Significance
Apolipoprotein E4 (APOE4) is the most influential genetic risk factor for late-onset Alzheimer’s disease (AD). Carrying APOE4 increases the risk of developing AD by 4-fold (one allele) to 14-fold (two alleles). Cognitive impairment in AD patients strongly correlates with the severity of tau pathology. APOE4-carrying AD patients suffer from significant cerebral atrophy, and animal studies have demonstrated that pathological tau drives the cerebral atrophy. However, the mechanisms that exacerbate tau pathology/hyperphosphorylation in APOE4-carriers are not well understood. Our data show that the astrocyte-secreted protein glypican-4 is a key driver of APOE4-mediated tau abnormal hyperphosphorylation.
Keywords: Alzheimer’s disease, APOE4, glypican-4, tau pathology, astrocytes
Abstract
Tau protein aggregates are a major driver of neurodegeneration and behavioral impairments in tauopathies, including in Alzheimer’s disease (AD). Apolipoprotein E4 (APOE4), the highest genetic risk factor for late-onset AD, has been shown to exacerbate tau hyperphosphorylation in mouse models. However, the exact mechanisms through which APOE4 induces tau hyperphosphorylation remains unknown. Here, we report that the astrocyte-secreted protein glypican-4 (GPC-4), which we identify as a binding partner of APOE4, drives tau hyperphosphorylation. We discovered that first, GPC-4 preferentially interacts with APOE4 in comparison to APOE2, considered to be a protective allele to AD, and second, that postmortem APOE4-carrying AD brains highly express GPC-4 in neurotoxic astrocytes. Furthermore, the astrocyte-secreted GPC-4 induced both tau accumulation and propagation in vitro. CRISPR/dCas9-mediated activation of GPC-4 in a tauopathy mouse model robustly induced tau hyperphosphorylation. In the absence of GPC4, APOE4-induced tau hyperphosphorylation was largely diminished using in vitro tau fluorescence resonance energy transfer-biosensor cells, in human-induced pluripotent stem cell-derived astrocytes and in an in vivo mouse model. We further show that APOE4-mediated surface trafficking of APOE receptor low-density lipoprotein receptor-related protein 1 through GPC-4 can be a gateway to tau spreading. Collectively, these data support that APOE4-induced tau hyperphosphorylation is directly mediated by GPC-4.
Alzheimer’s disease (AD) is the most common neurodegenerative disorder (1). The total cost of medical care for the treatment of AD in 2020 was estimated at $305 billion in the United States, and this cost is expected to increase to more than $1 trillion as the population ages (2). There is a critical need for therapeutic agents that may prevent the disease or slow down the rate of disease progression. Inherited autosomal-dominant forms of AD (familial AD) are caused by the presence of mutations in amyloid precursor protein gene (APP) or presenilin genes (PSEN1 and PSEN2) (3). Familial AD represents less than 1% of all AD cases, while the remaining AD cases are late-onset or sporadic AD (4). Although the exact causes of sporadic AD remain unclear, the apolipoprotein variant E4 (APOE4) is known as the highest genetic risk factor for sporadic AD (5).
APOE plays a major role in the circulation of high-density and very low-density lipoproteins and mediates the transport of lipids between the cells (6). Human APOE is expressed in three genetic variants: APOE2, APOE3, and APOE4. These variants differ in the position of two amino acid residues: APOE3 has a cysteine at position 112 and arginine at position 158, APOE2 has a cysteine at both positions, and APOE4 has arginine at both positions (7). Among these three APOE isoforms, APOE4 is the most influential genetic risk factor for late-onset AD. The increase in AD risk varies depending on ancestral background, sex, and multiple genetic or environmental factors (5). However, as a rough estimate, having a single APOE4 allele increases AD risk 2- to 4-fold, and having two APOE4 alleles ∼8- to 16-fold (5). APOE4 carriers also develop AD pathologies earlier compared to noncarriers (5, 8). In contrast, APOE2 carriers have a lower likelihood of developing AD (8–13). Amyloid-β (Aβ) plaques and hyperphosphorylated neurofibrillary tau tangles are characteristic features of AD pathology (14, 15). Hyperphosphorylated tau proteins are dissociated from microtubules and migrate from axons to somatodendritic compartments, where they are assembled into protofibrils, also called paired helical filaments (PHFs), and subsequently, into neurofibrillary tangles (16). Cognitive impairment in AD patients shows a strong correlation with tau pathology (17–23). AD patients homozygous for APOE4 suffer from significant cerebral atrophy (24–26), and animal studies have demonstrated that pathological tau drives cerebral atrophy (27, 28). However, the mechanisms that drive abnormal tau hyperphosphorylation in APOE4-carrying individuals are not well understood.
In the brain, APOE is secreted by glial cells, primarily astrocytes (5). Cholesterol and phospholipids produced by astrocytes in the form of APOE-containing high-density lipoprotein-like particles are vital for neuronal survival (29). Astrocytes play a critical functional role in the central nervous system, including maintaining synaptic connections and extracellular homeostasis, and the loss of physiological astrocytic functions can be a main contributor to neurodegeneration (30). Recent studies suggest that a gain of toxic functions leads to astrocyte-mediated neurodegeneration in AD (31–34). Although emerging studies suggest that neurotoxic astrocytes may be major drivers of neurodegeneration in AD, the molecular interconnection between astrocytes and APOE4-mediated tau pathology/hyperphosphorylation in neurons has yet to be resolved. Here, we report that astrocyte-secreted glypican 4 (GPC-4) strongly interacts with APOE4 and exacerbates APOE4-induced tau hyperphosphorylation.
Results
APOE Variants and Tau Pathology.
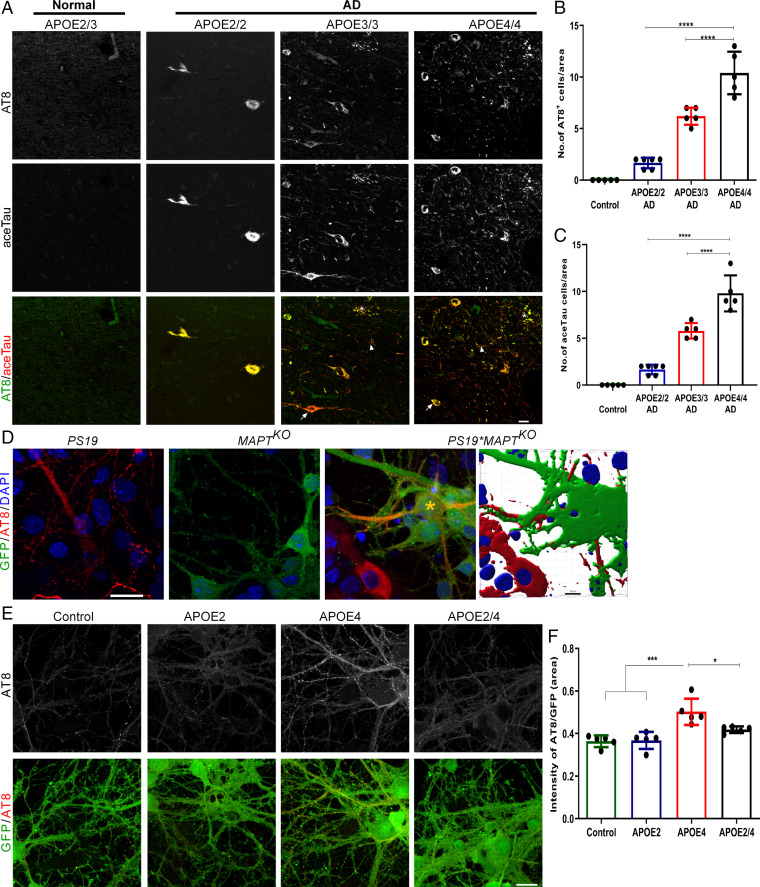
Tau hyperphosphorylation in the prefrontal cortical (PFC) regions correlates with clinical dementia rate in AD patients (23, 35). AT8 antibody, which recognizes phosphorylated Ser202 and Thr205 residues of tau proteins and correlates with disease progression, is one of the widely used antibodies to detect pathological tau proteins (27, 36). Therefore, we first examined tau pathology in the postmortem PFC regions (Broadmann 10) of human APOE variants using AT8 antibody. The sample details are given in SI Appendix, Table S1. We observed the presence of neurofibrillary tangles, neuropil threads, and neuritic plaques (Fig. 1A) that are different forms of neurofibrillary changes observed in the AD postmortem brain (37). The number of AT8 and aceTau+ (Lys174) neurons were significantly higher in APOE4/4 compared to APOE3/3 and APOE2/2 AD patients (Fig. 1B and C). All subjects had Aβ depositions (SI Appendix, Fig. S1A). We next investigated whether APOE2 and APOE4 differentially influenced tau spreading/uptake. To address this question, we cocultured neurons from MAPT knockout (KO) mice that expressed GFP and from PS19 mice (carrying the human P301S mutation in the MAPT gene) (SI Appendix, Fig. S1B and C). The tau+ signal in the GFP+-cocultured neurons from these two groups (PS19+MAPTKO) of mice is considered to be the tau protein released from PS19 neurons. Fig. 1D shows the primary neuronal cultures from PS19 (Fig. 1D, Left), MAPTKO (Fig. 1D, Center) and the coculture (Fig. 1D, Right). The presence of the tau+ signal in the GFP+ neurons (Fig. 1D, star) suggests that tau proteins that originated in PS19 neurons were taken up by the GFP+ neurons (Fig. 1D). To avoid any cross-reactivity during imaging, monocultures of PS19 and MAPTKO were used as baseline signals of GFP and AT8, respectively. We isolated APOE2 and APOE4 particles from frozen PFC regions of normal human APOE2/2 and APOE4/4 carrier brains (SI Appendix, Fig. S1D). When the neuronal cultures were treated with APOE isoforms, we observed that APOE4 particles robustly enhanced tau spreading/uptake (Fig. 1E and F). Upon adding APOE2 particles with APOE4 particles to the neuronal cultures, we observed no APOE4-mediated increase in tau spreading/uptake (Fig. 1E and F). Furthermore, we found that APOE4 particles induced significantly more AT8 levels in PS19 neuronal culture, but APOE4-induced AT8 levels were reduced in the presence of APOE2 particles (SI Appendix, Fig. S1E–G).
