Abstract
A filamentous soil bacterium, Streptomyces griseus 2247, carries a 7.8-Mb linear chromosome. We previously showed by macrorestriction analysis that mutagenic treatments easily caused deletions at both ends of its linear chromosome and changed the chromosome to a circular form. In this study, we confirmed chromosomal circularization by cloning and sequencing the junction fragments from two deletion mutants, 404-23 and N2. The junction sequences were compared with the corresponding right and left deletion end sequences in the parent strain, 2247. No homology and a 6-bp microhomology were found between the two deletion ends of the 404-23 and N2 mutants, respectively, which indicate that the chromosomal circularization was caused by illegitimate recombination without concomitant amplification. The circularized chromosomes were stably maintained in both mutants. Therefore, the chromosomal circularization might have occurred to prevent lethal deletions, which otherwise would progress into the indispensable central regions of the chromosome.
Streptomyces species are gram-positive soil bacteria with a high G+C base composition (70 to 74%) in their DNA (3). They display a complex morphological differentiation similar to that of fungi and produce a large number of secondary metabolites, such as medically useful antibiotics. Macrorestriction analysis by pulsed-field gel electrophoresis (PFGE) revealed that all of the hitherto-analyzed Streptomyces species carry an ∼8-Mb linear chromosome in spite of being classified as bacteria (11, 12, 15, 16, 19). It is also known that Streptomyces linear chromosomes are unstable and exhibit large deletions and amplifications (1, 2, 4). The sizes of deletions sometimes reach 2 Mb (5). Moreover, chromosomal circularization in some deletion mutants of Streptomyces lividans (20) and Streptomyces ambofaciens (11) was demonstrated by the detection of a new fusion macrorestriction fragment.
Streptomyces griseus, which is, physiologically, one of the best-studied Streptomyces species, produces the antibiotic streptomycin and forms spores even in liquid culture. It also produces A-factor, a bacterial hormone which positively regulates both streptomycin production and spore formation (6, 9). The linear topology of the chromosome of S. griseus 2247 was proved by the physical mapping of AseI and DraI fragments, the identification of a protein binding to the end fragments, and restriction analysis of the terminal inverted repeat (TIR) regions (12). The unstable afsA gene (7), which might code for a key enzyme in A-factor biosynthesis, was located 150 kb from the left end (13). Macrorestriction analysis showed that in two afsA-negative mutants, 404-23 and N2, the chromosomal ends were deleted and recombined to generate a circular chromosome (13).
To finally confirm chromosomal circularization in S. griseus, the junctions of the circularized chromosomes in deletion mutants 404-23 and N2 were cloned and sequenced. Comparison of their nucleotide sequences with those of the corresponding deletion ends in the parent strain, 2247, revealed that circularization occurred by nonhomologous recombination without amplification. Based on the structural and genetic properties of the two circularized chromosomes, the instability of Streptomyces chromosomes is discussed in relation to their linear and circular topologies.
MATERIALS AND METHODS
Bacterial strains, plasmid, cosmid library, and media.
S. griseus 2247 and its afsA-negative deletion mutants 404-23 and N2 were described previously (12, 13). Mutant 404-23 shows a bald appearance on solid MB medium (10 g of mannitol, 2 g of peptone, 1 g of yeast extract, 1 g of meat extract, and 5 g of MgSO4 per liter [pH 7.0]), while mutant N2 sporulates as the parent strain, 2247, does. Both mutants grow normally in solid and liquid cultures. pUC19 was used for all of the cloning experiments in this study. The cosmid library was constructed for the 2247 chromosome (12) and aligned at both terminal regions (13). Glucose-meat extract-peptone (GMP) medium contains 10 g of glucose, 4 g of peptone, 2 g of meat extract, 2 g of yeast extract, 5 g of NaCl, and 0.25 g of MgSO4 · 7H2O per liter (pH 7.0).
DNA manipulation, Southern hybridization, and nucleotide sequencing.
