Significance
The same signal can convey different information across an animal’s lifetime. High-density desert locusts avoid predation as juveniles by exhibiting striking warning coloration, which honestly advertises their unpalatability relative to their camouflaged, low-density conspecifics. Here, we show that by reusing their youthful “don’t touch me” yellow color upon sexual maturation, high-density adult male locusts also advertise unprofitability, but in this case to fellow amorous males. This three-way (developmental stage, population density, sex) control of a single carotenoid-binding protein toward multiple adaptive outcomes makes it an exciting model system for unravelling the molecular evolution of an animal signal.
Keywords: sexual dichromatism, sexual selection, phenotypic plasticity, locust swarming, male–male mounting
Abstract
Adaptive plasticity requires an integrated suite of functional responses to environmental variation, which can include social communication across life stages. Desert locusts (Schistocerca gregaria) exhibit an extreme example of phenotypic plasticity called phase polyphenism, in which a suite of behavioral and morphological traits differ according to local population density. Male and female juveniles developing at low population densities exhibit green- or sand-colored background-matching camouflage, while at high densities they show contrasting yellow and black aposematic patterning that deters predators. The predominant background colors of these phenotypes (green/sand/yellow) all depend on expression of the carotenoid-binding “Yellow Protein” (YP). Gregarious (high-density) adults of both sexes are initially pinkish, before a YP-mediated yellowing reoccurs upon sexual maturation. Yellow color is especially prominent in gregarious males, but the reason for this difference has been unknown since phase polyphenism was first described in 1921. Here, we use RNA interference to show that gregarious male yellowing acts as an intrasexual warning signal, which forms a multimodal signal with the antiaphrodisiac pheromone phenylacetonitrile (PAN) to prevent mistaken sexual harassment from other males during scramble mating in a swarm. Socially mediated reexpression of YP thus adaptively repurposes a juvenile signal that deters predators into an adult signal that deters undesirable mates. These findings reveal a previously underappreciated sexual dimension to locust phase polyphenism, and promote locusts as a model for investigating the relative contributions of natural versus sexual selection in the evolution of phenotypic plasticity.
Phenotypic plasticity is a universal adaptation to environmental heterogeneity in time and space (1). For plasticity to constitute a successful evolutionary strategy, it must address the consequences of environmental variation across a range of contexts. For example, conspecific population density covaries with numerous fitness benefits (like increased protection from predators) and costs [like increased sexual competition (2)]. Across taxa, plasticity involves covariation among different functions that likely reflects correlational selection on integrated suites of physiological, morphological, and behavioral traits (3). How are plastic responses integrated across contexts, and how might this lead to novel (4) signal–receiver systems? We focus on communication signals across two contexts with density-dependent effects on fitness. The first context is predator deterrence, where high densities of other unpalatable individuals favor conspicuous warning coloration. The second is sexual competition, where higher competitor densities increase selection on mechanisms to reduce sexual interference (2). We show that a novel visual signal–receiver system recruited from juvenile warning coloration functions to minimize interference from other males during high-density mating encounters.
Locust phase polyphenism is a well-known example of density-dependent phenotypic plasticity (5–7). The most obvious outward manifestation of this plasticity is behavior, with lone-living “solitarious” phase locusts being relatively sedentary and actively avoiding one another, while crowd-living “gregarious” phase locusts are far more active and form cohesive aggregations. This gregarious behavior can lead to collective migratory behaviors on a landscape scale, with gregarious juveniles marching in vast bands that turn into flying swarms upon molting to adulthood. The years 2019 to 2021 have seen a rise in swarming of the desert locust Schistocerca gregaria, with ongoing outbreaks across East Africa and the Middle East (8–10).
In some locust species, these density-dependent behavioral extremes are accompanied by similarly striking differences in coloration that have evolved to bolster their respective life histories (5, 6, 11, 12) (Fig. 1A). Solitarious phase locusts generally develop cryptic coloration. In juveniles of both S. gregaria and the migratory locust Locusta migratoria, this takes the form of background-matching “homochromy” in which nymphs are bright green when developing under humid conditions (=among vegetation) or a dull sand color under drier conditions (6, 13). Solitarious locusts of both sexes maintain cryptic coloration upon molting to adulthood, with solitarious S. gregaria becoming dull brown and L. migratoria maintaining their nymphal green/sand color; in both species, these solitarious phenotypes are then carried into sexual maturation. Gregarious juveniles, by contrast, have little use for camouflage in high-density aggregations, and nymphs of S. gregaria and L. migratoria instead develop bright yellow/orange and black aposematic coloration, which honestly advertises to would-be predators the increased deterrence that gregarious nymphs acquire, either by feeding on toxic plants (14–16) or endogenous toxin production (17). In S. gregaria, the bright yellow component of this aposematic signal is imparted by the expression of a carotenoid-binding “Yellow Protein” (YP), an olfactory-binding related “takeout-like” protein with a hydrophobic core that binds dietary β-carotene into the cuticle (18, 19). YP expression is also essential for the green color of humid-reared solitarious nymphs (18), in conjunction with elevated levels of the blue biliverdin pigment in the hemolymph (6, 20), and plays a minor role in producing the sand-colored phenotype of dry-reared solitarious nymphs (18).
Fig. 1.
Color phenotypes and YP expression in the desert locust. (A) Color phenotypes of the desert locust, with those expressing YP highlighted in yellow. (i) Solitarious juveniles of both sexes (male shown) exhibit cryptic coloration: green at high humidity (=among vegetation) or sand at low humidity (not shown; figure 2 in ref. 6). (ii) Upon reaching adulthood, solitarious males (shown) and females retain a dull cryptic coloration. (iii) Gregarious juveniles of both sexes (male shown) exhibit black and yellow aposematic warning coloration. (iv) Upon reaching adulthood, gregarious males (shown) and females are light pink while sexually immature. (v) Gregarious females become a beige-brown color upon sexual maturation (∼10 d). (vi) Gregarious males (including sham-injected controls in this paper) become bright yellow upon sexual maturation. (vii) RNAi of YP, via dsRNA injection into sexually immature males, leads to a nonyellow, beige coloration upon maturation. (B) Reflectance spectra for 10 each of mature gregarious females, mature gregarious males, and dsYP mature gregarious males (v, vi, and vii in A, respectively). Central line and surrounding color per strip represent the mean reflectance at that wavelength ±1 SE. Approximate spectral sensitivity curves of the three desert locust photoreceptors are also shown (73, 74) (SI Appendix, Results). (C) qRT-PCR of YP expression in the abdominal cuticle of adult male locusts. Boxplots show the relative expression (median ± IQR [interquartile range] and range) in control and dsYP males. RNAi led to a significant reduction in YP mRNA in dsYP males (Mann–Whitney U test, n = 6 per group, U = 36, P = 0.0022; **P < 0.01). Image credits: Tom Fayle and Steve Rogers, University of Cambridge, Cambridge, UK, and Timon Smeets, KU Leuven, Leuven, Belgium.
