Significance
Polymer upcycling converts plastic waste to high-value chemicals, representing a highly attractive approach to addressing the urgent environmental crisis. Existing upcycling methods are mediated by sophisticated catalysts and challenged by the low selectivity and economic value of the products, undercutting their practicality. This report reveals a highly efficient and resilient approach of degradation-upcycling (Deg-Up) to upcycling plastic wastes, by utilizing judiciously selected cascade reactions rather than complicated, sophisticated, and expensive catalysts. We find that the degradation-upcycling approach effectively upcycles polystyrene to high-value products, including diphenylmethane, benzophenone, 1,2-diphenylethane, or 4-oxo-4-phenyl-butyric acid. Techno-economic analysis shows high profitability and minor sensitivity to market fluctuation.
Keywords: polymer waste, sustainability, recycling, upcycling, cascade
Abstract
Plastic waste represents one of the most urgent environmental challenges facing humankind. Upcycling has been proposed to solve the low profitability and high market sensitivity of known recycling methods. Existing upcycling methods operate under energy-intense conditions and use precious-metal catalysts, but produce low-value oligomers, monomers, and common aromatics. Herein, we report a tandem degradation-upcycling strategy to exploit high-value chemicals from polystyrene (PS) waste with high selectivity. We first degrade PS waste to aromatics using ultraviolet (UV) light and then valorize the intermediate to diphenylmethane. Low-cost AlCl3 catalyzes both the reactions of degradation and upcycling at ambient temperatures under atmospheric pressure. The degraded intermediates can advantageously serve as solvents for processing the solid plastic wastes, forming a self-sustainable circuitry. The low-value-input and high-value-output approach is thus substantially more sustainable and economically viable than conventional thermal processes, which operate at high-temperature, high-pressure conditions and use precious-metal catalysts, but produce low-value oligomers, monomers, and common aromatics. The cascade strategy is resilient to impurities from plastic waste streams and is generalizable to other high-value chemicals (e.g., benzophenone, 1,2-diphenylethane, and 4-phenyl-4-oxo butyric acid). The upcycling to diphenylmethane was tested at 1-kg laboratory scale and attested by industrial-scale techno-economic analysis, demonstrating sustainability and economic viability without government subsidies or tax credits.
Plastic annual production has grown from <2 million tons in the 1950s to ∼367 million tons in 2020 (1), and it is predicted to double in the coming decades (2). About 40% of the plastics are for short-term use and quickly turn into waste (3). Based on the second law of thermodynamics, the plastic wastes, once deposited to the natural environment, will eventually reach every corner of the planet, threatening the inhabitants of the earth (4–8). To mitigate the plastic-waste crisis, a closed-loop plastic cycle is brewing with the efforts of major economies (9, 10). On the one hand, researchers have designed degradation-friendly polymers (11–15), but the market insertion of new polymers is a long-drawn process due to investment risks (16). On the other hand, substantial efforts are devoted to recycling legacy plastics. Although plastic quality irreversibly deteriorates with times of reuse (17), mechanical recycling occupies ∼88% of the recycling market (18). Alternatively, chemical recycling (∼6.5% of the market), such as depolymerization and repolymerization, complements mechanical recycling and retains the plastic quality (7, 18, 19). Nevertheless, both methods have uncertain profitability (16, 20) and are sensitive to market fluctuation (21, 22), thus often requiring help from government subsidies or tax returns. High profitability and low market sensitivity are urgently demanded to increase the incentive for plastic recycling, especially for “end-of-life” plastics.
Selective conversion of end-of-life plastic wastes (e.g., tertiary/quaternary plastic wastes) into value-added chemicals, or chemical upcycling, has the potential to bring plastic recycling out of the “low-end” dilemma. Great progress has been made for commodity polymers, such as polyethylene (PE) (23–25), polyethylene terephthalate (PET) (13, 26–28), polypropylene (PP) (29, 30), and polyesters (26, 27, 31), which have been successfully converted to monomers or aromatics through catalytic pyrolysis or enzymatic degradation. However, for practical deployment in the industry, these upcycling methods will be more attractive if the products have high economic value. In addition, the products are often a complex mixture, and their economic value fluctuates around the wholesale price of virgin plastics (32). Obtaining upcycling products with high economic values and high selectivity, preferably at mild reaction conditions to expand the profit margin, is thus a target of plastic chemical upcycling (7, 33). Tandem catalytic strategies have been recently adopted for PE to improve the product selectivity, especially toward alkyl aromatics (23, 25, 34). Despite the improved selectivity, the products are still a mixture of various alkyl aromatics, whose values are not significantly higher than the plastic wastes to make the process economically viable.
As an alternative to the development of sophisticated catalysts and innovative polymers, the selective conversion of plastics to high-value chemicals is achievable by engineering the pathways using common industrial catalysts, reagents, and reactions. Herein, we propose a tandem reaction strategy of degradation-upcycling (Deg-Up) to chemically upcycle end-of-life plastic wastes. Our strategy is to judiciously select two or more cascade reactions that can precisely regulate reaction pathways to produce desired products with much higher economic values, while inhibiting unwanted products, leading to potential profitable industrial implementation.
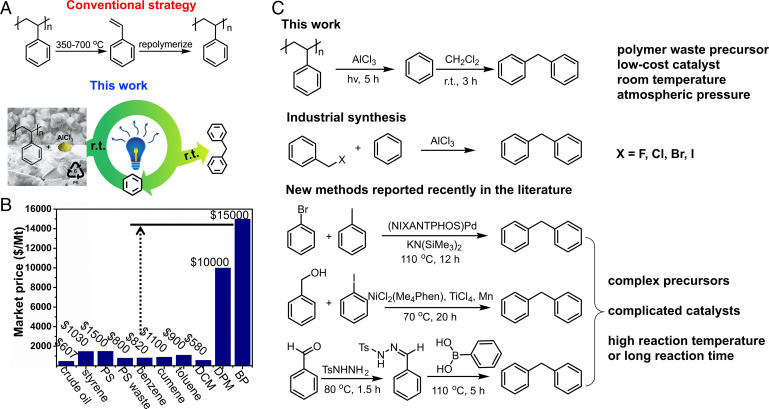
To demonstrate the feasibility of the Deg-Up strategy, we have custom-tailored an upcycling procedure for polystyrene (PS). PS was selected because it is rich in aromatics (74 wt.%), and it is widely used for utensils, packaging, and insulation. Yet, <10% of PS annual production is recycled, and no catalytic upcycling methods have been employed to harvest the valuable aromatic (16, 19, 21, 22, 35–38). Upcycling PS and polyolefins, in general, is challenging due to the lack of backbone heteroatoms as in PET and polyesters, which are susceptible to breakage and thus degrade relatively easily. Additionally, PS and especially expanded PS foam suffer from a high volume/mass ratio, increasing the collection, transportation, and storage costs and further compressing the already-narrow profit margin (17). In our Deg-Up procedure, PS waste was first photochemically degraded to aromatics over AlCl3, followed by a reaction with dichloromethane (DCM) to produce high-value diphenylmethane (DPM), which has a market price over 10 times that of commodity aromatics to compensate for the high costs of PS collection and transportation (Fig. 1 A and B). DCM is considered the least toxic simple chlorohydrocarbon and is used in the industries of food, pharmaceuticals, paint, metal cleaning, and others. According to the US Environmental Protection Agency report “Risk Evaluation for Methylene Chloride” [Chemical Abstracts Service Registry No. 75-09-2, 2020 (39)], DCM has no unreasonable risk for use in chemical production. The product DPM is an important high-value compound in the fragrance and medicine industries (SI Appendix, Fig. S1) and is usually made by a Friedel–Crafts reaction of benzene and halomethyl benzene in industry or via cross-coupling and benzylation in the laboratory (40–42). Compared to these methods that require specific precursors (e.g., aromatics with certain functional groups), expensive catalysts, and often elevated temperatures (Fig. 1C), our approach uses AlCl3 as the catalyst and polymer wastes as a low-cost feedstock. The approach can be easily inserted into the existing DPM industry by simply replacing expensive benzyl chloride (∼$2,300 per ton) with low-cost PS waste (average ∼$800 per ton) and DCM. The reaction conditions are mild, proceeding at ambient temperature and atmospheric pressure. The mild reaction conditions reduce energy consumption, remove the need for heat management, and, importantly, avoid coking and mitigate polyaromatic formation commonly seen in high-temperature processes. The Deg-Up method has been scaled up to 1 kg in the laboratory. Upon further scale-up to 100 metric tons, techno-economic analysis (TEA) shows high profitability and little sensitivity to market fluctuation, such as the PS waste cost.
