Abstract
A consensus-directed search for ςB promoters was used to locate potential candidates for new ςB-dependent genes in Bacillus subtilis. Screening of those candidates by oligonucleotide hybridizations with total RNA from exponentially growing or ethanol-stressed cells of the wild type as well as a sigB mutant revealed 22 genes that required ςB for induction by ethanol. Although almost 50% of the proteins encoded by the newly discovered ςB-dependent stress genes seem to be membrane localized, biochemical functions have so far not been defined for any of the gene products. Allocation of the genes to the ςB-dependent stress regulon may indicate a potential function in the establishment of a multiple stress resistance. AldY and YhdF show similarities to NAD(P)-dependent dehydrogenases and YdbP to thioredoxins, supporting our suggestion that ςB-dependent proteins may be involved in the maintenance of the intracellular redox balance after stress.
In Bacillus subtilis, the alternative sigma factor ςB tightly controls a large stationary-phase and stress regulon (7–10, 16, 17, 31). The functional characterization of members of the ςB regulon led to the assumption that the proteins encoded are involved in the protection of DNA, membranes, and proteins against oxidative damage, which might represent an important component within the complex stress response (6, 14). Moreover, general stress proteins appear to contribute to survival of extreme environmental conditions such as severe heat or osmotic stress, repeated freezing and thawing, as well as acid or alkaline shock of starving B. subtilis (15, 32). In summary, the expression of the ςB-dependent general stress regulon is expected to provide an unspecific, multiple and prospective stress resistance to nongrowing B. subtilis cells in anticipation of future stress (for a review, see reference 17).
The discovery and characterization of new ςB-dependent genes will certainly improve our understanding of the physiological role of the entire ςB regulon in B. subtilis. So far, members of the ςB regulon have been defined mainly on the basis of transposon mutagenesis or identification of protein spots from two-dimensional protein gels (5, 10, 11, 31). In this study, we used the combination of a consensus promoter-based search for new ςB targets and an oligonucleotide hybridization to detect new members of the general stress regulon of B. subtilis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The B. subtilis wild-type strain 168 (3) and its isogenic sigB mutant ML6 (22) were cultivated in a shaking water bath at 37°C in a minimal medium with glucose as the carbon source previously described (29). Ethanol stress was imposed by adding ethanol to exponentially growing cells to a final concentration of 4% (vol/vol).
Analysis of transcription.
Total RNA of both B. subtilis strains was isolated by the acid phenol method of Majumdar et al. (25), with modifications as previously described (31). Decreasing amounts of total RNA were transferred onto a positively charged nylon membrane by slot blotting. Hybridizations at 50°C with digoxigenin-end-labeled oligonucleotides specific for the various genes (Table 1) and detections were performed as instructed by the manufacturer (Boehringer Mannheim). For 3′-end labeling, 100 pmol of each oligonucleotide was incubated for 1 h in a 20-μl reaction mixture containing 12.5 nmol of digoxigenin-11-ddUTP, 2.5 mM cobalt chloride, 1× reaction buffer and 12.5 U of terminal transferase. The whole volume was used for the hybridization. Primer extensions were performed with radiolabeled primers as previously described (33) (Table 1). A DNA-sequencing ladder was generated with the same primers, using PCR products as a template (Table 1).
TABLE 1.
Oligonucleotides used in this study
| Gene-specific oligonucleotide or primer | Sequence (5′→3′) |
|---|---|
| Positive control oligonucleotides | |
| ctc | CATGTCCTGAAGTACGGATATTCC |
| gspA | TTACCTCTCTCTCCTGATCC |
| trxA | CAAGGTCCGCACCAAGGAGC |
| Oligonucleotides specific for tested genes | |
