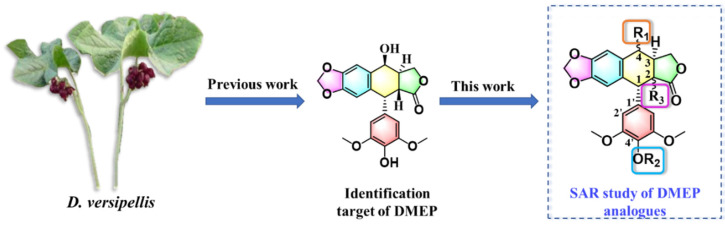
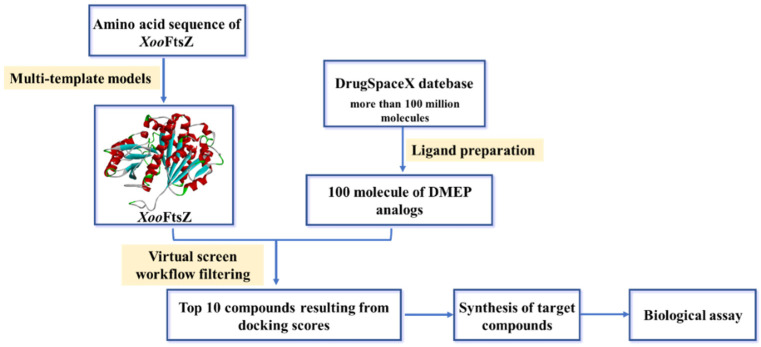
Abstract
The emergence of phytopathogenic bacteria resistant to antibacterial agents has rendered previously manageable plant diseases intractable, highlighting the need for safe and environmentally responsible agrochemicals. Inhibition of bacterial cell division by targeting bacterial cell division protein FtsZ has been proposed as a promising strategy for developing novel antibacterial agents. We previously identified 4′-demethylepipodophyllotoxin (DMEP), a naturally occurring substance isolated from the barberry species Dysosma versipellis, as a novel chemical scaffold for the development of inhibitors of FtsZ from the rice blight pathogen Xanthomonas oryzae pv. oryzae (Xoo). Therefore, constructing structure−activity relationship (SAR) studies of DMEP is indispensable for new agrochemical discovery. In this study, we performed a structure−activity relationship (SAR) study of DMEP derivatives as potential XooFtsZ inhibitors through introducing the structure-based virtual screening (SBVS) approach and various biochemical methods. Notably, prepared compound B2, a 4′-acyloxy DMEP analog, had a 50% inhibitory concentration of 159.4 µM for inhibition of recombinant XooFtsZ GTPase, which was lower than that of the parent DMEP (278.0 µM). Compound B2 potently inhibited Xoo growth in vitro (minimum inhibitory concentration 153 mg L−1) and had 54.9% and 48.4% curative and protective control efficiencies against rice blight in vivo. Moreover, compound B2 also showed low toxicity for non-target organisms, including rice plant and mammalian cell. Given these interesting results, we provide a novel strategy to discover and optimize promising bactericidal compounds for the management of plant bacterial diseases.
Keywords: natural products (NPs), structure-based virtual screening (SBVS), Xanthomonas oryzae pv. oryzae (Xoo), FtsZ inhibitors, plant bacterial diseases
1. Introduction
Plant diseases caused by phytopathogenic bacterium represent crucial threats to plant health and the productivity of agriculture crops [1,2,3]. The human population is predicted to increase to 10 billion by the year 2100, which will require a doubling or tripling of current agricultural productivity to ensure that adequate food supplies are maintained [4,5,6]. Modern agriculture has benefited substantially from the use of agrochemicals, but many, especially traditional agrochemicals, are hazardous to both the environment and human health [7,8]. For instance, bismerthiazol (BT), a commercial bactericide active against Xanthomonas oryzae pv. oryzae (Xoo), exhibits subchronic and chronic toxicity in humans upon oral consumption [9]. These drawbacks have highlighted the urgent need for safer and more environmentally responsible pesticides.
Natural product-based pesticides have many potential advantages over synthetic compounds, including lower toxicity and easier and more environmentally friendly degradation. Consequently, there has been exponential growth in the development of new agrochemicals originating from natural products and their derivatives, which are seen as an effective panacea for integrated pest management [10,11,12,13]. For instance, some natural β-methoxyacrylic acid fungicides and their synthetic strobilurin derivatives are extensively used to control fungal pathogens [14]. Similarly, natural pyrethrum and synthetic pyrethroids are used commercially to control insects [15]. These examples illustrate the potential for natural products and their derivatives to be developed as new pesticides.
4′-Demethylepipodophyllotoxin (DMEP) is an aryltetralin cyclolignan isolated from the barberry species Dysosma versipellis and represents a structural framework for many compounds shown to display various bioactivities [16,17,18]. For example, structural modification of DMEP has yielded many anticancer agents, including etoposide and teniposide [16,19], and various DMEP analogs and derivatives with insecticidal activity have been developed and shown to successfully control insect pests in recent years [20,21,22]. We recently employed the framework of DMEP to develop inhibitors of bacterial FtsZ, a tubulin homolog that possesses GTPase activity, that have bactericidal activity and control bacterial leaf blight of rice [23]. The results of that study suggested a new drug discovery strategy and application for DMEP to develop potent FtsZ-targeting compounds for controlling intractable bacterial diseases of plants.
In the present study, we utilized a structure-based virtual screening strategy to guide the design of candidate DMEP-derived compounds with bactericidal properties [24]. Structure-based virtual screening is an increasingly common and prominent strategy in drug discovery and facilitates the design of synthesizable and novel chemical structures with certain molecular targets and bioactivities. For example, candidate agents targeting the kinase discoidin domain receptor 1, which has been implicated in many human diseases, were identified using the DrugSpaceX platform (https://drugspacex.simm.ac.cn/, accessed on 1 October 2021), which catalogs features such as drug-likeness, synthesizability, diversity, and novelty of compounds within a three-dimensional chemical space [25]. In the present study, we designed and synthesized a panel of DMEP derivatives and evaluated their ability to inhibit recombinant XooFtsZ GTPase activity and XooFtsZ assembly, to induce morphological changes and inhibit Xoo growth in vitro, and to prevent or ameliorate rice bacterial leaf blight in vivo. We also summarize and highlight key aspects of the structure–activity relationship (SAR) of the DMEP scaffold. A summary of the approach is presented in Figure 1, and the corresponding workflow of virtual screening and bioassay is outlined in Figure 2.
Figure 1.
The research contents for target molecules in the current work.
Figure 2.
Summary of the workflow of virtual screening of XooFtsZ in the current work.
