Abstract
Although sepsis and acute kidney injury (AKI) have a bidirectional interplay, the pathophysiological mechanisms between AKI and sepsis are not clarified and worthy of a comprehensive and updated review. The primary pathophysiology of sepsis-associated AKI (SA-AKI) includes inflammatory cascade, macrovascular and microvascular dysfunction, cell cycle arrest, and apoptosis. The pathophysiology of sepsis following AKI contains fluid overload, hyperinflammatory state, immunosuppression, and infection associated with kidney replacement therapy and catheter cannulation. The preventive strategies for SA-AKI are non-specific, mainly focusing on infection control and preventing further kidney insults. On the other hand, the preventive strategies for sepsis following AKI might focus on decreasing some metabolites, cytokines, or molecules harmful to our immunity, supplementing vitamin D3 for its immunomodulation effect, and avoiding fluid overload and unnecessary catheter cannulation. To date, several limitations persistently prohibit the understanding of the bidirectional pathophysiologies. Conducting studies, such as the Kidney Precision Medicine Project, to investigate human kidney tissue and establishing parameters or scores better to determine the occurrence timing of sepsis and AKI and the definition of SA-AKI might be the prospects to unveil the mystery and improve the prognoses of AKI patients.
Keywords: acute kidney injury, infection, sepsis, pathophysiology, sepsis-associated acute kidney injury
1. Introduction
Acute kidney injury (AKI) is an essential but complex syndrome that results from heterogeneous mechanisms and carries considerable morbidity and mortality [1,2]. AKI occurs in 10%–15% of hospitalized patients and 50% in the intensive care unit (ICU) setting [3,4]. AKI is recognized as a decrease in glomerular filtration rate (GFR), and the contemporarily accepted system for definition and classification of AKI is the Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guideline for AKI 2012 [5], which is based on serum creatinine, GFR, and urine output. Although the KDIGO 2012 AKI guideline has many limitations, it could not be replaced by any other AKI definition/classification/staging systems [6]. Although there has been some advancement in AKI therapies, including kidney replacement therapy (KRT), the improvement in AKI patients’ outcomes has been limited over the decades [7,8]. The disappointing results call for a strategy to prevent the occurrence and progression of AKI at an earlier stage. For this purpose, a crucial step is to obtain a more profound and clear understanding of the association between AKI and other underlying clinical illness or factors that cause or precipitate AKI occurrence [9,10].
Sepsis, a life-threatening organ dysfunction resulting from a dysregulated host response to infection, causes heavy healthcare and economic burdens worldwide [4,11,12,13]. At the same time, sepsis is a primary and crucial precipitating factor of AKI in critically ill patients. It is known that sepsis increases the risk of AKI development [11], and an increasing body of evidence reveals higher risks of infection or sepsis following AKI [12]. Due to the close relationship between AKI and sepsis, some investigators suggested taking AKI as an early sign of sepsis [13]. As to the molecular-level aspect, existing evidence, including our previous work, has demonstrated procalcitonin’s optimal ability to detect sepsis and AKI [14,15]. Since procalcitonin is an infection biomarker, these findings indicate the complicated association between sepsis and AKI. However, the pathophysiological mechanisms between AKI and sepsis are not clearly understood and are worthy of a comprehensive and updated review.
The current narrative review focused on the bidirectional pathophysiologies between AKI and sepsis. We extended sepsis to a broader field to include severe infection to provide a more comprehensive review. Although most proposed theories are based on animal models, this knowledge helps physicians and researchers understand the underlying pathophysiology crucial for new perspectives to improve the patients’ prognoses of these populations.
2. Epidemiology
Although it is well known that sepsis increases the subsequent AKI risks, the incidence of sepsis-associated AKI (SA-AKI) has not been well determined [11]. The incidence rates of AKI among septic patients were reported as 54% in a multicenter prospective cohort study enrolling critically ill patients from 24 European countries [16] and 47.1% in a multicenter retrospective cohort study enrolling hospitalized patients across China [17]. According to the observation that AKI occurs in roughly one to two out of three septic patients, the SA-AKI has involved six to eleven million cases [11,18]. Besides, sepsis is also the leading cause of death in AKI patients [19].
On the other hand, AKI is associated with a higher risk of de novo infections and the progression of sepsis. Among the cardiac surgical patients, postoperative AKI influenced the risk of severe infections in a retrospective analysis of 24,660 patients [20], and even mild AKI was independently associated with postoperative infection [21]. Besides surgical patients, a higher infection risk following AKI was also noticed in non-surgical patients [12,22,23,24]. In a propensity score-matched cohort study studying hospitalized patients, Griffin BR et al. found that compared to the patients without AKI, those with recovered AKI whose creatinine returned to baseline were still associated with a 4.5-fold increased odds ratio for infection within 30 days following discharge [22]. The association between AKI and subsequent infection persistently exists in a nationwide population-based cohort study. A previous work of our study group demonstrated that patients with severe AKI requiring KRT had an approximately 3-fold higher risk of developing severe sepsis than the non-AKI group (6.84 versus 2.32 per 100 person-years) in the index hospitalization. The hazard ratios for severe sepsis in the comparisons between the KRT-requiring AKI group and non-AKI group were 2.05–3.44 in different adjustment models with various follow-up periods of the multivariate analyses. The subgroup analysis found that even the patients who recovered from the KRT-requiring AKI had significantly higher hazard ratios (1.61–1.68) of developing severe sepsis than those without KRT-requiring AKI [12]. Another previous work of our team also observed an increased risk of active tuberculosis following KRT-requiring AKI in the Taiwan National Health Insurance database. This association was also noted even in patients weaned from dialysis, suggesting that immune dysfunction in AKI is not limited in the short term [23]. A retrospective cohort study showed that the three most frequent infections in critical patients with AKI were pneumonia (54.3%), intra-abdominal infection (11.9%), and urinary tract infections (9.7%) [24].
3. Pathophysiology of SA-AKI
The pathophysiology of SA-AKI remains unclear, although scientists have made considerable effort in this field. Most current theories are based on animal models or autopsy results [11]; thus, the interpretation and application of these findings should be made cautiously. In an earlier era, renal hypoperfusion and associated ischemia were considered the main culprits of SA-AKI, but animal experiments have not entirely supported this traditional concept.
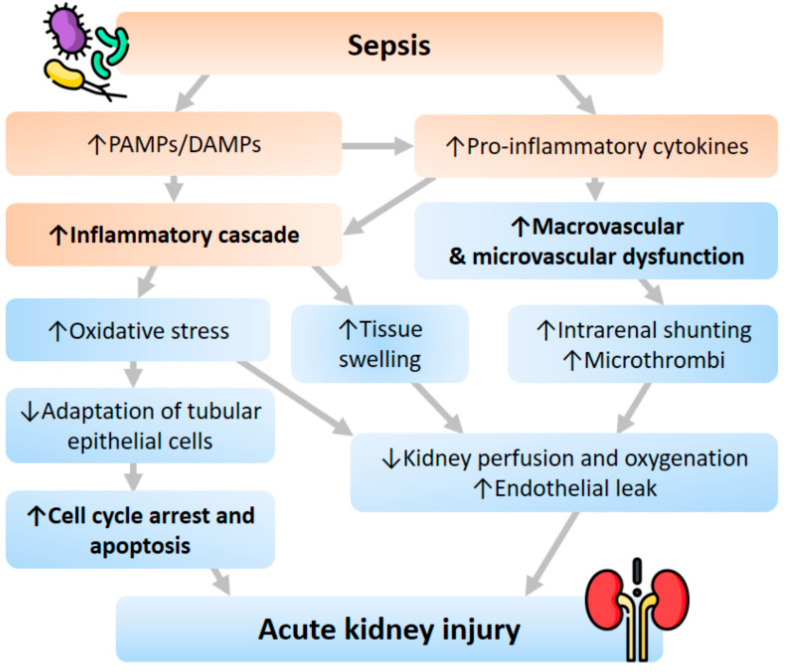
Several animal studies demonstrated that the subjects with Gram-negative bacteremia had significantly increased renal blood flow compared with the controlled group. In histology findings, the degree of tubular injury was mild, and there was no significant difference between the sepsis and non-sepsis groups [25,26,27]. Moreover, an animal model did not reveal a significant correlation between early SA-AKI and histopathologic lesions on renal biopsy [25]. Therefore, instead of the traditional concept, inflammatory cascade, macrovascular and microvascular dysfunction, and cell response abnormality are now believed to be the three cornerstones of the underlying pathophysiological mechanism of SA-AKI (Figure 1).
