Significance
Sepsis, the cause of one in five deaths worldwide, often induces vasodilatory shock requiring emergent treatment with catecholamine vasopressors, such as norepinephrine, to prevent rapidly fatal organ ischemia. However, immunosuppression is an increasingly recognized component of the dysregulated host-response in sepsis, and norepinephrine exacerbates immunosuppression. Angiotensin-II is an alternative vasopressor that is approved to treat hypotension in sepsis. Herein, we found that myeloid cell-dependent angiotensin-II type 1 receptor (AT1R) signaling enhanced bacterial clearance, increased innate phagocytosis, and modulated the systemic inflammatory response in a cecal ligation and puncture model of murine sepsis. These immune and pathogen-clearing effects of innate immune AT1R signaling suggest angiotensin-II may have therapeutic value in sepsis not just for cardiovascular support, but also as an immunomodulator.
Keywords: angiotensin II, renin-angiotensin system, sepsis, immunity, innate, vasoconstrictor agents
Abstract
Sepsis, defined as organ dysfunction caused by a dysregulated host-response to infection, is characterized by immunosuppression. The vasopressor norepinephrine is widely used to treat low blood pressure in sepsis but exacerbates immunosuppression. An alternative vasopressor is angiotensin-II, a peptide hormone of the renin-angiotensin system (RAS), which displays complex immunomodulatory properties that remain unexplored in severe infection. In a murine cecal ligation and puncture (CLP) model of sepsis, we found alterations in the surface levels of RAS proteins on innate leukocytes in peritoneum and spleen. Angiotensin-II treatment induced biphasic, angiotensin-II type 1 receptor (AT1R)-dependent modulation of the systemic inflammatory response and decreased bacterial counts in both the blood and peritoneal compartments, which did not occur with norepinephrine treatment. The effect of angiotensin-II was preserved when treatment was delivered remote from the primary site of infection. At an independent laboratory, angiotensin-II treatment was compared in LysM-Cre AT1aR−/− (Myeloid-AT1a−) mice, which selectively do not express AT1R on myeloid-derived leukocytes, and littermate controls (Myeloid-AT1a+). Angiotensin-II treatment significantly reduced post-CLP bacteremia in Myeloid-AT1a+ mice but not in Myeloid-AT1a− mice, indicating that the AT1R-dependent effect of angiotensin-II on bacterial clearance was mediated through myeloid-lineage cells. Ex vivo, angiotensin-II increased post-CLP monocyte phagocytosis and ROS production after lipopolysaccharide stimulation. These data identify a mechanism by which angiotensin-II enhances the myeloid innate immune response during severe systemic infection and highlight a potential role for angiotensin-II to augment immune responses in sepsis.
Sepsis, the cause of 20% of deaths worldwide (1), is life-threatening organ dysfunction caused by a dysregulated host response to infection (2). This dysregulated host response includes suppression of beneficial innate immune responses, which is associated with particularly poor outcomes (3, 4).
Catecholaminergic vasopressors, particularly norepinephrine, are widely used to maintain blood pressure but contribute to immunosuppression in sepsis (5, 6). Specifically, norepinephrine decreases blood levels of proinflammatory cytokines (e.g., tumor necrosis factor-α [TNF-α], interleukin [IL]-6) and chemokines (e.g., MCP-1, KC, macrophage inflammatory protein [MIP]-1α, MIP-2) and increases blood levels of antiinflammatory cytokines (e.g., IL-10) (5). In cecal-ligation and puncture (CLP), an animal model that mimics some aspects of human sepsis, norepinephrine increases systemic bacterial dissemination (5). Yet, current international sepsis management guidelines recommend norepinephrine as first-line treatment for septic shock (7, 8), in part due to a lack of adequate alternatives. The current dependence on norepinephrine as first-line treatment despite its negative immunologic effects highlights the need to investigate other approaches to increase blood pressure while maintaining beneficial immune responses (6).
In contrast to catecholamines, angiotensin-II is an endogenous vasoconstrictor and Food and Drug Administration (FDA)-approved vasopressor that displays well-established proinflammatory effects (9). Angiotensin-II induces the vascular endothelium to secrete proinflammatory cytokines and chemokines (10–12). Diverse types of leukocytes express angiotensin receptors, and the renin-angiotensin system (RAS) modulates the function of these cells in complex ways (13–16). Therefore, we tested the hypothesis that angiotensin-II augments the immune response in CLP.
Results
CLP Alters RAS-Related Protein Expression on Innate Leukocytes.
To explore whether murine CLP alters the expression of RAS proteins on the surface of innate immune cells, we measured the abundance of surface angiotensin-II type 1 receptors (AT1R) and angiotensin converting enzyme-1 (ACE-1) on splenic and peritoneal neutrophils (CD11b+/Ly6G+) and monocytes (CD11b+/Ly6G−/MHCII−/Ly6C+). AT1R expression on innate immune cells was higher after CLP (SI Appendix, Fig. S1). In the spleen, AT1R expression was progressively higher at each time point post-CLP on neutrophils (β = 66%/d [95%CI: 41 to 91%/d], P < 0.0001) and inflammatory (i.e., Ly6CHi) monocytes (β = 74%/d [27 to 121%/d], P = 0.0042). In the peritoneum, similar findings were observed on neutrophils (P = 0.0250) at 24 and 72 h post-CLP and at all post-CLP time points on inflammatory monocytes (P = 0.0462).
In contrast to previous studies of localized infection (17), we did not observe higher ACE-1 expression on neutrophils or inflammatory monocytes at later time points after CLP. Rather, at each post-CLP time point, there was progressively lower ACE-1 expression on splenic neutrophils (β = −15%/d [−4 to −26%], P = 0.0118) (SI Appendix, Fig. S1). In splenic inflammatory monocytes, post-CLP ACE-1 expression did not significantly differ from time 0 (T0) although variability was markedly greater. In the peritoneum, inflammatory monocyte ACE-1 expression followed a biphasic pattern where expression was higher at 24 h and lower by 72 h. In peritoneal neutrophils, ACE-1 expression over time trended similarly to that in peritoneal inflammatory monocytes but differences versus T0 were not statistically significant.
Angiotensin-II Alters the Immune Response to CLP.
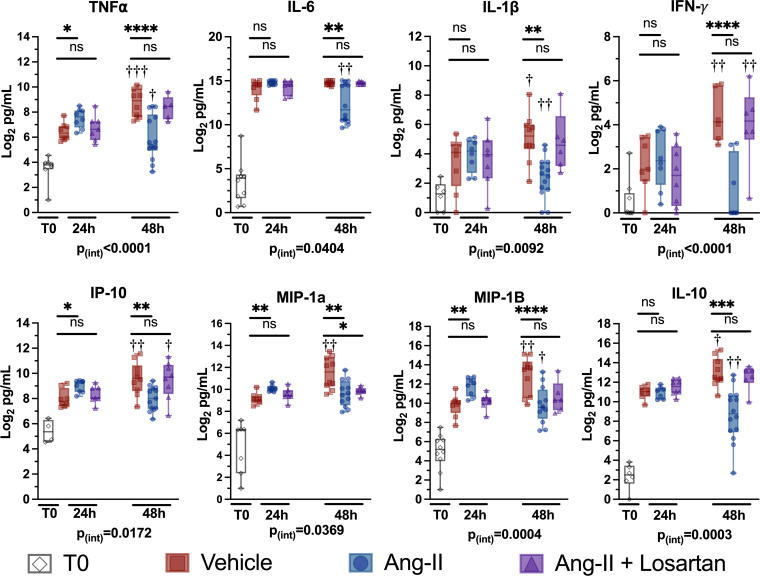
The altered leukocyte AT1R and ACE-1 expression suggested that exogenous angiotensin-II treatment might affect the innate immune response to CLP. Therefore, we measured serum cytokine levels at 24 and 48 h post-CLP in mice treated with perioperatively initiated intraperitoneal angiotensin-II. At 24 h post-CLP, levels of TNF-α, IL-6, IL-1β, interferon (IFN)-γ–induced protein 10 (IP-10), MIP-1α, MIP-1β, and IL-10 increased (Fig. 1). Compared to vehicle-treated mice, however, angiotensin-II–treated mice had significantly higher levels of TNF-α (P = 0.0340), IP-10 (P = 0.0128), MIP-1α (P = 0.0018), and MIP-1β (P = 0.0019) at the 24-h post-CLP time point. Addition of the selective AT1R antagonist, losartan, to angiotensin-II was associated with levels that were statistically indistinguishable from vehicle alone, suggesting that the effect of angiotensin-II depended on AT1R. At 24 h post-CLP, elevations in IL-6, IL-1β, and IL-10 were similar among the three groups.
