 2.
Postencoding sleep, but not wakefulness, decreases exploratory rearing at remote NOR testing. (A) Illustration of the two prominent exploratory behaviors on the NOR task. In addition to object exploration (object sniffing), rearing on the hind legs was analyzed to assess to what extent remote retrieval involved recall of environmental context, that is, distal spatial cues. (B) Percentage change in mean rearing duration (mean ± SEM) at first and third minutes of retrieval testing, compared to mean rearing duration during encoding (set to 100%). Only rats that had slept after encoding (Left) and retained hippocampus function (empty bars) showed a decrease in rearing duration at retrieval, whereas mean rearing duration in the rats that remained awake (Right) and in the rats whose hippocampi were inactivated during the postencoding interval (red bars) did not change between encoding and remote retrieval testing; n = 11, 11, 10, and 8 rats for sleep control, sleep muscimol, wake control, and wake muscimol groups, respectively. +++P < 0.001, for one-sample t tests against chance level; ***P < 0.001, **P < 0.01, for pairwise t tests (two-sided) between muscimol and control groups.
Inactivation of the Hippocampus Has Opposite Effects on Consolidation of NOR Memory during Postencoding Sleep and Wakefulness.
We then investigated whether hippocampal function makes an essential contribution to the formation of long-term NOR memory during sleep and wakefulness. To this end, the GABA-A receptor agonist muscimol was bilaterally infused (at a rate of 0.25 µL/min) into the dorsal hippocampus. In the sleep condition, the infusion started as soon as EEG recordings indicated signs of continuous sleep, that is, after 31.55 ± 4.36 min and 26.97 ± 4.48 min of the postencoding interval for muscimol and vehicle infusions, respectively. In the wake condition, infusions were started after comparable time intervals, that is, after 33.75 ± 3.88 min and 31.68 ± 4.64 min for muscimol and vehicle infusions (P = 0.394 and P = 0.675, for the differences between sleep and wake conditions, respectively). At remote retrieval testing 1 wk later, rats whose hippocampi had been inactivated during postencoding sleep showed a distinctly decreased NOR performance in comparison with the vehicle control rats (Fig. 1B; χ2[1] = 8.32, P = 0.004), although discrimination ratios for the first minute remained above chance level, indicating that some NOR memory was left (t(10) = 3.01, P = 0.013; discrimination index at third minute t(10) = 1.85, P = 0.094). In contrast, inactivating the hippocampus during postencoding wakefulness enhanced remote NOR performance, in comparison with the control rats in which hippocampal function was not perturbed (Fig. 1B; χ2[1] = 10.66, P = 0.001, t(16) = 2.68, P = 0.016 and t(16) = 3.90, P = 0.001, for post hoc pairwise comparisons between muscimol and control conditions at the first and third minutes of retrieval; χ2[1] = 18.51, P < 0.001 for sleep/wake × muscimol/control interaction, with exclusion of one rat; see Materials and Methods). NOR memory was well above chance level for discrimination scores at the first and third minutes (t(7) = 12.32, P < 0.001 and t(7) = 9.81, P < 0.001, respectively). Analyses of control variables revealed a consistent increase in total distance traveled at NOR retrieval for the muscimol condition (SI Appendix, Fig. S1). However, reanalysis of discrimination ratios using this parameter as covariate confirmed all of the significant effects of muscimol on NOR performance (all P < 0.01), thus confirming that these effects were not confounded by nonspecific motivational and locomotion-related factors. In combination, these data indicate that hippocampal activity influences the consolidation of NOR memory during sleep and wakefulness in opposite directions. In agreement with active systems consolidation, they confirm a supportive influence of hippocampal activity on sleep-dependent consolidation (7, 9). In contrast, rather than being independent of hippocampal function, wake consolidation processes appear to be disturbed by interfering hippocampal activity (12, 19, 20).
Inactivation of the hippocampus during postencoding sleep and wakefulness also differentially affected the rats’ rearing behavior during remote testing (χ2[1] = 6.05, P = 0.014, for sleep/wake × muscimol/control interaction effect). Inactivating the hippocampus during postencoding sleep prevented the decrease in mean rearing duration between encoding and remote testing that was observed for the vehicle control rats (Fig. 2 B, Left; change from encoding to retrieval for muscimol vs. control across minutes: χ2[1] = 24.63, P < 0.0001; first minute: −2.3 ± 7.7% vs. −29.7 ± 5.6%, t(10) = 5.08, P < 0.001; third minute: −5.5 ± 5.2% vs. −25.0 ± 5.0%, t(10) = 3.65, P = 0.0044). By contrast, when the hippocampus was inactivated during postencoding wakefulness, rearing duration at remote retrieval testing was not affected, and remained at the same high level as during encoding (Fig. 2 B, Right; change from encoding to retrieval for muscimol vs. control across minutes: χ2[1] = 0.03, P = 0.87; for first minute: −4.04 ± 7.36% vs. −2.90 ± 8.04%, t(16) = −0.10, P = 0.92; third minute: 0.99 ± 6.12% vs. 2.93 ± 8.94%, t(16) = −0.17, P = 0.87). Muscimol infusion did not affect the number of rearing events, and there were also no differences between muscimol and control conditions during encoding (all P > 0.25; SI Appendix, Fig. S2). Thus, the main finding of these experiments indicates that hippocampal inactivation selectively prevents habituation of rearing when induced during sleep, corroborating the view that sleep, but not wakefulness, promotes the parallel consolidation of spatial context features.
Remote NOR Memory Is Context Dependent after Postencoding Sleep but Not Wakefulness.
