Abstract
Salmonella typhimurium causes systemic and fatal infection in inbred mice, while the related serotype Salmonella typhi is avirulent for mammals other than humans. In order to identify genes from the virulent strain S. typhimurium ATCC 14028 that are absent in S. typhi Ty2, and therefore might be involved in S. typhimurium mouse virulence, a PCR-supported genomic subtractive hybridization procedure was employed. We have identified a novel putative fimbrial operon, stfACDEFG, located at centisome 5 of the S. typhimurium chromosome, which is absent in S. typhi, Salmonella arizonae, and Salmonella bongori but was detected in several other Salmonella serotypes. The fimbrial genes represent a genomic insertion in S. typhimurium compared to the respective region between fhuB and hemL in Escherichia coli K-12. In addition, the subtraction procedure yielded F plasmid-related sequences from the S. typhimurium virulence plasmid, a number of DNA fragments representing parts of lambdoid prophages and putative sugar transporters, and several fragments with unknown sequences. The majority of subtracted chromosomal sequences map to three distinct locations, around centisomes 5, 27, and 57.
The outcome of a Salmonella infection in vertebrates is determined by both the infecting serotype and the infected host. Thus, the host-adapted serotype Salmonella typhi causes typhoid fever in humans but is avirulent in mice and other mammals. On the contrary, Salmonella typhimurium infects a broad spectrum of mammalian hosts, in which it usually leads to a local and self-limiting gastroenteritis. However, S. typhimurium causes a fatal typhoid-like disease in susceptible inbred mice. In both humans and mice, typhoid fever is characterized by systemic dissemination of the bacteria, which, after crossing the intestinal barrier, replicate in organs of the reticuloendothelial system.
The genetic differences that account for the variance in the host ranges of S. typhimurium and S. typhi have been only partially elucidated. The few virulence factors known to differ between these serotypes are involved in early stages of infection rather than in the crucial systemic colonization. S. typhimurium contains several different fimbrial biosynthetic operons that are absent in S. typhi and which play a role in adherence to various types of epithelial cells (2–5). For example, the lpf-encoded long polar fimbriae appear to mediate tropism of S. typhimurium towards mouse small intestinal Peyer’s patches, facilitating the preferential invasion of Peyer’s patch-embedded M cells by S. typhimurium (5). In contrast, factors that are known to affect systemic infection are present in both S. typhimurium and S. typhi (11, 15, 18). The goal of this study was to identify DNA sequences that are present in S. typhimurium but are absent in the S. typhi genome and which therefore might encode factors involved in S. typhimurium mouse virulence. We have performed a genomic subtraction analysis and have identified a variety of DNA fragments that are present in S. typhimurium but lacking in S. typhi. In addition to several genes known to be absent in S. typhi, i.e., genes of the S. typhimurium virulence plasmid, we have identified an undescribed putative fimbrial operon, sequences which belong to at least two S. typhimurium-specific lambdoid prophages, several sequences with similarities to sugar transporters, and a large number of sequences with no homologs in the current databases. Interestingly, the majority of these sequences, including those of the prophages, map to three chromosomal regions, those at centisomes (cs) 5 and 25 to 30 and around cs 57.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
S. typhimurium ATCC 14028 and LT2 were from the American Type Culture Collection; S. typhi Ty2 was a gift of Carolyn Hardegree, Food and Drug Administration; and all other Salmonella strains were from the Salmonella Genetic Stock Center. Escherichia coli SM10λpir and CC118 were from the S. I. Miller laboratory strain collection. Bacteria were routinely grown in L broth and selected with 100 mg of ampicillin per liter when appropriate. Plasmid pGP704 (16) was used as a cloning vector in the π-positive cloning host E. coli SM10λpir.
Genomic subtraction procedure. (i) Preparation of driver and tester DNA.
To obtain random DNA fragmentation, 50 μg of whole genomic DNA from S. typhimurium ATCC 14028 or S. typhi Ty2 was sonicated in 30% glycerol until a homogeneous distribution of DNA fragments was obtained. Samples (5 μg) of each of the fragmented DNAs were treated with mung bean nuclease (1 U/μg of DNA) for 30 min at 30°C and purified after phenol-chloroform extraction. Portions (2.5 μg) of the purified DNAs were separated on agarose gels, and fractions ranging from 300 to 500 bp were eluted from the gels in a total volume of 20 μl. Adapter sequences were generated from mixtures of complementary oligonucleotides. Oligonucleotides were mixed, denatured, and slowly cooled to room temperature. S. typhimurium DNA fragments were ligated to adapter AD2, consisting of 300 pmol of OL27 (TCTCCTAGGAGATCTCCTGCATGCG) and 300 pmol of OL28 (CGCATGCAGGAGATCTCCTAGGA). Adapter AD16 for S. typhi DNA contained 300 pmol of OL43db (TbATbTCTTGCGCCTTAAACCAACC) (superscript “b” indicates biotinylated nucleotide) and 300 pmol of OL44 (GATCGGTTGGTTTAAGGCGCAAGAA). This adapter sequence contains a 5′ overhang and was filled in with Klenow polymerase to obtain blunt ends. Adapter sequences were ligated to eluted genomic DNA fragments for 14 h at 14°C with 400 U of T4 DNA ligase. Fragment libraries from S. typhimurium and S. typhi were individually amplified by PCR (30 cycles, annealing temperature of 68°C) with OL27 and OL43db, respectively, as the starter oligonucleotides. Biotinylated PCR products were purified with Promega PCR purification columns by using 1× SSC (0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate at 65°C for elution.
(ii) Subtractive hybridization.
