Abstract
Combining oral PI3K inhibitors with immunochemotherapy for indolent B-cell lymphoma has been associated with toxicity. In the Phase III CHRONOS-4 safety run-in, 21 patients received intravenous copanlisib plus rituximab-based immunochemotherapy. There were no dose-limiting toxicities, and preliminary objective response rates were 90% to 100%. Copanlisib is the first PI3K inhibitor to demonstrate safe, tolerable, and effective combinability with immunochemotherapy, with evaluation ongoing.
Background:
When treating indolent B-cell lymphoma, combining continuously administered oral phosphatidylinositol 3-kinase (PI3K) inhibitors with immunochemotherapy has been associated with toxicity. CHRONOS-4 (Phase III; NCT02626455) investigates the intravenous, intermittently administered pan-class I PI3K inhibitor copanlisib in combination with rituximab plus bendamustine (R-B) or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in patients with relapsed indolent B-cell lymphoma. We report safety run-in results.
Patients and Methods:
Patients aged ≥18 years with relapsed CD20-positive indolent B-cell lymphoma received copanlisib (45 mg, increasing to 60 mg if no dose-limiting toxicities) weekly on an intermittent schedule with R-B or R-CHOP. Primary objective was to identify a recommended Phase III dose (RP3D). We also assessed objective response, safety, and tolerability.
Results:
Ten patients received copanlisib plus R-B and 11 received copanlisib plus R-CHOP. No dose-limiting toxicities were reported; RP3D was 60 mg. All patients had ≥1 treatment-emergent adverse event (TEAE), most commonly (all grade/grade 3/4) for copanlisib plus R-B: decreased neutrophil count (80%/50%), nausea (70%/0%), decreased platelet count (60%/10%), hyperglycemia (60%/50%); for copanlisib plus R-CHOP: hyperglycemia (82%/64%), hypertension (73%/64%), decreased neutrophil count (64%/64%). Two and 8 patients had serious TEAEs with copanlisib plus R-B and R-CHOP, respectively. Among evaluable patients, objective response rates were 90% (5 complete, 4 partial) and 100% (3 complete, 7 partial) with copanlisib plus R-B and R-CHOP, respectively.
Conclusion:
Copanlisib is the first PI3K inhibitor to demonstrate safe, tolerable, and effective combinability with immunochemotherapy in patients with relapsed indolent B-cell lymphoma at full dose (60 mg). Further evaluation is ongoing.
Keywords: Bendamustine, CHRONOS-4, Phase III, R-CHOP, Safety run-in
Introduction
Phosphatidylinositol 3-kinase (PI3K) signaling is often dysregulated in indolent B-cell lymphomas.1 Patients with indolent disease typically respond to first-line immunochemotherapy, but the disease course is characterized by continuing relapses, shorter durations of response or remission, and risk of transformation into aggressive lymphoma.2,3 Several single-agent PI3K-isoform-specific inhibitors are in development or have been approved for the treatment of relapsed or refractory indolent B-cell lymphoma, including oral inhibitors of PI3K-δ,4-6 an oral inhibitor of PI3K-δ/PI3K-γ,7 and copanlisib,8 an intravenous, intermittently administered pan-PI3K inhibitor with predominant activity against the PI3K-α/PI3K-δ isoforms.9,10
Copanlisib received approval from the US Food and Drug Administration in the USA and in Taiwan as monotherapy for adult patients with relapsed indolent follicular lymphoma who have received at least 2 systemic therapies, based on the results from a Phase II study.11 In this study, a dose of copanlisib 60 mg was administered to patients intravenously over a 1-hour infusion, on an intermittent schedule of days 1, 8, and 15 of a 28-day cycle,11 which is the approved dose for copanlisib. Copanlisib has demonstrated target engagement and PI3K pathway modulation and inhibition in patients with lymphoma and solid tumors.12 In pooled safety data from 364 patients with hematologic malignancies receiving treatment with copanlisib in 8 Phase I and II studies, there were no late-onset toxicities or worsening of severity of treatment-emergent adverse events (TEAEs) and few severe gastrointestinal TEAEs.13
The Phase III CHRONOS-3 study recently identified copanlisib as the first PI3K inhibitor to be safely combined with rituximab, and the first to demonstrate broad and superior efficacy in combination with rituximab in patients with relapsed indolent B-cell lymphoma.14 Combining PI3K inhibitors with immunochemotherapy (rituximab plus bendamustine [R-B], or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone [R-CHOP]) in patients with indolent B-cell lymphoma who have progressed on rituximab-based treatment is an ongoing subject of investigation in this area of high unmet medical need. However, the continuous administration of the orally administered PI3K inhibitor idelalisib in combination with rituximab-based immunochemotherapy has been associated with severe toxicity, such as autoimmune dysfunction, opportunistic infections, diarrhea, and colitis, leading to the discontinuation of several clinical trials of this combination approach in patients with indolent B-cell lymphoma.15
We present results from the initial safety run-in (SRI) of CHRONOS-4, a Phase III, randomized, double-blind, placebo-controlled study to evaluate the safety, tolerability, and clinical indicators of efficacy of copanlisib in combination with standard immunochemotherapy in patients with relapsed indolent B-cell lymphoma.
Patients and Methods
This study was conducted in accordance with Good Clinical Practice guidelines and applicable local laws and regulations, and under the guiding principles detailed in the Declaration of Helsinki. The study protocol and all amendments were reviewed and approved by each site’s institutional ethical committee or review board. All participants provided written, informed consent.