| aldY | TTCCGTCGTGCTCTTGGCCCACTC |
| lctE | CGCAAATGCATAACTGCTTCC |
| yabJ | CCGCAAACTGTTCCATATCCGCG |
| yacL | CAGCTTCTTGTCTTGAACCTCATG |
| ycnH | TGAGGAATCGTTTTGATCTCCTCG |
| ydaS | GGCGAGGCTCGGTCCCCATGTGCC |
| ydaT | CCCATTCCTTCGCTTTGCTTGTCGC |
| ydbP | ATATTCATGCGTGTGCAGTCTGGG |
| ydhK | AGAGATAACATCAGAATTCCCAGTGC |
| yfhK | ATTTGAGCCCAATCGGCATTTACG |
| yflA | TAGCGGCGGACCCCACAGCCAATC |
| yhaR | GCGTGATGGGCATCAGGCCGATTC |
| ydhF | TGGAGATATCAGCCCCCTCTTTAGA |
| yjbC | CCCTCTTGCATCCTTAGACACATAC |
| yjgB | GGCATTTCCCCTTTATAGGCGGTG |
| ykgA | GTGCTGGTCGTTAGCGGTTTTTAC |
| yotK | CATCTTGTTTAACAGTGTTTGAATTGAATG |
| yoxA | GCCATACAATGTTGGTGTGTCGTG |
| yoxC | CTTGTTTTGGATCACGGTAACTCC |
| ypuB | CTTAGCACCCAGTTTAACTTTTCTTG |
| yqhA | TACAAACTTCCAGCCATTGTTGCG |
| yqhQ | TCCGTTCTTCTGATGGCTGTAACG |
| yqhZ | GCTTCCAATTCACCAGATGCTTGGAG |
| yqiS | CACTTCCTCATCCTCAGCATGAGC |
| yqxL | TCTTGCCTTACTTCTTTCTTGAGG |
| yrvD | CGGCCATTCTGATCTTCCAAACAGG |
| ysdB | TTAGGATTCGCGACATATTTGACC |
| yvrE | CTGTCATCCCGAAGATGATACAGG |
| yxkO | GGCAATGGCCCGGTACGTCTCTTC |
| yycD | TGTCGGCGCTACATTCTCCACCTC |
| Primers for primer extension and generation of PCR products | |
| yacL-PE | AATGAAGAACGCCTGAACTATTCG |
| yhdF-PE | TTCCTCGATAATCCTCGTCCTCTG |
| yjbC-PE | TCAATTGGAAAGTACTCGCTAAGC |
| yacL-forward | GGACAGAAGGGCATCTGATC |
| yacL-reverse | TCTTCTCCCTTTACAGCGCC |
| yhdF-forward | GTGGTGCGCCCATGTACTGG |
| yhdF-reverse | CACCACACAAAAAACCAGCTC |
| yjbC-forward | GCAGGAAGTTATTCCCGAGC |
| yjbC-reverse | CATCGTTGTTTTCCAAGACCTC |
General methods.
The BSOrf homology search tool of the database of the B. subtilis genome sequencing project was used for deriving of the primers. Database searches were performed with the Blast program (2).
RESULTS AND DISCUSSION
Knowledge of the sequence of the entire B. subtilis genome (24) provides an excellent basis for a comprehensive analysis of gene expression by using global approaches such as two-dimensional protein electrophoresis (proteome analysis) (5), chip technology (transcriptome analysis) (13), and consensus sequence-based searches for members of individual regulons (20). In this report, we used the genome sequence for the identification of new ςB-dependent genes.
After an initial manual evaluation, the whole genome was searched with the sequence pattern DGWKTNDN12–15GGRWAW (D = A, G, T; W = A, T; K = G, T; R = A, G; N = A, C, G, T). Only targets deviating not more than three nucleotides from the consensus of ςB-dependent promoters GTTTWWN12–15GGGWAW and lying within 400 bp upstream of predicted open reading frames were considered for further analyses. The rather large deviation from the consensus was intentionally permitted so that we could identify even weak ςB-dependent promoters.
To demonstrate that the presence of a putative ςB promoter indeed conferred ςB-dependent stress induction, total RNA from isogenic wild-type and sigB mutant cells was isolated before (control) and after ethanol stress (4% [vol/vol]) and hybridized with a digoxigenin-labeled oligonucleotide designed against the open reading frame downstream of the potential ςB target.
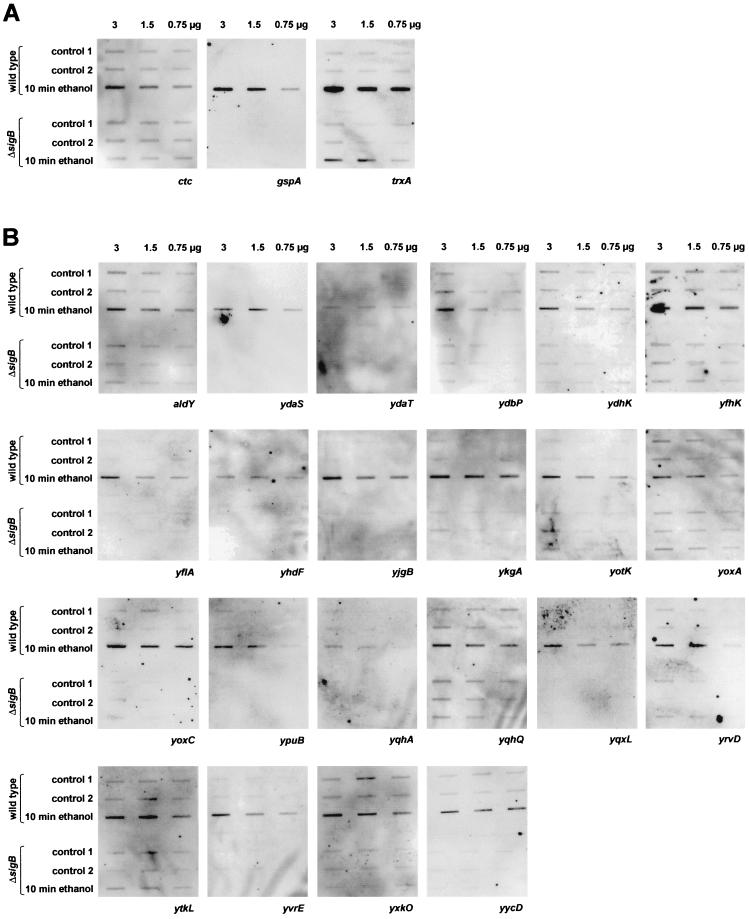
Prior to the screening, oligonucleotide probes against known ςB-dependent genes were used to prove the specificity of the hybridization assay. Rigorously ςB-dependent genes such as gspA displayed a signal only after treatment of the wild type with ethanol; no signal was observed with RNA from growing cells or from the sigB mutant as the template (Fig. 1A; Table 2, group A) (4). ctc illustrates the pattern for genes the transcription of which is driven by the vegetative sigma factor ςA in addition to ςB (18). Basal-level expression was easily detected for such genes in the wild type and the sigB mutant prior to stress, whereas induction by ethanol stress absolutely required ςB (Fig. 1A; Table 2, group A). Genes such as trxA, which are subject to a double or multiple control, may retain induction even in the sigB mutant albeit at a reduced level (Fig. 1A; Table 2, group A) (28).
