Abstract
Background: The purpose of this study was to evaluate the risk of cardiovascular mortality (CVM) among patients with bladder cancer (BC). Methods and Materials: Data were collected from the Surveillance, Epidemiology, and End Results (SEER) database for patients who were diagnosed with BC by pathology between 2000 and 2016. The standardized mortality rate (SMR) was calculated based on reference data from the general population. Nelson–Aalen cumulative hazard curves were used to assess the risk of experiencing CVM in BC patients. Multivariate competing risk models were performed. Results: In total, data from 237,563 BC patients were obtained from the SEER database for further analysis, of which 21,822 patients experienced CVM; the overall SMR for CVM in BC patients was 1.16 (95% CI: 1.14–1.17). Age, race, sex, year of diagnosis, histologic type, summary stage, surgery, marital status, and college education level were independent predictors of CVM in patients with BC. Conclusions: Patients with BC have a significantly increased risk of experiencing CVM compared to the general population. Pre-identification of high-risk groups and cardiovascular protection interventions are important measures to effectively improve survival in this group of patients.
Keywords: bladder cancer, cardiovascular mortality, SEER, competing risk regression
1. Introduction
It is estimated that there are more than 500,000 new cases of bladder cancer (BC) worldwide each year, with approximately 40% of these resulting in death. However, the US alone accounts for 16% of all new cases worldwide each year [1,2,3].
Cardiovascular diseases (CVDs) are one of the primary causes of death worldwide. According to a study published in The Lancet in 2018, cardiovascular mortality (CVM) increased by 2.1% over a 10-year period from 2007 [4]. A total of 17.9 million people died from CVD in 2019, contributing to 32% of all deaths worldwide, while only about 10 million people died from cancer in 2019. In 2019, Kochanek et al. reported that in the US, over 0.64 million deaths were due to heart disease, while nearly 0.6 million deaths were attributed to primary cancers [5].
With the improvement in the quality of life and medical care, patients’ life expectancy has increased, and mortality from primary cancers has gradually decreased, leading to the prominence of mortality factors for non-primary cancers, among which CVD is one of the leading causes of death for non-cancers [6]. Several published studies have indicated that cancer patients are at significantly higher risk of experiencing CVM than the general population for a variety of reasons [7,8,9].
A detailed literature search revealed no reports of CVM in patients with BC. Therefore, our discoveries may help to establish a more targeted follow-up strategy for BC patients as well as more effective CVM prevention measures.
2. Methods
2.1. Data Source and Patient Selection
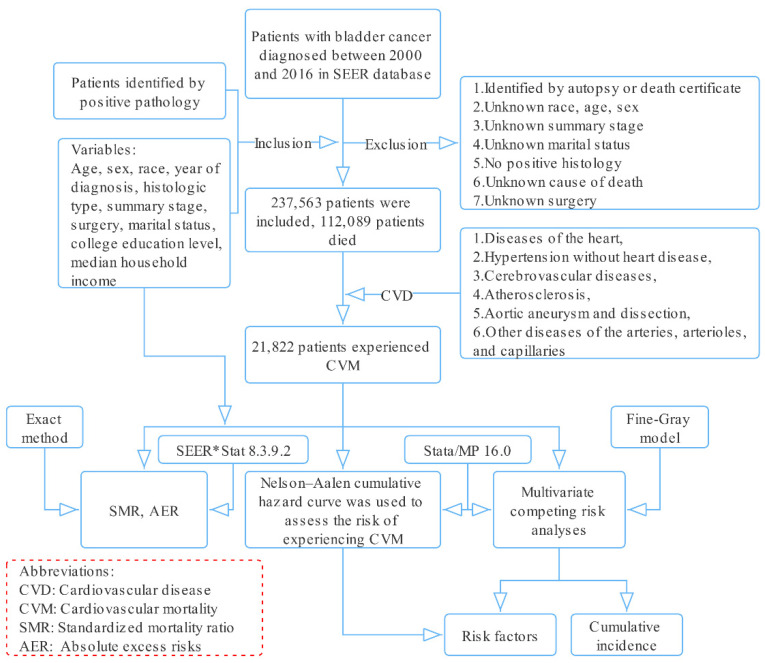
Information related to patients with BC diagnosed from 2000 to 2016 was downloaded from the Surveillance, Epidemiology, and End Results (SEER) database using SEER*Stat software (National Cancer Institute, Bethesda, MD, USA, version 8.3.9.2, Database: Incidence—SEER 18 Regs excluding AK Research Data, November 2018 Sub (2000–2016) for standardized mortality ratios (SMRs)) (Figure 1).
Figure 1.
Selection of eligible patients and study design.
Patients diagnosed with BC with positive pathology from 2000 to 2016 were included. The following histological codes were used: 8000, 8004, 8010, 8012, 8013, 8020–8022, 8030–8033, 8041, 8042, 8045, 8046, 8050–8052, 8070–8075, 8082, 8083, 8120–8122, 8130, 8131, 8240, 8244, 8246, 8490, 8507, 8542, 8560, 8570, 8574–8576, 8940, and 8980 (International Classification of Diseases for Oncology, 3rd edition).
Patients identified only by autopsy or death certificate and patients with incomplete data for certain variables (age, sex, race, etc.) were excluded.
CVM was the primary endpoint of interest, defined by the following six CVDs in the SEER database: (1) diseases of the heart, (2) hypertension without heart disease, (3) cerebrovascular diseases, (4) atherosclerosis, (5) aortic aneurysm and dissection, (6) other diseases of the arteries, arterioles, and capillaries [10,11], while competing events were deaths from BC, other cancers, and other non-cancer diseases.
2.2. Study Variables
The definitions and information regarding variables are as follows: age at diagnosis (0–50, 51–60, 61–70, 71–80, 81+), race (White, Black, American Indian/Alaska Native, Asian or Pacific Islander), sex (male, female), year of diagnosis, histologic type (Tcc: transitional cell carcinoma; Scc: squamous cell carcinoma; Ac: adenocarcinoma; Nec: neuroendocrine carcinoma; Oet: other epithelial tumors), summary stage (in situ, localized, regional, distant), surgery (no surgery; TURBT: transurethral resection of bladder tumor; PC: partial cystectomy; RC: radical cystectomy), marital status (married, separated, divorced, widowed, unmarried (unmarried or domestic partner, single)), college education level, median household income, cause of death, and follow-up time.
2.3. Statistical Analysis
The SMR is the ratio of the number of observed deaths to the number of expected deaths of CVM [12]. We used an exact method to calculate the 95% confidence intervals (95% CIs) for all SMRs. Absolute excess risks (AERs) were also calculated, which are a proxy for the excess number of deaths per 10,000 person-years in different subgroups [10,12]. The Nelson–Aalen cumulative hazard curve was used to assess the risk of experiencing CVM in different subgroups of BC patients. Multivariate competing risk analyses were conducted to identify risk factors associated with CVM [13].
All analyses were conducted using SEER*Stat software (version 8.3.9.2, National Cancer Institute, Bethesda, MD, USA), Stata/MP version 16.0 (Stata Corp, College Station, TX, USA), and Microsoft Excel 2019 (Microsoft, Redmond, WA 98052-6399, USA). A two-sided p-value < 0.05 was considered statistically significant.
