Abstract
Benign prostatic hyperplasia (BPH) and prostate cancer (PCa) belong to the most frequent diseases in ageing men. It has been proposed that prostate chronic inflammation is a risk factor for the development of both BPH and PCa. However, potential stimuli that cause or maintain inflammation in the prostate gland are still poorly characterized. Bacterial infections seems to be one of the potential sources of prostatitis. Recent studies show that Propionibacterium acnes (P. acnes) is the most prevalent microorganism in the prostate gland and may be a predisposing factor for inflammation of prostatic tissue. It indicates that P. acnes may contribute to cancer development by enhancing proinflammatory responses, as well as by modifying the prostate extracellular environment. In this review, we discuss the potential role of P. acnes in the development of BPH and PCa and highlight the importance of regulatory T CD4(+)FoxP3(+) (Treg) and Th17 cells in response to P. acnes infection in the context of both prostate diseases.
Keywords: prostate cancer, benign prostatic hyperplasia, prostate microbiome, P. acnes, inflammatory cells, Treg cells, Th17 cells, prostate chronic inflammation
1. Introduction
Benign prostatic hyperplasia (BPH) and prostate cancer (PCa) belong to the most frequent diseases in ageing men [1,2,3]. BPH is a nonmalignant enlargement of the prostate gland caused by unregulated hypertrophy of the epithelial and fibromuscular tissues of the transition zone (TZ) and periurethral area. It is a common cause of lower urinary tract symptoms (LUTS) in men. Patients with BPH may experience poor urinary flow, frequency, hesitancy initiating flow, post-void dribbling, and nocturia. The prevalence of the disease increases after the age of 40 years [2,3,4]. So far, emerging hypotheses to explain the pathogenesis of BPH have included androgen, estrogen, insulin, stem cell, proliferative reawakening, telomerase, and inflammatory pathways. Currently, the leading area of discussion and research on the etiology of this disease is chronic inflammation within the prostate gland, which causes growth factor production, stem cell activation, and cellular proliferation [4]. Potential stimuli for the inflammatory process and, consequently for the BPH, have been proposed. These include autoimmune responses, bacterial and viral infections, dietary factors, hormone changes, and urinary reflux into the collecting ducts of the prostate [5]. The mentioned stimuli may cause lymphocyte activation, cytokine release, and growth factor, which induce hyperplasia acts as a self-perpetuating cycle, leading to chronic inflammation and a progressive increase in prostate volume [6].
Prostate cancer (PCa) is the first most common malignancy in men and the second leading cause of cancer death among men worldwide (2021 estimate) [7]. It develops in elderly men and is rare in men under 40. The average age at diagnosis is about 66 [1]. In addition to ageing, well-established risk factors for PCa include family history of disease, certain inherited genetic conditions (e.g., Lynch syndrome and BRCA1 and BRCA2 mutations) and African ancestry [8]. There is also evidence that smoking and excess body weight may increase the risk of fatal PCa [9,10]. Early-stage disease is asymptomatic. In more advanced cases, PCa symptoms are similar to benign prostate conditions, such as BPH and prostatitis [1]. The high long-term survival is observed in patients with localized prostate cancer. However, metastatic prostate cancer remains largely incurable even despite the use of intensive multimodal therapy. The mortality of advanced disease is due to the lack of therapeutic regiments capable of generating durable responses in conditions of extreme tumor heterogeneity at the genetic and biological cell levels [11].
The three main causes of prostate-related morbidity are BPH, PCa, and prostatitis. Despite many years of scientific study, the etiology and pathogenesis of BPH and PCa have not been fully understood. Currently, researchers have focused their attention on the role of prostate chronic inflammation in the development of both diseases [12,13]. The frequent observation of inflammatory cells in the prostate microenvironment in adult men indicates that inflammation is involved in these conditions [14]. Recent evidence points to a role for inflammation and atrophy in the development of prostate diseases, and suggests that the prostate microbiome may be involved in establishing an inflammatory microenvironment of the prostate that may promote carcinogenesis and tumor progression [15,16]. Bacterial infections are one of the potential stimuli that cause or maintain tissue inflammation [17,18]. As research shows, Propionibacterium ances (P. acnes) is the most prevalent microorganism isolated from prostatic tissue. There are studies suggesting that P. acnes contributes to the development of prostate inflammation, and consequently, to prostate diseases. This bacterium is involved in the inflammatory response by producing chemotactic factors and attracting leucocytes. P. acnes arouses a particular interest in the discussed context. Therefore, in this review, we discuss the potential role of P. acnes in the development of BPH and PCa and highlight the importance of regulatory T CD4(+)FoxP3(+) (Treg) and Th17 cells in response to P. acnes infection in the context of both prostate diseases.
2. The Prostate Microbiome and Chronic Inflammation
Chronic inflammation is commonly observed in patients with BPH, preneoplastic and malignant prostates. Hence, it has been suggested that chronic inflammation is the risk factor for the development of BPH, prostate carcinogenesis and cancer progression. More than 150 years ago, the link between inflammation and cancer was hypothesized by Virchow after his discovery of leukocytes in neoplastic tissues. Research has shown that inflammation may contribute to the development of cancer in many organs, for instance, in the bladder, colon, liver, lungs, pancreas, and prostate [19]. The molecular evidence presented so far, from animal and human studies, points to the regulatory role of chronic inflammation in prostate cancer development and progression to advanced metastatic disease [19,20,21,22]. The presence of chronic prostatitis was identified to be an independent marker for Gleason score upgrade (GSU) by Guner et al. The researchers compared two groups with and without GSU in terms of chronic prostatitis. The study showed that the presence of chronic prostatitis associated with PCa was higher in the patient cohort with GSU in contrast to the other group [23].
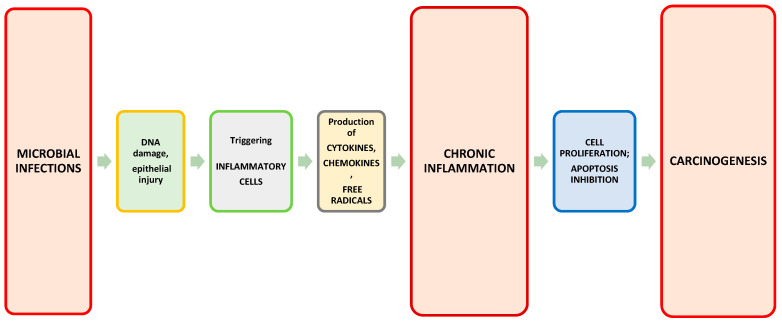
According to the researchers, there are some potential stimuli of prostatic inflammation: microbial infections, chemical irritations, diet, obesity, and physical traumas [24,25]. These factors can cause DNA damage and epithelial injury. The epithelial damage triggers an immune system response leading to the expansion and recruitment of the inflammatory cells to the prostate. These cells produce cytokines, chemokines, and free radicals which cause chronic inflammation, DNA damage, and further epithelial injury. In consequence, it leads to compensatory epithelial proliferation and the nuclear alternations. Thus, prostatic intraepithelial neoplasia may occur [24].
The effect of the prostate microbiome on prostate diseases is of particular interest. The microbiome can influence every stage of the disease from initiation to progression and treatment outcomes. This may occur as a result of direct interactions with a known microbial etiology, as well as modulation of the immune system, changes in metabolism, and effects on therapy. In many cases, both direct and indirect interactions with the microbiome are involved [26]. As many studies show, microbial inflammation is associated with the stimulation of the production of cytokines and chemokines. This can lead to cell proliferation and/or inhibition of apoptosis. Subsequently, carcinogenesis may be promoted (Figure 1) [27,28]. In healthy individuals, it has been observed that differentiated bacterial flora causes the production of inflammatory cytokines, including IFNγ, TNF-α, IL-1β, IL-6, IL-17, and IL-22 by myeloid and lymphoid cells [29]. Tumor progression can be promoted by these factors through various mechanisms, including IFNγ and IL-17 mediated tumor immune surveillance or the recruitment of immune cells into the tumor microenvironment via TNF-α, IL-1β, and IL-6 [28].