FIG. 1.
Screening of potential targets for ςB-dependent transcription by slot blot hybridization with digoxigenin-labeled oligonucleotides specific for the corresponding genes. The lanes contain decreasing amounts (3, 1.5, and 0.75 μg) of total RNA isolated from wild-type bacteria and sigB mutant cells before (controls 1 and 2, exponential growth) and 10 min after the imposition of ethanol stress. (A) Hybridization pattern of known ςB-dependent genes. Whereas the stress induction of ctc and gspA strictly requires ςB, induction of trxA can occur at both ςB-dependent and ςB-independent promoters. (B) New ςB-dependent genes requiring ςB for induction by ethanol stress. (C) Genes displaying induction by ethanol stress even in the absence of ςB. (D) Examples of target genes that lack induction by ethanol stress despite displaying a putative ςB promoter.
TABLE 2.
Defined ςB promoters of the control genes and promoters of the presumable new ςB-dependent genes found in this study
| Group and promoter sequencea | Gene | Protein
|
Presumed function and/or similarity | Probably cotranscribed gene(s) | |
|---|---|---|---|---|---|
| Molecular mass (kDa) | pI | ||||
| A (ςB-dependent genes [positive controls]) | |||||
| CGAGGTTTAAATCCTTATCGTTAT-GGGTATTGTTTGTAATAG-N33-ATG | ctc | 22.0 | 4.24 | Belongs to the L25P family of ribosomal proteins | |
| ACGTGTTTATTTTTTTGAAAAA---GGGTATGTAACTTG-TAC-N23-ATG | gspA | 33.5 | 5.19 | Similar to lipopolysaccharide-1,3-galactosyltransferases and to lipopolysaccharide-1,2-glucosyltransferases | |
| TCAGGTTTTAAAACAGCTCCGGCA-GGGCATGGTAAAGTACAT-N241-ATG | trxA | 11.39 | 4.34 | Thioredoxin | |
| B (genes controlled by ςB) | |||||
| ATTCGTTTACATATTGGCTTCAG--CGGAAATAGAAGAAGACA-N25-ATG | aldY | 52.8 | 5.07 | Probable aldehyde dehydrogenase, contains an aldehyde dehydrogenase signature (glutamic acid active site) | |
| AAGTGTTTCGAAATGATCAGGAGC-GGATATTGATTGGTAAAT-N21-ATG | ydaS | 8.64 | 12.22 | Membrane protein, unknown function | |
| ATGCGTTTTTATTTTTCACCTGC--GGGTACCATTTTTATAAA-N19-ATG | ydaT | 16.5 | 6.38 | Unknown | ydaS |
| GTTTGATTACAGCCAGAGCTGTCTGCAGGGAAACATTATGTTCTG-N193-ATG | ydbP | 12.4 | 4.42 | Similar to thioredoxins of A. fulgidus and S. cerevisiae | |
| TTATGTTTGGCTTTGCAAACAAAG-GGGAATAGGACAAACTGC-N76-TTG | ydhK | 22.5 | 7.81 | Protein with 1 transmembrane domain, unknown function | |
| ACATGTTTCACCAGCCTGTCAATCAGGGAATACCACTTATATC-N26-ATG | yfhK | 18.7 | 10.37 | Protein probably with signal sequence, similar to proteins with unknown function | |
| AAAGGTTTATGTTTTTCCATCTAT-GGGAAATGATTCATAAAC-N17-ATG | yflA | 50.6 | 10.4 | Integral membrane protein, similar to amino acid carrier protein | |
| TGGCGTTTATTCATTTCTGTCGTG-TGGTAACGTTCAGTATCA-N24-GTG | yhdF | 31.4 | 5.9 | Similar to glucose-1-dehydrogenase, belongs to the short-chain dehydrogenases/reductases family | |
| ACATGTTTCGTTGCAAAAACACG--GGGAAACGGAATGGTAGA-N19-ATG | yjgB | 20.8 | 6.68 | Membrane lipoprotein, unknown function | |
| AATGGTTTAATGATTTTCATGATGAGGGAATAATAAATGTATT-N25-ATG | ykgA | 29.6 | 4.88 | Function unknown, similar to streptococcal acid lipoprotein | ykgB |
| TCTAGTTTCTCTTTTTAAAAGAGTAGGGTATTGCAAACAACAG-N58-ATT | yotK | 7.2 | 7.69 | Unknown | |