2. Results and Discussion
2.1. Design and Synthesis of Target Compounds
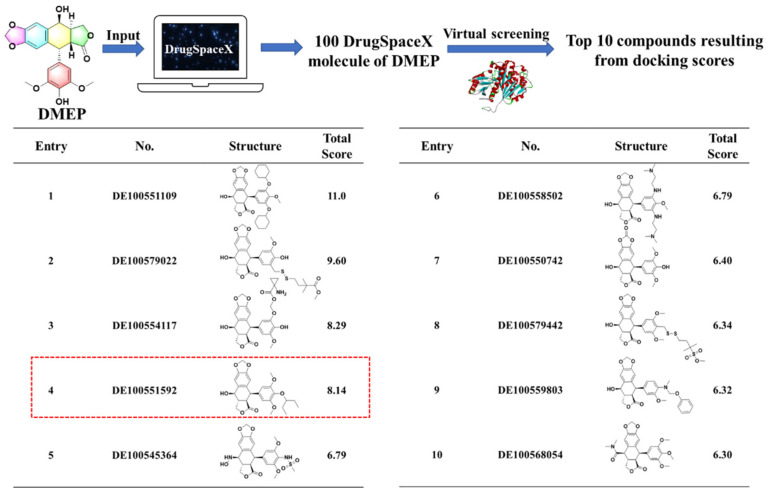
Encouraged by our previous work [23], DMEP was currently certified as a promising scaffold of XooFtsZ inhibitors, but discovering how to guide and prepare higher active compounds derived from DMEP quickly and with high efficiency is a crucial purpose of our current work. To maximize the identification of derivatives that would be effective, safe to non-target organisms, and easily synthesizable, we employed a ligand-based approach followed by reranking of molecular docking scores using structure-based virtual screening. Briefly, the structure of DMEP was submitted to DrugSpaceX and 100 drug-like DMEP analogs were downloaded and docked with reconstructed XooFtsZ using Sybyl-X 2.0 software. The top 10 analogs of DMEP were selected by ranking the docking scores, which were obtained for each analog in various positions, thereby providing an indication of the accuracy and stability of the docking simulations. Thus, the higher the score, the more stable was the predicted interaction. Notably, many of the selected compounds had similar characteristics, such as substitutions of the para and meta positions of the phenyl ring that could potentially increase the protein−compound interaction (Figure 3). Overall, these results predicted that 4′-substituted DMEP analogs would be easily synthesizable and may have better bactericidal properties than DMEP.
Figure 3.
Top 10 compounds resulting from docking scores.
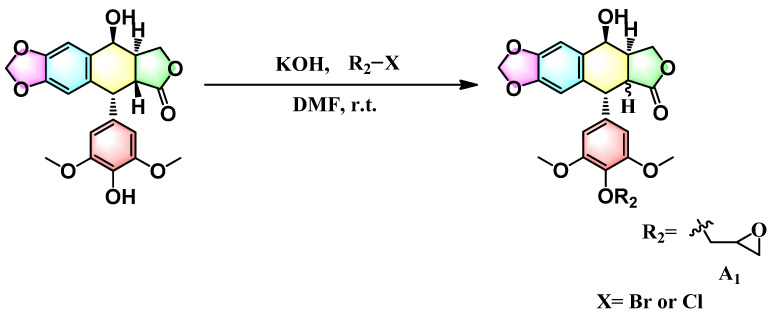
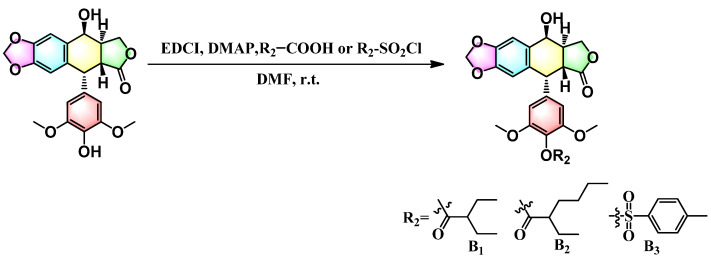
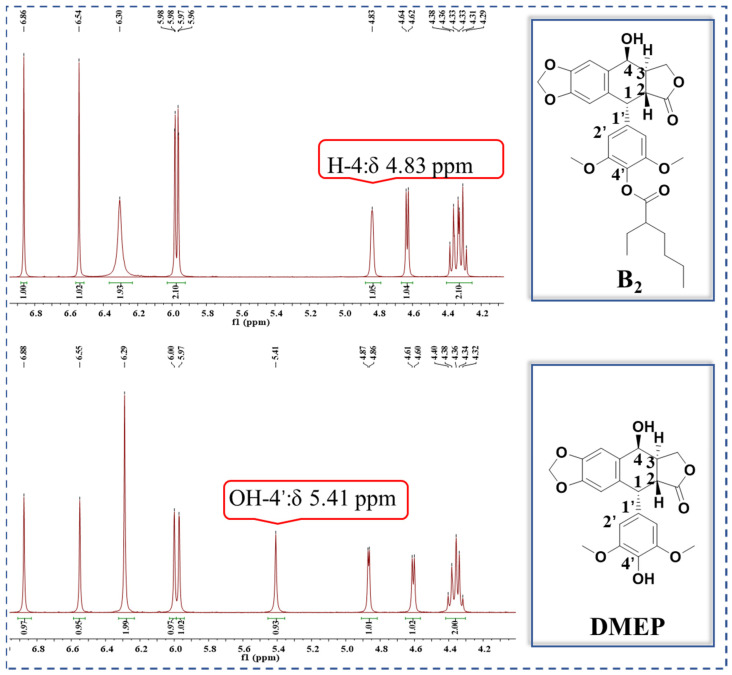
To evaluate the effects of the 4′-substituted DMEP analogues, a series of title compounds were synthesized, and their synthetic routes were displayed in Scheme 1 and Scheme 2. As shown in Figure 4, the substituent position of monoester derivatives was confirmed based on the chemical shifts of H-4 and OH-4′. Notably, for DMEP, the chemical shift of OH-4′ was identified at 5.41 ppm, and the chemical shift of H-4 was confirmed at 4.86–4.87 ppm. By contrast, the OH-4′ group of compounds B1, B2 and B3 were substituted by the acyloxy group or sulfuryl group, and the corresponding chemical shifts disappeared in the spectrum, respectively. Moreover, the chemical shift of H-4 of compounds B1, B2, and B3 remained at 4.83 ppm. Thus, this obviously demonstrated that the compounds B1, B2, and B3 were substituted by the acyloxy group or sulfuryl group at the OH-4′ group.
Scheme 1.
Synthesis route of compound A1.
Scheme 2.
Synthesis route of compounds B1–B3.
Figure 4.
Comparison of partial 1H NMR spectra of B2 with DMEP.
2.2. The Anti-Xoo Bioactivity of Title Compounds
The antibacterial potency of the title compounds was first evaluated by measuring the growth of Xoo in vitro in the presence of a range of compound concentrations (Table 1). Most of the compounds had low antibacterial activity and only compound B2 exhibited moderate activity. Thus, the 50% effective concentration (EC50) for inhibition of Xoo growth was 153 mg L−1 for compound B2 and >200 mg L−1 for the remaining DMEP derivatives, which compared with 39.7 mg L−1 for DMEP and 36.3 mg L−1 for the control antibacterial agent, bismerthiazol. To further examine the inhibitory activity of these compounds, we measured the GTPase activity of purified recombinant XooFtsZ in vitro in the presence of compound B2 or the control GTPase inhibitor berberine (Table 2). Compound B2 inhibited purified XooFtsZ GTPase activity by 54.8% at 200 µM and by 48.6% at 100 µM. Further screening yielded 50% inhibitory concentrations (IC50s) of 159.4 µM and 225.0 µM for compound B2 and berberine, respectively. Thus, although compound B2 inhibited XooFtsZ GTPase activity with slightly higher potency than berberine and DMEP (IC50 = 278.7 µM), as demonstrated in our previous study [23], compound B2 was less potent than DMEP for inhibition of Xoo growth. One possible explanation for this apparent discrepancy may be the relatively poor aqueous solubility of compound B2 compared with DMEP, which may have restricted the bactericidal activity of compound B2 to a greater extent compared with its GTPase-inhibiting activity. However, the LogP values of the compounds, as determined with ChemDraw Professional 17.0, predicted that DMEP would have a lower cLogP value compared with compound B2 (cLogP = 0.97 and 3.69, respectively). Taken together, these analyses indicated that compound B2 exerted moderate anti-Xoo activity and outstanding Xoo GTPase-inhibitory activity. Therefore, we selected compound B2 for further analysis of its potential bactericidal activity and mechanism of action.
Table 1.