Fig. 1.
The brains of postmortem APOE4 AD patients accumulate more tau proteins and APOE4 enhances tau propagation/uptake. (A) Representative IHC images of postmortem PFC tissues from APOE2/3(control), APOE2/2 (AD), APOE3/3 (AD), and APOE4/4 (AD) individuals stained with AT8 and aceTau (Lys174) antibodies (n = 5 to 6) show the presence of Neurofibrillary tangles (arrow), neuropil threads (arrowhead), and neuritic plaque (asterisk). (Scale bar, 20 µm.) (B and C) Compared to APOE3/3 and APOE2/2, APOE4/4 carriers show the presence of more neurofibrillary tangle-containing neurons. (D) Neuronal monocultures from PS19 (Left), MAPTKO (Center), and mixed neuronal culture of PS19*MAPTKO (Right) showing the expression of tau (AT8), GFP, and uptake of tau proteins, respectively. PS19*MAPTKO neurons were 3D-reconstructed using IMARIS software. (Scale bar, 20 µm.) (E) We treated the mixed neurons (PS19*MAPTKO) with APOE2 or APOE4 particles isolated from human brains, and accessed tau spreading after 4 d. (Scale bar, 20 µm.) (F) Representative IHC staining of APOE treated neuronal culture shows that APOE4 treatments enhanced tau spreading whereas APOE2 significantly reduced APOE4-mediated tau spreading. (Scale bar, 20 µm.) n = 5, one-way ANOVA, *P < 0.05, ***P < 0.001 and ****P < 0.0001.
Link between GPC-4 and APOE4.
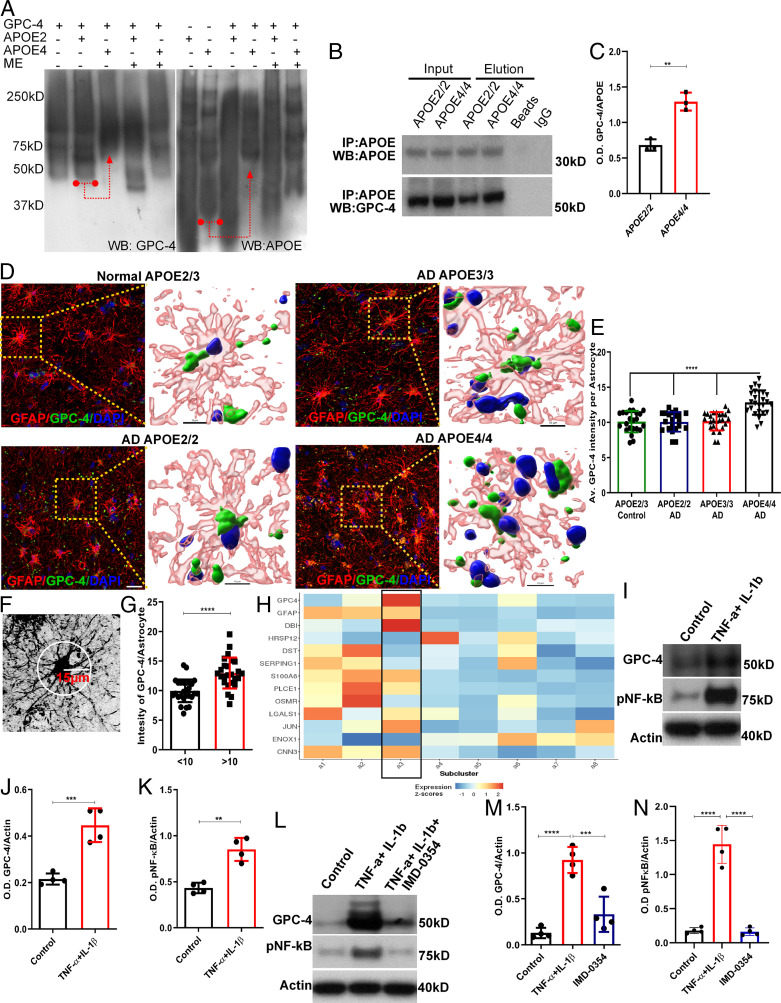
Arboleda-Velasquez et al. (18) recently reported that an AD autosomal dominant mutation carrier did not develop significant cognitive impairments until her 70s. She also had an unusual mutation in the APOE3 gene (Christchurch R136S mutation). Compared to purified human APOE3 proteins, APOE3 protein with R136S mutation had a weaker interaction with heparin. It is proposed that these distinct interactions of APOE R136S with heparin may be a potential reason that the aforementioned presenilin1 mutation carrier did not develop dementia and displayed less tau spreading compared to her counterparts (18). We therefore reasoned that heparan sulfate proteoglycans (HSPGs) may be involved in APOE4-mediated tau pathology/phosphorylation. We first screened a list of HSPGs and their interactions with APOE2 or APOE4, and found that GPC-4 strongly binds with APOE4 compared to APOE2 (Fig. 2A–C and SI Appendix, Fig. S2A). We incubated purified human GPC-4 protein either with purified human APOE2 or APOE4 protein at room temperature for 1 h, as outlined in Fig. 2A, and analyzed the samples with a nondenaturing gel. Compared to APOE2+GPC-4 combinations, the APOE4+GPC-4 combination showed a significant shift when probed with GPC-4 and APOE antibodies. Furthermore, treatment with 2-mercaptoethanol (ME) disturbed the shift of GPC-4+APOE4 (Fig. 2A). Nonetheless, APOE2+GPC-4 combinations showed the appearance of new bands compared to control, suggesting that APOE-2 somehow interacts with GPC-4 and future studies should explore further. We next validated APOE and GPC-4 interactions in postmortem human brain tissues. We coimmunoprecipitated GPC-4 proteins from APOE2/2 and APOE4/4 human brains with APOE antibodies (Fig. 2B) using Pierce Direct IP kit, which enables efficient antigen immunoprecipitations by covalently immobilizing purified antibodies onto agarose beads (see Materials and Methods for details). The levels of eluted GPC-4 proteins were normalized by corresponding eluted APOE proteins. Fig. 2C shows that the GPC-4/APOE complex is higher in APOE4/4 carriers compared to APOE2/2 carriers.
Fig. 2.
