Abstract
The aim of this study was to investigate the effects of different exercise modes on improving inflammatory response in the elderly. For the research methodology, databases such as CNKI (China National Knowledge Infrastructure), Wanfang Data, Pubmed, Web of Science, and EBSCO were selected for searching. The Cochrane Risk of Bias (ROB) tool was used to evaluate the methodological quality of the included studies, and RevMan5.4.1 analysis software was applied for the statistical analysis. A total of 31 studies (20 randomized controlled trials and 11 self-controlled trials) with 1528 subjects were included. The results of this meta-analysis showed that aerobic exercise, resistance exercise, aerobic + resistance exercise, and HIIT all significantly reduced the levels of IL-6, TNF-α, and CRP in the elderly, and the improvement effects of aerobic + resistance exercise on IL-6, HIIT on TNF-α, and resistance exercise on CRP in the elderly were better than those of the other three exercise modes, respectively. In conclusion, aerobic exercise, resistance exercise, aerobic + resistance exercise, and HIIT all contribute to ameliorating the inflammatory status of the elderly, among which resistance exercise is a noteworthy exercise mode for the elderly to improve inflammatory status.
Keywords: exercise modes, the elderly, inflammation, meta-analysis
1. Introduction
As elderly individuals age, the increase in inflammatory factors often leads to a systemic and chronic inflammatory response state [1], which ultimately leads to poor vaccine efficacy, increased incidence of opportunistic infections, and a rising rate of morbidity and mortality in the elderly [2]. Regular participation in physical activity can help to decrease the incidence of different types of chronic diseases. It has been reported that the protective effect of exercise is associated with the improvement of immune function, which may regulate the status and function of macrophage polarization and contribute to the promotion of wound healing and sleep quality. Furthermore, regular exercise has been shown to be associated with a decreased risk of cancer and delayed tumorigenesis in the elderly [3,4,5].
Immune senescence is a progressive degeneration of the immune system function associated with human aging, which has been described as an increase in circulating concentrations of classical pro-inflammatory cytokines. Regarding systemic inflammation, elevated levels of the acute-phase protein C-reactive protein (CRP) and cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) have been found in the elderly [6]. These inflammatory markers not only accelerate the aging process but are also associated with an increased risk of developing cardiovascular diseases and cancers [7]. Currently, some studies have found that exercise reduces the levels of IL-6, TNF-α, and CRP, while others have reported that exercise does not improve the inflammatory status of the body [7]. Previous meta-analyses of the inflammatory response to exercise in the elderly included insufficient studies and the methodological quality of the included studies was relatively low [8,9,10]. In addition, that only one form of exercise was discussed, the inclusion of non-exercise forms of interventions in the study, and the wide age range of subjects and inaccurate results illustrate the limitations of the previous meta-analyses [8,11].
Although exercise has been shown to be effective in lowering IL-6, TNF-α, and CRP levels, there is no consensus on the improvement effects of different exercise modes on IL-6, TNF-α, and CRP in the elderly [8]. The different exercise modes studied here included aerobic exercise, resistance exercise, aerobic + resistance exercise, and HIIT. Aerobic exercise is a series of exercises that rely primarily on aerobic energy-production processes. “Aerobic” is defined as “involving, relating to, or requiring oxygen” and refers to the adequate use of oxygen to meet energy requirements through aerobic metabolism during exercise. Aerobic exercise is performed by repeating a series of light- to moderate-intensity activities over a long period of time. Resistance exercise involves physical exercise designed to improve strength and endurance. It is usually associated with weightlifting, and it can also include various training techniques such as calisthenic, isometric, and plyometric techniques. High-Intensity Interval Training (HIIT) is a training regimen that alternates short periods of high-intensity or explosive anaerobic exercise with short recovery periods until depleted, thus relying almost maximally on the anaerobic energy release system [12]. The purpose of this meta-analysis was to compare the effects of different exercise modes on the inflammatory response level in the elderly, so as to provide a reliable theoretical reference for the rational selection of exercise modes for the related diseases caused by systemic chronic inflammatory response in the elderly.
2. Materials and Methods
2.1. Selection and Exclusion Criteria of Literature
Inclusion criteria: (1) study subjects: physically and mentally healthy middle-aged and elderly people, free of diabetes, cardiovascular disease, and cancer, aged from 51 to 91 years old; (2) study types: randomized controlled trials or self-controlled trials; (3) intervention: exercise intervention; (4) outcome indicators: include one or more of IL-6, TNF-α, and CRP.
2.2. Searching Strategy
The literature search was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The title, abstract, keywords, and search fields were searched in CNKI (China National Knowledge Infrastructure), Wanfang Data, PubMed, Web of Science, and EBSCO, and the search period was until May 2022. The search was performed using Boolean operators combined with the following terms: “elderly”, “older”, “inflammatory reaction”, “inflammatory response”, “inflammatory factors”, “inflammatory”, “aerobic exercise”, “resistance exercise”, “high-intensity interval exercise (HIIT)”, “HIIT”, and “exercise intervention”. Additional searches were conducted within the reference lists of the included records.
2.3. Data Extraction and Quality Evaluation
2.3.1. Data Extraction and Processing
The Endnote citation manager software was applied to conduct the literature screening. The first author of the literature, the year of publication, the sample-size number, the characteristics of the participants (gender, the number of male and female participants, age), the characteristics of the intervention (exercise modes, intervention protocol, duration, frequency), and the outcomes were extracted from the literature. Literature screening and data extraction were conducted independently by two researchers, who consulted each other after completion. If there was any disagreement, the studies would be submitted to a third researcher for further discussion and decision.
2.3.2. Literature Quality Evaluation
Two researchers independently conducted the evaluation of the methodological quality and the risk of bias of the included studies according to the Cochrane Handbook 5.1.0, and the studies were assessed by the following aspects: (1) random sequence generation; (2) allocation concealment; (3) selective reporting; (4) blinding of participants and personnel; (5) blinding of outcome assessment; (6) incomplete outcome data; and (7) other biases. Based on the evaluation criteria, the studies were classified as “low risk”, “medium risk”, “high risk” or “unknown risk”. For the self-controlled trials, the ROBINS-I 2.0 (Risk of Bias in Non-randomized Studies of Interventions) was used to evaluate the methodological quality and the risk of bias.