The Anti-Xoo activity of target compounds.
|
Inhibition (%) |
EC50 (mg L−1) |
MIC (mg L−1) |
||||
|---|---|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 | 100 (mg L−1) |
20 (mg L−1) |
||
| A1 |
|
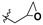
|
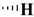
|
18.3 ± 2.15 | 10.6 ± 0.90 | >200 | >400 |
| B1 |
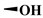
|
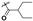
|
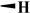
|
17.4 ± 6.00 | 5.33 ± 1.13 | >200 | >400 |
| B2 |
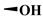
|
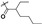
|
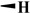
|
44.2 ± 3.20 | 7.30 ± 2.20 | 153 ± 9.20 | 400 |
| B3 |
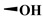
|
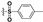
|
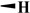
|
10.2 ± 1.20 | 4.33 ± 0.80 | >200 | >400 |
| DMEP |
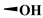
|
H |
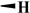
|
81.3 ± 10.2 | 26.2 ± 4.10 | 39.7 ± 0. 18 | 50 |
| BT | 92.7 ± 1.70 | 30.0 ± 2.20 | 36.3 ± 2.50 | 50 | |||
Table 2.
Inhibition effects of compound B2 and berberine on the XooFtsZ GTPase activity.
| Compounds | Inhibition Rate (%) | IC50 (μM) | |
|---|---|---|---|
| 200 μM | 100 μM | ||
| B2 | 54.8 ± 5.20 | 48.6 ± 4.10 | 159.4 ± 16.7 |
| Berberine hydrochloride | 48.7 ± 4.10 | 34.1 ± 2.60 | 225.0 ± 18.5 |
2.3. Investigation of Action Mechanism for Prepared Compound B2 Targeting XooFtsZ
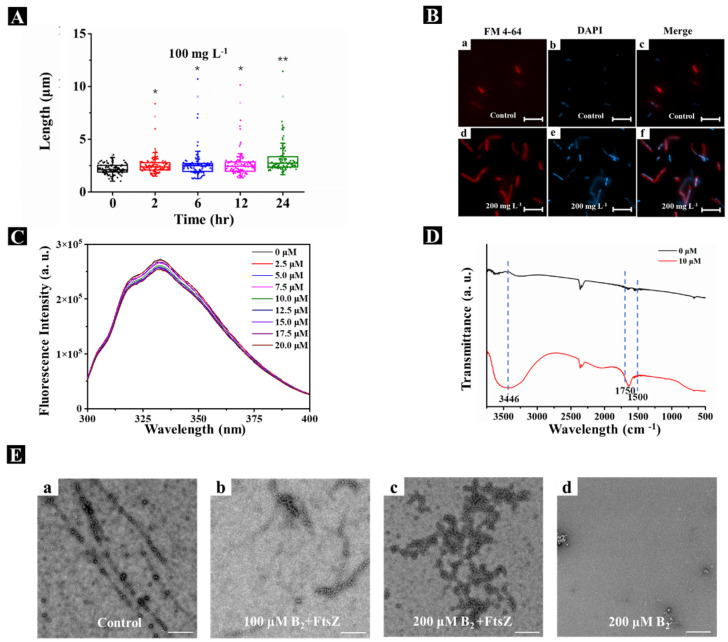
We next examined the effects of compound B2 on the morphology of Xoo cells using transmission electron microscopy (TEM) and fluorescence microscopy. Incubation of Xoo with 100 mg L−1 compound B2 significantly increased the average Xoo cell length from 2.05 ± 0.27 µM at 0 h to 4.03 ± 2.20 µM and 5.86 ± 3.23 µM at 12 h and 24 h, respectively (Figure 5A). Similarly, fluorescence microscopy of Xoo cells labeled with the lipophilic dye FM 4-64 and the DNA-intercalating dye 4′-6-diamidino-2-phenylindole revealed the elongated and filamentous appearance of the cells after incubation with compound B2 (Figure 5B), which confirmed the TEM results.
Figure 5.
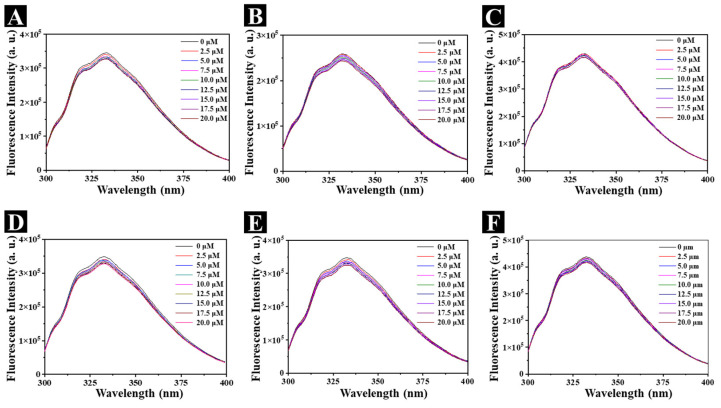
Mechanism of action for compound B2 targeting XooFtsZ. (A) Statistical data of the lengths of Xoo cells from TEM images after incubation with 100 mg L−1 compound B2 for different intervals of time. (B) The fluorescence patterns for the Xoo cells affected by 200 mg L−1 DMEP for 24 h. The cell membranes were visualized by FMTM 4−64FX and are shown in red; the DNA was visualized by DAPI and is shown in blue. Shown are control cells (a,b) and overlay (c); in the presence of 200 mg L−1 compound B2 (d,e) and overlay (f). The scale bars are 5 μm. (C) Fluorescence titration experiments of XooFtsZ (10 μM) with different concentrations (ranging from 0 μM to 20 μM) of compound B2, λex = 280 nm, λem = 334 nm. (D) FT−IR spectra of pure XooFtsZ (black line, 30 µM) and XooFtsZ-B2 complex (red line, 30 µM XooFtsZ was incubated with 10 µM compound B2). (E) Transmission electron micrographs of XooFtsZ polymers after incubation with different concentrations of compound B2. (a) 0 μM compound B2 + 20 μM XooFtsZ, (b) 100 μM compound B2 + 20 μM XooFtsZ, (c) 200 μM compound B2 + 20 μM XooFtsZ, (d) 200 μM compound B2 without XooFtsZ. Scale bars are 1 μm. Asterisks represented significant differences in comparison to control through used SPSS 20.0 software with Duncan (D) adjustment: (*) p < 0.05 and (**) p < 0.01.
Direct binding between compound B2 and recombinant XooFtsZ was evaluated by measuring the intrinsic fluorescence intensity of XooFtsZ before and after the addition of compound B2. As shown in Figure 5C, the emission fluorescence intensity decreased in the presence of compound B2 in an increasing, concentration-dependent manner. The KA of XooFtsZ–compound B2 complex formation was calculated as 103.22 M−1, which was similar to that of XooFtsZ–DMEP at 103.48 M−1 (Table 3). Potential conformational changes in XooFtsZ triggered by compound B2 binding were investigated using FT-IR. In the spectra shown in Figure 5D, 1600–1700 cm−1 represents the amide I band, which relates to the secondary structure of XooFtsZ. Compared with free XooFtsZ, complexes of XooFtsZ and compound B2 exhibited peaks in the 1600 cm−1 to 1700 cm−1 region, suggesting that compound B2 binding altered C-N stretching and N-H bending in XooFtsZ. The broader band at 3400 cm−1 also indicated that XooFtsZ–B2 complexes exhibited O-H and N-H stretching vibrations compared with free XooFtsZ. These interesting results suggested that compound B2 binding to XooFtsZ changed the protein conformation, which may be responsible for the change in the biological activity of XooFtsZ.
Table 3.