Coloration in gregarious locusts resets at adulthood; YP expression stops (21) and freshly molted S. gregaria adults of both sexes are light pink, which gives way to a beige color after 3 to 7 d as their cuticle fully sclerotizes. At the point of sexual maturation after a further 3 to 7 d (i.e., 10 to 14 d post molt), gregarious male S. gregaria reexpress YP and quickly develop a bright yellow color across most of the body (22) (Fig. 1A). This change in both YP reexpression and cuticular yellowing occurs in as little as 24 h, and is either substantially lower or entirely absent in contemporaneous gregarious females in laboratory cultures, and entirely absent in solitarious adults of both sexes (19, 21, 22). Yellowing of sexually mature male S. gregaria is accompanied by release of the volatile phenylacetonitrile (PAN; also known as benzyl cyanide), an antiaphrodisiac pheromone that repels rival males during copulatory mate guarding (23–27).
The function of sexual dichromatism in S. gregaria and other locust species (5, 6, 28) has remained unknown since phase polyphenism was first described in 1921 (22, 29, 30). Previous workers have speculated that sexually dimorphic yellowing might function to attract females (22), like carotenoid-based male colors in many other taxa. An additional possibility is that yellow coloration is the visual component of a multimodal signal that repels other males. Mature males will sometimes mount immature (i.e., nonyellow) males when deprived of females in the laboratory (31–34), and recent fieldwork has shown that mature males will quickly identify (and fiercely compete for) nonyellow gravid females as they arrive at open-ground lekking sites (35). Here, we tested the response of both male and female locusts to males naturally expressing yellow coloration, versus YP knockdown males expressing female-like beige coloration.
Results
Male Adult Yellowing Deters Other Males.
We performed behavioral assays of male–male mounting (MMM) to test the hypothesis that adult male reexpression of YP in S. gregaria serves as a male–male antiharassment signal. YP-RNA interference (RNAi) in immature adult males (dsYP) prevented yellowing upon sexual maturation, such that their resulting color more closely resembled that of mature females than that of the bright yellow green fluorescent protein (dsGFP)–injected (control) males (Fig. 1 A and B and SI Appendix, Results), and this could be attributed to a successful YP knockdown (Fig. 1C). For each of eight behavioral trials, 12 bright yellow control males and 12 beige-colored dsYP males were marked and observed together in a chamber for 3 h, with 24 mature females behind a perforated screen to provide the natural pheromone cues of a wild population (Fig. 2A). During each trial, we recorded all unambiguous occurrences of a male mounting another male by jumping or climbing on top of them, as usually observed during normal male–female mounting (5, 34, 36, 37); we also noted the identities of both males in the dyad (Methods).
Fig. 2.
Male yellowing is an intrasexual signal in S. gregaria. (A) Observation chamber and assay setup for MMM assays. Twelve dsYP males and 12 controls were placed in the main chamber and observed for 3 h (n = 8). Twenty-four mature virgin females in the smaller chamber served as an olfactory releaser of male sexual behavior, and ensured that the assay environment more closely achieved the normal balance of male and female pheromones that exists in natural high-density groups. (B) Outcome for all 192 males assayed, plotted as the number of mounts given against the number of mounts received. Treatment is indicated by color (yellow, control; blue, dsYP), trial number 1 to 8 is indicated by decreasing transparency, and count at each made/received combination is indicated by point size (see key, Inset). (C) Mounter choice. Irrespective of their own phenotype, what color conspecifics did male locusts mount throughout each 3-h trial? Males were categorized according to their behavior; boxplots show the number of males per category across eight trials (median ± IQR and range). Significantly more males mounted only a dsYP conspecific(s) than mounted either a control conspecific(s) only or mounted males of both phenotypes (Kruskal–Wallis χ2 = 24.829, n = 8 per group, degrees of freedom [d.f.] = 3, P < 0.001. Dunn’s test of multiple comparisons using rank sums with Bonferroni correction: significant differences [*P < 0.05, **P < 0.01, and ***P < 0.001]). Overall, 51.0% of the 192 males assayed mounted only a dsYP conspecific(s), compared with 2.1% that only mounted a control(s). (D) Signaler outcome. Boxplots show the number of males that were mounted by any other conspecific(s) throughout each of the eight trials (median ± IQR and range). Significantly more dsYP males were mounted per trial than were control males (Mann–Whitney U test, n = 8 per group, U = 0.5, P = 0.0011: significant difference **P < 0.01). Overall, 16.7% of the 96 control males assayed were mounted by another male, compared with 63.5% of the 96 dsYP males. Full breakdowns of all phenotype-by-phenotype interactions are given in SI Appendix, Figs. S2 and S4 and Tables S1–S4.
Control males were significantly more likely to mount a conspecific (of either color) than were dsYP males (Poisson generalized linear model [GLM], Z = −3.813, P = 0.00014; Fig. 2B and SI Appendix, Fig. S1). (There was also a significant effect of trial and trial*treatment interaction, which was caused by relatively inactive control males making fewer mounting attempts overall in the final four [September] trials [SI Appendix, Figs. S1 and S2].) To investigate this further, all 192 males were categorized according to the phenotype(s) of the other males they mounted, if at all. Significantly more males mounted only a dsYP conspecific(s) than mounted either a control conspecific(s) only, or mounted males of both phenotypes (Fig. 2C). Across the 115 males (59.9%) assayed in the eight trials that mounted at least one male, just 4 animals (3.5%) only mounted yellow males, while 98 individuals (85.2%) only mounted dsYP males, a 24.5-fold difference.
dsYP males performed significantly fewer mountings overall (probably due to being mounted themselves), but post hoc comparisons did not otherwise indicate that their target preferences significantly differed from those of control males (a full breakdown is in SI Appendix, Fig. S2 and Table S1; post hoc comparisons are in SI Appendix, Table S2). Control and dsYP males were therefore equally likely to choose to mount dsYP over control males, and therefore equally capable of receiving, processing, and evaluating the visual signal.
The consequence of this mounter preference was that dsYP males were significantly more likely to be mounted by any other male throughout the 3-h assay (negative binomial GLM, Z = 3.474, P = 0.00051; Fig. 2 B and D and SI Appendix, Fig. S3). Categorizing all 192 males according to whether—and by which other males—they were targeted for mounting showed that mounted dsYP males were not disproportionally targeted by males of either treatment, and neither were the mounted control males (a full breakdown is in SI Appendix, Fig. S4 and Table S3; post hoc comparisons are in SI Appendix, Table S4). Overall, all eight trials recorded more mounted dsYP males than mounted controls, for which a simple sign test was significant (P = 0.58 = 0.0039). Across all eight trials, 63.5% of the 96 dsYP males were mounted by at least one other male, compared with 16.7% of the 96 controls. dsYP males were therefore 3.8 times more likely to be mounted than were controls.
Males within both treatment groups exhibited a similarly broad spectrum of activity levels, ranging from individuals that moved around for most of the 3-h trial to others that remained perched in one spot. Our assays confirmed previous findings that male locusts tend to mount a moving target (32, 33, 38), with most mounting events occurring when a male walked past another male or jumped to a new location in the cage. Active dsYP males were therefore more frequently targeted than their inactive dsYP counterparts, and this imbalance explains the apparent discrepancy between mounter preference (24.5 times more likely to mount a dsYP male) and the observed disadvantage (3.8 times more likely to be mounted) for dsYP males. Males responded to being mounted in a variety of ways, including: immediately kicking the mounting conspecific away; standing still until the mounting male dismounted, which could take several minutes; or continuing to explore the assay chamber with the mounting male on top, which could again last several minutes. Mounted males would, on rare occasions, stack on top of other males to form triads. This range of behavioral responses was observed equally in dsYP and control males.