Study Design
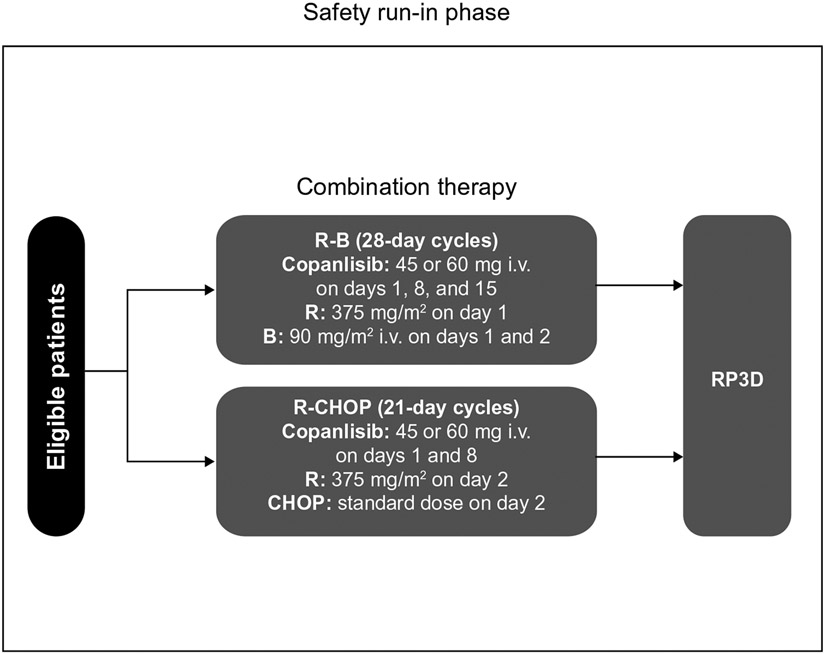
CHRONOS-4 (NCT02626455) is a Phase III, randomized, double-blind, placebo-controlled study conducted in 2 parts: the SRI (Figure 1) and a subsequent Phase III part (Supplementary Figure 1). The primary objective of the SRI was to determine the recommended Phase III dose of copanlisib in combination with standard immunochemotherapy (R-B or R-CHOP) to be used in the subsequent Phase III part of the study. Secondary objectives of the SRI were to evaluate the radiologic and clinical indicators of treatment efficacy, and the safety and tolerability of copanlisib in combination with R-B or R-CHOP.
Figure 1. Study design of the CHRONOS-4 Phase III study—safety run-in.
CHOP treatment includes cyclophosphamide 750 mg/m2 i.v., doxorubicin 50 mg/m2 i.v., and vincristine 1.4 mg/m2 i.v. (maximum dose 2.0 mg) on day 2 of a 21-day cycle, and prednisone 100 mg tablets on days 2 to 6 of a 21-day cycle. Abbreviations: B = bendamustine; CHOP = rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; i.v. = intravenous; R = rituximab; R-B = rituximab plus bendamustine; R-CHOP = rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; RP3D = recommended Phase III dose.
In the SRI, copanlisib dosing was based on an open-label, 2-dose level, 3 + 3 design. The 2 treatment combinations of copanlisib (with either R-B or R-CHOP) were each tested at 2 copanlisib dose levels (a first level of 45 mg and a second level of 60 mg) for safety and tolerability. It was planned that a minimum of 3 and a maximum of 6 patients were to be evaluable for dose-limiting toxicities (DLTs) per dose level. The design of the dose-finding steps for copanlisib and definitions of DLTs are shown in Supplementary Figure 2. Three patients were initially planned to receive copanlisib 45 mg, and dosing was escalated to 60 mg if no DLTs were observed in the initial cohort treated with 45 mg. All patients were evaluated for the occurrence of DLTs during the first treatment cycle; treatment was to be discontinued in any patient experiencing a DLT in the first cycle. Patients not fully evaluable for occurrence of DLTs during the first cycle of therapy were replaced.
Patients treated in the SRI who did not experience a DLT in the first cycle and who, in the investigator’s opinion, could benefit from further treatment could continue combination therapy for a maximum of 6 cycles followed by copanlisib monotherapy at the same dose of copanlisib that had been administered with immunochemotherapy. The maximum duration of treatment with copanlisib was 12 months (including combination therapy and monotherapy). Patients with benefit who remained on treatment with copanlisib in combination with R-B or R-CHOP after the first cycle were evaluated for treatment safety, tolerability, and efficacy.
Copanlisib was administered as a 1-hour intravenous infusion on days 1, 8, and 15 of a 28-day cycle in combination with R-B, or days 1 and 8 of a 21-day cycle with R-CHOP for ≤6 cycles. In the R-B combination, rituximab was administered intravenously at a dose of 375 mg/m2 of body surface area on day 1 of each cycle, and bendamustine was administered intravenously at a dose of 90 mg/m2 of body surface area on days 1 and 2 of each cycle. In the R-CHOP combination, rituximab was administered intravenously at a dose of 375 mg/m2 of body surface area on day 2 of each cycle along with intravenous cyclophosphamide 750 mg/m2, intravenous doxorubicin 50 mg/m2, and intravenous vincristine 1.4 mg/m2 (maximum dose of vincristine 2 mg), and oral prednisone or prednisolone 100 mg daily from day 2 to day 6 of each cycle. From cycle 7, copanlisib was administered as monotherapy on days 1, 8, and 15 of a 28-day cycle, irrespective of which immunochemotherapy regimen was received during cycles 1 to 6. The maximum duration of copanlisib therapy was 12 months.
Dosing was not to be reduced for rituximab or any components of CHOP or bendamustine in the first treatment cycle; except for rituximab, dose reductions were otherwise permitted from cycle 2 onwards according to local standards of care, prescribing information, and the investigator’s experience. After cycle 1, the copanlisib dose could be reduced from 60 mg to 45 mg, and further to 30 mg, if toxicities occurred. The use of myeloid growth factors in the prophylactic and therapeutic setting was allowed during study treatment based on local standard of care and at the investigator’s discretion.