Samples (1.5 μg) of driver DNA (S. typhi-AD16) were mixed with ca. 0.01 μg of tester DNA (S. typhimurium-AD2) in a total volume of 10 μl of hybridization buffer (50 mM HEPES [pH 7.5] 0.5 M NaCl, 0.1% sodium dodecyl sulfate, 1 mM EDTA). The mixture was denatured for 10 min at 100°C with mineral oil, slowly cooled to 65°C, and kept at this temperature for 48 h. Biotinylated homo- and heteroduplexes were removed from the mixture by organic-phase extraction with 10 μg of streptavidin dissolved in hybridization buffer in a total volume of 100 μl. After addition of streptavidin, the mixture was kept at room temperature for 10 min and extracted five times with 100 μl of phenol-chloroform (1:1 dilution) and twice with 200 μl of chloroform. The DNA was precipitated and dissolved in 5 μl of H2O. A portion (4 μl) of this solution was used for a second round of subtraction with 1.5 μg of driver DNA. After the second subtraction, subtracted S. typhimurium-AD2 DNA was PCR amplified with OL27. The PCR product was restricted with BglII and cloned into pGP704 cut with BglII. Amplification of individual cloned inserts was done with oligonucleotides P1 (CGAATTCCCCGAAAAGTGCCACCTG) and P2 (CAGAATTCCCGGGAGAGCTCG) as the PCR primers.
Genomic mapping of subtracted DNA fragments and Southern blot hybridization.
Subtracted S. typhimurium DNA fragments were labelled by using an enhanced chemiluminescence direct nucleic acid labelling and detection system (Amersham) and were hybridized to an ordered array of Mud-P22 mapping phages (7) or to restricted total genomic DNA according to standard protocols. The array of mapping phages was prepared by individually blotting 0.08 μg of purified DNA from 54 mapping phages. Phage lysate preparation and DNA preparation from phage lysates were done as described previously (7, 19).
DNA sequencing.
DNA sequencing was done by using an automated ABI DNA sequencer and fluorescent dye termination methods. The sequence of the stf chromosomal region of S. typhimurium was obtained by genomic primer walking with DNA from a Mud-P22 mapping phage purified from strain TT15226 (7) as the template. Phage isolation and DNA purification were done as described previously (7, 19).
Nucleotide sequence accession numbers.
The sequence of the putative stf operon has been deposited in GenBank under accession no. AF093503. The sequences of the S. typhimurium DNA fragments described here which are absent in S. typhi have been deposited in GenBank under accession no. AF175343 to AF175384.
RESULTS
Subtraction of S. typhimurium genomic DNA with DNA from S. typhi.
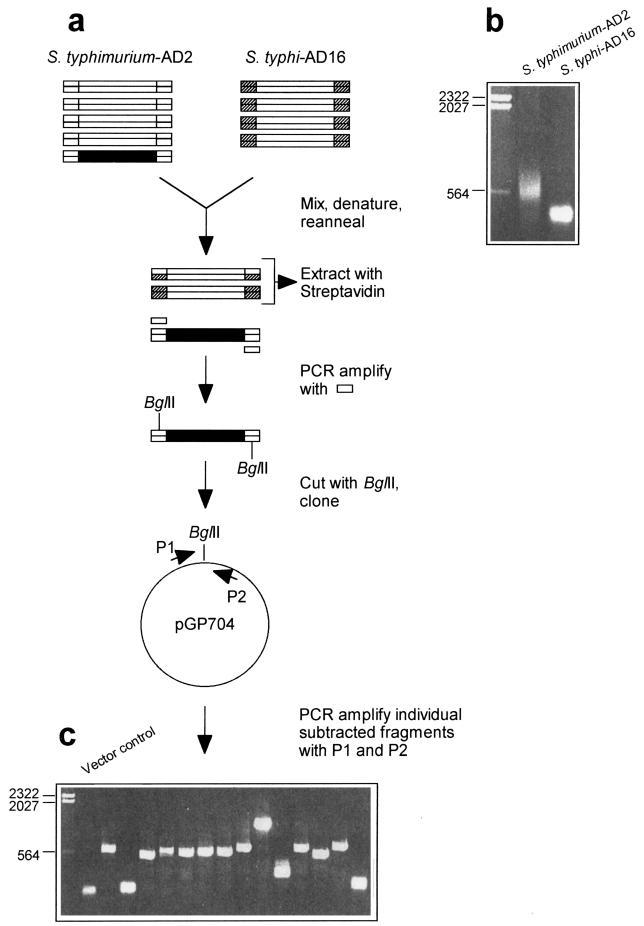
Figure 1 presents an overview of the procedure used for the isolation of S. typhimurium DNA fragments that are not present in the S. typhi genome. Total genomic DNA from both organisms was sheared by ultrasonication, and the resulting ends were blunted with mung bean nuclease (see Materials and Methods). Subsequently, the fragments were separated on agarose gels and fragments ranging from 300 to 500 bp were eluted from the gels. Size-selected fragments were ligated with double-stranded adapter oligonucleotides by using different adapter sequences for DNA fragments from S. typhimurium and S. typhi. Subsequently, these fragment pools were amplified by PCR. In the case of S. typhi DNA (driver), the oligonucleotides used for the adapter generation and PCR amplification were biotinylated. DNA fragments from S. typhimurium (tester) were hybridized to a 100-fold excess of driver DNA, and the biotinylated homo- and heteroduplexes were removed by organic-phase extraction with streptavidin (see Materials and Methods). The resulting nonbiotinylated DNA fragments were subjected to a second round of subtraction and were subsequently amplified with the tester-specific oligonucleotides. This subtracted and reamplified fraction was hybridized against Sau3A-restricted total genomic DNA from S. typhimurium or S. typhi. Almost no signal was obtained with DNA from S. typhi, while a strong signal was obtained with S. typhimurium DNA (data not shown).
FIG. 1.
Genomic subtraction procedure used to isolate S. typhimurium-specific DNA fragments. (a) Schematic representation of the subtraction procedure. A fragment of S. typhimurium DNA not present in S. typhi is shown in black. Biotinylated adapter sequences are indicated by hatching. (b) Agarose gel (sizes [in kilobases] of marker fragments are indicated) showing PCR-amplified S. typhimurium-AD2 fragments and S. typhi-AD16 fragments before subtraction. (c) Agarose gel showing individually amplified S. typhimurium DNA fragments after subtraction and cloning. The PCR product obtained by using the cloning vector without an insertion as the template is in the lane adjacent to the size marker lane. (See text for further details.)