Figure 1.
Pathophysiology of AKI following sepsis. Abbreviations: DAMPs, damage-associated molecular patterns; PAMPs, pathogen-associated molecular patterns.
3.1. Inflammatory Cascade
In the state of sepsis, inflammatory mediatory molecules such as pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) are released into the intravascular space and bind to receptors such as Toll-like receptors (TLRs) on the surface of immune cells. This reaction subsequently initiates a sequence of the signal cascade, producing and releasing pro-inflammatory cytokines. Besides, renal tubular epithelial cells also have expressed TLRs, especially TLR-2 and TLR-4. Thus, a similar pathway will be activated once PAMPs or DAMPs are filtered through the glomerulus, leading to increased oxidative stress, production of reactive oxygen species, and mitochondrial injury [11,28,29].
3.2. Macrovascular and Microvascular Dysfunction
At the same time, efferent arteriolar vasodilation and intrarenal shunting contribute to macrovascular dysfunction. The macrovascular dysfunction diverts the renal blood flow from the medulla to the cortex, causing decreased perfusion and oxygenation of the medulla, further worsening kidney function [30]. As for the microvascular system, elevated pro-inflammatory cytokines and activated leukocytes in sepsis might lead to microthrombi formation in renal capillaries [31]. The microthrombi formation results in decreased blood flow and diffusion to the inflamed and edematous tissue [32]. These vascular dysfunctions also cause the production of reactive oxygen species that further damage the epithelial barrier that ends up in an endothelial leak [33,34].
3.3. Cell Cycle Arrest and Apoptosis
To date, different theories have been proposed to explain the metabolic adaptation that tubular epithelial cells undergo in sepsis. The fundamental concepts of these theories include optimizing energy utilization, priority preserving vital cell functions, and avoiding cell death. During sepsis, the cell adaption downregulates aerobic glycolysis and increases oxidative phosphorylation to improve survival and make the host less vulnerable to developing AKI [35,36,37]. Since cell replication is a very energy-consuming process, several checkpoints are the sentinels to evaluate whether the cell has sufficient energy to undergo replication during the cell cycle. If the energy is deemed insufficient, the cell will proceed into cell cycle arrest to avoid energy failure and apoptosis. Any delay or inadequacy of this rescue action finally results in cell death. Both cell cycle arrest and apoptosis play essential roles in the initiation of SA-AKI.
Excellent examples to support the primary roles of cell cycle arrest and apoptosis in SA-AKI development are the two emerging biomarkers, the tissue inhibitor of metalloproteinase-2 (TIMP-2) and insulin-like growth factor-binding protein-7 (IGFBP-7). These two molecules are basically cell cycle arrest biomarkers, but they have optimal predictive ability for SA-AKI [11,25].
4. Pathophysiology of Sepsis Following AKI
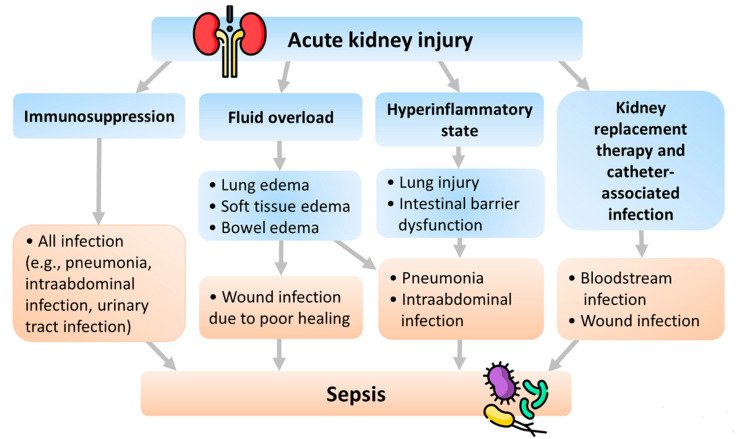
Several pathophysiologies of sepsis following AKI have been proposed. We summarize these pathophysiologies in Figure 2 and explain them below (Figure 2). Pneumonia is the most frequent infection that results from volume overload and inflammation in AKI patients. Moreover, there is a close and complex association between AKI, immune dysregulation, pneumonia, and lung injury. Therefore, we also include lung injury in addition to pneumonia in the following sections to provide readers with a more comprehensive understanding.
Figure 2.
Pathophysiology of sepsis following AKI.
4.1. Fluid Overload
Fluid overload is a common complication of oliguria or anuria in AKI that presents with tissue edema. Soft tissue edema causes poor tissue healing and wound infection [38]. Bowel edema impairs barriers to infection, allowing intestinal bacteria translocation and leading to the development of intraabdominal infection or even sepsis [38]. Besides, lung edema occurs due to fluid accumulation, increased vascular permeability, and down-regulation of the sodium-potassium pump and aquaporin in AKI. The reduced aquaporin activity resulting from AKI [39] also predisposes ventilator-induced lung injury [40]. Lung edema, along with another complication of fluid overload, pleural effusion, exacerbates subsequent lung atelectasis, pneumonia, and empyema [38].
4.2. Hyperinflammatory State
AKI presents a hyperinflammatory state with reduced cytokine clearance and increased systemic cytokine levels, such as interleukin (IL)-17A, IL-6, IL-8, IL-1 beta, IL-12, and tumor necrosis factor (TNF)-alpha [41,42,43,44]. During AKI, the increased IL-17A attributes to Paneth cells in the small intestines and results in neutrophil influx, T cell activation, IL-6 overproduction, hepatic injury, and intestinal barrier disruption. IL-1 beta, IL-2, IL-6, IL-8, IL-1 beta, IL-12, TNF-alpha, and their receptors escalate early after AKI and lead to pro-inflammatory, neutrophil activation, endothelial cell apoptosis, and endothelial dysfunction in animal models [41,42,43,44]. Besides, some other plasma cytokines (e.g., keratinocyte-derived chemokine and Granulocyte-macrophage colony-stimulating factor) are generated during AKI. Nevertheless, although the plasma IL-6 is elevated, the monocyte cytokine production of IL-1beta, TNF-alpha, and IL-6 is impaired in critically ill AKI patients. This cytokine pattern in AKI patients is more similar to critically ill patients without AKI than those non-critically ill patients with chronic kidney disease (CKD) [45]. This presentation is also consistent with the finding that the AKI-associated hyperinflammatory state suppresses the immune system function and impairs the clearance of infection [46,47].
Elevated serum inflammatory biomarkers, such as IL-6, plasminogen activator inhibitor-1, and TNF-alpha, have essential roles in AKI and pneumonia. The circulating cytokine levels are higher in the patients with both AKI and pneumonia than in those has only pneumonia, suggesting the influence of AKI on cytokine is independent of infection. Furthermore, higher immune response in patients with AKI and even mild pneumonia links with increased mortality risk [48]. IL-6 induces increased pulmonary chemokine (C-X-C motif) ligand 1 (CXCL1) expression in AKI, promoting lung neutrophil infiltration. On the contrary, blockade of the CXCL1 signal reduces neutrophil infiltration in the lung, and IL-6 knockout mice are resistant to lung injury following ischemic AKI [49]. Increased neutrophil infiltration, vascular permeability, salt and water transporters dysregulation, and inflammatory cytokines are crucially associated with AKI-induced lung injury [50]. Besides neutrophils, T lymphocytes and macrophages also mediate lung injury during AKI. T lymphocyte administration induces pulmonary cellular apoptosis and lung microvascular barrier dysfunction, whereas macrophage mediates increased pulmonary vascular permeability via chemokine production in the experimental AKI model. Furthermore, AKI causes lung injury by inducing a pro-inflammatory and proapoptotic lung endothelial cell response with TNF receptor 1-dependent caspase activation, programmed cell death, and microvascular barrier dysfunction [51,52,53].