APOE4 interacts with GPC-4 and APOE4 AD postmortem brains express more GPC-4 in neurotoxic astrocytes. (A) GPC-4 proteins were incubated with either APOE2 or APOE4 proteins at room temperature for 1 h, and then separated by a nondenaturing gel (without SDS). Western blot analysis with GPC-4 (Left) and APOE (Right) antibodies revealed that combinations of APOE4+GPC-4 were robustly shifted while some noticeable shifts were observed with APOE2+GPC-4. A red arrow (Left) indicates GPC-4 proteins were shifted when combined with APOE4, but not with APOE2. A red arrow (Right) indicates APOE4 proteins were shifted when combined with GPC-4, whereas APOE2 proteins were not shifted when combined with GPC-4. The samples treated with ME disturbed the GPC-4/APOE4 interactions. (B and C) Proteins isolated from APOE2/2 and APOE4/4 human brains were coimmunoprecipitated with APOE antibodies (n = 3) (B). The levels of immunoprecipitated GPC-4 proteins were normalized with corresponding APOE immunoreactive bands (C). It shows that the APOE/GPC-4 complex is significantly higher in APOE4/4 compared to APOE2/2. (D and E) Representative IHC staining of APOE2/3(control), APOE2/2(AD), APOE3/3 (AD) and APOE4/4 (AD) postmortem tissues (PFC) with GFAP and GPC-4 antibodies show that APOE4 carrying AD patients expressed significantly higher levels of GPC-4 in astrocytes compared to control and other APOE genotypes (E). n = 5 to 6. (Scale bars, 20 µm.) (F and G) Astrocytes from APOE4/4 AD brains were grouped into two categories based on the number of the branches at 15-μm radius (F). Group 1: fewer than 10 branches. Group 2: more than 10 branches. Astrocytes with more branches expressed significantly elevated levels of GPC-4 (G). (H) AD-associated astrocytic genes were selected from Habib et al. (43) and generated a heat map with subtypes of astrocytes from Grubman et al. (42). Disease-associated genes were enriched in subtypes 2 and 3, and GPC-4 was enriched in a subtype 3. (I–K) Western blot analysis from astrocyte culture shows that treatment with TNF-α and IL-1β significantly increased expression of GPC-4, and it also activated the NF-κB pathway. (L–N) Western blot analysis from astrocyte culture treated with NF-κB pathway blocker, IMD-0354, reversed TNF-α and IL-1β-induced expression of GPC-4. n = 4; one-way ANOVA or unpaired t test, **P < 0.01, ***P < 0.001, and ****P < 0.0001. O.D., optical density.
We next examined whether GPC-4 was differentially expressed in human APOE variants. Immunohistochemistry (IHC) staining revealed that APOE4-carrying AD postmortem brain tissue (Broadmann area 10) expressed more GPC-4 protein in astrocytes compared to APOE4 noncarriers (Fig. 2D and E). A three-dimensional (3D) structure of a highlighted astrocyte shows the expression of GPC-4 within the cells (Fig. 2D) and a traditional orthogonal view of the same cells are shown in SI Appendix, Fig. S2B. Astrocytes represent a diverse population of cells with varying complex morphologies and functions (38, 39). Astrocytes with fewer branches are classified as resting astrocytes, while astrocytes with more branches are classified as disease-associated astrocytes (DAAs) (31, 34, 40). We measured the level of astrocytic GPC-4 from APOE4/4 AD brains. Fig. 2F and G show that astrocytes with more branches express significantly higher levels of GPC-4 protein, suggesting that activated astrocytes express GPC-4. We analyzed previously published single-cell RNA-sequencing (scRNA-seq) studies from human and mouse AD brains to independently validate whether GPC-4 is expressed by DAAs. These scRNA-seq studies revealed the presence of DAAs in mice and humans (41–43). The genes related DAAs from a mouse scRNA-seq study was retrieved (43). These disease astrocytic genes were plotted along with the GPC-4 gene in human astrocytic subclusters (42) to examine whether human DAAs express GPC-4. The analysis was performed using a link provided by the authors (42). We found that DAA genes are mainly expressed in astrocyte subtypes 2 and 3. Interestingly, GPC-4 was expressed within astrocyte subcluster 3 (Fig. 2H). This finding is suggestive that GPC-4 is expressed by DAAs.
We next aimed to test whether DAAs produce more GPC-4, in vitro. Astrocytes, which are stimulated by proinflammatory factors, such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β, are considered as DAAs or neurotoxic astrocytes (44). We performed astrocyte culture from WT mice, and treated them with TNF-α and IL-1β to test whether DAAs secrete more GPC-4 proteins. As expected, TNF-α and IL-1β treatment induced significantly higher levels of GPC-4 protein in astrocytic culture (Fig. 2I–K). We next tested whether inflammatory pathway NF-κB plays a role in up-regulation of GPC-4. IMD-0354 is widely used to block the NF-κB pathway (44). We observed that TNF-α and IL-1β–induced GPC-4 expression was blocked in the presence of IMD-0354 (Fig. 2L–N), suggesting that the NF-κB–dependent pathway regulates expression of GPC-4 in DAAs or neurotoxic astrocytes. Findings by us and other groups show that GPC-4 is expressed by astrocytes, but not by neurons (42, 45, 46) (SI Appendix, Fig. S3A).
GPC-4 Induces Tau Hyperphosphorylation and Uptake In Vitro and In Vivo.
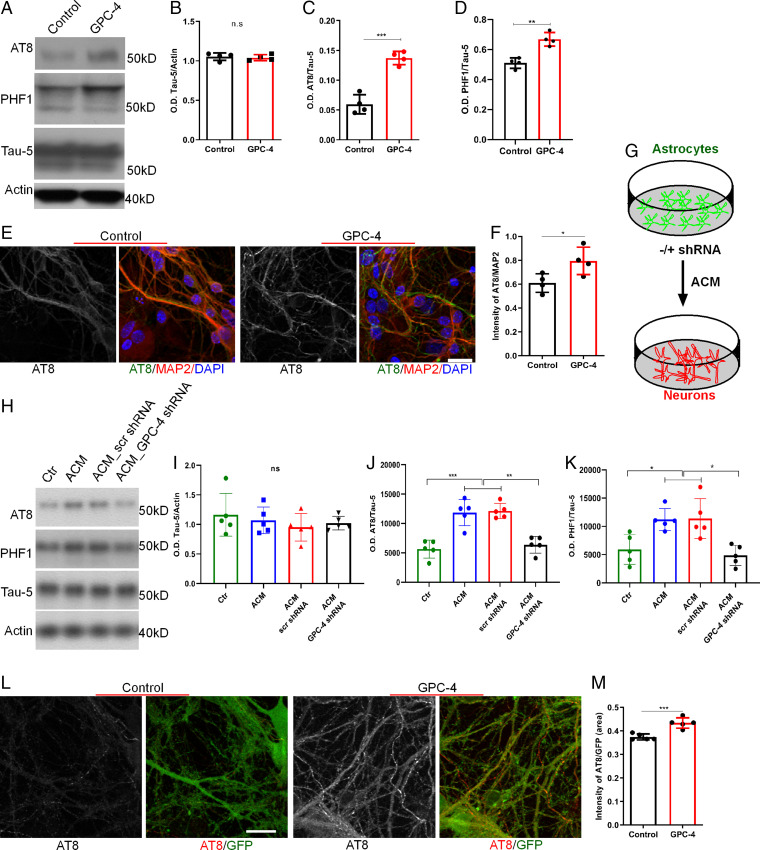
We next asked whether GPC-4 plays a role in tau hyperphosphorylation. We treated the PS19-derived neurons with purified human GPC-4 and found that it robustly induced AT8 and PHF1 (pSer396/Ser404) levels (Fig. 3A–D). The IHC experiments confirmed these findings (Fig. 3E and F). To test whether astrocyte-secreted GPC-4 is sufficient to induce tau hyperphosphorylation, we next treated the WT mouse astrocyte culture with control or GPC-4 shRNA, and collected astrocytes-conditioned medium (ACM) and added to PS19 mouse primary neuronal culture (Fig. 3G and SI Appendix, Fig. S3B–D). Like purified human GPC-4 protein, ACM-treated neurons showed significantly higher levels of AT8 and PHF1 (Fig. 3H–K). Interestingly, conditioned medium from GPC-4–deprived astrocytes failed to induce tau phosphorylation in neurons (Fig. 3H–K). We cocultured neurons of PS19 and MAPTKO mice to test the effect of GPC-4 on tau spreading/uptake, and found that the addition of GPC-4 increased tau spreading from PS19 neurons to MAPTKO neurons (Fig. 3L and M).
Fig. 3.
GPC-4 induces tau hyperphosphorylation in vitro. (A–D) Western blot analysis of proteins isolated from PS19 primary neuronal culture, which were treated with GPC-4 protein, shows that GPC-4 significantly enhanced pTau levels (C and D), whereas no changes were observed in total tau protein (B). (E and F) Representative IHC staining of GPC-4–treated neuronal culture with AT8 and MAP2 antibodies demonstrates that GPC-4 treatment enhanced pTau levels (E). (Scale bar, 20 µm.) (G) Schematic diagram shows that astrocytes (WT mice) were treated with control or GPC-4 shRNA and the resulting ACM was added to PS19 neuronal culture. (H–K) Addition of ACM to neuronal culture increased pTau (AT8 and PHF1) levels whereas total tau proteins were unaltered. On the other hand, addition of GPC-4–deprived ACM failed to induce tau phosphorylation in PS19 neurons. (L and M) Representative IHC staining of GPC-4 treated neuronal coculture (PS19*MAPTKO mice) shows that GPC-4 treatment enhanced tau spreading/uptake. (Scale bar, 20 µm.) n = 4 to 5, two-way ANOVA or unpaired t test. *P < 0.05, **P < 0.01, and ***P < 0.001.