Figure 1.
Microbial infections as a cause of chronic inflammation that can lead to carcinogenesis.
The prostate gland can be chronically exposed to a multitude of microorganisms. It indicates that there are associations between the microbiome composition and pathological conditions of prostatic tissue. Epidemiological, histopathological, and molecular data suggest that prostate chronic inflammation is connected with bacterial and viral infections. Research shows that as much as 10–20% of cancers are attributed to chronic inflammation involving microbes [22]. As studies show, DNA and RNA from bacteria, fungi, parasites, and viruses have been found in prostatectomy samples from men who suffer from BPH and PCa [30,31,32]. Specific microbes can cause genome instability and, consequently, influence carcinogenesis by producing tumor-promoting metabolites and inducing an immune response [16]. Therefore, studies have indicated that the prostate tissue microbiome can contribute to prostate inflammation in relation to benign prostate conditions such as BPH, as well as to tumor progression and the response to treatment [26,33,34].
As research shows, the prostatic tissue contains a variety of bacteria. The microbiome of the prostate tumor microenvironment was analyzed by Cavarretta et al. In this study, the authors noted that Propionibacterium spp. were the most abundant among other genera and that Staphylococcus spp. were more represented in the tumor tissues [35]. In other research, Feng et al. assessed the metagenome and metatranscriptome of prostatic tissue and they found that Propionibacterium, Escherichia, Acinetobacter, and Pseudomonas spp. were abundant and constituting the core of the prostate microbiome both in tumor and in adjacent benign tissues [36]. Yow et al. used 16S rRNA gene sequencing to detect bacterial agents in high-grade prostate cancer tissues. The authors identified Enterobacteriaceae spp. common to all examined samples and P. acnes in 95% of analyzed samples [37]. The presence of P. acnes. in the prostate gland appears to be crucial, as evidenced by numerous studies examining its potential role in the development of prostate diseases.
3. P. acnes in Patients with BPH and PCa
P. acnes has been reported as the most prevalent microorganism in normal and pathological prostate glands [32,35]. It is an anaerobic, Gram-positive, and opportunistic bacterium whose occurrence on the skin is the common cause of acne vulgaris [38]. However, as research shows, prostate-derived P. acnes isolates do not represent contamination from patient skin, the medical team, or the surgical environment. The researchers typed P. acnes isolates from radical prostatectomy tissue samples using multilocus sequence typing (MLST). They identified eight different sequence types (STs) among prostate-derived P. acnes isolates. Interestingly, these were not typical skin/acne STs, but rather characteristic STs associated with opportunistic infections and/or urethral flora [39]. It appears that urinary microbial studies are important in identifying prostate diseases [16,26,40,41].
As a ubiquitous slow-growing organism with the capacity to form biofilm, P. acnes has been also identified as the etiological agent in implant-associated infections, related to, for example, prosthetic heart valves, prosthetic joint devices, and neurosurgical shunts. However, the virulence of P. acnes is low. Therefore, infection symptoms occur after a long-term infection [42,43]. P. acnes may also be involved in sarcoidosis pathogenesis [44,45].
Recent research shows that P. acnes is isolated with a high frequency from prostatic tissue of patients with BPH and PCa [32,35,46]. Cavaretta et al. noticed the high abundance of Propionibacterium spp., mainly composed by P. acnes, in non-tumor and tumor prostatic tissue [35]. The study carried out by Davidsson et al. showed that P. acnes is more common in men with PCa (60% of cases with P. acnes) than in men without neoplastic lesions in the prostate gland (only 26% cases with P.acnes) [46]. Other researchers, using in situ immunofluorescence (ISIF), found P. acnes in 58 out of 71 (82%) tested cancerous prostate tissue samples. However, in the same study, P. acnes was absent in healthy prostate tissues (20 samples) [47]. Dadashi et al. detected P. acnes in 68% of PCa and 58% of BPH specimens [48]. P. acnes has also been shown by Alexeyev O. et al. as the predominant microorganism in prostatic tissue in a large cohort of BPH patients [32]. Other authors also confirmed the high frequency of P. acnes isolation from individuals with BPH (positive P. acnes in 41% of cases) [49]. Cohen et al. observed a significant higher degree of prostatic inflammation in prostate samples positive for P. acnes [50]. It has also been reported that the P. acnes detection in prostate tissue was associated with subsequent PCa diagnosis. However, in this study, no difference was found in the Gleason score between P. acnes positive and negative patients [32]. Kakegawa et al. reported that patients with high serum PSA level and initial biopsy negative for cancer progressed more frequently to PCa in subsequent biopsies if the initial biopsy was positive for the presence of P. acnes [51]. Interestingly, the potential pathogenic role of P. acnes was also assessed in the genitourinary tract by Manente et al. The researchers evaluated the presence of P. acnes DNA in urine or seminal fluid of patients with recurrent symptoms of urinary infection. The test results in these patients for the most common urinary tract pathogens and sexually transmitted infection (STI) agents were negative. In the conducted tests, the presence of P. acnes was detected in 56 urine samples (108 urine samples were examined) and in 17 semen samples (51 semen samples were examined). The authors suggested that P. acnes infection could be a cause of pathogenic cascade leading in the long term, an inflammatory process of the prostate tissue [41]. Any microorganism can infect the prostate gland when ascending the urethra or by reflux of urine into the prostatic duct [52].
4. P. acnes May Also Contribute to Other Cancers and Inflammatory Diseases
Recent studies show that P. acnes is also considered a contributing factor in the development of neoplasms in tissues other than the prostate gland [53,54,55,56]. Therefore, the potential role of this bacterium in carcinogenesis seems to be so important that it attracts the interest of more and more researchers. For instance, P. acnes was investigated also as a non-Helicobacter pylori (H. pylori) bacteria that can stimulate gastric cancer (GC) risk [53,56]. Researchers used 16S rRNA gene sequencing and fluorescent in situ hybridization (FISH) and they found that P. acnes significantly increased in GC tissues, especially in H. pylori–negative samples. Moreover, it has been investigated that the abundance of the bacteria correlated with TNM stages of GC patients. The same authors detailed the mechanism for the tumor-promoting effect of P. acnes. They used immunofluorescence, RT-qPCR, and Western-blotting analysis to detect that P. acnes triggers M2 polarization of macrophages via TLR4/PI3K/Akt signaling. Ultimately, the researchers identified P. acnes as a possible agent that could regulate the tumor microenvironment by enhancing immunosuppression and thus promoting GC progression [53]. It has been reported that that M2 polarization of macrophages in tumors could be driven by canonical M2 stimuli, such as IL-4, IL-10, and IL-13 [57]. It has been also found that IL-10 expression at mRNA level was greatly enhanced in macrophages stimulated with P. acnes [53]. On the other hand, Tzeng et al. examined the microbiome of human breast tissue, including breast cancer samples. They reported that benign tissue samples (healthy control and high-risk) have a similar microbiome composition with higher mean relative abundances of 11 genera, including Propionibacterium spp. However, in this study, Propionibacterium spp. was not found in cancer-associated samples (tumor and tumor adjacent normal) [55]. Kim et al. researched microbiome markers of pancreatic cancer based on bacteria-derived extracellular vesicles acquired from blood samples. The study showed that at the genus level, four species, including Propionibacterium, were less abundant, while the other six species were more abundant in pancreatic cancer samples [58]. Suprewicz et al. assessed the effect of P.acnes on the proliferation capability and mechanical features of gingival cells and cell lines derived from breast, lung, and ovarian cancer. It was observed that P. acnes had the highest growth-promoting abilities in relation to breast cancer MCF-7 and ovarian cancer SKOV-3 cells [59]. These studies suggest that P. acnes may be crucial agent for the development of cancer diseases. However, the role of this bacterium in carcinogenesis may depend on the type of tissue in which the tumor develops.