TLR-4 is essential in recognizing pathogens, such as lipopolysaccharide, heparan sulfate, heat shock proteins, and high-mobility group box protein B1 (HMGB1). TLR-4 actives humoral and cellular adaptive immune responses and is associated with neutrophil infiltration, increased neutrophil elastase activity, and vascular permeability in the lung [54]. HMGB1, a pro-inflammatory cytokine released from apoptotic cells, interacts with TLR-4 on target cells and activates nuclear factor kappa B and immunostimulatory responses [55]. In the ischemic AKI model, HMGB1 blockade reduced pulmonary neutrophil infiltration independent from TLR-4. These observations suggested that the TLR-4-HMGB1 pathway contributes to AKI-induced lung injury and has variable effects on different types of AKI [56].
4.3. Immunosuppression
Although AKI causes a pro-inflammatory response under general conditions, AKI may attenuate the neutrophil’s inflammatory effects under inflammatory states. Several reports found that neutrophil recruitment into inflamed organs decreased significantly during AKI, reducing the bacterial killing and promoting infection [57,58,59]. In animal models, mice with concomitant pneumonia and AKI had milder pulmonary inflammation than those with pneumonia only because AKI diminishes neutrophil recruitment into the lung during severe lung injury [57]. As in human investigation, the neutrophil function is more intensely suppressed in patients with AKI and sepsis than in patients with sepsis alone, suggesting that AKI interferes with neutrophil function [58].
A typical process of neutrophil recruitment includes capture, rolling, slow-rolling, firm adhesion, and transmigration. AKI impedes neutrophils’ slowing rolling and transmigration by abating E-selectin/intercellular adhesion molecule-1 and P-selectin/intercellular adhesion molecule-1. The selectin-mediated slow leukocyte rolling is inhibited by reduced phosphorylation of spleen tyrosine kinase, Akt, phospholipase C-γ2, and p38 mitogen-activated protein kinases [59,60]. The F-actin formation is a crucial process of neutrophil migration. AKI impedes neutrophil migration by impairing F-actin formation by interfering with the phosphatidylinositol 3-kinase-γ and phospholipase C-γ2-dependent pathways [57,58].
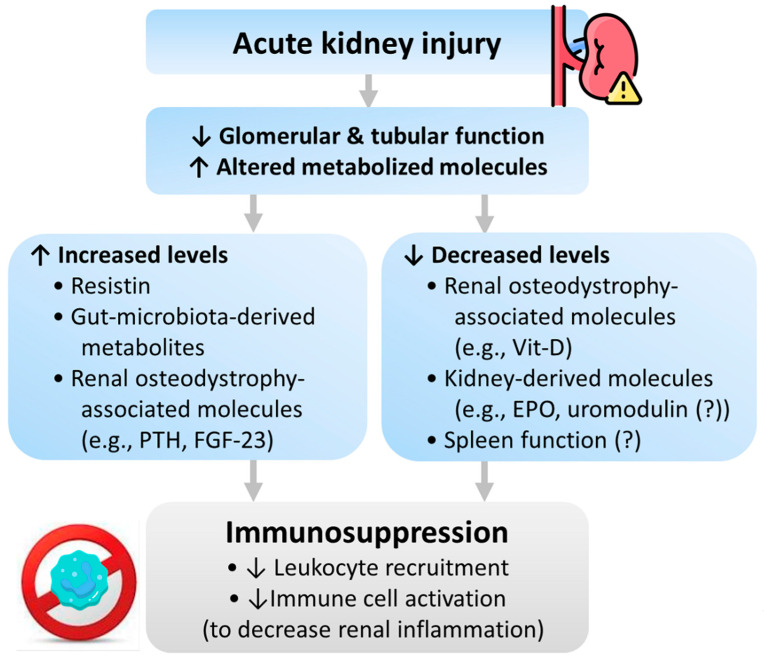
Besides, impaired monocyte function has also occurred in AKI. This kind of immunosuppression is more similar to patients with systemic inflammatory response syndrome rather than patients with CKD or end-stage kidney disease, suggesting that inflammation rather than kidney function is a significant determinant factor of immune dysregulation in AKI [45]. The AKI-associated immunosuppression might result from uremic toxins and abnormal metabolites molecules secondary to AKI. We summarize these pathophysiologies in Figure 3.
Figure 3.
Pathophysiology of immunosuppression following AKI. Abbreviations: EPO, erythropoietin; FGF-23, fibroblast growth factor-23; PTH, parathyroid hormone; Vit-D, vitamin D.
4.3.1. Resistin
Resistin is a 12-kDa uremic toxin and pro-inflammatory cytokine. Increased serum resistin concentrations positively correlate with sepsis severity [61]. The serum resistin level is less than 20 ng/mL in healthy individuals, which might increase to 30–40 ng/mL in end-stage kidney disease patients and further increase to higher than 100 ng/mL in patients with septic shock and AKI [62]. Under high concentrations (more than 20 ng/mL), resistin blocks phosphorylation of the phosphatidylinositol 3-kinase pathway, and subsequently inhibits neutrophil chemotaxis, neutrophil migration, and bacterial killing ability. Besides, resistin impairs phosphoinositide-dependent kinase 1, an essential protein for actin polymerization. Since actin polymerization is responsible for reactive oxygen species and neutrophil migration, resistin may contribute to the disturbed immune response by diminishing neutrophil migration [58,63]. The above mechanism is supported by a study showing that resistin causes neutrophil dysfunction, resembling AKI-associated neutrophil dysfunction [58].
4.3.2. Gut Microbiota-Derived Metabolites
AKI causes urea accumulation and increased urea influx into the gut, where urea is converted into ammonia that disrupts the epithelial tight junction of the gut and aggravates bacterial translocation [64]. During AKI, gut dysbiosis develops via both innate immunity (macrophages and neutrophils) and adaptive immunity (T-helper 17 cell) pathways [65]. Gut dysbiosis decreases short-chain fatty acids and increases gut-derived uremic toxins (e.g., indoxyl sulfate and p-cresyl sulfate), which alters immune homeostasis and further exacerbates AKI [65,66]. Besides, gut dysbiosis also contributes to intestinal inflammation and leaky gut [66]. The neutrophils, pro-inflammatory macrophages, and T-helper 17 cells accumulated in the gut impair barrier integrity and enhance bacterial translocation [67], and the increased plasma TNF-α, IL-17A, and IL-6 lead to endothelial apoptosis and epithelial necrosis in the small intestine [68,69]. All factors mentioned above contribute to immunosuppression and consequent infection.
4.3.3. Renal Osteodystrophy-Associated Molecules
Vitamin D, parathyroid hormone (PTH), and fibroblast growth factor-23 (FGF-23) are biomarkers of renal osteodystrophy. Their abnormal homeostases in AKI and CKD also influence immune function. The active vitamin D metabolite, 1,25-dihydroxy-vitamin D3 (calcitriol), is converted from vitamin D by the kidney; thus, vitamin D production is decreased in AKI. Vitamin D has been shown to promote macrophage phagocytosis, regulate monocyte immune function, and diminish inflammatory cytokine production. The regulation of monocyte function is mainly through the stimulation of the cathelicidin, an endogenous anti-microbial peptide produced by macrophages and neutrophils [70]. Besides, calcitriol markedly enhances tight junctions and decreases susceptibility to mucosal barrier damage and subsequent infection [71]. Clinically, a systemic review and meta-analysis revealed that vitamin D deficiency increases the risks of severe infections and mortality of critically ill patients [72].