S. griseus strains were reciprocally grown in liquid GMP medium in Sakaguchi flasks for 3 days and washed twice with 10.3% sucrose by centrifugation. Total DNA was prepared from the mycelia by the neutral method described by Tanaka et al. (21). Total DNA was digested with restriction enzymes, separated by conventional agarose gel electrophoresis, and transferred to nylon membrane filters by the capillary method. Hybridization was carried out with the digoxigenin system (Boehringer Mannheim) overnight at 70°C in standard buffer according to the supplier’s protocol. After hybridization, washing was done twice for 5 min each in 2× wash solution at room temperature and then twice for 15 min each in 0.1× wash solution at 70°C. Nucleotide sequences were determined by the dideoxy termination method with a Sequenase kit (Toyobo) and [32P]dCTP and with the dye terminator cycle sequencing kit (Amersham Pharmacia Biotech) and a Prism-373 sequencer (PE Applied Biosystems).
RESULTS
Restriction analysis of the chromosomal deletions in mutant 404-23.
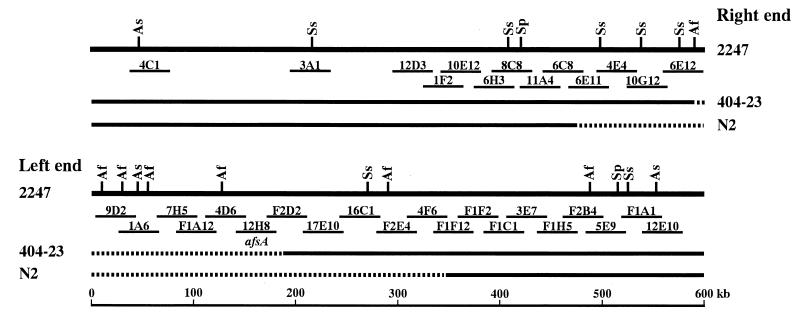
Various mutagenic treatments easily caused deletions at both ends of S. griseus 2247, producing afsA-negative mutants. In the afsA-negative deletion mutant 404-23, the right and left deletion ends of the chromosome were located on cosmids 6E12 and F2D2, respectively (13). The restriction and cosmid maps at both chromosomal ends of S. griseus 2247 and the deleted regions in the two afsA-negative mutants are shown in Fig. 1; one gap remaining at the right side of cosmid F2D2 was filled in by the newly isolated cosmids 17E10 and 16C1. In addition, the circularization of the deleted chromosome in 404-23 was indicated by the appearance of a new 90-kb SspI fusion fragment, which hybridized to both deletion end cosmids 6E12 and F2D2 (13). To confirm a circular topology of the mutant chromosome and to understand the driving force for circularization, the fusion junction in mutant 404-23 and the corresponding right and left deletion ends in strain 2247 were analyzed.
FIG. 1.
Restriction and cosmid maps at both chromosomal ends of S. griseus 2247 and deleted regions in two afsA-negative mutants. All the AflII (Af), AseI (As), SpeI (Sp), and SspI (Ss) sites in the terminal 600-kb regions are shown. Cosmids 17E10 and 16C1, which filled in the gap that remained at the right side of cosmid F2D2, are newly isolated. The deleted regions in afsA-negative mutants are indicated by dashed lines.
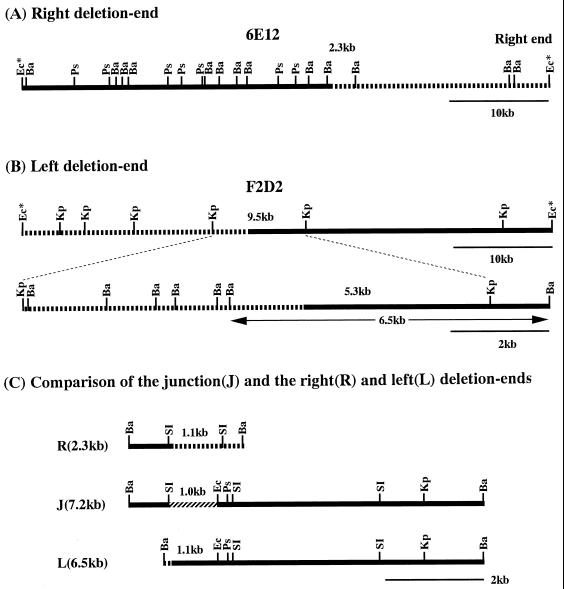
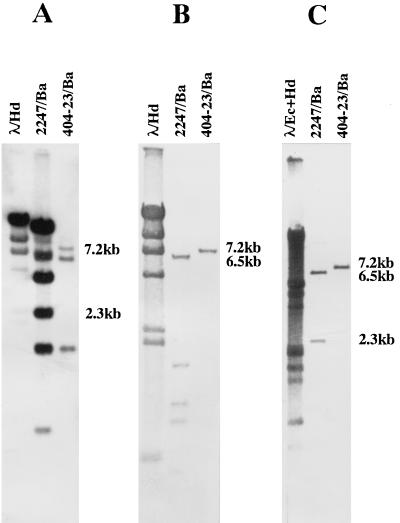
The BamHI restriction map of cosmid 6E12 (Fig. 2A) was constructed for the analysis of the right TIR of the 2247 chromosome (12). As shown in Fig. 3A, Southern hybridization analysis of the BamHI digests of the 2247 and 404-23 DNAs probed by the end PstI fragment of cosmid 6E12 revealed that four BamHI fragments from the right end of the chromosome had disappeared in the latter, and a new 7.2-kb BamHI fragment appeared instead. This result indicated that the chromosomal deletion at the right end progressed into the fourth 2.3-kb BamHI fragment, which in turn generated a 7.2-kb BamHI fusion fragment. The 2.3-kb BamHI fragment was subcloned from cosmid 6E12 for further analysis, and it hybridized to the 7.2-kb BamHI fragment of mutant 404-23 (data not shown).