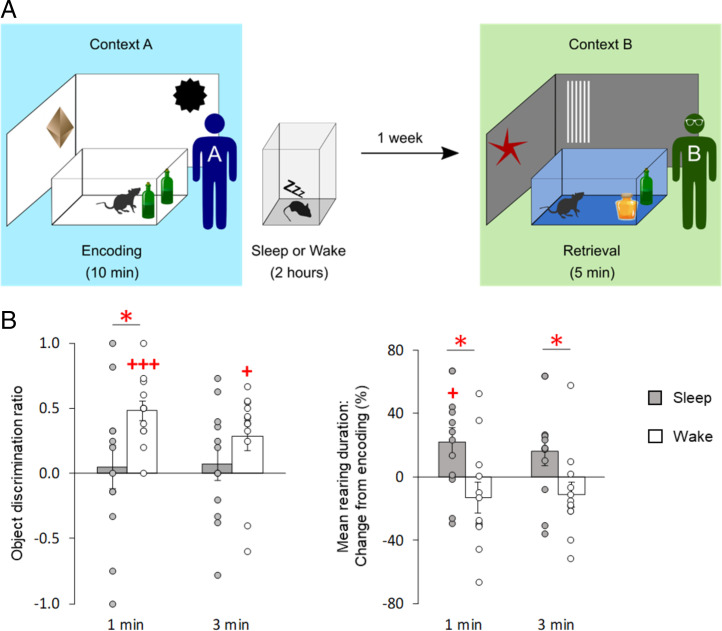
If only sleep-dependent consolidation of NOR memory, but not wake-associated consolidation, were coupled to the simultaneous formation of a hippocampus-dependent context memory, then recall of NOR memory in a context other than that presented during encoding would be expected to be more severely affected after sleep- than wake-dependent consolidation. In order to test this hypothesis, we tested two groups of rats on the NOR task after postencoding sleep and wake periods, respectively. However, this time NOR recall was tested 1 wk later in an entirely different experimental context by a different experimenter (Fig. 3A). Indeed, the rats that had slept after encoding showed no significant NOR memory at the remote retrieval test, whereas the rats in the wake group displayed robust NOR memory (Fig. 3 B, Left; mean ± SEM discrimination score; first minute: sleep, 0.05 ± 0.17, t(11) = 0.28, P = 0.78; wake, 0.48 ± 0.08, t(11) = 6.34, P < 0.001; third minute: sleep, 0.07 ± 0.13, t(11) = 0.57, P = 0.58; wake, 0.29 ± 0.11, t(11) = 2.59, P = 0.025) which was significantly stronger than in the sleep group (χ2[1] = 5.41, P = 0.020 for sleep/wake main effect). These findings suggest that memory for objects after wake-dependent consolidation is less well integrated with the learning context and can, therefore, be more easily retrieved in a different context.
Fig. 3.
Remote NOR memory after postencoding sleep, but not after postencoding wakefulness, is context dependent. (A) Experimental procedures. During encoding, rats explored two identical objects in an arena of context A for 10 min, followed by a 2-h interval in which the rats either slept or remained awake. Retrieval of NOR memory was tested a week later in a different (but familiar) context B. Contexts A and B differed with respect to distal and proximal cues, and olfactory and auditory stimulation, and also with regard to the experimenters who performed the experiments (see Materials and Methods). (B) (Left) Mean (±SEM) object discrimination ratios at first and third minutes of the retrieval phase for animals of the sleep (gray bars) and wake (empty bars) groups, and (Right) percentage change in mean rearing duration (mean ± SEM) at first and third minutes of retrieval phase, compared to mean rearing duration during encoding (set to 100%). Note that rats that slept after encoding (gray bars) showed a decrease in NOR memory performance, but an increase in rearing duration, relative to the wake rats that remained awake during the 2-h postencoding interval (empty bars); n = 12 rats each for sleep and wake groups. +++P < 0.001, +P < 0.05 for one-sample t tests against chance level; *P < 0.05 for pairwise t tests (two-sided) between groups.
This inference is corroborated by the finding that rats that had slept after encoding showed increased mean rearing duration during remote retrieval testing in comparison with the encoding phase (Fig. 3 B, Right; first minute: 22.03 ± 9.17%, t(11) = 2.40, P = 0.035; third minute: 16.07 ± 8.85%, t(11) = 1.82, P = 0.097), which indicates that these rats detected the contextual change of the NOR task (17). In contrast, the rats in the wake group displayed the same level of exploratory rearing upon remote retrieval testing as they did during encoding (Fig. 3 B, Right; first minute: −13.08 ± 9.65%, t(11) = −1.36, P = 0.20; third minute: −11.14 ± 7.88%, t(11) = −1.41, P = 0.19, χ2[1] = 7.57, P = 0.006 for the sleep/wake main effect). Rearing behavior in these wake-group rats was, in fact, very similar to that seen in the wake rats tested in unchanged context conditions (Fig. 1B). Overall, this suggests that postencoding wakefulness does not support the formation of a persistent memory for the environmental context.
Sleep Spindles and Slow Oscillation–Spindle Events Are Associated with Enhanced Remote NOR Memory.
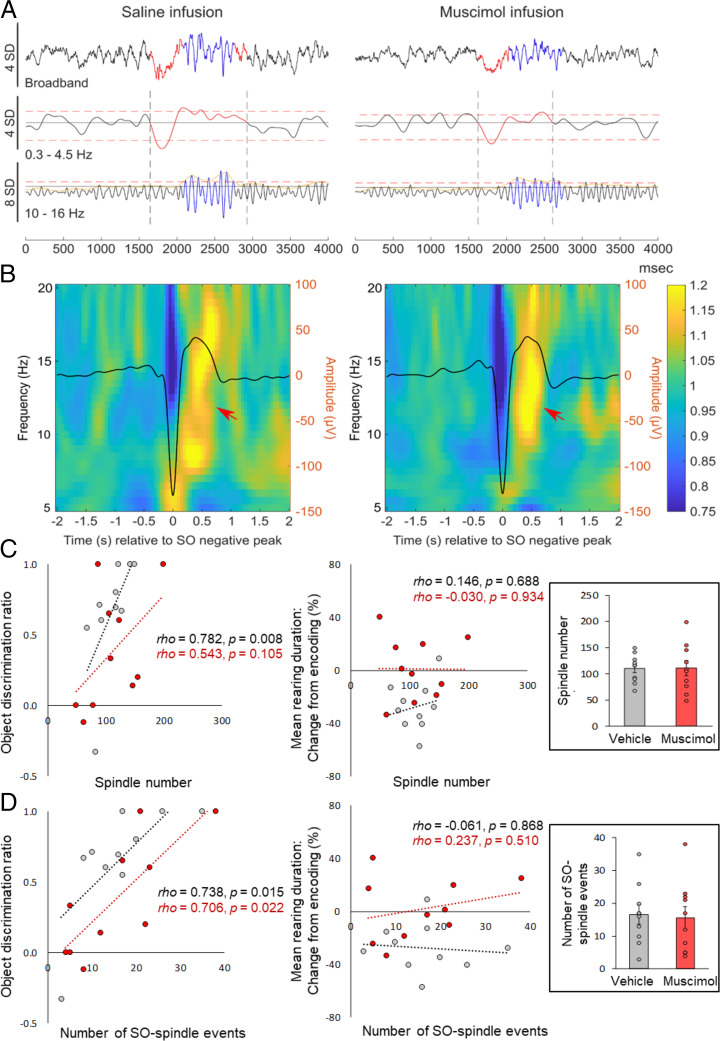
In order to identify specific mediators of sleep-dependent consolidation, we more closely analyzed sleep EEG recordings during postencoding sleep. Based on previous findings (21–23), we concentrated on sleep spindles and slow oscillations (SOs) associated with slow-wave sleep (SWS) and theta activity associated with rapid eye movement (REM) sleep (see Table 1 and 2 for sleep architecture and sleep parameters). This analysis revealed that the greater the numbers of SO or spindles recorded during postencoding SWS, the higher the NOR discrimination ratios were at 1 min during retrieval (rho = 0.730, P = 0.017, rho = 0.782, P = 0.008; Fig. 4; see SI Appendix, Table S1 for a summary of correlational analyses). The correlation between NOR performance and SO and spindle density showed the same trend but did not reach statistical significance (rho = 0.546 and rho = 0.534, respectively, both P = 0.11). In light of evidence suggesting that spindles nesting in the up state of the SO are of particular importance for memory consolidation (22, 24, 25), we separately analyzed such coupled SO–spindles events. Indeed, this analysis also revealed a positive association between the number of coupled SO–spindle events and remote NOR performance (Fig. 4 C and D; rho = 0.74, P = 0.015). The significance of this correlation was confirmed in regression analyses that controlled for the contribution of uncoupled spindles (occurring in the absence of an SO). Here, the number of coupled SO–spindle events significantly predicted NOR performance (β = 0.65, t(7) = 2.81, P = 0.026), whereas noncoupled spindles did not (β = 0.34, t(7) = 1.47, P = 0.185, with the two predictors explaining R2adj = 53% of the total variance, F(2,7) = 6.17, P = 0.029). Finally, NOR performance was also positively correlated with theta energy during REM sleep (rho = 0.72, P = 0.019). While we observed substantial associations with NOR performance, none of the EEG sleep measures was correlated with mean rearing duration or numbers of rearing events at remote recall testing (Fig. 4 C and D; all P < 0.69).