3. Results
3.1. Patient Characteristics
For this study, a total of 237,563 patients diagnosed with BC were identified from 2000 to 2016. The mean age was 70.82 ± 12.04 years, and the median follow-up time was 49 months. Most of the patients were over 71 years old (54.93%), White (90.40%), male (75.72%), married (64.58%), and had carcinoma in situ (52.79%). The histologic types of BC consisted of Tcc (95.63%), Scc (1.59%), Ac (0.97%), Nec (0.65%), and Oet (1.15%). A total of 201,433 (84.79%) patients underwent TURBT, 3,278 (1.38%) patients underwent PC, 20,999 (8.84%) patients underwent RC, and 11,853 (4.99%) patients did not undergo surgery. The largest number of deaths occurred during the follow-up period of <1 year (32.81%), followed by the 1- to 3-year follow-up period (28.07%). Among the 112,089 patients that died during the follow-up period, 21,822 patients experienced CVM, with the main cause being disease of the heart (70.14%), followed by cerebrovascular disease (12.31%), hypertension without heart disease (2.89%), atherosclerosis (0.97%), aortic aneurysm and dissection (1.14%), and other diseases of the arteries, arterioles, and capillaries (1.06%).
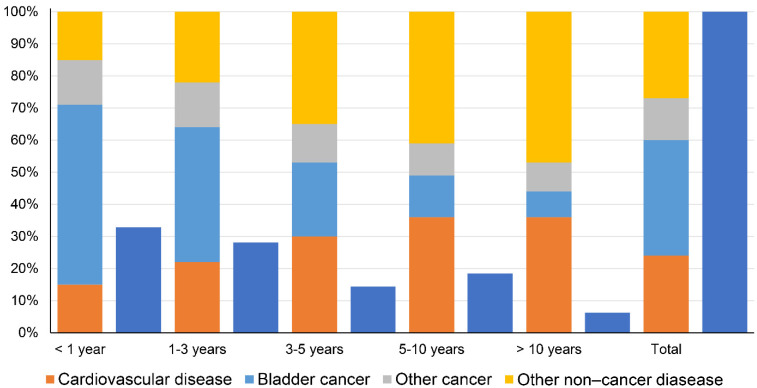
A total of 37,761 BC patients died within 1 year after diagnosis, including 20,954 (55.49%) deaths from BC, 5660 (14.99%) deaths from CVD, 5381 (14.25%) deaths from other cancers, and 5766 (15.27%) deaths from other non-cancer diseases. The proportion of cancer-related deaths decreased gradually at <1 year, 1–3 years, 3–5 years, 5–10 years, and >10 years (including BC and other cancers), while the proportion of non-cancer disease-related deaths increased gradually (including CVD and other non-cancer diseases) (Figure 2).
Figure 2.
Causes of death in each latency period following bladder cancer diagnosis (dark blue indicates the percentage of the total number of deaths for each latency period).
3.2. SMR and AER
The overall SMR for CVM was 1.16 (95% CI: 1.14–1.17), and the AER was 27.18/10,000 person-years in BC patients. Table 1 shows the baseline features and SMRs of CVM in patients with BC.
Table 1.
Baseline features and standardized mortality ratios of cardiovascular mortality in patients with bladder cancer.
| Observed Deaths | Expected Deaths | SMR [95% CI] | AER per 10,000 | Persons | Person-Years at Risk | |
|---|---|---|---|---|---|---|
| Total | 21,822 | 18,888.75 | 1.16 # [1.14–1.17] | 27.18 | 237,563 | 1,079,006.59 |
| Age (yrs) | ||||||
| 00–50 | 147 | 120.54 | 1.22 # [1.03–1.43] | 2.86 | 13,009 | 92,394.95 |
| 51–60 | 898 | 670.57 | 1.34 # [1.25–1.43] | 11.57 | 32,856 | 196,551.81 |
| 61–70 | 2921 | 2378.56 | 1.23 # [1.18–1.27] | 17.57 | 61,201 | 308,691.31 |
| 71–80 | 7874 | 7244.13 | 1.09 # [1.06–1.11] | 19.51 | 75,277 | 322,772.51 |
| 81+ | 9982 | 8474.95 | 1.18 # [1.15–1.20] | 95.02 | 55,220 | 158,596.01 |
| Race | ||||||
| White | 20,093 | 17,534.79 | 1.15 # [1.13–1.16] | 25.98 | 214,764 | 984,523.07 |
| Black | 1064 | 849.60 | 1.25 # [1.18–1.33] | 42.24 | 13,045 | 50,763.83 |
| AIAN | 39 | 17.98 | 2.17 # [1.54–2.97] | 86.85 | 619 | 2420.49 |
| API | 626 | 486.39 | 1.29 # [1.19–1.39] | 33.81 | 9135 | 41,299.21 |
| Sex | ||||||
| Male | 17,080 | 14,647.53 | 1.17 # [1.15–1.18] | 30.00 | 179,880 | 810,942.96 |
| Female | 4742 | 4241.22 | 1.12 # [1.09–1.15] | 18.68 | 57,683 | 268,063.63 |
| Year of diagnosis | ||||||
| 2000–2003 | 8044 | 7142.72 | 1.13 # [1.10–1.15] | 24.01 | 53,447 | 375,401.43 |
| 2004–2007 | 6745 | 5791.75 | 1.16 # [1.14–1.19] | 28.78 | 56,320 | 331,202.90 |
| 2008–2011 | 4649 | 3975.76 | 1.17 # [1.14–1.20] | 27.50 | 57,139 | 244,822.26 |
| 2012–2016 | 2384 | 1978.52 | 1.20 # [1.16–1.25] | 31.78 | 706,57 | 127,580.00 |
| Histologic Type | ||||||
| Tcc | 21,070 | 18,386.59 | 1.15 # [1.13–1.16] | 25.54 | 226,953 | 1,047,766.11 |
| Scc | 293 | 201.34 | 1.45 # [1.28–1.62] | 74.55 | 3926 | 12,026.36 |
| Nec | 66 | 44.71 | 1.48 # [1.14–1.88] | 81.80 | 1565 | 2602.70 |