FIG. 2.
Location of the chromosomal deletion ends and the fusion junction in mutant 404-23. The right and left deletion ends of the 404-23 chromosome were narrowed by stepwise physical mapping of the deletion end cosmids 6E12 (A) and F2D2 (B) and finally located by comparison with the junction fragment (C). The deleted and fused regions are shown by dashed and shaded lines, respectively. Ba, BamHI; Ps, PstI; Ec, EcoRI; Kp, KpnI; SI, SacI; Ec*, EcoRI sites derived from the cosmid vector Supercos1.
FIG. 3.
Southern hybridization analysis of the chromosomal deletion ends and the fusion junction in mutant 404-23. The 2247 and 404-23 DNAs were digested with BamHI, separated by agarose gel electrophoresis, transferred to nylon membranes, and hybridized with the following probes: the end PstI fragment of cosmid 6E12 (A), the 9.5-kb KpnI fragment of cosmid F2D2 (B), and the 7.2-kb BamHI fusion fragment (C).
The deletion at the left end of the chromosome was analyzed as follows. At first, a KpnI restriction map was constructed for the left deletion end cosmid F2D2 (Fig. 2B). By comparing the KpnI digests of the 2247 and 404-23 DNAs probed with F2D2, it was found that the left deletion end was located on the central 9.5-kb KpnI fragment (data not shown). Then, a precise BamHI restriction map was constructed for the 9.5-kb KpnI fragment (Fig. 2B). When the BamHI digests of the 2247 and 404-23 DNAs were probed by the 9.5-kb KpnI fragment, it was found that all of the BamHI fragments in 2247 had disappeared in 404-23 and a new 7.2-kb BamHI fragment appeared instead (Fig. 3B). This result showed that the deletion at the left end progressed into the rightmost 5.3-kb BamHI-KpnI fragment on the 9.5-kb KpnI fragment, which in turn generated a 7.2-kb BamHI fusion fragment. To analyze the fusion junction, a 6.5-kb BamHI fragment, which carries the 5.3-kb BamHI-KpnI deletion end fragment (Fig. 2B), and the 7.2-kb BamHI fusion fragment were cloned from the F2D2 DNA and total DNA of mutant 404-23, respectively. As expected, the cloned 7.2-kb BamHI fusion fragment hybridized to both the 2.3-kb BamHI right deletion end fragment and the 6.5-kb BamHI left deletion end fragment (Fig. 3C).
Nucleotide sequence of the fusion junction of the 404-23 chromosome.
The restriction maps of three cloned BamHI fragments, which carry the right deletion end, the fusion junction, and the left deletion end, were compared (Fig. 2C). As the map shows, the fusion junction was clearly located on the 1.0-kb SacI-EcoRI fragment on the 7.2-kb BamHI fusion fragment. Therefore, this fragment, the corresponding 1.1-kb SacI fragment at the right deletion end, and the 1.1-kb BamHI-EcoRI fragment at the left deletion end were subcloned and subjected to sequence analysis. Since the fusion junction was found to be close to the SacI cloning site on the left side of the fusion clone, the left BamHI-SacI fragment was also subcloned and sequenced.
