Abstract
The increased interest in nanomedicine and its applicability for a wide range of biological functions demands the search for raw materials to create nanomaterials. Recent trends have focused on the use of green chemistry to synthesize metal and metal-oxide nanoparticles. Bioactive chemicals have been found in a variety of marine organisms, including invertebrates, marine mammals, fish, algae, plankton, fungi, and bacteria. These marine-derived active chemicals have been widely used for various biological properties. Marine-derived materials, either whole extracts or pure components, are employed in the synthesis of nanoparticles due to their ease of availability, low cost of production, biocompatibility, and low cytotoxicity toward eukaryotic cells. These marine-derived nanomaterials have been employed to treat infectious diseases caused by bacteria, fungi, and viruses as well as treat non-infectious diseases, such as tumors, cancer, inflammatory responses, and diabetes, and support wound healing. Furthermore, several polymeric materials derived from the marine, such as chitosan and alginate, are exploited as nanocarriers in drug delivery. Moreover, a variety of pure bioactive compounds have been loaded onto polymeric nanocarriers and employed to treat infectious and non-infectious diseases. The current review is focused on a thorough overview of nanoparticle synthesis and its biological applications made from their entire extracts or pure chemicals derived from marine sources.
Keywords: marine, nanoparticles, infectious disease, antimicrobial, anticancer, antioxidant, antiinflammatory, antidiabetic
1. Introduction
Infectious diseases have the potential to contribute to an increase in the global death rate [1]. Infectious diseases can be caused by viruses, fungi, and bacteria [2]. These microorganisms cause a variety of diseases, including cholera, candidiasis, and COVID-19 [3,4]. COVID-19 is a recent example that has triggered a pandemic [5]. Multiple drug resistance in viruses, fungi, and bacteria has reached alarming levels that must be addressed promptly. Various health organizations throughout the world have stated that various drug-resistant pathogenic microorganisms must be eradicated quickly [6]. Furthermore, current drugs for treating infectious diseases to patients suffering from non-infectious illnesses, such as cancer, inflammation, obesity, and diabetes, might possibly harm the human body [7,8,9]. To meet this demand, novel molecules that can function as antimicrobials against pathogenic microbes must be investigated [10]. The terrestrial ecosystem has yet to investigate the marine environment [11]. Many applications for marine compounds have been documented [12]. Secondary metabolites produced by marine microorganisms have a wide range of applications [13]. The potential biological activity of marine organisms stems from communication and defensive systems in their natural habitat [13]. Many possible antimicrobial applications from marine sources have been investigated [14].
Furthermore, due to their biodiversity and production of various molecules with varying chemical structures, marine organisms can be exploited as valuable biologics to treat cancer, inflammation, and immune system diseases [15]. As a result of their diverse biological activities, natural compounds derived from marine resources have significantly contributed to disease treatment in place of conventional pharmaceuticals [16]. Nanotechnology is a developing technology with several applications in various sectors [17]. Recent research trends have demonstrated that nanoparticles have a wide range of therapeutic potential [18]. The biosynthesis of nanoparticles is a simple and inexpensive method [19]. Furthermore, the approach of synthesizing nanoparticles from diverse natural products is extensively employed as an eco-friendly method, since it does not produce toxic by-products [20]. Various techniques have been developed to synthesize different types of inorganic nanoparticles, such as gold, zinc, titanium, magnesium, and silver [21]. The biosynthesis of nanoparticles provides antibacterial, drug delivery, sensing, and anticancer treatment. Nanoparticles produced from pure compounds, in particular, outperform traditional drugs in terms of biological activity [22]. This review paper advances our understanding of marine-derived compound nanoparticles as possible therapeutics for a variety of biological roles.
2. Green Synthesis of Nanoparticles (NPs) for Its Application in the Field of Medicine
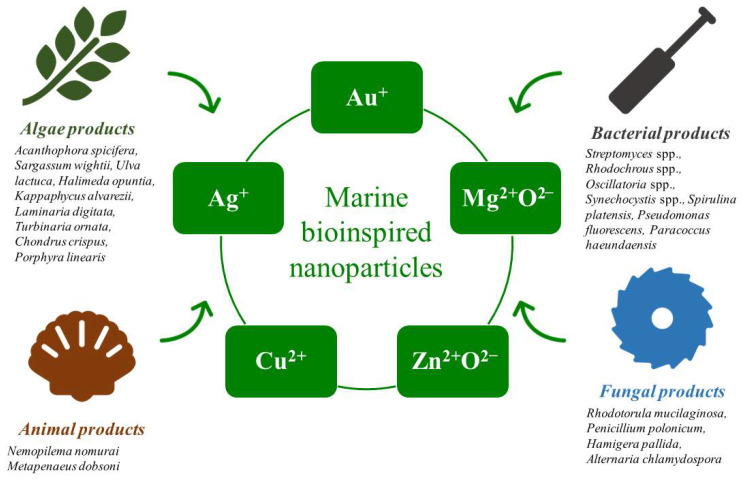
Nanotechnology is a new discipline of research that works with chemical, biological, and physical sciences to produce nanosized particles with various applications. The size range of nanoparticles has been investigated between 1–100 nm [23,24,25]. Because of their high surface area to volume ratio, nanoparticles have a substantially larger proportion of surface, which leads to enhanced reactivity [26]. Because of their small size, nanoparticles can have a variety of sizes and forms [27]. Nanoparticles have a wide range of applications, including the medicinal, diagnostic, drug discovery, biological sensor, and reagent industries [28]. These biologically active nanoparticles are produced by employing various biological fluids as reducing agents for metal and non-metal ions, such as gold, silver, copper, zinc oxide, platinum, and titanium oxide [29]. The diverse therapeutic applications of nanoparticles, as well as the outbreak of several infectious diseases, motivate this research [30]. The general approaches for nanoparticle production include bioassisted, chemical, and physical methods [31]. Researchers are currently more interested in biological entities than chemical approaches. Fungi, bacteria, plants, and algae from the marine have been found to produce nanoparticles [12]. Green synthetic nanoparticles can be easily decomposed using enzymes included in the nanoparticles, making them more environmentally benign than conventional agents [29]. The reduction of metal ions by reducing agents found in the organism is essential for the synthesis of metal nanoparticles [32]. These reactions are driven by phenolics, terpenoids, alkaloids, amines, carbonyl groups, flavanones, proteins, pigments, and amides found in the organism [33]. Because marine organisms dwell on the unexplored seabed, it is critical to understand the metabolic mechanisms leading to metal ion reduction by diverse types of marine organisms [33]. Figure 1 depicts the different marine organisms, such as algae, bacteria, fungi, and animals, employed in the synthesis of metal nanoparticles.
Figure 1.
Different types of metal and metal-oxide nanoparticles are synthesized using natural products from various marine organisms.