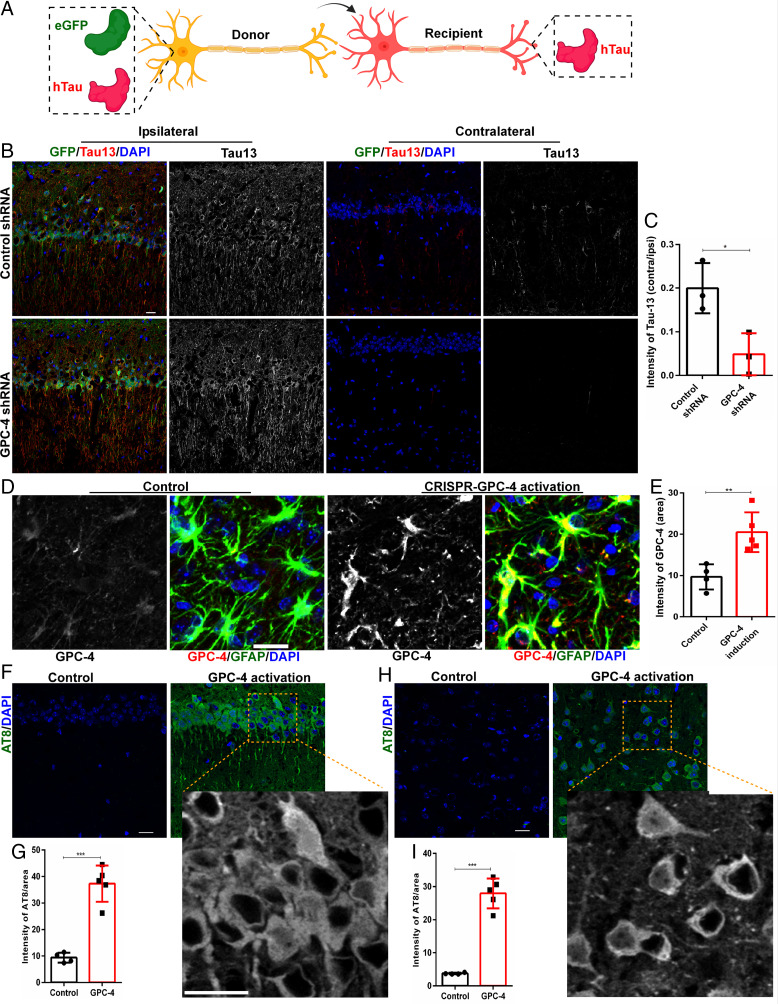
We next sought to investigate a possible link between GPC-4 and tau phosphorylation using 8-mo-old WT mice. An adeno-associated virus (AAV) that encodes eGFP-2PA-hTauP301L mRNA was recently used to study tau propagation from one neuron to another (47, 48). The 2PA sites will cause the translation of independent eGFP and hTauP301L proteins. While the donor (initially infected) neurons will express both eGFP and hTauP301L proteins, the released hTauP301L proteins will be taken up by synaptically connected neurons (the recipients) (Fig. 4A). We injected eGFP-2PA-hTauP301L AAV virus in the ipsilateral CA1 regions and control/GPC-4 short-hairpin RNA (shRNA) in the contralateral CA1 regions (SI Appendix, Fig. S4A), and examined them after 4 wk to investigate the effects of GPC-4 in tau spreading. As expected, we observed a robust expression of eGFP and hTauP301L in the ipsilateral sites (Fig. 4B). We also observed the propagation of hTauP301L proteins to the contralateral CA1 regions (Fig. 4B). Interestingly, GPC-4 shRNA treatment reduced the levels of hTauP301L in contralateral CA1 regions (Fig. 4B and C). These in vivo findings provide further support to our in vitro data on the role of GPC4 on tau propagation (Fig. 3L and M). We next induced expression of GPC-4 in 4-mo-old PS19 mice using a CRISPR/dCas9 system to investigate the role of GPC-4 in tau accumulation in vivo. After 1 wk of injection, we observed a tremendous expression of GPC-4 proteins in astrocytes (Fig. 4D and E), but not in neurons (SI Appendix, Fig. S4B). Following 3 wk of induction, we observed the presence of hyperphosphorylated tau in the hippocampal region (Fig. 4F and G and SI Appendix, Fig. S4C). Additionally, induction of GPC-4 in cortical regions also induced tau accumulation (Fig. 4H and I).
Fig. 4.
GPC-4 induces tau hyperphosphorylation in vivo. (A) Schematic diagram shows that donor cells express both GFP and hTau while synaptically connected neurons receive hTa. (B and C) We injected eGFP-2PA-hTauP301L AAV virus in the ipsilateral CA1 region and control/GPC-4 shRNA in the contralateral CA1 region (8-mo-old WT mice), and examined them after 4 wk. Representative IHC images human tau specific antibody Tau13 show that GPC-4 shRNA treatment significantly reduced tau spreading in the contralateral CA1 region. The levels of contralateral Tau13 were normalized by the levels of ipsilateral Tau13. (Scale bar, 20 µm.) (D and E) In order to induce the expression of GPC-4 proteins, we activated the GPC-4 gene by injecting GPC-4 CRISPR/dCas9 lentivirus activation systems in the cortex or hippocampus (4-mo-old WT mice). Representative IHC with GPC-4 and GFAP antibodies show that, following 1 wk of injection, GPC-4 CRISPR/dCas9 robustly induced GPC-4 expression compared to control lentiviral activation particles. (Scale bar, 20 µm.) (F and G). IHC images with the AT8 antibody show that GPC-4 induced significantly higher levels of pTau in the CA1 region of the hippocampus. (Scale bars, 20 µm.) (H and I). IHC images with AT8 antibody shows that GPC-4 induced significantly higher levels of pTau in the cortex. (Scale bar, 20 µm.) n = 4 to 5, unpaired t test, *P < 0.05, **P < 0.01, and ***P < 0.001.
GPC-4 Is Necessary for APOE4-Mediated Tau Uptake and Hyperphosphorylation.
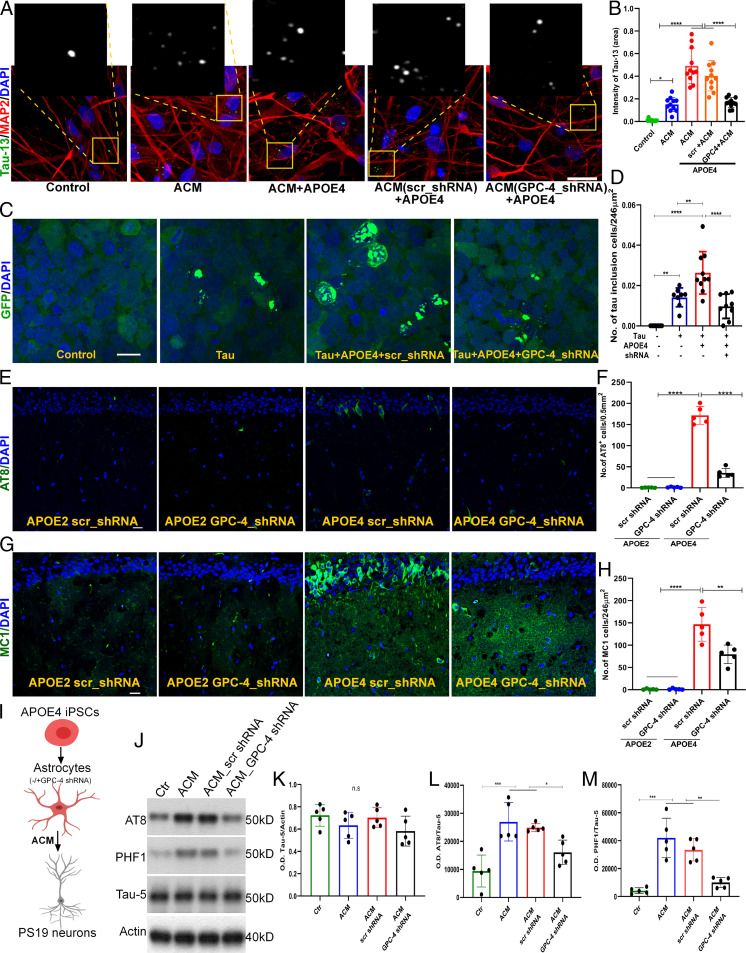
Given that GPC-4 induced tau phosphorylation and interacted with APOE4, we reasoned that GPC-4 would play an important role in APOE4-mediated tau phosphorylation. We treated primary neuronal culture (WT mice) with ACM (derived from WT astrocyte culture) alone, APOE4+ACM, and APOE4 with GPC-4–deprived ACM for 24 h and then incubated with 1 µg/mL of recombinant human tau protein for 1 h. IHC with human tau protein specific antibody (Tau13) revealed that APOE4 increased tau uptake and this increase was reversed in the absence of GPC-4 (Fig. 5A and B). This result indicates that GPC-4 regulates APOE4-induced tau uptake. We next examined a possible interaction between GPC-4 and APOE4 in tau aggregation in vitro. We used tau fluorescence resonance energy transfer (FRET)-biosensor cells to monitor tau protein aggregation (49). Human AD-derived insoluble tau protein treatment caused aggregation of endogenous tau fusion protein, confirming the seeding capacity of our isolated tau proteins (Fig. 5C and SI Appendix, Fig. S5A). Interestingly, addition of APOE4 particles increased tau aggregation, and treatment with GPC-4 shRNA diminished APOE4-induced tau aggregation (Fig. 5C and D and SI Appendix, S5 B and C).