Hyperparathyroidism, often occurring in AKI and CKD settings, is associated with abnormal immune function. High PTH levels inhibit T lymphocyte transformation and CD4/CD8 ratio and produce dose-dependent inhibition of B lymphocyte proliferation and immunoglobulin production. Besides, high PTH also influences polymorphonuclear leukocytes by inhibiting migration, phagocytosis, bactericidal activity, and chemotaxis [73,74].
FGF-23 is a bone-derived hormone whose serum levels increase in AKI and CKD [75]. FGF-23 impairs immune functions through direct interactions with myeloid cells, including macrophages and polymorphonuclear leukocytes. In murine kidney injury models, FGF-23 influences leukocyte recruitment and host defense, which are restored after FGF-23 neutralization [76]. Besides, FGF23 indirectly increases inflammation and infection by suppressing vitamin D production in the kidney’s proximal tubule [77]. As a result, a higher FGF-23 concentration is associated with increased infection risk in patients irrespective of kidney disease [78,79].
4.3.4. Kidney-Derived Molecules
Erythropoietin (EPO) and uromodulin are kidney-derived molecules that influence macrophage and sepsis progression. Experiments have shown that EPO level increases within the first 48 h of AKI and then drops progressively [80]. EPO benefits immunomodulation by normalizing activated CD4(+) T lymphocytes and their proliferative capacity, hastening efferocytosis, and suppressing inflammatory gene expression [18,80]. Therefore, decreased EPO levels in AKI might be associated with immunosuppression.
Uromodulin (also known as Tamm–Horsfall protein) is a high-molecular-weight polymer yielded by the kidney and excreted into the urine [81]. The polymeric uromodulin prevents bacteria from attachment to the urothelial surface. Meanwhile, uromodulin executes its immunomodulatory role by regulating macrophage number, phagocytic function, and neutrophil production [82]. Experimental studies found that uromodulin knockout mice had higher bladder and urinary tract infection risks [83,84]. Increased interstitial presence of uromodulin negatively regulates the inflammatory response in the murine’s proximal tubules and hastens kidney recovery, suggesting uromodulin as a prognostic biomarker of recovery from AKI [85]. Another experiment disclosed that circulating uromodulin dropped after AKI, which was associated with an increase in systemic reactive oxygen species. These findings suggested that uromodulin plays a crucial role in systemic oxidative stress and explained how uromodulin deficiency is associated with unfavorable outcomes [86]. As in humans, elevated serum or urinary uromodulin levels are beneficial against kidney function decline, cardiovascular events, and overall mortality [87,88,89].
4.3.5. Kidney and Spleen Interactions
The spleen is a component of the reticuloendothelial system accountable for host defense. Splenic IL-10 downregulates the pro-inflammatory response following ischemic AKI. Patients with asplenia have a higher risk for fulminant infection. In subjects with AKI, splenectomy exacerbates lung injury and pneumonia. In mice with sepsis and AKI, splenocyte apoptosis causes a vicious cycle of sepsis and spleen dysfunction [90,91], indicating the association between AKI, spleen dysfunction, and sepsis.
4.4. KRT and Catheter-Associated Infection
Critically ill patients with AKI treated with KRT are more susceptible to a nosocomial bloodstream infection, most commonly caused by Gram-positive species [92]. Similar findings were also observed in critically ill children treated with CKRT, demonstrating that more than four days of CKRT increased the risk of infection [93].
5. Potentially Preventive Strategies
The preventive strategies for SA-AKI are challenging and non-specific, mainly focusing on infection control and prevention of further kidney injury secondary to other problems. These strategies are summarized below. First, promptly prescribe proper antibiotics to treat infection and sepsis. Second, prescribe adequate hydration and vasopressor, with indication, to maintain a mean arterial blood pressure >65 mmHg and renal perfusion and autoregulation. Third, choose balanced crystalloids rather than 0.9% saline as intravenous fluid. The use of balanced crystalloids plays a protective role against major kidney adverse events, and the protective effect of balanced crystalloids is more significant in septic patients than in those without sepsis [94,95]. Fourth, avoid nephrotoxic agents in patients with high AKI risk. Some nephrotoxic agents, such as aminoglycosides and vancomycin, especially when in combination with piperacillin-tazobactam and amphotericin B, should be used with great caution. Besides, contrast-enhanced imaging studies should also be weighed carefully beforehand. Fifth, apply the KDIGO bundle to septic patients as possible. The KDIGO bundle is a package of preventive measures proposed by the KDIGO guideline. The bundle’s effect on reducing AKI occurrence and severity in the high-risk postoperative septic patient is under evaluation by a controlled, prospective, randomized clinical trial started in January 2020 (ClinicalTrials.gov, NCT04222361). Sixth, keep high awareness of “abdominal compartment syndrome” since high intra-abdominal pressure is a known deteriorating factor of SA-AKI, especially after trauma, surgery, or fluid resuscitation [96]. Although there is no consensus on whether early decompression or administration of diuretics helps prevent AKI, it is still worth attention to eliminate this potent trigger [97] (Table 1).
Table 1.
Potentially preventive strategies for SA-AKI.
| Strategies | Remarks |
|---|---|
| Prompt and proper antibiotics administration | Treatment of infection and sepsis |
| Vasopressor keeping a mean arterial blood pressure > 65 mmHg (norepinephrine preferred) | Maintain renal perfusion and autoregulation |
| Balanced crystalloid fluid administration | Avoidance of chloride overload. With benefit on major kidney adverse events |
| Avoidance of nephrotoxic agents | e.g., some antibiotics and contrast media |
| Application of the KDIGO bundle (?) | Effects under evaluation |
| High awareness of abdominal compartment syndrome | High intra- abdominal pressure is a deteriorating factor of SA-AKI |
Abbreviations: SA-AKI, sepsis-associated acute kidney injury; KDIGO, Kidney Disease Improving Global Outcomes.
On the other hand, several potentially preventive strategies for sepsis following AKI exist (Table 2). It is worth mentioning that the evidence of clinical benefits is weak or even lacking in most of the strategies. First, supplement probiotics and short-chain fatty acid [65], or administer AST-120 (an oral adsorbent) [98] to protect against kidney injury and to decrease gut microbiota-derived metabolites which alter immune homeostasis. Second, supplement vitamin D for an immunomodulation effect. A low serum calcitriol level is associated with a lower survival rate in human sepsis [99]. Besides, vitamin D pretreatment attenuates renal oxidative stress in lipopolysaccharide-induced AKI by regulating antioxidant enzyme genes and blocking nuclear factor kappa B-mediated cell apoptosis [100]. Third, avoid fluid overload during the oliguric period in AKI. The management includes decreasing fluid administration and increasing fluid removal by diuretics or KRT. Fourth, avoid unnecessary catheter cannulation to lower the bloodstream infection risk. Fifth, for severe AKI patients with KRT indications, consider using hemofiltration and hemoadsorption to remove some cytokine and molecules that are harmful to immunity. For example, continuous hemofiltration with a polyacrylonitrile dialysis membrane (AN69ST) showed a high adsorption capacity and significant clearance to adsorb HMGB1 [101], and a hemoadsorption corrects high serum resistin levels in patients with septic shock in vitro and restores anti-bacterial neutrophil function [102].
Table 2.
Potentially preventive strategies for sepsis following AKI.
| Strategies | Remarks |
|---|---|
| Probiotics and short chain fatty acid supplementation | Decrease gut-microbiota-derived metabolites and their immunosuppressive effects |
| AST-120 administration | |
| Vitamin D supplementation | Immunomodulation effect |
| Avoidance of fluid overload | Decrease tissue edema and infection risk |
| Avoidance of unnecessary catheter cannulation | Decrease bloodstream infection risk |
| KRT strategies | Hemofiltration and hemoadsorption for removing some cytokines and molecules that are harmful to immunity |
Abbreviations: KRT, kidney replacement therapy.