3. Marine Organisms and Compounds for the Green Synthesis of NPs
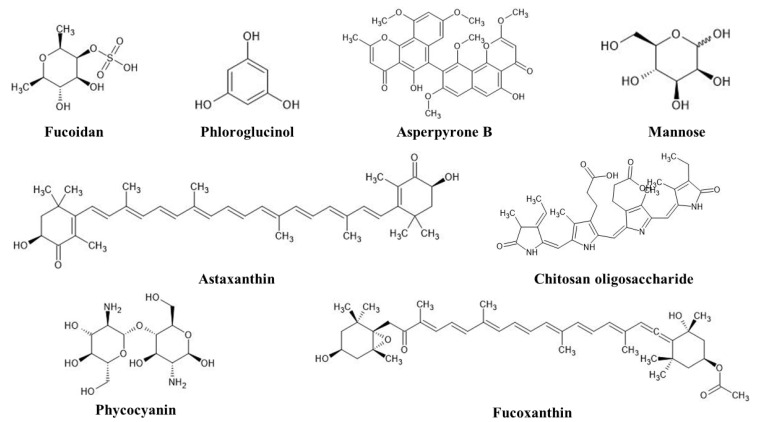
Current research and innovation in marine science are contributing to the exponential growth of numerous sectors, including pharmaceuticals, environmental trends, nanomedicine, and food [14]. The ocean covers around 70–71% of the earth’s surface [34]. Previous research studied the oceans, accounting for around 2.2 million distinct species [35]. The ocean contains an unimaginable number of marine-derived compounds with varied applications that are beneficial to humans, such as antimicrobial compounds [36]. The marine ecosystem contains around 25,000 physiologically active chemicals with various applications [37]. Currently, the marine environment paves the way for numerous antibacterial, antifungal, and antiviral compounds. Seaweeds, bacteria, and fungus are possible sources for combating infectious diseases [14,38]. According to prior research, the market for marine-derived compounds has surpassed 10 billion USD [39]. The production of marine-based nanoparticles from a variety of sources, including bacteria, fungus, seaweeds, and marine plants, has received considerable attention [33]. Algae with high cell growth rates, high stress tolerance, and an abundance of physiologically active substances, such as Ulva lactuca, Spirulina platensis, and Sargassum muticum, are regarded as promising biocatalysts for the synthesis of various types of nanomaterials [40,41,42]. Among pure algal compounds, phloroglucinol, eckol, phlorofucofuroeckol A, fucodiphlorethol G, 7-phloroeckol, 6,6′-bieckol, and dieckol act as effective reducing agents in the nanoparticle synthesis process [43]. Diverse marine microorganisms adapt to harsh marine environments as well as a broad variety of temperatures, salinity, and pH, making them suitable biological factories for green nanoparticle synthesis [44]. Bacteria and fungi produce intracellular or extracellular inorganic compounds that react with metal ions to form nanoparticles [45,46]. Furthermore, nanoparticles made from marine-derived animals show good biocompatibility [47]. Seafood waste, in particular, may be used to make a variety of biological products by utilizing its high value-added qualities during the purification process [48]. Figure 2 shows numerous pure compounds obtained from marine organisms that act as reducing agents in the nanoparticle synthesis process.
Figure 2.
Chemical structures of various pure compounds derived from marine organisms used as reducing agents in nanoparticle synthesis.
4. Marine Bioinspired NPs Used for Bacterial Infection
Table 1 summarizes a detailed review of marine-based nanoparticles utilized in treating various infectious diseases. Bacterial infection has a negative impact on public health [49]. Nanoparticles are attractive options since they have excellent bactericidal activity when treating pathogenic bacteria [50]. Several studies have been carried out to investigate the mechanisms of marine-inspired nanoparticles as antibacterial agents [51]. In general, marine antimicrobial macromolecules exhibit antibacterial mechanisms, such as (1) inhibition of DNA replication, (2) inhibition of expression of enzymes and other cellular proteins required for ATP production, (3) structural changes and damage to bacterial cell membranes, and (4) ROS production by inhibiting respiratory enzymes [52]. Several marine bacteria, including Vibrio spp., Pseudoalteromonas spp., and Ruegeria spp., generate antimicrobial compounds, a feature seen globally [53]. The marine bacterium Pseudomonas rhizosphaerae, in particular, has been shown to produce benzene-type secondary metabolites with potent antibacterial properties [54]. Secondary metabolites produced by marine algae, on the other hand, include polyphenols, terpenes, acetogenin, and aromatic compounds, which have a variety of biological functions, including antibacterial effects [55]. Silver nanoparticles derived from the marine cyanobacterium Chroococcus minutus showed antibacterial action against pathogenic strains of Escherichia coli and Streptococcus pyogenes, which have been discovered to be novel antibacterial for upper respiratory tract infection [56]. The synthesis of silver nanoparticles from cyanobacterium sources had improved control over pathogenic bacteria. Silver nanoparticles derived from the marine endophytic fungus Penicillium polonicum showed antibacterial activity against Acinetobacter baumanii, with MIC value of 15.62 µg/mL and MBC value of 31.24 µg/mL [44]. These findings were attributed to the activation of apoptosis by altering the osmotic pressure regulation of cells during the interaction of silver nanoparticles and bacteria. Silver nanoparticles using S. muticum extracts as a capping agent significantly suppressed the growth of Bacillus subtilis, E. coli, Klebsiella pneumoniae, and Salmonella Typhimurium [57]. These silver nanoparticles interacted with the bacterial membrane and penetrated the bacterium. Moreover, silver nanoparticles synthesized using S. swartzii showed antibacterial action by producing considerable deterioration in E. coli [58]. When combined with silver nanoparticles, S. wightii and Valonopsis pachynema demonstrated increased antibacterial activity against Micrococcus luteus and S. marcescens [59]. Silver nanoparticles produced from these seaweeds had a strong antibacterial activity because silver ions caused bacteria to release K+ ions. Silver nanoparticles produced from an aqueous extract of Gelidiella acerosa inhibited the growth of P. aeruginosa and B. subtilis [60]. These bacteria were discovered to absorb silver nanoparticles from the cell surface. Silver nanoparticles produced using a culture-free extract of marine Streptomyces sp. Al-Dhabi-87 had excellent antibacterial activity against wound-infecting microorganism strains such as Staphylococcus aureus, S. epidermidis, and Enterococcus faecalis [61]. These nanoparticles displayed antibacterial action by releasing intracellular components and altering the cellular structure. Secondary metabolites found in S. longifolium extract reduced CuSO4 to Cu2+, resulting in copper oxide nanoparticles [62]. These CuSO4 nanoparticles showed remarkable antibacterial activity against V. parahemolyticus, V. harvey, Aeromonas hydrophila, and Serratia marcescens.
Table 1.