Fig. 1.
Upcycling PS waste to DPM via UV-assisted degradation. (A) Comparison of the conventional depolymerization–repolymerization approach to our tandem Deg-Up strategy. Conventionally, PS is depolymerized at high temperatures to styrene, which is repolymerized to PS. Due to impurities in the recycled styrene, the repolymerized PS has inferior properties and reduced value. In our Deg-Up strategy, PS is degraded photochemically to yield mostly benzene and then converted to high-value DPM at room temperature. AlCl3 catalyzes both degradation and upcycling reactions and thus enables a one-pot process. Excess benzene is recycled as solvent to create a self-sustaining upcycling circuitry. (B) Market values of styrene, PS, and PS waste, common aromatic products from PS degradation, and upcycled products of DPM and benzophenone (BP), as of March 2021. Crude oil is included because it is the upstream feedstock to produce PS and many aromatics. DCM is a low-cost additive for PS upcycling. (C) Comparison of our DPM synthesis strategy with those used in industry and literature.
Results
In a proof-of-concept experiment, PS (∼1.0 g, Mw = 192 kDa, Ð = 2.15) was dissolved in benzene (∼10 mL) and combined with AlCl3 (∼1.0 g) in a three-neck quartz flask equipped with a stir bar, glass dashpot, gas inlet, and a stopper. Initially, the color of the solution in the reactor appeared light yellow. After degassing with argon (Ar), the solution was placed under an ultraviolet (UV) lamp (253.7 nm, 12.5 W⋅cm−2, ambient temperature ∼37 °C), and the color started turning into orange and then dark brown (SI Appendix, Fig. S2). The color change was likely induced by the coordination of AlCl3 with aromatics to form an aromatic/Al ionic liquid (43–45).
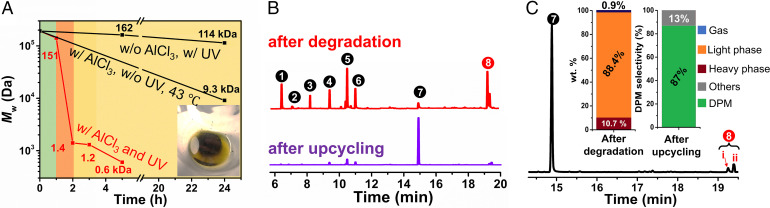
The PS degradation had an initiation stage of ∼1 h, in which the PS molecular mass decreased only slightly, to ∼151 kDa (Fig. 2A). This slow initiation is attributed to the incubation of the aromatic/Al halide complex (43, 44), as shown by the color change in the first hour. According to size-exclusion chromatography (SEC; Fig. 2A and SI Appendix, Fig. S3A), the PS underwent a sharp decrease in molecular mass from 151 kDa (degree of polymerization [DP] ∼1,500) to 1.4 kDa (DP ∼14) in the second hour. In the subsequent 3 h, the PS oligomers continued degrading into smaller molecules. Liquid chromatography (LC)-mass spectroscopy (MS) further supported the SEC results (SI Appendix, Fig. S4). All the eluents detected by time-of-flight (TOF) and UV detectors showed molecular masses <1,000 Da. In a control experiment using UV light in the absence of AlCl3 (Fig. 2A and SI Appendix, Fig. S3B, w/o AlCl3), PS was barely degraded after 24 h, showing Mw slightly lower than the virgin PS. In another control experiment using AlCl3, but no UV light (Fig. 2A and SI Appendix, Fig. S3B, w/o UV), even at a higher temperature of 43 °C, the PS was only partially degraded, even after 24 h of reaction. With both UV light and AlCl3, PS finished most of the degradation within 5 h.
Fig. 2.
Characterization and analysis of products from PS Deg-Up. (A) Mw time-evolution of PS upon degradation under various conditions. (A, Inset) A photograph of waste PS foam after Deg-Up. (B) GC traces of the liquid phase after waste PS foam degradation and upcycling in benzene. The solvent peak is omitted to draw focus to signals of the products. (C) Magnified view to illustrate the primary upcycled product DPM (7). C, Inset reports the measured weight fractions of the products and the reaction selectivity toward DPM. Mass spectra of the products (1 through 8) and their isomers are shown in SI Appendix, Fig. S7.
After degradation, the mixture in the reactor separated into light (88.4 wt.%) and heavy phases (10.7 wt.%). The light phase was primarily benzene and some by-products, including ethyl benzene (1), cumene (2), tert-butylbenzene (3), indane (4), 1-methylindane (5), 1-methylindene (6), DPM (7), phenylindene, and methyl phenylindene (8) (Fig. 2B). Indanes, indenes, and their derivatives were produced by PS backbiting reactions during degradation (44). Although DPM was produced upon PS degradation, its yield was low. The remaining heavy phase was a mixture of inorganic salts and organics, such as indanes, indenes, and heavy aromatics.
In the upcycling reaction, the mixture was injected with DCM (5.0 mL). Assisted by the readily available AlCl3, the phenyl groups were swiftly upgraded to DPM with a high selectivity (87%), as evidenced by the substantially stronger gas chromatography (GC) peak of DPM than any by-products (Fig. 2 B and C). Side products were evaluated, and they were minor (SI Appendix, Fig. S5). The conversion of DPM could be controlled by the upcycling reaction time (SI Appendix, Fig. S6). For example, after 3 h of upcycling, 658 mg of DPM was harvested, accounting for 97% of the phenyl groups recycled from PS waste, based on the evaluated benzene yield. Balancing the extracted and reacted benzene in the Deg-Up reactions enabled circulated utilization of benzene for processing additional PS, forming an upcycling circuitry of high self-sustainability.