Table 1.
Sleep architecture
| Sleep parameter | Latency (min) | Duration (min) | ||
|---|---|---|---|---|
| SWS | SWS | PreREM | REM | |
| Vehicle | 13.70 ± 3.21 | 49.67 ± 2.38 | 3.95 ± 0.59 | 6.00 ± 0.87 |
| Muscimol | 17.10 ± 2.17 | 50.62 ± 4.37 | 4.67 ± 0.92 | 3.95 ± 1.26 |
| Group comparison |
t (9) = −0.96, P = 0.36 |
t (9) = −0.22, P = 0.83 |
t (9) = −0.73, P = 0.48 |
t (9) = 1.68, P = 0.13 |
Fig. 4.
Associations between behavioral performance at remote retrieval and sleep spindle–related activity during postencoding SWS. (A) Individual EEG traces from one rat (over the left prefrontal cortex) after intrahippocampal infusion of vehicle (Left) or muscimol (Right) during postencoding sleep. Original recordings are shown before (Top) and after filtering in the slow oscillatory (0.3 Hz to 4.5 Hz, Middle) and spindle (10 Hz to 16 Hz, Bottom) frequency bands. (B) Time-frequency power plots −2 s to +2 s around the negative peak (0 s) of the EEG SOs (overlaid solid line, right y axis). Data represent averages across all identified SO events and all animals during the 2-h postencoding sleep interval after vehicle (Left) and muscimol infusion (Right), n = 10 rats in each condition. Power is color coded. Note the increase in spindle-related power nesting in the upstate of the SO in both conditions (arrows). (C) NOR performance (discrimination ratios; Left), but not mean rearing duration (indicating spatial context memory; Right) at retrieval testing, is positively correlated with the number of sleep spindles and (D) the number of coupled SO–spindle events. Vehicle, gray dot plots; muscimol, red dot plots. Note that the correlation of NOR with SO–spindle event numbers is also observed after muscimol infusion. Spearman correlation coefficients and P values (uncorrected for multiple comparison) are indicated. Inserted bar graphs indicate the mean (±SEM) number of identified spindles and SO–spindle events identified during the 2-h postencoding interval.
Inactivating the hippocampus by infusion of muscimol did not alter gross sleep architecture, that is, time spent in and onset latencies of SWS, preREM, or REM sleep (all P > 0.095; Table 1). Moreover, sleep oscillatory activity (SOs, spindles, coupled SO–spindle events) was comparable between muscimol and control conditions (all P > 0.162; Table 2) except that, in agreement with previous reports (9), the power of REM sleep theta was decreased during muscimol infusion (t(7) = −2.67, P = 0.032; Table 2). Overall, these findings exclude the possibility that impaired NOR performance after hippocampal inactivation is a consequence of gross changes in sleep patterns. Interestingly, the positive correlation observed between numbers of coupled SO–spindle events and NOR performance under conditions of a functioning hippocampus was similarly observed with intrahippocampal infusion of muscimol during postencoding sleep (rho = 0.71, P = 0.022, with P > 0.3 for the comparison with respective correlation in the control condition; see SI Appendix, Table S1 for respective correlations for SO and spindle parameters), suggesting that sleep spindles can contribute to consolidation processes that take place outside and independently of the hippocampus.
Table 2.
Sleep parameters
| Sleep parameter | SWS | REM | |||||
|---|---|---|---|---|---|---|---|
| SO density (number per minute) | SO amplitude (mV) | Spindle density (number per minute) | Spindle power (mV2/s) | Spindle mean duration (s) | Theta power (mV2/s) | Total theta energy (mV2/s) | |
| Vehicle | 5.63 ± 0.78 | 0.230 ± 0.020 | 2.37 ± 0.13 | 0.03 ± 0.002 | 0.58 ± 0.007 | 0.026 ± 0.002 | 8.33 ± 1.31 |
| Muscimol | 5.70 ± 0.81 | 0.225 ± 0.022 | 2.28 ± 0.12 | 0.03 ± 0.003 | 0.58 ± 0.01 | 0.021 ± 0.002 | 4.60 ± 1.60 |
| Group comparison |
t (9) = −0.92, P = 0.38 |
t (9) = 0.07, P = 0.95 |
t (9) = 0.70, P = 0.50 |
t (9) = 0.92, P = 0.37 |
t (9) = 0.07, P = 0.95 |
t (9) = 2.67, P = 0.032 |
t (9) = 2.03, P = 0.07 |
Sleep parameters for the postencoding sleep condition in rats with functioning (vehicle) and inactivated hippocampus (muscimol). Indicated are mean (±SEM) values of density and peak-to-peak amplitude of SOs, density, power, and mean duration of spindles during SWS, and theta power and energy during REM sleep; n = 10 rats for each condition (corresponding to the behavioral data in Figs. 1 and 2, one rat was excluded from the analyses due to technical issues with the recording). Note that, apart from a decrease in REM sleep theta power, muscimol infusion did not alter sleep.