List of marine-bioinspired metallic nanoparticles treating infectious diseases.
| Name of Marine-Derived Compound/Product | Sources/Organism | Name of NPs | Size Range of MNPs | Shape/Morphology | Antimicrobial Types | Microbial Pathogens | References |
|---|---|---|---|---|---|---|---|
| Extracts |
|
AgNPs | 12 nm | Spherical | Antibacterial |
|
[78] |
| Extracts | U. lactuca | AgNPs | 20–50 nm | - | Antibacterial |
|
[79] |
| Extracts |
|
SeNPs | 30, 80 nm | Spherical | Antibacterial |
|
[80] |
| Extracts | Spirulina platensis | SNPs | 200–450 nm | Spherical | Antibacterial | V. parahaemolyticus | [81] |
| Extracts | Chroococcus minutus | AgNPs | - | - | Antibacterial |
|
[56] |
| Extracts | U. lactuca | SeNP | 85 nm | Spherical | Antibacterial |
|
[82] |
| Extracts | Sargassum muticum | AgNPs | 20–54 nm | Spherical | Antibacterial |
|
[57] |
| Extracts | S. swartzii | AgNPs | 20–40 nm | Spherical | Antibacterial | E. coli | [58] |
| Extracts | Gelidium corneum | AgNPs | 20–50 nm | Spherical | Antibacterial | E. coli | [83] |
| Extracts | Laminaria ochroleuca | AgNPs | 10–20 nm | Spherical | Antibacterial |
|
[84] |
| Extracts | Streptomyces sp. Al-Dhabi-87 | AgNPs | 10–17 nm | Spherical | Antibacterial |
|
[61] |
| Extracts |
|
AgNPs | 30–40, 55–70 nm | - | Antibacterial |
|
[59] |
| Extracts |
|
AgNPs | 5.52, 35 nm | Spherical | Antibacterial |
|
[85] |
| Extracts | Gelidiella acerosa | AgNPs | - | - | Antibacterial |
|
[60] |
| Extracts | Acanthophora spicifera | AuNPs | <20 nm | Spherical | Antibacterial |
|
[86] |
| Extracts | G. amansii | AgNPs | 27–54 nm | Spherical | Antibacterial |
|
[87] |
| Extracts | S. wighitii | MgONPs | 68.06 nm | Flower | Antibacterial |
|
[88] |
| Extracts | Oscillatoria princeps | AgNPs | 3.30–17.97 nm | Spherical | Antibacterial |
|
[89] |
| Extracts | Nocardiopsis dassonvillei-DS013 | AgNPs | 30–80 nm | Circular | Antibacterial |
|
[90] |
| Extracts | Streptomyces sp. Al-Dhabi-87 | AgNPs | 11–21 nm | Cubic | Antibacterial |
|
[91] |
| Extracts | Penicillium polonicum | AgNPs | 10 nm | Spherical | Antibacterial | A. baumanii | [44] |
| Chitosan | Marine Seafood | AgNPs | 5–20 nm | Spherical | Antibacterial |
|
[92] |
| Chitosan |
|
|
|
Spherical | Antibacterial | S. aureus | [93] |
| Extracts |
|
AgNPs | 10.69, 12.83 nm | Spherical | Antibacterial |
|
[94] |
| Extracts | Cymodocea serrulata | AgNPs | 40.49–66.44 nm | - | Antibacterial | V. parahaemolyticus | [95] |
| Extracts | S. longifolium | CuONPs | 40–60 nm | - | Antibacterial |
|
[62] |
| Extracts | C. crinita | ZnONPs | 23–200 nm | Rectangular | Antibacterial |
|
[96] |
| Extracts | Synechocystis sp. | AgNPs | 10–35 nm | Spherical | Antibacterial | MRSA | [97] |
| Extracts | O. limnetica | AgNPs | 3.30–17.97 nm | Quasi-spherical | Antibacterial |
|
[98] |
| Extracts | Red algae | Co3O4NPs | 29.8 ± 8.6 nm | Spherical | Antibacterial |
|
[99] |
| Extracts | U. lactuca | AgNPs | 20–50 nm | - | Antiviral |
|
[79] |
| Extracts |
|
|
|
|
Antiviral | HSV-1 | [100] |
| Extracts |
|
AgNPs | 5.52–35.00 nm | Spherical | Antiviral | Poliovirus | [85] |
| Extracts |
|
AgNPs | 50–65, 15–30, and 40–50 nm | Spherical | Antiviral | Newcastle disease virus | [101] |
| Extracts | U. rigida | AgNPs | 12 nm | Spherical | Antifungal |
|
[78] |
| Extracts | S. griseus | AgNPs | 14.54 nm | Spherical | Antifungal | C. albicans | [102] |
| Extracts | G. corneum | AgNPs | 20–50 nm | Spherical | Antifungal | C. albicans | [83] |
| Extracts | P. fluorescens | AgNPs | - | - | Antifungal |
|
[103] |
| Extracts |
|
AgNPs | - | - | Antifungal |
|
[104] |
| Extracts |
|
AgNPs | 10.69,12.83 nm | Spherical | Antifungal |
|
[94] |
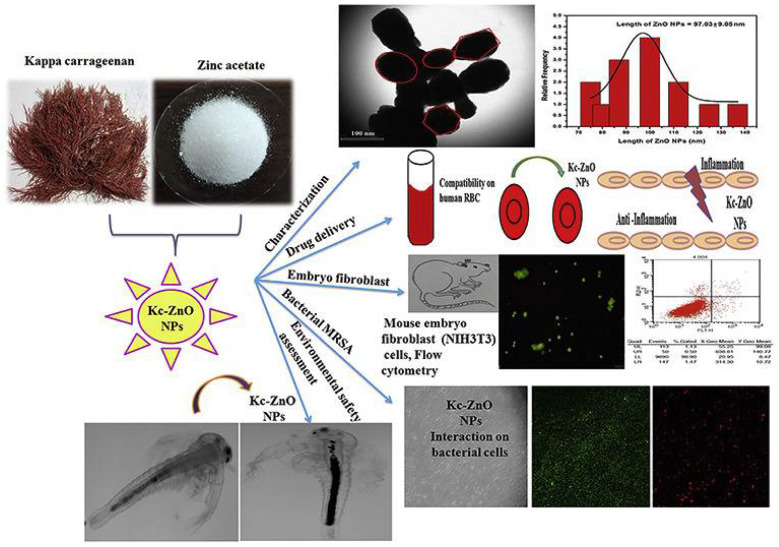
Carrageenan is a water-soluble, high-molecular-weight, sulfated polysaccharide isolated from many species of red algae. Carrageenan has been widely used in the pharmaceutical, medical, and food industries due to its high viscosity, gelling capacity, and biocompatibility [63,64]. Vijayakumar et al. [65] synthesized Kappa-carrageenan wrapped zinc-oxide nanoparticles (KC-ZnONPs) with antibacterial and antibiofilm activity against Methicillin-resistant S. aureus (MRSA) (Figure 3). Based on hemocompatibility studies on human RBCs and eco-safety studies using Artemia salina, the synthesized KC-ZnONPs showed high biocompatibility and were non-toxic to the environment.
Figure 3.
Synthesis of Kappa-Carrageenan wrapped Zinc-oxide nanoparticles (KC-ZnONPs) as an antibacterial agent against Methicillin-resistant Staphylococcus aureus. Reprinted with permission from reference [65]. Copyright, 2019 Elsevier B.V.
Fucoidan, a pure chemical derived from Fucus vesiculosus, was used to synthesize gold nanoparticles, which demonstrated antibacterial action against P. aeruginosa (MIC value of 512 µg/mL) [66]. In addition, the fucoidan-gold nanoparticles reduced the production of virulence factors, such as rhamnolipid, pyocyanin, and pyoverdine. Due to the presence of mannose, the capsular polymeric material isolated from marine B. altitudinis proved efficient as a stabilizer for CuO nanoparticle synthesis [67]. The MIC value of CuO nanoparticles containing mannose against P. aeruginosa was 1.0 µg/mL. Silver nanoparticles synthesized by the marine fungus Aspergillus flavus utilizing amylase showed antibacterial efficacy against Gram-positive and Gram-negative bacteria [68]. In particular, amylase-silver nanoparticles had the strongest antibacterial activity against A. hydrophila (MIC value of 1.6 µg/mL). Khan et al. [69] synthesized gold nanoparticles from chitosan oligosaccharide, a natural marine compound, to treat P. aeruginosa biofilm infections. Chitosan oligosaccharide-gold nanoparticles exhibited antibiofilm efficacy by lowering bacterial hemolysis and P. aeruginosa virulence factors. P. aeruginosa hemolysis and protease activity were reduced by a nanocomposite of chitosan and polypyrrole [70]. Moreover, the production of various virulence factors, such as rhamnolipid, pyoverdine, and pyocyanin was reduced by this nanocomposite.