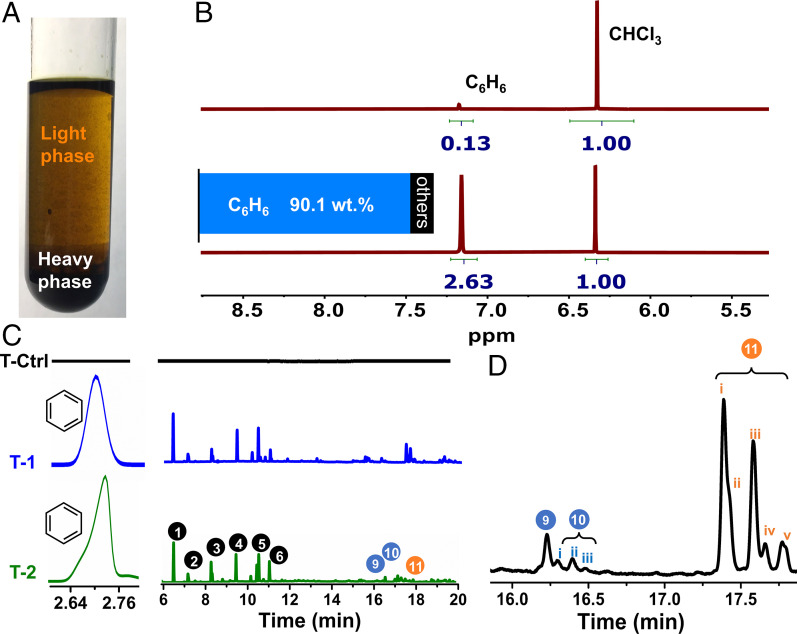
To determine the yield of benzene in PS degradation, isotope experiments were conducted in C6D6. The 1H-NMR revealed a benzene yield of 90.1 wt.% (or 89.0 mol.%) of the total aromatic carbon in PS (Fig. 3), indicating that PS underwent a dephenylation process. This result was further confirmed by a parallel PS degradation experiment in C6H6 using an external reference of toluene. The amount of increased benzene after degradation was determined by GC, showing a similar benzene yield of 91.9 wt.% (or 90.7 mol.%).
Fig. 3.
Characterization of benzene intermediate after PS degradation. (A) A photograph of the light and heavy phases after PS degradation. (B) The 1H-NMR spectra in C6D6 before and after PS degradation. (B, Inset) Benzene yield by the mass of phenyl group in PS. (C) GC traces of the light phase after PS degradation in toluene (T-Ctrl, blank experiment in toluene without PS, but with AlCl3 under UV; T-1, degradation of PS in toluene using AlCl3 under UV; T-2, degradation of waste PS foam in toluene using AlCl3 under UV). (D) Magnified view of the GC trace to distinguish isomers. Mass spectra of the products (1 through 11) and their isomers are in SI Appendix, Fig. S7.
To further investigate the degradation process of PS, we conducted experiments under identical conditions, but switching the solvent from benzene to toluene. Toluene served as a low-cost tracer molecule thanks to the extra methyl group relative to benzene, allowing for convenient tracing of the solvent during the degradation. Similar to the PS degradation in benzene, the degradation in toluene phase-separated into a light phase and a heavy phase (Fig. 3A). GC-MS (Fig. 3 C and D) revealed toluene and a primary product of benzene in the light phase. Other by-products resembled those in the benzene solvent system. Interestingly, GC-MS also detected benzyltoluenes (10) and ditolylmethanes (11), and both had a series of isomers (Fig. 3D), suggesting side reactions of the solvent during the PS degradation. There are two possible side reactions. First, toluene likely reacted with the backbone of PS and its oligomers to produce benzyltoluenes, which explained the formation of DPM during PS degradation in the benzene system. The reaction of solvent with polymer was further confirmed by the decreasing intensity of benzyltoluenes in a series of controlled experiments with increasing volumes of toluene (SI Appendix, Fig. S8). Another possible side reaction is the polymerization of aromatics over Lewis’ acid. Early research on Friedel–Craft reactions and poly(phenylene) showed that aromatics could undergo polymerization over AlCl3. For example, in the presence of AlCl3, ∼1 wt.% benzene could be polymerized to diphenyl, polyaromatics, and poly(phenylene) at 80 °C after 24 h (46, 47). However, the temperature of our reactions was much lower, and the participation of solvent in side reactions was minor; using an external reference (Materials and Methods), the amount of toluene loss during the degradation was found to be ∼2.2 wt.% on average, including both reacted and vaporized solvent (SI Appendix, Figs. S8 and S9).
After removing the light phase, the heavy phase was extracted by acetone. The resulting solution was primarily composed of toluene and benzene (SI Appendix, Fig. S10A). As confirmed by SEC, the acetone solution contained no detectable polymers. TOF-MS further confirmed that the molecular masses of the acetone extract were smaller than 680 Da, primarily smaller than 270 Da. The gray precipitate was delivered to Fourier-transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), and TOF-MS. The FTIR and XPS spectra showed that it was possibly a mixture of unreacted AlCl3 and Al chlorohydrates, but no organics, or the amount of organics was below the detection limit. TOF-MS also excluded the existence of polymers in the solid. The molecular mass of the solid was lower than 980 Da, primarily lower than 200 Da.