6. Limitations and Further Prospects
Several limitations exist in determining the bidirectional pathophysiological mechanisms between AKI and sepsis. First, the causality association between sepsis and AKI is hard to establish since sepsis and AKI are multifactorial clinical entities with complex pathophysiologies, and the precise occurrence time points of sepsis and AKI are often vague. Second, the pathologic information on SA-AKI is limited because kidney biopsy might not be suitable for SA-AKI patients in their critical situations. The existing pathophysiology is mainly from animal experiments. Nonetheless, the Kidney Precision Medicine Project, a multicenter prospective cohort study aiming to evaluate human kidney tissue, might provide valuable data to unveil the mystery [103]. Third, the current AKI diagnosis is based on function biomarkers (serum creatinine and urine amount) that delay the timing of AKI diagnosis and fail to diagnose the specific etiology of AKI differentially. Some parameters indicating sepsis (e.g., sepsis-3 or other scores) or kidney injury (e.g., biomarkers of kidney tubular damage, measurement of renal blood flow, or other scores) might potentially aid value in defining SA-AKI after the validation with patients’ prognoses.
7. Conclusions
The current review provided a comprehensive review of the pathophysiological interplay between sepsis and AKI. The pathophysiological of SA-AKI primarily includes inflammatory cascade, macrovascular and microvascular dysfunction, and cell cycle arrest and apoptosis. On the other hand, the pathophysiology of sepsis following AKI contains fluid overload, hyperinflammatory state, immunosuppression, and KRT and catheter-associated infection. The preventive strategies for SA-AKI are non-specific, mainly focusing on infection control and preventing further kidney insults. However, the preventive strategies for sepsis following AKI might focus on decreasing some metabolites, cytokines, or molecules harmful to our immunity, supplementing vitamin D3 for its immunomodulation effect, and avoiding fluid overload and unnecessary catheter cannulation. Several limitations make a clear understanding of the bidirectional pathophysiologies difficult. The prospects to unveil the mystery and improve the prognoses of AKI patients contain conducting studies, such as the Kidney Precision Medicine Project, to investigate human kidney tissue and establish parameters or scores better to determine the occurrence timing of sepsis and AKI and the definition of SA-AKI.
Acknowledgments
The authors thank Simone Yu for her professional English-edition.
Author Contributions
Conceptualization: Y.-M.C., Y.-T.C., W.-C.K. and C.-C.S.; writing—original draft preparation: Y.-M.C., Y.-T.C., W.-C.K. and C.-C.S.; writing—review and editing: W.-C.K. and C.-C.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This review work received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pannu N., James M., Hemmelgarn B., Klarenbach S. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin. J. Am. Soc. Nephrol. CJASN. 2013;8:194–202. doi: 10.2215/CJN.06480612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu V.C., Huang T.M., Lai C.F., Shiao C.C., Lin Y.F., Chu T.S., Wu P.C., Chao C.T., Wang J.Y., Kao T.W., et al. Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int. 2011;80:1222–1230. doi: 10.1038/ki.2011.259. [DOI] [PubMed] [Google Scholar]
- 3.Al-Jaghbeer M., Dealmeida D., Bilderback A., Ambrosino R., Kellum J.A. Clinical Decision Support for In-Hospital AKI. J. Am. Soc. Nephrol. 2018;29:654–660. doi: 10.1681/ASN.2017070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoste E.A., Bagshaw S.M., Bellomo R., Cely C.M., Colman R., Cruz D.N., Edipidis K., Forni L.G., Gomersall C.D., Govil D., et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 5.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pr. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 6.Yang S.Y., Chiou T.T., Shiao C.C., Lin H.Y., Chan M.J., Wu C.H., Sun C.Y., Wang W.J., Huang Y.T., Wu V.C., et al. Nomenclature and diagnostic criteria for acute kidney injury—2020 consensus of the Taiwan AKI-task force. J. Med. Assoc. 2022;121:749–765. doi: 10.1016/j.jfma.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Grams M.E., Rabb H. The distant organ effects of acute kidney injury. Kidney Int. 2012;81:942–948. doi: 10.1038/ki.2011.241. [DOI] [PubMed] [Google Scholar]
- 8.Druml W. Systemic consequences of acute kidney injury. Curr. Opin. Crit. Care. 2014;20:613–619. doi: 10.1097/MCC.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 9.Malhotra R., Siew E.D. Biomarkers for the Early Detection and Prognosis of Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. CJASN. 2017;12:149–173. doi: 10.2215/CJN.01300216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlton J.R., Portilla D., Okusa M.D. A basic science view of acute kidney injury biomarkers. Nephrol. Dial. Transplant. 2014;29:1301–1311. doi: 10.1093/ndt/gft510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peerapornratana S., Manrique-Caballero C.L., Gomez H., Kellum J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96:1083–1099. doi: 10.1016/j.kint.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai T.S., Wang C.Y., Pan S.C., Huang T.M., Lin M.C., Lai C.F., Wu C.H., Wu V.C., Chien K.L., National Taiwan University Hospital Study Group on Acute Renal Failure Risk of developing severe sepsis after acute kidney injury: A population-based cohort study. Crit. Care. 2013;17:R231. doi: 10.1186/cc13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manrique-Caballero C.L., Del Rio-Pertuz G., Gomez H. Sepsis-Associated Acute Kidney Injury. Crit. Care Clin. 2021;37:279–301. doi: 10.1016/j.ccc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu D., Su L., Han G., Yan P., Xie L. Prognostic Value of Procalcitonin in Adult Patients with Sepsis: A Systematic Review and Meta-Analysis. PLoS ONE. 2015;10:e0129450. doi: 10.1371/journal.pone.0129450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y.T., Lai M.Y., Kan W.C., Shiao C.C. Independent Predictive Ability of Procalcitonin of Acute Kidney Injury among Critically Ill Patients. J. Clin. Med. 2020;9:1939. doi: 10.3390/jcm9061939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent J.L., Sakr Y., Sprung C.L., Ranieri V.M., Reinhart K., Gerlach H., Moreno R., Carlet J., Le Gall J.R., Payen D., et al. Sepsis in European intensive care units: Results of the SOAP study. Crit. Care Med. 2006;34:344–353. doi: 10.1097/01.CCM.0000194725.48928.3A. [DOI] [PubMed] [Google Scholar]