Patients
Eligible patients were aged ≥18 years with a histologically confirmed diagnosis of CD20-positive indolent B-cell lymphoma (histology was limited to follicular lymphoma grades 1-3a, marginal zone lymphoma [splenic, nodal, or extranodal], small lymphocytic lymphoma, or lymphoplasmacytic lymphoma/Waldenström macroglobulinemia), relapsed or progressed after 1 to 3 lines of therapy, including rituximab and/or rituximab biosimilars, or other anti-CD20 monoclonal antibody-based immunochemotherapy and alkylating agents. Patients who experienced a lack of response or progression within 6 months of the last date of anti-CD20 monoclonal antibody-containing treatment were ineligible. Key additional inclusion and exclusion criteria are presented in Supplementary Table 1.
Endpoints and Assessments
The primary endpoint in the SRI was the occurrence of DLTs, which informed the primary objective of identifying the recommended Phase III dose (Supplementary Figure 2). The frequency and intensity of adverse events (AEs) were carefully monitored, together with the occurrence of new or unexpected AEs. Safety was assessed throughout the study and included the following secondary endpoints: toxicity/AEs; vital signs; clinical laboratory variables; and review of concomitant medications. AEs were reported and graded according to the Common Terminology Criteria for Adverse Events version 4.03. Secondary outcome measures were evaluation of indicators of treatment efficacy and safety and tolerability of copanlisib in combination with R-B or R-CHOP, assessed by the number of patients with TEAEs.
Screening tests were performed within 7 days before planned day 1 of cycle 1 and included a complete physical examination and laboratory values. At each visit before treatment, patients were evaluated for opportunistic infections, including any new onset or worsening of pulmonary symptoms (ie, cough, dyspnea, or fever). Cytomegalovirus was monitored by polymerase chain reaction on day 1 of every cycle during combination therapy. For patients with signs of cytomegalovirus, CD4 levels were monitored.
On copanlisib infusion days, blood pressure was measured before, during, and after the infusion. Predose blood pressure values had to be <150/90 mm Hg before copanlisib was administered. In addition, on day 1 in the R-B treatment group, blood pressure was measured during and after rituximab infusion.
On copanlisib infusion days, blood glucose was measured before dosing and at the end of the copanlisib infusion, at the end of the rituximab infusion, and at the end of the bendamustine infusion if the patient received corticosteroid medication before bendamustine infusion. On the day after the first copanlisib infusion of each cycle, patients receiving corticosteroid medication before the bendamustine or rituximab infusions had a blood glucose test within 30 minutes before bendamustine or rituximab infusions and at the end of the infusions.
Tumors were assessed radiologically at screening and every 12 weeks during treatment using contrast-enhanced computed tomography, magnetic resonance imaging, or positron emission tomography-computed tomography. Assessment of tumors in patients with Waldenström macroglobulinemia who did not have radiologically measurable lesions at screening was performed by laboratory and clinical tests. Response to treatment was assessed by the investigator according to the Lugano classification,16 or according to the Owen Criteria17 for patients with Waldenström macroglobulinemia.
Statistical Analysis
Safety data from the SRI were summarized by dose level. All patients who had received at least 1 dose of study treatment were included in analyses of safety (full analysis set).
Results
Patients and Treatment
Ten patients were enrolled and treated in the copanlisib plus R-B SRI, 7 with follicular lymphoma, 1 with lymphoplasmacytic lymphoma, 1 with Waldenström macroglobulinemia, and 1 with extranodal marginal zone lymphoma (Table 1); 3 patients received copanlisib at the 45 mg dose level and 7 patients received copanlisib at the 60 mg dose level. Eleven patients were enrolled and treated in the copanlisib plus R-CHOP SRI, all with follicular lymphoma; 5 patients received copanlisib at the 45 mg dose level and 6 patients received copanlisib at the 60 mg dose level. Eighty percent of patients in the R-B SRI and 45% of patients in the R-CHOP SRI were female; the median ages of the groups were 62 and 64 years, respectively (Table 1). Before study enrollment, 60% of patients in the R-B SRI and 82% of patients in the R-CHOP SRI had received 1 line of therapy (Table 1).
Table 1.
Patient Demographics and Baseline Disease Characteristics
| Copanlisib Plus R-B SRI (N = 10) |
Copanlisib Plus R-CHOP SRI (N = 11) |
Total (N = 21) | |
|---|---|---|---|
| Females | 8 (80.0) | 5 (45.5) | 13 (61.9) |
| Age (y), median (range) | 62 (41-82) | 64 (46-78) | 63 (41-82) |
| ECOG performance status | |||
| 0 | 5 (50.0) | 8 (72.7) | 13 (61.9) |
| 1 | 5 (50.0) | 3 (27.3) | 8 (38.1) |
| Histology of lymphoma | |||
| Follicular lymphoma | 7 (70.0) | 11 (100) | 18 (85.7) |
| Extranodal marginal zone lymphoma or MALT lymphoma | 1 (10.0) | 0 | 1 (4.8) |
| Lymphoplasmacytic lymphoma | 1 (10.0) | 0 | 1 (4.8) |
| Waldenström macroglobulinemia | 1 (10.0) | 0 | 1 (4.8) |
| Grade of follicular lymphomaa | |||
| 1 | 5 (50.0) | 0 | 5 (23.8) |
| 2 | 2 (20.0) | 7 (63.6) | 9 (42.9) |
| 3a | 0 | 4 (36.4) | 4 (19.0) |
| Stage at study entryb | |||
| I | 1 (10.0) | 2 (18.2) | 3 (14.3) |
| II | 0 | 1 (9.1) | 1 (4.8) |
| III | 2 (20.0) | 3 (27.3) | 5 (23.8) |
| IV | 6 (60.0) | 5 (45.5) | 11 (52.4) |
| Previous anticancer therapy lines | |||
| 1 | 6 (60.0) | 9 (81.8) | 15 (71.4) |
| 2 | 3 (30.0) | 2 (18.2) | 5 (23.8) |
| 3 | 1 (10.0) | 0 | 1 (4.8) |
Data are presented as n (%) unless otherwise indicated.