| Ac | 144 | 86.84 | 1.61 # [1.36–1.90] | 74.51 | 2388 | 7134.40 |
| Oet | 249 | 161.48 | 1.54 # [1.35–1.74] | 105.00 | 2731 | 8240.26 |
| Summary stage | ||||||
| In situ | 11,859 | 11,497.53 | 1.03 # [1.01–1.05] | 5.35 | 125,403 | 675,257.36 |
| Localized | 8744 | 6679.56 | 1.31 # [1.28–1.34] | 58.19 | 86,178 | 354,786.32 |
| Regional | 945 | 608.73 | 1.55 # [1.45–1.65] | 81.60 | 17,170 | 41,207.66 |
| Distant | 274 | 102.93 | 2.66 # [2.36–3.00] | 220.58 | 8812 | 7755.25 |
| Surgery | ||||||
| No | 1161 | 781.75 | 1.49 # [1.40–1.57] | 87.01 | 11,915 | 43,585.52 |
| TURBT | 19,521 | 17,082.51 | 1.14 # [1.13–1.16] | 25.53 | 201,251 | 955,166.63 |
| PC | 236 | 226.84 | 1.04 [0.91–1.18] | 6.81 | 3340 | 13,451.45 |
| RC | 904 | 797.66 | 1.13 # [1.06–1.21] | 15.92 | 21,057 | 66,803.00 |
| Marital status | ||||||
| Married | 12,906 | 12,352.70 | 1.04 # [1.03–1.06] | 7.43 | 153,408 | 744,788.58 |
| Separated | 109 | 83.52 | 1.31 # [1.07–1.57] | 38.29 | 1589 | 6654.38 |
| Divorced | 1431 | 936.75 | 1.53 # [1.45–1.61] | 59.77 | 19,239 | 82,686.26 |
| Widowed | 5566 | 4254.34 | 1.31 # [1.27–1.34] | 99.24 | 37,708 | 132,164.93 |
| Unmarried | 1810 | 1261.44 | 1.43 # [1.37–1.50] | 48.67 | 25,619 | 112,712.43 |
| Education level | ||||||
| College level ≦ 50% | 20,343 | 17,373.84 | 1.17 # [1.15–1.19] | 29.93 | 219,156 | 991,670.08 |
| College level > 50% | 1479 | 1514.49 | 0.98 [0.93–1.03] | −4.06 | 18,407 | 87,303.01 |
| Median household income | ||||||
| USD 0–50,000 | 4155 | 3206.62 | 1.30 # [1.26–1.34] | 49.17 | 44,104 | 192,881.08 |
| USD 50,000–100,000 | 16,783 | 14,742.45 | 1.14 # [1.12–1.16] | 24.49 | 18,2476 | 832,950.98 |
| Over USD 100,000 | 884 | 939.26 | 0.94 [0.88–1.01] | −10.4 | 10,983 | 53,141.02 |
Abbreviations: SMR, standardized mortality ratio; CI, confidence interval; AER, absolute excess risk; AIAN, American Indian/Alaska Native; API, Asian or Pacific Islander; Tcc, transitional cell carcinoma; Scc, squamous cell carcinoma; Nec, neuroendocrine carcinoma; Ac, adenocarcinoma; Oet, other epithelial tumors; TURBT, transurethral resection of bladder tumor; PC, partial cystectomy; RC, radical cystectomy. # p < 0.05.
The SMRs of the six causes of CVM in patients with BC are shown in Table 2. The most significant increase in the SMR was found for aortic aneurysm and dissection (SMR [95% CI]: 1.31 [1.15–1.47]), followed by other diseases of the arteries, arterioles, and capillaries (SMR [95% CI]: 1.20 [1.02–1.38]), atherosclerosis (SMR [95% CI]: 1.18 [1.09–1.28]), diseases of the heart (SMR [95% CI]: 1.16 [1.13–1.19]), cerebrovascular diseases (SMR [95% CI]: 1.13 [1.06–1.24]), and hypertension without heart disease (SMR [95% CI]: 1.05 [1.01–1.09]).
Table 2.
The standardized mortality ratios of all causes of cardiovascular mortality in patients with bladder cancer.
| CVD | Observed Deaths | Expected Deaths | SMR [95% CI] |
|---|---|---|---|
| Total | 21,822 | 18,888.75 | 1.16 # [1.14–1.17] |
| Diseases of the heart | 17,293 | 14,900.46 | 1.16 # [1.13–1.19] |
| Hypertension without heart disease | 712 | 678.07 | 1.05 # [1.01–1.09] |
| Cerebrovascular diseases | 3036 | 2675.16 | 1.13 # [1.06–1.24] |
| Atherosclerosis | 239 | 203.37 | 1.18 # [1.09–1.28] |
| Aortic aneurysm and dissection | 281 | 214.32 | 1.31 # [1.15–1.47] |
| Other diseases of the arteries, arterioles, and capillaries | 261 | 217.37 | 1.20 # [1.02–1.38] |
Abbreviations: CVD, cardiovascular disease; SMR, standardized mortality ratio. # p < 0.05.
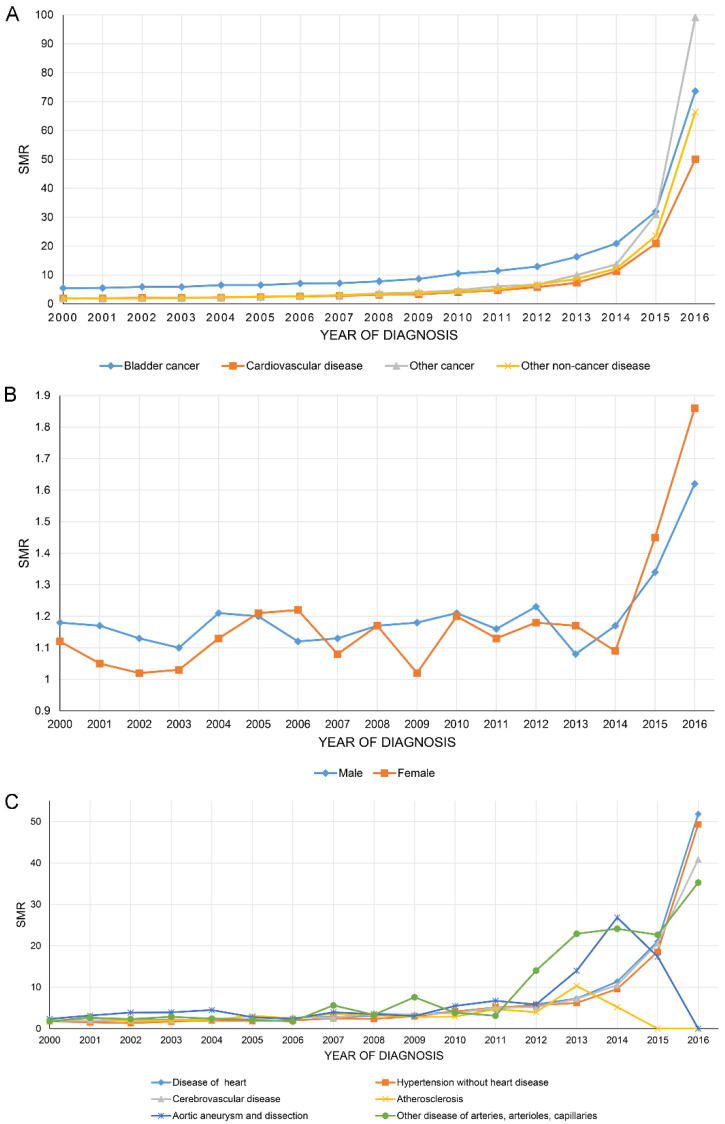
Figure 3A shows that the SMR of all causes of death of BC patients increased year by year, with BC having the highest SMR of all. Figure 3B,C show the SMR in CVD stratified by sex and all causes of CVM.
Figure 3.
The overall standardized mortality ratio (SMR) of all causes of death of bladder cancer patients increased year by year (A). SMR in cardiovascular disease stratified by sex (B) and all causes of cardiovascular mortality (C).