5. Marine Bioinspired NPs Used for Fungal Infection
Fungal infection is a constant cause of death [71]. The number of fungal infection cases is increasing, and it has been claimed that over 150 million fungal infections occur yearly, with a 1.5 million death rate from fungal infection [72]. Several secondary metabolites with antifungal action are produced by marine microorganisms, mammals, and algae, similar to antibacterial activity [73]. Antifungal chemicals are produced by a variety of marine species, including bacterial chitinases, lipopeptides, and lactones [74]. Brown algae phlorotannins, on the other hand, have antifungal activity by altering the composition of ergosterol in the yeast cell membrane [75]. The marine depsipeptidepapuamide A has been shown to trigger fungus apoptosis by binding to phosphatidylserine in the cell membrane and entering the plasma membrane [76]. Plakortide F acid, a polyketide endoperoxide produced from marine sponges, also had antifungal activity through affecting Ca2+ homeostasis [77]. As a result, many researchers continue to look for antifungal activity in a variety of marine organisms for application to nanoparticles. Green synthesis of silver nanoparticles from U. rigida had antifungal action against the fungus Trichophyton mantigrophytes and T. cutaneum, which are linked toskin infections [78].
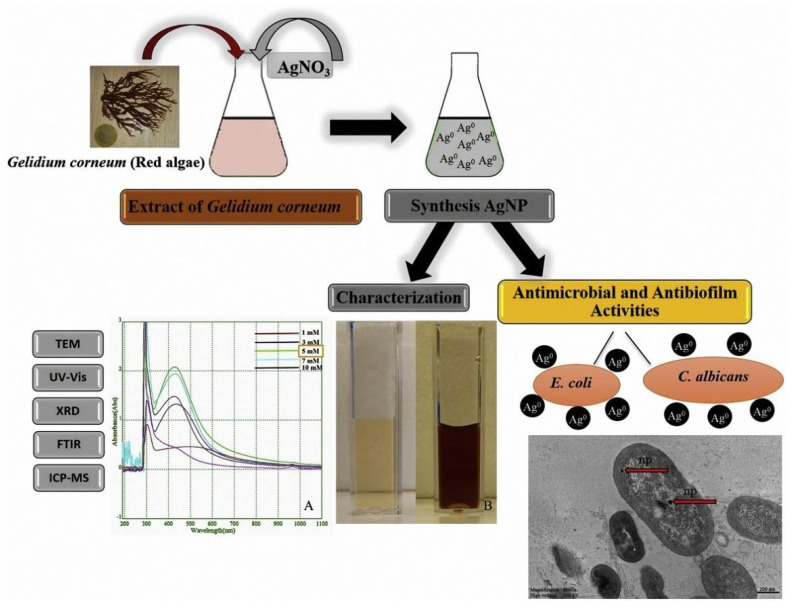
These silver nanoparticles produced an insoluble chemical that inactivated the fungal cell wall’s sulfhydryl group and disrupted the membrane, resulting in an antifungal effect. Silver nanoparticles synthesized from aqueous extracts of Cymodocea serrulata and Padina australis had antifungal action against plant fungi, including Pyriporia oryzea, Alternaria sp., Helminthisporium oryzea, Rhizoctonia solani, and Xanthomanas oryzae [104]. These antifungals were discovered as a consequence of cell wall disruption, DNA damage, and an increase in ROS. Silver nanoparticles (AgNPs) synthesized from Gelidium corneum extract, which served as a reducing agent had excellent antifungal and antibiofilm properties against Candida albicans [83]. Biosynthetic silver nanoparticles, in particular, demonstrated antifungal effectiveness by generating cell membrane and cell wall destruction, as well as cytoplasmic damage (Figure 4).
Figure 4.
Synthesis and characterization of AgNPs using extract of marine red algae Gelidium corneum with antimicrobial and antibiofilm inhibition characteristics towards Escherichia coli and Candida albicans. (A) UV-vis absorption spectra of AgNPs synthesized using extract in the presence of different silver salt concentrations and (B) Change in color of the reaction mixture indicates the formation of AgNPs. Reprinted with permission from reference [83]. Copyright, 2019 Elsevier Ltd.
6. Marine Bioinspired NPs for Treating Viral Infection
A viral particle is smaller than a live cell. Several viral infections have been documented to be caused by a pathogenic virus. Viruses cause a variety of diseases, leading to increased death rate. Viral infections include smallpox, polio, HIV, and hepatitis C [105]. Antiviral compounds produced by marine organisms include polyphenols, alkaloids, lipids, carbohydrates, steroids, terpenoids, exopolysaccharides, polyketides, zoanthoxanthins, and peptides [106]. Virus adsorption, penetration, capsid decoration, biosynthesis, virus assembly, and virus release are all inhibited or inactivated by marine polysaccharides [107]. One of the metabolites produced by marine organisms, phlorotannins, has been shown to interfere with viral attachment, penetration, and replication [15]. Silver nanoparticles derived from the seaweed U. lactuca showed cytotoxic efficacy against the vector-borne pathogens Aedes aegypti and Culex pipiens [79]. Because of their small particle size, silver nanoparticles synthesized from U. lactuca demonstrated more effective action than conventional insecticides. Additionally, these silver nanoparticles bonded to the insect cuticle and entered within the cell, disrupting additional cell functions. Silver nanoparticles mediated by Oscillatoria sp. and gold nanoparticles mediated by S. platensis displayed antiviral efficacy against herpesvirus [100]. These nanoparticles induced glycoprotein aggregation and surface changes, both of which might inhibit viral binding and penetration. Silver nanoparticles derived from extracellular extracts of the marine actinomycetes Rhodococcus rhodochrous and Streptomyces sp. inhibited poliovirus in RD cells [85]. The interaction of viral proteins with silver nanoparticles caused poliovirus inhibition. Silver nanoparticles derived from Dictyosphaerium sp., a freshwater microalgae, had substantial antiviral activity against the Newcastle disease virus [101]. These silver nanoparticles were bound to the viral glycoprotein envelope, limiting virus penetration.