Discussion
The significance of sleep for long-term memory formation is well recognized. But what about the waking state? Does the wakeful mode of brain activity also promote memory consolidation? The present study set out to characterize memory consolidation associated with the waking state by contrasting it with sleep-dependent consolidation. Our results in rats demonstrate that long-term NOR memory is present at a remote retrieval test performed 1 wk later, when the rats remain awake during the critical 2-h period after encoding of the memory. However, memories formed under these conditions differed from those formed during postencoding sleep, both in quantity and quality. First, in comparison with the memory traces formed during postencoding sleep, wake consolidation resulted in a weaker memory, that is, lower discrimination ratios upon remote retrieval testing. Second, unlike postencoding sleep, which produced accompanying memory for spatial environmental context—as indicated by a decrease in exploratory rearing on hind legs during remote retrieval—wake-associated consolidation apparently lacked such accompanying formation of context-related long-term memory. Thirdly, unlike sleep-dependent consolidation, consolidation associated with postencoding wakefulness was not impaired by inactivation of the hippocampus, but was instead enhanced, which implies that ongoing hippocampal activity interferes with consolidation during wakefulness. And fourthly, NOR memory recall after wake-associated consolidation was superior to that seen after postencoding sleep when the rats had to recognize the test objects in a context different from that used during encoding. These findings support the view that the brain’s wake state is associated with a distinct mode of long-term memory formation which is, in part, linked to different memory traces.
Our experimental manipulation of sleep and wakefulness focused on a 2-h interval following the encoding phase, which has been shown to be most important for sleep-dependent consolidation (23, 26–28). Nevertheless, considering our focus on the first 2 h after encoding, it could be argued that consolidation in the wake group originated from the sleep period that occurred after the 2-h postencoding interval, rather than from the preceding period of wakefulness, which would be compatible with our observation that NOR memory recall 1 wk after encoding was weaker in the wake than the sleep condition. However, this explanation is unlikely because, in previous studies, delaying sleep by more than 2 h after encoding was not found to restore consolidation more fully in similar tasks (4). More importantly, the assumption that the wake condition induces a delayed sleep-dependent consolidation process cannot explain the qualitative difference in wake-consolidated memory at remote testing, that is, the lack of accompanying context memory, as well as its enhancement by hippocampal inactivation. It should be noted, however, that our results do not exclude the possibility that the later periods of sleep occurring after the 2-h postencoding interval may also be required for maintaining systems level consolidation.
Our finding of lower discrimination ratios upon remote NOR testing after postencoding wakefulness relative to the sleep-based consolidation condition agrees with findings indicating that memory consolidated during wakefulness is also less persistent, decaying earlier in comparison to memory that was consolidated during a postencoding sleep epoch (9, 29). Nevertheless, we should be wary of premature generalization here, as wake-associated consolidation processes are still incompletely understood. Wake-associated consolidation might be strongly affected by organismic drives. For example, in fasted Drosophila fruit flies, consolidation of odor–taste associations during wakefulness was found to be as powerful as consolidation during postencoding sleep-like states (3).
Basically, two different mechanisms can, in principle, account for the relative weakness of NOR memory upon remote testing after wake-based vis-a-vis sleep-associated consolidation: Underlying synaptic consolidation and potentiation may be weaker, or the relevant object representations may be less accessible to recognition. Factors active during organismic states—such as neuropeptide Y during hunger or glucocorticoids during mild stress (3, 11, 30)—may promote wake-associated memory consolidation by enhancing synaptic consolidation processes. Enhanced accessibility of object memories, on the other hand, might be a consequence of a systems consolidation process that involves a transformation of the originally encoded episodic representation of the task, such that the objects are more readily retrievable. Indeed, sleep is considered to support such active systems consolidation of episodic memory for events experienced in a spatiotemporal context (31, 32), in which the hippocampus serves to bind the event representation into the spatial–temporal context. The repeated neuronal replay of spatial context information in hippocampal place cell ensembles during SWS epochs represents a core mechanism of sleep-dependent consolidation (7, 10, 33, 34) that might foster the gradual transformation of the representation, such that it becomes more stably integrated within preexisting neocortical long-term memory, as well as with contextual information (35). Such transformations would not only explain the enhanced retrieval of the objects on our NOR task after sleep but also our finding regarding exploratory rearing, which suggests that postencoding sleep, but not wake consolidation of NOR memory, occurs in parallel with the formation of spatial, contextual long-term memory. The better embedding in contextual long-term memory may thus, indeed, provide enhanced access to the object memory (36–38).
This view of sleep as actively consolidating and transforming the representation is further supported by our findings that enhanced SO and sleep spindle activity, in particular with spindles nesting in SO up states, predicted improved NOR performance at remote retrieval. There is increasing evidence that such SO–spindle events support hippocampal-to-neocortical information transfer, as well as extra-hippocampal storage processes during systems consolidation (22, 25, 39). Adding to this evidence, we found that SO–spindle events also predict remote NOR performance in rats in which hippocampus function is inactivated during postencoding sleep, suggesting that such events support synaptic consolidation of representations outside the hippocampus, regardless of whether or not hippocampal memory information is fed into these SO–spindle events (40–43). It is important to note that these correlational findings do not rule out other predicting factors on memory consolidation during sleep, given that the analyses comprised a small number of animals and hence possess only low statistical power.
Although these EEG findings basically agree with an active systems consolidation process during sleep, there is considerable debate as to whether active systems consolidation during sleep indeed supports embedding into, and concurrent strengthening of, contextual memory or, alternatively, favors the formation of a decontextualized representation of the object, or supports both types of processes in parallel (31, 34, 44–46). Our finding of a decrease in exploratory rearing activity—which points to enhanced context memory at remote retrieval testing after postencoding sleep—remains inconclusive in this regard, because it is not clear whether the rats actually relied on this context memory when they retrieved the objects. Rearing on hind legs is a spontaneous exploratory behavior, presumably to enhance the rat’s perception of distal stimuli, which is associated with an increase in hippocampal theta activity and information flow through the medial perforant pathway (15, 17). Exploratory rearing decreases after repeated exposures to a familiar environment, and increases in response to environmental novelty or a reconfiguration of familiar contextual stimuli (16, 47). It thus represents a valid indicator of contextual memory. However, upon remote retrieval, we did not find a correlation between the animal’s rearing behavior and NOR performance that would have provided a clue in support of the idea that postencoding sleep fosters the formation of an integrated object-in-context memory.
That sleep indeed supports the formation of such object-in-context memory is ultimately demonstrated by our context change experiments in which NOR retrieval was tested in a context distinctly different from the encoding context. Here, rats of the sleep group displayed the expected increase in exploratory rearing in response to the change in context (16), but, simultaneously, NOR performance was distinctly impaired; that is, the change in context apparently interfered with the retrieval of the NOR memory. This pattern supports the view that sleep consolidation strengthens the association between context and event in the episodic representation. Importantly, findings obtained in the context change experiments simultaneously underlined essential features of wake-associated consolidation, insofar as the rats in the wake group in these experiments showed no signs of an activated context memory (i.e., no change in exploratory rearing) in conjunction with a well-preserved object recognition memory. Thus, in contrast to sleep consolidation, consolidation during wakefulness favors the formation of a context-independent event memory.