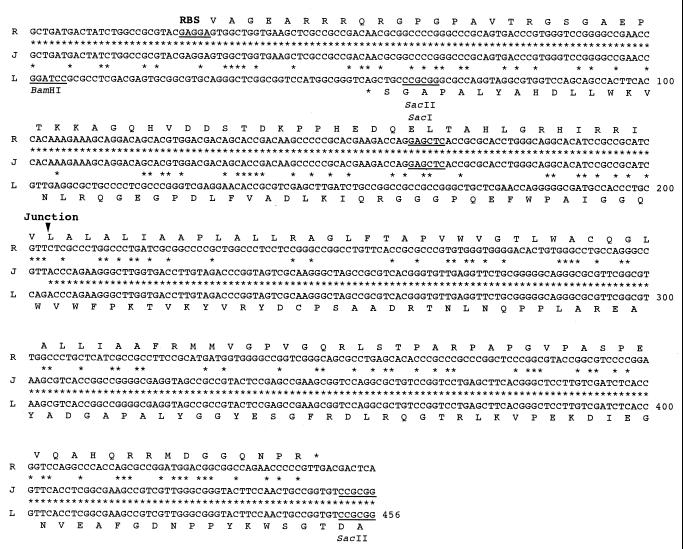
A total of 456 nucleotides (nt) from the three regions are compared in Fig. 4; the fusion junction is clearly located between nt 203 and 204. We anticipated, but did not find, homology between the right and left deletion end sequences, nor did we detect any amplification around the junction. These results suggest that the chromosomal circularization occurred by illegitimate recombination between the right and left deletion ends, which do not have any homology to each other.
FIG. 4.
Nucleotide sequences of the fusion junction (J) in mutant 404-23 and the corresponding right (R) and left (L) deletion ends in the parent strain, 2247. The fusion junction is located between nt 203 and 204. Putative ORFs and the corresponding protein sequences (in the one-letter code) are shown together with an RBS and a stop codon (*). The numbering for the nucleotide sequence is shown on the right.
The location of open reading frames (ORFs) was postulated by the identification of possible initiation and stop codons and a ribosome-binding site (RBS) and by frame analysis of the unique codon usage in Streptomyces DNA (24); namely, G+C content in the ORF increases in the codons in the following order: second, first, and third position, to more than 90% in the third position. Consequently, a putative ORF was found at the right deletion end, which is carried on the reading strand, starting at nt 30 and ending at nt 449 (Fig. 4). A possible RBS (GAGGA) is located immediately upstream of the initiation codon. Another putative ORF was found at the left deletion end, which is carried on the complementary strand and ends at nt 53. A homology search did not find any proteins with significant sequence similarity to either of the proteins predicted from these ORFs. Both proteins were completely destroyed by deletion and circularization of the chromosome in mutant 404-23. These results further support the idea that the chromosomal circularization was caused by illegitimate recombination and that the junction sequence itself did not play a significant role in circularization.
Restriction analysis of the chromosomal deletions in mutant N2.
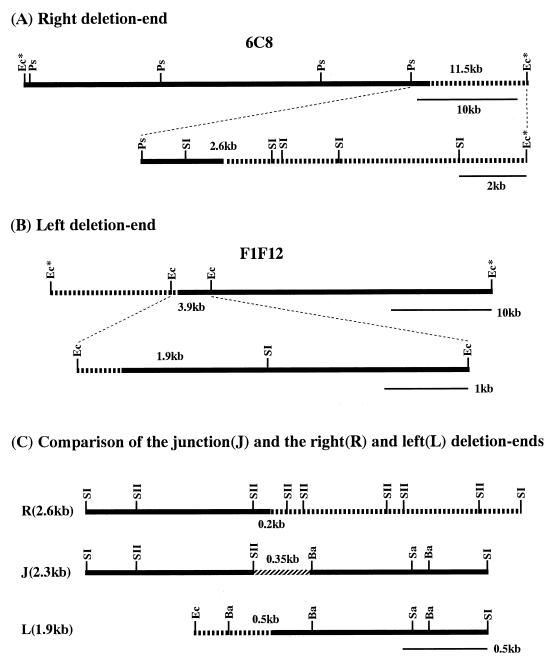
No specific sequence which could induce recombination was found at the fusion junction of the 404-23 chromosome. To find out if this was the case in other mutants, we next analyzed another deletion mutant, N2. The right and left deletion ends of the chromosome in mutant N2 were previously located on cosmids 6C8 and F1F12, respectively (13) (Fig. 1). Chromosomal circularization was also indicated by the positive hybridization of both deletion end cosmids to a newly visualized 200-kb SspI fusion fragment (13).