Sequences of S. typhimurium-specific DNA fragments.
In order to isolate individual DNA fragments from the pool of subtracted S. typhimurium fragments, the DNA was cut with BglII, for which a site had been originally included in the adapter sequence, and cloned into BglII-cut pGP704. After transformation into E. coli SM10λpir, cloned inserts were individually amplified by PCR with oligonucleotides (P1 and P2) corresponding to plasmid sequences adjacent to the cloned insert. In agreement with the original size selection, the majority of fragments had lengths of approximately 500 bp (Fig. 1). Individual fragments were blotted onto a membrane and hybridized with total genomic DNA from S. typhi, which was used as a probe. Of 150 DNA fragments tested, 87% gave no or only weak hybridization signals with S. typhi DNA (data not shown).
The 80 fragments which gave no signal with S. typhi DNA were sequenced. The clones fell into four groups: (i) fragments with sequence similarities to known genes from S. typhimurium and other organisms (15 fragments), (ii) fragments with sequence similarities to lambdoid phages (13 fragments), (iii) fragments with sequence similarities to genes from conjugative plasmids (15 fragments), and (iv) 37 unknown sequences.
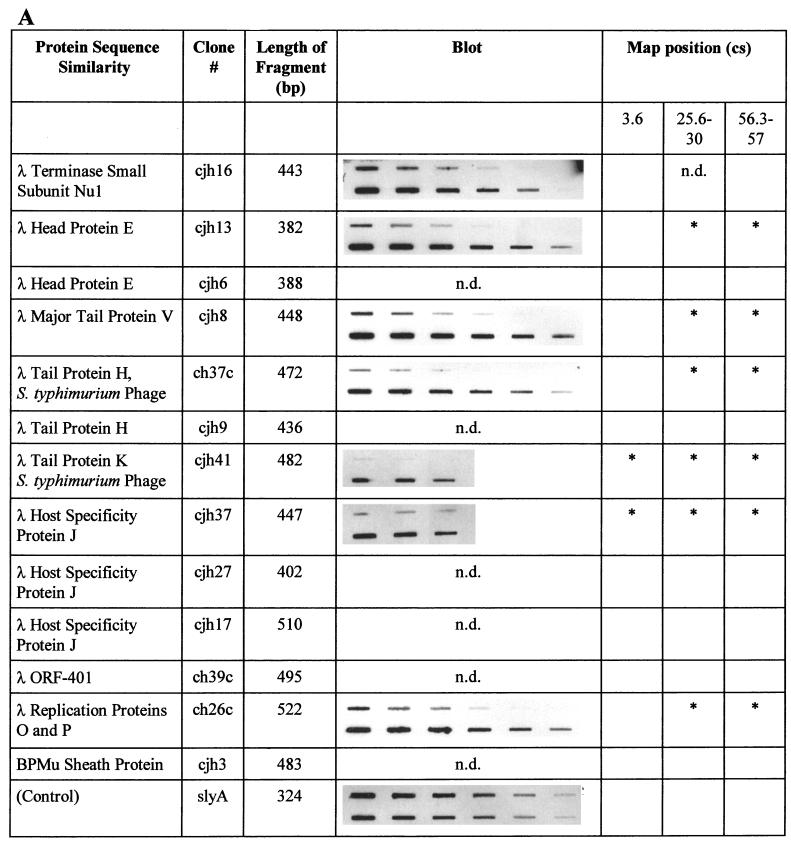
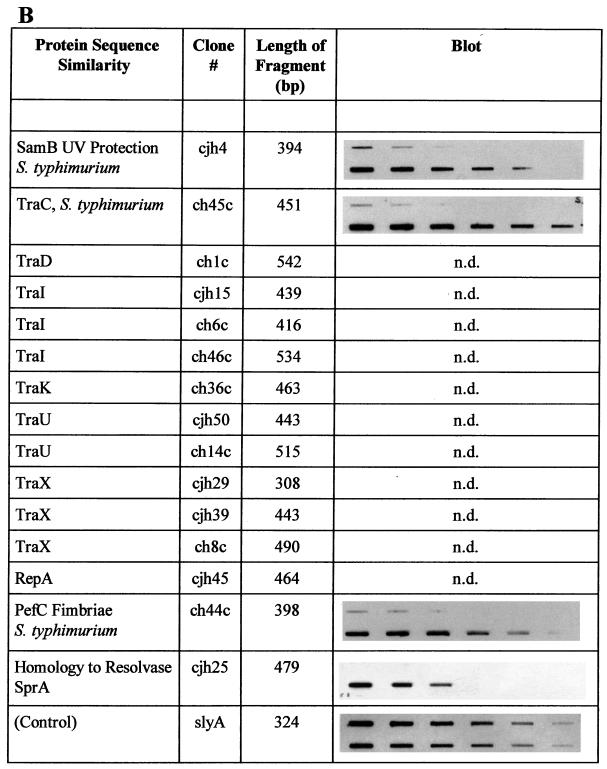
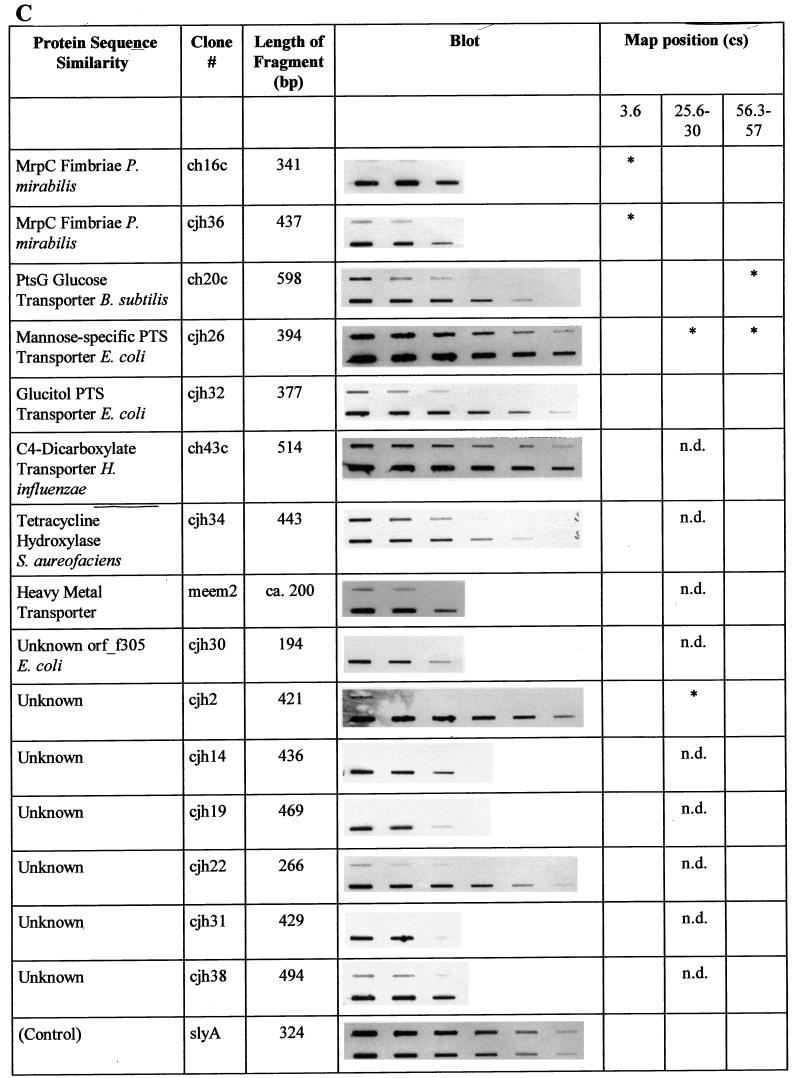
In the Southern blot analysis described above, total genomic S. typhi DNA was used as a probe. Therefore, a weak hybridization signal may artificially have been caused by a low probe-to-target ratio. In order to confirm the absence of these S. typhimurium sequences in the genome of S. typhi, selected individual fragments were used as probes and hybridized to both S. typhimurium and S. typhi total genomic DNA. Of 38 fragments analyzed, 26 gave no signal or reduced signals with S. typhi DNA. Figure 2 summarizes the results of this analysis for DNA fragments which proved to be specific to S. typhimurium.
FIG. 2.
Summary of the characterizations of S. typhimurium-specific subtracted DNA fragments. Protein sequence similarities were determined by BLAST analysis. Similarities were considered significant if BLAST scores were below 10−5. The slot blots shown demonstrate the absence of individual sequences in S. typhi. The upper row of each blot shows total genomic DNA from S. typhi Ty2 blotted in decreasing amounts, while the lower row contains blotted total genomic DNA from S. typhimurium ATCC 14028. The blots were individually hybridized with full-length DNA fragments isolated by subtraction (see text). The control blot was hybridized with a probe present in both organisms (slyA) and demonstrates equal amounts of target DNA in each row. Each row in each blot contains serial dilutions in the range of 2.5 to 0.04 μg of total genomic DNA, with equal amounts of DNA loaded in the corresponding upper and lower slots. The locations on