Binding parameters of different compounds with Xoo–FtsZ.
| Compounds | Stern–Volmer Quenching Constants | Binding Parameters | ||||
|---|---|---|---|---|---|---|
| Ksv (M−1) | Kq (M−1 S−1) | R | KA (M−1) | n | R2 | |
| B2 | 3.845 × 103 | 3.845 × 1011 | 0.96 | 103.22 | 0.92 | 0.95 |
| DMEP | 7.593 × 103 | 7.593 × 1011 | 0.98 | 103.48 | 0.92 | 0.98 |
| Berberine | 1.789 × 104 | 1.789 × 1012 | 0.91 | 103.44 | 0.81 | 0.98 |
Self-assembly of XooFtsZ was monitored by TEM and showed that, whereas free XooFtsZ formed single-stranded and uniform protofilaments, addition of compound B2 to XooFtsZ resulted in fewer single-stranded protofilaments and an increase in disordered and disorganized protein aggregation compared with the control sample. This finding demonstrated that compound B2 binding disorders the self-assembly of XooFtsZ via regulation of protein conformation, suggesting a mechanism for the inhibition of XooFtsZ GTPase activity.
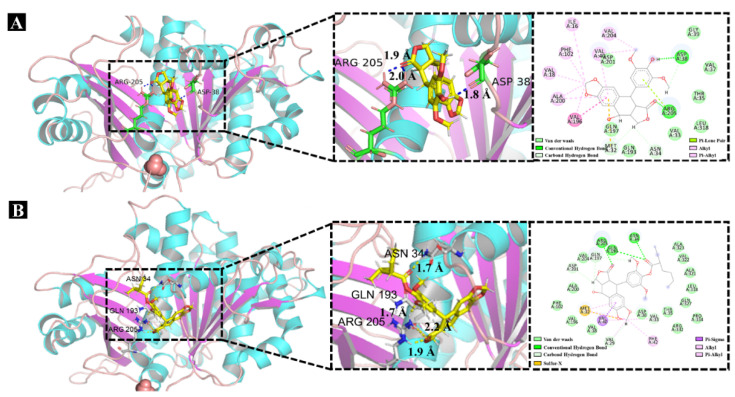
Molecular docking is an increasingly common and effective approach for predicting possible binding modes of small molecules complexed with proteins [26,27,28]. Investigation of XooFtsZ–B2 docking (Figure 6) showed that Asp38 and Arg205 were the main residues interacting with compound B2 to form hydrogen bonds. Sulfur-X, alkyl, π–δ, and Van der Waals bonds interaction also appeared crucial for complex formation. π-Alkyl or alkyl interactions were observed between compound B2 and Met32, Val33, Phe42, and Val40 residues; sulfur-X interaction was observed between compound B2 and Met32; and π–δ bonding was observed between compound B2 and Val40 (Figure 6). The docking scores are provided in Table S1. Collectively, these molecular docking results showed that the docking score for XooFtsZ interaction was higher for compound B2 than DMEP (6.34 vs. 5.92). These results further substantiated the results of our FT-IR spectra analysis.
Figure 6.
Molecular docking studies of XooFtsZ with DMEP or compound B2. (A) The docking results of XooFtsZ with DMEP. (B) The docking results of XooFtsZ with compound B2.
2.4. Potential Mechanism of Action for 4′-Demethylepipodophyllotoxin (DMEP) Analogues
DMEP and its derivatives represent a sustainable natural bioresource with antifungal [29], anticancer [30,31], and antiviral [32] activities, among other biological properties. To begin the SAR of the DMEP scaffold and XooFtsZ activity, we tested several commercially available DMEP analogs and found that they all exhibited weak anti-Xoo activity in vitro compared with the parent compound (Table 4). Determination of the minimum inhibitory concentrations (MICs), which represent the lowest concentrations that inhibit Xoo growth, showed that DMEP and bismerthiazol both had MICs of 50 mg L−1, whereas the remaining analogs tested had much poorer anti-Xoo activities (MICs > 200 mg L−1). Despite this, examination of the effects of these compounds on Xoo cell morphology showed that several compounds, including teniposide and etoposide, induced cellular elongation similar to DMEP and compound B2 (Figure 7). The binding parameters for these compounds and XooFtsZ were determined (Figure 8 and Table 5) and showed that the quenching mechanism between XooFtsZ and these compounds could format a weaker noncovalent complex than compound B2. The KA values for the interactions between XooFtsZ and podophyllotoxin, picropodophyllotoxin, 4′-demethylpodophyllotoxin (DMEOP), deoxypodophyllotoxin, teniposide, and etoposide were 101.34 M−1, 101.81 M−1, 102.26 M−1, 101.04 M−1, 101.73 M−1, and 102.50 M−1, respectively, all of which were lower than the KA of 103.48 M−1 for DMEP–XooFtsZ. Overall, these results indicated that the hydroxyl group of DMEP was crucial for its anti-Xoo activity as well as for its interaction with XooFtsZ.
Table 4.
The Anti-Xoo activity of DMEP derivativities.
|
Inhibition (%) | EC50 (mg L−1) |
MIC (mg L−1) |
||||
|---|---|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 | 100 (mg L−1) |
20 (mg L−1) |
||
| Podophyllotoxin |
|
CH3 |
|
- | - | >200 | >400 |
| Picropodophyllotoxin |
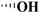
|
CH3 |
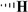
|
- | - | >200 | >400 |
| DMEOP |
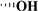
|
H |
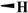
|
- | - | >200 | >400 |
| Deoxypodophyllotoxin | H | CH3 |
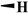
|
10.3 ± 3.50 | 12.2 ± 4.80 | >200 | >400 |
| Teniposide |
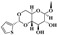
|
H |
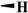
|
20.3 ± 9.21 | - | >200 | >400 |
| Etoposide |
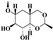
|
H |
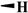
|
18.8 ± 9.60 | - | >200 | >400 |
| DMEP |
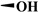
|
H |
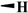
|
81.3 ± 10.2 | 26.2 ± 4.10 | 39.7 ± 0. 18 | 50 |
| BT | 92.7 ± 1.70 | 30.0 ± 2.20 | 36.3 ± 2.50 | 50 | |||
Figure 7.
TEM images of Xoo cells after incubating with DMEP analogues at 100 mg L−1 for 24 h. Scale bars are 5 μm.
Figure 8.
Fluorescence titration experiments of XooFtsZ (10 μM) with elevated concentrations (from 0 μM to 20 μM) of podophyllotoxin (A), picropodophyllotoxin (B), DMEOP (C), deoxypodophyllotoxin (D), teniposide (E), and etoposide (F), λex = 280 nm, λem = 334 nm.
Table 5.
Binding parameters of DMEP and DMEP analogues with XooFtsZ.