Fig. 1.
Angiotensin-II treatment modulates the systemic inflammatory response to CLP in a biphasic manner. Cytokine levels at T0 and at 24 and 48 h post-CLP. The y axis displays cytokine concentrations on a log2 scale. Dots represent individual mice, horizontal bars represent group median, box height represents interquartile range, error bars represent range. Asterisks indicate the (multiple comparison-adjusted) P values vs. the control (vehicle) group at the indicated time point as follows: ns, P > 0.05; *P < 0.05. **P < 0.005; ***P < 0.0005; ****P < 0.0001. Daggers indicate the (multiple comparison adjusted) P values for the indicated treatment group at 48 h vs. the respectively treated group at 24 h post-CLP as follows: †P < 0.05, ††P < 0.005; †††P < 0.0005, ††††P < 0.0001. The P value for the overall interaction effect of time with treatment group is displayed below each graph.
Angiotensin-II treatment significantly abrogated increases from 24 to 48 h post-CLP in the levels of several cytokines, including TNF-α (Pinteraction < 0.0001), IFN-γ (Pinteraction < 0.0001), IL-1β (Pinteraction = 0.0092), and IL-10 (Pinteraction = 0.0003) (Fig. 1). Similarly, among angiotensin-II–treated mice, the initially marked elevations in IL-6 levels were significantly lower at 48 h compared to both vehicle alone at 48 h and angiotensin-II at 24 h (Fig. 1). While IL-6 levels were significantly lower at 48 h vs. 24 h post-CLP for the angiotensin-II animals, there was no difference between time points in IL-6 levels for vehicle treatment (Pinteraction = 0.0404). These angiotensin-II effects depended on AT1R, as they were abolished by addition of losartan. In summary, angiotensin-II treatment was associated with proinflammatory cytokine levels that were higher at 24 h post-CLP, but lower at 48 h post-CLP.
To further understand the effects of angiotensin-II on immune responses to CLP, we determined the abundance of innate leukocyte populations, evaluating peritoneal cells as indicators of the local response in the infected compartment and splenic cells as indicators of systemic processes. At 24 h post-CLP, the numbers of neutrophils, macrophages (i.e., CD11b+/Ly6G−/MHCII−/CD64+), Ly6CHi inflammatory monocytes (CD11b+/Ly6G−/MHCII−/CD64−/Ly6CHi), and Ly6CLo monocytes (CD11b+/Ly6G−/MHCII−/CD64−/Ly6CLo) were significantly higher than baseline and were similar in the three treatment groups (SI Appendix, Fig. S2).
At 48 h post-CLP, although the numbers of peritoneal macrophages (Pinteraction = 0.0005), inflammatory monocytes (Pinteraction = 0.0003), and neutrophils (Pinteraction = 0.0468), but not Ly6CLo monocytes (Pinteraction = 0.58), remained significantly above baseline levels in all treatment groups, angiotensin-II–treated mice had significantly higher cell counts than vehicle alone. These differences associated with angiotensin-II treatment were, again, abrogated by addition of losartan, indicating dependence on AT1R signaling. Both monocyte-derived and CD11c+ dendritic cell numbers were, again, similar among all groups.
Angiotensin-II Enhances Bacterial Clearance in Peritoneal Fluid and Blood through AT1R-Dependent Signaling.
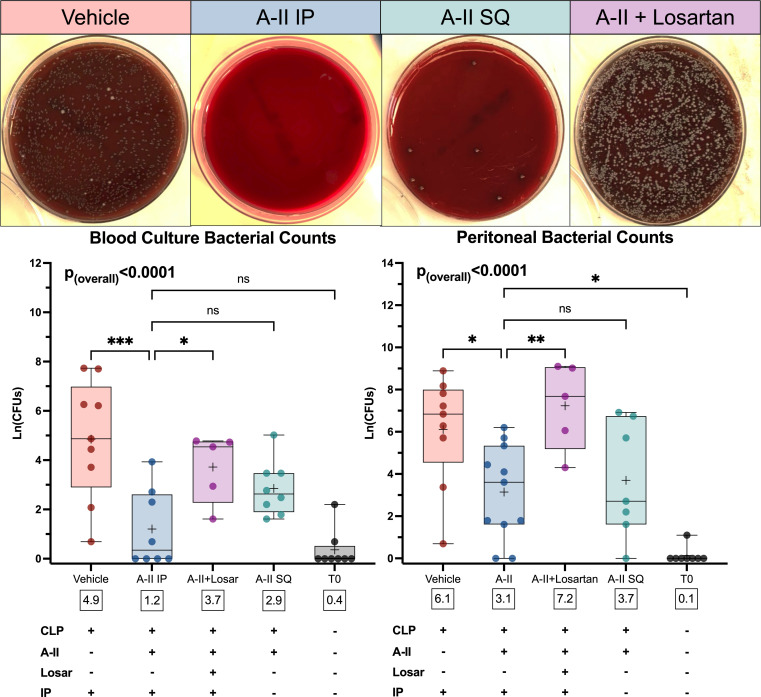
To test whether angiotensin-II treatment impacted clearance of infection, we measured bacterial counts in peritoneal lavage fluid and blood cultures collected 48 h post-CLP. Compared to vehicle, angiotensin-II–treated mice had significantly lower CLP-induced bacterial counts in both blood (Δmean: −3.7 log-CFUs, 95%CI: −5.7 to −1.7, P = 0.0002) and peritoneal fluid (Δmean: −3.0 log-CFUs, 95%CI: −5.5 to −0.4, P = 0.0169) (Fig. 2). This difference was not present in either blood (Δmean: 2.5, log-CFUs, 95%CI: 0.2 to 4.9, P = 0.0321) or peritoneal fluid (Δmean: 4.1, log-CFUs, 95%CI: 1.1 to 7.1, P = 0.0051) in animals where angiotensin and losartan were coadministered, indicating the observed effect of angiotensin-II was dependent on AT1R. The same pattern was seen for blood cultures obtained 24 h post-CLP (SI Appendix, Fig. S3). Peritoneal cultures from this timepoint showed only the angiotensin-II group had a significant reduction in peritoneal bacterial counts from 24 to 48 h postoperatively (SI Appendix, Fig. S3).
Fig. 2.
Angiotensin-II decreases bacterial growth in peritoneal and blood cultures in an AT1R-dependent manner 48-h after CLP. (Top) Images display representative peritoneal culture plates from each of the treatment groups. (Middle) Graphs display bacterial counts in treatment groups and T0 mice. The y axis displays bacterial counts on a natural log scale. Dots represent individual mice, horizontal bars represent group median, box height represents interquartile range, error bars represent range. Asterisks indicate the (multiple comparison-adjusted) P values vs. the control (vehicle) group as follows: ns, P > 0.05; *P < 0.05; **P < 0.005; ***P < 0.0005. White boxes below each box display the overall group mean. (Bottom) Table summarizes interventions for each graph column. Abbreviations: A-II, angiotensin-II; IP, intraperitoneal; Ln(CFUs), natural log of colony forming units; Losar, losartan.