The activity of P. acnes as a proinflammatory agent has also been researched in the case of multisystem inflammatory disorders such as sarcoidosis. Studies show that P. acnes can be involved in the pathogenesis of sarcoidosis. The bacterium has been detected in granulomas of some sarcoidosis patients, but not in any non-sarcoidosis ganulomas, such as tuberculosis and sarcoid reaction granulomas [60,61]. Beijer et al. investigated that the presence of P. acnes in granulomas is associated with chronic disease requiring treatment [44]. In patients with sarcoidosis, an increased immune response to P. acnes antigens was observed. Shupp et al. observed that BAL cells of sarcoidosis patients produce inflammatory cytokines (TNF-α and GM-CSF) upon stimulation with P. acnes [62]. This suggests that P. acnes can be an important factor causing hyperinflammatory status also in non-cancer diseases and in various organs.
5. P. acnes Induces Proinflammatory Response in Prostate Gland
P. acnes cause inflammatory diseases through their hemolytic, cytotoxic, and immunostimulatory activities [63,64]. It indicates that P. acnes may contribute to cancer development by enhancing proinflammatory responses, as well as by modifying the prostate extracellular environment. The proinflammatory response consists of the producing chemotactic factors, recruitment, and expansion of immune cells. The inflammatory infiltrate primarily includes T lymphocytes, macrophages and, less frequently, plasma cells and eosinophils [13,49,65,66]. Infiltrating cells are inducing to release pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), vascular endothelial growth factor (VEGF), IL-1β, IL-6, IL-8, IL-12, and IL-17 [67]. In a study by Shinohara et al., C57BL/6J mice were inoculated with a vehicle control or a prostatectomy-derived strain of P. acnes strain. Researchers have observed severe acute and chronic inflammation of the prostate gland. In addition, it was investigated that the inflammatory lesions were associated with an increase in the Ki-67 proliferative index, and a decrease in the production of Nkx3.1 and androgen receptor (AR). It was also reported that the observed response required live bacteria. This indicates the potential intracellular presence of P. acnes in prostate epithelial cells [68]. Davidsson et al. observed increased cell proliferation and cytokine/chemokine secretion in the prostate cells (PNT1A cell line) that were co-cultured with isolates of P. acnes [46]. Similar effects have been noted previously by Fassi Fehri et al. The microarray analysis they carried out showed a strong multifaceted inflammatory response of the prostate epithelial cell line RWPE1 that was co-cultured with live P. acnes isolated from cancerous prostates. The authors observed active secretion of cytokines and chemokines, such as IL-6 and IL-8 from infected cells. It has been suggested that the immune response included the activation of the COX2-prostaglandin, the plasminogen-matrix metalloproteinase pathways, as well as the activation of the transcriptional factors NF-κB and STAT3. It was also found that long-term exposure to P. acnes altered cell proliferation and initiated cellular transformation [48]. It should be noted that an increased level of IL-6 in the serum of patients with PCa is associated with advanced metastases. Moreover, IL-6 activates the JAK/STAT signaling pathway. Persistent activation of STAT3 transcription factor induces proliferation and tumor growth. In addition, the previously mentioned molecules such as VEGF and COX-2 are involved in angiogenesis [48,69,70,71,72].
6. Treg and Th17 Cells in the Tumor Environment
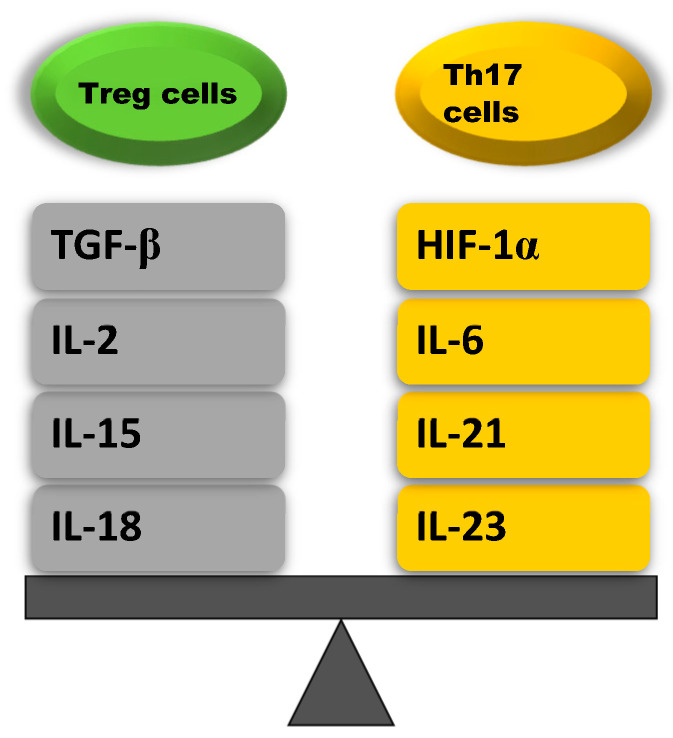
Although T helper type 17 (Th17) cells and Treg cells share a common precursor cell (the naïve CD4 T cell) and require a common tumor growth factor (TGF)-β signal for initial differentiation, they have opposite functions. Th17 cells induce autoimmunity and inflammation, whereas the role of Treg cells is to inhibit these activities and maintain immune homeostasis [73]. The balance between Th17 and Treg cells is mainly affected by TCR signaling, cytokines, costimulatory signals, microbiomes, and other factors. It has been found that mainly inflammation cytokines (IL-2, IL-6, IL-15, IL-18, IL-21, and IL-23), including transforming growth factor β (TGF-β) and hypoxia-inducible factor 1-α (HIF-1α), are involved in regulating the balance between Th17 and Treg cells. IL-2, IL-15, IL-18, and TGF-β affect Treg cells, whereas IL-6, IL-21, IL-23, and HIF-1α affect Th17 cells (Figure 2, Table 1) [71,74].
Figure 2.
Cytokines that maintain the balance between Treg and Th17 cells [75].
Table 1.
Effect of cytokines on the Treg/Th17 cells balance [75].