| Compounds | Stern–Volmer Quenching Constants | Binding Parameters | ||||
|---|---|---|---|---|---|---|
| Ksv (M−1) | Kq (M−1 S−1) | R | KA (M−1) | n | R2 | |
| Podophyllotoxin | 2.203 × 103 | 2.203 × 1011 | 0.94 | 101.34 | 0.54 | 0.94 |
| Picropodophyllotoxin | 3.603 × 103 | 3.603 × 1011 | 0.95 | 101.81 | 0.63 | 0.96 |
| DMEOP | 1.757 × 103 | 1.757 × 1011 | 0.98 | 102.26 | 0.79 | 0.96 |
| Deoxypodophyllotoxin | 2.276 × 103 | 2.276 × 1011 | 0.98 | 101.04 | 0.48 | 0.97 |
| Teniposide | 2.920 × 103 | 2.920 × 1011 | 0.98 | 101.73 | 0.61 | 0.97 |
| Etoposide | 2.396 × 103 | 2.396 × 1011 | 0.98 | 102.50 | 0.82 | 0.97 |
| DMEP | 7.593 × 103 | 7.593 × 1011 | 0.98 | 103.48 | 0.92 | 0.98 |
| Berberine | 1.789 × 104 | 1.789 × 1012 | 0.91 | 103.44 | 0.81 | 0.98 |
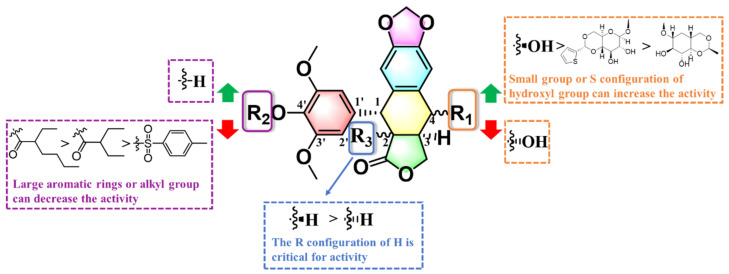
2.5. Outcome of SAR Study
To extend the SAR of compounds based on the DMEP, we systematically examined the antibacterial potency of DMEP analogs based on inhibition of Xoo growth in vitro. The results can be summarized as follows (Figure 9): (1) when the 4-position is in the S configuration, a bulky group at the 4-position was unfavorable to anti-Xoo activity: DMEP (EC50 = 38.7 mg L−1) > teniposide and etoposide (both EC50 > 200 mg L−1); (2) the S configuration of the hydroxyl group was excellent for anti-Xoo activity: DMEP (EC50 = 38.7 mg L−1) > deoxypodophyllotoxin and DMEOP (both EC50 > 200 mg L−1), which was in agreement with the docking results for these compounds (Figure 6); (3) the R configuration of the H group at the 2-position was beneficial for anti-Xoo activity: A1 (EC50 > 200 mg L−1) < B2 (EC50 = 153 mg L−1); and (4) the hydroxyl group at the 4′-position increased the anti-Xoo activity: DMEP (EC50 = 39.7 mg L−1) > B1 (EC50 > 200 mg L−1) and B2 (EC50 > 153 mg L−1).
Figure 9.
Overall SAR summary of the DMEP analogues.
2.6. In Vivo Trials against Rice Bacterial Leaf Blight Infected by Xoo
Encouraged by these in vitro results, we asked whether compound B2-mediated inhibition of XooFtsZ might provide an effective approach to controlling bacterial leaf blight diseases. Using pot experiments, we observed that compound B2 had good curative activity against rice bacterial leaf blight and gave a control efficiency of 54.9% at 200 mg mL−1, which was better than both commercial TC (31.2%) and, as previously reported, DMEP (50.0%) [23]. Similarly, compound B2 had superior protective activity (48.4%) against bacterial leaf blight compared with either TC (30.4%) or DMEP (46.8%) [23]. Thus, targeting of bacterial FtsZ by compound B2 holds promise for the management of plant bacterial diseases.
2.7. Assessment of Potential Risk of DMEP and Compound B2 through Phytotoxicity and Cytotoxicity Testing
Determining the potential off-target toxicity of novel agricultural and pest management agents is an important consideration in the development of safer and more environmentally responsible toxins. Therefore, we compared the potential phytotoxicity of DMEP and compound B2 against rice plants, as previously described [33]. Notably, compound B2 was non-toxic to rice plants at a concentration of 200 mg L−1, which was an effective dose for anti-Xoo activity in vivo. We also examined the cytotoxicity of DMEP and compound B2 against two representative mammalian cell lines in vitro using a standard MTT cytotoxicity assay [34,35]. We tested the normal rat kidney cell line NRK-52E and the human non-small cell lung cancer cell line (A549), which was included because several DMEP analogs are already in clinical use as anticancer agents. Interestingly, compound B2 was more cytotoxic than either DMEP or gefitinib, a small molecule clinical used for the treatment of lung cancer, against A549 cells, but was the least cytotoxic compound against NRK-52E cells (IC50 60.8 µM compared with 30.8 µM and 21.0 µM for DMEP and gefitinib, respectively), and corresponding results was showed in Figure S1. Furthermore, to illustrate the druggability of compound B2, we submitted the structure of compound B2 into the website http://www.swissadme.ch/index.php (accessed on 1 November 2021), and the corresponding results showed that compound B2 met the drug-likeness rules, including Lipinski, Veber, Egan, and Muegge, with a bioavailability score of 0.55 [36]. Notably, these data showed that compound B2 has high anti-Xoo activity, low phytotoxicity, high antiproliferative activity against the A549 cancer cell line, and low antiproliferative activity against the normal NRK-52E cell line.
3. Materials and Methods
3.1. Instruments and Chemicals
Instruments: NMR spectra of prepared title compounds were obtained on a Bruker Biospin AG-400 instrument (Bruker Optics, Ettlingen, Germany) using DMSO-d6/CDCl3 as solvent and tetramethylsilane as the internal standard; HRMS spectra were achieved using Waters Xevo G2-S QTOF MS (Waters MS Technologies, Manchester, UK). TEM images of Xoo’s morphological changes were visualized on a FEI Talos F200C electron microscope (FEI, Hillsboro, OR, USA) operating at a voltage of 200 kV. Fluorescence spectra data were performed on a FluoroMax®-4P (HORIBA Scientific, Paris, France). The FT-IR spectra data were recorded on a Nicolet iS50 instrument (Thermo Fisher Scientific, Waltham, MA, USA). Fluorescent images of Xoo cells were achieved using an Olympus-BX53-microscope (Olympus, Tokyo, Japan). The optical values were recorded on Cytation™5 multi-mode readers (BioTek Instruments, Inc., Winooski, VT, USA). Recombinant XooFtsZ was purified by a GE ÄKTA pure 25 system (GE Healthcare Bio-Sciences, Piscataway, NJ, USA).
Chemicals: All the chemicals were purchased from Bide Pharmatech Co., Ltd. (Shanghai, China) and Energy Chemical of Saen Chemical Technology Co., Ltd. (Shanghai, China). The Ni-NTA column (1 × 5 mL) and HiTrap desalting column (5 × 5 mL) were acquired from the GE Healthcare company (USA). IPTG (isopropyl β-D-thiogalactoside), HEPES, EDTA, disodium hydrogenphosphate, sodium dihydngen phoshate, imidazole, and NaCl were provided by the Bioengineering Co., Ltd. (Shanghai, China) and Solarbio Life Sciences & Technology Co., Ltd. (Beijing, China). GTP was ordered from ThermoFisher Scientific Vendor Co., Ltd. (Shanghai, China).
3.2. Experimental Section
The wild-type Xanthomonas oryzae pv. oryzae (Xoo) strain ZJ173 was kindly provided by Prof. Ming-Guo Zhou (Nanjing Agricultural University, Nanjing, China). The minimum inhibitory concentration (MIC) and in vivo of anti-Xoo bioactivity (in vitro and in vivo assay), and purification of recombinant XooFtsZ. The structures of the title compounds were characterized by 1H NMR, 13C NMR and HRMS, and corresponding data was provided as Figures S2–S13. All of the above-mentioned experimental details can be found in supplementary data.
3.3. The Strategy of Structure-Based Virtual Screening (SBVS)
Initially, the amino acid sequence of XooFtsZ was achieved from the website of the national center for biotechnology information, and its three-dimensional structure was reconstructed through using multi-template modeling. Particularly, modeling XooFtsZ’s protein backbone dihedral angle parameters was further refined through the GROMOS 54A7 force field. These details can be found in our previous work [37].
In the second stage of the virtual screening, a structure-based virtual screening (SBVS) approach was carried out through using the database of DrugSpaceX [25]. Notably, more than 100 million chemical products bearing synthesizable and drug-like properties were provided in the DrugSpaceX database. Briefly, the structure of DMEP was submitted to the DrugSpaceX website (https://drugspacex.simm.ac.cn/, accessed on 1 October 2021), and one hundred DMEP analogues were visualized in the website. Thereafter, these DMEP analogues were downloaded as a subset and further used for the virtual screening. Subsequently, the automated protein preparation protocol was used for docking by operating Sybyl-X 2.0 software (Tripos Associates, Saint Louis, MO, USA). Finally, according to the results of the docking score, the top 10 compounds with the best scores were listed and ranked in Figure 3.