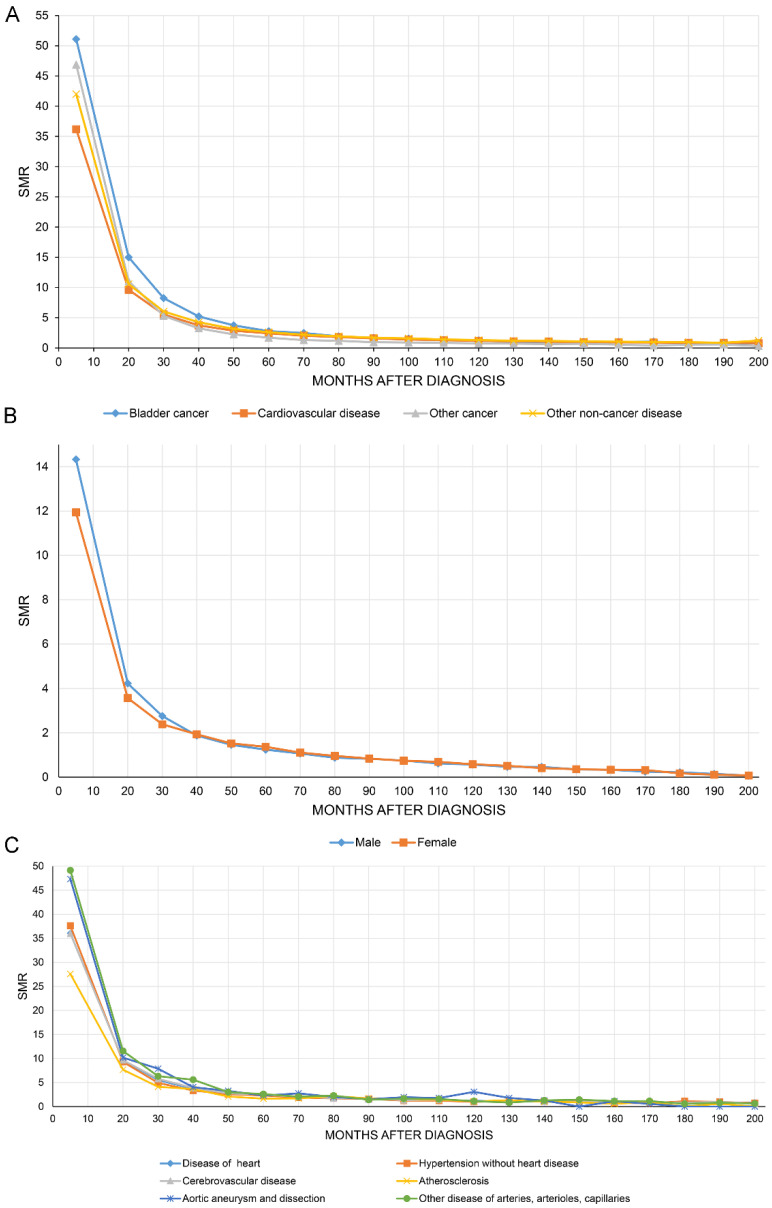
Figure 4A shows that the SMR of all causes of death of BC patients decreased with increasing follow-up time. Figure 4B,C show the SMR for CVD stratified by sex and all causes of CVM.
Figure 4.
The overall standardized mortality ratio (SMR) of all causes of death in bladder cancer patients decreased with increasing follow-up time (A). SMR in cardiovascular disease stratified by sex (B) and all causes of cardiovascular mortality (C).
3.3. Nelson–Aalen Cumulative Hazard Curve
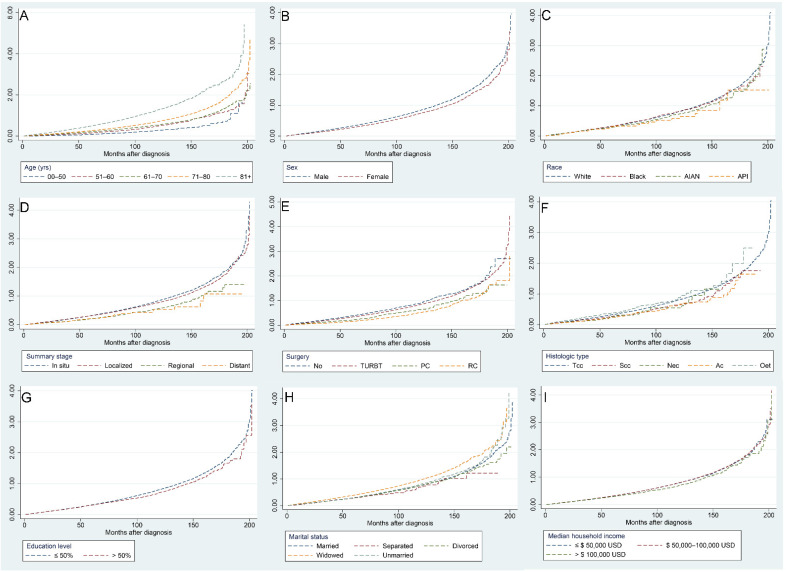
Figure 5 illustrates the risk of CVM with increasing follow-up time for different factors, with the following subgroups associated with a higher risk of CVM: age over 71 years, male sex, White, carcinoma in situ and localized tumors, no surgery, Tcc and Nec pathological types, a college education level less than 50%, widowed, and a median household income less than USD 100,000.
Figure 5.
Independent Nelson–Aalen cumulative hazard curves for various factors of cardiovascular mortality in bladder cancer patients: age (A), sex (B), race (C), summary stage (D), surgery (E), histologic type (F), education level (G), marital status (H), and median household income (I).
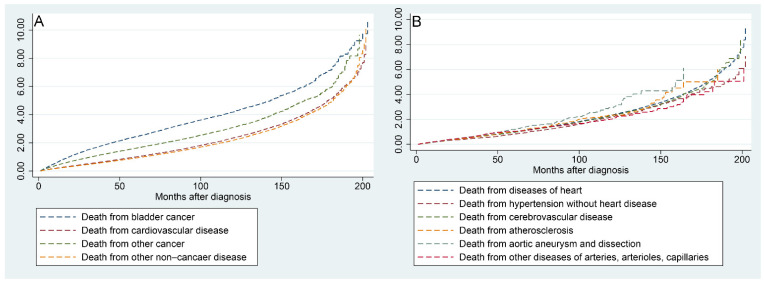
Figure 6A shows that the risk of all mortality factors in BC patients increased with the time to follow-up. Figure 6B shows that the risk of the six factors contributing to CVM increased progressively with the time to follow-up.
Figure 6.
Nelson–Aalen cumulative hazard curves for all causes of death in primary bladder cancer patients (A) and stratified by the six factors of cardiovascular disease (B).