| Treg Cells Upregulating Cytokines | Th17 Cells Upregulating Cytokines |
|---|---|
| TGF-β: stimulates naïve CD4+ T cells that induce SMAD2 and SMAD3 that activate the transcription factor Foxp3 | HIF-1α: promotes the differentation of Th17 cells by inducing ROR-γt transcription and inhibits the differentation of Treg cell in an active process aimed at degradation of the Foxp3 protein |
| IL-2: increases Foxp3 expression by phosphorylation of STAT5 which binds to the Foxp3 locus | IL-6: stimulates naïve CD4+ T cells to differentiate into Th17 via STAT3 phosphorylation which induces the upregulation of Th17-specific genes (ROR-γt, IL-17, IL-23) |
| IL-15: increases Foxp3 expression by activating STAT5 and inhibits Th17 cell differentation by reducing IL-17 secretion | IL-21: stimulates Th17 cell differentation by activating STAT3, which increases ROR-γt expression |
| IL-18: inhibits Th17 cell differentation by inhibiting MyD88- dependent IL-1R downstream signal | IL-23: maintains Th17 cell differentation by enhancing the transcription of Th17 specyfic cytokines such as ROR-γt |
The process of Th17 cells differentiation consists of three stages in which various factors are involved, such as TGF-β, IL-6, IL-21, and IL-23. The initiation of Th17 cell differentiation is mediated by TGF-β and IL-6. Then, IL-21 expands the differentiation state of these cells. Ultimately, IL-23 is responsible for maintaining the stable maturation of Th17 cells during the later stage of the differentiation process [76]. Interestingly, naïve CD4+ T cells in the absence of IL-6 or IL-21 differentiate into Treg cells [75]. Treg cells are chemoattracted to the tumor microenvironment by chemokine gradients. CCR4, CCR8, CCR10, and CXCR3 induce Treg cell migration to the tumor microenvironment in response to CC and CXC chemokines: CCR4 is bound by CCL17 and CCL22, CCR8 is bound by CCL1, CCR10 is bound by CCL28,and CXCR3 is activated by CXCL9/10/11 [77]. Treg cells are highly activated and immunosuppressive within the tumor microenvironment. These cells are characterized by upregulated levels of FoxP3 and Helios [78,79,80]. Thus, Treg cells play an essential role in maintaining immune tolerance and balance, whereas Th17 cells show proinflammatory activities [74]. Treg cells suppress effector cells, such as T effector (Teff) cells, monocytes, macrophages, natural killer (NK) cells, and antigen-presenting (APC) cells via various mechanisms that lead to the inhibition of effector cell activation and proliferation, as well as to the induction of apoptosis [81]. These mechanisms include increased consumption of IL-2 and Teff deprivation and upregulated levels of inhibitory immune checkpoints [82,83,84,85]. Treg cells suppress the activity of immune cells and thereby controlling inflammation, by secreting anti-inflammatory cytokines, such as TGF-β and IL-10 [86,87,88].
Th17 are the key mediators of many autoimmune diseases; therefore, they can be also involved in the inflammatory process of cancer. [89]. As research shows, Th17 cells have been found in various human cancers. It has been also reported that Th17 cells in cancer show both tumor-promoting and tumor-suppressing activity. Th17-derived cytokines: IL-17 and IL-22, promote transformed cell properties and neighboring stromal cell activity, thereby influence the tumor microenvironment. Moreover, these cytokines also modulate the activities of myeloid and T cells that are involved in regulation of the immune system [90]. Liu et al. investigated that age-related CD4+ T cells, especially Th17 cells-secreted factors, can contribute to prostate carcinogenesis. The researchers used a C57BL/6J (B6) mouse as an ageing animal model to determine the role of age-related Th17 response in PCa cell growth, migration, and invasion. It was observed that Th17 cells, Th17 cytokines, and Th17/Treg ratio were increased compared to young mice. In addition, factors secreted from Th17 cells (IL-17A, IL-17F, and IL-22) promoted PCa cell viability, migration, and invasion, as well as activated the NF-κB and ERK1/2 signaling in PCa cells compared to young mouse prostate tissues [91]. NF-κB has also been found as a critical link between inflammation and cancer. Many researchers have demonstrated a positive association between the activation of NF-κB and PCa [92,93,94]. Thus, research confirmed that the balance between Th17 and Treg cells may play an essential role in prostate carcinogenesis. As earlier studies show, IL-17A expression increased in more than 50% of prostate cancers [95,96]. It has also been revealed that IL-17 induces prostate adenocarcinoma via MMP7-mediated epithelial-to-mesenchymal transition [97]. Other researchers investigated the importance of Th17 cells and IL-17 in a Pten-null prostate cancer mouse model. They found that SR1001 and anti-IL-17 antibody treatment increased apoptosis and reduced proliferation, angiogenesis, and inflammatory cell infiltration in Pten-null mice [98]. Cunningham et al. also assessed the interleukin-17′s role in prostate cancer. They reported that co-injection of recombinant IL-17A and mouse PCa cells enhanced metastasis to the pelvic lymph nodes [99]. Research suggests that Th17 cells and IL-17 can not only contribute to tumor progression but can also increase metastasis in patients with PCa.
7. P. acnes Contributes to Prostate Infiltration by Treg and Th17 Cells
It has been found that the presence of P. acnes in the prostate gland in patients with BPH and PCa is connected with the higher infiltrating of prostatic tissue by regulatory T CD4(+)FoxP3(+) cells (Treg cells). The authors also noticed that the infiltration of Treg cells is dependent on the aggressiveness of cancer in the Gleason scale in patients with P. acnes [49]. It has been previously investigated that the number of Treg cells is significantly increased in both tumor tissue and in the peripheral blood of patients with PCa [100]. This may indicate the effect of P. acnes on promoting carcinogenesis in the prostate gland. Interestingly, studies have shown that Treg cells can suppress anti-tumor responses, which is directly related with increasing risk of cancer recurrence. These cells play a role in preserving self-tolerance and inhibiting extra immune responses. Hence, Treg cells may support tumor progression as tumor-associated antigens are mainly self-antigens [100]. The studies also discovered that PCa patients with elevated levels of Treg cells within the tumor microenvironment have poor prognosis and low survival rates [100,101]. As other research shows, the imbalance between Treg cells and CD4(+)IL-17(+) cells in the tumor environment may promote inflammation and cancer progression. Moreover, it can contribute to the development of acquired resistance to immunotherapy. On the other hand, targeting Treg and Th17 cells could improve clinical outcomes [101,102]. It should be noted that the role of Th17/Treg in chronic inflammation associated with various diseases has been highlighted in many other studies, for example, in obesity, inflammatory bowel disease, autoimmune, and metabolic diseases [74,103,104,105].
As research shows, IL-17 exerts strong pro-inflammatory effects and is an important mediator in inflammation-associated cancer [105]. Radej et al. observed that the infiltration of CD4(+)IL-17(+) cells was significantly higher in BPH patients with P. acnes compared to BPH patients without the presence of this bacterium in prostate tissue. However, this correlation was not found in patients with PCa [48]. On the other hand, in earlier research carried out by Steiner et al., the authors reported that IL-17 mRNA and protein expression was increased in 79% of BPH and 58% of PCa specimens. In this study, IL-17 expression was very weak and restricted to lymphocytes in the samples of normal prostate [95]. The results obtained by Agak et al. confirm that P. acnes can induce immune cells to release high levels of IL-17. The authors also noted that this bacterium can modulate the CD4(+) T cell response in various ways, leading to the generation of Th17 cells [106].
8. Conclusions
The microbiome of the prostate can contribute to the development of prostate chronic inflammation in relation to BPH, as well as to carcinogenesis and tumor progression. The research carried out on human samples so far has provided evidence for a link between BPH or PCa and P. acnes using various technical approaches, such as cultivation, in situ hybridization, immunohistochemistry, and PCR-based profiling of bacterial 16S rRNA. P. acnes as a most prevalent microorganism in the prostate gland may be a predisposing factor for inflammation that can lead to the development of BPH and PCa. The relationship between the immune response and the presence of P. acnes in prostate tissue shows the participation of this bacterium in the intensification of inflammation. It appears that Treg and Th17 cells can be factors that promote prostate disease in response to P. acnes infection. The balance between Treg and Th17 cells in BPH and PCa patients may have important implications for clinicians and clinical researchers seeking more reliable prognostic markers and more targeted therapeutic approaches. In light of the current research, the indication of P. acnes as the cause of prostate chronic inflammation, and thus, also the cause of prostate diseases, seems to be justified; however, it should be thoroughly investigated and clearly confirmed.