3.4. Determination of the Binding Constant (KA) of Compounds-XooFtsZ Interaction
The dissociation constants of compounds-XooFtsZ were determined by using typical fluorometric titration assays [38,39]. Briefly, 10 μM XooFtsZ was co-incubated with various concentrations (0, 2.5, 5.0, 7.5, 10.0, 12.5, 15.0, 17.5 and 20.0 μM) of test compounds in 20 mM phosphate buffer (pH 7.4) containing 150 mM KCl and 1 mM EDTA at 25 °C. Then, these samples were recorded using the FluoroMax®-4P instrument (Ex = 280 nm, slit widths = 3 nm). The corresponding binding constant (KA) of each sample was calculated by utilizing the Stern–Volmer method (F0/F = 1 + Kq τ0[Q] = 1 + Ksv [Q]) at 334 nm.
3.5. Morphological Studies Using Transmission Electron Microscopy (TEM)
Xoo cells (OD595 = 0.1) were co-incubated without/with 100 mg L−1 of compound B2 in nutrient broth for 24 h in a shaker (180 rpm, 28 ± 1 °C). After that, these samples were covered with Formvar-carbon-coated copper grids and then negatively stained using 1% phosphotungstic acid. Finally, prepared samples were photographed by operating a transmission electron microscope (TEM), and the corresponding Xoo length of each sample was measured using ImageJ software (NIH Image, Bethesda, MD, USA) [40,41].
3.6. Fourier Transform Infrared (FT-IR) Spectroscopy Analysis
The FT-IR spectra analysis was carried out by referring to previously reported methods [42,43]. Briefly, 30 µM of XooFtsZ was mixed without/with 10 µM compounds in 20 mM phosphate buffer (pH 7.4) containing 150 mM KCl and 1 mM EDTA at 25 °C for 10 min. Then, 2 µL of treated sample was covered on the new KBr disc. Finally, the spectra of each sample were scanned under a certain condition (Scanning area: 500–4000 cm−1, scans: 32, resolutions: 4 cm−1). Particularly, the background spectrum was pre-recorded. The FT-IR spectra of each sample were yielded using a Nicolet iS50 instrument (Thermo Fisher Scientific, Waltham, MA, USA) (n = 2 for every group).
3.7. Fluorescence Patterns for the Xoo Cells Triggered by Compounds
Xoo cells were precultured in the above condition (2.5) and also displayed their morphological changes through using a BX53 fluorescence microscope. Briefly, the Xoo cells were fixed with 7% formaldehyde for 10 min and further washed with phosphate-buffered saline buffer (PBS, 10 mM, pH 7.3). Thereafter, these samples were stained with FM™ 4-64 dye solution (3 mg L−1) for 20 min and subsequently washed by phosphate-buffered saline buffer (PBS, 10 mM, pH 7.3). Finally, these samples were spread on a glass slide and then stained with DAPI solution (2 mg L−1) for fluorescence imaging [44,45].
3.8. Statistical Analysis
Statistical analyses were executed with one-way ANOVA by using SPSS 20.0 software. The Duncan (D) adjustment was performed to determine the significant difference between different treatments. Asterisks represented significant differences in comparison to control: (*) p < 0.05 and (**) p < 0.01. In the section of anti-Xoo bioassay in vivo, different uppercase letters following the control efficiency values illustrated that there was a significant difference (p < 0.05) among different treatment groups. The results were presented as means ± SD.
4. Conclusions
As the cause of rice bacterial leaf blight, the vascular phytopathogenic bacterium Xoo is a major cause of reduced crop quality and quantity. Based on our previous work identifying DMEP as a novel chemical scaffold for XooFtsZ inhibitors, we used a combination of in silico, in vitro, and in vivo approaches to design and systematically test DMEP derivatives with potential anti-Xoo activity. Compound B2 was validated as a potential XooFtsZ inhibitor with an IC50 (159.4 µM) lower than that of the parent DMEP (278.0 µM). We also showed that compound B2 likely binds to XooFtsZ by interacting with residues Asp38, Arg205, Met32, Val33, Phe42, and Val40, and that the interaction disrupts FtsZ linear assembly and induces elongation of Xoo cells. Finally, we showed that compound B2 displayed good curative and protective activities against rice bacterial leaf blight in pot studies but displayed low general phytotoxicity against rice plants and low cytotoxicity against mammalian cell lines. Taken together, our results identify compound B2 as a promising FtsZ-targeting DMEP derivative that could be developed for the management of plant bacterial diseases.
Abbreviations
| DMEP | 4′-demethylepipodophyllotoxin |
| Xoo | Xanthomonas oryzae pv. Oryzae |
| FtsZ | filamentous temperature-sensitive protein Z |
| SBVS | Structure-based virtual screening |
| DMEOP | 4′-demethylpodophyllotoxin |
| D. versipellis | Dysosma versipellis |
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23169119/s1. References [46,47,48,49,50,51,52] are cited in the supplementary materials.
Author Contributions
Conceptualization, Y.-L.S. and S.Y.; methodology, Y.-L.S. and S.-S.L.; software, S.-S.L., J.Y. and J.X.; formal analysis, L.-W.L.; data curation, P.-Y.W.; writing—original draft preparation, X.Z. and S.Y.; writing—review and editing, X.Z. and S.Y.; visualization, Z.-B.W.; supervision, X.Z. and S.Y.; project administration, X.Z., and S.Y.; funding acquisition, X.Z. and S.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
We acknowledge the supports from National Natural Science Foundation of China (21877021, 32160661), the Guizhou Provincial S&T Project (2018[4007]), the Guizhou Province [Qianjiaohe KY number (2020)004]), Program of Introducing Talents of Discipline to Universities of China (D20023, 111 Program) and GZU (Guizhou University) Found for Newly Enrolled Talent (No. 202229).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li P., Hu D.Y., Xie D.D., Chen J.X., Jin L.H., Song B.A. Design, synthesis, and evaluation of new sulfone derivatives containing a 1,3,4-oxadiazole moiety as active antibacterial agents. J. Agric. Food Chem. 2018;66:3093–3100. doi: 10.1021/acs.jafc.7b06061. [DOI] [PubMed] [Google Scholar]
- 2.Yuan T., Li X.H., Xiao J.H., Wang S.P. Characterization of Xanthomonas oryzae-responsive cis-acting element in the promoter of rice race-specific susceptibility gene Xa13. Mol. Plant. 2011;4:300–309. doi: 10.1093/mp/ssq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stover E., Driggers R., Richardson M.L., Hall D.G., Lee R.F. Incidence and severity of asiatic citrus canker on diverse citrus and citrus-related germplasm in a florida field planting. Hortscience. 2014;49:4–9. doi: 10.21273/HORTSCI.49.1.4. [DOI] [Google Scholar]
- 4.Borlaug N.E. Feeding a world of 10 billion people: The miracle ahead. Biotechnol. Biotec. Eq. 1997;11:3–13. doi: 10.1080/13102818.1997.10818934. [DOI] [Google Scholar]