3. Results
3.1. General Results of Selected Research Literature
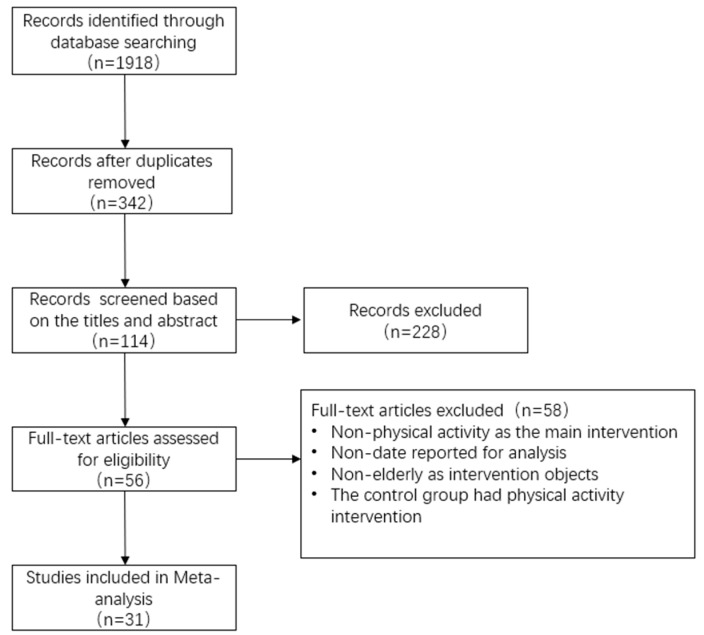
After screening the literature strictly according to the established uniform inclusion and exclusion criteria, we found that the sample size of the randomized controlled trials was limited and that many of the screened studies were self-controlled trials. Considering that the limited number of randomized controlled trials may reduce the reliability of the meta-analysis, the low-risk and high-quality self-controlled trials and studies with participants aged 51 years or older were also included in the study. For the self-controlled trials, meta-analyses were performed to compare the differences between “pre-exercise intervention” and “post-exercise intervention” data. After the final screening, 31 articles with a total sample size of 1528 were included, and the studies were mainly conducted in healthy elderly people without diabetes, cardiovascular disease, and cancer. The flow chart of the literature screening is shown in Figure 1.
Figure 1.
Literature selection process.
3.2. General Features of the Selected Research Literature
3.2.1. General Information of Each Study
Through rigorous screening according to the inclusion and exclusion criteria, 20 randomized controlled trials (RCT) and 11 self-controlled trials (SCT) ultimately met the inclusion criteria for the meta-analysis, and a total of 1528 subjects were included in these 31 trials. Among the included studies, 11 studies included aerobic exercise (AE), 16 studies included resistance exercise (RE), 3 studies included aerobic + resistance exercise (AE+RE), and 2 studies included HIIT (see Table 1).
Table 1.
General features of selected research literature.
| First Author | Year | Type of Study, Health Condition | Age (Years) | Sample Size (n) | Intervention | Frequency | Duration | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Control Group | Intervention Group | Control Group | Intervention Group | |||||||
| Rall and Roubenoff et al. [13] | 1996 | SCT, Healthy |
70.3 ± 5.0 | 8 | RE | 1/2W | 12 W | IL-6 | ||
| Arsenault and Côté et al. [14] | 2009 | RCT, Overweight/Obesity |
57.2 ± 6.1 | 57.3 ± 6.6 | 82 | 267 | AE | 3–4/W | 6 M | IL-6, TNF-α, CRP |
| Campbell and Campbell et al. [15] | 2009 | RCT, Overweight |
60.9 ± 6.8 | 60.5 ± 7.0 | 62 | 53 | AE | 12 M | CRP, IL-6 | |
| Beavers and Hsu et al. [16] | 2010 | RCT, Healthy |
77.0 ± 4.4 | 76.4 ± 4.1 | 186 | 183 | AE | 3/W | 12 M | IL-6, CRP |
| Friedenreich and Neilson et al. [17] | 2012 | RCT, Healthy |
60.6 ± 5.7 | 61.2 ± 5.4 | 160 | 160 | AE | 5/W | 12 M | IL-6, TNF-α, CRP |
| Irwin and Olmstead [18] | 2012 | SCT, Healthy |
70.7 ± 5.9 | 46 | AE | 2/W | 25 W | IL-6, CRP | ||
| Beavers and Ambrosius et al. [19] | 2013 | RCT, Overweight/Obesity |
60–79 | 60–79 | 93 | 97 | AE | 5/W | 18 M | IL-6 |
| Ho and Dhaliwal et al. [20] | 2013 | RCT, Overweight/Obesity |
52 (40–66) | 53 (43–64) | 16 | 17 | AE+RE | 3/W | 12 M | IL-6, TNF-α |
| So and Song et al. [21] | 2013 | RCT, Healthy |
68.4 ± 5.8 | 71.6 ± 5.5 | 22 | 18 | RE | 3/W | 12 W | IL-6, TNF-α |
| Wanderley and Moreira et al. [22] | 2013 | RCT, Healthy |
67.8 ± 5.5 | 67.3 ± 4.9 | 11 | 19 | RE | 2/W 8/M |
IL-6, TNF-α | |
| 69.9 ± 5.7 | 20 | AE | ||||||||