Author Contributions
Conceptualization, S.R.; writing—original draft preparation, M.S.; writing—review and editing, M.S.; visualization, S.R., M.S.; supervision, R.M.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rawla P. Epidemiology of Prostate Cancer. World J. Oncol. 2019;10:63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madersbacher S., Sampson N., Culig Z. Pathophysiology of Benign Prostatic Hyperplasia and Benign Prostatic Enlargement: A Mini-Review. Gerontology. 2019;65:458–464. doi: 10.1159/000496289. [DOI] [PubMed] [Google Scholar]
- 3.Bin Lim K. Epidemiology of clinical benign prostatic hyperplasia. Asian J. Urol. 2017;4:148–151. doi: 10.1016/j.ajur.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devlin C.M., Simms M.S., Maitland N.J. Benign prostatic hyperplasia–what do we know? Br. J. Urol. 2020;127:389–399. doi: 10.1111/bju.15229. [DOI] [PubMed] [Google Scholar]
- 5.Ficarra V., Sekulovic S., Zattoni F., Zazzera M., Novara G. Why and How to Evaluate Chronic Prostatic Inflammation. Eur. Urol. Suppl. 2013;12:110–115. doi: 10.1016/j.eursup.2013.08.002. [DOI] [Google Scholar]
- 6.Ficarra V., Rossanese M., Zazzara M., Giannarini G., Abbinante M., Bartoletti R., Mirone V., Scaglione F. The Role of Inflammation in Lower Urinary Tract Symptoms (LUTS) due to Benign Prostatic Hyperplasia (BPH) and Its Potential Impact on Medical Therapy. Curr. Urol. Rep. 2014;15:463. doi: 10.1007/s11934-014-0463-9. [DOI] [PubMed] [Google Scholar]
- 7.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 8.Rebbeck T.R., Devesa S.S., Chang B.-L., Bunker C.H., Cheng I., Cooney K., Eeles R., Fernandez P., Giri V.N., Gueye S.M., et al. Global Patterns of Prostate Cancer Incidence, Aggressiveness, and Mortality in Men of African Descent. Prostate Cancer. 2013;2013:560857. doi: 10.1155/2013/560857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foerster B., Pozo C., Abufaraj M., Mari A., Kimura S., D’Andrea D., John H., Shariat S. Association of Smoking Status With Recurrence, Metastasis, and Mortality Among Patients With Localized Prostate Cancer Undergoing Prostatectomy or Radiotherapy: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4:953–961. doi: 10.1001/jamaoncol.2018.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauby-Secretan B., Scoccianti C., Loomis D., Grosse Y., Bianchini F., Straif K., International Agency for Research on Cancer Handbook Working Group Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang G., Zhao D., Spring D.J., Depinho R.A. Genetics and biology of prostate cancer. Genes Dev. 2018;32:1105–1140. doi: 10.1101/gad.315739.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai T., Santi R., Tamanini I., Galli I.C., Perletti G., Bjerklund Johansen T.E., Nesi G. Current Knowledge of the Potential Links between Inflammation and Prostate Cancer. Int. J. Mol. Sci. 2019;20:3833. doi: 10.3390/ijms20153833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korniluk A., Koper O., Kemona H., Dymicka-Piekarska V. From inflammation to cancer. Ir. J. Med. Sci. 2017;186:57–62. doi: 10.1007/s11845-016-1464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verze P., Cai T., Lorenzetti S. The role of the prostate in male fertility, health and disease. Nat. Rev. Urol. 2016;13:379–386. doi: 10.1038/nrurol.2016.89. [DOI] [PubMed] [Google Scholar]
- 15.Sfanos K.S., Yegnasubramanian S., Nelson W.G., De Marzo A.M. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 2017;15:11–24. doi: 10.1038/nrurol.2017.167. [DOI] [PubMed] [Google Scholar]
- 16.Moghadam S.O., Momeni S.A. Human microbiome and prostate cancer development: Current insights into the prevention and treatment. Front. Med. 2020;15:11–32. doi: 10.1007/s11684-019-0731-7. [DOI] [PubMed] [Google Scholar]
- 17.Brüggemann H., Al-Zeer M.A. Bacterial signatures and their inflammatory potentials associated with prostate cancer. APMIS. 2020;128:80–91. doi: 10.1111/apm.13021. [DOI] [PubMed] [Google Scholar]
- 18.da Silva A.P.B., Alluri L.S.C., Bissada N.F., Gupta S. Association between oral pathogens and prostate cancer: Building the relationship. Am. J. Clin. Exp. Urol. 2019;18:1–10. [PMC free article] [PubMed] [Google Scholar]
- 19.Virchow R. An Address on the Value of Pathological Experiments. BMJ. 1881;2:198–203. doi: 10.1136/bmj.2.1075.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delongchamps N.B., de la Roza G., Chandan V., Jones R., Sunheimer R., Threatte G., Jumbelic M., Haas G.P. Evaluation of Prostatitis in Autopsied Prostates—Is Chronic Inflammation More Associated With Benign Prostatic Hyperplasia or Cancer? J. Urol. 2008;179:1736–1740. doi: 10.1016/j.juro.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stark T., Livas L., Kyprianou N. Inflammation in prostate cancer progression and therapeutic targeting. Transl. Androl. Urol. 2015;4:455–463. doi: 10.3978/j.issn.2223-4683.2015.04.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guner E., Danacioglu Y.O., Arikan Y., Seker K.G., Polat S., Baytekin H.F., Simsek A. The presence of chronic inflammation in positive prostate biopsy is associated with upgrading in radical prostatectomy. Arch. Ital. Urol. Androl. 2021;93:280–284. doi: 10.4081/aiua.2021.3.280. [DOI] [PubMed] [Google Scholar]
- 24.de Bono J.S., Guo C., Gurel B., De Marzo A.M., Sfanos K.S., Mani R.S., Gil J., Drake C.G., Alimonti A. Prostate carcinogenesis: Inflammatory storms. Nat. Cancer. 2020;20:455–469. doi: 10.1038/s41568-020-0267-9. [DOI] [PubMed] [Google Scholar]
- 25.Tong Y., Zhou R.-Y. Review of the Roles and Interaction of Androgen and Inflammation in Benign Prostatic Hyperplasia. Mediat. Inflamm. 2020;2020:7958316. doi: 10.1155/2020/7958316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter C.M., Shrestha E., Peiffer L.B., Sfanos K.S. The microbiome in prostate inflammation and prostate cancer. Prostate Cancer Prostatic Dis. 2018;21:345–354. doi: 10.1038/s41391-018-0041-1. [DOI] [PubMed] [Google Scholar]
- 27.Bultman S.J. Emerging roles of the microbiome in cancer. Carcinogenesis. 2013;35:249–255. doi: 10.1093/carcin/bgt392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosean C.M.B., Rutkowski M.R. The influence of the commensal microbiota on distal tumor-promoting inflammation. Semin. Immunol. 2017;32:62–73. doi: 10.1016/j.smim.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schirmer M., Smeekens S.P., Vlamakis H., Jaeger M., Oosting M., Franzosa E.A., Ter Horst R., Jansen T., Jacobs L., Bonder M.J., et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell. 2016;167:1125–1136.e8. doi: 10.1016/j.cell.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sfanos K.S., Sauvageot J., Fedor H.L., Dick J.D., De Marzo A.M., Isaacs W.B. A molecular analysis of prokaryotic and viral DNA sequences in prostate tissue from patients with prostate cancer indicates the presence of multiple and diverse microorganisms. Prostate. 2007;68:306–320. doi: 10.1002/pros.20680. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee S., Alwine J.C., Wei Z., Tian T., Shih N., Sperling C., Guzzo T., Feldman M.D., Robertson E.S. Microbiome signatures in prostate cancer. Carcinogenesis. 2019;40:749–764. doi: 10.1093/carcin/bgz008. [DOI] [PubMed] [Google Scholar]