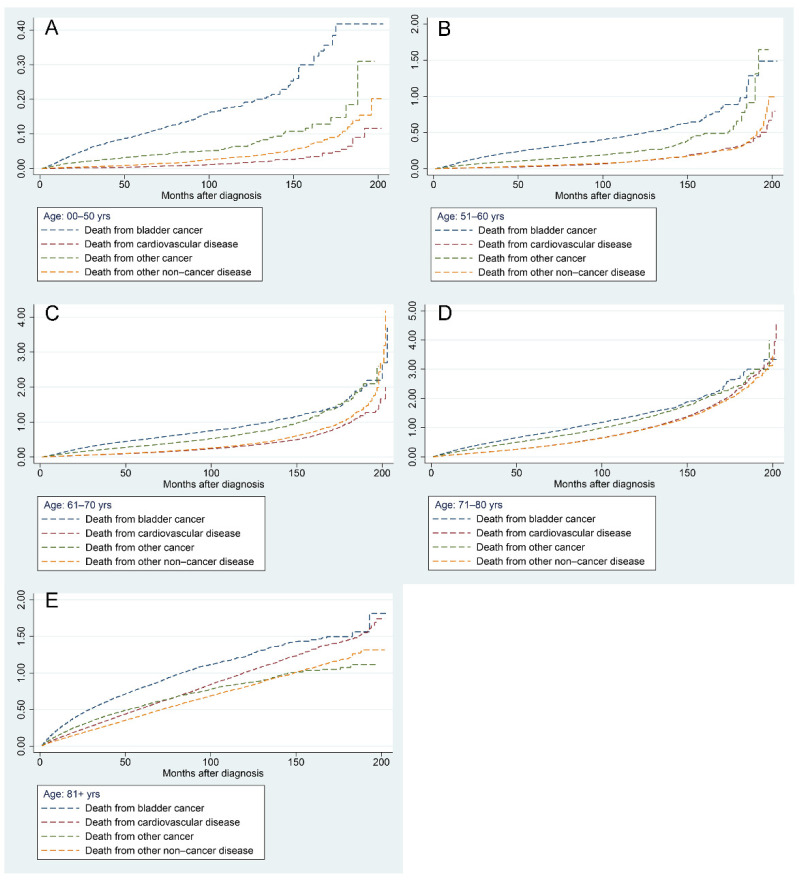
Figure 7 demonstrates the results of a progressive increase in the risk of all mortality factors in BC patients of different ages with increasing follow-up time. The risk of BC-related death was highest in patients aged less than 70 years, followed by other cancers, other non-cancer diseases, and CVDs (Figure 7A–C). In patients aged 71–80 years, the risk of CVD surpassed that of other non-cancer diseases and ranked third when the follow-up time exceeded 120 months (Figure 7D). In patients older than 81 years, the risk of CVD-related mortality surpassed that of other cancers as the second leading risk factor at approximately 80 months of follow-up (Figure 7E).
Figure 7.
Nelson–Aalen cumulative hazard curves for all causes of death in primary bladder cancer patients in different age groups: 0–50 years (A), 51–60 years (B), 61–70 years (C), 71–80 years (D), and 81+ years (E).
3.4. Predictors of Death from CVD
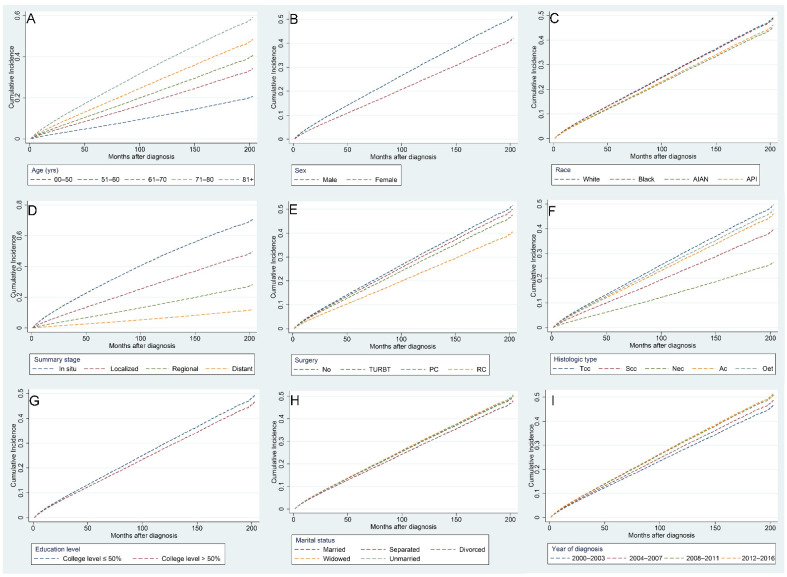
Risk factors associated with CVM in BC patients were identified using multivariate competing risk regression analysis (Table 3). We found that the following indicators were independently related to higher risks of CVM: age over 81 years (HR [95% CI]: 3.855 [3.285–4.525]), diagnosed between 2012 and 2016 (HR [95% CI]: 1.149 [1.094–1.208]), widowed (HR [95% CI]: 1.079 [1.043–1.116]), and unmarried (HR [95% CI]: 1.066 [1.016–1.119]). On the contrary, the following indicators were found to be independently related to lower risks of CVM: American Indian/Alaska Native (HR [95% CI]: 0.895 [0.827–0.968]), female (HR [95% CI]: 0.755 [0.73–0.78]), Scc histological type (HR [95% CI]: 0.446 [0.349–0.571]), distant summary stage (HR [95% CI]: 0.105 [0.093–0.119]), undergoing RC (HR [95% CI]: 0.715 [0.652–0.784]), and a college education level >50% (HR [95% CI]: 0.926 [0.87–0.986]). Figure 8 shows the CIF curves using Fine–Gray competing risk analyses.
Table 3.
Competing risk regression analysis for predictors of cardiovascular mortality in patients with bladder cancer.