7. Marine Bioinspired NPs for Treating Non-Infectious Diseases
Table 2 shows studies that have used marine bioinspired nanoparticles to treat a wide range of non-infectious diseases. Inflammation is the body’s natural response to tissue injury, infection, and genetic alterations [108]. The immune system is activated within the body under inflammatory circumstances, resulting in the release of various inflammatory mediators, such as bradykinins and prostaglandins [109]. Thus, reducing prostaglandin levels can aid in the prevention of chronic disease by controlling inflammation [110]. To reduce inflammation and inflammatory mediators, steroids and nonsteroidal antiinflammatory medications constitute one of the treatment paths for inflammatory diseases [111]. These synthetic antiinflammatory drugs, on the other hand, can have substantial negative effects [112]. As a result, it is required to use marine-based organisms with high biological activity to generate nanoparticles with similar therapeutic benefits and no negative effects. Silver nanoparticles produced from macroalgae such as Galaxaura elongate, Turbinaria ornate, and Enteromorpha flexuosa have shown considerable antiinflammatory action via membrane stabilization [113]. Silver nanoparticles, in particular, reduced prostaglandin production by inhibiting protein denaturation, cyclooxygenase, and 5-lipoxygenase. At 500 µg/mL, ZnO nanoparticles wrapped in Kappa-carrageenan demonstrated 82% antiinflammatory efficacy (Figure 3) [65]. Because of their high surface area to volume ratio, these nanoparticles were more effective than bulk materials in inhibiting cytokines and inflammatory coenzymes. Cancer is caused by the uncontrollable growth of cells and tissues [114]. Cancer treatment options include surgery, radiation, and potentially toxic medication therapy [115]. As a result, several investigations are being done to discover anticancer drugs that kill cancer cells without hurting humans [116]. Nanoparticles loaded with various physiologically active chemicals are one of the most effective drug delivery techniques for cancer therapy [117]. Marine-derived natural products, in particular, are potential molecules for the development of anticancer drugs because they may influence multiple pathways, such as immunity, cancer cell death, and tumor growth [118]. Silver nanoparticles derived from Caulerpa taxifolia showed antitumor efficacy against A549 lung cancer cells [119]. Necrosis and condensation of A549 cells were shown to be mediated by silver nanoparticles derived from marine algae, suggesting that nanomaterials are relevant for cancer cell research. Furthermore, gold nanoparticles inhibited phosphorylation of AKT and ERK, which are essential for cell growth in HeLa cancer cells [47]. Interestingly, these gold nanoparticles derived from jellyfish extract exhibited a significant lethal effect on HeLa cancer cells. Cu2O nanoparticles derived from Rhodotorula mucilaginosa showed anticancer activity against SKOV-3, MCF-7, HepG2, A549, SW620, and HT-29 [117]. In particular, reactive oxygen species production and oxidative stress enhanced the anticancer mechanism of Cu2O nanoparticles. Similarly, Shunmugam et al. [120] synthesized gold nanoparticles from the marine bacterium V. alginolyticus, which had antioxidant and anticancer activity (Figure 5). The anticancer activity was attributed to the treated cells’ nuclear condensation.
Table 2.
List of marine-bioinspired metallic nanoparticles for treating non-infectious diseases.
| Name of Marine-Derived Compound/Product | Organisms/Sources | Name of NPs | Size Range of MNPs | Shape/Morphology | Types of Non-Infectious Disease Treatment | Effects/Activities | References |
|---|---|---|---|---|---|---|---|
| Extracts |
|
AgNPs | 12 nm | Spherical | Anticancer | Human breast adenocarcinoma cell line | [78] |
| Extracts |
|
AgNPs | 5.52, 35 nm | Spherical |
|
|
[85] |
| Extracts | Acanthophora spicifera | AuNPs | <20 nm | Spherical | Anticancer | Human colon adenocarcinoma (HT-29) cells | [86] |
| Extracts | Sargassum wighitii | MgONPs | 68.06 nm | Flower | Anticancer | A549 | [88] |
| Extracts | Rhodotorula mucilaginosa | Cu2ONPs | 51.6–111.4 nm | Spherical | Anticancer |
|
[117] |
| Extracts | Pterocladia capillacea | CuONPs | 62 nm | Spherical | Anticancer | Breast cancer, ovarian cancer, and hepatocellular carcinoma cell lines | [121] |
| Extracts | Laminaria digitata | ZnONPs | 100–350 nm | Spindle | Anticancer | Fibroblasts cells and human colon cancer cells | [122] |
| Extracts | Hamigera pallidass | AgNPs | 5.85 ± 0.84, 3.69–16.11 nm | Spherical |
|
|
[123] |
| Extracts |
|
AgNPs | 30–90, 20–60, 30–90 nm | Spherical |
|
|
[113] |
| Extracts | U. lactuca | AgNPs | 8–14 nm | Spherical | Anticancer | Human colon cancer | [124] |
| Extracts | Alternaria chlamydospora | AuNPs | - | Spherical |
|
|
[125] |
| Extracts |
|
AuNPs | 16.9 ± 2.5, 15.0 ± 3.0, 44.2 ± 6.1 nm | Spherical |
|
|
[126] |
| Extracts | C. crinita | ZnONPs | 23–200 nm | Rectangular | Antioxidant | DPPH | [96] |
| Extracts | Synechocystis sp. | AgNPs | 10–35 nm | Spherical | Wound-healing | Diabetic wounded animals | [97] |
| Carrageenan &Carrageenan oligosaccharide | Marine red algae | AuNPs | 141 ± 6 nm | Spherical | Anticancer | HCT-116 and HepG2 cells | [127] |
| Extracts | Paracoccus haeundaensis | AuNPs | 20.93 ± 3.46 nm | Spherical |
|
|
[128] |
| Extracts | Caulerpa taxifolia | AgNPs | - | - | Anticancer | A549 lung cancer cells | [119] |
| Extracts | Nemopilema nomurai | AuNPs | 35.2 ± 8.7 nm | Spherical | Anticancer | HeLa cancer cells | [47] |
| Extracts | Oscillatoria limnetica | AgNPs | 3.30–17.97 nm | Quasi-spherical | Anticancer |
|
[98] |
| Extracts | Red algae | Co3O4NPs | 29.8 ± 8.6 nm | Spherical | Anticancer | HepG2 cancer cells | [99] |
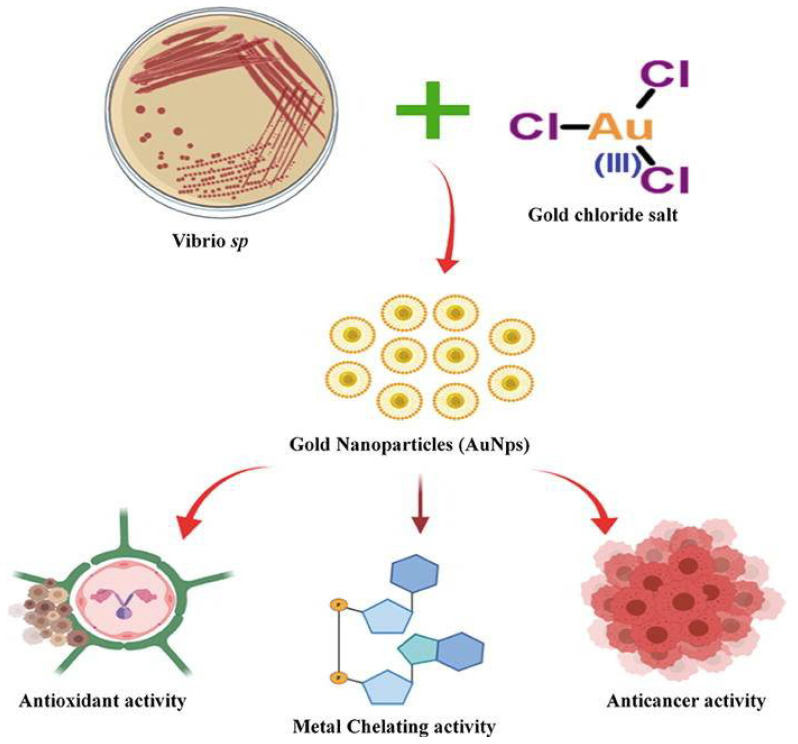
| Extracts | Vibrio alginolyticus | AuNPs | 50–100 nm | Monodispersed, irregular shape | Anticancer | HCA-7 cells | [120] |
Figure 5.