- 32.Alexeyev O., Bergh J., Marklund I., Thellenberg-Karlsson C., Wiklund F., Grönberg H., Bergh A., Elgh F. Association between the presence of bacterial 16S RNA in prostate specimens taken during transurethral resection of prostate and subsequent risk of prostate cancer (Sweden) Cancer Causes Control. 2006;17:1127–1133. doi: 10.1007/s10552-006-0054-2. [DOI] [PubMed] [Google Scholar]
- 33.Liss M.A., White J.R., Goros M., Gelfond J., Leach R., Johnson-Pais T., Lai Z., Rourke E., Basler J., Ankerst D., et al. Metabolic Biosynthesis Pathways Identified from Fecal Microbiome Associated with Prostate Cancer. Eur. Urol. 2018;74:575–582. doi: 10.1016/j.eururo.2018.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sfanos K.S., Markowski M.C., Peiffer L., Ernst S.E., White J.R., Pienta K.J., Antonarakis E.S., Ross A.E. Compositional differences in gastrointestinal microbiota in prostate cancer patients treated with androgen axis-targeted therapies. Prostate Cancer Prostatic Dis. 2018;21:539–548. doi: 10.1038/s41391-018-0061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavarretta I., Ferrarese R., Cazzaniga W., Saita D., Lucianò R., Ceresola E.R., Locatelli I., Visconti L., Lavorgna G., Briganti A., et al. The Microbiome of the Prostate Tumor Microenvironment. Eur. Urol. 2017;72:625–631. doi: 10.1016/j.eururo.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 36.Feng Y., Ramnarine V.R., Bell R., Volik S., Davicioni E., Hayes V.M., Ren S., Collins C.C. Metagenomic and metatranscriptomic analysis of human prostate microbiota from patients with prostate cancer. BMC Genom. 2019;20:146. doi: 10.1186/s12864-019-5457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yow M.A., Tabrizi S.N., Severi G., Bolton D.M., Pedersen J., Australian Prostate Cancer BioResource. Giles G.G., Southey M.C. Characterisation of microbial communities within aggressive prostate cancer tissues. Infect. Agents Cancer. 2017;12:4. doi: 10.1186/s13027-016-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Platsidaki E., Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Research. 2018;7:1953. doi: 10.12688/f1000research.15659.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mak T.N., Yu S.-H., De Marzo A., Brüggemann H., Sfanos K.S. Multilocus sequence typing (MLST) analysis ofPropionibacteriumacnesisolates from radical prostatectomy specimens. Prostate. 2012;73:770–777. doi: 10.1002/pros.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shrestha E., White J.R., Yu S.-H., Kulac I., Ertunc O., De Marzo A.M., Yegnasubramanian S., Mangold L.A., Partin A.W., Sfanos K.S. Profiling the Urinary Microbiome in Men with Positive versus Negative Biopsies for Prostate Cancer. J. Urol. 2018;199:161–171. doi: 10.1016/j.juro.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manente L., Gargiulo U., Gargiulo P., Dovinola G. Propionibacterium acnes in urine and semen samples from men with urinary infection. Arch. Ital. Urol. Androl. 2022;94:62–64. doi: 10.4081/aiua.2022.1.62. [DOI] [PubMed] [Google Scholar]
- 42.Achermann Y., Goldstein E.J., Coenye T., Shirtliff M.E. Propionibacterium acnes: From Commensal to Opportunistic Biofilm-Associated Implant Pathogen. Clin. Microbiol. Rev. 2014;27:419–440. doi: 10.1128/CMR.00092-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capoor M.N., Birkenmaier C., Wang J.C., McDowell A., Ahmed F.S., Brüggemann H., Coscia E., Davies D.G., Ohrt-Nissen S., Raz A., et al. A review of microscopy-based evidence for the association of Propionibacterium acnes biofilms in degenerative disc disease and other diseased human tissue. Eur. Spine J. 2019;28:2951–2971. doi: 10.1007/s00586-019-06086-y. [DOI] [PubMed] [Google Scholar]
- 44.Beijer E., Seldenrijk K., Eishi Y., Uchida K., Damen J., Grutters J.C., Veltkamp M. Presence of Propionibacterium acnes in granulomas associates with a chronic disease course in Dutch sarcoidosis patients. ERJ Open Res. 2020;7:486. doi: 10.1183/23120541.00486-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchida K., Furukawa A., Yoneyama A., Furusawa H., Kobayashi D., Ito T., Yamamoto K., Sekine M., Miura K., Akashi T., et al. Propionibacterium acnes-Derived Circulating Immune Complexes in Sarcoidosis Patients. Microorganisms. 2021;9:2194. doi: 10.3390/microorganisms9112194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidsson S., Mölling P., Rider J., Unemo M., Karlsson M.G., Carlsson J., Andersson S.-O., Elgh F., Söderquist B., Andrén O. Frequency and typing of Propionibacterium acnes in prostate tissue obtained from men with and without prostate cancer. Infect. Agents Cancer. 2016;11:26. doi: 10.1186/s13027-016-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.FassiFehri L., Mak T.N., Laube B., Brinkmann V., Ogilvie L.A., Mollenkopf H., Lein M., Schmidt T., Meyer T.F., Brüggemann H. Prevalence of Propionibacterium acnes in diseased prostates and its inflammatory and transforming activity on prostate epithelial cells. Int. J. Med. Microbiol. 2011;301:69–78. doi: 10.1016/j.ijmm.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 48.Dadashi M., Eslami G., Taghavi A., Goudarzi H., Hajikhani B., Goudarzi M., Ghazi M. Is Propionibacterium acnes a Causative Agent in Benign Prostate Hyperplasia and Prostate Cancer? Arch. Clin. Infect. Dis. 2018;13:e58947. doi: 10.5812/archcid.58947. [DOI] [Google Scholar]
- 49.Radej S., Płaza P., Olender A., Szewc M., Bar K., Maciejewski R. Infiltrating Treg and Th17 Cells of the Prostate Hypertrophy Gland Associated with Propionibacterium Acnes Infection. Res. Rep. Urol. 2020;12:593–597. doi: 10.2147/RRU.S284066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen R.J., Shannon B.A., McNEAL J.E., Shannon T., Garrett K.L. Propionibacterium acnes Associated with Inflammation in Radical Prostatectomy Specimens: A Possible Link to Cancer Evolution? J. Urol. 2005;173:1969–1974. doi: 10.1097/01.ju.0000158161.15277.78. [DOI] [PubMed] [Google Scholar]
- 51.Kakegawa T., Bae Y., Ito T., Uchida K., Sekine M., Nakajima Y., Furukawa A., Suzuki Y., Kumagai J., Akashi T., et al. Frequency of Propionibacterium acnes Infection in Prostate Glands with Negative Biopsy Results Is an Independent Risk Factor for Prostate Cancer in Patients with Increased Serum PSA Titers. PLoS ONE. 2017;12:e0169984. doi: 10.1371/journal.pone.0169984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gandaglia G., Zaffuto E., Fossati N., Cucchiara V., Mirone V., Montorsi F., Briganti A. The role of prostatic inflammation in the development and progression of benign and malignant diseases. Curr. Opin. Urol. 2017;27:99–106. doi: 10.1097/MOU.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 53.Li Q., Wu W., Gong D., Shang R., Wang J., Yu H. Propionibacterium acnes overabundance in gastric cancer promote M2 polarization of macrophages via a TLR4/PI3K/Akt signaling. Gastric Cancer. 2021;24:1242–1253. doi: 10.1007/s10120-021-01202-8. [DOI] [PubMed] [Google Scholar]