| Tomeleri and Ribeiro et al. [23] | 2016 | RCT, Healthy |
69.5 ± 4.7 | 66.8 ± 3.2 | 19 | 19 | RE | 3/W | 8 W | IL-6, TNF-α, CRP |
| Li Shugang et al. [24] | 2014 | SCT, Healthy |
65.65 ± 3.14 | 17 | RE | 2/W | 16 W | IL-6, TNF, CRP | ||
| Wang He et al. [25] | 2015 | RCT, Healthy |
63.39 ± 3.43 | 63.53 ± 3.12 | 20 | 25 | AE | 5/W | 6 M | IL-6, TNF |
| Li Shiguang er al. [26] | 2015 | SCT, Healthy |
70.63 ± 3.93 | 11 | RE | 3/W | 12 W | IL-6, TNF, CRP | ||
| Forti and Van Roie et al. [27] | 2016 | SCT, Healthy |
67.86 ± 4.36 | 17 | RE | 3/W | 12 W | IL-6 | ||
| Friedenreich and O’Reilly et al. [28] | 2016 | SCT, Healthy |
59.4 ± 4.8 | 200 | AE | 5/W | 12 M | IL-6, TNF-α, CRP | ||
| Rondanelli and Klersy et al. [29] | 2016 | SCT, Healthy |
80.21 ± 8.54 | 61 | AE + RE | 5/W | 12 W | CRP | ||
| Allen and Higham et al. [30] | 2017 | RCT, Healthy |
49.2 ± 6.1 | 14 | 20 | HIIT | 3/W | 9 W | TNF-α, CRP | |
| Chupel and Minuzzi et al. [31] | 2018 | SCT, Healthy |
83.5 ± 7.3 | 13 | RE | 2/W | 14 W | IL-6, TNF-α | ||
| Chen and Wu et al. [32] | 2018 | RCT, Healthy |
68.3 ± 2.8 | 66.7 ± 5.3 | 16 | 17 | RE | 2/W | 8 W | CRP, IL-6, TNF-α |
| Tomeleri and Ribeiro et al. [33] | 2018 | RCT, Healthy |
68.8 ± 4.6 | 71.0 ± 5.4 | 22 | 24 | RE | 3/W | 12 W | IL-6, TNF-α, CRP |
| Tomeleri and Souza et al. [34] | 2018 | RCT, Healthy |
68.8 ± 4.9 | 72.1 ± 6.3 | 23 | 22 | RE | 3/W | 18 W | IL-6, TNF-α, CRP |
| Abd and Al-Shreef [7] | 2018 | SCT, Healthy |
65.96 ± 3.42 | 40 | RE | 3/W | 6 M | IL-6, TNF-α | ||
| Abd and Al-Jiffri [35] | 2019 | RCT, Healthy |
61–67 | 25 | 25 | AE | 3/W | 6 M | IL-6, TNF-α | |
| Nunes and Martins et al. [36] | 2019 | RCT, Healthy |
62.9 ± 2.25 | 62.3 ± 2.08 | 13 | 13 | HIIT | 3/W | 12 W | IL-6 |
| Urzi and Marusic et al. [37] | 2019 | RCT, Healthy |
88.9 ± 5.3 | 84.4 ± 7.7 | 9 | 11 | RE | 3/W | 12 W | CRP |
| Chen Tiantian et al. [38] | 2020 | RCT, Healthy |
63.59 ± 2.21 | 64.75 ± 2.89 | 10 | 20 | AE | 6/W | 12 M | IL-6, CRP |
| de Castro and Da et al. [39] | 2020 | SCT, Healthy |
67.36 ± 7.13 | 14 | RE | Dis | IL-6, TNF-α | |||
| Despeghel and Reichel et al. [40] | 2021 | RCT, Healthy |
69.8 ± 4.4 | 70.4 ± 5.3 | 10 | 30 | AE + RE | - | 6 W | IL-6, TNF-α |
| Timon and Martínez-Guardado et al. [41] | 2021 | RCT, Healthy |
70.5 ± 4.0 | 70.3 ± 3.3 | 19 | 18 | RE | 3/W | 24 W | IL-6, CRP |
| Xiao Youding et al. [42] | 2022 | SCT, Healthy |
70.79 ± 4.91 | 23 | RE | 3/W | 16 W | TNF-α, CRP | ||
W: week(s); M: month(s); Dis: disposable.
3.2.2. Quality Evaluation of the Selected Literature
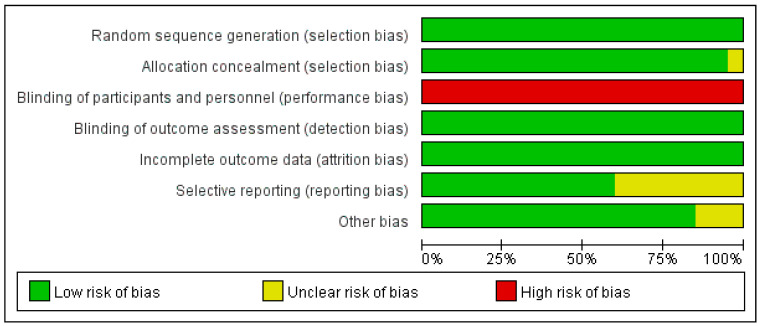
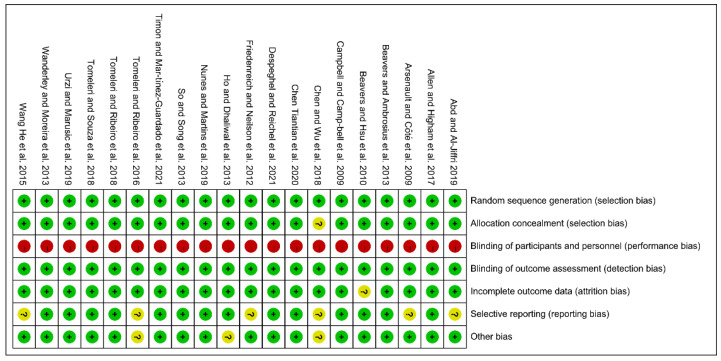
A total of 31 studies, including 20 randomized controlled trials and 11 self-controlled trials, were included in the study. Due to the characteristics of the exercise intervention, the assessment of performance bias (blinding of participants and personnel) was excluded from the evaluation. Finally, the risk of bias in all of the included 20 RCT studies was moderate, with 1 study having a moderate risk of selection bias, 8 studies having a moderate risk of reporting bias, and 3 studies having a moderate risk of other bias. After the exclusion of the bias due to deviations from the intended interventions, all 11 self-controlled trials had a low-to-moderate risk of bias. (Figure 2 and Figure 3 and Table 2).