Male Adult Yellowing Does Not Influence Male–Female Mating Outcomes.
We performed female mate-choice assays to test the hypothesis that female locusts would preferentially copulate with males exhibiting a brighter yellow phenotype. Individual mature virgin females were offered one each of control and dsYP males in 2-h trials in a small terrarium (n = 45; Fig. 3A). Four trials did not result in any copulatory mountings, and in two of these trials the females jumped around and kicked off the mounting male(s). On 21 occasions the control male was the first to mount and copulate with the female, and on 20 occasions the dsYP male was the first to mount and copulate with the female. This small difference was not significant (Fig. 3B; raw data are in SI Appendix, Table S5). Latency to initiate copulation with females ranged widely between a few seconds and 92 min from the start of the assay, but did not significantly differ by treatment group (Fig. 3B). The only male–male interactions that we observed in these trials occurred after a male had already mounted the female. In each of these instances (four when the control male was first to mount the female, and six when the dsYP male was first to mount the female; SI Appendix, Table S5), the second male stacked on top of the first male and attempted to mate with the female below. In every case the first male was the one to achieve copulation which, as in most other trials, they maintained until the end of the trial in a typical mate-guarding behavior (5). On no occasion, among all 41 successful trials, did we observe both males successfully copulate with the female within the 2-h trial duration.
Fig. 3.
Mate-choice assay. (A) Observation chamber and assay setup for female mate-choice assays. One mature virgin female and one each of dsYP and control virgin males were observed for 2 h (n = 45). (B) Female mate choice. Out of 41 trials that resulted in mating, control males mounted and copulated with the females on 21 occasions while dsYP males mounted and copulated with the females on 20 occasions. This difference was not significant (χ2 = 0.02439, d.f. = 1, P = 0.8759; n.s., not significant, P > 0.05). Boxplots show the latency to copulate with the female in minutes (median ± IQR and range), which did not significantly differ between the two groups (Mann–Whitney U test, n = 21 for controls, 20 for dsYP, U = 248, P = 0.3278; raw data are in SI Appendix, Table S5).
Release of the Antiaphrodisiac Pheromone PAN Covaries with Male–Male Harassment.
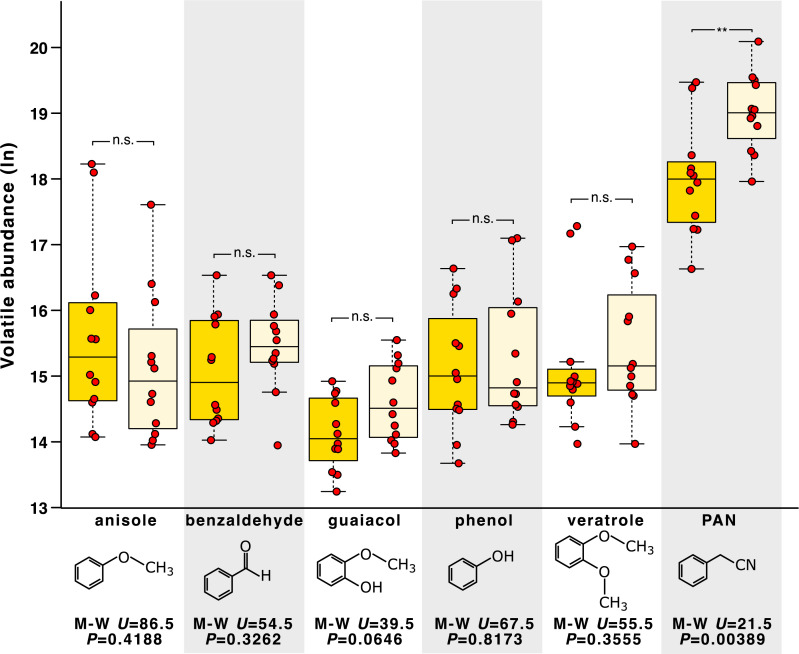
We performed gas chromatography–mass spectroscopy (GC-MS) to test the hypothesis that dsYP males would release an elevated titer of the antiaphrodisiac pheromone PAN in response to increased harassment by conspecifics. Twelve each of dsYP and control males were kept in separate cages prior to GC-MS analysis where—consistent with our behavioral assays—MMM occurred far more frequently among the dsYP males than it did among the yellow control animals in the other cage. dsYP males produced significantly more antiaphrodisiac PAN than did controls (median PAN abundance 2.7 times greater in the dsYP males; Fig. 4). We also tested for five other well-characterized components of the male volatile profile [anisole, benzaldehyde, guaiacol, phenol, and veratrole (39)], none of which showed a significant difference between groups (Fig. 4; raw abundances are given in SI Appendix, Table S6).
Fig. 4.
Effect of YP-RNAi (or its resulting color phenotype) on release of male volatiles, as measured by GC-MS. Boxplots (yellow [Left] for controls; beige [Right] for dsYP) show the absolute abundance (log scale) per treatment (median ± IQR and range, with values farther than 1.5× IQR from the median presented as outliers). Chemical structure, Mann–Whitney U, and P are given for each volatile (n.s., P > 0.05; **P < 0.01). Previous studies showed that these six volatiles form the bulk of the mature male-specific pheromone in S. gregaria, with PAN accounting for ∼80% of the total emission (39). This is supported by our analysis. YP-RNAi led to a significant increase in PAN emission (Mann–Whitney U test, n = 12 of each group, U = 21.5, P = 0.0039), and did not significantly affect any of the other volatiles measured (raw data are in SI Appendix, Table S6).
Discussion
The term “aposematism” typically refers to interspecific warning signals between unprofitable prey and their potential predators, but the word was originally intended as a far more general descriptor of unprofitability (40). This was noted by Sherratt and Forbes, whose modeling (41) indicated that bright male coloration in coenagrionid damselflies likely functions as an intraspecific form of male–male “antiharassment aposematism.” Our MMM assays demonstrate that vivid adult male yellowing in gregarious S. gregaria constitutes a density-dependent example of antiharassment aposematism, which reduces mistaken MMM during scramble mating at high population densities. This honest advertisement of sexual identity benefits all males in a large breeding scramble (35), by enabling individuals to target their reproductive efforts toward females while simultaneously avoiding harassment from same-sex conspecifics. The potential cost of this harassment in a wild population was demonstrated in our assays by the fact that dsYP males were significantly less able to perform copulatory mounts on perceived females due to being mounted themselves. The unambiguous nature of yellow pigmentation as a visual signal makes it far more reliable at extremely high densities than olfactory or auditory signals, which are harder to localize among many thousands of nearby individuals. Solitarious males do not compete for mates in high-density aggregations and therefore have no need for the signal. Further, the fact that yellowing is not a simple ontogenetic color change in all male locusts suggests that yellowing comes at some cost (42)—potentially an increased conspicuousness to predators—which is mitigated for gregarious animals by the usual benefits of group living which are not available to their lone-living counterparts (43). Our study manipulates locust color phenotypes toward a clear behavioral response and provides a parsimonious function for this sex- and density-dependent trait.