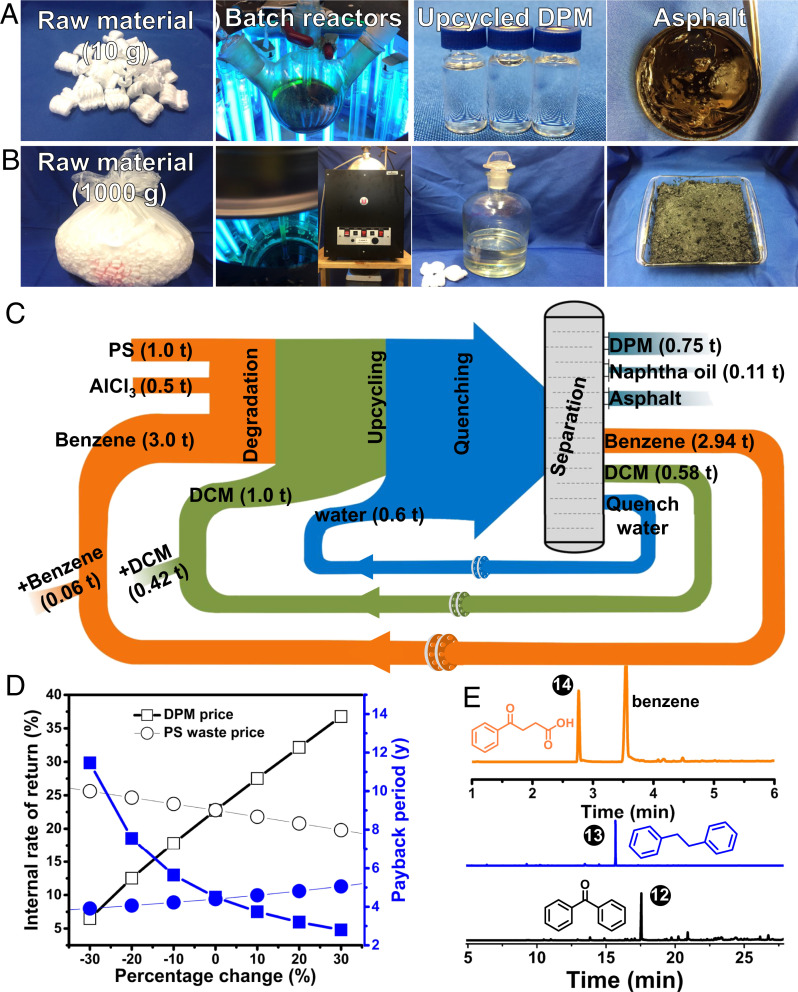
Both scalability and economic viability are cardinal to practical deployment of PS Deg-Up. To demonstrate the scalability, Deg-Up was scaled up by a factor of 10 and 1,000 to process 10 g and 1 kg of PS wastes from both municipal and laboratory sources (SI Appendix, Fig. S11), respectively, in batch using slightly modified procedures (SI Appendix). PS wastes were successfully degraded and upcycled at both scales (Fig. 4). Notably, both large-scale reactions showed resilience to impurities, such as pigments, dirt, adhesives, and other polymers, including PE, PP, and polyvinyl chloride. The degradation at the 1-kg scale was slower than that at the smaller scales because we used an open photoreactor and low-power UV lamp. After upcycling, the reaction was quenched by ice water, and the high-value DPM was characterized and distilled (SI Appendix, Figs. S12 and S13). The remainder pitch oil was collected and blended with Al waste to form a hard asphalt by-product (Fig. 4B). The 1-kg scale reaction served as the foundation for another 1,000 times scale-up in TEA (SI Appendix, Figs. S12–S18).
Fig. 4.
Scale-up and TEA. Photographs depicting the scaling-up of the 1-g proof-of-concept reactions to 10-g (A) and 1-kg (B) reactions. (C) Sankey diagram of a 1-ton-scale PS Deg-Up reaction. The scaled-up TEA is based on the experimental scale up from 1 g to 1,000 g. (D) Sensitivity analysis of profitability with respect to PS waste and DPM prices. (E) Versatility of “Deg-Up” to produce diverse high-value chemicals. (E, Upper) LC trace of 4-oxo-4-phenyl butyric acid upcycled by succinic anhydride. (E, Lower) GC traces of benzophenone and 1,2-diphenylethane upcycled by oxalyl chloride and 1,2-dichrloroethane, respectively.
Economic viability of Deg-Up was analyzed by TEA based on a 100-ton/year and 1-ton/batch waste PS capacity (Fig. 4C). The TEA is based on a system that included a reactor, UV lamps, distillation tower, and vacuum pump. The total fixed capital investment (i.e., factory setup cost) is estimated at $1.28 million (M), including $0.17M working capital. The annual revenue is $0.765M, with an annual variable product cost of $0.414M (without depreciation), which leads to an annual net profit of $0.221M, resulting in an internal rate of return (IRR) of 22.75%, a payback period of 4.49 y, and an average return of investment of 17.25%. Even without any government subsidies or tax returns usually provided to the recycling industry, our Deg-Up approach shows high profitability and, thus, is highly attractive for industrial investment.
Discussion
The profitability is robust against changes in the market. Sensitivity analysis shows that the biggest influencers on the investment are DPM output and price (Fig. 4D and SI Appendix, Figs. S19–S21). Effects of labor cost, utility (including the cost of UV light), and other raw materials (including PS waste cost) on the investment are minor. The TEA confirms that our Deg-Up strategy circumvents the common challenges facing plastic recycling (i.e., oil price, PS price, PS waste price, and the additional collection, transportation, and storage costs associated with foamy plastics) (SI Appendix, Figs. S22 and S23). A decrease of DPM output or price by 30% will drop the IRR to 6.48%, while an increase of 30% will boost the IRR to 36.77%. A change in the equipment cost by −30 to 30% results in an IRR range of 13.27 to 35.05% (SI Appendix, Fig. S19). The profitability is less sensitive to other factors. Therefore, our upcycling method can still be profitable, even in a volatile market, unlike the decomposition of PS to styrene monomers (SI Appendix).
In case the consumption volume of DPM is not comparable to that of PS, adjusting the two cascade reactions can enable the upcycling of PS to other high-volume, high-value specialty chemicals, such as benzophenone, 1,2-diphenylethane, and 4-oxo-4-phenyl butyric acid (Fig. 4E and SI Appendix, Fig. S24). Additionally, further nitration and reduction of DPM can produce methylenedianiline and then synthesize methylene diphenyl diisocyanate, a bulk chemical for producing polyurethane that is usually twice more valuable than PS. Lastly, our Deg-Up strategy can be extended to other commodity polymers and help address the global plastic waste challenge jointly with other methods.
In conclusion, our study demonstrates an economically attractive strategy to upcycle a commodity polyolefin into high-value specialty chemicals at high selectivity. The upcycling strategy tandemly combines two highly selective Deg-Up reactions to convert PS to DPM, which can be used as fragrant wearables and medicinal edibles and have one-order-of-magnitude higher market value than plastic wastes. The Deg-Up reactions proceed under mild conditions and remove the need for high-temperature, high-pressure, and expensive catalysts required in conventional catalytic degradation (23, 24, 31). The Deg-Up strategy is highly profitable and compensates for the polymer waste collection, transportation, and storage costs. We anticipate the process to complement existing methods (e.g., mechanical recycling and chemical recycling to styrene), and it is particularly suited for eliminating end-of-life plastics due to the high tolerance of low-quality plastics.
Materials and Methods
Materials.
All chemicals were purchased from Sigma-Aldrich with a purity of >99% unless otherwise stated. Deuterated benzene (C6D6, 99.8%, Cambridge Isotope Laboratories, Inc.) and waste PS packaging foam (Mw ∼152 kDa, polydispersity index [PDI] = 2.55) were used as received.
Characterization.
Photodegradation was conducted in three-neck quartz flask (KangBo, 0.3 L, transmittance ∼70% for UVC) under a UV reactor (Rayonet RPR-100) equipped with 12 light bulbs (peak wavelength, 253.7 nm). The maximum light intensity of each lamp was 12.5 W⋅cm−2.
All GC analyses were performed on a 6890 GC equipped with a DB-5 capillary column (30 m long × 250 µm inner diameter with a film thickness of 0.25 µm) from J&W Scientific and a flame ionization detector. The following operating parameters were used for each GC analysis: injection port temperature, 280 °C; purge valve, 3 mL⋅min−1; purge time, 1 min; total flow, 11 mL⋅min−1; constant flow, 0.8 mL⋅min−1; injection volume, 1 µL, split 1:10; column oven initial temperature, 40 °C; column oven initial time, 3 min; column oven ramp rate, 10 °C⋅min−1 to 280 °C; column oven final temperature, 280 °C; column oven final time, 1 min.