| AACGGTGTTTTTTTATTTGATAG--GGGAAAATATAAAATGGA-N64-ATG | yoxA | 37.2 | 6.00 | Similar to hypothetical proteins of B. subtilis | pbp |
| TTCTGATTAAAAAAACGGATACA--GGGTAATGACATAAGAAA-N12-ATG | yoxC | 11.39 | 10.37 | Protein probably with signal or transmembrane sequence, similar to the general stress protein YtxG | yoxB, yoaA |
| TGCTGATTATACAAAAAGTGGATT-GGGAATGATAAAAGAACA-N21-ATG | ypuB | 7.23 | 4.24 | Protein with 1 transmembrane domain, unknown function | ypuC |
| TTCGGTTTTTCACCTGTCCAGAAACTGGGCTAGCTGGATTCGAAC-N136-ATG | yqhA | 31.8 | 5.17 | Membrane protein with 1 transmembrane domain, similar to RsbR of B. subtilis | |
| CTGCGAGTAAAATTTGAAAATAAC-GGGTATAATGCATGTAGG-N118-ATG | yqhQ | 36.0 | 9.71 | Integral membrane protein with unknown function | yqhP |
| ACTGGTTTAGTGACGCGGTTATT--GGGCAATTAAAGAATAAA-N25-ATG | yqxL | 37.7 | 10.00 | Membrane protein, similar to divalent cation transport proteins | |
| CCATGTTTAAAACCAGTCTTGATT-GGGAAAGTTACCTCAATA-N21-ATG | yrvD | 12.2 | 9.16 | Membrane protein with unknown function | yrvE, apt |
| ATAAGTTTTTCAGCTTTTTAAAAA-GGGAAAATAAAAAAAACA-N24-ATG | ytkL | 10.9 | 6.41 | Similar to proteins with unknown function | |
| AGTGGTTTGGACACCTCTTTGCC--GGGAATAACAATATATAG-N24-ATG | yvrE | 33.2 | 4.65 | Similar to the eukaryotic SMP-30/CGR1 protein family | |
| TTTTGTTTGAAAAAGAAAAGGGAC-AGGAAAAATAGGAAAAGA-N19-ATG | yxkO | 29.9 | 5.8 | Membrane protein with unknown function, similar to hypothetical family of uncharacterized proteins (UPF0031) | |
| GATCGTTTCGGACAGTAACAAGGC-GGGAAAAATGCAATAAAA-N19-ATG | yycD | 7.4 | 4.81 | Unknown | |
| C (complex-regulated genes, ςB could be involved) | |||||
| AATTGAATTGACCGGATCTTGGCC-TGGAAAACCAATGACTAA-N363-ATG | lctE | 34.9 | 5.64 | l-Lactate dehydrogenase | lctP |
| TTTCGGTTAAAACCTTATGAATAC-GGGTATATTAATGTTGGT-N74-ATG | yacL | 40.8 | 5.01 | Membrane protein, similar to hypothetical proteins | yacM, yacN, gltX |
| AACGGATTACTTTTGCTGACAGC--GGGAATTAACGGTAATAT-N153-ATG | ycnH | 50.2 | 4.8 | Similar to the succinate-semialdehyde dehydrogenase GabD of E. coli, contains an aldehyde dehydrogenase signature (cysteine active site) | |
| GGCTGTTTAAACAAGAAGAAAATG-GGGTATATCTAAAAGTAT-N30-ATG | yjbC | 23.1 | 5.22 | Unknown | yjbD |
| TTCGGAATTTATATTACAAAAT---GGATAAATATGGCCTTGC-N143-ATG | yqiS | 31.7 | 8.18 | Protein with 1 transmembrane domain, similar to phosphate butyryltransferases, belongs to the phosphate acetyltransferase and butyryltransferase family | yqiT, yqiV, yqiW, bfmBAA, bfmBAB, bfmBB |
| ATACGACTATTTCACTTGAAAATC-GGGTATATGTTTTTACAG-N83-ATG | ysdB | 15.6 | 9.85 | Membrane protein with unknown function | |
| D (noninducible genes preceded by a putative ςB promoter sequence) | |||||
| ATGAGTTTAACGCAAATGTGGC---GGGAATCGGCGTCTTAGT-N157-ATG | yabJ | 13.6 | 5.24 | Unknown, similar to proteins with unknown function | |
| AAAGGTTTATTTGGGGATGGCG---GGGAATGTATAGGAGAAA-N34-ATG | yhaR | 29.5 | 6.27 | Protein with 1 transmembrane domain, similar to 3-hydroxybutyryl-coenzyme A-dehydratase | yhaQ, yhaP |
| AGGTGTGTGAAAATATTCGGTAATAGGGTAAAAAAACCTTGAT-N86-ATG | yqhZ | 14.8 | 4.8 | Unknown, similar to NusB (transcription termination) | folD |
Boldface sequences represent, from left to right, −35 region, −10 region, and transcriptional start site (+1). Start codons are indicated on the far right.