Abbreviations: ECOG = Eastern Cooperative Oncology Group; MALT = mucosa-associated lymphoid tissue; R-B = rituximab plus bendamustine; R-CHOP = rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; SRI = safety run-in.
Data for 3 patients in the R-B SRI were missing/not applicable.
Data for 1 patient in the R-B SRI were missing/not applicable.
In the R-B SRI, the median number of copanlisib treatment cycles was 3 (range, 2-5), and the mean dose of copanlisib administered per cycle was 135.2 mg (range, 80-180 mg). Eight patients had dose interruptions or delays with copanlisib therapy, which were caused by AEs. Copanlisib dose reduction occurred in 1 patient receiving 45 mg and no patients receiving 60 mg in the R-B SRI. At the time of data cut-off, no patients had yet received copanlisib monotherapy following the protocol-specified ≤6 cycles of R-B. The mean dose of rituximab was 375 mg/m2 per cycle (range, 375-375 mg/m2), and the mean dose of bendamustine was 82.0 mg/m2 per cycle (range, 67.5-90.0 mg/m2); overall, patients received a median of 3.6 cycles of R-B (range, 2-5). Seven patients had dose interruptions or delays from R-B therapy, which were caused by AEs. At the time of data cut-off, 6 patients in the R-B SRI were still receiving treatment with copanlisib.
In the R-CHOP SRI, the median number of copanlisib cycles was 8 (range, 2-15), and the mean copanlisib dose administered per cycle was 106.0 mg (range, 68-136 mg). Nine patients had dose interruptions or delays with copanlisib therapy, which were caused by AEs. Copanlisib dose reduction occurred in 1 patient receiving 45 mg and no patients receiving 60 mg in the R-CHOP SRI. Six patients received copanlisib monotherapy following the protocol-specified ≤6 cycles of R-CHOP. Mean chemotherapy doses per cycle were as follows: rituximab 380.5 mg/m2 (range, 367-428 mg/m2), cyclophosphamide 750 mg/m2 (range, 750-750 mg/m2), doxorubicin 50 mg/m2 (range, 50-50 mg/m2), vincristine 1.4 mg/m2 (range, 1-2 mg/m2), and prednisone/prednisolone 118.5 mg (range, 58-340 mg). Patients received a median of 6 cycles of R-CHOP (range, 2-6). Dose interruptions or delays were recorded in 5 patients for rituximab, 4 patients each for cyclophosphamide, doxorubicin, and vincristine, and 3 patients for prednisone/prednisolone; AEs were the primary cause of all interruptions and delays. At the time of data cut-off, 3 patients in the R-CHOP SRI were still receiving treatment with copanlisib.
Safety
No DLTs were reported at either dose of copanlisib (45 or 60 mg) in the R-B SRI or the R-CHOP SRI. The recommended Phase III dose of copanlisib was therefore defined as 60 mg for both immunochemotherapy combinations.
All patients in both treatment groups (100%) experienced at least 1 TEAE (Table 2). Nine patients (90%) in the R-B SRI and 11 patients (100%) in the R-CHOP SRI experienced TEAEs considered related to copanlisib, and all patients in both groups experienced AEs considered related to R-B or R-CHOP. Serious TEAEs were recorded in 2 patients (20%) in the R-B SRI and 8 patients (73%) in the R-CHOP SRI; all were considered related to study treatment (Table 2).
Table 2.
Overview of TEAEs (Safety Analysis Set)
| Copanlisib Plus R-B SRI (N = 10) | Copanlisib Plus R-CHOP SRI (N = 11) | |
|---|---|---|
| Any TEAE | 10 (100) | 11 (100) |
| Grade ≥3 | 7 (70.0) | 10 (90.9) |
| Any copanlisib-related TEAE | 9 (90.0) | 11 (100) |
| Any R-B- or R-CHOP-related TEAE | 10 (100) | 11 (100) |
| Any serious TEAE | 2 (20.0) | 8 (72.7) |
| Grade ≥3 | 2 (20.0) | 6 (54.5) |
| Copanlisib-related | 2 (20.0) | 8 (72.7) |
Data are presented as n (%).
Abbreviations: AE = adverse event; R-B = rituximab plus bendamustine; R-CHOP = rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; SRI = safety run-in; TEAE = treatment-emergent adverse event.
The most common TEAEs of any grade in patients receiving copanlisib plus R-B were decreased neutrophil count (80%), nausea (70%), decreased platelet count (60%), and hyperglycemia (60%); in patients receiving copanlisib plus R-CHOP, the most common all-grade TEAEs were hyperglycemia (82%), hypertension (73%), and decreased neutrophil count (64%) (Table 3).
Table 3.