Figure 2.
Risk of bias graph.
Figure 3.
Risk of bias summary [14,15,16,17,19,20,21,22,25,30,32,33,34,35,36,37,38,40,41].
Table 2.
Results of quality evaluation of included self-controlled trial literature.
| Study | Bias Due to Confounding | Bias in Selection of Participants into the Study | Bias in Classification of Interventions | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result |
|---|---|---|---|---|---|---|---|
| Abd and Al-Shreef [7] | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| Chupel and Minuzzi et al. [31] | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| de Castro and Da et al. [39] | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| Forti and Van Roie et al. [27] | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| Friedenreich and O’Reilly et al. [28] | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| Irwin and Olmstead [18] | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| Rall and Roubenoff et al. [13] | Medium risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| Rondanelli and Klersy et al. [29] | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| Li Shiguang et al. [26] | Low risk | Low risk | Low risk | High risk | Low risk | Medium risk | Low risk |
| Li Shugang et al. [24] | Low risk | Low risk | Low risk | High risk | Low risk | Medium risk | Low risk |
| Xiao Youding et al. [42] | Medium risk | Low risk | Low risk | High risk | Low risk | Medium risk | Low risk |
3.3. Effects of Different Exercise Modes on IL-6 in the Elderly
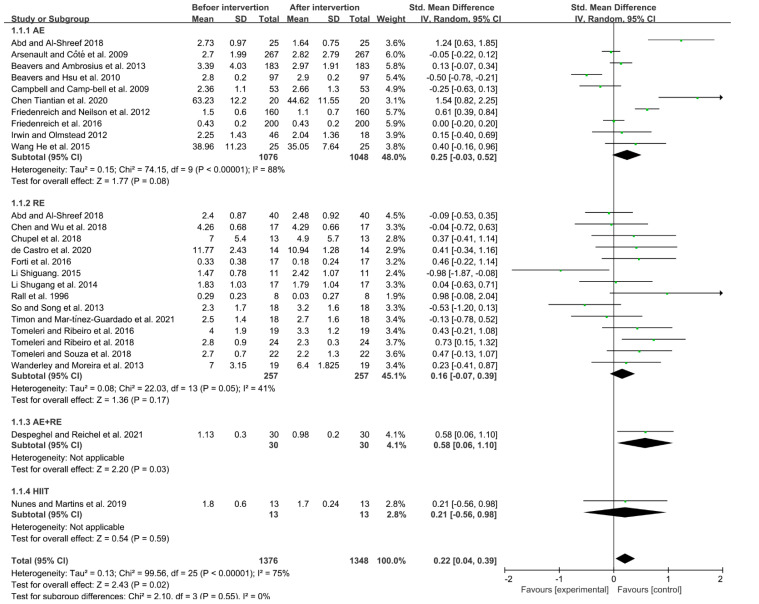
Due to the high heterogeneity between the studies (I2 = 75%, p < 0.05), a random-effects model was used for analysis. The results indicated that exercise could significantly reduce the level of IL-6 in the elderly (SMD = 0.22, 95% CI = 0.04~0.39, p < 0.05). Subgroup analysis showed that aerobic exercise, resistance exercise, aerobic + resistance exercise, and HIIT could all reduce the level of IL-6 in the elderly (aerobic exercise: SMD = 0.25, 95% CI = −0.03~0.52, p = 0.08; resistance exercise: SMD = 0.16, 95% CI = −0.07~0.39, p = 0.17). Meanwhile, both aerobic + resistance exercise and HIIT could lower the level of IL-6 in the elderly (aerobic + resistance exercise: SMD = 0.58, 95% CI = 0.06~1.10, p < 0.05; HIIT: SMD = 0.21, 95% CI = −0.56~0.98, p = 0.59). The aerobic + resistance exercise mode had a better effect than the other exercise modes in reducing IL-6 in the elderly (Figure 4).
Figure 4.
Effects of different exercise modes on IL-6 in the elderly [7,13,14,15,16,17,18,19,21,22,24,25,26,27,28,31,32,33,34,36,38,39,40,41].
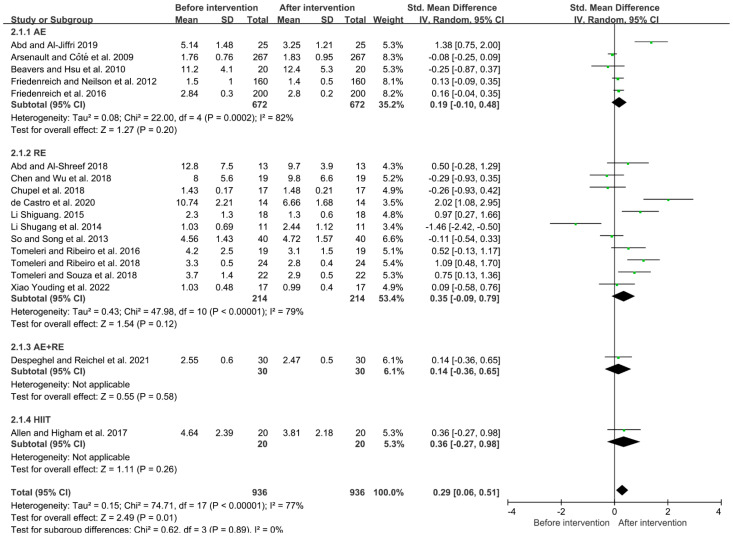
3.4. Effects of Different Exercise Modes on TNF-α in the Elderly
Due to the high heterogeneity among the studies (I2 = 77%, p < 0.05), the random-effects model was used for analysis. The results showed that exercise could significantly reduce the level of TNF-α in the elderly (SMD = 0.29, 95% CI = 0.06~0.51, p < 0.05). Subgroup analysis showed that aerobic exercise, resistance exercise, aerobic + resistance exercise, and HIIT could all reduce the level of TNF-α in the elderly (aerobic exercise: SMD = 0.19, 95% CI = −0.10~0.48, p = 0.20; resistance exercise: SMD = 0.35, 95% CI = −0.09~0.79, p = 0.12; aerobic + resistance exercise: SMD = 0.14, 95% CI = −0.36~0.65, p = 0.58; HIIT: SMD = 0.36, 95% CI = −0.27~0.98, p = 0.26). The HIIT mode had a better effect on the reduction in TNF-α in the elderly compared to other exercise modes (Figure 5).
