Abstract
Pneumonia is a pathological process of interstitial lung tissue and distal airway and alveolar infection and infiltration. SMART-COP (systolic blood pressure, multilobar infiltrates, albumin, respiratory rate, tachycardia, confusion, oxygen, and pH) is a severity score method designed to identify individuals who require intensive respiratory or vasopressor support (IRVS) support due to pneumonia. Therefore, it is important for management decisions in pneumonia. This meta-analysis was conducted to determine the performance of the SMART-COP score in predicting the prognosis and severity of patients presenting with community-acquired pneumonia (CAP). The current meta-analysis was performed using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A systematic search was conducted using Medline, Embase, and CINAHL to identify relevant studies assessing the validity of the SMART-COP score in predicting the severity of patients with CAP. Overall, nine studies were included in the current meta-analysis. A pooled sensitivity of the SMART-COP score to predict the use of IRVS is 89% (95% CI: 84%-92%) while its specificity is 68% (95% CI: 65%-70%). The pooled sensitivity of the SMART-COP score to predict 30-day mortality is 92% (95% CI: 89%-94%) while its specificity is 39% (95% CI: 37%-42%). To summarize, SMART-COP is a new, eight-variable instrument that appears to accurately identify patients with CAP who will require IRVS and 30-day mortality. Our findings show that SMART-COP will be a valuable tool for clinicians in accurately predicting illness severity in CAP patients as compared to other scoring systems. SMART-COP can be useful to identify patients who need urgent management.
Keywords: diagnostic test accuracy, prognosis, severity, community-acquired pneumonia (cap), smart-cop
Introduction and background
Pneumonia is a pathological process of interstitial lung tissue and distal airway and alveolar infection and infiltration [1]. Its clinical definition is a group of symptoms, including tachypnea, increased sputum production, productive cough, chills, increased bronchial lung sounds, fever, or pleuritic chest discomfort, all of which are followed by chest X-ray infiltration (CXR) [2]. The incidence of pneumonia is 20% to 30% in low- and middle-income countries while in developed countries, its incidence is 3% to 4% [3-4]. It is one of the most common causes of mortality and morbidity. Based on studies, it is one of the top five causes of death in old age people. Patients who require hospital admission had the highest morbidity rates from community-acquired pneumonia (CAP), with a 30-day mortality rate of up to 13% recorded in those patients [5-6].
Even though most patients have mild symptoms, 5% present with shock, multiorgan dysfunction, or hypoxaemic respiratory failure [7]. Identification of patients who will require advanced support or who are at risk of poor prognosis is important. Several assessment tools have been developed and validated to guide physicians in managing patients with CAP. CURB-65 (confusion, uremia, respiratory rate, BP, age > 65 years) and pneumonia severity index (PSI) are two popular tools [8-9]. SMART-COP (systolic blood pressure, multilobar infiltrates, albumin, respiratory rate, tachycardia, confusion, oxygen, and pH), developed by a group of Australian academics, is one of the most recent methods for assessing pneumonia. SMART-COP is a severity score method designed to identify individuals who require intensive respiratory or vasopressor support (IRVS) support and intensive care unit (ICU) admission due to pneumonia. The SMART-COP score includes tachycardia, systolic blood pressure (SBP), oxygen saturation (SpO2), potential hydrogen (pH), and acute confusion [10]. In comparison to previous scoring systems, this score is more sensitive and specific in identifying patients at risk of severe disease and predicting the requirement for ICU care based on the likelihood of requiring intense respiratory or vasopressor support [2].
As compared to other scoring systems, SMART-COP can be useful in identifying or recognizing patients with increased risk for a severe form of the disease [2]. Therefore, it is important for management decisions in pneumonia. It is different from CURB-65 and PSI in that an important goal of these tools is the identification of seriously ill patients who need to be referred to ICU while the SMART-COP score has greater effectiveness in identifying patients who require IRVS support [10]. Because ICU admission criteria differ between regions, the current study focused on features associated with vasopressor support or intensive respiratory instead of simple ICU admission because these are more likely to be objective markers of CAP severity across institutions and health care systems.