TEAEs Occurring in ≥2 Patients in Either Group
| Copanlisib Plus R-B SRI (N = 10) |
Copanlisib Plus R-CHOP SRI (N = 11) |
|||
|---|---|---|---|---|
| All Grades | Grade 3 or 4 | All Grades | Grade 3 or 4 | |
| Blood and lymphatic system disorders | ||||
| Anemia | 4 (40.0) | 0 | 5 (45.5) | 1 (9.1) |
| Febrile neutropenia | 0 | 0 | 2 (18.2) | 2 (18.2) |
| Gastrointestinal disorders | ||||
| Nausea | 7 (70.0) | 0 | 4 (36.4) | 0 |
| Mucositis oral | 5 (50.0) | 1 (10.0) | 3 (27.3) | 0 |
| Diarrhea | 4 (40.0) | 1 (10.0) | 6 (54.5) | 1 (9.1) |
| Constipation | 3 (30.0) | 0 | 3 (27.3) | 0 |
| Decreased appetite | 2 (20.0) | 0 | 0 | 0 |
| Other gastrointestinal disorders | 0 | 0 | 2 (18.2) | 1 (9.1) |
| Vomiting | 2 (20.0) | 0 | 0 | 0 |
| General disorders and administration site conditions | ||||
| Fatigue | 5 (50.0) | 0 | 4 (36.4) | 0 |
| Fever | 4 (40.0) | 0 | 5 (45.5) | 0 |
| Flu-like symptoms | 0 | 0 | 3 (27.3) | 1 (9.1) |
| Immune system disorders | ||||
| Allergic reaction | 2 (20.0) | 0 | 2 (18.2) | 1 (9.1) |
| Infections and infestations | ||||
| Lung infection | 1 (10.0) | 1 (10.0) | 3 (27.3) | 2 (18.2) |
| Other infections and infestations | 0 | 0 | 2 (18.2) | 1 (9.1) |
| Upper respiratory infection | 0 | 0 | 3 (27.3) | 0 |
| Urinary tract infection | 0 | 0 | 2 (18.2) | 0 |
| Investigations | ||||
| Decreased neutrophil count | 8 (80.0) | 5 (50.0) | 7 (63.6) | 7 (63.6) |
| Decreased platelet count | 6 (60.0) | 1 (10.0) | 6 (54.5) | 2 (18.2) |
| Decreased white blood cell count | 4 (40.0) | 2 (20.0) | 2 (18.2) | 2 (18.2) |
| Increased ALT | 3 (30.0) | 0 | 0 | 0 |
| Increased AST | 3 (30.0) | 0 | 0 | 0 |
| Decreased lymphocyte count | 3 (30.0) | 3 (30.0) | 3 (27.3) | 3 (27.3) |
| Metabolism and nutrition disorders | ||||
| Hyperglycemia | 6 (60.0) | 5 (50.0) | 9 (81.8) | 7 (63.6) |
| Hypokalemia | 0 | 0 | 2 (18.2) | 2 (18.2) |
| Musculoskeletal and connective tissue disorders | ||||
| Other musculoskeletal and connective tissue disorders | 0 | 0 | 2 (18.2) | 0 |
| Nervous system disorders | ||||
| Dysgeusia | 3 (30.0) | 0 | 2 (18.2) | 0 |
| Headache | 3 (30.0) | 0 | 3 (27.3) | 0 |
| Peripheral sensory neuropathy | 0 | 0 | 3 (27.3) | 0 |
| Respiratory, thoracic, and mediastinal disorders | ||||
| Cough | 0 | 0 | 5 (45.5) | 0 |
| Dyspnea | 0 | 0 | 2 (18.2) | 0 |
| Skin and subcutaneous tissue disorders | ||||
| Rash maculopapular | 4 (40.0) | 0 | 1 (9.1) | 0 |
| Pruritus | 3 (30.0) | 0 | 2 (18.2) | 0 |
| Vascular disorders | ||||
| Hypertension | 2 (20.0) | 2 (20.0) | 8 (72.7) | 7 (63.6) |
| Hypotension | 1 (10.0) | 0 | 2 (18.2) | 1 (9.1) |
Data are presented as n (%).
Abbreviations: ALT = alanine aminotransferase; AST = aspartate aminotransferase; R-B = rituximab plus bendamustine; R-CHOP = rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; SRI = safety run-in; TEAE = treatment-emergent adverse event.
TEAEs of worst grade 3 or 4 occurring in 2 or more patients receiving copanlisib plus R-B were decreased neutrophil count and hyperglycemia (5/10 [50%] each), decreased lymphocyte count (3/10 [30%]), and decreased white blood cell count and hypertension (2/10 [20%] each). In patients receiving copanlisib plus R-CHOP, the TEAEs of worst grade 3 or 4 occurring in 2 or more patients were hyperglycemia, hypertension, and decreased neutrophil count (7/11 [64%] each), decreased lymphocyte count (3/11 [27%]), and febrile neutropenia, hypokalemia, lung infection, decreased platelet count, and decreased white blood cell count (2/11 [18%] each) (Table 3).
The 2 serious TEAEs in the R-B SRI were eosinophilia and lung infection, occurring in 1 patient receiving copanlisib 45 mg; the case of eosinophilia was considered related to copanlisib, and the case of lung infection was considered related to copanlisib and R-B therapy. No patients receiving copanlisib 60 mg plus R-B had serious TEAEs. Serious TEAEs in the R-CHOP SRI occurring in 2 or more patients were lung infection (3 patients [27%], all receiving copanlisib 60 mg), febrile neutropenia (2 patients [18%], 1 receiving copanlisib 45 mg and 1 receiving 60 mg), fever (2 patients [18%], 1 receiving copanlisib 45 mg and 1 receiving 60 mg), and hyperglycemia (2 patients [18%], both receiving copanlisib 60 mg). In the R-CHOP SRI, 2 serious TEAEs of lung infection were considered related to copanlisib, and 1 was considered related to R-CHOP. The serious TEAEs of febrile neutropenia, fever, and hyperglycemia were considered related to R-CHOP, with fever and hyperglycemia also considered related to copanlisib.
In the R-B SRI, 4 patients experienced TEAEs leading to discontinuation of copanlisib treatment, and 3 patients had TEAEs leading to discontinuation of R-B. In the R-CHOP SRI, 5 patients experienced TEAEs leading to discontinuation of copanlisib treatment, and 3 patients had TEAEs leading to discontinuation of R-CHOP.
One patient (10%) experienced a TEAE of special interest (grade 3 pneumonitis) in the R-CHOP SRI; no patients experienced pneumonitis in the R-B SRI or colitis in either treatment group.
No patients in either treatment group received granulocyte colony-stimulating factor.
Efficacy
Response to treatment was evaluated in the 10 patients in the R-B SRI and in 10 of the 11 patients in the R-CHOP SRI. Overall, 5 and 3 patients achieved complete responses in the R-B and R-CHOP SRIs, respectively, with 4 and 7 patients achieving partial responses (Table 4); the objective response rates were 90% with copanlisib plus R-B and 100% with copanlisib plus R-CHOP. One patient in the R-B SRI had stable disease, and 1 patient receiving copanlisib plus R-CHOP was not included in the efficacy analysis because the first postbaseline tumor assessment, at which a partial response was achieved, was after the date of data cut-off.