Sexual dimorphisms typically evolve via the two proximate mechanisms of sexual selection: male–male competition (intrasexual selection) and female mate choice (intersexual selection) (44–48). The evolution of bright male-specific colors is frequently driven by intersexual selection, but our female mate-choice assays clearly demonstrate that female S. gregaria do not prefer yellow males over nonyellow dsYP ones. It remains possible that gregarious female locusts exercise some form of cryptic mate choice, but this seems unlikely; it is inefficient to spend many hours in copula only to then reject the mate’s spermatozoa, especially since 1) females are fully capable of physically repelling males by jumping and kicking (32, 37), which we observed in two trials that did not result in mating, and 2) there is no shortage of alternative mates in a swarm. It therefore seems that intersexual selection plays little, if any, proximate or ultimate role in the expression of male yellowing.
By contrast, our MMM assays suggest that yellow coloration functions as an intrasexual signal. Intrasexual communication usually occurs when there is competition for mates, and involves both conflict and cooperation: conflict, because actors are competing for the same resource, and cooperation, because it is in each party’s interest to minimize the escalating costs of physical interactions (49). In this case, however, yellow coloration could easily evolve as a two-way byproduct benefit (50): Signalers and receivers both benefit by staying apart. This strategy of mutual tolerance might at first seem anathema to life in a highly competitive swarm (51, 52), but it fits with previously established relationships between male–male interactions and population density. At low population densities, aggression is usually unnecessary and investment in traits related to mate searching should be favored whereas, at high densities, males that fight for access to females would likely incur excessive costs because of the persistent presence of a large number of rivals (53). Swarm-specific sexual dichromatism is therefore a simple way of avoiding aggressive interactions at extremely high densities, by maximizing the likelihood of accurate mate recognition.
Male sexual dichromatism also serves as a male–male avoidance signal in damselflies, another competitive scramble breeder, for which the males of many species are more brightly colored and ornately patterned than females (54–56). Several workers have shown a similar function for carotenoid-mediated male coloration in scramble-breeding frogs (57–62). Despite these clear parallels, the breeding systems in these taxa sit across a spectrum of density-dependent plastic control. Sexual dichromatism in damselflies is a constitutive ontogenetic trait, and apparently not a plastic trait at all; similarly, their scramble-breeding aggregations appear to be the default (or at least most common) strategy. Dynamic dichromatism in frogs differs in that the color change in males is, in fact, density-dependent, though explosive breeding again appears to be the default/dominant strategy. In locusts, however, the entire breeding strategy is density-dependent, along with most other aspects of locust life history. Low-density populations can and do exist for many generations in the solitarious phase, in which large breeding scrambles never occur and males never turn yellow. Male yellowing only occurs in high-density gregarious populations, which is by no means the “default” state for S. gregaria or any other locust. As such, density-dependent antiharassment aposematism as a strategy mediated entirely by the environment appears to be unique to S. gregaria (and possibly other locust species).
Yellowing and the Pheromone PAN Are a Multimodal Signal.
YP-mediated yellowing in gregarious male S. gregaria is accompanied by the synthesis and release of a suite of volatile compounds, with the pheromone PAN accounting for around 80% of the total emission (39). PAN is a well-studied volatile in locusts (6, 17, 26, 63); in L. migratoria it is released by gregarious juveniles of both sexes and gregarious adult males, but in S. gregaria its release is limited to mature gregarious males and only then in the presence of other mature males, with mature females having no releaser effect (23). Seidelmann and colleagues previously demonstrated that PAN acts as a “courtship-inhibition pheromone” or “male-specific repellent” in S. gregaria, which prevents both mistaken MMM and harassment from competing males during copulatory mate guarding (23–27). Given the clear overlap of the male–male repellent function between PAN and yellow coloration, we hypothesized that the increased MMM resulting from YP-RNAi would lead to a compensatory increase in PAN emission in dsYP males. Furthermore, the tight coupling between yellowing and PAN emission led to a previous suggestion that the color might be an unimportant side effect of PAN production (22), and it was therefore important to check that YP-RNAi did not cause a confounding reduction of PAN in our behavioral assays. dsYP males released significantly more PAN than did the yellow control males. Given that PAN is only released in the presence of other males (23), the observed increase in PAN was most likely in response to elevated MMM behavior prior to GC-MS analysis.
It remains possible that MMM-based harassment was not the only proximate mechanism underlying the elevated PAN emission from dsYP males; for example, a compensatory feedback mechanism might exist between the YP and PAN synthesis pathways, leading to an overexpression of PAN in dsYP males. However, this seems unlikely given the weak tissue overlap: YP-mediated yellowing is strongest in the cuticle of the head, thorax, and abdomen, whereas PAN is predominantly emitted from the wings and legs (25). In any case, these results show that 1) PAN was not a confounding factor in our MMM assays, because dsYP males released more of it, and 2) this increased PAN release was still not sufficient to prevent intrasexual harassment. This second point builds on Seidelmann’s (27) previous finding that, upon encountering a mounting male–female pair, previously female-deprived males will often ignore PAN and attempt to aggressively usurp the other male. Males in our experiments were similarly female-deprived, though we did not measure or control their initial sexual response threshold beyond rearing them in single-sex colonies into maturity, so we cannot quantify the extent to which sexual responsiveness played a role in releasing MMM behavior. (Seasonal variation in sexual response threshold might also explain the observed differences between March and September MMM trials.) This should be addressed by future research, along with the question of whether PAN is specifically released in response to MMM, or if olfactory/visual male cues are sufficient. Nevertheless, female-deprived males in our MMM experiments not only ignored PAN but also preferentially mounted female-colored (or perhaps, less male-colored) dsYP conspecifics. This clearly indicates that yellow color is the stronger of the two male–male stimuli, which function together as a multimodal signal. We argue that yellow coloration has evolved to be the dominant male–male signal because it would be more reliable than an olfactory cue when males and females meet during dense daytime aggregations (35), while PAN likely serves as an additional close-range “backup signal” (64) for males that are mounting and mate guarding females, particularly under low-light conditions.
Yellow Protein Is a Multifunctional Signaling Gene.
Our study confirms an intraspecific aposematic role for yellow coloration in locusts, with clear adaptive benefits for breeding males. Notably, however, the bright yellow and black patterning in gregarious nymphs (of both sexes) has already been shown to be an interspecific (=antipredator) aposematic warning signal (14–16). In contrast to the present study in adults, Sword and Simpson (65) could not find an intraspecific role for this patterning in juveniles, either in terms of gregarizing solitarious conspecifics or aggregating gregarious ones. Intriguingly, it was recently shown that YP mediates the yellow component of this gregarious nymphal phenotype, as well as the yellow component of the green solitarious nymphal phenotype (18). It therefore follows that context-dependent expression of YP underpins at least three separate density-dependent signaling strategies in S. gregaria: antipredator aposematism in gregarious juveniles of both sexes; antipredator camouflage in solitarious juveniles of both sexes; and antiharassment aposematism in mature gregarious males.