The use of the SMART-COP score in pneumonia patients to assist Emergency Physicians in determining disease severity and predicting the need for early intensive respiratory or vasopressor support. It will reduce their ED length of stay by allowing for an earlier decision of disposition, resulting in more effective and efficient use of resources in developing countries. Therefore, this meta-analysis was conducted to determine the performance of the SMART-COP score in predicting the prognosis and severity of patients presenting with CAP.
Review
Methodology
The current meta-analysis was performed using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Search Strategy
A systematic search was conducted using Medline (PubMed), Embase, and CINAHL to identify relevant studies assessing the validity of the SMART-COP score in predicting the severity of patients with CAP. Citation searching was also done on Web of Science, Scopus, and Google Scholar to maximize sensitivity. Key terms used to search relevant articles included “SMART-COP”, “validity”, “community-acquired pneumonia”, “IRVS”, and “severity outcomes”. Boolean operators (AND, OR) were also used while searching for relevant articles. The search was performed for articles in English, and no restrictions were placed on the year of publication. Reference lists of retrieved articles were also screened to make sure a comprehensive search.
Eligibility Criteria
Eligible studies were retrospective or prospective, assessing the validity of the SMART-COP score in predicting IRVS use and 30-day mortality. The evaluation had to be done within 24 hours of hospital admission. Studies assessing the validity of SMART-COP scores in children were excluded from the current meta-analysis. Two investigators independently evaluated all studies. Non-relevant studies were excluded based on the study title and abstract. The full text was obtained of all relevant articles, and two investigators independently assessed the eligibility criteria and extracted the data from articles based on first author name, year of publication, study type, outcomes, study setting, mean age, and inclusion criteria. Any disagreement that occurred between investigators was resolved through discussion or the involvement of a third investigator.
Quality Assessment
The quality of studies was assessed by two authors independently utilizing the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool. This tool assessed the risk of bias about applicability by assessing four important domains that included the flow of patients, reference standard, index text, and patient selection through the study and timing of tests.
Data Abstraction
The primary outcome of interest was IRVS usage while secondary outcomes included admission to the ICU and 30-day mortality, which was counted per event and defined as reported in the included articles. Diagnostic accuracy measures were recorded, such as the area under the curve (AUC), specificity, sensitivity, negative predictive values (NPV), and positive predictive values (PPV), to perform a diagnostic meta-analysis.
Statistical Analysis
Data analysis was done using STATA version 16.0 (metadata and metan packages; Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC) and review manager (RevMan) software (version 5.4.1; Review Manager (RevMan) [Computer program]. The Cochrane Collaboration, 2020). The pooled sensitivity, specificity, and negative and positive likelihood ratios, along with their 95% confidence intervals, were obtained using a bivariate random-effects model. Because several specificities and sensitivities were reported in the articles at different cut-offs, a linear mixed model was utilized with a correlation structure in order to take the dependence of the measures into account. Forest plots of the specificity and sensitivity were utilized for the graphical representation of the results. The I-square index was used to measure heterogeneity, the Cochran test was used for testing heterogeneity, and a p-value less than 0.05 was considered statistically significant.