Table 4.
Tumor Response Evaluation (Investigator Assessment; Full Analysis Set With Postbaseline Tumor Assessment)
| Copanlisib Plus R-B SRI (N = 10) | Copanlisib Plus R-CHOP SRI (N = 10a) | |
|---|---|---|
| Best overall response | ||
| Complete response | 5 (50.0)b [18.7, 81.3] | 3 (30.0) [6.7, 65.2] |
| Partial response | 4 (40.0)c [12.2, 73.8] | 7 (70.0)b [34.8, 93.3] |
| Stable disease | 1 (10.0) [0.3, 44.5] | 0 [0.0, 30.8] |
| Progressive disease | 0 [0.0, 30.8] | 0 [0.0, 30.8] |
| Objective response rate | 9 (90.0) [55.5, 99.7] | 10 (100) [69.2, 100] |
Data are presented as n (%) [95% CI].
Abbreviations: CI = confidence interval; R-B = rituximab plus bendamustine; R-CHOP = rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; SRI = safety run-in.
11 patients were treated, but 1 patient was not included because their first postbaseline tumor assessment was reached after the data cut-off date.
1 patient had their first postbaseline tumor assessment outside of the protocol-required 12-week (± 1 week) assessment period.
2 patients had their first postbaseline tumor assessment outside of the protocol-required 12-week (± 1 week) assessment period.
Discussion
This SRI of the CHRONOS-4 study evaluated the safety and tolerability of copanlisib in combination with R-B or R-CHOP in patients with relapsed indolent B-cell lymphoma who had relapsed after 1 to 3 lines of treatment, including rituximab and alkylating agents. The recommended Phase III dose of copanlisib was defined as 60 mg for both immunochemotherapy combinations, consistent with the current full dose of copanlisib approved for the treatment of relapsed follicular lymphoma.18 Copanlisib was generally well tolerated in both immunochemotherapy combinations, and no DLTs or fatal AEs were reported at either dose of copanlisib (45 or 60 mg) in either treatment group. Only 2 patients required a copanlisib dose reduction due to a TEAE; 1 patient in each arm had a dose reduction from 45 mg to 30 mg.
At the time of data cut-off, 6 patients in the R-B SRI and 3 patients in the R-CHOP SRI were still receiving study treatment. The most common TEAEs following a median of 3 copanlisib treatment cycles in the R-B SRI and 8 in the R-CHOP SRI were consistent with those reported in previous studies of copanlisib monotherapy and, overall, included decreased neutrophil count, nausea, decreased platelet count, hyperglycemia, and hypertension.11,19 Hyperglycemia is a known on-target class effect of PI3K pathway inhibition20 and is one of the most common TEAEs observed in clinical studies of copanlisib monotherapy, usually seen as a transient, infusion-related effect that has been reported to be manageable, with blood glucose levels returning to normal levels within a day of copanlisib infusion.21 Management of copanlisib-related hyperglycemia frequently required adequate hydration alone in the Phase II CHRONOS-1 study in patients with indolent B-cell lymphoma, with insulin or oral glucose-lowering agents required in a proportion of patients.11 Hematologic toxicities in this study are most likely a consequence of the accompanying immunochemotherapy regime, having been observed at similar frequencies in other immunochemotherapy trials.22,23 However, these side effects have also been observed with targeted PI3K inhibition in malignant B-cells, as previously reported in several monotherapy studies of different agents (copanlisib, all-grade neutropenia 28.9%19; duvelisib, all-grade neutropenia 28.7%24; umbralisib, grade 3 or 4 neutropenia 8%5). Rituximab alone is also associated with hematologic AEs in patients with indolent lymphoma, with neutropenia and thrombocytopenia common events with either rituximab alone or in combination with chemotherapy.25 No AEs of colitis were reported in the SRI of CHRONOS-4, which is encouraging given the high rates of severe gastrointestinal toxicities and liver enzyme elevations observed in patients in a Phase II study of idelalisib plus ofatumumab26 and a Phase I study of duvelisib plus rituximab or bendamustine/rituximab.27 The intermittent dose schedule and intravenous administration of copanlisib could explain this finding, as it results in reduced exposure in the gastrointestinal tract compared with daily oral regimens. Serious TEAEs were observed in both SRI treatment groups—1 case of eosinophilia and 1 case of lung infection considered related to copanlisib—whereas the remaining serious TEAEs were considered related to combination therapy or to immunochemotherapy alone. Lung infection has been reported as a serious TEAE in studies of other PI3K inhibitors in monotherapy4 and was observed in a previous report of copanlisib monotherapy.11 Opportunistic infections in the respiratory tract and autoimmune toxicities such as pneumonitis have been reported with monotherapy with the PI3K inhibitors idelalisib and duvelisib,28-30 with a black box warning for fatal and/or severe pneumonitis in place for both.6,7 Severe opportunistic infections, among other toxicities, have been observed with idelalisib plus rituximab-based chemotherapy, leading to the discontinuation of several studies.15 Eosinophilia has not been previously reported with copanlisib but may fall within the expected hematologic toxicity profile of PI3K inhibitors, as seen with other agents in monotherapy.31 Longer-term study of the copanlisib plus R-B or R-CHOP regimens in a larger number of patients is ongoing to gain a broader view of the safety profile of each combination; conclusive interpretations are not yet possible from the few patients evaluated in the CHRONOS-4 SRI.
Preliminary evaluation of response to copanlisib plus rituximab-based immunochemotherapy in these small cohorts of patients demonstrated encouraging evidence of efficacy, with objective response rates of 90% in the R-B SRI and 100% in the R-CHOP SRI.