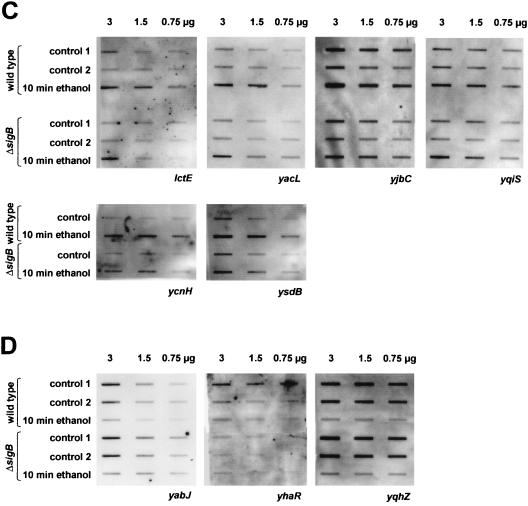
Of the 28 genes induced by ethanol stress, 22 were induced only in the wild type and required ςB for induction (Fig. 1B). The number of new ςB-dependent genes may even exceed 22 since some of the promoters were located in front of operons containing probably one or two additional genes (e.g., ykgA/ykgB, ypuB/ypuC, yqhQ/yqhP, and yoxC/yoxB/yoaA). Although these oligonucleotide hybridizations clearly establish the ςB dependency of the induction, they do not provide information about the precise location of the ςB-dependent promoter. Therefore, primer extension experiments were performed for selected genes in order to map the 5′ ends of the corresponding transcripts and to ascertain that transcription initiated downstream of the potential ςB-dependent promoters after the imposition of ethanol stress. Figure 2A displays the results obtained for yhdF as a representative example. The 5′ ends of yhdF (Fig. 2A) were barely detectable with RNA from exponentially growing bacteria, but the intensity of the signal dramatically increased after stress. In agreement with the ςB dependency, the signal was not observed with RNA isolated from a sigB mutant.
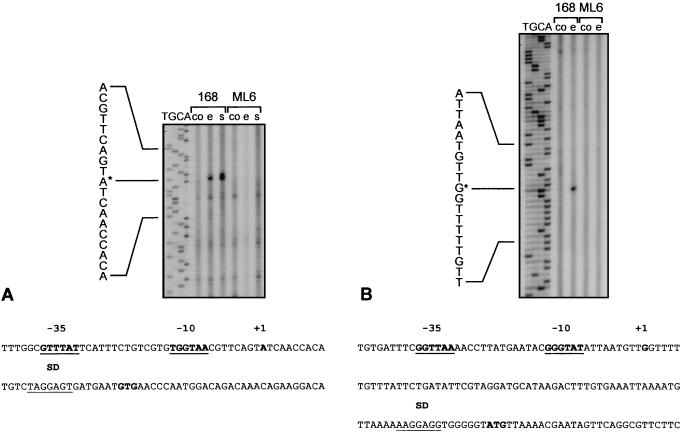
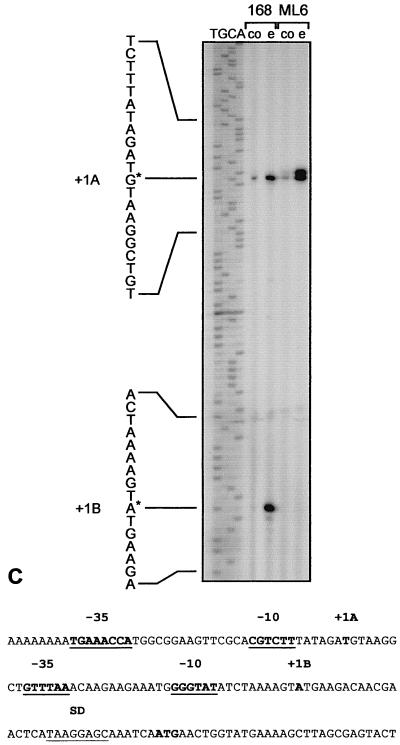
FIG. 2.