Results
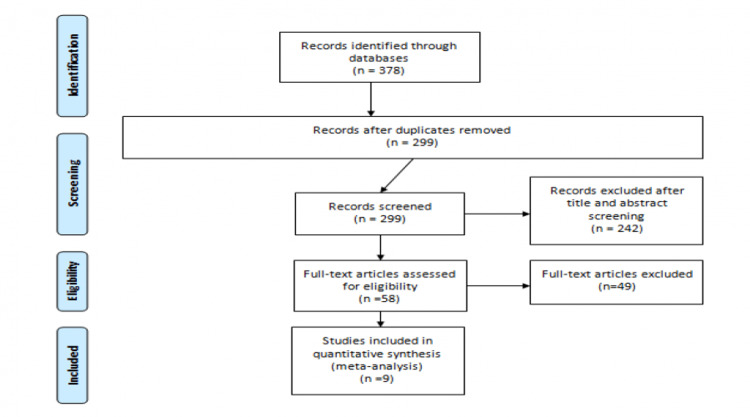
The PRISMA flow chart presenting the articles selected for this meta-analysis is shown in Figure 1. Through a systematic search of Medline, Embase, and CINAHL, 378 articles were retrieved, among which 79 were duplicates. Of the 299 articles, 242 articles were excluded based on abstract and title. The full text of 58 articles was obtained and reviewed for eligibility criteria. Overall, eight articles fulfilled the eligibility criteria. One article was identified by manual searching of the references. Overall, nine articles were included in the current meta-analysis. Table 1 shows the characteristics of the included studies. Among all included studies, eight were prospective [2,10-16] while one was conducted retrospectively [17]. Most of the included studies used three as a cut-off of the SMART-COP score to predict IRVS need and 30-day mortality in patients [10-15,17].
Table 1. Characteristics of included studies.
CAP: community-acquired pneumonia; IRVS: intensive respiratory or vasopressor support
| Author | Year of Publication | Study Design | Outcomes | Sample Size | Cut-off of SMART-COP Score | Mean Age | Inclusion Criteria |
| Alici et al [11] | 2015 | Prospective | Need of IRVS | 84 | More than or equal to 3 | 58.6 Years | - |
| Chalmer et al [12] | 2008 | Prospective | Need of IRVS | 335 | More than or equal to 3 | <50 years | Patients less than 50 years of age and presenting with a new infiltrate on a chest radiograph. |
| Charles et al [10] | 2008 | Prospective | Need of IRVS, 30-day mortality | 862 | More than or equal to 3 | - | Age of at least 18 years, at least 1 symptom of CAP, CXR changes |
| Davis et al [13] | 2010 | Prospective | Need of IRVS | 184 | More than or equal to 3 | 50.1 Years | Adult patients with sepsis |
| Ehsanpoor et al [2] | 2019 | Prospective | Need of IRVS, 30-day mortality | 143 | More than or equal to 5 | 68.13 Years | Patients with age older than 18 years old, having at least 3 specific clinical presentations and signs of pneumonia |
| Fukuyama et al [14] | 2011 | Prospective | 30-day mortality | 298 | More than or equal to 3 | 76 Years | Patients with age greater than or equal to 18 patients and admitted to the hospital with pneumonia |
| Hamza et al [15] | 2019 | Prospective | 30-day mortality | 76 | More than or equal to 3 | 59.32 Years | Patients with age greater than or equal to 18 patients and admitted to the hospital with pneumonia |
| Masuduzzaman et al [16] | 2020 | Prospective | 30-day mortality | 54 | More than or equal to 4 | 46.74 Years | Patients with age greater than or equal to 18 patients and admitted to the hospital with pneumonia |
| Williams et al [17] | 2018 | Retrospective | 30-day mortality | 618 | More than or equal to 3 | 56 Years | Patients with age greater than or equal to 18 patients and admitted to the hospital with pneumonia |
Figure 1. PRISMA flow diagram showing the selection of articles for review.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Risk of Bias
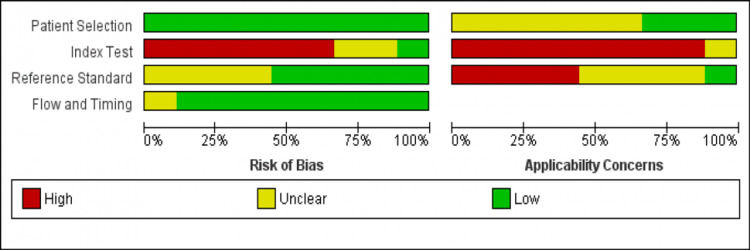
The results of quality assessment using the QUADAS-2 tool are represented in Figure 2. Overall, the applicability of included studies was moderate. All included studies did not use a pre-assigned index test cut-off.