the chromosomal map of S. typhimurium (17) (Fig. 3), as determined by hybridization of specific fragments to an ordered array of mapping phages (7), are indicated. An asterisk indicates a hybridization signal obtained with mapping phages representing one of three different chromosomal locations near cs 3.6, cs 25.6 to 30, and cs 56.3 to 57. n.d., not determined. Characterizations of sequences with similarities to phage proteins (A), sequences of the S. typhimurium virulence plasmid pSLT (B), and other sequences (C) are summarized. B. subtilis, Bacillus subtilis; H. influenzae, Haemophilus influenzae; S. aureofaciens, Streptomyces aureofaciens.
Genomic mapping of S. typhimurium-specific DNA fragments.
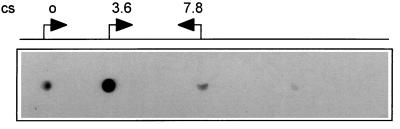
S. typhimurium DNA fragments which did not strongly hybridize to S. typhi genomic DNA were individually located on the genomic map of S. typhimurium by hybridization to an ordered array of Mud-P22 mapping phages (7). Figure 3 shows a representative result for clone cjh36 (which represents an internal part of the novel fimbrial gene stfC [see below]). The strong signal obtained with DNA from the phage which packages chromosomal DNA in the clockwise direction starting at cs 3.6 and the weak signals at cs 0 and 7.8 (clockwise and counterclockwise packaging directions, respectively) indicate that the probe hybridizes to a region between cs 3.6 and 7.8 in proximity to cs 3.6. As described in detail below, sequences adjacent to the DNA region detected by this probe, fhuB and hemL, map to cs 4.9 and 5, respectively (17).
FIG. 3.
Chromosomal mapping of the stf region. An internal fragment of stfC was hybridized to an ordered array of Mud-P22 mapping phages which individually package defined genomic segments (7). The signals obtained are correlated with the chromosomal start point (o) of DNA packaging, and the direction of DNA packaging of individual phages is indicated.
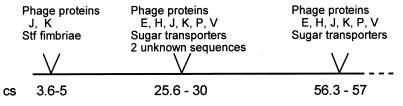
For various clones, the results obtained by Mud-P22 phage blot mapping were confirmed by hybridization to blots of XbaI- and BlnI-restricted genomic DNA separated by pulsed-field gel electrophoresis (PFGE) (13, 14). The combined results of these experiments are shown in Fig. 2 and are schematically summarized in Fig. 4. Interestingly, the majority of fragments tested map to three different chromosomal regions, at cs 3.6, 25.6 to 30, and 56.3 to 57. These regions contain sequences of lambdoid prophages. Some of the phage sequences tested map to all three locations (encoding phage proteins J and K), while others (encoding phage proteins E, H, P, and V) are located at cs 25.6 to 30 and at cs 56.3 to 57 but are absent in the cs 3.6 position. Furthermore, sequences with similarity to sequences encoding sugar transport enzymes (Fig. 2) map to both cs 25.6 to 30 and cs 56.3 to 57. Interestingly, none of the sequences tested mapped to a further location.