- 5.Guo S.X., He F., Song B.A., Wu J. Future direction of agrochemical development for plant disease in China. Food Energy Secur. 2021;10:e293. doi: 10.1002/fes3.293. [DOI] [Google Scholar]
- 6.Sakschewski B., Bloh W.V., Huber V., Müller C., Bondeau A. Feeding 10 billion people under climate change: How large is the production gap of current agricultural systems? Ecol. Model. 2014;288:103–111. doi: 10.1016/j.ecolmodel.2014.05.019. [DOI] [Google Scholar]
- 7.Campos E.V., Proença P.L., Oliveira J.L., Bakshi M., Abhilash P.C., Fraceto L.F. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecol. Indic. 2019;105:483–495. doi: 10.1016/j.ecolind.2018.04.038. [DOI] [Google Scholar]
- 8.Donatelli M., Magarey R.D., Bregaglio S., Willocquet L., Whish J.P.M., Savary S. Modelling the impacts of pests and diseases on agricultural systems. Agr. Syst. 2017;155:213–224. doi: 10.1016/j.agsy.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y.L., Zhang Y., Pei R.J., Xie Y.F., Yao W.R., Guo Y.H., Qian H. Fast detection of bismerthiazol in cabbage based on fluorescence quenching of protein-capping gold nanoclusters. Anal. Sci. 2018;34:415–419. doi: 10.2116/analsci.17P347. [DOI] [PubMed] [Google Scholar]
- 10.Oguh C.E., Okpaka C.O., Ubani C.S., Okekeaji U., Joseph P.S., Amadi E.U. Natural pesticides (biopesticides) and uses in pest management—A critical review. [(accessed on 1 October 2021)];AJBGE. 2019 2:1–18. Available online: https://sdiarticle4.com/prh/doc/Ms_AJBGE_53356.pdf. [Google Scholar]
- 11.Wu Y.X., Ren D., Gao C., Li J.Y., Du B., Wang Z.Y., Qian S. Recent advances for alkaloids as botanical pesticides for use in organic agriculture. Int. J. Pest Manage. 2021:1–11. doi: 10.1080/09670874.2021.1917723. [DOI] [Google Scholar]
- 12.Cantrell C.L., Dayan F.E., Duke S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012;75:1231–1242. doi: 10.1021/np300024u. [DOI] [PubMed] [Google Scholar]
- 13.Marrone P.G. Pesticidal natural products–status and future potential. Pest Manag. Sci. 2019;75:2325–2340. doi: 10.1002/ps.5433. [DOI] [PubMed] [Google Scholar]
- 14.Aly A.H., Debbab A., Proksch P. Fifty years of drug discovery from fungi. Fungal Divers. 2011;50:3–19. doi: 10.1007/s13225-011-0116-y. [DOI] [Google Scholar]
- 15.Ravula A.R., Yenugu S. Pyrethroid based pesticides–chemical and biological aspects. Crit. Rev. Toxicol. 2021;51:117–140. doi: 10.1080/10408444.2021.1879007. [DOI] [PubMed] [Google Scholar]
- 16.Yu X., Che Z.P., Xu H. Recent advances in the chemistry and biology of podophyllotoxins. Chem. Eur. J. 2017;23:4467–4526. doi: 10.1002/chem.201602472. [DOI] [PubMed] [Google Scholar]
- 17.Zhu X.K., Guan J., Xiao Z., Mark Cosentino L., Lee K.H. Anti-AIDS agents. Part 61: Anti-HIV activity of new podophyllotoxin derivatives. Bioorg. Med. Chem. 2004;12:4267–4273. doi: 10.1016/j.bmc.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q.Y., Zhao W., Tang Y.J. Discover the leading compound of 4β-S-(5-fluorobenzoxazole)-4-deoxy-4′-demethylepipodophyllotoxin with millimolar-potency toxicity by modifying the molecule structure of podophyllotoxin. Eur. J. Med. Chem. 2018;158:951–964. doi: 10.1016/j.ejmech.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 19.Zhao W., Cong Y., Li H.M., Li S.Y., Shen Y.M., Qi Q.S., Zhang Y.M., Li Y.Z., Tang Y.J. Challenges and potential for improving the druggability of podophyllotoxin-derived drugs in cancer chemotherapy. Nat. Prod. Rep. 2021;38:470–488. doi: 10.1039/D0NP00041H. [DOI] [PubMed] [Google Scholar]
- 20.Zhi X.Y., Yang C., Zhang R., Hu Y., Ke Y.Z., Xu H. Natural products-based insecticidal agents 13. Semisynthesis and insecticidal activity of novel phenazine derivatives of 4β-acyloxypodophyllotoxin modified in the E-ring against Mythimna separata Walker in vivo. Ind. Crop. Prod. 2013;42:520–526. doi: 10.1016/j.indcrop.2012.06.045. [DOI] [Google Scholar]
- 21.Zhi X.Y., Yu X., Yang C., Ding G.D., Chen H., Xu H. Synthesis of 4β-acyloxypodophyllotoxin analogs modified in the C and E rings as insecticidal agents against Mythimna separata Walker. Bioorg. Med. Chem. Lett. 2014;24:765–772. doi: 10.1016/j.bmcl.2013.12.105. [DOI] [PubMed] [Google Scholar]
- 22.Huang J.L., Xu M., Li S.C., He J.N., Xu H. Synthesis of some ester derivatives of 4′-demethoxyepipodophyllotoxin/2′-chloro-4′-demethoxyepipodophyllotoxin as insecticidal agents against oriental armyworm, Mythimna separata Walker. Bioorg. Med. Chem. Lett. 2017;27:511–517. doi: 10.1016/j.bmcl.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X., Ye H.J., Gao X.H., Feng Y.M., Shao W.B., Qi P.Y., Wu Z.B., Liu L.W., Wang P.Y., Yang S. The discovery of natural 4′-demethylepipodophyllotoxin from renewable dysosma versipellis species as a novel bacterial cell division inhibitor for controlling intractable diseases in rice. Ind. Crop. Prod. 2021;174:114182. doi: 10.1016/j.indcrop.2021.114182. [DOI] [Google Scholar]
- 24.Wang Z., Sun H.Y., Shen C., Hu X.P., Gao J.B., Li D., Cao D.S., Hou T.J. Combined strategies in structure-based virtual screening. Phys. Chem. Chem. Phys. 2020;22:3149–3159. doi: 10.1039/C9CP06303J. [DOI] [PubMed] [Google Scholar]
- 25.Yang T.B., Li Z.J., Chen Y.J., Feng D., Wang G.C., Fu Z.Y., Ding X.Y., Tan X.Q., Zhao J.H., Luo X.M., et al. DrugSpaceX: A large screenable and synthetically tractable database extending drug space. Nucleic Acids Res. 2021;49:D1170–D1178. doi: 10.1093/nar/gkaa920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng X.Y., Zhang H.X., Mezei M., Cui M. Molecular Docking: A powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des. 2011;7:146–157. doi: 10.2174/157340911795677602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novikov F.N., Chilov G.G. Molecular docking: Theoretical background, practical applications and perspectives. Mendeleev Commun. 2009;19:237–242. doi: 10.1016/j.mencom.2009.09.001. [DOI] [Google Scholar]
- 28.Ferreira L.G., dos Santos R.N., Oliva G., Andricopulo A.D. Molecular Docking and Structure-Based Drug Design Strategies. Molecules. 2015;20:13384–13421. doi: 10.3390/molecules200713384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anil Kumar K., Kumar Singh S., Siva Kumar B., Doble M. Synthesis, anti-fungal activity evaluation and QSAR studies on podophyllotoxin derivatives. Cent. Eur. J. Chem. 2007;5:880–897. doi: 10.2478/s11532-007-0036-6. [DOI] [Google Scholar]