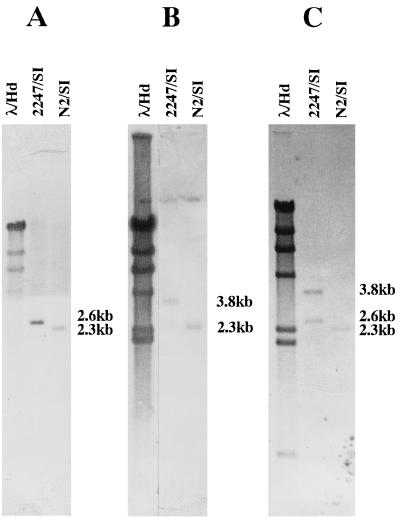
As summarized in Fig. 5A and B, the right deletion end was located by restriction mapping and Southern hybridization first on the rightmost 11.5-kb PstI-EcoRI fragment of cosmid 6C8 and then on the 2.6-kb SacI fragment. Similarly, the left deletion end was located on the central 3.9-kb EcoRI fragment of cosmid F1F12 and then on the 1.9-kb EcoRI-SacI fragment. Both the 2.6-kb SacI fragment and the 1.9-kb EcoRI-SacI fragment were subcloned for sequencing analysis. As shown in Fig. 6A and B, the fragments hybridized to the same 2.3-kb fragment of the N2 DNA digested with SacI, which indicates that the right and left deletion ends were combined to form a fusion fragment in mutant N2, too. This was confirmed by the cloning of the 2.3-kb SacI fusion fragment and its positive hybridization to the 2.6- and 3.8-kb fragments of 2247 DNA digested with SacI (Fig. 6C).
FIG. 5.
Locations of the chromosomal deletion ends and the fusion junction in mutant N2. The right and left deletion ends and the fusion junction were determined by stepwise physical mapping of the deletion end cosmids 6C8 (A) and F1F12 (B) and by comparison with the fusion fragment (C). SII, SacII; Sa, SalI. Other abbreviations are the same as for Fig. 2.
FIG. 6.
Southern hybridization analysis of the chromosomal deletion ends and the fusion junction in mutant N2. The SacI digests of the 2247 and N2 DNAs were hybridized with the following probes: the 2.6-kb SacI fragment of cosmid 6C8 (A), the 1.9-kb EcoRI-SacI fragment of F1F12 (B), and the 2.3-kb SacI fusion fragment (C).
Nucleotide sequence of the fusion junction of the N2 chromosome.
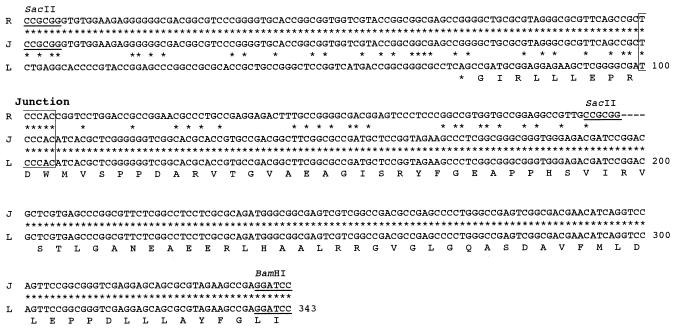
Detailed restriction maps of three fragments, which carry the right deletion end, the fusion junction, and the left deletion end, were constructed and are compared in Fig. 5C. The fusion junction was clearly located on the 0.35-kb SacII-BamHI fragment. At the same time, the right and left deletion ends were located on the 0.2-kb SacII fragment and the 0.5-kb BamHI fragment, respectively. These fragments were subcloned and sequenced.