- 54.Liu X., Shao L., Liu X., Ji F., Mei Y., Cheng Y., Liu F., Yan C., Li L., Ling Z. Alterations of gastric mucosal microbiota across different stomach microhabitats in a cohort of 276 patients with gastric cancer. eBioMedicine. 2018;40:336–348. doi: 10.1016/j.ebiom.2018.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tzeng A., Sangwan N., Jia M., Liu C.-C., Keslar K.S., Downs-Kelly E., Fairchild R.L., Al-Hilli Z., Grobmyer S.R., Eng C. Human breast microbiome correlates with prognostic features and immunological signatures in breast cancer. Genome Med. 2021;13:60. doi: 10.1186/s13073-021-00874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gunathilake M.N., Lee J., Choi I.J., Kim Y.-I., Ahn Y., Park C., Kim J. Association between the relative abundance of gastric microbiota and the risk of gastric cancer: A case-control study. Sci. Rep. 2019;9:13589–13611. doi: 10.1038/s41598-019-50054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang N., Liang H., Zen K. Molecular Mechanisms That Influence the Macrophage m1-m2 Polarization Balance. Front. Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim J., Han K., Han Y., Kang N., Shin T.-S., Park H., Kim H., Kwon W., Lee S., Kim Y.-K., et al. Microbiome Markers of Pancreatic Cancer Based on Bacteria-Derived Extracellular Vesicles Acquired from Blood Samples: A Retrospective Propensity Score Matching Analysis. Biology. 2021;10:219. doi: 10.3390/biology10030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suprewicz Ł., Tokajuk G., Cieśluk M., Deptuła P., Sierpińska T., Wolak P., Wollny T., Tokajuk J., Głuszek S., Piktel E., et al. Bacteria Residing at Root Canals Can Induce Cell Proliferation and Alter the Mechanical Properties of Gingival and Cancer Cells. Int. J. Mol. Sci. 2020;21:7914. doi: 10.3390/ijms21217914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Negi M., Takemura T., Guzman J., Uchida K., Furukawa A., Suzuki Y., Iida T., Ishige I., Minami J., Yamada T., et al. Localization of Propionibacterium acnes in granulomas supports a possible etiologic link between sarcoidosis and the bacterium. Mod. Pathol. 2012;25:1284–1297. doi: 10.1038/modpathol.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Isshiki T., Homma S., Eishi Y., Yabe M., Koyama K., Nishioka Y., Yamaguchi T., Uchida K., Yamamoto K., Ohashi K., et al. Immunohistochemical Detection of Propionibacterium acnes in Granulomas for Differentiating Sarcoidosis from Other Granulomatous Diseases Utilizing an Automated System with a Commercially Available PAB Antibody. Microorganisms. 2021;9:1668. doi: 10.3390/microorganisms9081668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schupp J.C., Tchaptchet S., Lützen N., Engelhard P., Müller-Quernheim J., Freudenberg M.A., Prasse A. Immune response to Propionibacterium acnes in patients with sarcoidosis–in vivo and in vitro. BMC Pulm. Med. 2015;15:75. doi: 10.1186/s12890-015-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong Q., Nelson D.E., Toh E., Diao L., Gao X., Fortenberry J.D., Van Der Pol B. The Microbial Communities in Male First Catch Urine Are Highly Similar to Those in Paired Urethral Swab Specimens. PLoS ONE. 2011;6:e19709. doi: 10.1371/journal.pone.0019709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alexeyev O.A., Marklund I., Shannon B., Golovleva I., Olsson J., Andersson C., Eriksson I., Cohen R., Elgh F. Direct Visualization of Propionibacterium acnes in Prostate Tissue by Multicolor Fluorescent In Situ Hybridization Assay. J. Clin. Microbiol. 2007;45:3721–3728. doi: 10.1128/JCM.01543-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calcinotto A., Spataro C., Zagato E., Di Mitri D., Gil V., Crespo M., De Bernardis G., Losa M., Mirenda M., Pasquini E., et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature. 2018;559:363–369. doi: 10.1038/s41586-018-0266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Escamilla J., Schokrpur S., Liu C., Priceman S.J., Moughon D., Jiang Z., Pouliot F., Magyar C., Sung J.L., Xu J., et al. CSF1 Receptor Targeting in Prostate Cancer Reverses Macrophage-Mediated Resistance to Androgen Blockade Therapy. Cancer Res. 2015;75:950–962. doi: 10.1158/0008-5472.CAN-14-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kistowska M., Meier B., Proust T., Feldmeyer L., Cozzio A., Kuendig T., Contassot E., French L.E. Propionibacterium acnes Promotes Th17 and Th17/Th1 Responses in Acne Patients. J. Investig. Dermatol. 2015;135:110–118. doi: 10.1038/jid.2014.290. [DOI] [PubMed] [Google Scholar]
- 68.Shinohara D.B., Vaghasia A.M., Yu S.-H., Mak T.N., Brüggemann H., Nelson W.G., De Marzo A.M., Yegnasubramanian S., Sfanos K.S. A mouse model of chronic prostatic inflammation using a human prostate cancer-derived isolate of Propionibacterium acnes. Prostate. 2013;73:1007–1015. doi: 10.1002/pros.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palayoor S.T., Youmell M.Y., Calderwood S.K., Coleman C.N., Price B.D. Constitutive activation of IκB kinase α and NF-κB in prostate cancer cells is inhibited by ibuprofen. Oncogene. 1999;18:7389–7394. doi: 10.1038/sj.onc.1203160. [DOI] [PubMed] [Google Scholar]
- 70.Lin Q., Lai R., Chirieac L.R., Li C., Thomazy V.A., Grammatikakis I., Rassidakis G.Z., Zhang W., Fujio Y., Kunisada K., et al. Constitutive Activation of JAK3/STAT3 in Colon Carcinoma Tumors and Cell Lines: Inhibition of JAK3/STAT3 Signaling Induces Apoptosis and Cell Cycle Arrest of Colon Carcinoma Cells. Am. J. Pathol. 2005;167:969–980. doi: 10.1016/S0002-9440(10)61187-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Durant L., Watford W.T., Ramos H.L., Laurence A., Vahedi G., Wei L., Takahashi H., Sun H.-W., Kanno Y., Powrie F., et al. Diverse Targets of the Transcription Factor STAT3 Contribute to T Cell Pathogenicity and Homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adekoya T.O., Richardson R.M. Cytokines and Chemokines as Mediators of Prostate Cancer Metastasis. Int. J. Mol. Sci. 2020;21:4449. doi: 10.3390/ijms21124449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee G.R. The balance of Th17 versus treg cells in autoimmunity. Int. J. Mol. Sci. 2018;19:730. doi: 10.3390/ijms19030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan J.-B., Luo M.-M., Chen Z.-Y., He B.-H. The Function and Role of the Th17/Treg Cell Balance in Inflammatory Bowel Disease. J. Immunol. Res. 2020;2020:8813558. doi: 10.1155/2020/8813558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 76.Ueno A., Jeffery L., Kobayashi T., Hibi T., Ghosh S., Jijon H. Th17 plasticity and its relevance to inflammatory bowel disease. J. Autoimmun. 2018;87:38–49. doi: 10.1016/j.jaut.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 77.Ohue Y., Nishikawa H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019;110:2080–2089. doi: 10.1111/cas.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shitara K., Nishikawa H. Regulatory T cells: A potential target in cancer immunotherapy. Ann. N. Y. Acad. Sci. 2018;1417:104–115. doi: 10.1111/nyas.13625. [DOI] [PubMed] [Google Scholar]