FIG. 4.
Schematic representation of the locations of subtracted DNA fragments on the chromosomal map of S. typhimurium. Map positions are indicated according to reference (17).
Localization of an F-related gene fragment to the S. typhimurium virulence plasmid.
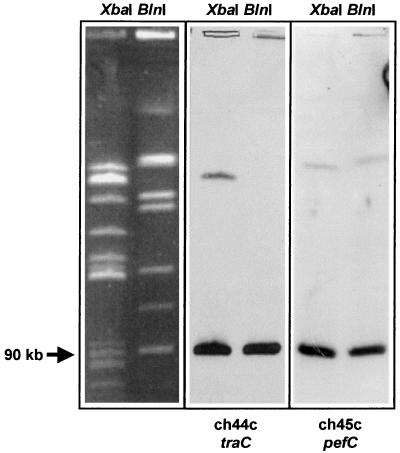
It was recently shown that S. typhimurium contains sequences similar to portions of the F-related conjugative plasmid from E. coli, and it was speculated that these sequences may be part of the S. typhimurium virulence plasmid pSLT (8). In order to analyze whether the tra gene fragments isolated in this study (Fig. 2) are localized on pSLT, we mapped the genomic location of the traC fragment by PFGE (13, 14). As shown in Fig. 5, fragment ch44c, representing traC, hybridized to the same 90-kb fragment as did the known plasmid-borne pefC fragment in both XbaI- and BlnI-restricted genomic DNA from S. typhimurium ATCC 14028.
FIG. 5.
Genomic mapping of subtracted S. typhimurium DNA fragments representing parts of the S. typhimurium virulence plasmid genes traC and pefC. Probes were hybridized to a blot of total genomic DNA of S. typhimurium ATCC 14028 restricted with either XbaI or BlnI and separated by PFGE. Assignment of DNA fragment sizes was done by using a size standard (not shown) as well as by comparing fragment patterns to published S. typhimurium PFGE analyses (13, 14).
Identification of a novel putative fimbrial operon in S. typhimurium.
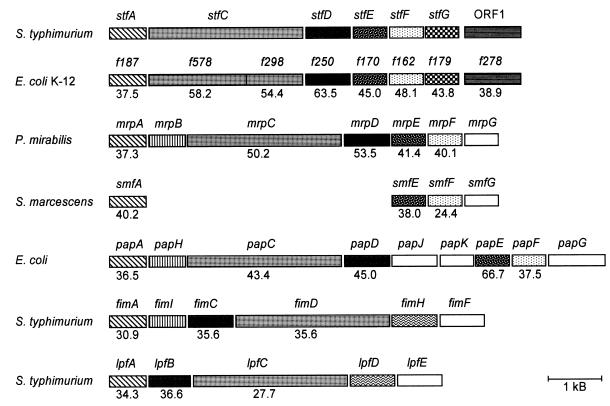
The subtracted fragments ch16c and cjh36 are homologous to the mrpC fimbrial biosynthesis gene of Proteus mirabilis (Fig. 2). In order to analyze whether these fragments represent a fimbrial biosynthetic gene cluster, DNA flanking the isolated fragments was sequenced by genomic primer walking (see Materials and Methods). In a total DNA region of 7.5 kb, six open reading frames (ORF) which potentially encode fimbriae with close similarity to proteins encoded by the mannose-resistant fimbrial operon (mrp) of P. mirabilis (1) were identified. The genes were designated stf (for S. typhimurium fimbriae) and suffixed A to G according to the suffixes of the mrp genes from P. mirabilis. In addition to its close similarity to the mrp operon, the putative stf operon is closely related in structure and deduced protein sequences to an uncharacterized gene cluster from E. coli K-12. The similarity to these E. coli genes comprises an ORF (ORF1) with similarity to ORF f278 from E. coli K-12, which is located downstream from stfG. Figure 6 shows the overall genetic organization of this chromosomal region in comparison to the genetic organization of other selected fimbrial operons. The lengths and putative functions of the stf-encoded proteins are given in Table 1.
FIG. 6.
Comparison of genetic organization and similarities of deduced proteins of the stf genes and other fimbrial operons from various organisms. Direction of transcription is from left to right. Similarities of deduced proteins are indicated by different patterns. The identities of amino acid sequences with the stf-encoded proteins are given as percentages. S. marcescens, Serratia marcescens.
TABLE 1.
Lengths and putative functions of proteins encoded by the stf genes
| Protein | Length (amino acids) | Putative function(s) |
|---|---|---|
| StfA | 186 | Major component of pilus rod |
| StfC | 885 | Outer membrane usher |
| StfD | 250 | Periplasmic chaperone |
| StfE | 170 | Minor pilin |
| StfF | 158 | Minor pilin |
| StfG | 173 | Minor pilin, putative adhesin |
| ORF1 | 279 | Unknown |
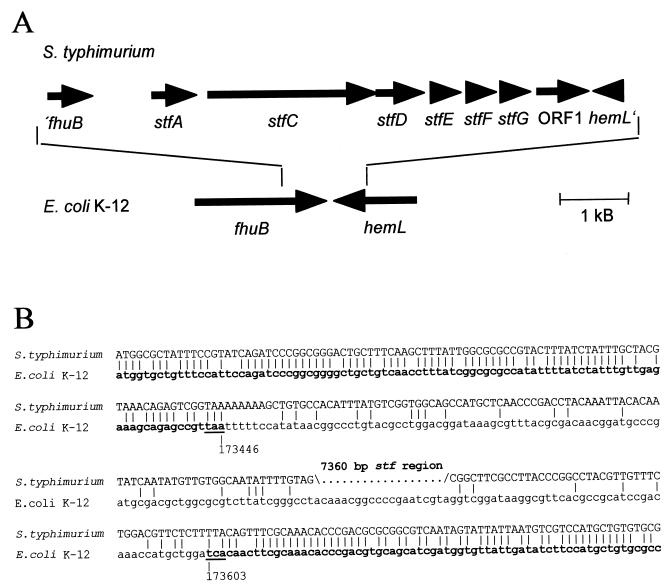
The stf region represents a genomic insertion in S. typhimurium relative to the corresponding region in the E. coli genome.