Fig. 5.
GPC-4 drives APOE4-mediated tau hyperphosphorylation. (A and B) Cultured neurons (WT) were treated with ACM alone, ACM with APOE4, and GPC-4–deprived (shRNA treated) ACM with APOE4. After 24 h, neurons were incubated with purified human tau proteins for 1 h. Representative IHC images of APOE4+ACM treated neuronal culture shows that APOE4 treatment enhanced tau uptake, but in the absence of GPC-4 (GPC-4 shRNA-treated ACM) APOE4-induced tau uptake was significantly reduced (B). Magnification of insets in (A): 60×. (Scale bar, 20 µm.) (C and D) Tau FRET-biosensor cells were used to monitor seeding activity of AD-tau proteins in the presence of APOE4 and GPC-4. We incubated Tau FRET-biosensor cells with 0.6 μg/mL tau proteins and 1 μg/mL APOE4 with/without GPC-4 shRNA for 2 d, and then counted the number of tau inclusion containing cells/area. Compared to tau alone, tau with APOE4 induced significantly higher numbers of tau inclusions in Tau FRET biosensor cells. Interestingly, APOE4-mediated tau inclusions were reduced in the presence of GPC-4 shRNA. n = 8 to 10. (Scale bar, 20 µm.) (E and F) We injected APOE2 or APOE4 particles isolated from corresponding human brains, in the absence or presence of GPC-4 shRNA. Following 3 wk of injections, no tau phosphorylation (AT8) was detected with APOE2. APOE4 robustly induced tau phosphorylation, but APOE4 failed to induce tau phosphorylation in the absence of GPC-4. (Scale bar, 20 µm.) (G and H) IHC staining with MC1 antibodies show that APOE4-mediated tau pathology was diminished in the presence of GPC-4 shRNA. (Scale bar, 20 µm.) (I–M) Human APOE4 iPSCs were differentiated into astrocytes, and the ACM was collected after 3 d of shRNA treatments. The PS19 neurons were treated with ACM for 4 d. Western blot analysis shows that ACM significantly increased AT8 and PHF1 levels, and the ACM-induced tau pathologies were reduced in the absence of GPC-4. n = 4 to 5, two-way ANOVA, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
We next investigated the role of GPC-4 in APOE4-induced tau phosphorylation in vivo. We injected APOE2 or APOE4 particles in the CA1 regions of the 4-mo-old PS19 mice and examined this group with age-matched controls after 3 wk. As expected, APOE4 robustly induced tau accumulation in comparison to control groups (Fig. 5E–H). Interestingly, APOE4-mediated tau accumulation was dramatically reduced in the absence of GPC-4 (Fig. 5E–H). Next, we performed an additional experiment to validate the role of GPC-4 in APOE4-mediated tau phosphorylation in human-induced pluripotent stem cells (iPSCs)-derived astrocytes. Human APOE4 iPSCs cells were differentiated into mature astrocytes, as previously described (50, 51) (Fig. 5I). The astrocytes were treated with GPC-4 shRNA (SI Appendix, Fig. S5D and E), and ACM was collected and added to PS19-derived neurons. GPC-4 shRNA treatment did not show significant changes in the APOE levels of iPSCs-derived astrocytes (SI Appendix, Fig. S5F and G). While the ACM treatment from APOE4 astrocytes increased tau phosphorylation, the GPC-4 shRNA treatment hampered the APOE4-ACM–induced tau phosphorylation (Fig. 5J–M).
GPC-4 Regulates APOE4-Mediated Membrane Trafficking of the LRP1 Receptor.
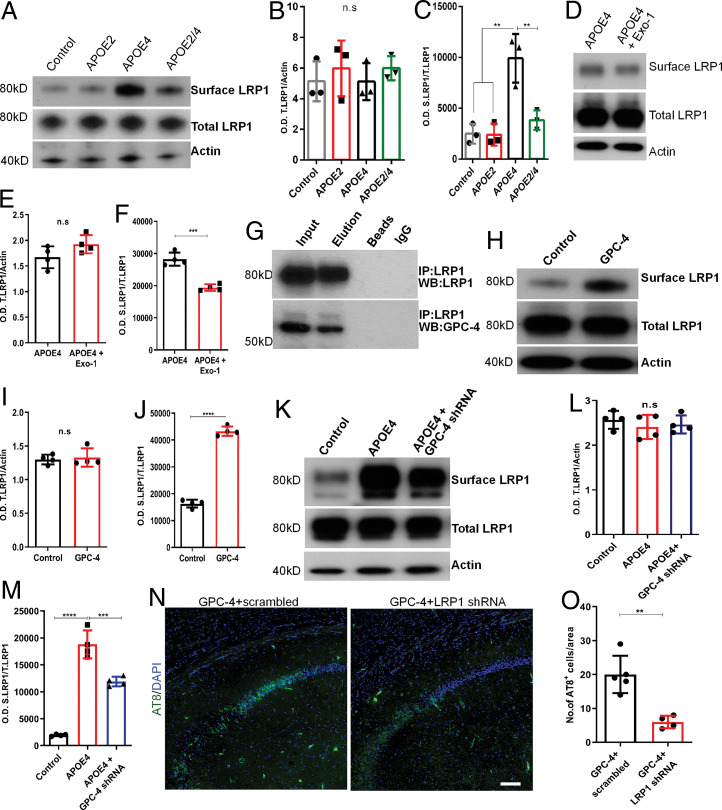
Finally, we aimed to understand the molecular interaction between APOE4 and GPC4 in inducing tau phosphorylation. Low-density lipoprotein receptor-related protein 1 (LRP1) is a major APOE receptor, and it has been suggested that it is involved in tau uptake and spreading (47). We therefore examined whether APOE2 and APOE4 have differential effects on the LRP1 receptor. We monitored the effects of APOE2 and APOE4 on the total and surface LRP1 levels in primary neuronal culture (WT mice). We used the Pierce Cell Surface Protein Isolation Kit to isolate surface proteins. We found that the addition of APOE2 had no effect on either the total or surface LRP1 levels (Fig. 6A–C). In contrast, APOE4 enhanced the trafficking of surface LRP1 levels (Fig. 6A–C). Interestingly, APOE4-mediated surface trafficking of LRP1 was blocked by APOE2 (Fig. 6A–C). To validate the APOE4-mediated surface trafficking of LRP1, we treated the neurons with an exocytic pathway blocker, Exo-1. As expected, inhibition of exocytosis significantly reduced APOE4-mediated surface trafficking of LRP1 receptors (Fig. 6D–F). Since GPC-4 is an astrocyte-secretory factor and an APOE4 binding partner, we tested whether GPC-4 would interact with neuronal LRP1. We first examined the likelihood of the GPC-4/LRP1 interaction to test this notion. A coimmunoprecipitation assay from human PFC postmortem brain tissue revealed that GPC-4 directly interacts with LRP1 (Fig. 6G). In the primary neuronal culture (WT mice), the addition of GPC-4 had no effect on the total LRP1, whereas the surface LRP1 levels increased greatly (Fig. 6H–J). We next investigated whether APOE4 is dependent on GPC-4 to induce surface LRP1 levels. We treated neurons with either ACM with additional APOE4 (WT mouse astrocytic culture) or ACM from GPC-4 shRNA-treated astrocytes with additional APOE4. In the absence of GPC-4, APOE4 addition resulted in a significant reduction in surface trafficking of LRP1 (Fig. 6K–M). Additionally, in the absence of LRP1 (LRP1 shRNA), GPC-4–induced tau phosphorylation was significantly reduced in the CA1 regions of PS19 mouse (Fig. 6N and O and SI Appendix, Fig. S6).