By inactivating the hippocampus, we explored the contribution of this structure to postencoding wake- and sleep-associated consolidation. Our findings confirm the dependence of sleep-dependent consolidation on this brain structure, which has previously been demonstrated even for memories like NOR that can be encoded and retrieved in the absence of a functioning hippocampus (9, 48). Against this backdrop, the important finding of the present study is that, rather than remaining unaffected, postencoding wake consolidation processes were actually enhanced by inactivation of the hippocampus. This finding is, indeed, reminiscent of human studies showing that ethanol enhances memory, but only when administered after learning, which was attributed to a GABA agonistic effect of ethanol preventing new hippocampal encoding and, thereby, any retroactive interference that would weaken the recently learned information (49, 50). Assuming that wakefulness itself, even in conditions of quiescence, is characterized by enhanced hippocampal encoding activity, in comparisons with sleep, our finding appears to fit also with the view proposed by Mednick et al. (50) that the hippocampus contributes to memory consolidation in an “opportunistic” manner, that is, whenever there are moments of reduced interference due to encoding. The hippocampus plays a binding role in episodic memory formation which, in the NOR task, requires the integration of object-related information from the perirhinal cortex and spatial information from, among other structures, the parahippocampal (postrhinal in rats) cortex, that is, two structures that are reciprocally connected to the hippocampus via the entorhinal cortex (51, 52). Accordingly, in hippocampally lesioned rats, severe memory deficits are observed in tasks requiring the rat to discriminate objects presented in a particular place and/or a particular context (13, 53–55). Coordinate hippocampal neuronal reactivations in this system, which occur repeatedly during postencoding SWS and reach these and other cortical areas (including the medial prefrontal cortex), thus represent a mechanism that can potentially explain the enhancing effect of hippocampal activity on sleep-dependent NOR memory consolidation. Perhaps it is this hippocampally driven system whose activation during wakefulness retroactively interferes with consolidation processes taking place outside of the hippocampus (12, 19, 20, 56).
Overall, our findings are consonant with the idea of two distinct memory systems that can competitively interact, one being hippocampus dependent and the other relying on extra-hippocampal, striatal, and cortical regions (57–60). The present data add to this idea by demonstrating that the interaction between these memory systems extends to the postencoding consolidation process, and does so in a brain state–dependent manner. Thus, against the background of our understanding of sleep-dependent memory consolidation, this study begins to delineate essential features of wake-dependent memory consolidation. Unlike sleep consolidation, wake consolidation of experience is shown to lack accompanying formation of long-term contextual memory, and is disturbed rather than enhanced by ongoing hippocampal activity, likely affecting the traces that were directly encoded into cortical and other extra-hippocampal networks (61).
Materials and Methods
Experimental Design and General Procedures.
Each experiment comprised an encoding phase, a subsequent 2-h postencoding period, and a retrieval phase that took place 1 wk after the encoding phase. The main experiments were designed 1) to differentiate between the effects of postencoding sleep and wakefulness and 2) to assess the effects of hippocampal inactivation (by the infusion of muscimol) during the respective postencoding intervals of sleep and wakefulness on the consolidation of NOR memory. Thus, four experimental conditions, that is, sleep control, sleep muscimol, wake control, and wake muscimol groups (Fig. 1A), were employed. Comparisons between the effects of postencoding sleep and wakefulness were based on two groups of rats (sleep group, n = 11; wake group, n = 10). To define the contributions of hippocampal function to consolidation during postencoding sleep, each animal in the sleep group was tested on two occasions, that is, once under intrahippocampal infusion of muscimol and once under infusion of vehicle, during the 2-h postencoding period. The order of muscimol and vehicle conditions was counterbalanced across animals of the group, and the two conditions were separated by an interval of at least 2 wk (i.e., twice as long as the tested 1-wk retention interval). Hippocampal contributions during wakefulness were evaluated in two separate groups of rats, one of which received intrahippocampal infusions of muscimol (n = 8 rats), while the other was infused with vehicle (n = 6 rats) during the postencoding wake period. We added another sample of n = 4 untreated rats, which had not undergone surgical implantation of cannulas, as the strictest control condition because, in these rats, the hippocampus was assuredly intact. Since exploratory behavior and memory performance were virtually the same in these untreated and in the vehicle-treated rats, the two groups were merged into one wake control group (n = 10 rats).
In a further experiment (“context change”), we investigated the context dependency of remote NOR memory retrieval in two (separate) groups of rats, a sleep group (n = 12 rats) and a wake group (n = 12 rats). Here, the rats were tested, at the 1-wk retention interval, in an experimental context that differed from that employed in the encoding phase (Fig. 3A). In all experiments, animals were randomly assigned to a given experimental condition before the experiment. The experimenters were not blinded to the experimental condition during data collection. However, all behavioral and electrophysiological recordings were analyzed offline, with the experimenters blinded to the experimental conditions. All experiments took place during the animal’s rest phase (i.e., between 08:00 and 14:00 h).
NOR Task.
Twenty-four hours after the last habituation session (see SI Appendix, Supplementary Methods for habituation procedure), the rats were again brought into the test room for the encoding phase of the NOR task (Fig. 1A). Two identical objects were placed in the open field (see SI Appendix, Supplementary Methods for a description of behavioral apparatus), and the rats were allowed to explore them for 10 min. The objects were made of glass, with different shapes and colors, and heavy enough not to be moved by the rat (height: 15 cm to 30 cm; base diameter: 7 cm to 12 cm). They were placed at least 10 cm equidistant from the walls. The encoding phase was followed by a 2-h postencoding period, during which the rats were placed in the resting box, and either left undisturbed (sleep condition) or kept awake (wake condition). In the wake condition, wakefulness was enforced by gentle handling. This procedure has been shown to minimize stress and confounding influences of locomotion when applied over a longer period (62) (SI Appendix, Supplementary Methods). It involves gently tapping on the “resting box” and, if necessary, gently shaking the box. No intense stimulation was used, and video recordings ensured that no signs of startle or freezing behavior occurred. After the postencoding period, the rats were brought back to their home cages, and kept under routine conditions until retrieval testing 1 wk later. For remote memory testing on the NOR task, the rats were again placed in the open field containing two objects. In this retrieval phase, one of the objects used in the encoding phase was replaced by a novel object, and the rats were allowed to explore the objects and open field for 5 min. For the “context change” experiments, the rats were placed in context A for the encoding phase of the NOR task and context B for retrieval testing SI Appendix, Supplementary Methods. Types of objects and their locations in the open field, as well as the order of contexts used for the context change experiments, were counterbalanced across the experimental groups. After each phase, the objects and open field were cleaned with water containing 70% ethanol. The behavior of the rats was monitored by a video camera and analyzed offline by experienced experimenters using ANY-maze software (Stoelting Europe).