We assessed whether the observed angiotensin-II–dependent increases in serum chemokines and some peritoneal innate cells might be due to its known prochemotaxis effects (10, 12, 18), specifically testing whether the post-CLP increase in leukocyte abundance might reflect a direct chemotactic effect of intraperitoneal angiotensin-II. However, when we infused angiotensin-II subcutaneously (i.e., in a compartment remote from the site of infection) bacterial counts were statistically equivalent to those upon intraperitoneal angiotensin-II infusion for both peritoneal lavage fluid (Δmean: 0.6 log-CFUs, 95%CI: −2.2 to 3.3, P = 0.14) and blood cultures (Δmean: 1.7 log-CFUs, 95%CI: −0.4 to 3.7, P = 0.96) (Fig. 2). Thus, infusing angiotensin-II in a body compartment remote from the site of infection did not reverse the effect of treatment on bacterial clearance.
Angiotensin-II Has No Direct Antimicrobial Activity.
To determine whether direct antibacterial activity explained the effects of angiotensin-II on bacterial numbers in blood or peritoneum following CLP, we performed Kirby–Bauer disk diffusion testing using several different doses of angiotensin-II. We saw marked radial clearing surrounding ampicillin and imipenem controls, but angiotensin-containing disks did not affect bacterial growth (SI Appendix, Fig. S4).
Myeloid-Lineage Cells Mediate AT1R-Dependent Effects of Angiotensin-II on Bacterial Clearance after CLP.
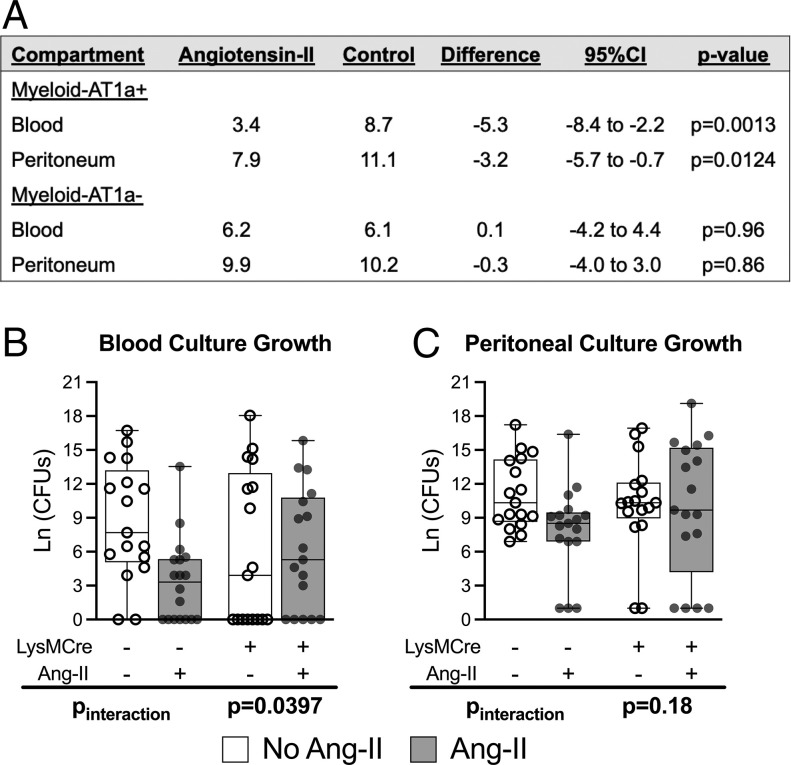
Because myeloid-lineage leukocytes (i.e., macrophages, neutrophils, and monocytes) express AT1R, these cell types could mediate the AT1R-dependent effect of angiotensin-II on bacterial burden in CLP. To test this, CLP with or without angiotensin-II treatment was performed in LysMCre(+)/AT1aRfl/fl (Myeloid-AT1a−) mice, which do not express AT1R on myeloid-derived cells. To simultaneously test whether the findings were independently reproducible, these studies were performed in an independent laboratory. Myeloid specificity of the knockout model was previously demonstrated (19), and genetics were confirmed for all animals.
Among the Myeloid-AT1a+ group, angiotensin-II–treated mice displayed significantly lower blood bacterial counts than untreated mice at 24 h post-CLP (3.4 vs. 8.7 log-CFUs, Δmean: −5.3, 95%CI: −8.4 to −2.2, P = 0.0013). In contrast, the Myeloid-AT1a− group demonstrated no difference in blood bacterial counts with angiotensin-II treatment (6.2 vs. 6.1 log-CFUs, Δmean: 0.1, 95%CI: −4.2 to 4.4) with a statistically significant interaction-effect between groups (difference-in-difference: −5.4 log-CFUs, Pinteraction = 0.0397) (Fig. 3), indicating that the effect of angiotensin-II depended on AT1R. The trend was similar for peritoneal cultures, with Myeloid-AT1a+ mice displaying significantly lower bacterial counts with angiotensin-II treatment (7.9 vs. 11.1 log-CFUs, Δmean: −3.2, 95%CI: −5.7 to −0.7, P = 0.0124), which was dependent on the presence of AT1R, as Myeloid-AT1a− mice did not show lower peritoneal bacterial counts with angiotensin-II treatment (9.9 vs. 10.2 log-CFUs, Δmean: −0.3, 95%CI: −4.0 to 3.0); however, for peritoneal cultures, the interaction effect for angiotensin-II treatment between genotypes was not statistically significant (difference-in-difference: −2.9, Pinteraction = 0.18) (Fig. 3). In addition to confirming the external reproducibility of our prior experiments, these findings indicate that the effect of angiotensin-II on blood bacterial burden was dependent on myeloid-lineage leukocytes that express AT1R.
Fig. 3.
AT1R deletion from myeloid-lineage leukocytes reverses the effect of angiotensin-II treatment to reduce blood culture growth 24 h post-CLP. (A–C) Bacterial counts by treatment and genotype. LysMCre+ and LysMCre− designate the Myeloid-AT1a− and Myeloid-AT1a+ genotypes, respectively. The y axis displays bacterial counts on a natural log scale. Dots represent individual mice, horizontal bars indicate the group median, box height represents the interquartile range, error bars represent the range. The Pinteraction indicates the interaction effect P value for the difference-in-difference: That is, a significant interaction effect indicates the difference between angiotensin-II treated and untreated mice within the Myeloid-AT1a− group is significantly different from the difference between treated and untreated mice in the Myeloid-AT1a+ group.
Angiotensin-II Enhances Innate Cell Phagocytosis and Modulates Reactive Oxygen Species Production in Response to Lipopolysaccharide Stimulation via AT1R in CLP.