| Characteristics | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Adjusted HR [95%CI] | p * | Adjusted HR [95%CI] | p * | |
| Age (yrs) | ||||
| 00–50 | Ref. | Ref. | ||
| 51–60 | 2.036 [1.715–2.418] | <0.001 | 1.804 [1.523–2.137] | <0.001 |
| 61–70 | 2.937 [2.495–3.459] | <0.001 | 2.249 [1.913–2.644] | <0.001 |
| 71–80 | 4.223 [3.596–4.960] | <0.001 | 2.858 [2.436–3.354] | <0.001 |
| 81+ | 5.788 [4.929–6.796] | <0.001 | 3.855 [3.285–4.525] | <0.001 |
| Race | ||||
| White | Ref. | Ref. | ||
| Black | 0.765 [0.720–0.814] | <0.001 | 0.989 [0.929–1.052] | 0.723 |
| AIAN | 0.848 [0.784–0.917] | <0.001 | 0.895 [0.827–0.968] | 0.006 |
| API | 0.696 [0.511–0.946] | 0.021 | 0.916 [0.682–1.232] | 0.563 |
| Sex | ||||
| Male | Ref. | Ref. | ||
| Female | 0.739 [0.716–0.763] | <0.001 | 0.755 [0.730–0.780] | <0.001 |
| Year of diagnosis | ||||
| 2000–2003 | Ref. | Ref. | ||
| 2004–2007 | 1.044 [1.013–1.077] | 0.006 | 1.062 [1.030–1.095] | <0.001 |
| 2008–2011 | 1.003 [0.968–1.039] | 0.886 | 1.133 [1.092–1.174] | <0.001 |
| 2012–2016 | 0.816 [0.779–0.855] | <0.001 | 1.149 [1.094–1.208] | <0.001 |
| Histologic Type | ||||
| Tcc | Ref. | Ref. | ||
| Scc | 0.459 [0.409–0.515] | <0.001 | 0.734 [0.655–0.823] | <0.001 |
| Nec | 0.244 [0.192–0.311] | <0.001 | 0.446 [0.349–0.571] | <0.001 |
| Ac | 0.399 [0.338–0.471] | <0.001 | 0.896 [0.758–1.059] | 0.197 |
| Oet | 0.686 [0.604–0.778] | <0.001 | 0.942 [0.830–1.069] | 0.358 |
| Summary stage | ||||
| In situ | Ref. | Ref. | ||
| Localized | 0.553 [0.538–0.568] | <0.001 | 0.562 [0.546–0.579] | <0.001 |
| Regional | 0.204 [0.190–0.218] | <0.001 | 0.271 [0.251–0.292] | <0.001 |
| Distant | 0.087 [0.077–0.098] | <0.001 | 0.105 [0.093–0.119] | <0.001 |
| Surgery | ||||
| No | Ref. | Ref. | ||
| TURBT | 1.291 [1.217–1.370] | <0.001 | 0.953 [0.898–1.011] | 0.11 |
| PC | 0.665 [0.579–0.764] | <0.001 | 0.889 [0.773–1.023] | 0.099 |
| RC | 0.393 [0.361–0.429] | <0.001 | 0.715 [0.652–0.784] | <0.001 |
| Marital status | ||||
| Married | Ref. | Ref. | ||
| Separated | 0.730 [0.605–0.879] | 0.001 | 0.999 [0.828–1.205] | 0.993 |
| Divorced | 0.807 [0.765–0.851] | <0.001 | 1.055 [0.999–1.113] | 0.052 |
| Widowed | 1.119 [1.085–1.154] | <0.001 | 1.079 [1.043–1.116] | <0.001 |
| Unmarried | 0.808 [0.770–0.848] | <0.001 | 1.066 [1.016–1.119] | 0.01 |
| Education level | ||||
| College level ≤50% | Ref. | Ref. | ||
| College level >50% | 0.929 [0.883–0.979] | 0.006 | 0.926 [0.870–0.986] | 0.017 |
| Median household income | ||||
| ≤USD 50,000 | Ref. | Ref. | ||
| USD 50,000–100,000 | 1.025 [0.991–1.059] | 0.151 | 0.999 [0.966–1.033] | 0.955 |
| >USD 100,000 | 0.975 [0.908–1.046] | 0.473 | 0.963 [0.885–1.048] | 0.383 |
Abbreviations: HR, hazard ratio; CI, confidence interval; AIAN, American Indian/Alaska Native; API, Asian or Pacific Islander; Tcc, transitional cell carcinoma; Scc, squamous cell carcinoma; Nec, neuroendocrine carcinoma; Ac, adenocarcinoma; Oet, other epithelial tumors; TURBT, transurethral resection of bladder tumor; PC, partial cystectomy; RC, radical cystectomy. * A two-sided p-value < 0.05 was considered statistically significant.
Figure 8.
Cumulative incidence curves for various factors of cardiovascular mortality in bladder cancer patients after competing risk regression analysis: age (A), sex (B), race (C), summary stage (D), surgery (E), histologic type (F), education level (G), marital status (H), and year of diagnosis (I).
4. Discussion
In this large population study based on the SEER database, we analyzed the long-term CVM of patients with BC. Although the number of diagnoses of BC is increasing every year, the number of people who develop CVM is decreasing. This may be related to the advancement of BC treatment strategies and the improved quality of comprehensive cancer management. At the same time, the treatment of CVD and the ability to cope with cardiovascular events have also improved, which has effectively reduced the incidence of CVM.
Published studies have illustrated that the risk of CVM varies considerably between patients with cancer at different primary sites [14,15,16]. In this study, we focused only on CVM in patients with BC. By studying 21,822 patients, we found that the risk of CVM in patients with BC was approximately 16% higher than that in the US general population (SMR [95% CI]: 1.16 [1.14–1.17]). Over the entire follow-up period, patients with BC had an increased risk of CVM from all causes. Our study identified age, race, sex, year of diagnosis, histologic type, summary stage, surgery, marital status, college education level, and median household income as independent predictors for the development of CVM in patients with BC.
Similar to the results previously published by Zaorsky et al. [17], we found that the risk of CVM in BC patients was highest in the first 10 months after diagnosis. Meanwhile, the study by Fang and Ye et al. suggested that newly diagnosed cancer may lead to psychological and emotional distress in cancer patients, which in part promotes the development of CVM [18,19]. Therefore, psychiatric assessment and psychological support are necessary for newly diagnosed BC patients. Using the Nelson–Aalen hazard curves, we found that the risk of CVM in BC patients gradually increased with increasing age at diagnosis. Primary cancer is the most common cause of death for most cancer patients. However, our study found that the risk of CVM ranked first when cancer patients were diagnosed at an age of >71 years. These results suggest that clinicians should focus not only on BC itself, but also on the risk factors for experiencing CVM in patients of advanced age.
Multivariate competing risk regression analysis was used to identify risk factors associated with CVM in BC patients. We also observed that patients with BC aged over 81 years had the highest CVM (HR [95% CI]: 3.855 [3.285–4.525]) and a lower SMR (SMR [95% CI]: 1.18 [1.15–1.20]), but patients aged 51–60 years had the highest SMR (SMR [95% CI]: 1.34 [1.25–1.43]), which is similar to the findings of Zaorsky et al. [17]. Men have a higher risk of CVM, which may be related to smoking, alcohol consumption, or higher work stress, all of which are independent risk factors for CVD [20,21,22,23]. Additionally, our study demonstrated that unmarried (HR [95% CI]: 1.066 [1.016–1.119]) and widowed (HR [95% CI]: 1.079 [1.043–1.116]) BC patients are at a higher risk of CVM, which may be associated with the fact that married patients are more likely to receive encouragement and support from their spouses, both emotionally and physically [24]. Additionally, some studies pointed out that marriage helps improve cardiovascular, endocrine, and immune function as well as cancer prognosis [25,26]. Patients with a lower socioeconomic status have been reported to be at higher risk for CVM [12,27], and our findings demonstrate that patients with lower levels of college education had a higher risk of CVM, in accordance with previous results.
In this study, the majority of patients underwent surgery (94.98%), including TURBT (84.71%), PC (1.41%), and RC (8.86%). Although only 1161 (5.30%) patients in this study did not receive surgery, the SMR (SMR [95% CI]:1.49 [1.40–1.57]) was the highest. Multivariate competing risk analysis showed that patients with BC who underwent RC had the lowest risk of developing CVM (HR [95% CI]: 0.715 [0.652–0.784]). This is probably explained by the fact that most patients who underwent RC surgery had an advanced tumor stage and did not have enough life expectancy to experience a CVM event (median survival time: 28 months for TURBT, 20 months for PC, 16 months for RC, and 11 months for no surgery). Our results show that patients with BC who are not treated with surgery have the highest risk of CVM, although the median survival time was only 11 months. One possible reason is that the diagnosis of BC often causes a longer period of psychological and emotional distress, and untreated BC often progresses rapidly, which would result in patients being at a higher risk of experiencing CVM [28,29].