Context Change Manipulation.
The two contexts (A and B) used in the context change experiments differed with respect to their visual, tactile, auditory, and olfactory features. The two different square, open-field arenas (80 cm × 80 cm; height of walls: 40 cm) were placed in adjacent rooms separated by a thick black curtain. In context A, the open-field surface was covered with light gray adhesive film. Three sides of the open field were surrounded by a white circular curtain with various objects attached to it (two three-dimensional [3D] multicolored circular objects, two black posters with white stripes, and two black posters). On the fourth side was a black curtain with a white poster and two 3D rectangular objects. The ceiling was also covered by a white curtain with a black and blue tube attached to a video camera mounting frame. In context B, the open-field floor was in a darker gray and covered with a rough, transparent plastic sheet. There were two large posters on a black curtain on the south side of the arena, and two other posters on the north wall. Several blue paper stripes were attached to the west wall. Two shelves with distinct objects on them, which differed in shape, size, and number, were placed on the opposite side in the room. Two different masking noise sounds were used in each context. Lighting conditions also differed between contexts, but light intensity did not exceed 30 lx. The resting box used for the postencoding interval was, in context A, the same as that described for the main experiment, but, in context B, another was used that differed in both size (39 × 46 cm; height: 41 cm) and material (Plexiglas). When used, they were placed in different experimental rooms. Finally, in each context, the rat was handled by a different experimenter.
Hippocampal Inactivation during the Postencoding Period.
We used the GABA-A receptor agonist muscimol to temporarily inactivate the dorsal hippocampus during sleep. The procedures were the same as described in Sawangjit et al. (9). Guide cannulae were surgically implanted bilaterally into the dorsal hippocampus. Dummy cannulae were inserted into the guide cannulae to prevent contamination. During a recovery period (at least 8 d), the dummy cannulae were removed and reinserted once a day to avoid occlusion of the guide cannulae. Muscimol (Sigma, 0.5 μg dissolved in 0.5 μL of 0.9% saline per hemisphere) or an equivalent volume of saline solution was infused bilaterally into the dorsal hippocampus through two 30-gauge injection cannulae. The injection cannulae protruded 1 mm beyond the tip of the guide cannulae. Each injection cannula was connected to a 10-μL Hamilton microsyringe (Hamilton) via 1-m polyethylene cannula tubing, and the substance was infused over 2 min by an automated syringe pump (PHD ULTRA, Harvard Apparatus). Substance administration started immediately upon visual online detection of continuous SWS for at least 10 s (sleep condition) or a roughly equivalent postencoding time in the wake condition. The times of substance administration were comparable among experimental conditions (sleep muscimol 31.55 ± 4.36 min, sleep vehicle 26.97 ± 4.48 min, wake muscimol 33.75 ± 3.88 min, wake vehicle 31.68 ± 4.64 min; P = 0.723).
Surgical Procedures and Histology.
Before surgery, rats received an intraperitoneal injection of anesthetic mixture (0.005 mg/kg fentanyl, 2 mg/kg midazolam and 0.15 mg/kg medetomidine). The surgery was carried out under general isoflurane anesthesia (induction: 1 to 2%, maintenance: 0.8 to 1.2% in 0.35 L/min O2). Rats were placed in the stereotaxic frame, and the skull was exposed. For substance infusion, two stainless steel guide cannulae (23 gauge, 7 mm long, Plastics One) were bilaterally implanted into the dorsal hippocampus (anterior–posterior [AP]: −4.3 mm, mediolateral [ML]: ±2.8 mm, dorsoventral [DV]: −1.4 mm under the skull surface, relative to bregma). The cannulae were introduced at this position, laterally tilted by 9° with respect to the vertical axis, and were affixed to the skull with four stainless steel bone screws and cold polymerizing dental resin. Dummy cannulae (7 mm long, Plastics One) were inserted into the guide cannulae and removed only during experiments.
For EEG recordings, four stainless steel screw electrodes (Plastics One) were implanted: two frontal electrodes (AP: +2.6 mm, ML: ±1.8 mm, relative to bregma) and two occipital electrodes (AP: −10.0 mm, ML: ±1.8 mm), serving as reference and ground electrodes, respectively. Two stainless steel wire electrodes were also implanted bilaterally in the neck muscles to record EMG signals. All electrodes were connected to a Mill-Max pedestal (Mill-Max Mfg. Corp.) and fixed to the skull with the cold polymerizing dental resin. After surgery, carprofen (5 mg/kg) was injected subcutaneously, and the rats were allowed to recover for at least 8 d before starting behavioral experiments. For EEG and EMG recordings, the electrodes were connected through a preamplifier headstage (HS-18MM, Neuralynx) to a Digital Lynx SX acquisition system (Neuralynx), amplified, filtered (EEG: 0.1 Hz to 50.0 Hz; EMG: 30.0 Hz to 300.0 Hz), and sampled at a rate of 1,000 Hz.
After completion of the experiments, histological verification of the infusion sites was performed (SI Appendix, Fig. S3). The rats were perfused intracardially with 0.9% saline followed by 4% paraformaldehyde (PFA). After decapitation, the brains were removed and immersed in the 4% PFA for 48 h to 72 h. Coronal sections of 50 μm to 70 μm were cut on a vibratome, stained with toluidine blue and examined under a light microscope. The infusion sites in all rats were confirmed to be in the dorsal hippocampus.
Analysis of Memory Performance.
Object exploration was defined as the rat being within 1 cm of an object, directing its nose toward the object, and engaging in active exploration behaviors such as sniffing. Leaning on the object without sniffing the object closely (>1 cm) was not counted as object exploration behavior. To assess NOR memory retrieval, a discrimination ratio was calculated according to the following general formula: (exploration time at novel object − exploration time at familiar object)/(exploration time at novel object + exploration time at familiar object). Novel object preference, that is, a positive value of the discrimination ratio, indicates memory for the familiar object (of the encoding phase), whereas a value of zero indicates no exploration preference. Moreover, the total time of object exploration (across both objects) and the distance traveled in the open field were utilized as indicators of locomotion and motivation. Data from one rat exposed to the wake muscimol condition was excluded from analyses owing to its very low object exploration time at encoding (0.6 s), deviating by more than 1.5 SD from the mean total object exploration time across all experimental groups. This exclusion criterion was chosen as it corresponded to the SD value closest to 0 s exploration time. The rat excluded by this criterion showed also very little object exploration (0.4 s) during retrieval testing.