Conclusion
Patients with relapsed indolent B-cell lymphoma represent a population who urgently require additional safe and effective treatment options. Overall, this analysis from the CHRONOS-4 R-B and R-CHOP SRIs demonstrated no unexpected safety concerns or DLTs when combining copanlisib with immunochemotherapy. Copanlisib plus R-B and copanlisib plus R-CHOP were generally well tolerated, and the safety profiles were consistent with reports of copanlisib monotherapy and immunochemotherapy. Copanlisib is the first PI3K inhibitor, including oral agents, to demonstrate preliminary safe, tolerable, and effective combinability with standard immunochemotherapy in this rituximab-sensitive pretreated population at full dose (60 mg). These results support further evaluation of copanlisib 60 mg plus R-B or R-CHOP vs. placebo plus R-B or R-CHOP in the Phase III part of the CHRONOS-4 study, which is currently ongoing, in order to determine the superiority of copanlisib vs. placebo in combination with immunochemotherapy in relapsed indolent B-cell lymphoma.
Clinical Practice Points
Combining continuously administered oral phosphatidylinositol 3-kinase (PI3K) inhibitors (eg, idelalisib and duvelisib) with rituximab-based immunochemotherapy in patients with indolent B-cell lymphoma, for whom there is a high unmet need, has been associated with severe toxicity, such as autoimmune dysfunction, opportunistic infections, diarrhea, and colitis. Copanlisib is an intravenous, intermittently administered pan-PI3K inhibitor that is approved as monotherapy for adult patients with relapsed indolent follicular lymphoma who have received at least 2 systemic therapies.
The safety run-in of the Phase III CHRONOS-4 study enrolled patients with relapsed indolent lymphoma who had relapsed after 1 to 3 lines of treatment; 10 patients received copanlisib with rituximab plus bendamustine (R-B) and 11 patients received copanlisib with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). The most common treatment-emergent adverse events in both groups were consistent with those reported in previous studies of copanlisib monotherapy, and included decreased neutrophil count, nausea, decreased platelet count, hyperglycemia, and hypertension. Preliminary efficacy results demonstrated encouraging evidence of efficacy, with objective response rates of 90% in patients treated with copanlisib plus R-B and 100% with copanlisib plus R-CHOP.
Copanlisib is the first PI3K inhibitor to demonstrate safe, tolerable, and effective combinability with immunochemotherapy in patients with relapsed indolent B-cell lymphoma at full dose (60 mg) and may soon represent a new treatment option in this underserved population. Further evaluation is ongoing to determine the superiority of copanlisib 60 mg plus R-B or R-CHOP vs. placebo plus R-B or R-CHOP in patients with relapsed indolent B-cell lymphoma.
Supplementary Material
Acknowledgments
This study is supported by Bayer AG. Bayer AG was involved in study design, data analysis and interpretation, writing of the report, and in the decision to submit the paper for publication. The funder had no role in data collection.
Lisa Lovelidge, PhD and Tanja Torbica, PhD, CMPP at Complete HealthVizion, McCann Health Medical Communications, provided medical writing support in the development of this manuscript, based on direction from the authors. This assistance was funded by Bayer AG.
Footnotes
Disclosure
MJM: consultancy: Bayer, Daiichi Sankyo, Roche, Genentech, Juno Therapeutics, Merck, Rocket Medical, Seattle Genetics, Takeda, Teva; honoraria: Bayer, Roche, Genentech, GlaxoSmithKline, ImmunoVaccine Technologies, Janssen, Pharmacyclics, Seattle Genetics, Takeda; research funding: Bayer, Roche, Genentech, GlaxoSmithKline, ImmunoVaccine Technologies, Janssen, Pharmacyclics, Rocket Medical, Seattle Genetics. MD: scientific advisory boards: Amgen, AstraZeneca, Bayer, Beigene, BMS/Celgene, Genmab, Gilead/Kite, Janssen, Novartis, Roche; speaker honoraria: Amgen, AstraZeneca, Bayer, BMS/Celgene, Gilead/Kite, Janssen, Roche; institutional research support: AbbVie, Bayer, Celgene, Janssen, Roche. SL: consultancy: Celgene, CHO Pharma USA, Incyte, Gilead, Janssen, Merck, Novartis, Roche, Takeda; honoraria: Merck, Roche, Takeda; research funding: Bayer, Celgene, Genmab, Janssen, Nanovector, Novartis, Roche, Takeda. AS: speaker bureau: AbbVie, Amgen, ArQule, AstraZeneca, Bayer, Bristol Myers Squibb, Celgene, Eisai, Gilead, Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, Servier, Takeda; advisory boards: Bayer, Bristol Myers Squibb, Eisai, Gilead, MSD, Pfizer, Servier; consultancy: ArQule. VB, BHC: employees of Bayer HealthCare Pharmaceuticals, Inc. MF: employee of Bayer AG. PLZ: honoraria: AbbVie, ADC Therapeutics, Bristol Myers Squibb, EUSA Pharma, Gilead, Incyte, Janssen, Kyowa Kirin, Merck, MSD, Roche, Servier, Takeda, TG Therapeutics, Verastem; board of directors or advisory committee memberships: AbbVie, ADC Therapeutics, Bristol Myers Squibb, Celgene, Celltrion, EUSA Pharma, Gilead, Immune Design, Incyte, Janssen-Cilag, Kyowa Kirin, Merck, MSD, Portola, Roche, Sandoz, Servier, Takeda, Verastem; speaker bureau: AbbVie, ADC Therapeutics, Bristol Myers Squibb, Celgene, Celltrion, EUSA Pharma, Gilead, Immune Design, Incyte, Janssen, Janssen-Cilag, Kyowa Kirin, Merck, MSD, Portola, Roche, Servier, Takeda, TG Therapeutics, Verastem; consultancy: EUSA Pharma, Janssen, MSD, Sanofi, Verastem; research funding: Portola. MP: none.
Supplementary material
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.clml.2021.06.021.