Many risk factors are shared between cancer and CVD, such as smoking, radiation, air pollution, and metabolic syndrome [30]. Recent studies have shown that there is also a direct interplay between cancer and CVD, with anthracyclines having significant cardiotoxic effects that can lead to CVD such as heart failure or atherosclerosis during or years after anticancer treatment [31,32,33]. In addition to risk factors, the genetic background plays an important role in the interaction between cancer and CVD [30]. Studies have shown that mutations in age-related clonal hematopoiesis of indeterminate potential accelerate the development of CVD such as atherosclerosis or coronary artery disease [34,35,36], further worsening the prognosis of patients with heart failure [37]. Recent studies have demonstrated that heart failure can promote the transition to the pre-tumor stage and tumor growth [38,39,40]. In cancer patients, there is a high incidence of cardiomyopathy-related mutations, such as the DNA damage response/repair system, and mutations in the DNA damage response/repair system gene increase the risk of cardiotoxicity with anticancer therapy [41]. Meanwhile, pathophysiological alterations in hereditary cardiomyopathy can promote cancer development and progression and may further increase the cardiotoxic effects of anticancer therapies [30,42,43].
There are still some shortcomings in our study. First, information related to CVD, such as smoking, alcohol consumption, and the presence of congenital diseases, was not recorded in the SEER database. Second, there was no further analysis of the effects of chemotherapy, radiotherapy, and some other new therapeutic strategies on CVM. Meanwhile, some studies reported that the causes of CVM on death certificates might have been overestimated [44], which might have affected the accuracy of our study to some extent.
5. Conclusions
In summary, patients with BC have a significantly increased risk of developing CVM compared to the general population. This suggests that early screening for CVD and the assessment and monitoring of risk factors for CVM should be performed after the diagnosis of BC. It also provides important guidance on how BC patients should be followed up and educated about their associated health risks. In addition, further investigations are needed to understand the mechanisms by which BC patients develop CVD, and to design effective prevention and monitoring strategies.
Acknowledgments
The authors would like to thank all working group members for their contribution to this study.
Abbreviations
BC: bladder cancer; CVM: cardiovascular mortality; SMR: standardized mortality ratio; CVD: cardiovascular disease; 95% CI: 95% confidence interval; SEER: Surveillance, Epidemiology, and End Results; CIF: cumulative incidence function; API: Asian or Pacific Islander; AIAN: American Indian/Alaska Native; Tcc: transitional cell carcinoma; Scc: squamous cell carcinoma; Ac: adenocarcinoma; Nec: neuroendocrine carcinoma; Oet: other epithelial tumors; TURBT: transurethral resection of bladder tumor; PC: partial cystectomy; RC: radical cystectomy.
Author Contributions
S.W. had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. S.W., C.G. and J.Z. contributed to the writing of the manuscript. C.G. and J.Z. conceptualized the research, supervised the work, and contributed to the editing and writing of the final manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This public information provided by the SEER program does not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found in the SEER database (https://seer.cancer.gov/, accessed on 22 November 2021).
Conflicts of Interest
The authors declare that they have no conflict of interest that might be relevant to the contents of this manuscript.
Funding Statement
This research was funded by the Kuanren Talents Program of Chongqing Medical University (Grants No. KY2019Y026) and the National Natural Science Foundation of China (Grants No. 81803057) from Junyong Zhang.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Richters A., Aben K., Kiemeney L. The global burden of urinary bladder cancer: An update. World J. Urol. 2020;38:1895–1904. doi: 10.1007/s00345-019-02984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Lenis A.T., Lec P.M., Chamie K., Mshs M.D. Bladder Cancer: A Review. JAMA. 2020;324:1980–1991. doi: 10.1001/jama.2020.17598. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochanek K.D., Murphy S.L., Xu J., Arias E. Deaths: Final Data for 2017. Natl. Vital Stat. Rep. 2019;68:1–77. [PubMed] [Google Scholar]
- 6.Sturgeon K.M., Deng L., Bluethmann S.M., Zhou S., Trifiletti D.M., Jiang C., Kelly S.P., Zaorsky N.G. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur. Heart J. 2019;40:3889–3897. doi: 10.1093/eurheartj/ehz766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaitanidis A., Spathakis M., Tsalikidis C., Alevizakos M., Tsaroucha A., Pitiakoudis M. Risk factors for cardiovascular mortality in patients with colorectal cancer: A population-based study. Int. J. Clin. Oncol. 2019;24:501–507. doi: 10.1007/s10147-018-01382-x. [DOI] [PubMed] [Google Scholar]
- 8.Felix A.S., Bower J.K., Pfeiffer R.M., Raman S.V., Cohn D.E., Sherman M.E. High cardiovascular disease mortality after endometrial cancer diagnosis: Results from the Surveillance, Epidemiology, and End Results (SEER) Database. Int. J. Cancer. 2017;140:555–564. doi: 10.1002/ijc.30470. [DOI] [PubMed] [Google Scholar]
- 9.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung C., Fossa S.D., Milano M.T., Sahasrabudhe D.M., Peterson D.R., Travis L.B. Cardiovascular Disease Mortality After Chemotherapy or Surgery for Testicular Nonseminoma: A Population-Based Study. J. Clin. Oncol. 2015;33:3105–3115. doi: 10.1200/JCO.2014.60.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun S., Wang W., He C. Cardiovascular Mortality Risk among Patients with Gastroenteropancreatic Neuroendocrine Neoplasms: A Registry-Based Analysis. Oxid. Med. Cell Longev. 2021;2021:9985814. doi: 10.1155/2021/9985814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q., Jiang C., Zhang Y., Zhang Y., Yue B., Zheng-Lin B., Zhao Y., Mauro M.J. Cardiovascular mortality among chronic myeloid leukemia patients in the pre-tyrosine kinase inhibitor (TKI) and TKI eras: A surveillance, epidemiology and end results (SEER) analysis. Leuk. Lymphoma. 2020;61:1147–1157. doi: 10.1080/10428194.2019.1711074. [DOI] [PubMed] [Google Scholar]
- 13.Austin P.C., Fine J.P. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat. Med. 2017;36:4391–4400. doi: 10.1002/sim.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye Y., Otahal P., Marwick T.H., Wills K.E., Neil A.L., Venn A.J. Cardiovascular and other competing causes of death among patients with cancer from 2006 to 2015: An Australian population-based study. Cancer Am. Cancer Soc. 2019;125:442–452. doi: 10.1002/cncr.31806. [DOI] [PubMed] [Google Scholar]