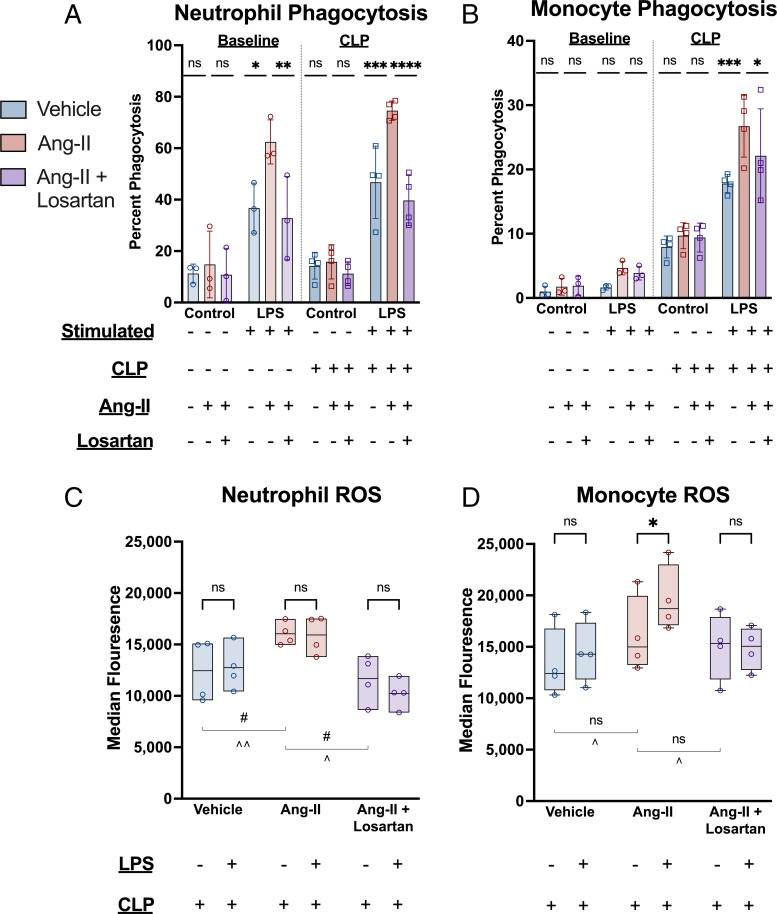
To investigate possible mechanisms for angiotensin-II’s effect on bacterial burden in CLP, we measured phagocytosis performed by innate cells isolated from spleen 48 h post-CLP, with and without ex vivo lipopolysaccharide (LPS) stimulation. In the absence of LPS stimulation, angiotensin-II treatment did not significantly alter the proportion of neutrophils or monocytes obtained from either baseline or post-CLP mice that had phagocytosed IgG-conjugated fluorescently tagged beads. However, when stimulated by LPS, the proportion of neutrophils that had phagocytosed beads was 1.5-fold higher with angiotensin-II treatment vs. vehicle in both baseline (P = 0.0011) and post-CLP (P = 0.0001) (Fig. 4). Losartan treatment prevented the increased phagocytosis observed with angiotensin-II treatment (P < 0.0001). Additionally, median fluorescence intensity was highest in the angiotensin-II–treated cells, indicating a higher degree of phagocytosis per cell (SI Appendix, Fig. S5). In contrast, angiotensin-II treatment induced a similarly higher monocyte fraction to perform phagocytosis after CLP (P = 0.0003) but did not significantly alter phagocytosis by these cells in baseline animals (P = 0.12). Losartan again reversed the angiotensin-II–induced increase in monocyte phagocytosis post-CLP (P = 0.0390). These results indicate that angiotensin-II promotes innate immune phagocytosis in response to LPS stimulation through AT1R.
Fig. 4.
Angiotensin-II enhances ex vivo phagocytosis and ROS production in response to LPS stimulation after CLP via AT1R. (A and B) Ex vivo phagocytosis with and without LPS stimulation. They y axis indicates the proportion of the indicated cell type positive for having phagocytized fluorescent-tagged IgG-conjugated beads. Bar height indicates mean, error bars the SD. Individual dots represent the average of the technical replicates for each biological replicate. Asterisks indicate the (multiple comparison adjusted) P values vs. the angiotensin-II group as follows: ns, P > 0.05; *P < 0.05; **P < 0.005; ***P < 0.0005; ****P < 0.0001. (C and D) Ex vivo ROS levels with and without LPS stimulation in splenic cells isolated post-CLP. Bar height indicates interquartile range, error bar height indicates the range. The horizontal black lines indicate the median. Each dot represents the average of the technical replicates for an individual biological replicate. Asterisks above the bars indicate the (multiple comparison-adjusted) P values for LPS stimulated vs. unstimulated as follows: ns, P > 0.05; *P < 0.05. The “#” symbols indicates P values for the difference between the indicated treatment groups without LPS stimulation (#P < 0.05) while “^” symbols indicate P values for the difference between the indicated treatment groups with LPS stimulation (^P < 0.05; ^^P < 0.005).
In the absence of ex vivo LPS stimulation, monocyte reactive oxygen species (ROS) production was not significantly higher in angiotensin-treated mice versus mice that received vehicle (P = 0.49) or angiotensin-II with losartan (P = 0.68) (Fig. 4). However, whereas monocytes from vehicle-treated or losartan-treated mice did not increase ROS production in response to LPS, LPS-stimulated monocytes from angiotensin-II–treated mice had significantly higher ROS levels than unstimulated monocytes (P = 0.0226). Neutrophils did not significantly increase ROS production after LPS stimulation in any of the three groups, but overall neutrophil ROS levels were significantly higher in the angiotensin-II group versus both controls. These results indicate that after CLP, angiotensin-II does not increase basal monocytic ROS production, but does increase monocyte ROS in the presence of bacterial stimulus.
Angiotensin-II Attenuates CLP-Induced Metabolic Disturbance.
To interrogate whether benefits from angiotensin-II enhancement of immune function after CLP were offset by increased inflammatory injury, we measured markers of organ dysfunction and clinical status. Consistent with our previous studies (20), angiotensin-II treatment reduced kidney injury molecule-1 (KIM-1) levels compared to vehicle and losartan controls (SI Appendix, Fig. S6), indicating decreased renal injury after CLP. Additionally, no increased trafficking of neutrophils or monocytes to kidney with angiotensin-II treatment was observed (SI Appendix, Fig. S7). Angiotensin-II significantly attenuated CLP-induced hypernatremia and hypocalcemia (SI Appendix, Fig. S6). Echocardiography showed no reduction in left ventricular ejection fraction or fractional shortening with angiotensin-II versus vehicle treatment (SI Appendix, Fig. S6). Furthermore, when murine sepsis scores were determined by a blinded assessor, the angiotensin-II group appeared holistically less ill than vehicle and angiotensin-II plus losartan groups (SI Appendix, Fig. S6). Overall, these data indicate that increased innate immune function induced by angiotensin-II in CLP is not associated with greater organ dysfunction or injury.
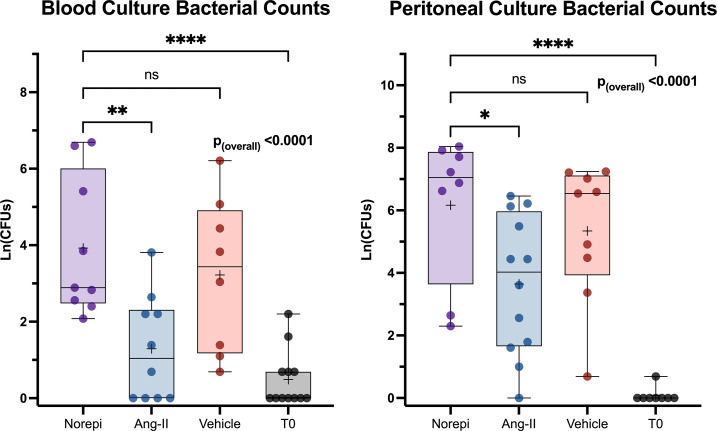
Peritoneal and Blood Bacterial Counts Post-CLP Are Higher with Norepinephrine than Angiotensin-II.