Figure 5.
Effects of different exercise modes on TNF-α in the elderly [7,14,16,17,21,23,24,26,28,30,31,32,33,34,35,39,40,42].
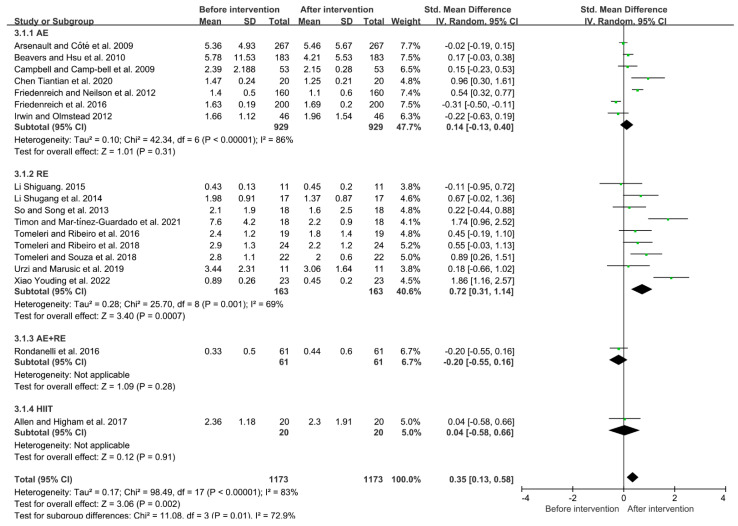
3.5. Effects of Different Exercise Modes on CRP in the Elderly
Due to the high heterogeneity between the studies (I2 = 83%, p < 0.01), the random-effects model was used for analysis. The results showed that exercise could significantly reduce the level of CRP in the elderly (SMD = 0.26, 95% CI = 0.08~0.43, p < 0.05). Subgroup analysis showed that aerobic exercise, resistance exercise and HIIT could all reduce the level of CRP in the elderly (aerobic exercise: SMD = 0.14, 95% CI = −0.13~0.40, p = 0.31; resistance exercise: SMD = 0.72, 95% CI = 0.31~1.14, p < 0.01; aerobic + resistance exercise: SMD = −0.20, 95% CI = −0.55~0.16, p = 0.28; HIIT: SMD = 0.04, 95% CI = −0.58~0.66, p = 0.91). The resistance exercise mode had a better effect than the other exercise modes in decreasing CRP in the elderly (Figure 6).
Figure 6.
Effects of different exercise modes on CRP in the elderly [14,15,16,17,18,21,23,24,26,28,29,30,33,34,37,38,41,42].
4. Discussion
The results showed that both aerobic exercise and resistance exercise reduced the levels of IL-6, TNF-α, and CRP in the elderly and that resistance exercise had a better effect than aerobic exercise, which is consistent with previous studies [8]. These results may be due to the fact that resistance exercise can increase the antioxidant capacity of skeletal muscle in the elderly [9] through up-regulating superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX), thus improving the anti-inflammatory capacity [43].
Aerobic exercise, resistance exercise, and aerobic + resistance exercise all have a better effect than HIIT in reducing the level of IL-6 in the elderly, which is similar to previous studies [44]. It is speculated that HIIT may lead to an increase in the expression level of the pro-inflammatory factor IL-6 (Table 3). However, it was found that the increase in IL-6 induced by HIIT had no adverse effects on health [45], as the increase in IL-6 induced by HIIT may have anti-inflammatory properties. This speculation was confirmed in the subsequent discussion on TNF-α.
Table 3.
Subgroup analysis of the effect of different modes on inflammatory response in the elderly.
| Subgroup | IL-6 | TNF-α | CRP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Effect Size | 95% CI | n | I2 | Effect Size | 95% CI | n | I2 | Effect Size | 95% CI | n | I2 |
| AE | 0.25 | −0.03, 0.52 | 1076 | 88% | 0.19 | −0.10, 0.48 | 672 | 82% | 0.14 | −0.13, 0.40 | 929 | 86% |
| RE | 0.16 | −0.07, 0.39 | 257 | 41% | 0.35 | −0.09, 0.79 | 214 | 79% | 0.72 | 0.31, 1.14 | 163 | 89% |
| AE + RE | 0.58 | 0.06, 1.10 | 30 | — | 0.14 | −0.36, 0.65 | 30 | — | −0.2 | −0.55, 0.16 | 61 | — |
| HIIT | 0.21 | −0.56, 0.98 | 13 | — | 0.36 | −0.27, 0.98 | 20 | — | 0.04 | −0.58, 0.66 | 20 | — |
| Total | 0.22 | 0.04, 0.39 | 1376 | 75% | 0.29 | 0.06, 0.51 | 936 | 77% | 0.35 | 0.13, 0.58 | 1173 | 83% |
As for TNF-α, we found that different exercise modes could all reduce the level of TNF-α in the elderly, of which HIIT had the most significant effect (Table 2). In addition to the increase in anti-inflammatory factor IL-6 induced by HIIT, there is also a prolonged excessive oxygen consumption following the end of HIIT [46]. In the state of excessive oxygen consumption, the rate of lipid metabolism increases. Given that adipose tissue continuously secretes inflammatory factors, we speculate that HIIT can reduce the level of TNF-α by improving lipid metabolism in the elderly. In addition, we observed that aerobic exercise did not have a significant effect on ameliorating the level of TNF-α; this may be because of the short intervention period (6 months to 12 months) included in the studies. Some studies suggested that aerobic exercise takes at least one year to result in a significant reduction in the level of TNF-α [47].