All GC-MS analyses were performed on a 6890 GC equipped with a 5973 Mass Selective Detector (MSD) from Agilent. The MS Wiley library was used to identify the peaks. Separations were performed by using the same GC column at the same operation conditions. The MSD transfer line temperature was 260 °C.
MS analysis was performed by using an Agilent 6220 Accurate-Mass mass spectrometer with an electrospray ionization probe in a positive mode and Agilent 1200 high-performance LC system (HPLC/MS) using isocratic 70/30 MeOH/H2O + 0.1% fluoroacetic acid as the solvent. Samples were dissolved and then injected directly into the MS by using a 1367B Agilent Autosampler.
SEC (TOSOH multidetector EcoSEC HLC-8320GPC) was utilized to determine the number average molecular weight (Mn), weight average molecular weight (Mw), and PDI. The instrument was equipped with two TSKgel SuperHM-H columns, a refractive index detector, and a multiangle light-scattering detector (WYATT, MiniDAWN TREOS). The oven temperature was kept at 50 °C, and the eluent flow rate was 0.5 mL⋅min−1. The mobile phase was DMF containing 0.05 M LiBr.
Proton NMR (1H NMR) spectroscopy was performed on a Bruker Avance II 500 spectrometer at 500 MHz inC6D6 with 64 scans.
FTIR was performed at room temperature by using a PerkinElmer attenuated total reflectance-FTIR (model Spectrum 100) in the range of 4,000 to 1,000 cm−1 with 256 scans at a resolution of 4 cm−1.
XPS spectra were conducted on a PHI VersaProbe III scanning XPS microscope with a monochromatic Al Kα X-ray source (1,486.6 eV). XPS spectra were acquired with 200 μm/50 W/15 kV X-ray settings and dual-beam charge neutralization.
Experimental Methods.
Deg-Up of PS in benzene solvent.
Degradation.
In a three-neck quartz flask equipped with a stir bar, glass dashpot, gas inlet, and a glass stopper (SI Appendix, Fig. S2A), PS (∼1.0 g) was dissolved in benzene (∼10.0 mL), followed by the addition of AlCl3 (∼1.0 g). The total mass of the reactor and reactants were recorded (m0). Immediately after loading, the reactor was purged with Ar (20 mL⋅s−1 for 0.5 min and then 5 mL⋅s−1 for 15 min) to remove air in the flask. The reactor was sealed, and the total mass was recorded again (m1), from which the change in mass (m1 − m0) was determined. The mass change was attributed to the loss of solvent and replacement of atmosphere from air to Ar. To evaluate the loss of solvent during purging, the mass difference between air and Ar was evaluated by using their densities at 25 °C (in a 0.3-L flask containing ∼10 mL of liquid; after replacing the air in the flask to Ar, the mass increase was ∼132 mg) (48, 49). The loss of solvent was thus (132 + m0 − m1) mg, and the actual solvent mass was tabulated in SI Appendix, Table S1. The quartz flask was then placed under a UV lamp (working temperature ∼37 °C) for 5 h. After photodegradation, the gases in the dashpot were released, and the total mass of the reactor was weighed again (m2) to evaluate the mass of gas in the dashpot (m2 − m1). The mass of the gas phase (gases in the flask and dashpot) was evaluated based on the mass in the dashpot and the volume ratio of gas in the dashpot and flask, excluding the mass of AR (SI Appendix, Table S1). The fractions of the light and heavy phases were roughly estimated. The total mass of the reactor was measured (m3). The light phase was pipetted out from the reactor. The potential residue was removed by a vacuum pump at room temperature, and the heavy phase remained because it is nonvolatile. The mass of the reactor was weighted (m4) to evaluate the recovery of the light phase (m3 − m4) and heavy phase. The light phase was then characterized by GC-MS (Fig. 2).
Determination of benzene yield from PS degradation.
Two methods were used to determine the benzene yield from degradation: 1) toluene as an external reference method and 2) the C6D6 isotope method.
Toluene as an external reference.
After degradation of PS in C6H6, the reaction was quenched by acetone (∼50 mL). A glass vial (∼2 mL), cleaned at 600 °C in air for 12 h, was filled with a controlled amount of toluene as an external reference (SI Appendix, Table S2). Carefully handled with a tweezer, the vial containing toluene was dropped into the quenched mixture. After sonication for 5 min, the mixture was sampled, filtered, and then characterized by using GC. The mass of benzene product from PS degradation () was determined by Eq. 1.
| [1] |
where is the peak area ratio of benzene over toluene; is the slope of the GC calibration curve ( = 58,531 and = 61,736; SI Appendix, Fig. S6C); is the mass of the toluene external reference (SI Appendix, Table S2); is the mass of solvent benzene before degradation (SI Appendix, Table S1); and is the mass of benzene vapor and is estimated using Eq. 2. based on Raoult’s law and the ideal gas equation,
| [2] |
where is the molar concentration of benzene in the liquid phase; is the saturated vapor pressure of benzene (13.33 kPa) at the postreaction temperature T (i.e., 293 K); is the gas volume in the reactor (∼0.3 L); is the ideal gas constant; and is the molar mass of benzene. Under the experimental condition, the mass of benzene vapor is ∼121.7 mg.
Isotope method.
PS (∼1 g) was degraded in C6D6 over AlCl3 under UV light following the same setup as above. After 5 h, the mixture was quenched by ice water (∼9 molar equivalent of AlCl3), resulting in solid precipitates of Al compounds, an aqueous solution, and an organic solution of C6D6. Chloroform (CHCl3) was added as a reference for 1H NMR analysis. The mass of benzene product () from PS degradation was calculated by using Eq. 3.
| [3] |
where is mass of chloroform; r is the 1H NMR peak area ratio of benzene to chloroform; is the number of hydrogen in C6H6; and are the molar mass of chloroform and benzene, respectively; and is the volume of C6D6, is the concentration of C6H6 in C6D6 (3.273 mg/g; Fig. 3B). C6H6 vapor was omitted due to a small molar ratio of C6H6 to C6D6 (∼0.07) in the solution.
Upcycling.
DCM (5.0 mL) was then injected to the reactor to upcycle the degradation products into DPM. The liquid products in the reactor separated into two phases. The light liquid phase was sampled and analyzed by GC-MS. In a parallel experiment, the light phase was sampled at 9, 25, 60, and 180 min after the injection of DCM, using an Ar-degassed syringe (∼0.5 mL) equipped with a long needle. Each sample was diluted with acetone (∼5.0 mL) and delivered for GC analysis. The yield of DPM was estimated by using Eq. 4, based on the GC peak ratio of benzene (∼2.7 min) and DPM (∼14.7 min) (SI Appendix, Table S3).
| [4] |
where is the GC peak area ratio of DPM over benzene; is the mass of benzene solvent; is the mass of DPM; and is the slope of the GC calibration curve ( = 73,675 and = 61,736) (SI Appendix, Fig. S6 C and E). The DPM vapor was ignored due to the low vapor pressure (SI Appendix, Table S4).