Mapping of the 5′ ends of yhdF (A), yacL (B), and yjbC (C) during exponential growth (co), after exposure to ethanol stress (e) and salt stress (s), by primer extension analyses with RNA from wild-type (168) and sigB mutant (ML6) cells. The transcriptional start sites are marked with asterisks. The −35 and −10 regions (underlined), the 5′ ends of the messages, and the translational start sites are in boldface; putative ςW-recognition sequences in front of yjbC are underlined.
The remaining six genes were induced by ethanol in the wild type as well as in the sigB mutant, indicating a more complex control (Fig. 1C). However, induction seemed to be more pronounced in the wild type than in the sigB mutant, implying an involvement of ςB (Fig. 1C). Evidence for a role of ςB in ethanol induction was obtained by primer extension experiments, two of which are displayed in Fig. 2B and C. One 5′ end of the yacL mRNA was mapped to a site which is preceded by a putative ςB-dependent promoter. The signal was exclusively detected after ethanol stress in the wild type and was missing with RNA from the sigB mutant as template (Fig. 2B). A second ςB-independent start site was mapped further downstream (data not shown).
Using a yjbC-specific primer, we mapped two 5′ ends of the yjbC mRNA. The intensity of both reverse transcripts increased after ethanol treatment (Fig. 2C). The sequence preceding the downstream signal resembled a typical ςB-dependent promoter, and this start site was not used in the sigB mutant. The promoter sequence located in front of the upstream start site resembled ςW-dependent promoters (19), and the intensity of the signal increased even in a sigB mutant after ethanol stress (Fig. 2C). ysdB, a second gene displaying partially ςB-dependent ethanol induction, also possesses a ςW-dependent promoter (21) and moreover contains fairly well conserved recognition sequences for ςB (Table 2,C). ςW is a new extracytoplasmic function sigma factor recently described by Huang et al. (19–21). The authors suggest that ςW activates a large stationary-phase regulon that functions in detoxification or production of antimicrobial compounds. Our data indicate that in addition to entry into stationary phase, ethanol stress induces transcription at ςW-dependent promoters (Fig. 1C and 2C) and that genes such as yjbC seem to be subject to a control by ςB and ςW.
The new presumably ςB-dependent promoters as well as the defined promoters of the control genes are summarized in Table 2. An alignment of all the ςB-dependent promoters currently available yields consensus sequences GTTTaa and GGG(A/T)A(A/T) for the −35 and −10 regions, respectively, which are separated by 13 to 15 nucleotides. Capital letters indicate bases which are conserved in more than 80% of the 58 promoters analyzed. Residues −36 (G), −33 (T), −15 (G), and −12 (A) seem to be particularly important since they are absolutely conserved in all 58 promoters. All of these residues exception the T at position −33 have previously been recognized as being critical for ςB promoter activity (27, 30). Less than 10% of all known ςB-dependent promoters deviate in more than two residues from this consensus sequence.
For most of the proteins encoded by the new ςB-dependent genes, biochemical functions have not been determined although similarities to sequences found in databases suggested putative functions for some of them. aldY and yhdF encode gene products with similarities to aldehyde and glucose dehydrogenases, respectively. Among the gene products which are induced by ethanol stress in the sigB mutant are two more dehydrogenases: LctE, an l-lactate dehydrogenase, and YcnH, similar to succinate-semialdehyde dehydrogenases. These data appear to confirm our earlier suggestion that several general stress proteins with similarities to NAD- or NAD(P)-dependent dehydrogenases might be involved in the maintenance of the redox balance during stress (17). YdbP, which needed ςB for induction, is similar to thioredoxins of Archaeoglobus fulgidus and Saccharomyces cerevisiae (44% identity in a 85-amino-acid overlap and 42% identity in a 75-amino-acid overlap, respectively) and could therefore also be involved in the protection against oxidative stress.
YqhA is highly similar to modulators of sigma factor activity such as RsbR and IspU of B. subtilis (1, 26). RsbR functions as a positive regulator of ςB activity by interaction with the antagonist protein RsbS (1). YqhA and RsbR share a high degree of identity in their C-terminal portion, which also comprises a conserved phosphorylation site (1). Consequently, it would be attractive to analyze whether YqhA belongs to the family of antagonist proteins in the partner-switching regulatory mechanism and might be involved in conveying environmental signals in the ςB signal transduction network.
Gaidenko and Price pointed out that ςB-dependent stress proteins may be involved in the maintenance of the cell envelope integrity during stress (15). Supporting their hypothesis, many new ςB-dependent genes described in this study seem to code for integral membrane proteins. The high proportion of membrane proteins found can partially be attributed to the fact that two-dimensional protein electrophoresis as one of the two approaches of identification of stress genes failed to detect this class of proteins due to their alkaline isoelectric point and solubilization problems (5). Induction of all of those genes by the stress sigma factor ςB provides only a first hint for their involvement in stress protection. Knockout mutations in the genes need to be analyzed for resistance to oxidative, acid, alkaline, heat or salt stress in order to gather more information on their function.