Previous studies indicate that norepinephrine, the vasopressor most often used to treat septic shock, is immunosuppressive (5). Therefore, we measured bacterial counts 48 h post-CLP in mice treated with intraperitoneally infused norepinephrine and compared the results to animals that received angiotensin-II. Angiotensin-II–treated mice displayed significantly lower bacterial counts in blood (Δmean: 2.6 log-CFUs, 95%CI: 0.9 to 4.3, P = 0.0014) and in peritoneal fluid (Δmean: 2.5 log-CFUs, 95%CI: 0.2 to 4.8, P = 0.0280) compared to norepinephrine-treated mice. Counts in the norepinephrine group were not significantly different from those for animals treated with vehicle alone (blood, Δmean: 0.7 log-CFUs, 95%CI: −1.1 to 2.5, P = 0.65; peritoneal fluid, Δmean: 0.8 log-CFUs, 95%CI: −1.6 to 3.3, P = 0.79) (Fig. 5).
Fig. 5.
Peritoneal and blood culture growth is decreased with angiotensin-II treatment but not norepinephrine treatment 48 h after CLP. Bacterial counts in treatment groups and T0 mice. The y axis displays bacterial counts on a natural log scale. Dots represent individual mice. Box height represents interquartile range, error bars represent range. Asterisks indicate the (multiple comparison adjusted) P values vs. the norepinephrine group at the indicated time point as follows: ns, P > 0.05; *P < 0.05; **P < 0.005; ****P < 0.0001. Norepi, Norepinephrine.
Discussion
Angiotensin-II treatment increased bacterial clearance and modulated the host immune response after CLP. This effect of angiotensin-II, not previously described in sepsis or CLP, was not seen in norepinephrine-treated mice. Pharmacologic AT1R antagonism reversed the effect of angiotensin-II in CLP, and selective AT1R deletion in myeloid-lineage leukocytes abrogated the effect of angiotensin-II on bacteremia burden in CLP, indicating that the observed angiotensin-II–mediated innate immune effects were dependent on AT1R. Angiotensin-II, through AT1R, augmented innate phagocytosis as well as monocytic ROS production after LPS-stimulation but did not increase organ injury.
Angiotensin-II is FDA-approved for the treatment of hypotension in catecholamine-refractory distributive shock and sepsis (21). When administered at clinically relevant doses to mice undergoing CLP, we found that angiotensin-II treatment was reproducibly associated with increased bacterial clearance from both the peritoneal cavity and the blood (Fig. 2). Selective knockout from myeloid-lineage cells of AT1R, the angiotensin-II type 1 receptor, abrogated the effect of angiotensin-II on bacteremia post-CLP, indicating that the effect of angiotensin-II depended on this receptor on myeloid cells (Fig. 3). The effect of angiotensin-II on bacterial burden was also abrogated by coadministering losartan, an AT1R blocker, further indicating that the effect was mediated through AT1R (Fig. 2). Angiotensin-II, again through AT1R, increased monocyte phagocytosis and ROS production but only in the presence of LPS: unstimulated monocyte phagocytosis and ROS levels were unaffected (Fig. 5). We did not observe increased innate cells trafficking to kidney, nor any increase in organ dysfunction. In fact, several physiologic measures were improved in this study, and we previously showed angiotensin-II markedly attenuates renal dysfunction after CLP via AT1R (20). Together, these results suggest angiotensin-II enhances myeloid bacterial clearance through AT1R by promoting phagocytosis and ROS production in the presence of a bacterial stimulus.
Our findings also indicate that angiotensin-II alters the systemic inflammatory response after CLP through AT1R signaling. Extensive literature documents the proinflammatory effects of angiotensin-II in chronic disease states, including renal hypertension and atherosclerosis (22). Similarly, AT1R blockade decreases inflammatory cytokines and organ injury in acute sterile inflammation (11, 23). However, our data indicate that describing angiotensin-II infusion in CLP as “proinflammatory” is overly simplistic. While angiotensin-II increased serum levels of several proinflammatory type 1 cytokines at 24 h post-CLP, angiotensin-II reduced TNF-α, IL-6, and IL-1β levels by 48 h post-CLP in an AT1R-dependent fashion (Fig. 1). These findings are consistent with prior studies that report AT1R deletion from kidney macrophages and CD4+ T lymphocytes increases rather than suppresses production of these and other inflammatory cytokines (19, 24, 25). A similar phenomenon was noted in Agtr1a−/− bone marrow chimeras (26). In bovine bacteremia, angiotensin-II–treated animals displayed lower levels of both IL-6 and IL-10 versus vehicle-treated controls (27). In our study, mice that received angiotensin-II alone displayed no elevations in IFN-γ, a cytokine that we and others have shown to be an important mediator of tissue autoinjury in the acute response to systemic inflammatory stimulus (28)(29). One interpretation of the serum cytokine levels is that angiotensin-II alters the kinetics of the inflammatory response to CLP, initially augmenting and subsequently limiting systemic inflammation, promoting initial bacterial defense, and limiting subsequent collateral organ dysfunction. Angiotensin-II’s effect to increase innate phagocytosis and ROS production specifically after LPS stimulation, and the absence of increased organ injury with angiotensin-II treatment support this notion. Given that high cytokine levels are maladaptive in the absence of a pathogen, such an effect would be teleologically beneficial. Alternatively, or in addition, the delayed decrement in cytokines could result from earlier augmentation of pathogen clearance and the associated reduction in bacterial stimuli. The latter interpretation is consistent with the observation that bacterial counts were lower at 48 h vs. 24 h post-CLP among angiotensin-II–treated mice but not among controls (SI Appendix, Fig. S3).
Angiotensin-II treatment altered the distribution of innate leukocytes in the peritoneal cavity. By 48 h post-CLP, angiotensin-II–treated mice displayed markedly higher numbers of neutrophils, macrophages, and Ly6CHi (inflammatory and antimicrobial) monocytes but not Ly6CLo (so called “patrolling”) monocytes or CD11c+ dendritic antigen-presenting cells. The increased inflammatory leukocyte abundance seems most likely to represent an epiphenomenon of the known chemotactic effects of angiotensin-II (10, 12), rather than a mechanism of bacterial clearance. Given that infusing angiotensin-II subcutaneously (i.e., outside the infected body compartment) delivered a similar change in peritoneal bacterial counts, a purely chemotactic effect is less likely than a systemically mediated one. Instead, as suggested by the effect on monocyte and neutrophil phagocytosis and ROS production, angiotensin-II appears to augment processes directly related to bacterial killing after CLP.
For the following reasons, differences in bacterial burden with angiotensin-II treatment are unlikely to result from the hormone’s cardiovascular effects. First, Myeloid-AT1a− mice lack myeloid AT1R responsiveness to angiotensin-II but still express AT1R on vascular cells, corroborated by the normal blood pressure response to angiotensin-II in these animals (19). Second, treatment with another vasopressor, norepinephrine, was not associated with enhanced bacterial clearance (Fig. 4). Third, coadministration of losartan with angiotensin-II in CLP does not induce ischemic injury in the kidneys (20), which are among the most physiologically sensitive organs to hypoperfusion. Yet, losartan was sufficient to reverse the effects of angiotensin-II on bacterial burden post-CLP (Fig. 4). Finally, we observed increased phagocytosis by LPS-stimulated innate cells that were treated with ex vivo angiotensin-II, which cannot influence systemic perfusion.
In contrast to our observation that angiotensin-II induced an AT1R-dependent increase in bacterial clearance, Khan et al. (17) identified an AT1R-independent neutrophil ACE-1–dependent enhancement in bacterial clearance. A similar effect is reported in myeloid-lineage monocytes (30). These findings, together with the present study, indicate that both systemic angiotensin-II and myeloid leukocyte-bound ACE-1 promote defense against pathogens and do so via distinct mechanisms.