Fig. 6.
GPC-4 regulates trafficking of APOE receptor LRP1. Using a Pierce Cell Surface Protein Isolation Kit, the total surface proteins were isolated from primary neuronal culture (WT mice). (A–C) Western blot analysis from primary neuronal culture treated with APOE (s) shows that addition of APOE4 significantly enhanced the surface LRP1 (S.LRP1) (C). There were no changes in total LRP1 levels (T.LRP1) (B). (D–F) Western blot analysis from primary neuronal culture treated with APOE4 or APOE4 with exocytosis inhibitor, Exo-1, suggest that active exocytosis is required for APOE4-mediated surface trafficking of LRP1. (G) Coimmunoprecipitation of human postmortem brain protein samples with LRP1 antibodies and subsequent Western blotting revealed that LRP1 and GPC-4 are in the same complex. (H–J) Western blot analysis from primary neuronal culture treated with GPC-4 protein shows that GPC-4 significantly enhanced trafficking of surface LRP1 levels (J), whereas total LRP1 levels were not affected (I). (K–M) Western blot analysis from neuronal culture treated with ACM alone (control), APOE4+ACM, and with APOE4+GPC-4 shRNA treated ACM show that APOE4-induced surface expression of LRP1 is likely mediated through GPC-4. The GPC-4 shRNA treatment reduced the surface expression of LRP1 in the presence of APOE4 (J). (N and O) Representative IHC images with AT8 antibodies show that GPC-4-induced tau phosphorylation was reduced in the absence of LRP1. (Scale bar, 20 µm.) n = 4 to 5, one-way ANOVA or unpaired t test, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Discussion
In addition to an earlier observation that suggested the presence of a strong correlation between tau pathology and dementia (23), recent studies have demonstrated that tau accumulation/spreading is associated with neurodegeneration and dementia (18, 22, 27). Tau-based therapy has recently become attractive for clinical trials. At this juncture, it is critical to understand every step in tau pathology/phosphorylation. Human APOE4 transgene in a PS19 mouse model displayed more tau accumulation (27, 52), and human tau PET studies in APOE4 carriers agree with these observations (13, 53, 54). Nevertheless, the mechanism through which APOE4 induces tau pathology/phosphorylation remains unknown. Here, we found that the astrocytic protein GPC-4 preferably interacts with APOE4, and the brains of postmortem APOE4 AD patients highly expressed GPC-4 in neurotoxic astrocytes. We showed that GPC-4 induced tau accumulation and propagation in vitro. CRISPR/dCAS9-mediated activation of GPC-4 induced tau phosphorylation in vivo. In the absence of GPC-4, APOE4-mediated tau phosphorylation was greatly diminished in tau FRET biosensor cells and PS19 neurons. We further demonstrated a molecular interlink between GPC-4, APOE4, and its receptor LRP1, which is also implicated in tau propagation.
GPC-4 is one of the six members of the glypican family. GPC-4 is an astrocyte-secreted protein that has been shown to regulate synaptic plasticity in the developing brain (45, 46). We found that GPC-4 preferentially binds with the APOE4 isoform, a risk allele to developing AD over the APOE2 isoform, a protective allele to AD. A subtype of activated A1 astrocytes are considered neurotoxic astrocytes, whose secretory molecules may be involved in the worsening of AD pathology (38, 44). Our data show that GPC-4 is mainly expressed by a neurotoxic astrocytic population in APOE4-carrying AD postmortem brains. This result is consistent with the scRNA-seq analysis of AD patients and AD mouse models (42, 43). We also found that the expression of GPC-4 was regulated via the NF-κB pathway in the presence of proinflammatory factors. In fact, a mouse study showed that human APOE4-expressing PS19 tau mouse model displayed increased microglial activity (27), which is known to secrete these factors.
The role of HSPGs in amyloid pathology is well documented (55). It has been shown that HSPGs are involved in the early stage of amyloid pathology in AD (56–58). However, the role of HSPGs in tau pathology/phosphorylation is poorly understood. While normal APOE3 strongly interacted with HSPGs in vitro, a mutation in the APOE3 allele (R136S) showed a weak interaction (18). This APOE3 Christchurch mutation was found to be present in an autosomal dominant AD mutation (presenilin1) carrier who had not developed cognitive impairments for several decades before expected (18). This suggests that HSPGs and their interactions with APOE can potentially have an important impact on tau pathology/phosphorylation. After demonstrating that GPC-4 preferentially binds with APOE4 over APOE2, we showed that GPC-4 enhances the phosphorylation of tau proteins and their propagation in vitro. Further activation of GPC-4 in vivo using a CRISPR/dCas9 system induced increased tau hyperphosphorylation in PS19 mice. Given that mice do not express APOE isoforms, injection of APOE particles isolated from the human brain more closely resembles human conditions, enabling the study of their role in AD pathologies. Interestingly, APOE4-induced tau phosphorylation was greatly diminished in the absence of GPC-4, suggesting that GPC-4 plays a critical role in tau phosphorylation. It has been shown that GPC-4 enhances neuronal excitability (46). Similarly, it is also known that APOE4-carrying AD patients and animal models display hyperexcitability (59, 60). Given that neuronal activity is proposed to enhance tau propagation (61), future studies are warranted to examine the link between neuronal activity and GPC-4/APOE4 in tau pathology.
It has been recently proposed that the APOE receptor LRP1 binds with tau proteins and internalizes them (47). We show that APOE4 induces cellular surface trafficking of LRP1 and tau phosphorylation. Notably, the addition of APOE2 did not alter the surface levels of LRP1 or tau phosphorylation; instead, it attenuated the APOE4-induced trafficking of LRP1 and tau phosphorylation. These results suggest that APOE2 is involved in rescue mechanisms in the presence of APOE4. Our data further suggest that GPC-4 is in a complex with LRP1 and APOE4, and that APOE4-mediated surface trafficking of LRP1 is likely dependent on GPC-4. This differential action of APOE isoforms in tau pathology thereby likely relies on the presence of associated factors, such as GPC-4, which interacts more strongly with APOE4 than APOE2.
A limitation of our study, in fact in the AD field itself, is the lack of animal models for sporadic AD. As a result, researchers utilize either dominant mutations containing Aβ animal models (such as 5xFAD) or frontotemporal dementia tau animal models (such as PS19) to understand Aβ and tau pathology/phosphorylation, respectively (27, 62, 63). However, in sporadic AD, tau and Aβ accumulate without mutations in the MAPT or APP/PSEN genes. The lack of genetically modifiable animal models to study sporadic AD has been a major setback for the field. Therefore, while other models (including injection of AD brain-derived tau species) have dramatically improved our understanding of tau pathology (64), modeling human sporadic AD-related organisms or organoids will likely improve understanding of the disease and therapeutic options for AD patients. Furthermore, our in vitro studies showed that APOE2 reverses APOE4-induced tau phosphorylation and surface trafficking of LRP1 receptors. However, our studies did not address how APOE2 ameliorates APOE4-mediated effects. Furthermore, most of current studies compare individual APOE variants, while heterozygous APOE carriers represent the majority of AD cases compared to homozygous APOE carriers (5, 65). Therefore, understanding how APOE4-mediated activity is affected in the presence of other APOE isoforms is important. In conclusion, we propose that GPC-4 interacts with APOE4 and its receptor LRP1 to induce tau hyperphosphorylation, and targeting GPC4/APOE4/LRP1 complexes could potentially open therapeutic windows against tau pathology in AD.
Materials and Methods
Human Postmortem Brains.
Human autopsy brain tissue (frozen) from PFC regions (Brodmann area 10) were obtained from the Mount Sinai NIH NeuroBioBank and Banner Sun Health Research Institute. The sample details are given in SI Appendix, Table S1. Pathologically confirmed AD cases were selected (Aβ deposits in neocortex and tau accumulation in the temporal/parietal cortical regions) (66). In addition, age-matched cognitively normal individuals were used as control in the experiments. Cases were not presorted based on the levels of tau or Aβ pathologies, race, sex, and education.
Animals.