Interestingly, the DNA region comprising stfACDEFG-ORF1 is flanked by sequences that are highly similar to the 3′ ends of fhuB and hemL. fhuB and hemL are located in direct proximity to each other in E. coli K-12. The sequence similarities between E. coli and S. typhimurium stop abruptly after the stop codons of fhuB and hemL (Fig. 7). Therefore, the stfACDEFG-ORF1 region represents a genomic insertion in S. typhimurium, compared to the corresponding chromosomal region in E. coli K-12. The G+C content of the stf region is 50.6 mol%, while the 660 bp which overlap fhuB exhibit a G+C content of 60.8 mol%, and the 150 bp which overlap hemL show a G+C content of 51.3 mol%.
FIG. 7.
(A) Genomic organization of the stf region, located between fhuB and hemL in S. typhimurium. The genes encoding the stf fimbriae and their positions relative to fhuB and hemL in E. coli are indicated by arrows. (B) Alignment of DNA sequences flanking the stf region in S. typhimurium with fhuB and hemL sequences of E. coli. The 3′ ends of the E. coli fhuB and hemL genes are shown in bold. The exact positions of the underlined stop codons of the complete E. coli K-12 genome sequence are given in base pairs. Identical bases are indicated by vertical bars.
In Fig. 6, a putative fimbrial operon from E. coli K-12 which is similar at the protein level to the stf region is shown. These E. coli fimbrial genes are located around bp 2449000 of the complete E. coli genome sequence in a chromosomal position different from that of fhuB and hemL, which are located at bp 173500.
Distribution of subtracted S. typhimurium sequences among various Salmonella serotypes.
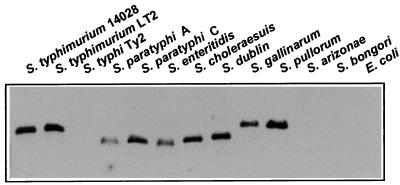
In order to determine whether stf sequences were absent only in S. typhi or whether they were also missing in other Salmonella serotypes, a Southern blot analysis was performed with a probe derived from stfC. Figure 8 shows that, in addition to being absent from S. typhi and E. coli, stfC is absent in serotypes S. arizonae and S. bongori but is present in all other serotypes tested.
FIG. 8.
Distribution of stf sequences among different Salmonella serotypes and E. coli. PstI-restricted genomic DNA of the organisms indicated was hybridized with an internal probe of the stfC gene (see Materials and Methods).
Similarly, sequences of lambdoid S. typhimurium prophages and a number of sequences with no homologs in current databases were hybridized to dot blots of total genomic DNAs from various Salmonella serotypes. The results of these analyses are summarized in Table 2. The two phage genes, E and J, are missing from S. arizonae, S. bongori, and E. coli in addition to S. typhi. The phage J gene is also lacking in S. paratyphi A but is present in the other serotypes tested. In contrast, the phage head gene E fragment localizes to S. typhimurium and S. choleraesuis but is absent in other serotypes.
TABLE 2.
Distribution of subtracted S. typhimurium fragments among selected Salmonella serotypes
| Serotype | Hybridization signala
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cjh13 phage E | cjh37 phage J | cjh35 | ch4c | ch13c | ch15c | ch23c | ch25c | ch29c | ch48c | |
| S. typhimurium 14028 | + | + | + | + | + | + | + | + | + | + |
| S. typhimurium LT2 | + | + | + | + | + | + | − | + | + | + |
| S. typhi Ty2 | − | − | − | − | − | − | − | − | − | − |
| S. paratyphi A | − | − | − | − | − | − | − | − | − | − |
| S. paratyphi C | − | + | + | − | + | − | − | − | + | + |
| S. enteritidis | − | + | + | − | − | − | − | − | − | − |
| S. choleraesuis | + | + | + | − | +/− | + | − | − | − | + |
| S. dublin | − | + | + | − | + | + | + | + | +/− | + |
| S. gallinarum | − | + | + | − | − | − | + | − | − | − |
| S. pullorum | − | + | + | − | + | − | + | − | + | + |
| S. arizonae | − | − | − | − | − | − | − | − | − | − |
| S. bongori | − | − | − | − | − | − | − | − | − | − |
| E. coli CC118 | − | − | ND | − | − | − | − | − | ND | ND |
| E. coli K-12 | ND | ND | − | ND | ND | ND | ND | ND | − | − |
+, strong; +/−, weak; −, none; ND, not determined.
The other sequences tested are variably present in S. paratyphi C, Salmonella enteritidis, S. choleraesuis, S. dublin, S. gallinarum, and S. pullorum but lacking in S. typhi, S. paratyphi A, S. arizonae, S. bongori, and E. coli (Table 2).
DISCUSSION
S. typhimurium systemically and lethally infects inbred mice, whereas the human-adapted S. typhi, the causative agent of human typhoid fever, is avirulent for mice. Therefore, S. typhimurium genes which are absent in the S. typhi genome might have a role in S. typhimurium mouse virulence. To identify S. typhimurium genes that are lacking in S. typhi, we applied a PCR-supported genomic subtractive hybridization procedure with short genomic DNA fragments from both organisms. Among the identified fragments are genes known to be absent in S. typhi, i.e., genes located on the S. typhimurium virulence plasmid. Furthermore, we have identified fragments of at least two S. typhimurium prophages and a hitherto-undescribed fimbrial operon. A fourth large group of S. typhimurium fragments that are not present in S. typhi have no homologs in the current databases.