An effect of angiotensin-II to promote pathogen clearance could have broad clinical implications. Early and effective source-control is a critical element of resuscitation for sepsis (31). We found angiotensin-II–treated mice had substantially lower blood and peritoneal bacterial burdens early after CLP than those treated with norepinephrine. Consistent with this observation, prior studies found that norepinephrine treatment increased bacterial dissemination in CLP and suppressed immune function in human sepsis (5, 6). Sepsis-induced immunosuppression is an increasingly recognized clinical entity with poor prognostic implications (3). More than one in five sepsis patients surviving to hospital discharge will be readmitted within 30 d (32). Nearly half of these admissions are for new or recurrent infection (33). Recent studies identified strongly up-regulated immunosuppressive monocyte expression programs in early clinical sepsis vs. nonseptic infection (34). Notably, these immunosuppressive monocytes are inducible ex vivo through IL-6 and IL-10 stimulation (35). We found that at 48 h post-CLP, these two cytokines were suppressed by angiotensin-II, raising the possibility that angiotensin-II–associated enhancement of bacterial clearance is mediated by modulation of the abundance of specific immunosuppressive and immunopotentiating immune cell subsets.
The efficacy of norepinephrine as a vasopressor has long driven its use to treat fluid-refractory hypotension in sepsis. For this reason, current clinical guidelines continue to recommend norepinephrine as the first-line vasopressor in septic shock (36). However, in a prespecified analysis of the ATHOS-III trial (37), a large subset of patients—identified by elevated plasma renin levels at enrollment—demonstrated both a robust hemodynamic response to angiotensin-II vasopressor therapy and increased survival (38). Post hoc analyses of the trial found that angiotensin-II treatment decreased mortality both in patients with ACE-1 dysfunction and in patients with acute renal failure at enrollment (39, 40). Our study identifies immunologic effects that could also contribute to advantages of angiotensin-II over norepinephrine in appropriately selected patients with septic shock.
Our study has important limitations. First, we studied only the first 48 h post-CLP. While clinically identified immunosuppressive monocyte programs up-regulate early in disease course (34), in sepsis, immunosuppression develops over time (3). Although CLP has well-documented limitations as a model of clinical sepsis (41, 42), it also has key advantages over many other sepsis models used in rodents and more reliably recapitulates several aspects of human disease (43–45). Extrapolating rodent immunology to human disease has limitations (41), as laboratory rodents possess perfunctory T cell repertoires compared to feral mice (42). An additional limitation is that treatment began at the end of surgery, whereas clinical treatment is often delayed of several hours from the onset of infection. Furthermore, while administering antibiotics would have increased clinical relevance, it would have substantially interfered with bacterial culture growth (43), a critical experimental measurement. Additionally, while we characterize the reduction in bacterial counts with angiotensin-II treatment after CLP as enhanced clearance, we did not measure pre-CLP bacterial levels. While we cannot conclusively exclude pretreatment differences in bacterial burden in blood or peritoneum, both compartments are sterile under physiologic conditions; explanations for reduced bacterial counts that do not invoke clearance are therefore far less likely.
Additional laboratory investigations into the specific molecular pathways that mediate angiotensin-II’s effect to promote phagocytosis and bacterial clearance after CLP would be welcomed. Furthermore, before the present results may be translated to the bedside, clinical studies should interrogate the effect of angiotensin-II on immune function in patients with sepsis. Specifically, embedding these inquiries within a clinical trial testing the effect of angiotensin-II treatment in septic shock on patient-centered outcomes, such as shock resolution, recovery of organ function, intensive care unit length-of-stay, and mortality would yield the highest impact for biological understanding and patient care.
Conclusion.
Angiotensin-II treatment augmented the systemic immune response and enhanced systemic and local bacterial clearance after CLP in an AT1R-dependent manner. In the blood compartment, this effect was mediated through the myeloid-specific AT1R. Given the high mortality and morbidity of septic shock, its dominant treatment with norepinephrine for hemodynamic support, and the observation that angiotensin-II treatment increased bacterial clearance compared to norepinephrine treatment, these findings suggest the immunomodulatory properties of angiotensin-II warrant further exploration in clinical sepsis.
Methods
Ethics Statement Regarding Animal and Human Experiments.
All animal studies were approved by the Institutional Animal Care and Use Committee and adhered to NIH guidelines as well as “Animal research: Reporting in vivo experiments: The ARRIVE guidelines” criteria (44).
Study Design.
This was an animal study conducted in four randomized cohorts. Each set of cohort experiments was performed three times. In the first descriptive cohort, untreated mice were a priori randomized to a time of killing—0, 24, 48, or 72 h—before undergoing CLP. In a second interventional cohort, mice undergoing CLP were randomized to treatment at the time of CLP to explore the effects of pharmacologic RAS modulation on immune function. In a third independent laboratory cohort, mice that selectively do not express AT1R on myeloid-derived cells and littermate controls were randomized to angiotensin-II or nontreatment to confirm results from the above experiments and to interrogate the cell-specific mechanisms. Finally, a fourth interventional cohort randomized mice undergoing CLP to a vasopressor therapy to compare the effects of angiotensin-II vs. norepinephrine.
No prior sample-size calculations were performed. Sample size was determined to be adequate based on the degree and consistency of differences between groups. The number of biological replicates are indicated in each figure. All ELISAs report each data point as the average of three technical replicates. Individual mice (the biological replicates) were the unit of analysis throughout. Investigators were blinded during experiments, as described below.
Mice.
Male C57BL/6 mice aged 12 to 14 wk (Jackson Labs) were maintained in a conventional, light-cycled facility. To obtain mice that lack AT1R exclusively on myeloid cells, we used an established breeding strategy to generate myeloid-specific deletion of AT1a (19). Briefly, first-step breeding crossed LysM-Cre(+) with AT1afl/fl mice to generate LysM-Cre(+)/AT1afl/wt progeny. Second-step breeding crossed first-step progeny with AT1afl/fl mice [LysM-Cre(+)/AT1afl/wt × AT1afl/fl] to generate LysM-Cre(+)/AT1afl/fl progeny. The final, experimental breeding step crossed second-step progeny with AT1afl/fl [LysM-Cre(+)/AT1afl/fl × AT1afl/fl]. All final-step progeny therefore express the floxed AT1a gene and are either LysM-Cre(−) or LysM-Cre(+). This strategy ensures all LysM-Cre(+) progeny are heterozygous in the LysM allele, which was verified by genotyping, in order to avoid a homozygous deficiency in the lysozyme M gene, which could otherwise affect bacterial clearance (45).
CLP.
CLP surgeries were performed by an author, blinded to treatment allocation, as previously described (20, 46). Briefly, mice underwent laparotomy under isoflurane. The cecum was ligated, double-punctured with a 22-gauge needle, and returned to the peritoneum. Mice were allowed to recover and resuscitated with subcutaneous 0.9% saline (50 mL/kg) immediately following surgery and again at 24 h postintervention. Experiments at both the study sites adhered to a common CLP protocol.
In the descriptive cohort (Laboratory 1), all mice underwent CLP without treatment and were killed at the prespecified time (0, 24, 48, or 72 h postsurgery). Two interventional cohorts (Laboratory 1) randomized mice to treatment as below. In the independent laboratory cohort (Laboratory 2), Myeloid-AT1a- and littermate control mice were simultaneously randomized to CLP with or without angiotensin-II treatment and were killed 24 h postsurgery.
Study Drugs.