- 79.Chougnet C., Hildeman D. Helios—controller of Treg stability and function. Transl. Cancer Res. 2016;5:S338–S341. doi: 10.21037/tcr.2016.07.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu W.-Q., Ji N.-F., Gu C.-J., Wang Y.-L., Huang M., Zhang M.-S. Coexpression of Helios in Foxp3+ Regulatory T Cells and Its Role in Human Disease. Dis. Markers. 2021;2021:5574472. doi: 10.1155/2021/5574472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saleh R., Elkord E. Acquired resistance to cancer immunotherapy: Role of tumor-mediated immunosuppression. Semin. Cancer Biol. 2019;65:13–27. doi: 10.1016/j.semcancer.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 82.Thornton A.M., Lu J., Korty P.E., Kim Y.C., Martens C., Sun P.D., Shevach E.M. Helios+ and Helios− Treg subpopulations are phenotypically and functionally distinct and express dissimilar TCR repertoires. Eur. J. Immunol. 2019;49:398–412. doi: 10.1002/eji.201847935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chinen T., Kannan A.K., Levine A.G., Fan X., Klein U., Zheng Y., Gasteiger G., Feng Y., Fontenot J.D., Rudensky A.Y. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 2016;17:1322–1333. doi: 10.1038/ni.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cao X., Cai S.F., Fehniger T.A., Song J., Collins L.I., Piwnica-Worms D.R., Ley T.J. Granzyme B and Perforin Are Important for Regulatory T Cell-Mediated Suppression of Tumor Clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 85.Sasidharan Nair V., Elkord E. Immune checkpoint inhibitors in cancer therapy: A focus on T-regulatory cells. Immunol. Cell Biol. 2018;96:21–33. doi: 10.1111/imcb.1003. [DOI] [PubMed] [Google Scholar]
- 86.Mayne C.G., Williams C.B. Induced and Natural Regulatory T Cells in the Development of Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2013;19:1772–1788. doi: 10.1097/MIB.0b013e318281f5a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taylor A., Verhagen J., Blaser K., Akdis M., Akdis C.A. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: The role of T regulatory cells. Immunology. 2006;117:433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sullivan J.A., Tomita Y., Jankowska-Gan E., Lema D.A., Arvedson M.P., Nair A., Bracamonte-Baran W., Zhou Y., Meyer K.K., Zhong W., et al. Treg-Cell-Derived IL-35-Coated Extracellular Vesicles Promote Infectious Tolerance. Cell Rep. 2020;30:1039–1051.e5. doi: 10.1016/j.celrep.2019.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Y., Mikrani R., Xie D., Wazir J., Shrestha S., Ullah R., Baig M.M.F.A., Ahmed A., Srivastava P.K., Thapa K.B., et al. Chronic prostatitis/chronic pelvic pain syndrome and prostate cancer: Study of immune cells and cytokines. Fundam. Clin. Pharmacol. 2019;34:160–172. doi: 10.1111/fcp.12517. [DOI] [PubMed] [Google Scholar]
- 90.Chang S.H. T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Arch. Pharmacal. Res. 2019;42:549–559. doi: 10.1007/s12272-019-01146-9. [DOI] [PubMed] [Google Scholar]
- 91.Liu S., Liu F., Zhang B., Yan P., Rowan B.G., Dvm A.B.A., Steele C., Jazwinski S.M., Moroz K., Norton E.B., et al. CD4 + T helper 17 cell response of aged mice promotes prostate cancer cell migration and invasion. Prostate. 2020;80:764–776. doi: 10.1002/pros.23990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.You Z., Ge D., Liu S., Zhang Q., Borowsky A.D., Melamed J. Interleukin-17 Induces Expression of Chemokines and Cytokines in Prostatic Epithelial Cells but Does Not Stimulate Cell Growth In Vitro. Int. J. Med. Biol. Front. 2012;18:629–644. [PMC free article] [PubMed] [Google Scholar]
- 93.Li Q., Liu L., Zhang Q., Liu S., Ge D., You Z. Interleukin-17 Indirectly Promotes M2 Macrophage Differentiation through Stimulation of COX-2/PGE2 Pathway in the Cancer Cells. Cancer Res. Treat. 2014;46:297–306. doi: 10.4143/crt.2014.46.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shukla S., MacLennan G.T., Fu P., Patel J., Marengo S.R., Resnick M.L., Gupta S. Nuclear Factor-κB/p65 (Rel A) Is Constitutively Activated in Human Prostate Adenocarcinoma and Correlates with Disease Progression. Neoplasia. 2004;6:390–400. doi: 10.1593/neo.04112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Steiner G.E., Newman M.E., Paikl D., Stix U., Memaran-Dagda N., Lee C., Marberger M.J. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate. 2003;56:171–182. doi: 10.1002/pros.10238. [DOI] [PubMed] [Google Scholar]
- 96.Sfanos K.S., Bruno T.C., Maris C.H., Xu L., Thoburn J.C., DeMarzo A.M., Meeker A.K., Isaacs W.B., Drake C.G. Phenotypic Analysis of Prostate-Infiltrating Lymphocytes Reveals TH17 and Treg Skewing. Clin. Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Q., Liu S., Parajuli K.R., Zhang W., Zhang K., Mo Z., Liu J., Chen Z., Yang S., Wang A.R., et al. Interleukin-17 promotes prostate cancer via MMP7-induced epithelial-to-mesenchymal transition. Oncogene. 2016;36:687–699. doi: 10.1038/onc.2016.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Q., Liu S., Ge D., Cunningham D.M., Huang F., Ma L., Burris T.P., You Z. Targeting Th17-IL-17 Pathway in Prevention of Micro-Invasive Prostate Cancer in a Mouse Model. Prostate. 2017;77:888–899. doi: 10.1002/pros.23343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cunningham D., Zhang Q., Liu S., Parajuli K.R., Nie Q., Ma L., Zhang A., Chen Z., You Z. Interleukin-17 promotes metastasis in an immunocompetent orthotopic mouse model of prostate cancer. Am. J. Clin. Exp. Urol. 2018;6:114–122. [PMC free article] [PubMed] [Google Scholar]
- 100.Miller A.M., Lundberg K., Özenci V., Banham A.H., Hellström M., Egevad L., Pisa P. CD4+CD25high T Cells Are Enriched in the Tumor and Peripheral Blood of Prostate Cancer Patients. J. Immunol. 2006;177:7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 101.Saleh R., Elkord E. FoxP3+ T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 2020;490:174–185. doi: 10.1016/j.canlet.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 102.Liu J., Duan Y., Cheng X., Chen X., Xie W., Long H., Lin Z., Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem. Biophys. Res. Commun. 2011;407:348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 103.Zhang S., Gang X., Yang S., Cui M., Sun L., Li Z., Wang G. The Alterations in and the Role of the Th17/Treg Balance in Metabolic Diseases. Front. Immunol. 2021;12:678355. doi: 10.3389/fimmu.2021.678355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shan J., Jin H., Xu Y. T Cell Metabolism: A New Perspective on Th17/Treg Cell Imbalance in Systemic Lupus Erythematosus. Front. Immunol. 2020;11:1027. doi: 10.3389/fimmu.2020.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Crouser E.D. Role of imbalance between Th17 and regulatory T-cells in sarcoidosis. Curr. Opin. Pulm. Med. 2018;24:521–526. doi: 10.1097/MCP.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 106.Agak G.W., Kao S., Ouyang K., Qin M., Moon D., Butt A., Kim J. Phenotype and Antimicrobial Activity of Th17 Cells Induced by Propionibacterium acnes Strains Associated with Healthy and Acne Skin. J. Investig. Dermatol. 2018;138:316–324. doi: 10.1016/j.jid.2017.07.842. [DOI] [PMC free article] [PubMed] [Google Scholar]