This sex-specific adult repurposing of a juvenile signaling mechanism toward a different end raises a number of questions regarding the involvement of YP in the shared evolutionary history of the three strategies: Which came first, and what were the relative contributions of natural versus sexual selection? And are the signals mutually exclusive? For example, are yellow male adults also signaling unpalatability to predators, like their juvenile counterparts? Empirical data on toxicity in adult locusts are lacking, but the highly mobile nature of adult swarms makes their diet far less predictable than that of juveniles, which have been shown to feed on toxicity-conferring host plants in the sub-Saharan “recession areas” where outbreaks originate (15). However, adults are well-known to be palatable to humans to the point of being a kosher and halal delicacy (66, 67), and they are also predated by well over a hundred African bird species (68, 69). It also seems unlikely that male yellowing is a dishonest aposematism (akin to a Batesian mimicry of the toxic juvenile state) since 1) the contrasting black patterning is absent [though the yellow component might of course be sufficient, and thermoregulation in open-ground lekking sites (35) might present a selective pressure against retaining the black patterning]; 2) male yellow coloration is not constant but has a break during early adulthood, and 3) as has been previously noted (22), antipredator strategies should also be expected to benefit females, but they show a relative lack of yellowing.
This leads to an important avenue for future research: Given the effectiveness of yellow coloration at repelling both predators (as juveniles, at least) and harassing males, why is yellowing so uncommon in females? Female yellowing can be entirely absent in laboratory colonies (22), while fieldwork in Mauritania estimated that bright yellow individuals accounted for up to 36.7% of females at two sites, as opposed to 97.1% of conspecific males (70). The absence of yellow may reduce aggression (as distinct from sexual harassment) from males. Further, selection on females to minimize sexual harassment may be counterbalanced by selection to attract suitable mates. Support for this hypothesis comes from the observation that female yellowing only begins well after maturation, after females have already mated (22, 71). Maeno et al. (35) clearly showed that females avoid harassment from males by occupying separate roosting sites, and only enter male-dominated lekking sites when gravid and sexually receptive. It therefore seems that this “group separation” behavior, coupled with cryptic coloration to avoid predation during oviposition, may have alleviated selection pressure toward female mimicry of the yellow male phenotype.
Might there be instances in other locust species where YP-mediated yellowing serves as a signal of unprofitability? And what epigenetic mechanisms have combined to regulate YP expression in a density-, ontogenetic-, and sex-specific manner? Future comparative studies with other locust species (11, 12) will be greatly facilitated by the recent publication of a draft genome sequence for S. gregaria (10), and will give insights into the relative contributions of sexual and natural selection during the evolution of multifunctional signaling and phenotypic plasticity.
Methods
Locusts.
Desert locusts (Schistocerca gregaria gregaria, Forskål 1775) were taken from the long-term culture maintained in the Zoological Institute at KU Leuven. Locusts were reared in crowded conditions (>250 insects per cage, 40 × 43 × 85 cm), at a constant temperature of 32 ± 1 °C, ambient relative humidity between 40 and 60%, and a light:dark cycle of 14:10 h. Locusts were fed daily with fresh cabbage leaves and dry rolled oats. Mature adults were supplied with cylindrical oviposition pots (15 cm high × 10 cm diameter) filled with seven parts sand, three parts peat, and one part water that were removed once a week and placed into fresh cages to start the next generation. Experimental locusts were age-synchronized by removing freshly molted adults to separate cages, with males and females kept apart to ensure virginity for all experiments. Adults for all experiments were used ∼2 to 3 wk post imaginal molt, when control males were bright yellow and contemporaneous adults in mixed-sex groups were mating and ovipositing.
Molecular Cloning of YP.
A complementary DNA (cDNA) sequence coding for S. gregaria YP (SgYP), containing a complete open reading frame, was identified in our in-house S. gregaria transcriptome database using the known protein (19) and partial transcript (21) sequences (SI Appendix, Methods and Fig. S5). The identified transcript was used for in silico analysis (SI Appendix, Methods and Figs. S5–S7) and primer design (SI Appendix, Table S7) with Geneious 9 (Biomatters). The sequence was amplified from cDNA derived from yellow adult male cuticle with attached epidermis by PCR, cloned into a pCR4-TOPO vector (TOPO TA Cloning Kit, Invitrogen), and confirmed via Sanger sequencing (LGC Genomics).
RNAi.
Double-stranded RNA (dsRNA) for RNAi was produced using the MEGAscript RNAi Kit (Ambion), according to the manufacturer’s instructions. Briefly, pCR4-TOPO plasmid containing the SgYP fragment formed the template for a PCR with REDTaq PCR Mix (Sigma-Aldrich), using primers appended at each 5′ end with the T7 RNA polymerase recognition site (Sigma-Aldrich; see SI Appendix, Table S7 for primer sequences). This PCR produced a single 796-bp fragment, consisting of 750 bp of the SgYP transcript sequence flanked at either end by 23 bp of T7 sequence. This PCR product was further cloned into a pCR4-TOPO vector, plasmid-purified (QIAprep Spin Miniprep Kit, Qiagen), and Sanger-sequenced to confirm sequence specificity. Purified plasmid was used as the template in an overnight MEGAscript transcription reaction at 37 °C. The final dsRNA against SgYP (dsYP) was purified using the columns supplied with the MEGAscript Kit, quantified with a NanoDrop ND-1000 spectrophotometer, and diluted to 65 ng/μL in locust saline (1 L: 8.766 g NaCl, 0.188 g CaCl2, 0.746 g KCl, 0.407 g MgCl2, 0.336 g NaHCO3).
Virgin males were injected 7 d post imaginal molt, at which point their cuticle had fully sclerotized but they had not yet started to turn yellow. Each male was injected with 650 ng dsYP (in 10 μL locust saline) into the thoracic cavity using a glass microsyringe and needle (Hamilton), which was inserted laterally between the second and third abdominal tergites and directed along the anterior–posterior axis so as to avoid piercing the gut. Control males were instead injected with dsRNA against the nonendogenous dsGFP but otherwise treated in the same way. All males received an identical booster injection 5 d later (day 12 post imaginal molt), by which time the control group had started to turn yellow. Age-matched virgin adult females used in behavioral trials were not injected at any stage.
qRT-PCR.
qRT-PCR analysis of the YP transcript was performed 16 d post imaginal molt (and therefore 4 d since their second and last dsRNA injections), at which point control males were bright yellow. Abdominal cuticle with attached epidermis (∼2 × 1 cm) was dissected from six each of control and dsYP males, and immediately homogenized in 1 mL QIAzol Lysis Reagent (Qiagen) using a MagNA Lyser and Green Beads (both Roche) for 2 min at 6,500 rpm. Total RNA was purified from this homogenate using the RNeasy Lipid Tissue Mini Kit (Qiagen) according to the manufacturer’s instructions, including the on-column DNase step (RNase-Free DNase, Qiagen). Concentration and purity of the eluted RNA were determined using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific), before cDNA synthesis with the PrimeScript RT Kit (Takara). cDNA was subsequently diluted 15-fold with molecular-grade water; 4 μL of each dilution was used as template in a 10-μL PCR, which also contained 5 μL Fast SYBR Green Master Mix (Applied Biosystems) and 0.5 μL each of forward and reverse primers (10 μM) established in previous studies (18, 21) (SI Appendix, Table S7). The same template samples were also amplified using primers against transcripts of the housekeeping genes elongation factor 1α (Ef1α) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in parallel reference reactions (18). All reactions were performed in duplicate on a StepOne System (ABI Prism, Applied Biosystems), using a thermal cycling profile consisting of 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s. A final melt-curve analysis was performed to confirm the presence of a single amplicon species, with no primer dimers or off-target amplification products.
Colorimetric Analysis.