The subtraction method employed in this analysis used ultrasonication for the fragmentation of the genomic DNA of both the tester (S. typhimurium) and the driver (S. typhi). In contrast, commonly used subtraction procedures rely on enzymatic restriction of genomic DNA and therefore may miss certain tester-specific DNA fragments due to the absence of respective restriction sites. As a result of the size selection of sheared DNA, the average length of subtracted S. typhimurium fragments identified in this study was 430 bp (Fig. 2). The procedure can easily be adapted for the identification of even shorter genomic differences. The efficiency of subtraction can be estimated by combining the results obtained from two hybridization analyses. In the first control, S. typhi total DNA was used to probe 150 subtracted fragments from S. typhimurium that had been individually blotted onto a membrane. Of the 130 S. typhimurium fragments which did not strongly hybridize with the S. typhi probe, 38 were individually used to probe S. typhi total DNA and 26 (68%) proved to give no signal or only weak signals with the S. typhi target. Thus, of the total of 150 DNA fragments, 88 fragments (68% of 130) were estimated to be unique to S. typhimurium, which represents a subtraction efficiency in the range of 60%.
Interestingly, the majority of the S. typhimurium-specific DNA sequences identified represent several distinct regions of the S. typhimurium genome: (i) 15 DNA fragments comprising a total of 6.8 kb are localized to the S. typhimurium virulence plasmid, which is absent in S. typhi; (ii) 13 fragments represent at least two cryptic prophages located at cs 25 and 57; and (iii) 2 fragments (0.8 kb) represent the stf fimbrial operon. The virulence plasmid is 90 kb long and the stf operon is 7.5 kb long, while the size of the prophages is not known. Therefore, 17 isolated fragments with a total length of 7.6 kb represent a total of 97.5 kb of genomic DNA from S. typhimurium, indicating that we have identified approximately 8% of all DNA fragments with a minimum length of ca. 500 bp which are present in S. typhimurium but lacking or highly divergent in S. typhi. Although we cannot exclude the possibility that the subtraction procedure is not truly representative, the number of S. typhimurium-specific DNA fragments identified in this study was likely limited by the number of fragments sequenced rather than by a restriction in the subtractive process. However, it is possible that the independent identification of several fragments from the same genes (i.e., stfC, traI, traU, and traX [Fig. 2]) may have resulted from a preferential amplification of these fragments during the preparation of tester DNA.
The total length of S. typhimurium-specific DNA fragments identified in this study is 11.2 kb (Fig. 2). Therefore, if these sequences represent 8% of S. typhimurium-specific DNA, it can be estimated that S. typhimurium ATCC 14028 carries at least 140 kb of genomic DNA which are absent in S. typhi Ty2. This value differs from an estimation given by Lan and Reeves, who reported that the LT2 strain of S. typhimurium contains between 300 and 600 kb which are absent in S. typhi (12). These authors calculated the amount of LT2 DNA absent in S. typhi from results obtained by probing a cosmid library of LT2 with a pool of LT2 DNA fragments obtained after subtraction with S. typhi DNA. However, Lan and Reeves reported that S. typhimurium LT2 contains about 100 kb of DNA which are absent from another S. typhimurium strain, SARA 21 (6). S. typhimurium ATCC 14028, which was used in this study, may also contain less DNA than LT2, rendering our estimation more in line with the one given by Lan and Reeves.
The subtraction procedure yielded two fragments that represent different parts of the stfC gene, which was found to be part of a novel putative fimbrial operon. Interestingly, the stf region is a genomic insertion in S. typhimurium compared to the respective region between the fhuB and hemL genes in E. coli. However, a putative fimbrial operon similar to stf in gene order and encoded proteins is present in a different chromosomal location in E. coli. Whether these fimbrial genes are functional and whether stf has a role in Salmonella host range determination are currently being investigated.
A group of subtracted fragments, including samB, pefC, and several tra genes, localizes to the 90-kb S. typhimurium virulence plasmid, pSLT (17). Not much is known about the genetic structure of the plasmid, apart from what is known of the samAB genes and the par and spv-pef regions (17). Recently, it has been suggested that pSLT is an F-related plasmid encoding conjugative transfer functions (8). Our results demonstrate that several genes of the F plasmid transfer region are present in S. typhimurium (Fig. 2). Furthermore, fragments ch44c and ch45c, representing traC and pefC, respectively, strongly hybridize to the 90-kb plasmid fragment in XbaI- and BlnI-restricted genomic DNA of S. typhimurium ATCC 14028, demonstrating that both genetic regions are located on the plasmid. Thus, pSLT is indeed F related and may be transferable by conjugation.
The subtraction procedure yielded a number of fragments located at cs 25 and 57 which appear to be parts of two lambdoid prophages that are absent in S. typhi. Recently, two cryptic S. typhimurium prophages designated Gifsy-2 (at cs 25) and Gifsy-1 (at cs 57) have been described (10). Since several fragments with unknown sequences map to the same chromosomal location as the prophage gene fragments do, we assume that these fragments are part of the prophages. Thus, Gifsy-1 and Gifsy-2 are possible vehicles for horizontally acquired genes that may have important functions in the biology of S. typhimurium. Indeed, the sodC gene, which, by facilitating resistance to the macrophage oxidative burst, is involved in mouse virulence, is located between the genes encoding the phage tail proteins M and L, which appear to be part of Gifsy-2 (9). Interestingly, two subtracted phage DNA fragments representing the phage genes J and K hybridized to a chromosomal region around cs 3.6 in addition to hybridizing to Gifsy-1 and Gifsy-2. Furthermore, the phage DNA fragments H, P, and V also gave signals of hybridization with the 90-kb virulence plasmid (8a). These data suggest that S. typhimurium ATCC 14028 carries at least four prophages or parts thereof.