Finally, the results showed that the improvement effect of resistance exercise on CRP is more significant than in other exercise modes (Table 2). Unlike IL-6 and TNF-α, CRP is an acute-phase plasma protein not only synthesized by the liver but also by adipocytes. One of its functions is to send signals to the immune system, instructing which cells participate in the defense response to infections and the inflammatory response of the body [48]. Like other cytokines, CRP is associated with cardiovascular disease and diabetes and is one of the factors of chronic inflammatory response in the human body. Resistance exercise can stimulate skeletal muscle metabolism by activating the 5′ adenosine monophosphate-activated protein kinase (AMPK) system [9]. Through this, it can help regulate hepatic glycogen synthesis and fat decomposition, delay muscle reduction, and increase body metabolism throughout the day, thus reducing CPR levels and ameliorating inflammation levels.
5. Limitations of Current Research
Despite our strict adherence to the procedures set out in PRISMA, the literature related to AE + RE and HIIT interventions is limited for our study, resulting in the possibility of heterogeneity in the results.
The elderly population itself may have elevated levels of inflammatory factors due to aging, which may have interfered with the assessment of inflammatory factor changes; the exercise interventions involved multiple movements and forms, and the training environment was not fixed, which may have biased the results due to other factors.
6. Conclusions
Based on the results of this meta-analysis, we can draw several conclusions. Aerobic exercise, resistance exercise, aerobic + resistance exercise, and HIIT can all contribute to ameliorating the level of inflammation in the elderly and reduce the levels of pro-inflammatory factors IL-6, TNF-α and CRP.
In particular, aerobic exercise, resistance exercise, and aerobic + resistance exercise all have a better effect than HIIT on reducing the level of IL-6 in the elderly, while HIIT is superior to other exercise methods in improving the anti-inflammatory characteristics of the body. Meanwhile, resistance exercise was superior to other exercise modalities in improving the inflammatory response, which may be a noteworthy exercise modality for improving inflammatory status in the elderly.
Acknowledgments
The authors are grateful to all the participants who were involved in this study.
Author Contributions
Conceptualization, H.Z., Z.H. and H.Y.; methodology, H.Z.; Data curation, H.Z. and Z.H.; Writing—the original draft, H.Z., Z.H. and H.Y.; Writing—reviewing and editing, H.Z., Z.H., R.W. and C.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sun J.Y., Shuangguan R.N., Yan Y., Ye J.P., Xie M.H. Research progress on the correlation between cardiopulmonary fitness and inflammatory markers and its mechanism. Chin. J. Rehabil. Med. 2016;31:228–234. [Google Scholar]
- 2.Noordam R., Oudt C.H., Bos M.M., Smit R. High-sensitivity C-reactive protein, low-grade systemic inflammation and type 2 diabetes mellitus: A two-sample Mendelian randomization study. Nutr. Metab. Cardiovasc. Dis. NMCD. 2018;28:795–802. doi: 10.1016/j.numecd.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Nuvagah F.L., Rose N., Ingo B., Elke E., Romain M., Tony M., Ivan B. Strength training reduces circulating interleukin-6 but not brain-derived neurotrophic factor in community-dwelling elderly individuals. Age. 2014;36:9704. doi: 10.1007/s11357-014-9704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J., Chen P.H., Xiao W.H. Obesity leads to skeletal muscle insulin resistance—Mediated by inflammatory factors and ameliorative effects of exercise. Chin. J. Sports Med. 2020;39:226–231. [Google Scholar]
- 5.Cong M.H., Shi H.P. Consensus of Chinese experts on exercise therapy for cancer patients. Sci. Sin. Vitae. 2022;52:587–602. doi: 10.1360/SSV-2022-0028. (In Chinese) [DOI] [Google Scholar]
- 6.Bruunsgaard H., Skinhøj P., Pedersen A.N., Schroll M., Pedersen B. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin. Exp. Immunol. 2000;121:255–260. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abd E.S., Al-Shreef F.M. Inflammatory cytokines and immune system modulation by aerobic versus resisted exercise training for elderly. Afr. Health Sci. 2018;18:120–131. doi: 10.4314/ahs.v18i1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monteiro-Junior R.S., Maciel-Pinheiro P.D., Portugal E.D.M., Figueiredo L.F.D., Terra R., Carneiro L.S.F., Rodrigues V.D., Nascimento O.J.M., Deslandes A.C., Laks J. Effect of Exercise on Inflammatory Profile of Older Persons: Systematic Review and Meta-Analyses. J. Phys. Act. Amp. Health. 2018;15:64–71. doi: 10.1123/jpah.2016-0735. [DOI] [PubMed] [Google Scholar]
- 9.Johnson M.L., Irving B.A., Lanza I.R., Vendelbo M.H., Konopka A.R., Robinson M.M., Henderson G.C., Klaus K.A., Morse D.M., Carrie H. Differential Effect of Endurance Training on Mitochondrial Protein Damage, Degradation, and Acetylation in the Context of Aging. J. Gerontol. 2015;70:1386. doi: 10.1093/gerona/glu221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yousefabadi H.A., Niyazi A., Alaee S., Fathi M., Rahimi G.R.M. Anti-Inflammatory Effects of Exercise on Metabolic Syndrome Patients: A Systematic Review and Meta-Analysis. Biol. Res. Nurs. 2020;22:1185452866. doi: 10.1177/1099800420958068. [DOI] [PubMed] [Google Scholar]