The nucleotide sequences around the right deletion end, the fusion junction, and the left deletion end are compared in Fig. 7. In contrast to findings for mutant 404-23, an identical 6-bp sequence, TCCCAC (nt 100 to 105), was found at both the right and left deletion ends, which formed the fusion junction in mutant N2. As was true for mutant 404-23, no amplification was found around the junction in mutant N2. A putative ORF was found at the left deletion end, which is carried on the complementary strand and ends at nt 63. The portion of this ORF corresponding to the carboxyl end of the predicted product was disrupted by deletion and circularization of the N2 chromosome. No protein homologous to the protein predicted from this ORF was found in the databases.
FIG. 7.
Nucleotide sequences of the fusion junction (J) in mutant N2 and the right (R) and left (L) deletion ends in the parent strain, 2247. The fusion junction is composed of 6-bp nucleotides with the sequence TCCCAC, shown by a box. A putative ORF is carried on the complementary strand near the left deletion end, and the corresponding protein sequence is shown, in the one-letter code.
DISCUSSION
Genetic instability is common in Streptomyces species. In most cases, large deletions of chromosomal DNA, which are often associated with large-scale DNA amplification, are detected. The reason for this instability has only recently been clarified. The demonstration of the linearity of Streptomyces chromosomes by macrorestriction analysis with PFGE (15) was a landmark and brought new insights into the genetic instability of Streptomyces (10, 23). Since then, three types of chromosomal rearrangements in Streptomyces have been revealed (5). (i) The linear chromosome is deleted at both ends and circularized by recombination of the deletion ends. (ii) Deletion and amplification occur at one chromosomal end and the other end is conserved intact. In this case, the chromosome keeps a linear topology, although the new end created has not yet been isolated or analyzed, due to amplification. (iii) A large deletion occurs inside of the chromosome, which therefore still keeps both ends intact.
In spite of extensive studies of chromosomal rearrangements in Streptomyces, the newly generated junctions have not been analyzed at the nucleotide sequence level, except in a few cases. It has been suggested that the junctions might be formed by the recombination of two direct repeats, such as transposons located at both deletion ends (23). Birch et al. (2) and Piendl et al. (17) reported the nucleotide sequences of the junctions of chromosomal rearrangement in Streptomyces glaucescens and S. lividans, respectively. In both studies, no transposable element was detected but illegitimate recombination events were deduced to have occurred, because no homology and only microhomologies of 2 to 5 bp were detected at the junctions in S. glaucescens and S. lividans, respectively. Neither study used macrorestriction analysis with PFGE to analyze the junctions. However, the junctions seem, in retrospect, to have been formed by an internal deletion in the former (type iii, described above) (5) and at the boundary with amplification in the latter (type ii).
In this study, we have analyzed the fusion junctions of the circularized chromosomes in S. griseus 404-23 and N2 by cloning and sequencing. The results confirmed the circularization of the S. griseus linear chromosome at the nucleotide sequence level. Similar to the cases of S. glaucescens and S. lividans, no homology was detected between the right and left deletion ends in mutant 404-23 and only a 6-bp homology was found in mutant N2. Such microhomologies are numerous in DNA; for example, 46 6-bp direct repeats were found between the sequence at the right deletion end and the corresponding 196-bp sequence at the left deletion end (Fig. 7). Therefore, it is unlikely that the junction sequence, TCCCAC, played a specific role in chromosomal circularization. In addition, no amplification was detected around the junctions in both mutants. So, in these cases too, illegitimate recombination between both deletion ends resulted in the circularization of the chromosome.
Volff and Altenbuchner (22) and Lin and Chen (14) studied the instability of artificially circularized chromosomes of S. lividans, which were constructed by the targeted recombination of two terminal regions of the chromosome with a kanamycin resistance gene cassette. The circularized chromosomes constructed either kept some part of the TIR regions or lost them completely. In either case, the circular chromosomes showed deletions and amplifications similar to or more extensive than the parent linear chromosome. In the artificially circularized chromosomes, two terminal regions are forced into close proximity, whether they keep part of the TIRs or not. This unusual circular structure might affect the stability of the chromosome. Therefore, the artificially circularized chromosomes tend to be deleted further, to the points where they regain stability.