Catalyst reusability.
Waste PS foam (∼1.0 g) was dissolved in benzene (∼10.0 mL) and photodegraded for 5 h by using AlCl3 (∼1.0 g) under UV light in a quartz three-neck flask. DCM (1.0 mL) was injected to convert the degradation products to DPM. After stirring at room temperature for 3 h, the light phase was collected and characterized by using SEC to confirm PS degradation (SI Appendix, Fig. S3C). The residual heavy phase, protected by Ar, was mixed with another gram of PS in benzene (10 mL) that was subjected to three freeze–pump–thaw cycles and Ar protection. After purging the flask with Ar (5 min, 5 mL/min), the mixture was placed under UV light for degradation. After 5 h, the light phase in the quartz flask was collected again for SEC characterization (SI Appendix, Fig. S3C).
PS Deg-Up to other products.
To exhibit the versatility of Deg-Up for producing other high-value chemicals other than DPM, we adjusted the upcycling step to obtain a list of products including 1,2-diphenylmethane, benzophenone, and 4-oxo-4-phenyl butyric acid.
Deg-Up of PS using halide.
The degradation followed a similar procedure. After degradation, the reactor was placed in an ice bath, followed by the addition of 0.012 mol halide (0.95 mL of 1,2-dichloroethane or 1.02 mL of oxalyl chloride). The reactor was then returned to 60 °C and stirred for 24 h. The reaction was quenched by ice water (∼0.5 mL). The upper layer was sampled and diluted in acetone (∼4 mL) for GC-MS characterization.
Deg-Up of PS using acid anhydride.
The degradation followed a similar procedure, and the upcycling was modified due to the low solubility of acid anhydrides in benzene. After degradation for 5 h, the reactor was placed in an ice bath. Under vigorous stirring, succinic anhydride (0.012 mol, 1.2 g) was added slowly. The mixture was kept stirring in the ice bath for 1 h to afford a suspension in benzene. The reactor was then transferred to an oil bath at 60 °C. After 24 h, the reaction was stopped and quenched by acetone (∼50 mL). Because the product was poorly soluble in neutral aqueous solution and benzene, acetone was utilized to extract the product for LC-MS characterization.
Additional discussion of PS Deg-Up to other products.
To exhibit the versatility of Deg-Up, additives such as oxalyl chloride, 1,2-dichloromethane, and succinic anhydride were used to obtain Deg-Up products benzophenone, 1,2-diphenylmethane, and 4-phenyl-4oxo butyric acid, respectively. We note that these processes are not optimized, and they only serve as examples to demonstrate the versatility.
Benzophenone.
The classic industrial process to obtain benzophenone is reacting benzene and phosgene over Lewis acid (50). However, for laboratory safety, the acutely toxic phosgene was substituted by oxalyl chloride to upcycle PS to benzophenone (51). The product of benzophenone was confirmed by GC-MS (Fig. 4E and SI Appendix, Fig. S7).
1,2-diphenylethane.
Similarly, a drug precursor 1,2-diphenylethane was prepared through Friedel–Craft alkylation and confirmed by GC-MS (Fig. 4E and SI Appendix, Fig. S7).
4-phenyl-4-oxo butyric acid.
Friedel–Craft acylation was utilized to Deg-Up PS to 4-phenyl-4-oxo butyric acid, the precursor of BuPhenyl (a Food and Drug Administration-approved drug for urea disorder). Due to the low solubility of 4-phenyl-4-oxo butyric acid in benzene, the product precipitated in the reactor after the reaction. The product of 4-phenyl-4-oxo butyric acid was confirmed by LC-MS using a 4-phenyl-4-oxo butyric acid standard (Fig. 4E and SI Appendix, Fig. S7 and S24). The solvent benzene signal (3.5 min) appeared later than the 4-phenyl-4-oxo butyric acid (3.1 min) due to the stronger affinity of benzene to the column than the hydrophilic mobile phase.
Degradation of PS in toluene solvent.
PS (∼1.0 g, Mw ∼192 kDa) was dissolved in toluene (∼10.0 mL) and combined with AlCl3 (∼1.0 g) in a three-neck quartz flask equipped with a stir bar, glass dashpot, gas inlet, and a glass stopper (SI Appendix, Fig. S2A). The flask was placed under UV light to initiate the degradation. After 1, 2, 3, and 5 h of UV exposure, the light and heavy phases were sampled (50 µL each) by using an automatic pipet and then diluted in ∼4 mL of DMF/LiBr. The diluted solutions were filtered before SEC characterization.
Control experiments.
Toluene without AlCl3.
PS was dissolved in toluene (∼10.0 mL) in a three-neck quartz flask equipped with a stir bar, glass dashpot, gas inlet, and a glass stopper (SI Appendix, Fig. S2A). After degassing with Ar, the reactor was placed under UV light to initiate the degradation. After 5 and 24 h of UV exposure, the liquid phase was sampled for SEC characterization.
Toluene without UV.
PS was dissolved in toluene (∼10.0 mL) and combined with AlCl3 (∼1.0 g) in a three-neck quartz flask equipped with a stir bar, glass dashpot, gas inlet, and a glass stopper. After degassing with Ar, the reactor was placed in an oil bath at 43 °C to initiate the reaction. After 24 h, the light and heavy phases were sampled (50 µL each) using a pipet and then characterized by SEC.
Characterization of the degradation products.
The analysis of the light- and heavy-phase products was performed using the GC/MS, SEC, FTIR, XPS, and TOF-MS.
Analysis of the gas phase.
The gas products were collected, and the composition was characterized by using GC-MS.
Analysis of the light phase.
The remaining products in the reactor separated into a light phase at the top and a heavy phase at the bottom. The total mass of the reactor was measured (m3). The light phase was then pipetted out and characterized by GC/MS, followed by toluene wash (10.0 mL) five times to ensure that the light phase was fully removed. The potential residue was removed by a vacuum pump. The mass of the reactor was weighted (m4) to evaluate the recovery of the light phase (m3 − m4) and heavy phase.
Analysis of the heavy phase.
A part of the heavy phase in the reactor was extracted by acetone (50.0 mL), forming a chartreuse acetone solution with insoluble precipitates. A part of this mixture was transferred into a colorimetric tube (SI Appendix, Fig. S2G). The acetone solution was sampled (100 µL) and diluted by additional fresh acetone (∼2 mL) before characterization using GC/MS (SI Appendix, Fig. S10A). Another ∼1 mL of the acetone solution was air-dried and dispensed into a DMF solution containing 0.05 M LiBr for SEC analysis (SI Appendix, Fig. S10B). In addition, another ∼2 mL of the acetone solution was sampled for TOF-MS without treatment (SI Appendix, Fig. S10C). Afterward, the acetone solution was carefully decanted to obtain a gray precipitate. The gray precipitate was dried at 60 °C under reduced pressure to remove any volatiles. The insoluble precipitate was then analyzed by FTIR and TOF-MS (SI Appendix, Fig. S10 D and E).