Anticancer and antioxidant properties of gold nanoparticles synthesized using marine microbe Vibrio alginolyticus. Reprinted with permission from reference [120]. Copyright 2020, Elsevier B.V.
Silver nanoparticles derived from shrimp shell chitin acted as a reducing agent and had anticancer action against human hepatocarcinoma [129]. In HepG2 cells, chitin-silver nanoparticles increased the expression of apoptosis-related proteins Bax, PARP, cytochrome-c, caspase-3, and caspase-9, while decreasing the expression of antiapoptosis proteins Bcl-2 and Bcl-xl. Phloroglucinol-encapsulated starch biopolymer exhibited dose-dependent anticancer activities against the HepG2 liver cancer cell line [130]. These findings were ascribed to the biopolymer’s hydrophobicity, which increased adhesion and adsorption capability to the cancer cell surface.
Cells produce potentially harmful ROS as a result of oxygen metabolism, which involves enzymatic and non-enzymatic reactions [131]. High levels of ROS caused by oxidative stress induce a variety of diseases in the body, including diabetes, hypertension, and Alzheimer’s [132]. Antioxidants, on the other hand, have a role in delaying, regulating, and avoiding the oxidative process that leads to the beginning and progression of the disease [133]. Through SOD enzymes, which catalyze the recombination of oxygen radicals, these antioxidants counteract the consequences of oxidative stress [134]. Currently, research is being performed to investigate natural substances capable of controlling oxidative stress, which leads to the investigation of nanoparticles with antioxidant activities. Many species with antioxidant activity in marine organisms, in particular, have been found and have piqued the interest of researchers due to their potential biological activity [135]. The antioxidant activity of gold nanoparticles produced by the marine fungus A. chlamydospora (inhibition of DPPH radicals) was dose-dependent [125]. Furthermore, in a concentration-dependent way, gold nanoparticles mediated by the marine bacteria Paracoccus haeundaensis cell-free supernatant demonstrated strong reducing power via DPPH scavenging activity [128]. Selenium nanoparticles produced from Spirulina phycocyanin protected INS-1E rat insulinoma cells against palmitic acid-induced cell death [136]. Phycocyanin and selenium shielded cells from oxidative damage and signaling pathways downstream. These findings indicate that marine-derived nanoparticles can be employed as effective natural antioxidants.
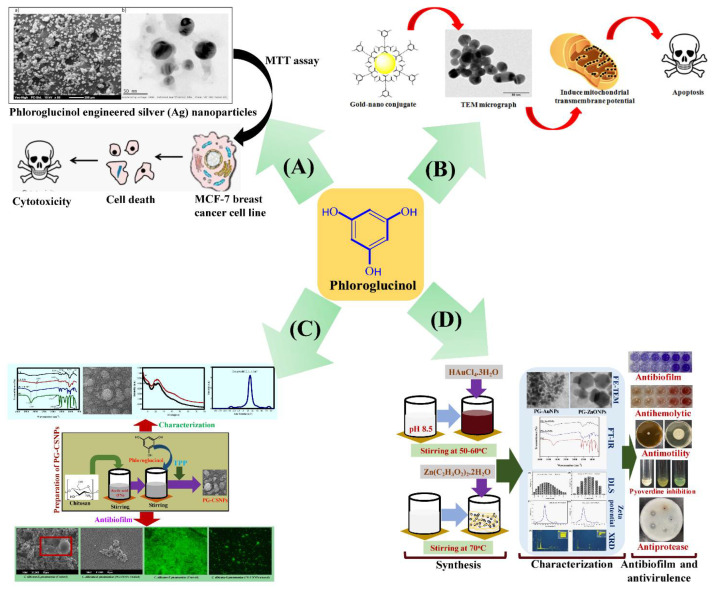
Figure 6 depicts the biological activity of nanoparticles synthesized using phloroglucinol. Silver nanoparticles synthesized using phloroglucinol showed anticancer efficacy against the MCF-7 breast cancer cell line [137]. Silver ions from phloroglucinol silver nanoparticles entered cancer cells and interacted with intracellular macromolecules, such as organelles, proteins, and DNA, to trigger apoptosis. Another study found that phloroglucinol-gold nanoparticles triggered death in HeLa cancer cells via enhancing mitochondrial membrane permeability [138]. Phloroglucinol-encapsulated chitosan nanoparticles showed antibiofilm action against single-species biofilms, such as K. pneumoniae, S. aureus, S. mutans, and C. albicans, and mixed-species biofilms, such as C. albicans-S. aureus/K. pneumoniae/S. mutans [139]. Gold and zinc oxide nanoparticles produced with phloroglucinol showed significant antibacterial action against P. aeruginosa [140]. Moreover, these nanoparticles inhibited P. aeruginosa twitching, swimming, and swarming motility, all of which have virulence features. Similarly, several marine-derived pure compounds are employed in synthesizing nanoparticles and encapsulating drugs for application in the field of medicine (Table 3).
Figure 6.
Application of phloroglucinol in the form of nanoparticles for treating infectious and non-infectious diseases. (A) The cytotoxicity action of phloroglucinol-engineered AgNPs towards MCF-7 breast cancer cell lines. (a) SEM image of AgNPs and (b) TEM image of AgNPs. Reproduced with permission from reference [137]. (B) Synthesis of the phloroglucinol-conjugated gold nanoparticles, which exhibit therapeutic potential towards cancer cells. The action mechanism involved apoptosis of cancer cells by promoting mitochondrial transmembrane permeation, as evident by fluorescence staining and gene expression studies. Reprinted with permission from reference [138], (C) Encapsulation of phloroglucinol into the chitosan nanoparticles. The PG-CSNPs exhibit antibiofilm properties towards single- and mixed-species biofilms of C. albicans-S. aureus/S. mutans/K. penumoniae. Reprinted with permission from reference [139], and (D) Synthesis of metal (AuNPs) and metal oxide (ZnONPs) nanoparticles using phloroglucinol. The synthesized PG-AuNPs and PG-ZnONPs showed antibiofilm and antivirulence properties towards P. aeruginosa. Reproduced with permission from reference [140]. Copyright 2021 by the authors and licensee MDPI, Basel, Switzerland.
Table 3.