Reflectance spectra were collected for 10 each of control males, dsYP males, and mature females using an S2000 spectrometer, PX-2 xenon strobe lamp, and 2.5-mm-diameter coaxial reflectance probe (Ocean Optics). Measurements were performed 16 d post imaginal molt. The probe was held at a 45° angle to the surface for each measurement to avoid collecting cuticular “glare” from the strobe (72), which illuminated a patch of cuticle ∼2 mm in diameter. Reflectance was calculated relative to a 99% white Spectralon reference block and measured every 0.382 nm across the 300- to 700-nm range at four locations per animal: left side of the abdomen; right side of the abdomen; ventral surface of the abdomen; and the front of the head. The four reflectance measurements at each recorded wavelength were averaged per individual, and these average values were smoothed across the entire 400-nm range by taking a rolling average of every 5-nm block (i.e., every 13 frames), giving individual reflectance spectra for each animal.
Male–Male Mounting Assays.
MMM trials were performed in a custom-made observation chamber (Fig. 2A) constructed from transparent 4-mm Perspex, with a perforated floating floor (1-mm-thick stainless steel with 2-mm holes) to provide ventilation and allow feces to drop through. A dividing perch made from the same perforated stainless steel created two chambers. For each 3-h MMM trial (n = 8), 24 adult virgin females were placed into the smaller of the two chambers as an olfactory releaser of male sexual behavior; 24 adult virgin males were then placed into the larger chamber: 12 control males, which were each labeled with a letter A to M (omitting the 1-like “I”) on both the ventral surface of the thorax and the distal end of each forewing using permanent marker; and 12 dsYP males, marked 1 to 12 in the same manner. It was assumed that males could smell the females through the partition (and vice versa), but visual contact was highly obscured by the divider and tactile interaction was impossible. All trials commenced at 10:00 AM, under the same environmental conditions that the locusts were bred in. Four trials were performed in March 2017, and another four in September 2017; all eight trials were completely independent, with no animals (male or female) being used in more than one trial.
Throughout each 3-h trial, the identities of both males for each mounting event were noted by one of three observers (R.C., D.A.C., L.M.). We only counted mounting events that achieved the stereotypical copulatory position, in which the mounting male (signal receiver, referred to as “mounter” for clarity) jumped or climbed onto the back of the mounted male (signaler) before holding onto its pronotum and thorax with the front two pairs of legs, accompanied by copulatory searching behavior with the abdomen (figure 187A in ref. 5). Mounting was almost invariably preceded by a range of well-studied precopulatory behaviors (34, 36) including antennation and visual scanning.
Mate-Choice Assays.
Female copulatory mate-choice trials were performed in 3-L plastic terraria (Savic; 14 × 14 × 20 cm; Fig. 3A). All 2-h trials commenced at 10:00 AM, and took place in the same environmental conditions that the locusts were bred in. White paper dividers were used to maintain visual isolation between trial cages, allowing 15 trials to be run simultaneously on 3 different days (total n = 45). For each trial, one yellow control male and one nonyellow dsYP male were added to a container simultaneously, followed by a mature virgin female (t = 0 min). For each trial, we recorded the first male to mount and mate with the female, and the time taken for them to do so. We also noted any female rejections of mounting males, and any male–male interactions.
Gas Chromatography–Mass Spectroscopy.
GC-MS was performed to measure abundance of the male-specific pheromone PAN (23), as well as five minor volatiles that were previously shown to be coreleased with PAN: anisole, benzaldehyde, guaiacol, phenol, and veratrole (39). Volatile emission profiles were obtained for 12 each of control and dsYP males. Males were kept as separate crowded groups in two 3-L plastic terraria (Savic; 14 × 14 × 20 cm) with food at 30 °C until GC-MS analysis. Individuals were removed from the holding containers and incubated at 30 °C for 1 h in 60-mL screw-neck headspace vials sealed with polytetrafluoroethylene (PTFE) 3.2-mm screw caps (Macherey-Nagel). Volatiles were then extracted at 30 °C for 1 h with a divinylbenzene-carboxen-polydimethylsiloxane solid phase microextraction (SPME) fiber (Supelco), before identification with a 7890A gas chromatograph coupled to a 5975C VL MSD (mass selective detector) (both Agilent Technologies), which was also equipped with a Multipurpose Sampler 2 (Gerstel). After extraction from the locust-containing vials, compounds were thermally revolatilized by heating to 220 °C in a split/splitless injector (splitless mode) equipped with an SPME liner (0.75-mm internal diameter [i.d.]; Supelco). The fiber was thermally desorbed for 5 min. Separation was performed on an HP-5MS column (30 m × 0.25 mm i.d. × 0.25 μm film thickness; Agilent Technologies) using helium as the carrier gas at a constant flow rate of 1 mL⋅min−1. Oven temperature was held at 40 °C for 4 min and then ramped at 10 °C⋅min−1 up to 240 °C, where it was held for a further 10 min, for a total GC run time of 34 min. Mass spectra in the 35- to 350-m/z range were recorded at a scanning speed of 4.17 scan cycles per second. Chromatograms and mass spectra obtained from the GC-MS were deconvoluted and analyzed using MSD ChemStation (Agilent Technologies) and the Automated Mass Spectral Deconvolution and Identification System, software v.2.2 (National Institute of Standards and Technology [NIST], USA). Volatile compounds were identified by matching with the NIST 14 mass spectral library, and volatile composition was compared by using absolute peak areas.
Statistical Analyses.
Statistical analyses were performed in R v.4.0.3, Bunny-Wunnies Freak Out. Count data from MMM behavioral assays (N = 192 male locusts) were tested using GLMs, with Poisson correction for “mounts given” data (using the standard GLM in R) and negative binomial correction for “mounts received” data (using the MASS package). “Treatment” (control or dsYP) and “trial” (N = 8) were treated as fixed effects in the GLM; generalized linear mixed models (glmer and glmer.nb) were attempted using the lme4 package with trial as a random effect, but eight levels led to singularity within the model. Residual diagnostics for both GLMs were assessed using the DHARMa package. Trial-level comparisons of count data were also performed using a Kruskal–Wallis test (n = 8, each containing 12 dsYP males and 12 control males) with Dunn’s post hoc testing for multiple comparisons with Bonferroni correction. Female choice assays were first assessed using χ2 analysis of count data to test for a female bias toward control or dsYP males in assays that resulted in mating, followed by a Mann–Whitney U test to compare female mounting latencies between control (n = 21) and dsYP males (n = 20). Mann–Whitney U tests were used to test for the effects of RNAi on YP messenger RNA (mRNA) (measured by qRT-PCR; n = 6 for both control and dsYP groups) and pheromone emission (measured by GC-MS; n = 12 for both control and dsYP groups).