A portion of the heavy phase was transferred to a colorimetric tube. Chloroform was injected into the colorimetric tube to dissolve the liquid components in the heavy phase. Because AlCl3 has a lower solubility in chloroform than in acetone, AlCl3 was preserved during solvent washing. A yellow solid residue (SI Appendix, Fig. S2H) was obtained after decanting the liquid. The remaining solid residue in the colorimetric tube was washed with chloroform (10 mL, 3 times) and then delivered for FTIR and XPS analysis (SI Appendix, Fig. S10 E–H).
Determination of solvent participation during PS degradation.
To determine the average solvent loss during PS degradation, three parallel experiments in toluene were conducted by using an external reference method. After 5 h of UV exposure, acetone (∼50 mL) was added to quench the degradation. An external reference DPM was measured in a glass vial that was precleaned at 600 °C in air for 12 h. Subsequently, the vial containing DPM was dropped into the solution in the reactor. After sonication for ∼5 min, the solution was sampled for GC. The amount of reacted toluene was calculated by using Eq. 5,
| [5] |
where is the toluene reacted; is the GC peak area ratio of toluene over DPM; is the mass of toluene solvent; is the mass of DPM external reference; and is the slope of the GC calibration curves (SI Appendix, Fig. S6 D and E; = 58,531 and = 73,675).
Scale-up reactions.
To reduce the usage of the solvents (benzene and DCM) and catalyst (AlCl3), we designed an improved procedure for the scale-up reactions and TEA.
Deg-Up of PS waste at 10-g scale.
PS waste foam (∼10 g) was dissolved in benzene (∼30.0 mL) and photodegraded in a quartz three-neck flask using AlCl3 (∼5.0 g) under UV light. After degradation for 5 h, DCM (∼7.5 mL) was injected into the reactor to upcycle the extracted phenyl groups from PS. After 12 h at room temperature, the reaction was quenched by using ice water. The product DPM was collected by vacuum distillation at 130 °C under ∼10 mmHg.
Deg-Up of PS waste at 1-kg scale.
Due to the limited size of our UV photoreactor, the Deg-Up reaction of PS waste at 1-kg scale was conducted in a 6-L cylindrical quartz beaker. The batch photoreactor was custom-built by assembling the quartz beaker, a UV lamp, a glass mechanical stir rod, an aluminum lid, and a polytetrafluoroethylene stir bar (SI Appendix, Fig. S14). As a semiopen system, a continuous Ar flow (∼100 mL/min) was applied to keep low levels of oxygen and moisture in the reactor. Waste PS recycled from packaging and laboratory supplies (SI Appendix, Fig. S11) was dissolved in benzene (∼3.5 L) without cleaning (i.e., glue, tapes, dirt, and other impurities were not removed). Because of the semiopen reactor and the constant gas purge, the solution volume decreased ∼500 mL after purging. AlCl3 powder was added to the reactor, which was later covered by the aluminum lid. After purging the reactor with Ar for another 2 h, the UV lamp was illuminated to trigger the reaction. The degradation reaction stopped after 41 h. Solution in the beaker was sampled for SEC to confirm the degradation (SI Appendix, Fig. S3D).
In the upcycling step, DCM (∼750 mL) was slowly added into the quartz beaker and stirred in an ice bath. After the addition of DCM, the beaker was kept at room temperature and sealed with a PE film to hinder solvent evaporation. The production of DPM was monitored by using GC (SI Appendix, Fig. S15). After 12 h, the light phase was decanted into a flask, and the residual heavy phase was quenched by ice water (∼0.6 kg). The remaining organic phase after quenching was combined with the light phase and distillated to harvest DPM.
Supplementary Material
Acknowledgments
G.L. acknowledges support from the Deans Discovery Fund at Virginia Tech and insightful discussions with Dr. Mark P. Stoykovich. This material is based on the work supported by NSF Award DMR-1752611 through the CAREER award. We acknowledge the Chemistry Chromatography Center at Virginia Tech, especially Dr. Mehdi Ashraf-Khorassani, for providing GC, GC-MS, and LC-MS support that has contributed to the results reported within this paper.
Footnotes
Competing interest statement: A provisional patent has been filed by Virginia Tech on findings reported here.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2203346119/-/DCSupplemental.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Plastics Europe, Plastics—the facts 2021: An analysis of European plastics production, demand and waste data (Report, Plastics Europe, Brussels, Belgium, 2021). [Google Scholar]
- 2.Lebreton L., Andrady A., Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 5, 6 (2019). [Google Scholar]
- 3.Geyer R., Jambeck J. R., Law K. L., Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stubbins A., Law K. L., Muñoz S. E., Bianchi T. S., Zhu L., Plastics in the Earth system. Science 373, 51–55 (2021). [DOI] [PubMed] [Google Scholar]
- 5.MacLeod M., Arp H. P. H., Tekman M. B., Jahnke A., The global threat from plastic pollution. Science 373, 61–65 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Santos R. G., Machovsky-Capuska G. E., Andrades R., Plastic ingestion as an evolutionary trap: Toward a holistic understanding. Science 373, 56–60 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Korley L. T. J., Epps T. H. III, Helms B. A., Ryan A. J., Toward polymer upcycling-adding value and tackling circularity. Science 373, 66–69 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Jambeck J. R., et al. , Marine pollution. Plastic waste inputs from land into the ocean. Science 347, 768–771 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Pearce D. W., Turner R. K., Turner P. R. K., “The circular economy” in Economics of Natural Resources and the Environment, Segerson K., Ed. (Johns Hopkins University Press, Baltimore, 1990), pp. 29–42. [Google Scholar]
- 10.Meys R., et al. , Achieving net-zero greenhouse gas emission plastics by a circular carbon economy. Science 374, 71–76 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Häußler M., Eck M., Rothauer D., Mecking S., Closed-loop recycling of polyethylene-like materials. Nature 590, 423–427 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Zhu J. B., Watson E. M., Tang J., Chen E. Y., A synthetic polymer system with repeatable chemical recyclability. Science 360, 398–403 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Tournier V., et al. , An engineered PET depolymerase to break down and recycle plastic bottles. Nature 580, 216–219 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y., Romain C., Williams C. K., Sustainable polymers from renewable resources. Nature 540, 354–362 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Abel B. A., Snyder R. L., Coates G. W., Chemically recyclable thermoplastics from reversible-deactivation polymerization of cyclic acetals. Science 373, 783–789 (2021). [DOI] [PubMed] [Google Scholar]
- 16.De Smet M., et al. , A circular economy for plastics—insights from research and innovation to inform policy and funding decisions (Report, European Commission, Brussels, Belgium, 2019). [Google Scholar]
- 17.Schyns Z. O. G., Shaver M. P., Mechanical recycling of packaging plastics: A review. Macromol. Rapid Commun. 42, e2000415 (2021). [DOI] [PubMed] [Google Scholar]
- 18.IDTechEx, Green Technology and Polymer Recycling: Market Analysis 2020-2030 (Report, IDTechEx, Cambridge, UK, 2021). [Google Scholar]
- 19.Achilias D. S., Kanellopoulou I., Megalokonomos P., Antonakou E., Lappas A. A., Chemical recycling of polystyrene by pyrolysis: Potential use of the liquid product for the reproduction of polymer. Macromol. Mater. Eng. 292, 923–934 (2007). [Google Scholar]
- 20.United Nations, Plastic recycling—an underperforming sector ripe for a remake (Report, United Nations, New York, 2019). [Google Scholar]
- 21.Tullo A. H., Will plastics recycling meet its deadline. C&EN 99, 28–33 (2021). [Google Scholar]
- 22.Organisation for Economic Co-Operation and Development, Improving plastics management: Trends, policy responses, and the role of international co-operation and trade (Report, Organisation for Economic Co-Operation and Development, Paris, 2018).