- 17.Xu X., Nie S., Liu Z., Chen C., Xu G., Zha Y., Qian J., Liu B., Han S., Xu A., et al. Epidemiology and Clinical Correlates of AKI in Chinese Hospitalized Adults. Clin. J. Am. Soc. Nephrol. CJASN. 2015;10:1510–1518. doi: 10.2215/CJN.02140215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellum J.A., Chawla L.S., Keener C., Singbartl K., Palevsky P.M., Pike F.L., Yealy D.M., Huang D.T., Angus D.C., ProCESS et al. The Effects of Alternative Resuscitation Strategies on Acute Kidney Injury in Patients with Septic Shock. Am. J. Respir. Crit. Care Med. 2016;193:281–287. doi: 10.1164/rccm.201505-0995OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selby N.M., Kolhe N.V., McIntyre C.W., Monaghan J., Lawson N., Elliott D., Packington R., Fluck R.J. Defining the cause of death in hospitalised patients with acute kidney injury. PLoS ONE. 2012;7:e48580. doi: 10.1371/journal.pone.0048580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thakar C.V., Yared J.P., Worley S., Cotman K., Paganini E.P. Renal dysfunction and serious infections after open-heart surgery. Kidney Int. 2003;64:239–246. doi: 10.1046/j.1523-1755.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 21.Griffin B.R., Teixeira J.P., Ambruso S., Bronsert M., Pal J.D., Cleveland J.C., Reece T.B., Fullerton D.A., Faubel S., Aftab M. Stage 1 acute kidney injury is independently associated with infection following cardiac surgery. J. Thorac. Cardiovasc. Surg. 2021;161:1346–1355.e1343. doi: 10.1016/j.jtcvs.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffin B.R., You Z., Holmen J., SooHoo M., Gist K.M., Colbert J.F., Chonchol M., Faubel S., Jovanovich A. Incident infection following acute kidney injury with recovery to baseline creatinine: A propensity score matched analysis. PLoS ONE. 2019;14:e0217935. doi: 10.1371/journal.pone.0217935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu V.C., Wang C.Y., Shiao C.C., Chang C.H., Huang H.Y., Huang T.M., Lai C.F., Lin M.C., Ko W.J., Wu K.D., et al. Increased risk of active tuberculosis following acute kidney injury: A nationwide, population-based study. PLoS ONE. 2013;8:e69556. doi: 10.1371/journal.pone.0069556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynvoet E., Vandijck D.M., Blot S.I., Dhondt A.W., De Waele J.J., Claus S., Buyle F.M., Vanholder R.C., Hoste E.A. Epidemiology of infection in critically ill patients with acute renal failure. Crit. Care Med. 2009;37:2203–2209. doi: 10.1097/CCM.0b013e3181a03961. [DOI] [PubMed] [Google Scholar]
- 25.Maiden M.J., Otto S., Brealey J.K., Finnis M.E., Chapman M.J., Kuchel T.R., Nash C.H., Edwards J., Bellomo R. Structure and Function of the Kidney in Septic Shock. A Prospective Controlled Experimental Study. Am. J. Respir. Crit. Care Med. 2016;194:692–700. doi: 10.1164/rccm.201511-2285OC. [DOI] [PubMed] [Google Scholar]
- 26.Lankadeva Y.R., Kosaka J., Iguchi N., Evans R.G., Booth L.C., Bellomo R., May C.N. Effects of Fluid Bolus Therapy on Renal Perfusion, Oxygenation, and Function in Early Experimental Septic Kidney Injury. Crit. Care Med. 2019;47:e36–e43. doi: 10.1097/CCM.0000000000003507. [DOI] [PubMed] [Google Scholar]
- 27.Kosaka J., Lankadeva Y.R., May C.N., Bellomo R. Histopathology of Septic Acute Kidney Injury: A Systematic Review of Experimental Data. Crit. Care Med. 2016;44:e897–e903. doi: 10.1097/CCM.0000000000001735. [DOI] [PubMed] [Google Scholar]
- 28.Kalakeche R., Hato T., Rhodes G., Dunn K.W., El-Achkar T.M., Plotkin Z., Sandoval R.M., Dagher P.C. Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. J. Am. Soc. Nephrol. 2011;22:1505–1516. doi: 10.1681/ASN.2011020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dellepiane S., Marengo M., Cantaluppi V. Detrimental cross-talk between sepsis and acute kidney injury: New pathogenic mechanisms, early biomarkers and targeted therapies. Crit. Care. 2016;20:61. doi: 10.1186/s13054-016-1219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calzavacca P., Evans R.G., Bailey M., Bellomo R., May C.N. Variable responses of regional renal oxygenation and perfusion to vasoactive agents in awake sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;309:R1226–R1233. doi: 10.1152/ajpregu.00228.2015. [DOI] [PubMed] [Google Scholar]
- 31.Ronco C., Bellomo R., Kellum J.A. Acute kidney injury. Lancet. 2019;394:1949–1964. doi: 10.1016/S0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- 32.Post E.H., Kellum J.A., Bellomo R., Vincent J.L. Renal perfusion in sepsis: From macro- to microcirculation. Kidney Int. 2017;91:45–60. doi: 10.1016/j.kint.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 33.Chelazzi C., Villa G., Mancinelli P., De Gaudio A.R., Adembri C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit. Care. 2015;19:26. doi: 10.1186/s13054-015-0741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guerci P., Ergin B., Ince C. The macro- and microcirculation of the kidney. Best Pr. Res. Clin. Anaesthesiol. 2017;31:315–329. doi: 10.1016/j.bpa.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Yang L., Xie M., Yang M., Yu Y., Zhu S., Hou W., Kang R., Lotze M.T., Billiar T.R., Wang H., et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat. Commun. 2014;5:4436. doi: 10.1038/ncomms5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escobar D.A., Botero-Quintero A.M., Kautza B.C., Luciano J., Loughran P., Darwiche S., Rosengart M.R., Zuckerbraun B.S., Gomez H. Adenosine monophosphate-activated protein kinase activation protects against sepsis-induced organ injury and inflammation. J. Surg. Res. 2015;194:262–272. doi: 10.1016/j.jss.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Opal S.M., Ellis J.L., Suri V., Freudenberg J.M., Vlasuk G.P., Li Y., Chahin A.B., Palardy J.E., Parejo N., Yamamoto M., et al. Pharmacological Sirt1 Activation Improves Mortality and Markedly Alters Transcriptional Profiles That Accompany Experimental Sepsis. Shock. 2016;45:411–418. doi: 10.1097/SHK.0000000000000528. [DOI] [PubMed] [Google Scholar]
- 38.Brandstrup B., Tonnesen H., Beier-Holgersen R., Hjortso E., Ording H., Lindorff-Larsen K., Rasmussen M.S., Lanng C., Wallin L., Iversen L.H., et al. Effects of intravenous fluid restriction on postoperative complications: Comparison of two perioperative fluid regimens: A randomized assessor-blinded multicenter trial. Ann. Surg. 2003;238:641–648. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He J., Yang B. Aquaporins in Renal Diseases. Int. J. Mol. Sci. 2019;20:366. doi: 10.3390/ijms20020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hales C.A., Du H.K., Volokhov A., Mourfarrej R., Quinn D.A. Aquaporin channels may modulate ventilator-induced lung injury. Respir. Physiol. 2001;124:159–166. doi: 10.1016/S0034-5687(00)00193-6. [DOI] [PubMed] [Google Scholar]
- 41.Andres-Hernando A., Dursun B., Altmann C., Ahuja N., He Z., Bhargava R., Edelstein C.E., Jani A., Hoke T.S., Klein C., et al. Cytokine production increases and cytokine clearance decreases in mice with bilateral nephrectomy. Nephrol. Dial. Transplant. 2012;27:4339–4347. doi: 10.1093/ndt/gfs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faubel S., Edelstein C.L. Mechanisms and mediators of lung injury after acute kidney injury. Nat. Rev. Nephrol. 2016;12:48–60. doi: 10.1038/nrneph.2015.158. [DOI] [PubMed] [Google Scholar]
- 43.Hoke T.S., Douglas I.S., Klein C.L., He Z., Fang W., Thurman J.M., Tao Y., Dursun B., Voelkel N.F., Edelstein C.L., et al. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J. Am. Soc. Nephrol. 2007;18:155–164. doi: 10.1681/ASN.2006050494. [DOI] [PubMed] [Google Scholar]
- 44.Bijuklic K., Jennings P., Kountchev J., Hasslacher J., Aydin S., Sturn D., Pfaller W., Patsch J.R., Joannidis M. Migration of leukocytes across an endothelium-epithelium bilayer as a model of renal interstitial inflammation. Am. J. Physiol. Cell Physiol. 2007;293:C486–C492. doi: 10.1152/ajpcell.00419.2006. [DOI] [PubMed] [Google Scholar]
- 45.Himmelfarb J., Le P., Klenzak J., Freedman S., McMenamin M.E., Ikizler T.A., Group P. Impaired monocyte cytokine production in critically ill patients with acute renal failure. Kidney Int. 2004;66:2354–2360. doi: 10.1111/j.1523-1755.2004.66023.x. [DOI] [PubMed] [Google Scholar]