Fischer et al. (5) studied mutants of S. ambofaciens which carried a naturally circularized chromosome. The mutants also exhibited genetic instability at higher rates than the wild-type strain, even though the deletion sizes were several hundred kilobases long at both ends and the terminal regions were completely removed. On the other hand, the circularized chromosomes in strains 404-23 and N2 and other mutants in our study were stably maintained. The deletion sizes in these S. griseus mutants were much smaller than those in the S. ambofaciens mutants (13). Therefore, the genetic instability of circularized chromosomes could not be explained only by the effect of remaining terminal regions. During the analysis of the S. griseus 2247 mutants, we did not detect any extensive amplification, which is common in other Streptomyces species in addition to deletion. One possibility is that the genetic instability in S. griseus is less intense than in other Streptomyces species, whether it carries a linear or circular chromosome.
Fischer et al. (5) suggested the absence of a terminator for bidirectional replication from oriC as a reason for the instability of circular chromosomes. However, the deletion sizes and the junction points were totally different in the two S. griseus mutants studied, 404-23 and N2. Therefore, it seems unlikely that the same terminator was generated in the mutants. Considering all the data, it could at least be said that the chromosomal circularization in S. griseus occurred to stabilize unstable deleted linear chromosomes. They escaped from lethal deletions by circularization, which was achieved by incidental illegitimate recombination between two deletion ends.
All the Streptomyces species hitherto analyzed carry a linear chromosome, in spite of its instability. Therefore, a linear chromosome should have some advantages over a circular chromosome. Qin and Cohen (18) suggested that in replication from the end of the linear plasmid pSLA2, the tertiary foldback structure formed by the single-strand overhang at the 3′ end may play an important role. Similar tertiary structures may also be formed at the ends of linear chromosomes and plasmids from many Streptomyces species (8). We are now studying a deletion mutant of S. griseus 2247 whose chromosome has lost both telomeres but still keeps a linear topology. Analysis of its new ends will give some hints of the essential structure and function of Streptomyces telomeres.
ACKNOWLEDGMENT
This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Altenbuchner J, Cullum J. Structure of an amplifiable DNA sequence in Streptomyces lividans 66. Mol Gen Genet. 1985;201:192–197. doi: 10.1007/BF00425659. [DOI] [PubMed] [Google Scholar]
- 2.Birch A, Hausler A, Rottener C, Hutter R. Chromosomal deletion and rearrangement in Streptomyces glaucescens. J Bacteriol. 1991;173:3531–3538. doi: 10.1128/jb.173.11.3531-3538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chater K F, Hopwood D A. Streptomyces. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 83–99. [Google Scholar]
- 4.Dyson P, Schrempf H. Genetic instability and DNA amplification in Streptomyces lividans 66. J Bacteriol. 1987;169:4796–4803. doi: 10.1128/jb.169.10.4796-4803.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer G, Decaris B, Leblond P. Occurrence of deletions, associated with genetic instability in Streptomyces ambofaciens, is independent of the linearity of the chromosomal DNA. J Bacteriol. 1997;179:4553–4558. doi: 10.1128/jb.179.14.4553-4558.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horinouchi S, Beppu T. Autoregulatory factors and communication in actinomycetes. Annu Rev Microbiol. 1992;46:377–398. doi: 10.1146/annurev.mi.46.100192.002113. [DOI] [PubMed] [Google Scholar]
- 7.Horinouchi S, Suzuki H, Nishiyama M, Beppu T. Nucleotide sequence and transcriptional analysis of the Streptomyces griseus gene (afsA) responsible for A-factor biosynthesis. J Bacteriol. 1989;171:1206–1210. doi: 10.1128/jb.171.2.1206-1210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C-H, Lin Y-S, Yang Y-L, Huang S-W, Chen C W. The telomeres of Streptomyces chromosomes contain conserved palindromic sequences with potential to form complex secondary structures. Mol Microbiol. 1998;28:905–916. doi: 10.1046/j.1365-2958.1998.00856.x. [DOI] [PubMed] [Google Scholar]