The distribution in various Salmonella serotypes of two phage gene fragments, cjh37 (phage gene J) and cjh13 (phage gene E), and of a number of fragments with unknown sequences was analyzed. The results, summarized in Table 2, show that the phage head gene E is present in S. typhimurium and S. choleraesuis but lacking in all other strains tested. On the other hand, the phage J gene was found in all Salmonella serotypes except S. typhi, S. paratyphi A, S. arizonae, and S. bongori. In S. typhimurium, cjh37 (phage gene J) colocalizes with cjh13 (phage gene E) to cs 25 and 57. Therefore, both subtracted DNA fragments appear to represent parts of Gifsy-1 and Gifsy-2. However, cjh37 also localizes to a chromosomal position near cs 3.6 representing a third prophage-like element. The distribution of these DNA fragments among Salmonella serotypes thus indicates that Gifsy-1 and Gifsy-2 or parts thereof are absent in S. typhi, S. paratyphi A and C, S. enteritidis, S. dublin, S. gallinarum, S. pullorum, S. arizonae, S. bongori, and E. coli, while the prophage element located near cs 3.6 in S. typhimurium is present in the majority of serotypes tested. The fragments with unknown sequences show no regular pattern of distribution among different Salmonella serotypes. Fragment cjh35 localizes to the same serotypes as does the phage gene J, indicating that cjh35 may be part of the prophage located at cs 3.6. In addition, colocalization of ch13c and ch48c to various serotypes indicates that these fragments may also be part of a common genetic region.
In summary, we have identified several S. typhimurium DNA fragments, including a novel fimbrial operon and several prophage-like elements, which are absent in S. typhi. The isolation of DNA sequences that are present in S. typhimurium but absent in S. typhi provides us with the tools necessary to assess the genetic basis of the differential mouse virulence and host ranges of these two important pathogens.
ACKNOWLEDGMENTS
M. E. and C.J.H. were supported by a research grant from the Deutsche Forschungsgemeinschaft, and C.J.H. was additionally supported by a personal grant from the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie.
REFERENCES
- 1.Bahrani F K, Mobley H L T. Proteus mirabilis MR/P fimbrial operon: genetic organization, nucleotide sequence, and conditions for expression. J Bacteriol. 1994;176:3412–3419. doi: 10.1128/jb.176.11.3412-3419.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bäumler A J, Heffron F. Identification and sequence analysis of lpfABCDE, a putative fimbrial operon of Salmonella typhimurium. J Bacteriol. 1995;177:2087–2097. doi: 10.1128/jb.177.8.2087-2097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bäumler A J, Tsolis R M, Bowe F A, Kusters J G, Hoffmann S, Heffron F. The pef fimbrial operon of Salmonella typhimurium mediates adhesion to murine small intestine and is necessary for fluid accumulation in the infant mouse. Infect Immun. 1996;64:61–68. doi: 10.1128/iai.64.1.61-68.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bäumler A J, Tsolis R M, Heffron F. Contribution of fimbrial operons to attachment to and invasion of epithelial cell lines by Salmonella typhimurium. Infect Immun. 1996;64:1862–1865. doi: 10.1128/iai.64.5.1862-1865.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bäumler A J, Tsolis R M, Heffron F. The lpf fimbrial operon mediates adhesion of Salmonella typhimurium to murine Peyer’s patches. Proc Natl Acad Sci USA. 1996;93:279–283. doi: 10.1073/pnas.93.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beltran P, Plock S A, Smith N H, Whittam T S, Old D C, Selander R K. Reference collection of strains of the Salmonella typhimurium complex from natural populations. J Gen Microbiol. 1991;137:601–606. doi: 10.1099/00221287-137-3-601. [DOI] [PubMed] [Google Scholar]
- 7.Benson N R, Goldman B S. Rapid mapping in Salmonella typhimurium with Mud-P22 prophages. J Bacteriol. 1992;174:1673–1681. doi: 10.1128/jb.174.5.1673-1681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyd E F, Hartl D L. Salmonella virulence plasmid. Modular acquisition of the spv virulence region by an F-plasmid in Salmonella enterica subspecies I and insertion into the chromosome of subspecies II, IIIa, IV and VII isolates. Genetics. 1998;149:1183–1190. doi: 10.1093/genetics/149.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Emmerth, M., and C. J. Hueck. Unpublished results.
- 9.Farrant J L, Sansone A, Canvin J R, Pallen M J, Langford P R, Wallis T S, Dougan G, Kroll J S. Bacterial copper- and zinc-cofactored superoxide dismutase contributes to the pathogenesis of systemic salmonellosis. Mol Microbiol. 1997;25:785–796. doi: 10.1046/j.1365-2958.1997.5151877.x. [DOI] [PubMed] [Google Scholar]
- 10.Figueroa-Bossi N, Coissac E, Netter P, Bossi L. Unsuspected prophage-like elements in Salmonella typhimurium. Mol Microbiol. 1997;25:161–173. doi: 10.1046/j.1365-2958.1997.4451807.x. [DOI] [PubMed] [Google Scholar]
- 11.Hohmann E L, Oletta C A, Killeen K P, Miller S I. phoP/phoQ-deleted Salmonella typhi (TY800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J Infect Dis. 1996;173:1408–1414. doi: 10.1093/infdis/173.6.1408. [DOI] [PubMed] [Google Scholar]
- 12.Lan R, Reeves P R. Gene transfer is a major factor in bacterial evolution. Mol Biol Evol. 1996;13:47–55. doi: 10.1093/oxfordjournals.molbev.a025569. [DOI] [PubMed] [Google Scholar]
- 13.Liu S-L, Hessel A, Sanderson K E. The XbaI-BlnI-CeuI genomic cleavage map of Salmonella typhimurium LT2 determined by double digestion, end labelling, and pulsed-field gel electrophoresis. J Bacteriol. 1993;175:4104–4120. doi: 10.1128/jb.175.13.4104-4120.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S-L, Sanderson K E. A physical map of the Salmonella typhimurium LT2 genome made by using XbaI analysis. J Bacteriol. 1992;174:1662–1672. doi: 10.1128/jb.174.5.1662-1672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller S I, Kukral A M, Mekalanos J J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanderson K E, Hessel A, Rudd K E. Genetic map of Salmonella typhimurium, edition VIII. Microbiol Rev. 1995;59:241–303. doi: 10.1128/mr.59.2.241-303.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shea J E, Hensel M, Gleeson C, Holden D. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youderian P, Sugiono P, Brewer K L, Higgins N P, Elliott T. Packaging specific segments of the Salmonella chromosome with locked-in Mud-P22 prophages. Genetics. 1988;118:581–592. doi: 10.1093/genetics/118.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]