Exploratory rearing behavior was analyzed to investigate contextual memory (17). Exploratory rearing was scored whenever the rat was standing on its hind legs in an upright position, lifting the forelegs off the ground. Rearing to an object together with sniffing within 1 cm of the object was not scored as rearing behavior. The mean duration of each rearing event, the number of rearing events, and the total rearing time were analyzed for both encoding and retrieval phases. We focus here on mean duration of rearing, as this measure proved to sensitively reflect spatial contextual memory in previous studies (17) and was most sensitive to contextual changes in our own experiments. In contrast, rearing numbers were less affected by contextual changes (SI Appendix, Fig. S2A). For analyses, the percentage change in mean rearing time during retrieval relative to that observed during encoding was calculated, and mean rearing time during the 10-min encoding phase was set to 100%.
Analysis of Sleep EEG.
Sleep stage classification was performed offline by an experienced experimenter using 10-s epochs according to standard criteria (63). The stages identified were SWS, preREM sleep, REM sleep, and wakefulness. Classification criteria for the wake stage were predominant low-amplitude fast activity associated with increased EMG tonus; for SWS were predominant high-amplitude delta activity (<4.0 Hz) and reduced EMG activity; and, for REM sleep, were predominant theta activity (4.0 Hz to 8.0 Hz), phasic muscle twitches, and minimum EMG activity. PreREM sleep was identified by a decrease in delta activity, a progressive increase of theta activity, and the presence of sleep spindles (10.0 Hz to 16.0 Hz).
SOs and spindles were identified in the EEG over the left frontal cortex. Procedures were adapted from Sawangjit et al. (9) and are described in detail in SI Appendix, Detection of Slow Oscillations and Spindles.
To analyze theta-band frequency activity during REM sleep epochs, the EEG signal was filtered between 5.0 and 10.0 Hz (Butterworth filter of third order), and the Hilbert transform of the signal was calculated. Mean theta power was calculated over all REM sleep epochs by taking the mean of the absolute values of the transformed signal from all REM sleep epochs. Total theta energy was calculated by taking the integral of the absolute values of the transformed signal from all REM sleep epochs. All EEG data were processed in Matlab (Version 2019b). Because of a technical failure during recording, data from one rat (vehicle control) were excluded from EEG analyses.
Statistical Analyses.
Statistical analyses of behavioral parameters concentrated on cumulative data at minutes 1 and 3 of the retrieval phase, because this initial period most sensitively reflects novelty discrimination–based memory (14, 18), especially for long-term memory retrieval (9). Note also that the essential results reported here did not change when concentrating the analyses on the first minute of the test phase. All statistical comparisons of experimental groups were done by fitting linear mixed-effects models with individual rats as random effect (random intercept only), and significance was tested by comparing models using likelihood ratio tests. Post hoc analyses employed two-sided Welch two-sample t tests or paired t tests. Correlational analyses report Spearman rank coefficients. No correction for multiple testing was used in the analyses. For a more detailed description of the analysis strategy, see SI Appendix, Statistical Analyses.
Statistical analyses were calculated in R (Version 1.3.1.1093) using the lme4 package (Version 1.1-23) and SPSS (Version 26.0, IBM). A P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Ilona Sauter for valuable technical support. This study was supported by grants from the Deutsche Forschungsgemeinschaft to M.I. (Grant DFG In 279/1-1) and the European Research Council to J.B. (Grant ERC AdG 883098 SleepBalance). M.I. is supported by the Hertie Foundation (Hertie Network of Excellence in Clinical Neuroscience).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2203165119/-/DCSupplemental.
Data Availability
Behavioral Data and code to run the analysis have been uploaded to Github (https://github.com/MedPsychTuebingen/Two-distinct-ways-to-form-long-term-object-recognition-memory-during-sleep-and-wakefulness) (64).
References
- 1.Rasch B., Born J., About sleep’s role in memory. Physiol. Rev. 93, 681–766 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stickgold R., Sleep-dependent memory consolidation. Nature 437, 1272–1278 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Chouhan N. S., Griffith L. C., Haynes P., Sehgal A., Availability of food determines the need for sleep in memory consolidation. Nature 589, 582–585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inostroza M., Binder S., Born J., Sleep-dependency of episodic-like memory consolidation in rats. Behav. Brain Res. 237, 15–22 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa H., Yamada K., Pavlides C., Ichitani Y., Sleep deprivation impairs spontaneous object-place but not novel-object recognition in rats. Neurosci. Lett. 580, 114–118 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Studte S., Bridger E., Mecklinger A., Nap sleep preserves associative but not item memory performance. Neurobiol. Learn. Mem. 120, 84–93 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Klinzing J. G., Niethard N., Born J., Mechanisms of systems memory consolidation during sleep. Nat. Neurosci. 22, 1598–1610 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Wills T. J., Lever C., Cacucci F., Burgess N., O’Keefe J., Attractor dynamics in the hippocampal representation of the local environment. Science 308, 873–876 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawangjit A., et al. , The hippocampus is crucial for forming non-hippocampal long-term memory during sleep. Nature 564, 109–113 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Wilson M. A., McNaughton B. L., Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679 (1994). [DOI] [PubMed] [Google Scholar]
- 11.Kelemen E., Bahrendt M., Born J., Inostroza M., Hippocampal corticosterone impairs memory consolidation during sleep but improves consolidation in the wake state. Hippocampus 24, 510–515 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliveira A. M. M., Hawk J. D., Abel T., Havekes R., Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn. Mem. 17, 155–160 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker G. R. I., Warburton E. C., When is the hippocampus involved in recognition memory? J. Neurosci. 31, 10721–10731 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winters B. D., Forwood S. E., Cowell R. A., Saksida L. M., Bussey T. J., Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: Heterogeneity of function within the temporal lobe. J. Neurosci. 24, 5901–5908 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barth A. M., Domonkos A., Fernandez-Ruiz A., Freund T. F., Varga V., Hippocampal network dynamics during rearing episodes. Cell Rep. 23, 1706–1715 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunsaker M. R., Rosenberg J. S., Kesner R. P., The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus 18, 1064–1073 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Lever C., Burton S., O’Keefe J., Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev. Neurosci. 17, 111–133 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Dix S. L., Aggleton J. P., Extending the spontaneous preference test of recognition: Evidence of object-location and object-context recognition. Behav. Brain Res. 99, 191–200 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., et al. , Hippocampal activation of Rac1 regulates the forgetting of object recognition memory. Curr. Biol. 26, 2351–2357 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Villar M. E., Martinez M. C., Lopes da Cunha P., Ballarini F., Viola H., Memory consolidation and expression of object recognition are susceptible to retroactive interference. Neurobiol. Learn. Mem. 138, 198–205 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Boyce R., Glasgow S. D., Williams S., Adamantidis A., Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 352, 812–816 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Latchoumane C. V., Ngo H.-V. V., Born J., Shin H.-S., Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron 95, 424–435.