Figure 2. Quality assessment.
Predictive Value of the SMART-COP Score to Predict the Need for IRVS
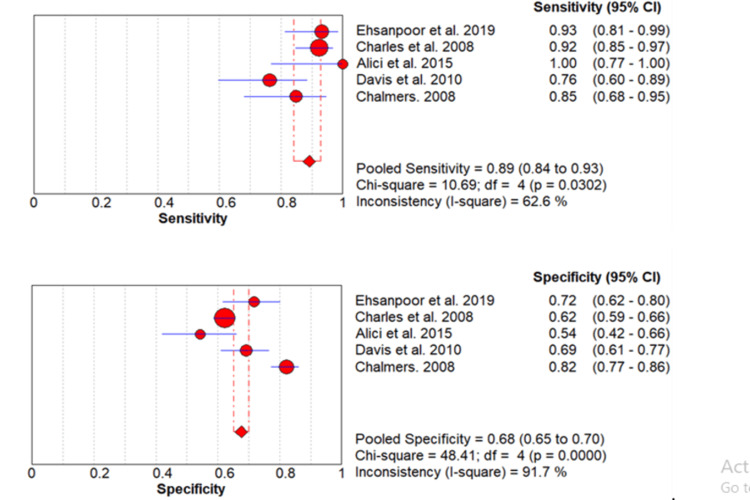
The rate of IRVS use in the included studies ranged from 6.38% to 65.48% [2,10-13] as shown in Table 2, and the pooled incidence of IRVS use for the five articles was 12% (95% CI: 11%-14%). There was significant heterogeneity (p-value=0.001). A meta-analysis of the predictive value of SMART-COP score for IRVS use was performed by including five studies. A pooled sensitivity of the SMART-COP score to predict the use of IRVS is 89% (95% CI: 84%-93%) while its specificity is 68% (95% CI: 65%-70%) as shown in Table 3. The forest plot is shown in Figure 3. The pooled AUC is 0.84.
Table 2. Rate of IRVS need and 30-day mortality in included studies.
IRVS: intensive respiratory or vasopressor support
| Studies | IRVS (%) | Mortality (%) |
| Alici et al, 2015 [11] | 65.48 | 7.14 |
| Chalmers et al, 2008 [12] | 16.42 | - |
| Charles et al, 2008 [10] | 6.38 | - |
| Davis et al, 2010 [13] | 29.89 | - |
| Ehsanpoor et al, 2019 [2] | 38.46 | 20.28 |
| Fukuyama et al, 2011 [14] | - | 10.07 |
| Hamza et al, 2019 [15] | - | 22.37 |
| Masuduzzaman et al, 2020 [16] | - | 5.56 |
| Williams et al, 2021 [17] | - | 23.48 |
Table 3. Meta-analysis of predictive data for IRVS and 30-day mortality.
Presented with a 95% confidence interval
IRVS: intensive respiratory or vasopressor support
| Measures | Outcomes | |
| IRVS need | 30-day mortality | |
| Pooled Sensitivity | 89 (84-93) | 92 (89-94) |
| Pooled Specificity | 68 (65-70) | 39 (37-42) |
| Pooled positive LR | 4.14 (2.68-6.39) | 1.95 (1.56-2.44) |
| Pooled negative LR | 0.39 (0.31-0.49) | 0.07 (0.02-0.20) |
| Pooled AUC | 0.84 (0.79-0.89) | 0.51 (0.44-0.57) |
| Diagnostic odds ratio | 17.89 (9.67-33.09) | 15.89 (4.67-54.02) |
Figure 3. Pooled sensitivity and specificity for IRVS.