Supplementary Material
Acknowledgments
We thank Evelien Herinckx for rearing the experimental locusts, Andrea Manica and Carl Soulsbury for statistical advice, Steve Rogers for commenting on an earlier draft of the manuscript, and three anonymous reviewers for their constructive comments. D.A.C. was funded by a postdoctoral fellowship from the Research Foundation of Flanders (FWO); J.V.B. was funded by the Special Research Fund of KU Leuven and FWO; G.A.S. was supported in part by a grant from the US NSF Biology Integration Institute Program (DBI-2021795) Behavioral Plasticity Research Institute: Transforming the Study of Phenotypic Plasticity through Biological Integration.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2200759119/-/DCSupplemental.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Pigliucci M., Phenotypic Plasticity: Beyond Nature and Nurture (Johns Hopkins University Press, 2001). [Google Scholar]
- 2.Kokko H., Rankin D. J., Lonely hearts or sex in the city? Density-dependent effects in mating systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 319–334 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svensson E. I., et al. , Correlational selection in the age of genomics. Nat. Ecol. Evol. 5, 562–573 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Broder E. D., et al. , Evolutionary novelty in communication between the sexes. Biol. Lett. 17, 20200733 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uvarov B. P., Grasshoppers and Locusts (Cambridge University Press, Cambridge, UK, 1966), vol. 1. [Google Scholar]
- 6.Pener M. P., Simpson S. J., Locust phase polyphenism: An update. Adv. Insect Physiol. 36, 1–272 (2009). [Google Scholar]
- 7.Cullen D. A., et al. , From molecules to management: Mechanisms and consequences of locust phase polyphenism. Adv. Insect Physiol. 53, 167–285 (2017). [Google Scholar]
- 8.Food and Agriculture Organization of the United Nations, Locust watch: Desert locust. https://www.fao.org/ag/locusts/en/info/info/index.html. Accessed 14 December 2021.
- 9.Kimathi E., et al. , Prediction of breeding regions for the desert locust Schistocerca gregaria in East Africa. Sci. Rep. 10, 11937 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verlinden H., et al. , First draft genome assembly of the desert locust, Schistocerca gregaria. F1000 Res. 9, 775 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song H., Foquet B., Mariño-Pérez R., Woller D. A., Phylogeny of locusts and grasshoppers reveals complex evolution of density-dependent phenotypic plasticity. Sci. Rep. 7, 6606 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilpatrick S. K., et al. , Revealing hidden density-dependent phenotypic plasticity in sedentary grasshoppers in the genus Schistocerca Stål (Orthoptera: Acrididae: Cyrtacanthacridinae). J. Insect Physiol. 118, 103937 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Pener M. P., Locust phase polymorphism and its endocrine relations. Adv. Insect Physiol. 23, 1–79 (1991). [Google Scholar]
- 14.Sword G. A., Density-dependent warning coloration. Nature 397, 217 (1999). [Google Scholar]
- 15.Sword G. A., Simpson S. J., El Hadi O. T. M., Wilps H., Density-dependent aposematism in the desert locust. Proc. Biol. Sci. 267, 63–68 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Despland E., Simpson S. J., Surviving the change to warning colouration: Density-dependent polyphenism suggests a route for the evolution of aposematism. Chemoecology 15, 69–75 (2005). [Google Scholar]
- 17.Wei J., et al. , Phenylacetonitrile in locusts facilitates an antipredator defense by acting as an olfactory aposematic signal and cyanide precursor. Sci. Adv. 5, eaav5495 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugahara R., Tanaka S., Environmental and hormonal control of body color polyphenism in late-instar desert locust nymphs: Role of the yellow protein. Insect Biochem. Mol. Biol. 93, 27–36 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Wybrandt G. B., Andersen S. O., Purification and sequence determination of a yellow protein from sexually mature males of the desert locust, Schistocerca gregaria. Insect Biochem. Mol. Biol. 31, 1183–1189 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Mahamat H., Hassanali A., Munyinyi D., Haemolymph pigment composition as a chemometric indicator of phase in the desert locust, Schistocerca gregaria. Int. J. Trop. Insect Sci. 17, 199–204 (1997). [Google Scholar]
- 21.Sas F., et al. , Development of a real-time PCR assay for measurement of yellow protein mRNA transcription in the desert locust Schistocerca gregaria: A basis for isolation of a peptidergic regulatory factor. Peptides 28, 38–43 (2007). [DOI] [PubMed] [Google Scholar]
- 22.De Loof A., et al. , Sexual differentiation in adult insects: Male-specific cuticular yellowing in Schistocerca gregaria as a model for reevaluating some current (neuro)endocrine concepts. J. Insect Physiol. 56, 919–925 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Seidelmann K., Luber K., Ferenz H.-J., Analysis of release and role of benzyl cyanide in male desert locusts, Schistocerca gregaria. J. Chem. Ecol. 26, 14 (2000). [Google Scholar]
- 24.Seidelmann K., Ferenz H.-J., Courtship inhibition pheromone in desert locusts, Schistocerca gregaria. J. Insect Physiol. 48, 991–996 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Seidelmann K., Weinert H., Ferenz H.-J., Wings and legs are production sites for the desert locust courtship-inhibition pheromone, phenylacetonitrile. J. Insect Physiol. 49, 1125–1133 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Seidelmann K., Warnstorff K., Ferenz H.-J., Phenylacetonitrile is a male specific repellent in gregarious desert locusts, Schistocerca gregaria. Chemoecology 15, 37–43 (2005). [Google Scholar]
- 27.Seidelmann K., The courtship-inhibiting pheromone is ignored by female-deprived gregarious desert locust males. Biol. Lett. 2, 525–527 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faure J. C., The life-history of the brown locust. J. Dep. Agric. South Africa 7, 205–224 (1923). [Google Scholar]
- 29.Uvarov B. P., A revision of the genus Locusta L. (= Pachytylus, Fieb.), with a new theory as to the periodicity and migration of locusts. Bull. Entomol. Res. 12, 135–163 (1921). [Google Scholar]
- 30.Uvarov B. P., Notes on locusts of economic importances, with some new data on the periodicity of locust invasion. Bull. Entomol. Res. 14, 31–39 (1923). [Google Scholar]
- 31.Norris M. J., Group effects on the activity and behaviour of adult males of the desert locust (Schistocerca gregaria Forsk.) in relation to sexual maturation. Anim. Behav. 10, 275–291 (1962). [Google Scholar]
- 32.Loher W., Contributions to the study of the sexual behaviour of Schistocerca gregaria Forskål (Orthoptera: Acrididae). Proc. R. Entomol. Soc. Lond. Ser. Gen. Entomol. 34, 49–56 (1959). [Google Scholar]
- 33.Amerasinghe F. P., Pheromonal effects on sexual maturation, yellowing, and the vibration reaction in immature male desert locusts (Schistocerca gregaria). J. Insect Physiol. 24, 309–314 (1978). [Google Scholar]
- 34.Inayatullah C., El Bashir S., Hassanali A., Sexual behavior and communication in the desert locust, Schistocerca gregaria (Orthoptera: Acrididae): Sex pheromone in solitaria. Environ. Entomol. 23, 1544–1551 (1994). [Google Scholar]
- 35.Maeno K. O., et al. , Density-dependent mating behaviors reduce male mating harassment in locusts. Proc. Natl. Acad. Sci. U.S.A. 118, e2104673118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uvarov B. P., Grasshoppers and Locusts (Centre for Overseas Pest Research, London, 1977), vol. 2. [Google Scholar]