- 46.Murugan R., Wen X., Keener C., Pike F., Palevsky P.M., Unruh M., Finkel K., Vijayan A., Elder M., Chen Y.F., et al. Associations between Intensity of RRT, Inflammatory Mediators, and Outcomes. Clin. J. Am. Soc. Nephrol. CJASN. 2015;10:926–933. doi: 10.2215/CJN.04560514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horiguchi H., Loftus T.J., Hawkins R.B., Raymond S.L., Stortz J.A., Hollen M.K., Weiss B.P., Miller E.S., Bihorac A., Larson S.D., et al. Innate Immunity in the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome and Its Implications for Therapy. Front. Immunol. 2018;9:595. doi: 10.3389/fimmu.2018.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murugan R., Karajala-Subramanyam V., Lee M., Yende S., Kong L., Carter M., Angus D.C., Kellum J.A., Genetic and Inflammatory Markers of Sepsis (GenIMS) Investigators Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77:527–535. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahuja N., Andres-Hernando A., Altmann C., Bhargava R., Bacalja J., Webb R.G., He Z., Edelstein C.L., Faubel S. Circulating IL-6 mediates lung injury via CXCL1 production after acute kidney injury in mice. Am. J. Physiol. Ren. Physiol. 2012;303:F864–F872. doi: 10.1152/ajprenal.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Awad A.S., Rouse M., Huang L., Vergis A.L., Reutershan J., Cathro H.P., Linden J., Okusa M.D. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int. 2009;75:689–698. doi: 10.1038/ki.2008.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lie M.L., White L.E., Santora R.J., Park J.M., Rabb H., Hassoun H.T. Lung T lymphocyte trafficking and activation during ischemic acute kidney injury. J. Immunol. 2012;189:2843–2851. doi: 10.4049/jimmunol.1103254. [DOI] [PubMed] [Google Scholar]
- 52.Altmann C., Andres-Hernando A., McMahan R.H., Ahuja N., He Z., Rivard C.J., Edelstein C.L., Barthel L., Janssen W.J., Faubel S. Macrophages mediate lung inflammation in a mouse model of ischemic acute kidney injury. Am. J. Physiol. Ren. Physiol. 2012;302:F421–F432. doi: 10.1152/ajprenal.00559.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White L.E., Cui Y., Shelak C.M., Lie M.L., Hassoun H.T. Lung endothelial cell apoptosis during ischemic acute kidney injury. Shock. 2012;38:320–327. doi: 10.1097/SHK.0b013e31826359d0. [DOI] [PubMed] [Google Scholar]
- 54.Kumar H., Kawai T., Akira S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 55.Wang H., Bloom O., Zhang M., Vishnubhakat J.M., Ombrellino M., Che J., Frazier A., Yang H., Ivanova S., Borovikova L., et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 56.Park J.S., Svetkauskaite D., He Q., Kim J.Y., Strassheim D., Ishizaka A., Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 57.Singbartl K., Bishop J.V., Wen X., Murugan R., Chandra S., Filippi M.D., Kellum J.A. Differential effects of kidney-lung cross-talk during acute kidney injury and bacterial pneumonia. Kidney Int. 2011;80:633–644. doi: 10.1038/ki.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singbartl K., Miller L., Ruiz-Velasco V., Kellum J.A. Reversal of Acute Kidney Injury-Induced Neutrophil Dysfunction: A Critical Role for Resistin. Crit. Care Med. 2016;44:e492–e501. doi: 10.1097/CCM.0000000000001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rossaint J., Spelten O., Kassens N., Mueller H., Van Aken H.K., Singbartl K., Zarbock A. Acute loss of renal function attenuates slow leukocyte rolling and transmigration by interfering with intracellular signaling. Kidney Int. 2011;80:493–503. doi: 10.1038/ki.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nourshargh S., Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41:694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 61.Macdonald S.P., Stone S.F., Neil C.L., van Eeden P.E., Fatovich D.M., Arendts G., Brown S.G. Sustained elevation of resistin, NGAL and IL-8 are associated with severe sepsis/septic shock in the emergency department. PLoS ONE. 2014;9:e110678. doi: 10.1371/journal.pone.0110678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koch A., Gressner O.A., Sanson E., Tacke F., Trautwein C. Serum resistin levels in critically ill patients are associated with inflammation, organ dysfunction and metabolism and may predict survival of non-septic patients. Crit. Care. 2009;13:R95. doi: 10.1186/cc7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cohen G., Ilic D., Raupachova J., Horl W.H. Resistin inhibits essential functions of polymorphonuclear leukocytes. J. Immunol. 2008;181:3761–3768. doi: 10.4049/jimmunol.181.6.3761. [DOI] [PubMed] [Google Scholar]
- 64.Zhang J., Ankawi G., Sun J., Digvijay K., Yin Y., Rosner M.H., Ronco C. Gut-kidney crosstalk in septic acute kidney injury. Crit. Care. 2018;22:117. doi: 10.1186/s13054-018-2040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chou Y.T., Kan W.C., Shiao C.C. Acute Kidney Injury and Gut Dysbiosis: A Narrative Review Focus on Pathophysiology and Treatment. Int. J. Mol. Sci. 2022;23:3658. doi: 10.3390/ijms23073658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang J., Kim C.J., Go Y.S., Lee H.Y., Kim M.G., Oh S.W., Cho W.Y., Im S.H., Jo S.K. Intestinal microbiota control acute kidney injury severity by immune modulation. Kidney Int. 2020;98:932–946. doi: 10.1016/j.kint.2020.04.048. [DOI] [PubMed] [Google Scholar]
- 67.Jo S.K. Kidney-Gut Crosstalk in AKI. Kidney360. 2021;2:886–889. doi: 10.34067/KID.0007722020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park S.W., Chen S.W., Kim M., Brown K.M., Kolls J.K., D’Agati V.D., Lee H.T. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab. Invest. 2011;91:63–84. doi: 10.1038/labinvest.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park S.W., Kim M., Kim J.Y., Ham A., Brown K.M., Mori-Akiyama Y., Ouellette A.J., D’Agati V.D., Lee H.T. Paneth cell-mediated multiorgan dysfunction after acute kidney injury. J. Immunol. 2012;189:5421–5433. doi: 10.4049/jimmunol.1200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jeng L., Yamshchikov A.V., Judd S.E., Blumberg H.M., Martin G.S., Ziegler T.R., Tangpricha V. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J. Transl. Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kong J., Zhang Z., Musch M.W., Ning G., Sun J., Hart J., Bissonnette M., Li Y.C. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G208–G216. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 72.de Haan K., Groeneveld A.B., de Geus H.R., Egal M., Struijs A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: Systematic review and meta-analysis. Crit. Care. 2014;18:660. doi: 10.1186/s13054-014-0660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castellanos M., Jung E., Park S.Y., Schuller-Levis G., Odaimi M., Elsayegh S., Kleiner M., Elsoueidi R., Shtaynberg N., Park E. Effect of parathyroid hormone and teriparatide on immune function of human adherent and non-adherent leukocytes. Clin. Nephrol. 2010;74:83–90. doi: 10.5414/CNP74083. [DOI] [PubMed] [Google Scholar]
- 74.Alexiewicz J.M., Smogorzewski M., Fadda G.Z., Massry S.G. Impaired phagocytosis in dialysis patients: Studies on mechanisms. Am. J. Nephrol. 1991;11:102–111. doi: 10.1159/000168284. [DOI] [PubMed] [Google Scholar]
- 75.Christov M. Fibroblast growth factor 23 in acute kidney injury. Curr. Opin. Nephrol. Hypertens. 2014;23:340–345. doi: 10.1097/01.mnh.0000447021.51722.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rossaint J., Oehmichen J., Van Aken H., Reuter S., Pavenstadt H.J., Meersch M., Unruh M., Zarbock A. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J. Clin. Invest. 2016;126:962–974. doi: 10.1172/JCI83470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fitzpatrick E.A., Han X., Xiao Z., Quarles L.D. Role of Fibroblast Growth Factor-23 in Innate Immune Responses. Front. Endocrinol. 2018;9:320. doi: 10.3389/fendo.2018.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chonchol M., Greene T., Zhang Y., Hoofnagle A.N., Cheung A.K. Low Vitamin D and High Fibroblast Growth Factor 23 Serum Levels Associate with Infectious and Cardiac Deaths in the HEMO Study. J. Am. Soc. Nephrol. 2016;27:227–237. doi: 10.1681/ASN.2014101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nowak K.L., Bartz T.M., Dalrymple L., de Boer I.H., Kestenbaum B., Shlipak M.G., Garimella P.S., Ix J.H., Chonchol M. Fibroblast Growth Factor 23 and the Risk of Infection-Related Hospitalization in Older Adults. J. Am. Soc. Nephrol. 2017;28:1239–1246. doi: 10.1681/ASN.2016040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamashita T., Noiri E., Hamasaki Y., Matsubara T., Ishii T., Yahagi N., Nangaku M., Doi K. Erythropoietin concentration in acute kidney injury is associated with insulin-like growth factor-binding protein-1. Nephrology. 2016;21:693–699. doi: 10.1111/nep.12656. [DOI] [PubMed] [Google Scholar]