- 11.Kim S.D., Yeun Y.R. Effects of Resistance Training on C-Reactive Protein and Inflammatory Cytokines in Elderly Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health. 2022;19:3434. doi: 10.3390/ijerph19063434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Y., Sun Z., Ya X., Zhou L., Wang M., Wang X., Liu Y. Effect of exercise training on arterial stiffness in obese and overweight children: A meta-analysis. Eur J. Pediatr. 2022;181:2633–2642. doi: 10.1007/s00431-022-04489-6. [DOI] [PubMed] [Google Scholar]
- 13.Rall L.C., Roubenoff R., Cannon J.G., Abad L.W., Meydani S.N. Effects of progressive resistance training on immune response in aging and chronic inflammation. Med. Amp. Ence. Sports Amp. Exerc. 1996;28:1356–1365. doi: 10.1097/00005768-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Arsenault B.J., Côté M., Cartier A., Lemieux I., Després J.P., Ross R., Earnest C.P., Blair S.N., Church T.S. Effect of exercise training on cardiometabolic risk markers among sedentary, but metabolically healthy overweight or obese post-menopausal women with elevated blood pressure. Atherosclerosis. 2009;207:530–533. doi: 10.1016/j.atherosclerosis.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell P.T., Campbell K.L., Wener M.H., Wood B.L., Potter J.D., McTiernan A., Ulrich C.M. A yearlong exercise intervention decreases CRP among obese postmenopausal women. Med. Sci. Sports Exerc. 2009;41:1533–1539. doi: 10.1249/MSS.0b013e31819c7feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beavers K.M., Hsu F.C., Isom S., Kritchevsky S.B., Church T., Goodpaster B., Pahor M., Nicklas B.J. Long-term physical activity and inflammatory biomarkers in older adults. Med. Sci. Sports Exerc. 2010;42:2189–2196. doi: 10.1249/MSS.0b013e3181e3ac80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedenreich C.M., Neilson H.K., Woolcott C.G., Wang Q., Stanczyk F.Z., McTiernan A., Jones C.A., Irwin M.L., Yasui Y., Courneya K.S. Inflammatory marker changes in a yearlong randomized exercise intervention trial among postmenopausal women. Cancer Prev. Res. 2012;5:98–108. doi: 10.1158/1940-6207.CAPR-11-0369. [DOI] [PubMed] [Google Scholar]
- 18.Irwin M.R., Olmstead R. Mitigating cellular inflammation in older adults: A randomized controlled trial of Tai Chi Chih. Am. J. Geriatr. Psychiatry. 2012;20:764–772. doi: 10.1097/JGP.0b013e3182330fd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beavers K.M., Ambrosius W.T., Nicklas B.J., Rejeski W.J. Independent and combined effects of physical activity and weight loss on inflammatory biomarkers in overweight and obese older adults. J. Am. Geriatr. Soc. 2013;61:1089–1094. doi: 10.1111/jgs.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho S.S., Dhaliwal S.S., Hills A.P., Pal S. Effects of chronic exercise training on inflammatory markers in Australian overweight and obese individuals in a randomized controlled trial. Inflammation. 2013;36:625–632. doi: 10.1007/s10753-012-9584-9. [DOI] [PubMed] [Google Scholar]
- 21.So W.Y., Song M., Park Y.H., Cho B.L., Lim J.Y., Kim S.H., Song W. Body composition, fitness level, anabolic hormones, and inflammatory cytokines in the elderly: A randomized controlled trial. Aging Clin. Exp. Res. 2013;25:167–174. doi: 10.1007/s40520-013-0032-y. [DOI] [PubMed] [Google Scholar]
- 22.Wanderley F.A., Moreira A., Sokhatska O., Palmares C., Moreira P., Sandercock G., Oliveira J., Carvalho J. Differential responses of adiposity, inflammation and autonomic function to aerobic versus resistance training in older adults. Exp. Gerontol. 2013;48:326–333. doi: 10.1016/j.exger.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Tomeleri C.M., Ribeiro A.S., Souza M.F., Schiavoni D., Schoenfeld B.J., Venturini D., Barbosa D.S., Landucci K., Sardinha L.B., Cyrino E.S. Resistance training improves inflammatory level, lipid and glycemic profiles in obese older women: A randomized controlled trial. Exp. Gerontol. 2016;84:80–87. doi: 10.1016/j.exger.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Li S.G., Zhou Y. Effect of resistance training on muscle content and serum inflammatory factors in the elderly. Chin. J. Gerontol. 2014;34:6659–6661. [Google Scholar]
- 25.Wang H., Gao H.N., Zhao Z.L. Effects of Long-term Winter Swimming Exercise on Elderly People’s Sex Hormone and Serum Inflammatory Factors. J. Shenyang Sport Univ. 2015;34:97–100. [Google Scholar]
- 26.Li S.G., Li W., Gao Q.J., Wang Y.W. Effects of strength training on aging vascular endothelial function and related mechanisms. Chin. J. Rehabil. Med. 2015;30:147–151. [Google Scholar]
- 27.Forti L.N., Van Roie E., Njemini R., Coudyzer W., Beyer I., Delecluse C., Bautmans I. Load-Specific Inflammation Mediating Effects of Resistance Training in Older Persons. J. Am. Med. Dir. Assoc. 2016;17:547–552. doi: 10.1016/j.jamda.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Friedenreich C.M., O’Reilly R., Shaw E., Stanczyk F.Z., Yasui Y., Brenner D.R., Courneya K.S. Inflammatory Marker Changes in Postmenopausal Women after a Year-long Exercise Intervention Comparing High Versus Moderate Volumes. Cancer Prev. Res. 2016;9:196–203. doi: 10.1158/1940-6207.CAPR-15-0284. [DOI] [PubMed] [Google Scholar]
- 29.Rondanelli M., Klersy C., Terracol G., Talluri J., Maugeri R., Guido D., Faliva M.A., Solerte B.S., Fioravanti M., Lukaski H., et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am. J. Clin. Nutr. 2016;103:830–840. doi: 10.3945/ajcn.115.113357. [DOI] [PubMed] [Google Scholar]