Values are shown with 95% confidence intervals.
Red circles are showing the sensitivity and specificity of individual studies while diamonds are showing pooled sensitivity and specificity. Lines are showing a 95% confidence interval.
IRVS: intensive respiratory or vasopressor support
Predictive Value of the SMART-COP Score to Predict 30-Day Mortality
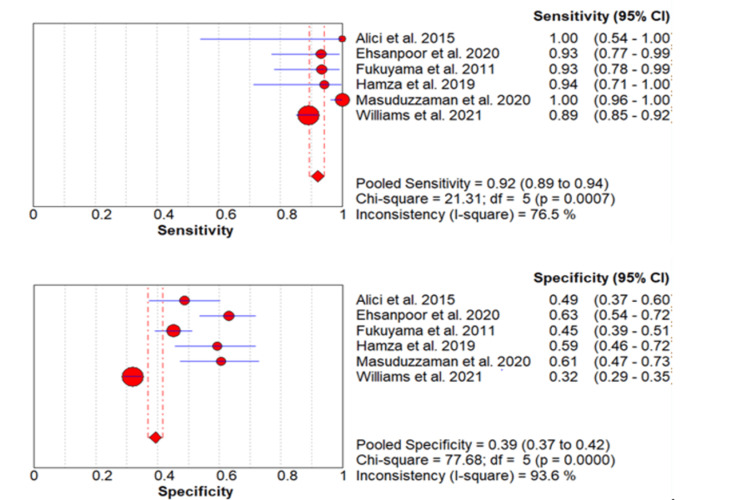
The incidence of 30-day mortality in the included studies ranged from 5.56% to 23.48% [2,11,14-17] as shown in Table 2. The pooled incidence of 30-day mortality for the six studies was 18% (95% CI: 16%-19%). A meta-analysis of the predictive value of the SMART-COP score for 30-day mortality was performed by including six studies. The pooled sensitivity of the SMART-COP score to predict 30-day mortality is 92% (95% CI: 89%-94%) while its specificity is 39% (95% CI: 37%-42%) as shown in Table 3. The forest plot is shown in Figure 4. The pooled AUC is 0.51.
Figure 4. Pooled sensitivity and specificity for 30-day mortality.
Values are shown with 95% confidence intervals
Red circles are showing the sensitivity and specificity of individual studies while diamonds are showing pooled sensitivity and specificity. Lines are showing a 95% confidence interval.
IRVS: intensive respiratory or vasopressor support
Discussion
Current assessment tools to predict pneumonia severity, like CURB-65 and PSI, are used to predict ICU admission and 30-day mortality among pneumonia patients. However, this outcome is based on the comorbid illness and age of the patients. Therefore, these tools cannot be used to predict the need for IRVS among patients with CAP [18]. In reality, clinicians may use such characteristics to determine whether a patient's situation may be classified as NFR, meaning it is not fit for vigorous medical treatment. It was identified from the current meta-analysis that the sensitivity of the SMART-COP score to predict IRVS need and 30-day mortality is 89% and 92%, respectively. The current meta-analysis also found that increasing the SMART-COP score was associated with an increased likelihood of IRVS need and 30-day mortality.
A meta-analysis was conducted by Marti et al. to compare various scoring systems in determining the prognosis of pneumonia. The study concluded that new severity scores of CAP in predicting ICU admission and need for IRVS, including SMART-COP score, SCAP (severe community-acquired pneumonia) score, and ATS/IDSA (American Thoracic Society/Infectious Diseases Society of America) 2007 minor criteria, had better discrimination performance as compared to the CURB-65 and PSI [19]. The optimal cut-off for the SMART-COP score for predicting the need for IRVS has varied in different studies [2,10-17]. Certain studies have identified optimal cut-off based on ROC curves and the Youden index, but the values range anywhere from 3 to 5, with slight variation in specificity and sensitivity.