- 81.Micanovic R., LaFavers K., Garimella P.S., Wu X.R., El-Achkar T.M. Uromodulin (Tamm-Horsfall protein): Guardian of urinary and systemic homeostasis. Nephrol. Dial. Transplant. 2020;35:33–43. doi: 10.1093/ndt/gfy394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Micanovic R., Khan S., Janosevic D., Lee M.E., Hato T., Srour E.F., Winfree S., Ghosh J., Tong Y., Rice S.E., et al. Tamm-Horsfall Protein Regulates Mononuclear Phagocytes in the Kidney. J. Am. Soc. Nephrol. 2018;29:841–856. doi: 10.1681/ASN.2017040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bates J.M., Raffi H.M., Prasadan K., Mascarenhas R., Laszik Z., Maeda N., Hultgren S.J., Kumar S. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: Rapid communication. Kidney Int. 2004;65:791–797. doi: 10.1111/j.1523-1755.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 84.Raffi H.S., Bates J.M., Jr., Laszik Z., Kumar S. Tamm-horsfall protein protects against urinary tract infection by proteus mirabilis. J. Urol. 2009;181:2332–2338. doi: 10.1016/j.juro.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.El-Achkar T.M., McCracken R., Liu Y., Heitmeier M.R., Bourgeois S., Ryerse J., Wu X.R. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am. J. Physiol. Ren. Physiol. 2013;304:F1066–F1075. doi: 10.1152/ajprenal.00543.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.LaFavers K.A., Macedo E., Garimella P.S., Lima C., Khan S., Myslinski J., McClintick J., Witzmann F.A., Winfree S., Phillips C.L., et al. Circulating uromodulin inhibits systemic oxidative stress by inactivating the TRPM2 channel. Sci. Transl. Med. 2019;11:eaaw3639. doi: 10.1126/scitranslmed.aaw3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Delgado G.E., Kleber M.E., Scharnagl H., Kramer B.K., Marz W., Scherberich J.E. Serum Uromodulin and Mortality Risk in Patients Undergoing Coronary Angiography. J. Am. Soc. Nephrol. 2017;28:2201–2210. doi: 10.1681/ASN.2016111162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garimella P.S., Katz R., Ix J.H., Fried L.F., Kritchevsky S.B., Devarajan P., Bennett M.R., Parikh C.R., Shlipak M.G., Harris T.B., et al. Association of urinary uromodulin with kidney function decline and mortality: The health ABC study. Clin. Nephrol. 2017;87:278–286. doi: 10.5414/CN109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leiherer A., Muendlein A., Saely C.H., Ebner J., Brandtner E.M., Fraunberger P., Drexel H. Serum uromodulin is a predictive biomarker for cardiovascular events and overall mortality in coronary patients. Int. J. Cardiol. 2017;231:6–12. doi: 10.1016/j.ijcard.2016.12.183. [DOI] [PubMed] [Google Scholar]
- 90.Andres-Hernando A., Altmann C., Ahuja N., Lanaspa M.A., Nemenoff R., He Z., Ishimoto T., Simpson P.A., Weiser-Evans M.C., Bacalja J., et al. Splenectomy exacerbates lung injury after ischemic acute kidney injury in mice. Am. J. Physiol. Ren. Physiol. 2011;301:F907–F916. doi: 10.1152/ajprenal.00107.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Doi K., Hu X., Yuen P.S., Leelahavanichkul A., Yasuda H., Kim S.M., Schnermann J., Jonassen T.E., Frokiaer J., Nielsen S., et al. AP214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int. 2008;73:1266–1274. doi: 10.1038/ki.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoste E.A., Blot S.I., Lameire N.H., Vanholder R.C., De Bacquer D., Colardyn F.A. Effect of nosocomial bloodstream infection on the outcome of critically ill patients with acute renal failure treated with renal replacement therapy. J. Am. Soc. Nephrol. 2004;15:454–462. doi: 10.1097/01.ASN.0000110182.14608.0C. [DOI] [PubMed] [Google Scholar]
- 93.Santiago M.J., Lopez-Herce J., Vierge E., Castillo A., Bustinza A., Bellon J.M., Sanchez A., Fernandez S. Infection in critically ill pediatric patients on continuous renal replacement therapy. Int. J. Artif. Organs. 2017;40:224–229. doi: 10.5301/ijao.5000587. [DOI] [PubMed] [Google Scholar]
- 94.Self W.H., Semler M.W., Wanderer J.P., Wang L., Byrne D.W., Collins S.P., Slovis C.M., Lindsell C.J., Ehrenfeld J.M., Siew E.D., et al. Balanced Crystalloids versus Saline in Noncritically Ill Adults. N. Engl. J. Med. 2018;378:819–828. doi: 10.1056/NEJMoa1711586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Semler M.W., Self W.H., Wanderer J.P., Ehrenfeld J.M., Wang L., Byrne D.W., Stollings J.L., Kumar A.B., Hughes C.G., Hernandez A., et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N. Engl. J. Med. 2018;378:829–839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Honore P.M., Jacobs R., Hendrickx I., Bagshaw S.M., Joannes-Boyau O., Boer W., De Waele E., Van Gorp V., Spapen H.D. Prevention and treatment of sepsis-induced acute kidney injury: An update. Ann. Intensive Care. 2015;5:51. doi: 10.1186/s13613-015-0095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kirkpatrick A.W., Roberts D.J., De Waele J., Jaeschke R., Malbrain M.L., De Keulenaer B., Duchesne J., Bjorck M., Leppaniemi A., Ejike J.C., et al. Intra-abdominal hypertension and the abdominal compartment syndrome: Updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fujii H., Yonekura Y., Yamashita Y., Kono K., Nakai K., Goto S., Sugano M., Goto S., Fujieda A., Ito Y., et al. Anti-oxidative effect of AST-120 on kidney injury after myocardial infarction. Br. J. Pharm. 2016;173:1302–1313. doi: 10.1111/bph.13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nguyen H.B., Eshete B., Lau K.H., Sai A., Villarin M., Baylink D. Serum 1,25-dihydroxyvitamin, D. An outcome prognosticator in human sepsis. PLoS ONE. 2013;8:e64348. doi: 10.1371/journal.pone.0064348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Graidis S., Papavramidis T.S., Papaioannou M. Vitamin D and Acute Kidney Injury: A Two-Way Causality Relation and a Predictive, Prognostic, and Therapeutic Role of Vitamin D. Front. Nutr. 2020;7:630951. doi: 10.3389/fnut.2020.630951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doi K., Ishizu T., Tsukamoto-Sumida M., Hiruma T., Yamashita T., Ogasawara E., Hamasaki Y., Yahagi N., Nangaku M., Noiri E. The high-mobility group protein B1-Toll-like receptor 4 pathway contributes to the acute lung injury induced by bilateral nephrectomy. Kidney Int. 2014;86:316–326. doi: 10.1038/ki.2014.62. [DOI] [PubMed] [Google Scholar]
- 102.Bonavia A., Miller L., Kellum J.A., Singbartl K. Hemoadsorption corrects hyperresistinemia and restores anti-bacterial neutrophil function. Intensive Care Med. Exp. 2017;5:36. doi: 10.1186/s40635-017-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Boer I.H., Alpers C.E., Azeloglu E.U., Balis U.G.J., Barasch J.M., Barisoni L., Blank K.N., Bomback A.S., Brown K., Dagher P.C., et al. Rationale and design of the Kidney Precision Medicine Project. Kidney Int. 2021;99:498–510. doi: 10.1016/j.kint.2020.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.