- 30.Allen N.G., Higham S.M., Mendham A.E., Kastelein T.E., Larsen P.S., Duffield R. The effect of high-intensity aerobic interval training on markers of systemic inflammation in sedentary populations. Eur. J. Appl. Physiol. 2017;117:1249–1256. doi: 10.1007/s00421-017-3613-1. [DOI] [PubMed] [Google Scholar]
- 31.Chupel M.U., Minuzzi L.G., Furtado G., Santos M.L., Hogervorst E., Filaire E., Teixeira A.M. Exercise and taurine in inflammation, cognition, and peripheral markers of blood-brain barrier integrity in older women. Appl. Physiol. Nutr. Metab. 2018;43:733–741. doi: 10.1139/apnm-2017-0775. [DOI] [PubMed] [Google Scholar]
- 32.Chen H.T., Wu H.J., Chen Y.J., Ho S.Y., Chung Y.C. Effects of 8-week kettlebell training on body composition, muscle strength, pulmonary function, and chronic low-grade inflammation in elderly women with sarcopenia. Exp. Gerontol. 2018;112:112–118. doi: 10.1016/j.exger.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Tomeleri C.M., Ribeiro A.S., Cavaglieri C.R., Deminice R., Schoenfeld B.J., Schiavoni D., Dos S.L., de Souza M.F., Antunes M., Venturini D., et al. Correlations between resistance training-induced changes on phase angle and biochemical markers in older women. Scand. J. Med. Sci. Sports. 2018;28:2173–2182. doi: 10.1111/sms.13232. [DOI] [PubMed] [Google Scholar]
- 34.Tomeleri C.M., Souza M.F., Burini R.C., Cavaglieri C.R., Ribeiro A.S., Antunes M., Nunes J.P., Venturini D., Barbosa D.S., Sardinha L.B., et al. Resistance training reduces metabolic syndrome and inflammatory markers in older women: A randomized controlled trial. J. Diabetes. 2018;10:328–337. doi: 10.1111/1753-0407.12614. [DOI] [PubMed] [Google Scholar]
- 35.Abd E.S., Al-Jiffri O.H. Aerobic exercise modulates cytokine profile and sleep quality in elderly. Afr. Health Sci. 2019;19:2198–2207. doi: 10.4314/ahs.v19i2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nunes P., Martins F.M., Souza A.P., Carneiro M., Orsatti C.L., Michelin M.A., Murta E., de Oliveira E.P., Orsatti F.L. Effect of high-intensity interval training on body composition and inflammatory markers in obese postmenopausal women: A randomized controlled trial. Menopause. 2019;26:256–264. doi: 10.1097/GME.0000000000001207. [DOI] [PubMed] [Google Scholar]
- 37.Urzi F., Marusic U., Ličen S., Buzan E. Effects of Elastic Resistance Training on Functional Performance and Myokines in Older Women-A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2019;20:830–834. doi: 10.1016/j.jamda.2019.01.151. [DOI] [PubMed] [Google Scholar]
- 38.Chen T.T., Li N.C., Wang H.W., He L., Li S.Y., Hu Y.L. Effects of Tai-Chi Exercise on Blood Lipids, Inflammatory Factors and BaPWV of Middle-Aged and Elderly People. J. Anhui Sports Sci. 2020;41:63–66. [Google Scholar]
- 39.de Castro D., Da C.N.D., Orsano V., de Sousa N.I., Beal F., Stone W., Dos S.R.T., Prestes J. Effect of high-velocity and traditional resistance exercise on serum antioxidants and inflammation biomarkers in older women: A randomized crossover trial. Exp. Gerontol. 2020;139:111026. doi: 10.1016/j.exger.2020.111026. [DOI] [PubMed] [Google Scholar]
- 40.Despeghel M., Reichel T., Zander J., Krüger K., Weyh C. Effects of a 6 Week Low-Dose Combined Resistance and Endurance Training on T Cells and Systemic Inflammation in the Elderly. Cells. 2021;10:843. doi: 10.3390/cells10040843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Timon R., Martínez-Guardado I., Camacho-Cardeñosa A., Villa-Andrada J.M., Olcina G., Camacho-Cardeñosa M. Effect of intermittent hypoxic conditioning on inflammatory biomarkers in older adults. Exp. Gerontol. 2021;152:111478. doi: 10.1016/j.exger.2021.111478. [DOI] [PubMed] [Google Scholar]
- 42.Xiao Y.D., Wei S.M. The effects of resistance training on cardiovascular efficiency in older adults and related mechanisms: A pilot of study. Chin. J. Rehabil. Med. 2022;37:202–209. [Google Scholar]
- 43.Rybka J., Kupczyk D., Kędziora-Kornatowska K., Pawluk H., Czuczejko J., Szewczyk-Golec K., Kozakiewicz M., Antonioli M., Carvalho L.A., Kędziora J. Age-related changes in an antioxidant defense system in elderly patients with essential hypertension compared with healthy controls. Redox Rep. 2011;16:71–77. doi: 10.1179/174329211X13002357050897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welsh C., Celis-Morales C.A., Brown R., Mackay D.F., Lewsey J., Mark P.B., Gray S.R., Ferguson L.D., Anderson J.J., Lyall D.M., et al. Comparison of Conventional Lipoprotein Tests and Apolipoproteins in the Prediction of Cardiovascular Disease. Circulation. 2019;140:542–552. doi: 10.1161/CIRCULATIONAHA.119.041149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leggate M. Ph.D. Thesis. Loughborough University; Loughborough, UK: 2012. The IL-6 system and its interaction with chronic low-grade inflammation and high intensity intermittent exercise. [Google Scholar]
- 46.Liu J.Q., Lu J.D., Liang T.Q., Chen S.N., Su H. Effect of resistance training on interleukin-6 and C-reactive protein in middle-age and elderly people: A Meta-analysis. Chin. J. Tissue Eng. Res. 2022;43:45–52. doi: 10.16835/j.cnki.1000-9817.2022.01.011. [DOI] [Google Scholar]
- 47.Shi M.L. Effect of Different Modes of Exercise Training on IL-6, TNF-α and hs-CRP. J. Xi’an Phys. Educ. Univ. 2011;28:481–488. [Google Scholar]
- 48.Bremer A.A., Jialal I. Adipose Tissue Dysfunction in Nascent Metabolic Syndrome. J. Obes. 2013;2013:393192. doi: 10.1155/2013/393192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.