References
- 1.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dreyling M, Ghielmini M, Rule S, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:298–308. [DOI] [PubMed] [Google Scholar]
- 3.Batlevi CL, Sha F, Alperovich A, et al. Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups. Blood Cancer J. 2020;10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burris HA 3rd. Flinn IW, Patel MR, et al. Umbralisib, a novel PI3Kδ and casein kinase-1ε inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study. Lancet Oncol. 2018;19:486–496. [DOI] [PubMed] [Google Scholar]
- 5.Fowler NH, Samaniego F, Jurczak W, et al. Umbralisib monotherapy demonstrates efficacy and safety in patients with relapsed/refractory marginal zone lymphoma: a multicenter, open label, registration directed phase II study. J Clin Oncol. 2019;37:7506. [Google Scholar]
- 6.US Food and Drug Administration. ZYDELIG (idelalisib) highlights of prescribing information. 2014. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206545lbl.pdf. Accessed March 1, 2021.
- 7.US Food and Drug Administration. COPIKTRA (duvelisib) highlights of prescribing information. 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211155s000lbl.pdf. Accessed March 1,2021.
- 8.US Food and Drug Administration. ALIQOPA (copanlisib) highlights of prescribing information. 2020. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209936s004lbl.pdf. Accessed March 1, 2021.
- 9.Liu N, Haegebarth A, Bull C, et al. BAY 80-6946, a highly potent and efficacious PI3K class I inhibitor, induces complete tumor regression or tumor stasis in tumor xenograft models with PIK3CA mutant or PTEN deletion. Poster 4478 presented at 101st Annual Meeting of the American Association for Cancer Research; 2010. [Google Scholar]
- 10.Seiler T, Hutter G, Dreyling M. The emerging role of PI3K inhibitors in the treatment of hematological malignancies: preclinical data and clinical progress to date. Drugs. 2016;76:639–646. [DOI] [PubMed] [Google Scholar]
- 11.Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017;35:3898–3905. [DOI] [PubMed] [Google Scholar]
- 12.Morschhauser F, Machiels JP, Salles G, et al. On-target pharmacodynamic activity of the PI3K inhibitor copanlisib in paired biopsies from patients with malignant lymphoma and advanced solid tumors. Mol Cancer Ther. 2020;19:468–478. [DOI] [PubMed] [Google Scholar]
- 13.Zinzani PL, Santoro A, Mollica L, et al. Copanlisib, a PI3K inhibitor, demonstrates a favorable long-term safety profile in a pooled analysis of patients with hematologic malignancies. Blood. 2019;134(suppl 1):4009. [Google Scholar]
- 14.Matasar MJ, Capra M, Özcan M, et al. Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma (CHRONOS-3): a randomised, phase 3 trial. Lancet Oncol. 2021;22:678–689. [DOI] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration. FDA alerts healthcare professionals about clinical trials with Zydelig (idelalisib) in combination with other cancer medicines. 2016. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm490618.htm. Accessed March 1, 2021.
- 16.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owen RG, Kyle RA, Stone MJ, et al. Response assessment in Waldenström macroglobulinaemia: update from the VIth International Workshop. Br J Haematol. 2013;160:171–176. [DOI] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration. ALIQOPA (copanlisib) highlights of prescribing information. 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209936s000lbl.pdf. Accessed March 1, 2021.
- 19.Dreyling M, Santoro A, Mollica L, et al. Long-term safety and efficacy of the PI3K inhibitor copanlisib in patients with relapsed or refractory indolent lymphoma: 2-year follow-up of the CHRONOS-1 study. Am J Hematol. 2020;95:362–371. [DOI] [PubMed] [Google Scholar]
- 20.Busaidy NL, Farooki A, Dowlati A, et al. Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J Clin Oncol. 2012;30:2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patnaik A, Appleman LJ, Tolcher AW, et al. First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin’s lymphomas. Ann Oncol. 2016;27:1928–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiddemann W, Barbui AM, Canales MA, et al. Immunochemotherapy with obinutuzumab or rituximab for previously untreated follicular lymphoma in the GALLIUM study: influence of chemotherapy on efficacy and safety. J Clin Oncol. 2018;36:2395–2404. [DOI] [PubMed] [Google Scholar]
- 23.Salles G. Seymour JF. Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42–51. [DOI] [PubMed] [Google Scholar]
- 24.Flinn IW, Miller CB, Ardeshna KM, et al. DYNAMO: a phase II study of duvelisib (IPI-145) in patients with refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2019;37:912–922. [DOI] [PubMed] [Google Scholar]
- 25.Salles G. Barrett M, Foà R, et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017;34:2232–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lampson BL, Kim HT, Davids MS, et al. Efficacy results of a phase 2 trial of first-line idelalisib plus ofatumumab in chronic lymphocytic leukemia. Blood Adv. 2019;3:1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flinn IW, Cherry MA, Maris MB, Matous JV, Berdeja JG, Patel M. Combination trial of duvelisib (IPI-145) with rituximab or bendamustine/rituximab in patients with non-Hodgkin lymphoma or chronic lymphocytic leukemia. Am J Hematol. 2019;94:1325–1334. [DOI] [PubMed] [Google Scholar]
- 28.Greenwell IB, Ip A, Cohen JB. PI3K inhibitors: understanding toxicity mechanisms and management. Oncology (Williston Park). 2017;31:821–828. [PubMed] [Google Scholar]
- 29.Cuneo A, Barosi G, Danesi R, et al. Management of adverse events associated with idelalisib treatment in chronic lymphocytic leukemia and follicular lymphoma: a multidisciplinary position paper. Hematol Oncol. 2019;37:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flinn IW, Hillmen P, Montillo M, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132:2446–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soria JC, LoRusso P, Bahleda R, et al. Phase I dose-escalation study of pilaralisib (SAR245408, XL147), a pan-class I PI3K inhibitor, in combination with erlotinib in patients with solid tumors. Oncologist. 2015;20:245–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.