Another interesting problem raised in this study is the question of why genes with presumable ςB promoters, which are conserved in all the positions known to be critical for promoter recognition by ςB (27, 30), still lacked stress induction (Fig. 1D). A potential reason for such a failure of induction can be the presence of operator elements blocked by repressor molecules. Recently, we obtained evidence that CtsR, a global repressor of class III heat stress genes (12, 23), prevents the ςB-dependent induction of the clpC operon by glucose starvation (23). A detailed transcriptional analysis is needed to determine whether the genes in question are controlled by ςB only under special circumstances or not at all.
Although the number of ςB-dependent genes is now more than 80, the use of sophisticated approaches such as DNA chip technology and detailed analysis of genes subject to complex transcriptional regulation by several networks will help to define still undiscovered members of this important regulon.
ACKNOWLEDGMENTS
We thank M. Messenger (University of Western Ontario, London, Ontario, Canada) for help at the beginning of this study and A. Harang for technical assistance.
This work was supported by grants from the Deutsche Forschungsgemeinschaft, the Fonds der Chemischen Industrie, and the EU Biotechnology Programme (BIO 4-CT95-0278) to M.H.
REFERENCES
- 1.Akbar S, Kang C M, Gaidenko T A, Price C W. Modulator protein RsbR regulates environmental signalling in the general stress pathway of Bacillus subtilis. Mol Microbiol. 1997;24:567–578. doi: 10.1046/j.1365-2958.1997.3631732.x. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Anagnostopoulos C, Spizizen J. Requirements for transformation in Bacillus subtilis. J Bacteriol. 1961;81:741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antelmann H, Bernhardt J, Schmid R, Hecker M. A gene at 333° on the Bacillus subtilis chromosome encodes the newly identified ςB-dependent general stress protein GspA. J Bacteriol. 1995;177:3540–3545. doi: 10.1128/jb.177.12.3540-3545.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antelmann H, Bernhardt J, Schmid R, Mach H, Völker U, Hecker M. First steps from a two-dimensional protein index towards a response-regulation map for Bacillus subtilis. Electrophoresis. 1997;18:1451–1463. doi: 10.1002/elps.1150180820. [DOI] [PubMed] [Google Scholar]
- 6.Antelmann H, Engelmann S, Schmid R, Sorokin A, Lapidus A, Hecker M. Expression of a stress- and starvation-induced dps/pexB homologous gene is controlled by the alternative sigma factor ςB in Bacillus subtilis. J Bacteriol. 1997;179:7251–7256. doi: 10.1128/jb.179.23.7251-7256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benson A K, Haldenwang W G. Characterization of a regulatory network that controls ςB expression in Bacillus subtilis. J Bacteriol. 1992;174:749–757. doi: 10.1128/jb.174.3.749-757.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benson A K, Haldenwang W G. The ςB-dependent promoter of the Bacillus subtilis sigB operon is induced by heat shock. J Bacteriol. 1993;175:1929–1935. doi: 10.1128/jb.175.7.1929-1935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boylan S A, Redfield A R, Brody M S, Price C W. Stress-induced activation of the ςB transcription factor of Bacillus subtilis. J Bacteriol. 1993;175:7931–7937. doi: 10.1128/jb.175.24.7931-7937.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boylan S A, Redfield A R, Price C W. Transcription factor ςB of Bacillus subtilis controls a large stationary-phase regulon. J Bacteriol. 1993;175:3957–3963. doi: 10.1128/jb.175.13.3957-3963.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boylan S A, Thomas M D, Price C W. Genetic method to identify regulons controlled by nonessential elements: isolation of a gene dependent on alternate transcription factor ςB of Bacillus subtilis. J Bacteriol. 1991;173:7856–7866. doi: 10.1128/jb.173.24.7856-7866.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derre I, Rapoport G, Msadek T. CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in Gram-positive bacteria. Mol Microbiol. 1999;31:117–132. doi: 10.1046/j.1365-2958.1999.01152.x. [DOI] [PubMed] [Google Scholar]
- 13.Desaizieu A, Certa U, Warrington J, Gray C, Keck W, Mous J. Bacterial transcript imaging by hybridization of total RNA to oligonucleotide arrays. Nat Biotechnol. 1998;16:45–48. doi: 10.1038/nbt0198-45. [DOI] [PubMed] [Google Scholar]
- 14.Engelmann S, Hecker M. Impaired oxidative stress resistance of Bacillus subtilis sigB mutants and the role of katA and katE. FEMS Microbiol Lett. 1996;145:63–69. doi: 10.1111/j.1574-6968.1996.tb08557.x. [DOI] [PubMed] [Google Scholar]