- 15.Oh C.M., Lee D., Kong H.J., Lee S., Won Y.J., Jung K.W., Cho H. Causes of death among cancer patients in the era of cancer survivorship in Korea: Attention to the suicide and cardiovascular mortality. Cancer Med. 2020;9:1741–1752. doi: 10.1002/cam4.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Man D., Wu J., Shen Z., Zhu X. Prognosis of patients with neuroendocrine tumor: A SEER database analysis. Cancer Manag. Res. 2018;10:5629–5638. doi: 10.2147/CMAR.S174907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaorsky N.G., Churilla T.M., Egleston B.L., Fisher S.G., Ridge J.A., Horwitz E.M., Meyer J.E. Causes of death among cancer patients. Ann. Oncol. 2017;28:400–407. doi: 10.1093/annonc/mdw604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang F., Keating N.L., Mucci L.A., Adami H.O., Stampfer M.J., Valdimarsdottir U., Fall K. Immediate risk of suicide and cardiovascular death after a prostate cancer diagnosis: Cohort study in the United States. J. Natl. Cancer Inst. 2010;102:307–314. doi: 10.1093/jnci/djp537. [DOI] [PubMed] [Google Scholar]
- 19.Huang W., Aune D., Ferrari G., Zhang L., Lan Y., Nie J., Chen X., Xu D., Wang Y., Rezende L.F.M. Psychological Distress and All-Cause, Cardiovascular Disease, Cancer Mortality Among Adults with and without Diabetes. Clin Epidemiol. 2021;13:555–565. doi: 10.2147/CLEP.S308220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong M.D., Chung A.K., Boscardin W.J., Li M., Hsieh H.J., Ettner S.L., Shapiro M.F. The contribution of specific causes of death to sex differences in mortality. Public Health Rep. 2006;121:746–754. doi: 10.1177/003335490612100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu F., Liu Y., Sun X., Yin Z., Li H., Deng K., Zhao Y., Wang B., Ren Y., Liu X., et al. Race- and sex-specific association between alcohol consumption and hypertension in 22 cohort studies: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2020;30:1249–1259. doi: 10.1016/j.numecd.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., Himmelfarb C.D., Khera A., Lloyd-Jones D., McEvoy J.W., et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messner B., Bernhard D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014;34:509–515. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 24.Dhindsa D.S., Khambhati J., Schultz W.M., Tahhan A.S., Quyyumi A.A. Marital status and outcomes in patients with cardiovascular disease. Trends Cardiovas. Med. 2020;30:215–220. doi: 10.1016/j.tcm.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Aizer A.A., Chen M.H., McCarthy E.P., Mendu M.L., Koo S., Wilhite T.J., Graham P.L., Choueiri T.K., Hoffman K.E., Martin N.E., et al. Marital status and survival in patients with cancer. J. Clin. Oncol. 2013;31:3869–3876. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallo L.C., Troxel W.M., Matthews K.A., Kuller L.H. Marital status and quality in middle-aged women: Associations with levels and trajectories of cardiovascular risk factors. Health Psychol. 2003;22:453–463. doi: 10.1037/0278-6133.22.5.453. [DOI] [PubMed] [Google Scholar]
- 27.Williams J., Allen L., Wickramasinghe K., Mikkelsen B., Roberts N., Townsend N. A systematic review of associations between non-communicable diseases and socioeconomic status within low- and lower-middle-income countries. J. Glob. Health. 2018;8:020409. doi: 10.7189/jogh.08.020409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kubzansky L.D., Huffman J.C., Boehm J.K., Hernandez R., Kim E.S., Koga H.K., Feig E.H., Lloyd-Jones D.M., Seligman M.E.P., Labarthe D.R. Positive Psychological Well-Being and Cardiovascular Disease: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018;72:1382–1396. doi: 10.1016/j.jacc.2018.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esler M. Mental stress and human cardiovascular disease. Neurosci. Biobehav. Rev. 2017;74:269–276. doi: 10.1016/j.neubiorev.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Pfeffer T.J., Pietzsch S., Hilfiker-Kleiner D. Common genetic predisposition for heart failure and cancer. Herz. 2020;45:632–636. doi: 10.1007/s00059-020-04953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohan N., Jiang J., Dokmanovic M., Wu W.J. Trastuzumab-mediated cardiotoxicity: Current understanding, challenges, and frontiers. Antib. Ther. 2018;1:13–17. doi: 10.1093/abt/tby003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choueiri T.K., Mayer E.L., Je Y., Rosenberg J.E., Nguyen P.L., Azzi G.R., Bellmunt J., Burstein H.J., Schutz F.A.B. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J. Clin. Oncol. 2011;29:632–638. doi: 10.1200/JCO.2010.31.9129. [DOI] [PubMed] [Google Scholar]
- 33.Aghel N., Delgado D.H., Lipton J.H. Cardiovascular toxicities of BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia: Preventive strategies and cardiovascular surveillance. Vasc. Health Risk Manag. 2017;13:293–303. doi: 10.2147/VHRM.S108874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans M.A., Sano S., Walsh K. Cardiovascular Disease, Aging, and Clonal Hematopoiesis. Annu. Rev. Pathol. 2020;15:419–438. doi: 10.1146/annurev-pathmechdis-012419-032544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaiswal S., Ebert B.L. Clonal hematopoiesis in human aging and disease. Science. 2019;366:eaan4673. doi: 10.1126/science.aan4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park S.J., Bejar R. Clonal Hematopoiesis in Aging. Curr. Stem Cell Rep. 2018;4:209–219. doi: 10.1007/s40778-018-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Libby P., Sidlow R., Lin A.E., Gupta D., Jones L.W., Moslehi J., Zeiher A., Jaiswal S., Schulz C., Blankstein R., et al. Clonal Hematopoiesis: Crossroads of Aging, Cardiovascular Disease, and Cancer: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019;74:567–577. doi: 10.1016/j.jacc.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meijers W.C., Maglione M., Bakker S.J.L., Oberhuber R., Kieneker L.M., de Jong S., Haubner B.J., Nagengast W.B., Lyon A.R., van der Vegt B., et al. Heart Failure Stimulates Tumor Growth by Circulating Factors. Circulation. 2018;138:678–691. doi: 10.1161/CIRCULATIONAHA.117.030816. [DOI] [PubMed] [Google Scholar]
- 39.Hasin T., Gerber Y., McNallan S.M., Weston S.A., Kushwaha S.S., Nelson T.J., Cerhan J.R., Roger V.L. Patients with heart failure have an increased risk of incident cancer. J. Am. Coll. Cardiol. 2013;62:881–886. doi: 10.1016/j.jacc.2013.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banke A., Schou M., Videbaek L., Møller J.E., Torp-Pedersen C., Gustafsson F., Dahl J.S., Køber L., Hildebrandt P.R., Gislason G.H. Incidence of cancer in patients with chronic heart failure: A long-term follow-up study. Eur. J. Heart Fail. 2016;18:260–266. doi: 10.1002/ejhf.472. [DOI] [PubMed] [Google Scholar]
- 41.Broustas C.G., Lieberman H.B. DNA damage response genes and the development of cancer metastasis. Radiat. Res. 2014;181:111–130. doi: 10.1667/RR13515.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui H., Zuo S., Liu Z., Liu H., Wang J., You T., Zheng Z., Zhou Y., Qian X., Yao H., et al. The support of genetic evidence for cardiovascular risk induced by antineoplastic drugs. Sci. Adv. 2020;6:eabb8543. doi: 10.1126/sciadv.abb8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Habibian M., Lyon A.R. Monitoring the heart during cancer therapy. Eur. Heart J. Suppl. 2019;21:M44–M49. doi: 10.1093/eurheartj/suz230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Mahroos R. Validity of death certificates for coding coronary heart disease as the cause of death in Bahrain. East. Mediterr. Health J. 2000;6:661–669. doi: 10.26719/2000.6.4.661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found in the SEER database (https://seer.cancer.gov/, accessed on 22 November 2021).