Application of marine-derived compounds in the synthesis of nanoparticles and encapsulation of drugs for application in the field of medicine.
| Classification of Sources | Natural Pure Compounds | Types of Nanomaterial | Size | Morphology | Biological Activity | Action Mechanism | References |
|---|---|---|---|---|---|---|---|
| Algae | Fucoidan | AuNPs | ~53 nm | Spherical | Antibacterial activity against Pseudomonas aeruginosa |
|
[66] |
| Algae | Phloroglucinol | AuNPs and ZnONPs | 41.6 ± 3.9, 52.7 ± 3.8 nm | Spherical and hexagonal | Antibacterial activity against P. aeruginosa |
|
[140] |
| Algae | Phycocyanin | SeNPs | 165, 235, 371, 815 nm | Spherical | Antioxidant | Protected INS-1E cells against palmitic acid-induced cell death by reducing oxidative stress and signaling pathways downstream | [136] |
| Algae | Fucoxanthin | AgNPs | 20–25 nm | Spherical | Antibacterial activity against Escherichia coli, Bacillus stearothermophilus, and Streptococcus mutans | - | [141] |
| Algae | Phloroglucinol | Starch biopolymer | 1–100 nm | Spherical | Anticancer | Adhesion and adsorption on the surfaces of cancer cells are enhanced | [130] |
| Algae | Phloroglucinol | CSNPs | 414.0 ± 48.5 nm | Spherical | Antibiofilm activity against Klebsiella pneumoniae, Staphylococcus aureus, Candida albicans, S. mutans, and mixed-species such as C. albicans-S. aureus/K. pneumoniae/S. mutans | The positive charge of CSNPs allows for easy biofilm penetration and binding | [139] |
| Algae | Usnic acid | Nanofibrous poly(ε-caprolactone)/decellularized extracellular matrix scaffolds | 3.89 ± 2.52, 4.95 ± 2.19, 5.00 ± 2.05 μm | Fusion of the fiber junctions |
|
|
[142] |
| Algae | Carrageenan | ZnONPs | 97.03 ± 9.05 nm | Hexagonal wurtzite phase |
|
|
[65] |
| Bacteria | Mannose | CuONPs | 108 nm | Spherical | Antibacterial activity against P. aeruginosa | Entered the cell membrane, causing lysis and cell rupture | [67] |
| Fungi |
|
AgNPs | 8–30 nm | Spherical | Acetylcholine esterase inhibitory activity | Enzyme structural alterations | [143] |
| Fungi | α-amylase | AgNPs | 22.88–26.35 nm | Spherical | Antibacterial activity against Aeromonas hydrophila, P. aeruginosa, Vibrio anguillarum, S. faecium, S. agalactiae, and Listeria spp. | Damage to cell membranes, oxidative stress, and protein and DNA damage | [68] |
| Animal | Chitin | AgNPs | 17–49 nm | Spherical | Anticancer activity in human hepatocellular carcinoma HepG2 cells |
|
[129] |
| Animal | Astaxanthin | AuNPs | 58.2 ± 4.6 nm | Polygonal and spherical | Antioxidant | Reduced ROS and increased antioxidant enzyme activity in rice plants treated to Cd to alleviate oxidative stress | [144] |
| Animal | Chitosan oligosaccharide | AuNPs | 56.01 ± 3.48 nm | Spherical | Antibacterial activity against P. aeruginosa |
|
[69] |
| Animal | Thiol chitosan | AuNSs | 185 ± 19 nm | Spherical | Antibacterial activity against E. coli, P. aeruginosa, and S. aureus | - | [145] |
| Animal | Chitosan | Polypyrrole nanocomposites | 55.77 ± 3.48 nm | Spherical | Antibiofilm activity against P. aeruginosa |
|
[70] |
8. Conclusions and Future Perspectives
In conclusion, because of their potential biological activity, marine-derived products have been widely used in the pharmaceutical industry. With an increased understanding of their biological functions, various marine-derived compounds have been used in the synthesis of nanoparticles. These products comprised polymers, organic compounds, and extract, which act as a powerful reducing agent in synthesizing metal and metal-oxide nanoparticles. Furthermore, certain polymeric material is used to effectively deliver the drug in the treatment of infectious and non-infectious diseases. The review detailed the list of the marine organism from which the extract was extracted and which was used to synthesize several forms of nanoparticles. Furthermore, these nanoparticles have been shown to have antimicrobial properties against bacterial, fungal, and viral pathogens. Antimicrobial mechanisms include the breakdown of cell membranes, as well as damage to cell walls and DNA. These marine-inspired nanoparticles have also shown promise in the treatment of non-infectious diseases, such as diabetes, cancer, wounds, inflammatory reactions, and leishmanial infections. Though significant progress has been made in the production of nanoparticles utilizing extracts from marine sources, relatively little information is known on the synthesis of nanoparticles using pure active compounds. This is because of the fact that there are several variations in extract preparation due to a number of environmental factors. As a result, future research should prioritize the use of pure active compounds for nanoparticle synthesis. Most antimicrobial research involving these nanoparticles has been conducted at the phenotypic level; however, investigations at the gene level are highly needed to explain the molecular mechanism.
Author Contributions
G.-J.J.: Literature Search, Writing—original draft and Editing, S.K.; Literature Search, Writing—original draft, and Editing, N.T.: Literature Search, Writing—original draft and Editing, F.K.: Conceptualization, Literature Search, Writing—original draft, and Writing—review and Editing, Y.-M.K.: Supervision, Funding, Writing—review and Editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education (2021R1A6A1A03039211 and 2022R1A2B5B01001998).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization . Global Health Estimates 2015: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2015. WHO; Geneva, Switzerland: 2016. [Google Scholar]
- 2.Hoffmann A.R., Proctor L., Surette M., Suchodolski J. The microbiome: The trillions of microorganisms that maintain health and cause disease in humans and companion animals. Vet. Pathol. 2016;53:10–21. doi: 10.1177/0300985815595517. [DOI] [PubMed] [Google Scholar]
- 3.Deen J., Mengel M., Clemens J. Epidemiology of Cholera. Vaccine. 2020;38((Suppl. S1)):A31–A40. doi: 10.1016/j.vaccine.2019.07.078. [DOI] [PubMed] [Google Scholar]
- 4.Pappas P.G., Lionakis M.S., Arendrup M.C., Ostrosky-Zeichner L., Kullberg B.J. Invasive candidiasis. Nat. Rev. Dis. Primers. 2018;4:18026. doi: 10.1038/nrdp.2018.26. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Responding to Community Spread of COVID-19. Reference WHO/COVID-19/Community_Transmission/2020.1. WHO; Geneva, Switzerland: 2020. [Google Scholar]
- 6.World Health Organization . Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2021. WHO; Geneva, Switzerland: 2021. [Google Scholar]
- 7.Chakraborty S., Rahman T. The difficulties in cancer treatment. Ecancermedicalscience. 2012;6:ed16. doi: 10.3332/ecancer.2012.ed16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurt R.T., Kulisek C., Buchanan L.A., McClave S.A. The obesity epidemic: Challenges, health initiatives, and implications for gastroenterologists. Gastroenterol. Hepatol. 2010;6:780. [PMC free article] [PubMed] [Google Scholar]