To determine whether angiotensin-II altered the immune response to CLP, we allocated mice to four treatments: angiotensin-II given intraperitoneally, angiotensin-II intraperitoneally + losartan (a selective AT1R antagonist), angiotensin-II given subcutaneously, or vehicle. Synthetic angiotensin-II (La Jolla Pharmaceuticals) was diluted in 0.9% saline and infused continuously (10 ng/kg/min at 1 µL/h) (20) using intraperitoneal micro-osmotic pumps (Alzet 1003D, ALZET Osmotic Pumps). Infusion pumps were implanted immediately prior to fascial closure. Control mice had vehicle-infusing pumps implanted. Pumps were primed for 6 h to ensure a constant infusion rate beginning at the time of implantation. We administered losartan (15 mg/kg subcutaneously) at the time of surgery and again 24 h later. We additionally included a group of mice who received subcutaneous angiotensin-II to help determine if the effects of angiotensin-II were mediated by locally induced chemotaxis or systemic effects. Unoperated mice serviced as additional controls.
For experiments in AT1a Myeloid-AT1a+ and Myeloid-AT1a− mice, angiotensin-II (Sigma Aldrich) was diluted in 0.9% saline and infused continuously (300 ng/kg/min at 1 µL/h) (47) using intraperitoneal micro-osmotic pumps (Alzet 1003D, ALZET Osmotic Pumps) implanted as above.
To determine if the effects of angiotensin-II on the immune response to CLP differed from that of other clinically relevant vasopressors, we allocated mice to either norepinephrine intraperitoneally (Sigma Aldrich, 0.1 μg/kg/min at 1 µL/h) vs. angiotensin-II or vehicle as above.
Post-CLP and Terminal Point Procedures.
Blinding of treatment allocation was maintained for post-CLP procedures. After killing, the peritoneum was washed with 5 mL of sterile PBS. The lavage was collected in a sterile receptacle. After vortexing, 20 µL of lavage was diluted to 1:10,000, plated onto solid media, and incubated overnight at 37 °C. The remaining lavage was processed and stained for flow cytometry. After incubation, bacterial colonies on each plate were counted using plate-counting software (OpenCFU) with standardized sensitivity settings.
Spleens were collected, immediately weighed, and subjected to digestion with DNase (100 µg/mL) and Collagenase A (1 mg/mL) in complete media for 30 min at 37 °C. Cells were resuspended following filtration through a 70-µm filter. Red blood cells were lysed. Spleen and peritoneal cells were then counted using a Countess-II Automated Cell Counter (ThermoFisher).
Blood was obtained by cardiac puncture at the time of killing. Twenty microliters of blood were diluted and cultured as above. The rest was spun for serum and frozen.
Flow Cytometry.
After single-cell suspensions were obtained, cells were stained without permeabilization for flow cytometry. We used LIVE/DEAD fixable viability dye (Life Technologies) and the fluorophore-conjugated antibodies as described in detail in SI Appendix, Table S1. Flow cytometric data were obtained from a BD LSR Fortessa 16-color cell analyzer and analyzed using FlowJo v10 (BD Bioscience). Gating strategies are listed in figure captions. We illustrate gating strategies in SI Appendix, Figs. S8 and S9.
Phagocytosis and ROS Production.
Splenic cells were homogenized into a single-cell suspension and incubated for 3 h in complete media with or without LPS, along with fluorescently labeled IgG conjugated latex beads to assess for phagocytosis. Following incubation, cells were labels for flow cytometry and percent of cells that had phagocytosed-labeled beads as indicated by fluorescence were assessed. In a separate assay, ROS levels were assessed as above with CellRox Green Reagent (5 μM; ThermoFisher) added 30 min prior to staining.
Ex Vivo Antibacterial Activity of Angiotensin-II.
We assessed whether angiotensin-II had ex vivo antibacterial activity using Kirby–Bauer disk diffusion methods. Angiotensin-II was dissolved in normal saline at either 0.1 µg/mL, 1.0 µg/mL, or 2.5 µg/mL. Control solutions were ampicillin at 0.8 µg/mL and imipenem at 0.4 µg/mL. Filter-paper disks were then impregnated with 25 µL of solution. Culture plates were then flooded with 2 mL of cecal aspirate, obtained from an untreated mouse, 24 h post-CLP. Impregnated disks were then added to plates, allowed to sit at room temperature for 2 h, and incubated overnight at 37 °C.
Cytokine Analysis.
Serum cytokine levels were measured by ELISA (Eve Technologies). Targets of interest included TNF-α, IL-6, IL-1β, IFN-γ, IL-10, IP-10, MIP-1α, MIP-1β.
Organ Dysfunction.
To assess renal injury, we measured plasma KIM-1 levels by ELISA (Eve Technologies). Cardiac function was interrogated with echocardiography, performed (Vevo 3100, Fujifilm Visual Sonics) by a single, blinded, experienced cardiologist according to a standardized image acquisition protocol. Images were obtained under isoflurane titrated to target a heart rate of 400 beats per minute to standardize anesthetic effects on cardiac function during imaging. Standard two-dimensional (B-mode, M-mode), Doppler and pulsed-tissue Doppler studies were performed. The B-mode parasternal long axis view was used to measure end-diastolic volume, end-systolic volume, and ejection fraction. The M-mode parasternal short-axis view was used to measure fractional shortening. Blood sodium and ionized calcium levels were measured from whole blood immediately upon collection using iStat Chem8+ cartridges, a point-of-care measurement device (Abbott Point of Care Inc.) that has excellent correlation (R2 = 0.99) with clinical core laboratory values (48). Additionally, blinded assessors recorded murine sepsis scores, which assign scores from 0 to 4 on the basis of appearance, behavior, and respiration. This score was specifically developed and validated in C57BL/6 sepsis models, including CLP: it shows high internal consistency, interrater reliability, and predictive accuracy for organ dysfunction and mortality (49, 50).
Statistical Analysis.
Individual mice were the unit of analysis throughout. Data distributions were assessed prior to statistical hypothesis testing and an appropriate transformation was applied if found to violate assumptions. Bacterial counts were natural log-transformed for analysis. Group differences over multiple time points were identified with generalized linear models. For analysis comparing different treatment groups over multiple time points, models included terms for treatment, time, and their interaction effect. Time was treated continuously or categorically based on the observed data distribution. Figures indicate the method used to correct for multiple comparisons. Analyses were conducted in SAS: University Edition (SAS Institute) and figures were produced with Prism v9 (GraphPad).
Supplementary Material
Acknowledgments
This work was supported by NIH Grants K08 GM132689 (to J.R.P.), R01 DK118019 (to S.D.C.), R01 AI153142 (to M.B.G.), R01 GM121102 (to C.S.D.), and K08 GM132794 (to M.D.T.); and US Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Grant BX000893 (to S.D.C.).
Footnotes
Competing interest statement: Synthetic angiotensin-II was generously provided by La Jolla Pharmaceutical Company. La Jolla Pharmaceutical Company had no access to any of the data in this study, was not involved in any part of its analysis or interpretation, and had no role in the drafting or editing of this manuscript. Additionally, C.S.D. is a Scientific Editor of Critical Care Medicine (yearly honorarium) for the Society of Critical Care Medicine; is a consultant for studies on sepsis (stock options) with Enlivex, Jerusalem, Israel; is a paid member of Data and Safety Monitoring Board, ICON Clinical Research LLC; is a paid consultant on sepsis research with Pfizer; and receives royalties for Evidence-Based Critical Care from Elsevier.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2211370119/-/DCSupplemental.
Data, Materials, and Software Availability
All study data are included in the main text and SI Appendix.