- 23.Boerner L. K., One-pot method turns polyethylene into valuable detergent precursor. C&EN 98 (2020). https://cen.acs.org/synthesis/catalysis/One-pot-method-turns-polyethylene/98/i41. [Google Scholar]
- 24.Rorrer J. E., Beckham G. T., Román-Leshkov Y., Conversion of polyolefin waste to liquid alkanes with Ru-based catalysts under mild conditions. JACS Au 1, 8–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guironnet D., Peters B., Tandem catalysts for polyethylene upcycling: A simple kinetic model. J. Phys. Chem. A 124, 3935–3942 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Allen R. D., Bajjuri K. M., Breyta G., Hedrick J. L., Larson C. E., “Methods and materials for depolymerizing polyesters.” US Patent 9914816 (2015)
- 27.Allen R. D., Bajjuri K. M., Hedrick J. L., Breyta G., Larson C. E., “Methods and materials for depolymerizing polyesters.” US Patent 9255194 (2016)
- 28.Yoshida S., et al. , A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 351, 1196–1199 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Vollmer I., Jenks M. J. F., Mayorga González R., Meirer F., Weckhuysen B. M., Plastic waste conversion over a refinery waste catalyst. Angew. Chem. Int. Ed. Engl. 60, 16101–16108 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eagan J. M., et al. , Combining polyethylene and polypropylene: Enhanced performance with PE/iPP multiblock polymers. Science 355, 814–816 (2017). [DOI] [PubMed] [Google Scholar]
- 31.DelRe C., et al. , Near-complete depolymerization of polyesters with nano-dispersed enzymes. Nature 592, 558–563 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Vogt B. D., Stokes K. K., Kumar S. K., Why is recycling of postconsumer plastics so challenging? ACS Appl. Polym. 3, 4325–4346 (2021). [Google Scholar]
- 33.Yeung C. W. S., Teo J. Y. Q., Loh X. J., Lim J. Y. C., Polyolefins and polystyrene as chemical resources for a sustainable future: Challenges, advances, and prospects. ACS Mater. Lett. 3, 1660–1676 (2021). [Google Scholar]
- 34.Tennakoon A., et al. , Catalytic upcycling of high-density polyethylene via a processive mechanism. Nat. Catal. 3, 893–901 (2020). [Google Scholar]
- 35.Lau W. W. Y., et al. , Evaluating scenarios toward zero plastic pollution. Science 369, 1455–1461 (2020). [DOI] [PubMed] [Google Scholar]
- 36.UN, The State of Plastics, World Environment Day Outlook 2018 (United Nations, New York, 2018). [Google Scholar]
- 37.Rudolph N., Kiesel R., Aumnate C., Understanding Plastics Recycling (Hanser Publishers, Liberty Township, OH, 2017). [Google Scholar]
- 38.Ukei H., et al. , Catalytic degradation of polystyrene into styrene and a design of recyclable polystyrene with dispersed catalysts. Catal. Today 62, 67–75 (2000). [Google Scholar]
- 39.Environmental Protection Agency, “Risk evaluation for methylene chloride” (Report, Environmental Protection Agency, Washington, DC, 2020).
- 40.Sha S. C., et al. , Cation-π interactions in the benzylic arylation of toluenes with bimetallic catalysts. J. Am. Chem. Soc. 140, 12415–12423 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suga T., Ukaji Y., Nickel-catalyzed cross-electrophile coupling between benzyl alcohols and aryl halides assisted by titanium co-reductant. Org. Lett. 20, 7846–7850 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Barluenga J., Tomás-Gamasa M., Aznar F., Valdés C., Metal-free carbon-carbon bond-forming reductive coupling between boronic acids and tosylhydrazones. Nat. Chem. 1, 494–499 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Scholz F., Himmel D., Eisele L., Unkrig W., Krossing I., The superacid HBr/AlBr(3): Protonation of benzene and ordered crystal structure of [C(6)H(7)](+)[Al(2)Br(7)](-). Angew. Chem. Int. Ed. Engl. 53, 1689–1692 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Nanbu H., Sakuma Y., Ishihara Y., Takesue T., Ikemura T., Catalytic degradation of polystyrene in the presence of aluminum chloride catalyst. Polym. Degrad. Stabil. 19, 61–76 (1987). [Google Scholar]
- 45.Tarakeshwar P., Kim K. S., A theoretical investigation of benzene−AlX3 and ethene−AlX3 (X = H, F, Cl) interactions. J. Phys. Chem. A 103, 9116–9124 (1999). [Google Scholar]
- 46.Ipatieff V. N., Komarewsky V. I., The action of aluminum chloride on benzene and cyclohexane. J. Am. Chem. Soc. 56, 1926–1928 (1934). [Google Scholar]
- 47.Kovacic P., Wu C., Reaction of ferric chloride with benzene. J. Polym. Sci. 47, 45–54 (1960). [Google Scholar]
- 48.Lide D. R., “Physical constants of inorganic compounds” in CRC Handbook of Chemistry and Physics, Lide D. R., Ed. (CRC Press LLC, Boca Raton, FL, 2003), pp. 43–101. [Google Scholar]
- 49.Crittenden J. C., Trussell R. R., Hand D. W., Howe K. J., Tchobanoglous G., “Appendix B” in MWH’s Water Treatment, J. C. Crittenden, Ed. (John Wiley & Sons, Hoboken, NJ, 2012), pp. 1857–1858. [Google Scholar]
- 50.Ryan T. A., Seddon E. A., Seddon K. R., Ryan C., “Industrial manufacture and uses” in Phosgene: And Related Carbonyl Halides, T. Anthony Ryan, Elaine A. Seddon, Kenneth R. Seddon, and Christine Ryan, Ed. (Elsevier Science, Amsterdam, 1996), pp. 167–221. [Google Scholar]
- 51.Alexandrou N. E., Friedel–Crafts reaction of oxalyl chloride with pentamethylbenzene. J. Chem. Soc. C: Organic, 536–537 (1969). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.