We noticed that the present scores did not include any acute-phase inflammatory markers, but preliminary data suggested that certain markers, such as procalcitonin, could improve the risk validity and strength of the score, hence boosting its value in predicting disease prognosis [20]. Researchers are expected to assess topographies specifically associated with receiving intensive respiratory support (i.e., mechanical ventilation (invasive or noninvasive) or vasopressors therapy to support blood pressure), not just ICU admission, because the principles for ICU admission differ among hospitals, ICUs, and countries [21]. These factors are expected to play a role in determining the severity of CAP in hospitals and care facilities.
While clinical judgment is the most important factor in predicting the severity of pneumonia, clinicians can use high-sensitivity scoring systems for pneumonia risk stratification to know about patients who may need closer monitoring or more aggressive treatment [14]. In the current meta-analysis, it was discovered that SMART-COP was effective in predicting the need for IRVS in our sample. The patient population was diverse, with the majority of them having numerous serious underlying conditions such as diabetes, renal, hepatic, or cardiac failure, obstructive lung disease, and so on. We can use SMART-COP with confidence to identify patients who are at high risk of severe pneumonia and require immediate treatment.
The current meta-analysis has certain limitations. First, studies included in this meta-analysis used different cut-offs, as only two studies used a cut-off of 4 and 5 while other studies used a cut-off of 3 to predict the outcomes of CAP. As only two studies used different cut-offs, we were not able to perform a sub-group analysis. In the future, more studies need to be conducted prospectively, including a larger sample size to determine the cut-off applicable to the general population with CAP. In addition, owing to the differences in study design, methodology, and patient population, this meta-analysis is limited by the heterogeneity of the included studies
Conclusions
In the end, the current study found that the pooled sensitivity of the SMART-COP score to predict the need for IRVS and 30-day mortality is 89% and 92%, respectively. To summarize, SMART-COP is a new, eight-variable instrument that appears to accurately identify patients with CAP who will require IRVS. Our findings show that SMART-COP will be a valuable tool for clinicians in accurately predicting illness severity in CAP patients. Predicting outcomes in CAP is a major safety concern, and physicians can intervene correctly and quickly by using severity risk categorization.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.New perspectives on community-acquired pneumonia in 388 406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Ewig S, Birkner N, Strauss R, et al. Thorax. 2009;64:1062–1069. doi: 10.1136/thx.2008.109785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Validity of SMART-COP score in prognosis and severity of community acquired pneumonia in the emergency department. Ehsanpoor B, Vahidi E, Seyedhosseini J, Jahanshir A. Am J Emerg Med. 2019;37:1450–1454. doi: 10.1016/j.ajem.2018.10.044. [DOI] [PubMed] [Google Scholar]
- 3.Prognostic factors for serious morbidity and mortality from community-acquired lower respiratory tract infections among the elderly in primary care. Hak E, Bont J, Hoes AW, Verheij TJ. Fam Pract. 2005;22:375–380. doi: 10.1093/fampra/cmi020. [DOI] [PubMed] [Google Scholar]
- 4.Validation of the Infectious Diseases Society of America/American Thoracic Society criteria to predict severe community-acquired pneumonia caused by Streptococcus pneumoniae. Kontou P, Kuti JL, Nicolau DP. Am J Emerg Med. 2009;27:968–974. doi: 10.1016/j.ajem.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 5.Community-acquired pneumonia guidelines and resident behavior. Flannery MT, McCool MJ. Am J Med. 2005;118:929–930. doi: 10.1016/j.amjmed.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 6.Guideline 'Diagnosis and treatment of community-acquired pneumonia' from the Dutch Thoracic Society [Article in Dutch] Aleva RM, Boersma WG. https://pubmed.ncbi.nlm.nih.gov/16304887/ Ned Tijdschr Geneeskd. 2005;1:2501–2507. [PubMed] [Google Scholar]