- 15.Gaidenko T A, Price C W. General stress transcription factor ςB and sporulation transcription factor ςH each contribute to survival of Bacillus subtilis under extreme growth conditions. J Bacteriol. 1998;180:3730–3733. doi: 10.1128/jb.180.14.3730-3733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hecker M, Schumann W, Völker U. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 17.Hecker M, Völker U. Non-specific, general and multiple stress resistance of growth-restricted Bacillus subtilis cells by the expression of the ςB regulon. Mol Microbiol. 1998;29:1129–1136. doi: 10.1046/j.1365-2958.1998.00977.x. [DOI] [PubMed] [Google Scholar]
- 18.Hilden I, Krath B N, Hove-Jensen B. Tricistronic operon expression of the genes gcaD (tms), which encodes N-acetylglucosamine 1-phosphate uridyltransferase, prs, which encodes phosphoribosyl diphosphate synthetase, and ctc in vegetative cells of Bacillus subtilis. J Bacteriol. 1995;177:7280–7284. doi: 10.1128/jb.177.24.7280-7284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X, Fredrick K L, Helmann J D. Promoter recognition by Bacillus subtilis ςW: autoregulation and partial overlap with the ςX regulon. J Bacteriol. 1998;180:3765–3770. doi: 10.1128/jb.180.15.3765-3770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang X, Helmann J D. Identification of target promoters for the Bacillus subtilis ςX factor using a consensus-directed search. J Mol Biol. 1998;279:165–173. doi: 10.1006/jmbi.1998.1765. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Gaballa A, Cao M, Helmann J D. Identification of target promoters for the Bacillus subtilis extracytoplasmic function ς factor, ςW. Mol Microbiol. 1999;31:361–371. doi: 10.1046/j.1365-2958.1999.01180.x. [DOI] [PubMed] [Google Scholar]
- 22.Igo M, Lampe M, Ray C, Schafer W, Moran C P, Jr, Losick R. Genetic studies of a secondary RNA polymerase sigma factor in Bacillus subtilis. J Bacteriol. 1987;169:3464–3469. doi: 10.1128/jb.169.8.3464-3469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krüger E, Hecker M. The first gene of the Bacillus subtilis clpC operon, ctsR, encodes a negative regulator of its own operon and other class III heat shock genes. J Bacteriol. 1998;180:6681–6688. doi: 10.1128/jb.180.24.6681-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunst F, Ogasawara N, Moszer I, Albertini A M, Danchin A. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 25.Majumdar D, Avissar Y J, Wyche J H. Simultaneous and rapid isolation of bacterial and eukaryotic DNA and RNA: a new approach for isolating DNA. BioTechniques. 1991;11:94–101. [PubMed] [Google Scholar]
- 26.Mizuno M, Masuda S, Takemaru K, Hosono S, Sato T, Takeuchi M, Kobayashi Y. Systematic sequencing of the 283 kb 210°–232° region of the Bacillus subtilis genome containing the skin element and many sporulation genes. Microbiology. 1996;142:3103–3111. doi: 10.1099/13500872-142-11-3103. [DOI] [PubMed] [Google Scholar]
- 27.Ray C, Hay R E, Carter H L, Moran C P., Jr Mutations that affect utilization of a promoter in stationary-phase Bacillus subtilis. J Bacteriol. 1985;163:610–614. doi: 10.1128/jb.163.2.610-614.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scharf C, Riethdorf S, Ernst H, Engelmann S, Völker U, Hecker M. Thioredoxin is an essential protein induced by multiple stresses in Bacillus subtilis. J Bacteriol. 1998;180:1869–1877. doi: 10.1128/jb.180.7.1869-1877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stülke J, Hanschke R, Hecker M. Temporal activation of beta-glucanase synthesis in Bacillus subtilis is mediated by the GTP pool. J Gen Microbiol. 1993;139:2041–2045. doi: 10.1099/00221287-139-9-2041. [DOI] [PubMed] [Google Scholar]
- 30.Tatti K M, Moran C P., Jr Promoter recognition by sigma-37 RNA polymerase from Bacillus subtilis. J Mol Biol. 1984;175:285–297. doi: 10.1016/0022-2836(84)90349-8. [DOI] [PubMed] [Google Scholar]
- 31.Völker U, Engelmann S, Maul B, Riethdorf S, Völker A, Schmid R, Mach H, Hecker M. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology. 1994;140:741–752. doi: 10.1099/00221287-140-4-741. [DOI] [PubMed] [Google Scholar]
- 32.Völker U, Maul B, Hecker M. Expression of the ςB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J Bacteriol. 1999;181:3942–3948. doi: 10.1128/jb.181.13.3942-3948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wetzstein M, Völker U, Dedio J, Lobau S, Zuber U, Schiesswohl M, Herget C, Hecker M, Schumann W. Cloning, sequencing, and molecular analysis of the dnaK locus from Bacillus subtilis. J Bacteriol. 1992;174:3300–3310. doi: 10.1128/jb.174.10.3300-3310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]