All animal procedures were performed according to the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee guidelines. Mice were deeply anesthetized with ketamine/xylazine and placed in a stereotaxic frame. After, a craniotomy 1 mm in diameter was made with a motorized mini drill, and the particles (below) were injected through a Hamilton syringe. They were injected at a rate of ∼0.5 μL/min, and the needles were kept in position for 5 min before slow withdrawal to prevent leakage of the liquid infused.
First, 8-mo-old WT mice (both male and female) were used for tau propagation study using AAV9 eGFP-2PA-hTauP301L. We produced AAV eGFP-2PA-hTauP301L virus in collaboration with Penn Vector Core. The virus titer was measured using ddPCR against the poly-A tail by the same core; 1 μL of AAV9 eGFP-2PA-hTauP301L (titers [GC/mL]: 2.74*1013) were injected in the CA1 regions (AP-2, ML-1.3 and DV-1.5) of the hippocampus and examined after 4 wk.
Second, 4-mo-old PS19 mice were used for the following experiments. To induce the expression of GPC-4, 2 μL of GPC-4 CRISPR/dCas9 lentiviral activation particles (sc-420640-LAC-2, Santa Cruz) were injected in the right CA1 region (AP-2, ML-1.3 and DV-1.5). The same volume of control lentiviral activation particles (sc-437282, Santa Cruz) was injected in a separate cohort of mice for comparison. After 1 wk of incubation period, mice were killed and processed for IHC to access the expression of GPC-4. After 3 wk of injection, the tau phosphorylation was examined.
Third, to study the effect of APOEs in tau pathology, 2 μg (1 μL) of APOE4 or APOE2 particles were unilaterally injected into CA1 regions (AP-2, ML-1.3 and DV-1.5). After 24 h, 2 μL of control shRNA lentiviral particles (sc-37007, Santa Cruz) or 2 μL of GPC-4 shRNA lentiviral particles (sc-145457-V, Santa Cruz) were injected. The APOE particles and lentiviral particles were injected in the same coordinates through the same injection site. Mice were killed after 3 wk of injection for further analysis.
Fourth, to test whether LRP1 plays a role in GPC-4–mediated tau phosphorylation, after 1 wk of GPC-4 activation (above) we injected 2 μL of LRP1 shRNA (sc-40101-V, Santa Cruz) or 2 μL of control shRNA (sc-37007, Santa Cruz) into CA1 regions (AP-2, ML-1.3 and DV-1.5). Mice were killed after 3 wk of shRNA injection for further analysis.
All of the lentivirus (above steps 2 to 4) received from Santa Cruz had 5,000 particles per microliter (1 × 106 in 200 μL).
Astrocyte Culture.
The procedures were adapted from a previous publication (46). This was performed as described in SI Appendix, Supplementary Text.
Neuronal Culture.
Primary cortical neuronal culture was performed from WT or PS19 or MAPTKO P1 pups as indicated throughout the report. The procedures were performed described previously with minor modifications (67) as in SI Appendix, Supplementary Text.
Isolation of Surface Proteins.
To isolate cell surface proteins, WT mouse neurons were cultured in 24-well plates as stated above. The cells from three wells/conditions were pooled together. This was performed as described in SI Appendix, Supplementary Text.
Protein Binding Assays.
To investigate whether GPC-4 differentially interacts with APOE2 and APOE4, the protein binding assay was performed. This was performed as described in SI Appendix, Supplementary Text.
Immunoprecipitation.
The total protein was isolated from frozen human brain samples using 1% Triton in a 50 mM Tris buffer. Pierce Direct IP kit (#26148, Thermo Fisher) was used to isolate proteins of interest as per the manufacturer’s instructions. This was performed as described in SI Appendix, Supplementary Text.
Electrophoresis and Western Blot.
This was performed as described in SI Appendix, Supplementary Text.
Tau Uptake Assay.
On day 11 of neuronal culture, mouse primary neurons were treated either with ACM with APOE4 or GPC-4 shRNA treated ACM with APOE4 for 24 h, as described in the report. The neurons were incubated with 1 μg/mL of human tau protein (#842501, Labome) for 1 h, washed with PBS three times and fixed with 4% PFA for 1 h. Any possible extracellular tau aggregates were eliminated by a trypsin wash, as described previously (68). Briefly, cells were incubated with 0.01% trypsin in DMEM for 1 min, and the trypsin was immediately deactivated with 10% FBS in DMEM. After three washes with PBS, it was processed for IHC.
Isolation of Tau Seeds.
Insoluble tau proteins from frozen postmortem AD brain tissue (Broadmann area 10) were isolated using a Sarkosyl-detergent method, as previously described (69). This was performed as described in SI Appendix, Supplementary Text.
Tau Seeding Assay.
This was performed as described in SI Appendix, Supplementary Text.
Human APOE iPSC-Astrocytes.
The consent for reprogramming human somatic cells to hiPSC was carried out on hSCRO protocol 19-04 at Mount Sinai (J. TCW). CRISPR/Cas9 genome-edited isogenic APOE lines (TCW1 APOE 44 lines derived from an AD patient) were utilized for this study (50). Human APOE4-iPSCs were differentiated into astrocytes, as described previously (50, 51). Once the cells reached about 80% of confluence, cells were washed with PBS, added conditioned medium with shRNAs, and incubated for 3 d. At the end of day 3, the medium was collected, concentrated/dialyzed using 10-kDa Amicon Ultra Centrifugal filter tubes. The concentrated ACM was added to PS19 neuronal culture and incubated for 4 d.
IHC and Analysis.
This was performed as described in SI Appendix, Supplementary Text.
Statistics.
All statistical details of the experiments can be found in either the main text figure legends or SI Appendix figure legends, including the n value, the P value, and statistical test being used. For all purposes, P ≤ 0.05 was considered as statistically significant. Statistical analyses were performed with Graphpad Prism software. Unpaired t test was used to compare two groups. For more than two groups, one/two-way ANOVA with Bonferroni correction was used, as indicated in the figure legends.
Supplementary Material
Acknowledgments
We thank Mount Sinai Brain Bank and Banner Sun Health Research Institute for providing human brain samples; Patrick Hof, Diede Broekaart, Saraswathi Subramaniyan, Kathryn Bowles, Anjalika Chongtham, Joon Ho Seo, and Sam Gandy for insightful comments; and Neeva Shafiian for assisting with quantification analysis. This work was supported by NIH Grants R01 AG063819 and R01 AG064020 (to A.C.P.), K01AG062683 (to J. TCW), U01AG058635 (to A.M.G.), and U19AG069701 (to J. TCW and A.M.G.); Paul B. Beeson Emerging Leaders Career Development Award in Aging K76 AG054772 (to A.C.P.); the Bright Focus Foundation (to A.C.P.); the DANA Foundation (to A.C.P.); the Alzheimer’s Drug Discovery Foundation (to A.C.P.); the Alzheimer’s Association (to A.C.P.); the Carolyn and Eugene Mercy Research Gift (to A.C.P.); the Karen Strauss Cook Research Scholar Award (to A.C.P.); and the Robert J. and Claire Pasarow Foundation (to A.C.P).
Footnotes
Competing interest statement: A.C.P. has patent applications 17/026,181 and PCT/2021/053403 that are unrelated to this work.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2108870119/-/DCSupplemental.
Data Availability
All study data are included in the main text and SI Appendix. Data will be shared and made available by request from a qualified academic investigator.