- 30.Xiao L., Zhao W., Li H.M., Wan D.J., Li D.S., Chen T., Tang Y.J. Design and synthesis of the novel DNA topoisomerase II inhibitors: Esterification and amination substituted 4′-demethylepipodophyllotoxin derivates exhibiting anti-tumor activity by activating ATM/ATR signaling pathways. Eur. J. Med. Chem. 2014;80:267–277. doi: 10.1016/j.ejmech.2014.03.082. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z.W., Zhang J.Q., Hui L., Chen S.W., Tian X. First synthesis and biological evaluation of novel spin-labeled derivatives of deoxypodophyllotoxin. Eur. J. Med. Chem. 2010;45:1673–1677. doi: 10.1016/j.ejmech.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 32.Castro M.A., Corral J.M.M.D., Gordaliza M., Go´mez-Zurita M.A., Puente M.L.D.L., Betancur-Galvis L.A., Sierra J., Feliciano A.S. Antiviral Synthesis, cytotoxicity and antiviral activity of podophyllotoxin analogues modified in the E-ring. Eur. J. Med. Chem. 2003;38:899–911. doi: 10.1016/j.ejmech.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Yang X.E., Long X.X., Ni W.Z., Ye Z.Q., He Z.L., Stoffella P.J., Calvert D.V. Assessing copper thresholds for phytotoxicity and potential dietary toxicity in selected vegetable crops. J. Environ. Sci. Health B. 2002;37:625–635. doi: 10.1081/PFC-120015443. [DOI] [PubMed] [Google Scholar]
- 34.Morgan D.M.L. Tetrazolium (MTT) Assay for Cellular Viability and Activity. Methods Mol. Biol. 1998;79:179–183. doi: 10.1385/0-89603-448-8:179. [DOI] [PubMed] [Google Scholar]
- 35.Mosmann T.J. Rapid colorimetic assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 36.Daina A., Michielin O., Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. [(accessed on 1 October 2021)];Sci. Rep. 2017 7:42717. doi: 10.1038/srep42717. Available online: https://www.nature.com/articles/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou X., Feng Y.M., Qi P.Y., Shao W.B., Wu Z.B., Liu L.W., Wang Y., Ma H.D., Wang P.Y., Li Z., et al. Synthesis and Docking Study of N-(Cinnamoyl)-N′-(substituted) acryloyl Hydrazide Derivatives Containing Pyridinium Moieties as a Novel Class of Filamentous Temperature-Sensitive Protein Z Inhibitors against the Intractable Xanthomonas oryzae pv. oryzae Infections in Rice. J. Agric. Food Chem. 2020;68:8132–8142. doi: 10.1021/acs.jafc.0c01565. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z.C., Li X.Y., Wang W.L., Zhang W.Y., Yu L., Hu D.Y., Song B.A. Interaction research on the antiviral molecule Dufulin targeting on southern rice black streaked dwarf virus P9-1 nonstructural protein. Viruses. 2015;7:1454–1473. doi: 10.3390/v7031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bi S.Y., Ding L., Tian Y., Song D.Q., Zhou X., Liu X., Zhang H.Q. Investigation of the interaction between flavonoids and human serum albumin. J. Mol. Struct. 2004;703:37–45. doi: 10.1016/j.molstruc.2004.05.026. [DOI] [Google Scholar]
- 40.Zhou X., Ye Y.Q., Liu S.S., Shao W.B., Liu L.W., Yang S., Wu Z.B. Design, synthesis and anti-TMV activity of novel α-aminophosphonate derivatives containing a chalcone moiety that induce resistance against plant disease and target the TMV coat protein. Pestic. Biochem. Phys. 2021;172:104749. doi: 10.1016/j.pestbp.2020.104749. [DOI] [PubMed] [Google Scholar]
- 41.Li Y., Zhou X., Wu H.G., Yu Z.Z., Li H., Yang S. Nanospheric heterogeneous acid-enabled direct upgrading of biomass feedstocks to novel benzimidazoles with potent antibacterial activities. Ind. Crop. Prod. 2020;150:112406. doi: 10.1016/j.indcrop.2020.112406. [DOI] [Google Scholar]
- 42.Liu C., Cheng F.F., Yang X.Q. Inactivation of soybean trypsin inhibitor by epigallocatechin gallate: Stopped-flow/fluorescence, thermodynamics, and docking studies. J. Agric. Food Chem. 2017;65:921–929. doi: 10.1021/acs.jafc.6b04789. [DOI] [PubMed] [Google Scholar]
- 43.Pu P., Zheng X., Jiao L.N., Chen L., Yang H., Zhang Y.H., Liang G.Z. Six flavonoids inhibit the antigenicity of β-lactoglobulin by noncovalent interactions: A spectroscopic and molecular docking study. Food Chem. 2021;339:128106. doi: 10.1016/j.foodchem.2020.128106. [DOI] [PubMed] [Google Scholar]
- 44.Beuria T.K., Santra M.K., Panda D. Sanguinarine blocks cytokinesis in bacteria by inhibiting FtsZ assembly and bundling. Biochemistry. 2005;44:16584–16593. doi: 10.1021/bi050767+. [DOI] [PubMed] [Google Scholar]
- 45.Singh D., Bhattacharya A., Rai A., Dhaked H.P.S., Awasthi D., Ojima I., Panda D. SB-RA-2001 inhibits bacterial proliferation by targeting FtsZ assembly. Biochemistry. 2014;53:2979–2992. doi: 10.1021/bi401356y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang P.Y., Xiang M., Luo M., Liu H.W., Zhou X., Wu Z.B., Liu L.W., Li Z., Yang S. Novel piperazine-tailored ursolic acid hybrids as significant antibacterial agents targeting phytopathogens Xanthomonas oryzae pv. oryzae and X. axonopodis pv. citri probably directed by activation of apoptosis. Pest Manag. Sci. 2020;76:2746–2754. doi: 10.1002/ps.5822. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y.L., Huang X., Liu L.W., Wang P.Y., Long Q.S., Tao Q.Q., Li Z., Yang S. Identification of racemic and chiral carbazole derivatives containing an isopropanolamine linker as prospective surrogates against plant pathogenic bacteria: In vitro and in vivo assays and quantitative proteomics. J. Agric. Food Chem. 2019;67:7512–7525. doi: 10.1021/acs.jafc.9b02036. [DOI] [PubMed] [Google Scholar]
- 48.Liu H.W., Ji Q.T., Ren G.G., Wang F., Su F., Wang P.Y., Zhou X., Wu Z.B., Li Z., Yang S. Antibacterial functions and proposed modes of action of novel 1,2,3,4-tetrahydro-β-carboline derivatives that possess an attractive 1,3-diaminopropan-2-ol pattern against rice bacterial blight, kiwifruit bacterial canker, and citrus bacterial canker. J. Agric. Food Chem. 2020;68:12558–12568. doi: 10.1021/acs.jafc.0c02528. [DOI] [PubMed] [Google Scholar]
- 49.Tao Q.Q., Liu L.W., Wang P.Y., Long Q.S., Zhao Y.L., Jin L.H., Xu W.M., Chen Y., Li Z., Yang S. Synthesis and in vitro and in vivo biological activity evaluation and quantitative proteome profiling of oxadiazoles bearing flexible heterocyclic patterns. J. Agric. Food Chem. 2019;67:7626–7639. doi: 10.1021/acs.jafc.9b02734. [DOI] [PubMed] [Google Scholar]
- 50.Zeng D., Wang M.W., Xiang M., Liu L.W., Wang P.Y., Li Z., Yang S. Design, synthesis, and antimicrobial behavior of novel oxadiazoles containing various N-containing heterocyclic pendants. Pest Manag. Sci. 2020;76:2681–2692. doi: 10.1002/ps.5814. [DOI] [PubMed] [Google Scholar]
- 51.Xiang M., Song Y.L., Ji J., Zhou X., Liu L.W., Wang P.Y., Wu Z.B., Li Z., Yang S. Synthesis of novel 18β-glycyrrhetinic piperazine amides displaying significant in vitro and in vivo antibacterial activities against intractable plant bacterial diseases. Pest Manag. Sci. 2020;76:2959–2971. doi: 10.1002/ps.5841. [DOI] [PubMed] [Google Scholar]
- 52.Cai S.Y., Yuan W.C., Li Y., Huang X.H., Guo Q., Tang Z.W., Fang Z.Y., Lin H., Wong W.L., Wong K.Y., et al. Antibacterial activity of indolyl-quinolinium derivatives and study their mode of action. Bioorg. Med. Chem. 2019;27:1274–1282. doi: 10.1016/j.bmc.2019.02.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.