- 37.Golov Y., Rillich J., Harari A., Ayali A., Precopulatory behavior and sexual conflict in the desert locust. PeerJ 6, e4356 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norris M. J., Sexual maturation in the desert locust (Schistocerca gregaria Forskål) with special reference to the effects of grouping. Anti-Locust Bull. 18, 1–44 (1954). [Google Scholar]
- 39.Hassanali A., Njagi P. G. N., Bashir M. O., Chemical ecology of locusts and related acridids. Annu. Rev. Entomol. 50, 223–245 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Poulton S. E. B., The Colours of Animals: Their Meaning and Use, Especially Considered in the Case of Insects (D. Appleton, New York, 1890). [Google Scholar]
- 41.Sherratt T. N., Forbes M. R., Sexual differences in coloration of coenagrionid damselflies (Odonata): A case of intraspecific aposematism? Anim. Behav. 62, 653–660 (2001). [Google Scholar]
- 42.Olson V. A., Owens I. P. F., Costly sexual signals: Are carotenoids rare, risky or required? Trends Ecol. Evol. 13, 510–514 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Sword G. A., Lorch P. D., Gwynne D. T., Insect behaviour: Migratory bands give crickets protection. Nature 433, 703–703 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Moore A. J., The evolution of sexual dimorphism by sexual selection: The separate effects of intrasexual selection and intersexual selection. Evolution 44, 315–331 (1990). [DOI] [PubMed] [Google Scholar]
- 45.Kokko H., Jennions M. D., Brooks R., Unifying and testing models of sexual selection. Annu. Rev. Ecol. Evol. Syst. 37, 43–66 (2006). [Google Scholar]
- 46.Fitze P. S., Cote J., Martínez-Rica J. P., Clobert J., Determinants of male fitness: Disentangling intra- and inter-sexual selection. J. Evol. Biol. 21, 246–255 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Rosenthal G. G., Mate Choice: The Evolution of Sexual Decision Making from Microbes to Humans (Princeton University Press, Princeton, NJ, 2017). [Google Scholar]
- 48.Zuk M., Simmons L. W., Sexual Selection: A Very Short Introduction (Oxford University Press, Oxford, UK, 2018). [Google Scholar]
- 49.Guilford T., Dawkins M. S., What are conventional signals? Anim. Behav. 49, 1689–1695 (1995). [Google Scholar]
- 50.Sachs J. L., Mueller U. G., Wilcox T. P., Bull J. J., The evolution of cooperation. Q. Rev. Biol. 79, 135–160 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Bazazi S., et al. , Collective motion and cannibalism in locust migratory bands. Curr. Biol. 18, 735–739 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Simpson S. J., Sword G. A., Lorch P. D., Couzin I. D., Cannibal crickets on a forced march for protein and salt. Proc. Natl. Acad. Sci. U.S.A. 103, 4152–4156 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knell R. J., Population density and the evolution of male aggression. J. Zool. (Lond.) 278, 83–90 (2009). [Google Scholar]
- 54.Beatty C. D., Andrés J. A., Sherratt T. N., Conspicuous coloration in males of the damselfly Nehalennia irene (Zygoptera: Coenagrionidae): Do males signal their unprofitability to other males? PLoS One 10, e0142684 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grether G. F., Drury J. P., Berlin E., Anderson C. N., The role of wing coloration in sex recognition and competitor recognition in rubyspot damselflies (Hetaerina spp.). Ethology 121, 674–685 (2015). [Google Scholar]
- 56.Khan M. K., Herberstein M. E., Sexually dimorphic blue bands are intrasexual aposematic signals in nonterritorial damselflies. Anim. Behav. 156, 21–29 (2019). [Google Scholar]
- 57.Bell R. C., Zamudio K. R., Sexual dichromatism in frogs: Natural selection, sexual selection and unexpected diversity. Proc. Biol. Sci. 279, 4687–4693 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bell R. C., Webster G. N., Whiting M. J., Breeding biology and the evolution of dynamic sexual dichromatism in frogs. J. Evol. Biol. 30, 2104–2115 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Kindermann C., Hero J.-M., Rapid dynamic colour change is an intrasexual signal in a lek breeding frog (Litoria wilcoxii). Behav. Ecol. Sociobiol. 70, 1995–2003 (2016). [Google Scholar]
- 60.Sztatecsny M., et al. , Don’t get the blues: Conspicuous nuptial colouration of male moor frogs (Rana arvalis) supports visual mate recognition during scramble competition in large breeding aggregations. Behav. Ecol. Sociobiol. 66, 1587–1593 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doucet S. M., Mennill D. J., Dynamic sexual dichromatism in an explosively breeding Neotropical toad. Biol. Lett. 6, 63–66 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rehberg-Besler N., Mennill D. J., Doucet S. M., Dynamic sexual dichromatism produces a sex signal in an explosively breeding Neotropical toad: A model presentation experiment. Behav. Processes 121, 74–79 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Guo X., et al. , 4-Vinylanisole is an aggregation pheromone in locusts. Nature 584, 584–588 (2020). [DOI] [PubMed] [Google Scholar]
- 64.Johnstone R., Multiple displays in animal communication: ‘Backup signals’ and ‘multiple messages.’ Philos. Trans. R. Soc. Lond. B Biol. Sci. 351, 329–338 (1996). [Google Scholar]
- 65.Sword G. A., Simpson S. J., Is there an intraspecific role for density-dependent colour change in the desert locust? Anim. Behav. 59, 861–870 (2000). [DOI] [PubMed] [Google Scholar]
- 66.Samejo A. A., Sultana R., Kumar S., Soomro S., Could entomophagy be an effective mitigation measure in desert locust management? Agronomy (Basel) 11, 455 (2021). [Google Scholar]
- 67.Isman M. B., Cohen M. S., Kosher insects. Am. Entomol. (Lanham Md.) 41, 100–103 (1995). [Google Scholar]
- 68.Mullié W. C., “Birds, locusts and grasshoppers” in Living on the Edge. Wetlands and Birds in a Changing Sahel, Zwarts L., Bijlsma R., van der Kamp J., Wymenga E., Eds. (KNNV Publishing, 2009), pp. 202–223. [Google Scholar]
- 69.Mullié W. C., Cheke R. A., Young S., Ibrahim A. B., Murk A. J., Increased and sex-selective avian predation of desert locusts Schistocerca gregaria treated with Metarhizium acridum. PLoS One 16, e0244733 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanaka S., Maeno K., Ould Mohamed S., Ould Ely S., Babah Ebbe M. A., Upsurges of desert locust populations in Mauritania: Body coloration, behavior and morphological characteristics. Appl. Entomol. Zool. 45, 641–652 (2010). [Google Scholar]
- 71.Nishide Y., Tanaka S., Yellowing, morphology and behaviour in sexually mature gynandromorphs of the desert locust Schistocerca gregaria. Physiol. Entomol. 37, 379–383 (2012). [Google Scholar]
- 72.Finkbeiner S. D., Fishman D. A., Osorio D., Briscoe A. D., Ultraviolet and yellow reflectance but not fluorescence is important for visual discrimination of conspecifics by Heliconius erato. J. Exp. Biol. 220, 1267–1276 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Schmeling F., et al. , Opsin expression, physiological characterization and identification of photoreceptor cells in the dorsal rim area and main retina of the desert locust, Schistocerca gregaria. J. Exp. Biol. 217, 3557–3568 (2014). [DOI] [PubMed] [Google Scholar]
- 74.Schmeling F., Tegtmeier J., Kinoshita M., Homberg U., Photoreceptor projections and receptive fields in the dorsal rim area and main retina of the locust eye. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 201, 427–440 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.