References
- 1.Iqbal K., Liu F., Gong C. X., Tau and neurodegenerative disease: The story so far. Nat. Rev. Neurol. 12, 15–27 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Wong W., Economic burden of Alzheimer disease and managed care considerations. Am. J. Manag. Care 26 (8, suppl.), S177–S183 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Karch C. M., Goate A. M., Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry 77, 43–51 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falcon B., et al. , Tau filaments from multiple cases of sporadic and inherited Alzheimer’s disease adopt a common fold. Acta Neuropathol. 136, 699–708 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belloy M. E., Napolioni V., Greicius M. D., A quarter century of APOE and Alzheimer’s disease: Progress to date and the path forward. Neuron 101, 820–838 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahley R. W., Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science 240, 622–630 (1988). [DOI] [PubMed] [Google Scholar]
- 7.Weisgraber K. H., S. C. Rall, Jr, Mahley R. W., Human E apoprotein heterogeneity. Cysteine-arginine interchanges in the amino acid sequence of the apo-E isoforms. J. Biol. Chem. 256, 9077–9083 (1981). [PubMed] [Google Scholar]
- 8.Liddell M., Williams J., Bayer A., Kaiser F., Owen M., Confirmation of association between the e4 allele of apolipoprotein E and Alzheimer’s disease. J. Med. Genet. 31, 197–200 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunders A. M., et al. , Association of apolipoprotein E allele ε 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 43, 1467–1472 (1993). [DOI] [PubMed] [Google Scholar]
- 10.Strittmatter W. J., et al. , Apolipoprotein E: High-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 90, 1977–1981 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corder E. H., et al. , Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat. Genet. 7, 180–184 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Pericak-Vance M. A., et al. , Linkage studies in familial Alzheimer disease: Evidence for chromosome 19 linkage. Am. J. Hum. Genet. 48, 1034–1050 (1991). [PMC free article] [PubMed] [Google Scholar]
- 13.Montagne A., et al. , APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 581, 71–76 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iqbal K., et al. , Protein changes in senile dementia. Brain Res. 77, 337–343 (1974). [DOI] [PubMed] [Google Scholar]
- 15.Masters C. L., et al. , Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. U.S.A. 82, 4245–4249 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leuzy A., et al. , Tau PET imaging in neurodegenerative tauopathies—Still a challenge. Mol. Psychiatry 24, 1112–1134 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C., Götz J., Tau-based therapies in neurodegeneration: Opportunities and challenges. Nat. Rev. Drug Discov. 16, 863–883 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Arboleda-Velasquez J. F., et al. , Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: A case report. Nat. Med. 25, 1680–1683 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolb H. C., Andrés J. I., Tau positron emission tomography imaging. Cold Spring Harb. Perspect. Biol. 9, a023721 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanseeuw B. J., et al. , Association of amyloid and tau with cognition in preclinical Alzheimer disease: A longitudinal study. JAMA Neurol. 76, 915–924 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziontz J., et al. , Tau pathology in cognitively normal older adults. Alzheimers Dement. (Amst.) 11, 637–645 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La Joie R., et al. , Prospective longitudinal atrophy in Alzheimer’s disease correlates with the intensity and topography of baseline tau-PET. Sci. Transl. Med. 12, eaau5732 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arriagada P. V., Growdon J. H., Hedley-Whyte E. T., Hyman B. T., Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42, 631–639 (1992). [DOI] [PubMed] [Google Scholar]
- 24.Agosta F., et al. , Apolipoprotein E ε4 is associated with disease-specific effects on brain atrophy in Alzheimer’s disease and frontotemporal dementia. Proc. Natl. Acad. Sci. U.S.A. 106, 2018–2022 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ossenkoppele R., et al. , Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 139, 1551–1567 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hohman T. J., et al. ; Alzheimer’s Disease Genetics Consortium and the Alzheimer’s Disease Neuroimaging Initiative, Sex-specific association of apolipoprotein e with cerebrospinal fluid levels of tau. JAMA Neurol. 75, 989–998 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Y., et al. ; Alzheimer’s Disease Neuroimaging Initiative, ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549, 523–527 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Therriault J., et al. , Association of Apolipoprotein e ϵ4 with medial temporal tau independent of Amyloid-β. JAMA Neurol. 77, 470–479 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lane-Donovan C., Herz J., ApoE, ApoE receptors, and the synapse in Alzheimer’s disease. Trends Endocrinol. Metab. 28, 273–284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahley R. W., Central nervous system lipoproteins: ApoE and regulation of cholesterol metabolism. Arterioscler. Thromb. Vasc. Biol. 36, 1305–1315 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liddelow S. A., et al. , Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith H. L., et al. , Astrocyte unfolded protein response induces a specific reactivity state that causes non-cell-autonomous neuronal degeneration. Neuron 105, 855–866.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richetin K., et al. , Tau accumulation in astrocytes of the dentate gyrus induces neuronal dysfunction and memory deficits in Alzheimer’s disease. Nat. Neurosci. 23, 1567–1579 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Chun H., et al. , Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H2O2- production. Nat. Neurosci. 23, 1555–1566 (2020). [DOI] [PubMed] [Google Scholar]
- 35.De Calignon A., et al. , Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Neuron 73, 685–697 (2012).22365544 [Google Scholar]
- 36.Dujardin S., et al. , Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat. Med. 26, 1256–1263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braak H., Del Tredici-Braak K., “Neural basis of Alzheimer’s disease” in International Encyclopedia of the Social and Behavioral Sciences Wright J. D., Ed. (ed. 2, 2015), pp. 591–596. [Google Scholar]
- 38.Khakh B. S., Deneen B., The emerging nature of astrocyte diversity. Annu. Rev. Neurosci. 42, 187–207 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Litchman W. M., Alei M., Florin A. E., Identification of diverse astrocyte populations and their malignant analogs. J. Chem. Phys. 50, 1897–1898 (1969). [Google Scholar]
- 40.Wilhelmsson U., et al. , Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc. Natl. Acad. Sci. U.S.A. 103, 17513–17518 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y., et al. , Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med. 26, 131–142 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grubman A., et al. , A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat. Neurosci. 22, 2087–2097 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Habib N., et al. , Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 23, 701–706 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdi K., et al. , Uncovering inherent cellular plasticity of multiciliated ependyma leading to ventricular wall transformation and hydrocephalus. Nat. Commun. 9, 1655 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farhy-Tselnicker I., et al. , Astrocyte-secreted Glypican 4 regulates release of neuronal Pentraxin 1 from axons to induce functional synapse formation. Neuron 96, 428–445.e13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allen N. J., et al. , Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486, 410–414 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rauch J. N., et al. , LRP1 is a master regulator of tau uptake and spread. Nature 580, 381–385 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wegmann S., et al. , Experimental evidence for the age dependence of tau protein spread in the brain. Sci. Adv. 5, eaaw6404 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holmes B. B., et al. , Proteopathic tau seeding predicts tauopathy in vivo. Proc. Natl. Acad. Sci. U.S.A. 111, E4376–E4385 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.TCW J., et al. , Cholesterol and matrisome pathways dysregulated in human APOE ∊4 glia. Cell 185, 10.2139/ssrn.3435267 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.TCW J., et al. , An efficient platform for astrocyte differentiation from human induced pluripotent stem cells. Stem Cell Reports 9, 600–614 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin Y. T., et al. , APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron 98, 1141–1154.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ossenkoppele R., et al. , Assessment of demographic, genetic, and imaging variables associated with brain resilience and cognitive resilience to pathological tau in patients with Alzheimer disease. JAMA Neurol. 77, 632–642 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mattsson N., et al. , Greater tau load and reduced cortical thickness in APOE ε4-negative Alzheimer’s disease: A cohort study. Alzheimers Res. Ther. 10, 77 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang G. L., Zhang X., Wang X. M., Li J. P., Towards understanding the roles of heparan sulfate proteoglycans in Alzheimer’s disease. BioMed Res. Int. 2014, 516028 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sandwall E., et al. , Heparan sulfate mediates amyloid-beta internalization and cytotoxicity. Glycobiology 20, 533–541 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Kanekiyo T., et al. , Heparan sulphate proteoglycan and the low-density lipoprotein receptor-related protein 1 constitute major pathways for neuronal amyloid-β uptake. J. Neurosci. 31, 1644–1651 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu C. C., et al. , Neuronal heparan sulfates promote amyloid pathology by modulating brain amyloid-β clearance and aggregation in Alzheimer’s disease. Sci. Transl. Med. 8, 332ra44 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nuriel T., et al. , Neuronal hyperactivity due to loss of inhibitory tone in APOE4 mice lacking Alzheimer’s disease-like pathology. Nat. Commun. 8, 1464 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koelewijn L., et al. , Oscillatory hyperactivity and hyperconnectivity in young APOE-ɛ4 carriers and hypoconnectivity in Alzheimer’s disease. eLife 8, e36011 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu J. W., et al. , Neuronal activity enhances tau propagation and tau pathology in vivo. Nat. Neurosci. 19, 1085–1092 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dujardin S., et al. , Tau molecular diversity contributes to clinical heterogeneity in Alheimer’s disease. Nat. Med. 26, 1256–1263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He Z., et al. , Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat. Med. 24, 29–38 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Narasimhan S., et al. , Pathological tau strains from human brains recapitulate the diversity of tauopathies in nontransgenic mouse brain. J. Neurosci. 37, 11406–11423 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corder E. H., et al. , Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923 (1993). [DOI] [PubMed] [Google Scholar]
- 66.Braak H., Braak E., Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991). [DOI] [PubMed] [Google Scholar]
- 67.Risher W. C., et al. , Astrocytes refine cortical connectivity at dendritic spines. eLife 3, e04047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Michel C. H., et al. , Extracellular monomeric tau protein is sufficient to initiate the spread of tau protein pathology. J. Biol. Chem. 289, 956–967 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo J. L., et al. , Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J. Exp. Med. 213, 2635–2654 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the main text and SI Appendix. Data will be shared and made available by request from a qualified academic investigator.