- 9.Khokhlov A S, Anisova L N, Tovarova I I, Kleiner E M, Kovalenko I V, Krasilnikova O I, Kornitskaya E Y, Pliner S A. Effect of A-factor on the growth of asporogenous mutants of Streptomyces griseus, not producing this factor. Z Allg Mikrobiol. 1973;13:647–655. doi: 10.1002/jobm.3630130803. [DOI] [PubMed] [Google Scholar]
- 10.Leblond P, Decaris B. New insight into the genetic instability of Streptomyces. FEMS Microbiol Lett. 1994;123:225–232. doi: 10.1111/j.1574-6968.1994.tb07229.x. [DOI] [PubMed] [Google Scholar]
- 11.Leblond P, Fischer G, Francou F-X, Berger F, Guerineau M, Decaris B. The unstable region of Streptomyces ambofaciens includes 210-kb terminal inverted repeats flanking the extremities of the linear chromosomal DNA. Mol Microbiol. 1996;19:261–271. doi: 10.1046/j.1365-2958.1996.366894.x. [DOI] [PubMed] [Google Scholar]
- 12.Lezhava A, Mizukami T, Kajitani T, Kameoka D, Redenbach M, Shinkawa H, Nimi O, Kinashi H. Physical map of the linear chromosome of Streptomyces griseus. J Bacteriol. 1995;177:6492–6498. doi: 10.1128/jb.177.22.6492-6498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lezhava A, Kameoka D, Sugino H, Goshi K, Shinkawa H, Nimi O, Horinouchi S, Beppu T, Kinashi H. Chromosomal deletions in Streptomyces griseus that remove the afsA locus. Mol Gen Genet. 1997;253:478–483. doi: 10.1007/s004380050346. [DOI] [PubMed] [Google Scholar]
- 14.Lin Y-S, Chen C W. Instability of artificially circularized chromosomes of Streptomyces lividans. Mol Microbiol. 1997;26:709–719. doi: 10.1046/j.1365-2958.1997.5991975.x. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y-S, Kieser H M, Hopwood D A, Chen C W. The chromosomal DNA of Streptomyces lividans 66 is linear. Mol Microbiol. 1993;10:923–933. doi: 10.1111/j.1365-2958.1993.tb00964.x. [DOI] [PubMed] [Google Scholar]
- 16.Pandza K, Pfalzer G, Cullum J, Hranueli D. Physical mapping shows that the unstable oxytetracycline gene cluster of Streptomyces rimosus lies close to one end of the linear chromosome. Microbiology. 1997;143:1493–1501. doi: 10.1099/00221287-143-5-1493. [DOI] [PubMed] [Google Scholar]
- 17.Piendl W, Kochl S, Flett F, Cullum J. Analysis of large deletions and characterization of the deletion endpoints associated with amplifiable DNA region in Streptomyces lividans. In: Baumberg S, Krugel H, Noack D, editors. Genetics and product formation in Streptomyces. New York, N.Y: Plenum Press; 1991. pp. 273–287. [Google Scholar]
- 18.Qin Z, Cohen S N. Replication at the telomeres of the Streptomyces linear plasmid pSLA2. Mol Microbiol. 1998;28:893–903. doi: 10.1046/j.1365-2958.1998.00838.x. [DOI] [PubMed] [Google Scholar]
- 19.Redenbach M, Kieser H M, Denapaite D, Eichner A, Cullum J, Kinashi H, Hopwood D A. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolor A3(2) chromosome. Mol Microbiol. 1996;21:77–96. doi: 10.1046/j.1365-2958.1996.6191336.x. [DOI] [PubMed] [Google Scholar]
- 20.Redenbach M, Flett F, Piendl W, Glocker I, Rauland U, Wafzig O, Kliem R, Leblond P, Cullum J. The Streptomyces lividans 66 chromosome contains a 1 MB deletogenic region flanked by two amplifiable regions. Mol Gen Genet. 1993;241:255–262. doi: 10.1007/BF00284676. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka T, Kuroda M, Sakaguchi K. Isolation and characterization of four plasmids from Bacillus subtilis. J Bacteriol. 1977;129:1487–1494. doi: 10.1128/jb.129.3.1487-1494.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volff J-N, Altenbuchner J. Artificial circularization of the chromosome with concomitant deletion of its inverted repeats enhances genetic instability and genome rearrangement in Streptomyces lividans. Mol Gen Genet. 1997;253:753–760. doi: 10.1007/s004380050380. [DOI] [PubMed] [Google Scholar]
- 23.Volff J-N, Altenbuchner J. Genetic instability of the Streptomyces chromosome. Mol Microbiol. 1998;27:239–246. doi: 10.1046/j.1365-2958.1998.00652.x. [DOI] [PubMed] [Google Scholar]
- 24.Wright F, Bibb M J. Codon usage in the G+C-rich Streptomyces genome. Gene. 1992;113:55–65. doi: 10.1016/0378-1119(92)90669-g. [DOI] [PubMed] [Google Scholar]