- 7.Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Wu Z, McGoogan JM. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.A prediction rule to identify low-risk patients with community-acquired pneumonia. Fine MJ, Auble TE, Yealy DM, et al. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 9.Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Lim WS, van der Eerden MM, Laing R, et al. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Charles PG, Wolfe R, Whitby M, et al. Clin Infect Dis. 2008;47:375–384. doi: 10.1086/589754. [DOI] [PubMed] [Google Scholar]
- 11.Comparison of severity scoring systems in community-acquired pneumonia. Alıcı İO, Çapan N, Ertürk A, Canbakan S. Eurasian J Pulmonol. 2015;17:15–21. [Google Scholar]
- 12.Predicting the need for mechanical ventilation and/or inotropic support for young adults admitted to the hospital with community-acquired pneumonia. Chalmers JD, Singanayagam A, Hill AT. Clin Infect Dis. 2008;47:1571–1574. doi: 10.1086/593195. [DOI] [PubMed] [Google Scholar]
- 13.Pneumonia risk stratification in tropical Australia: does the SMART-COP score apply? Davis JS, Cross GB, Charles PG, Currie BJ, Anstey NM, Cheng AC. Med J Aust. 2010;192:133–136. doi: 10.5694/j.1326-5377.2010.tb03450.x. [DOI] [PubMed] [Google Scholar]
- 14.Validation of scoring systems for predicting severe community-acquired pneumonia. Fukuyama H, Ishida T, Tachibana H, et al. Intern Med. 2011;50:1917–1922. doi: 10.2169/internalmedicine.50.5279. [DOI] [PubMed] [Google Scholar]
- 15.Evaluation of two different scores in assessing the severity of community acquired pneumonia: a cross-sectional study in Ismailia, Egypt. Hamza AH, El-Mokadem AM, Sallam EM, Mahmoud ME. https://mjmr.journals.ekb.eg/article_221963_01c815af2876d772907004204e0fa842.pdf Minia Journal of Medical Research. 2019;30:288–297. [Google Scholar]
- 16.Comparative study of CURB-65, expanded CURB-65, PSI and SMART-COP scoring in the severity assessment of community acquired pneumonia. Masuduzzaman SM, Islam MS, Anam MK, et al. https://www.banglajol.info/index.php/CHJ/article/view/56974 Chest & Heart Journal. 2020;44:73–81. [Google Scholar]
- 17.Utility of community-acquired pneumonia severity scores in guiding disposition from the emergency department: intensive care or short-stay unit? Williams JM, Greenslade JH, Chu KH, Brown AF, Lipman J. Emerg Med Australas. 2018;30:538–546. doi: 10.1111/1742-6723.12947. [DOI] [PubMed] [Google Scholar]
- 18.A prospective comparison of severity scores for identifying patients with severe community acquired pneumonia: reconsidering what is meant by severe pneumonia. Buising KL, Thursky KA, Black JF, MacGregor L, Street AC, Kennedy MP, Brown GV. Thorax. 2006;61:419–424. doi: 10.1136/thx.2005.051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Marti C, Garin N, Grosgurin O, Poncet A, Combescure C, Carballo S, Perrier A. https://link.springer.com/article/10.1186/cc11447. Crit Care. 2012;16:0. doi: 10.1186/cc11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assessment of clinical applicability of pneumonia scores to determine patients with community-acquired pneumonia who will need hospital admission. Mohamed EE, Abd Allah AE. https://www.ejcdt.eg.net/article.asp?issn=0422-7638;year=2019;volume=68;issue=2;spage=224;epage=230;aulast=Mohamed Egypt J Chest Dis Tuberc. 2019;68:224. [Google Scholar]
- 21.Validation of a predictive rule for the management of community-acquired pneumonia. Capelastegui A, España PP, Quintana JM, Areitio I, Gorordo I, Egurrola M, Bilbao A. Eur Respir J. 2006;27:151–157. doi: 10.1183/09031936.06.00062505. [DOI] [PubMed] [Google Scholar]