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Sawangjit A., Oyanedel C. N., Niethard N., Born J., Inostroza M., Deepened sleep makes hippocampal spatial memory more persistent. Neurobiol. Learn. Mem. 173, 107245 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Helfrich R. F., et al. , Bidirectional prefrontal-hippocampal dynamics organize information transfer during sleep in humans. Nat. Commun. 10, 3572 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niethard N., Ngo H.-V. V., Ehrlich I., Born J., Cortical circuit activity underlying sleep slow oscillations and spindles. Proc. Natl. Acad. Sci. U.S.A. 115, E9220–E9229 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eschenko O., Mölle M., Born J., Sara S. J., Elevated sleep spindle density after learning or after retrieval in rats. J. Neurosci. 26, 12914–12920 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eschenko O., Ramadan W., Mölle M., Born J., Sara S. J., Sustained increase in hippocampal sharp-wave ripple activity during slow-wave sleep after learning. Learn. Mem. 15, 222–228 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiffelholz T., Aldenhoff J. B., Novel object presentation affects sleep-wake behavior in rats. Neurosci. Lett. 328, 41–44 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Lutz N. D., Diekelmann S., Hinse-Stern P., Born J., Rauss K., Sleep supports the slow abstraction of gist from visual perceptual memories. Sci. Rep. 7, 42950 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilhelm I., Wagner U., Born J., Opposite effects of cortisol on consolidation of temporal sequence memory during waking and sleep. J. Cogn. Neurosci. 23, 3703–3712 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Inostroza M., Born J., Sleep for preserving and transforming episodic memory. Annu. Rev. Neurosci. 36, 79–102 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Oyanedel C. N., Sawangjit A., Born J., Inostroza M., Sleep-dependent consolidation patterns reveal insights into episodic memory structure. Neurobiol. Learn. Mem. 160, 67–72 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Fernández-Ruiz A., et al. , Long-duration hippocampal sharp wave ripples improve memory. Science 364, 1082–1086 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gridchyn I., Schoenenberger P., O’Neill J., Csicsvari J., Assembly-specific disruption of hippocampal replay leads to selective memory deficit. Neuron 106, 291–300.e6 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Lewis P. A., Durrant S. J., Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn. Sci. 15, 343–351 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Gulli R. A., et al. , Context-dependent representations of objects and space in the primate hippocampus during virtual navigation. Nat. Neurosci. 23, 103–112 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Hasselmo M. E., Eichenbaum H., Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw. 18, 1172–1190 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ritchey M., Montchal M. E., Yonelinas A. P., Ranganath C., Delay-dependent contributions of medial temporal lobe regions to episodic memory retrieval. eLife 4, e05025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staresina B. P., et al. , Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat. Neurosci. 18, 1679–1686 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dickey C. W., et al. , Travelling spindles create necessary conditions for spike-timing-dependent plasticity in humans. Nat. Commun. 12, 1027 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez L. M. J., Lüthi A., Sleep spindles: Mechanisms and functions. Physiol. Rev. 100, 805–868 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Peyrache A., Seibt J., A mechanism for learning with sleep spindles. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20190230 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Timofeev I., et al. , Short- and medium-term plasticity associated with augmenting responses in cortical slabs and spindles in intact cortex of cats in vivo. J. Physiol. 542, 583–598 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borquez M., Born J., Navarro V., Betancourt R., Inostroza M., Sleep enhances inhibitory behavioral control in discrimination learning in rats. Exp. Brain Res. 232, 1469–1477 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bryant N. B., Nadel L., Gómez R. L., Associations between sleep and episodic memory updating. Hippocampus 30, 794–805 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Niethard N., Born J., A backup of hippocampal spatial code outside the hippocampus? New light on systems memory consolidation. Neuron 106, 204–206 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Anderson M. I., et al. , Behavioral correlates of the distributed coding of spatial context. Hippocampus 16, 730–742 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Schapiro A. C., et al. , The hippocampus is necessary for the consolidation of a task that does not require the hippocampus for initial learning. Hippocampus 29, 1091–1100 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parker E. S., et al. , The alcohol facilitation effect on memory: A dose-response study. Psychopharmacology (Berl.) 74, 88–92 (1981). [DOI] [PubMed] [Google Scholar]
- 50.Mednick S. C., Cai D. J., Shuman T., Anagnostaras S., Wixted J. T., An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 34, 504–514 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eichenbaum H., Yonelinas A. P., Ranganath C., The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 30, 123–152 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kealy J., Commins S., The rat perirhinal cortex: A review of anatomy, physiology, plasticity, and function. Prog. Neurobiol. 93, 522–548 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Barbosa F. F., Pontes I. M., Ribeiro S., Ribeiro A. M., Silva R. H., Differential roles of the dorsal hippocampal regions in the acquisition of spatial and temporal aspects of episodic-like memory. Behav. Brain Res. 232, 269–277 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Drieskens D. C., et al. , CA1 inactivation impairs episodic-like memory in rats. Neurobiol. Learn. Mem. 145, 28–33 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Langston R. F., Wood E. R., Associative recognition and the hippocampus: Differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus 20, 1139–1153 (2010). [DOI] [PubMed] [Google Scholar]
- 56.Martínez M. C., Villar M. E., Ballarini F., Viola H., Retroactive interference of object-in-context long-term memory: Role of dorsal hippocampus and medial prefrontal cortex. Hippocampus 24, 1482–1492 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Albouy G., et al. , Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron 58, 261–272 (2008). [DOI] [PubMed] [Google Scholar]
- 58.Hartley T., Burgess N., Complementary memory systems: Competition, cooperation and compensation. Trends Neurosci. 28, 169–170 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Packard M. G., Goodman J., Factors that influence the relative use of multiple memory systems. Hippocampus 23, 1044–1052 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Poldrack R. A., Packard M. G., Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia 41, 245–251 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Brodt S., et al. , Fast track to the neocortex: A memory engram in the posterior parietal cortex. Science 362, 1045–1048 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Hagewoud R., et al. , Coping with sleep deprivation: Shifts in regional brain activity and learning strategy. Sleep 33, 1465–1473 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neckelmann D., Olsen Ø. E., Fagerland S., Ursin R., The reliability and functional validity of visual and semiautomatic sleep/wake scoring in the Møll-Wistar rat. Sleep 17, 120–131 (1994). [DOI] [PubMed] [Google Scholar]
- 64.Sawangjit A., et al., Two distinct ways to form long-term object recognition memory during sleep and wakefulness. Github. https://github.com/MedPsychTuebingen/Two-distinct-ways-to-form-long-term-object-recognition-memory-during-sleep-and-wakefulness. Deposited 25 July 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Behavioral Data and code to run the analysis have been uploaded to Github (https://github.com/MedPsychTuebingen/Two-distinct-ways-to-form-long-term-object-recognition-memory-during-sleep-and-wakefulness) (64).