References
- 1.Rudd K. E., et al. , Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 395, 200–211 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M., et al. , The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315, 801–810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotchkiss R. S., Monneret G., Payen D., Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 13, 862–874 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boomer J. S., et al. , Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306, 2594–2605 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stolk R. F., et al. , Norepinephrine dysregulates the immune response and compromises host defense during sepsis. Am. J. Respir. Crit. Care Med. 202, 830–842 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Stolk R. F., et al. , Potentially inadvertent immunomodulation: Norepinephrine use in sepsis. Am. J. Respir. Crit. Care Med. 194, 550–558 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Levy M. M., Evans L. E., Rhodes A., The surviving sepsis campaign bundle: 2018 update. Crit. Care Med. 46, 997–1000 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Rhodes A., et al. , Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Crit. Care Med. 45, 486–552 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Chappell M. C., Nonclassical renin-angiotensin system and renal function. Compr. Physiol. 2, 2733–2752 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nabah Y. N., et al. , Angiotensin II induces neutrophil accumulation in vivo through generation and release of CXC chemokines. Circulation 110, 3581–3586 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Miyoshi M., et al. , ANG II is involved in the LPS-induced production of proinflammatory cytokines in dehydrated rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R1092–R1097 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Mateo T., et al. , Angiotensin II-induced mononuclear leukocyte interactions with arteriolar and venular endothelium are mediated by the release of different CC chemokines. J. Immunol. 176, 5577–5586 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Rasini E., et al. , Angiotensin II type 1 receptor expression on human leukocyte subsets: A flow cytometric and RT-PCR study. Regul. Pept. 134, 69–74 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Owen C. A., Campbell E. J., Angiotensin II generation at the cell surface of activated neutrophils: Novel cathepsin G-mediated catalytic activity that is resistant to inhibition. J. Immunol. 160, 1436–1443 (1998). [PubMed] [Google Scholar]
- 15.Jurewicz M., et al. , Human T and natural killer cells possess a functional renin-angiotensin system: Further mechanisms of angiotensin II-induced inflammation. J. Am. Soc. Nephrol. 18, 1093–1102 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Bernstein K. E., et al. , Angiotensin-converting enzyme in innate and adaptive immunity. Nat. Rev. Nephrol. 14, 325–336 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan Z., et al. Angiotensin-converting enzyme enhances the oxidative response and bactericidal activity of neutrophils. Blood 130, 328–339 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joffre J., et al. Catecholaminergic vasopressors reduce Toll-like receptor agonist-induced microvascular endothelial cell permeability but not cytokine production. Crit. Care Med. 49, e315–e326 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J. D., et al. , Type 1 angiotensin receptors on macrophages ameliorate IL-1 receptor-mediated kidney fibrosis. J. Clin. Invest. 124, 2198–2203 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leisman D. E., et al. , Impaired angiotensin II type 1 receptor signaling contributes to sepsis induced acute kidney injury. Kidney Int. 99, 148–160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senatore F., et al. , FDA approval of angiotensin II for the treatment of hypotension in adults with distributive shock. Am. J. Cardiovasc. Drugs 19, 11–20 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Simões e Silva A. C., Silveira K. D., Ferreira A. J., Teixeira M. M., ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br. J. Pharmacol. 169, 477–492 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagiwara S., et al. , Antagonist of the type-1 ANG II receptor prevents against LPS-induced septic shock in rats. Intensive Care Med. 35, 1471–1478 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Zhang J. D., et al. , A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circ. Res. 110, 1604–1617 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J., et al. Competing actions of type 1 angiotensin II receptors expressed on T lymphocytes and kidney epithelium during cisplatin-induced AKI. J. Am. Soc. Nephrol. 27, 2257–2264 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowley S. D., et al. , A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension 55, 99–108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lankadeva Y. R., Kosaka J., Evans R. G., Bellomo R., May C. N., Urinary oxygenation as a surrogate measure of medullary oxygenation during angiotensin II therapy in septic acute kidney injury. Crit. Care Med. 46, e41–e48 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Taylor M. D., Fernandes T. D., Kelly A. P., Abraham M. N., Deutschman C. S., CD4 and CD8 T cell memory interactions alter innate immunity and organ injury in the CLP sepsis model. Front. Immunol. 11, 563402 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor M. D.et al., . T cell activation and IFNγ modulate organ dysfunction in LPS-mediated inflammation. J. Leukoc. Biol. 112, 221–232 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okwan-Duodu D., et al. , Angiotensin-converting enzyme overexpression in mouse myelomonocytic cells augments resistance to Listeria and methicillin-resistant Staphylococcus aureus. J. Biol. Chem. 285, 39051–39060 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivers E., et al. ; Early Goal-Directed Therapy Collaborative Group, Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 345, 1368–1377 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Meyer N., et al. , Temporal trends in incidence, sepsis-related mortality, and hospital-based acute care after sepsis. Crit. Care Med. 46, 354–360 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeMerle K. M., Royer S. C., Mikkelsen M. E., Prescott H. C., Readmissions for recurrent sepsis: New or relapsed infection? Crit. Care Med. 45, 1702–1708 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reyes M., et al. An immune-cell signature of bacterial sepsis. Nat. Med. 26, 333–340 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reyes M., et al. Plasma from patients with bacterial sepsis or severe COVID-19 induces suppressive myeloid cell production from hematopoietic progenitors in vitro. Sci. Transl. Med. 13, eabe9599 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans L., et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit. Care Med. 49, e1063–e1143 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Khanna A., et al. Angiotensin II for the treatment of vasodilatory shock. New Engl. J. Med. 377, 419–430 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Bellomo R., et al. Renin and survival in patients given angiotensin II for catecholamine-resistant vasodilatory shock. A clinical trial. Am. J. Respir. Crit. Care Med. 202, 1253–1261 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellomo R., et al. Angiotensin I and angiotensin II concentrations and their ratio in catecholamine-resistant vasodilatory shock. Crit. Care 24, 43 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tumlin J. A., et al. Outcomes in patients with vasodilatory shock and renal replacement therapy treated with intravenous angiotensin II. Crit. Care Med. 46, 949–957 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osuchowski M. F., et al. Minimum quality threshold in pre-clinical sepsis studies (MQTiPSS): An international expert consensus initiative for improvement of animal modeling in sepsis. Shock 50, 377–380 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abolins S., et al. The comparative immunology of wild and laboratory mice, Mus musculus domesticus. Nat. Commun. 8, 14811 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng MP, et al. Blood culture results before and after antimicrobial administration in patients with severe manifestations of sepsis: A diagnostic study. Ann. Intern. Med. 171, 547–554 (2019). [DOI] [PubMed] [Google Scholar]
- 44.NC3Rs Reporting Guidelines Working Group, Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Exp. Physiol. 95, 842–844 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Gong K. Q., Frevert C., Manicone A. M., Deletion of LysM in LysMCre recombinase homozygous mice is non-contributory in LPS-induced acute lung injury. Lung 197, 819–823 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abraham M. N., Jimenez D. M., Fernandes T. D., Deutschman C. S., Cecal ligation and puncture alters glucocorticoid receptor expression. Crit. Care Med. 46, e797–e804 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Lu X., Rudemiller N. P., Wen Y.et al. A20 in myeloid cells protects against hypertension by inhibiting dendritic cell-mediated T-cell activation. Circ. Res. 125, 1055–1066 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gbinigie O., Price C. P., Heneghan C., Van den Bruel A., Plüddemann A., Creatinine point-of-care testing for detection and monitoring of chronic kidney disease: Primary care diagnostic technology update. Br. J. Gen. Pract. 65, 608–609 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shrum B., et al. , A robust scoring system to evaluate sepsis severity in an animal model. BMC Res. Notes 7, 233 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mai S. H. C., et al. , Body temperature and mouse scoring systems as surrogate markers of death in cecal ligation and puncture sepsis. Intensive Care Med. Exp. 6, 20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the main text and SI Appendix.