- 9.Drożdżal S., Lechowicz K., Szostak B., Rosik J., Kotfis K., Machoy-Mokrzyńska A., Białecka M., Ciechanowski K., Gawrońska-Szklarz B. Kidney damage from nonsteroidal anti-inflammatory drugs—Myth or truth? Review of selected literature. Pharmacol. Res. Perspect. 2021;9:e00817. doi: 10.1002/prp2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman P.S. Antibacterial discovery: 21st century challenges. Antibiotics. 2020;9:213. doi: 10.3390/antibiotics9050213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sedeek A.M., Ismail M.M., Elsayed T.R., Ramadan M.A. Recent methods for discovering novel bioactive metabolites, specifically antimicrobial agents, from marine-associated microorganisms. Lett. Appl. Microbiol. 2022 doi: 10.1111/lam.13728. [DOI] [PubMed] [Google Scholar]
- 12.Patil M.P., Kim G.-D. Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloids Surf. B Biointerfaces. 2018;172:487–495. doi: 10.1016/j.colsurfb.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Montuori E., de Pascale D., Lauritano C. Recent Discoveries on Marine Organism Immunomodulatory Activities. Mar. Drugs. 2022;20:422. doi: 10.3390/md20070422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yosri N., Khalifa S.A., Guo Z., Xu B., Zou X., El-Seedi H.R. Marine organisms: Pioneer natural sources of polysaccharides/proteins for green synthesis of nanoparticles and their potential applications. Int. J. Biol. Macromol. 2021;193:1767–1798. doi: 10.1016/j.ijbiomac.2021.10.229. [DOI] [PubMed] [Google Scholar]
- 15.Khan F., Jeong G.-J., Khan M.S.A., Tabassum N., Kim Y.-M. Seaweed-Derived Phlorotannins: A Review of Multiple Biological Roles and Action Mechanisms. Mar. Drugs. 2022;20:384. doi: 10.3390/md20060384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang Y., Li H., Ji B., Cheng K., Wu B., Li Z., Zheng C., Hua H., Li D. Renieramycin-type alkaloids from marine-derived organisms: Synthetic chemistry, biological activity and structural modification. Eur. J. Med. Chem. 2021;210:113092. doi: 10.1016/j.ejmech.2020.113092. [DOI] [PubMed] [Google Scholar]
- 17.Bayda S., Adeel M., Tuccinardi T., Cordani M., Rizzolio F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules. 2019;25:112. doi: 10.3390/molecules25010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalil M.A., El-Shanshoury A.E.-R.R., Alghamdi M.A., Alsalmi F.A., Mohamed S.F., Sun J., Ali S.S. Biosynthesis of silver nanoparticles by Marine actinobacterium Nocardiopsis dassonvillei and exploring their therapeutic potentials. Front. Microbiol. 2021;12:705673. doi: 10.3389/fmicb.2021.705673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vala A., Shah S. Rapid synthesis of silver nanoparticles by a marine-derived fungus Aspergillus niger and their antimicrobial potentials. Int. J. Nanosci. Nanotechnol. 2012;8:197–206. [Google Scholar]
- 20.Hammami I., Alabdallah N.M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Univ. Sci. 2021;33:101560. doi: 10.1016/j.jksus.2021.101560. [DOI] [Google Scholar]
- 21.Spirescu V.A., Chircov C., Grumezescu A.M., Vasile B.Ș., Andronescu E. Inorganic nanoparticles and composite films for antimicrobial therapies. Int. J. Mol. Sci. 2021;22:4595. doi: 10.3390/ijms22094595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balakumaran M., Ramachandran R., Balashanmugam P., Mukeshkumar D., Kalaichelvan P. Mycosynthesis of silver and gold nanoparticles: Optimization, characterization and antimicrobial activity against human pathogens. Microbiol. Res. 2016;182:8–20. doi: 10.1016/j.micres.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Gnanajobitha G., Paulkumar K., Vanaja M., Rajeshkumar S., Malarkodi C., Annadurai G., Kannan C. Fruit-mediated synthesis of silver nanoparticles using Vitis vinifera and evaluation of their antimicrobial efficacy. J. Nanostructure Chem. 2013;3:67. doi: 10.1186/2193-8865-3-67. [DOI] [Google Scholar]
- 24.Song X., Shi X., Yang M. Dual application of Shewanella oneidensis MR-1 in green biosynthesis of Pd nanoparticles supported on TiO2 nanotubes and assisted photocatalytic degradation of methylene blue. IET Nanobiotechnol. 2018;12:441–445. doi: 10.1049/iet-nbt.2017.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar D.A., Palanichamy V., Roopan S.M. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014;127:168–171. doi: 10.1016/j.saa.2014.02.058. [DOI] [PubMed] [Google Scholar]
- 26.Jin R. The impacts of nanotechnology on catalysis by precious metal nanoparticles. Nanotechnol. Rev. 2012;1:31–56. doi: 10.1515/ntrev-2011-0003. [DOI] [Google Scholar]
- 27.Nagarajan R. Nanoparticles: Building Blocks for Nanotechnology. ACS Publications; Washington, DC, USA: 2008. [Google Scholar]
- 28.Dash D.K., Panik R.K., Sahu A.K., Tripathi V. Applications of Nanobiotechnology. IntechOpen; London, UK: 2020. Role of nanobiotechnology in drug discovery, development and molecular diagnostic. [Google Scholar]
- 29.Mahmood Ansari S., Saquib Q., De Matteis V., Awad Alwathnani H., Ali Alharbi S., Ali Al-Khedhairy A. Marine Macroalgae Display Bioreductant Efficacy for Fabricating Metallic Nanoparticles: Intra/Extracellular Mechanism and Potential Biomedical Applications. Bioinorg. Chem. Appl. 2021;2021:5985377. doi: 10.1155/2021/5985377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponnuchamy K., Jacob J.A. Metal nanoparticles from marine seaweeds—A review. Nanotechnol. Rev. 2016;5:589–600. doi: 10.1515/ntrev-2016-0010. [DOI] [Google Scholar]
- 31.Daraio C., Jin S. Nanotechnology for Biology and Medicine. Springer; Berlin/Heidelberg, Germany: 2012. Synthesis and patterning methods for nanostructures useful for biological applications; pp. 27–44. [Google Scholar]
- 32.Sardar M., Mazumder J.A. Environmental Nanotechnology. Springer; Berlin/Heidelberg, Germany: 2019. Biomolecules assisted synthesis of metal nanoparticles; pp. 1–23. [Google Scholar]
- 33.Asmathunisha N., Kathiresan K. A review on biosynthesis of nanoparticles by marine organisms. Colloids Surf. B Biointerfaces. 2013;103:283–287. doi: 10.1016/j.colsurfb.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 34.Ehrlich H. Marine Biological Materials of Invertebrate Origin. Springer; Berlin/Heidelberg, Germany: 2019. [Google Scholar]
- 35.Mora C., Tittensor D.P., Adl S., Simpson A.G., Worm B. How many species are there on Earth and in the ocean? PLoS Biol. 2011;9:e1001127. doi: 10.1371/journal.pbio.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russo P., Kisialiou A., Lamonaca P., Moroni R., Prinzi G., Fini M. New drugs from marine organisms in Alzheimer’s disease. Mar. Drugs. 2015;14:5. doi: 10.3390/md14010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2016;33:382–431. doi: 10.1039/C5NP00156K. [DOI] [PubMed] [Google Scholar]
- 38.Cardoso J., Nakayama D.G., Sousa E., Pinto E. Marine-derived compounds and prospects for their antifungal application. Molecules. 2020;25:5856. doi: 10.3390/molecules25245856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Šimat V., Elabed N., Kulawik P., Ceylan Z., Jamroz E., Yazgan H., Čagalj M., Regenstein J.M., Özogul F. Recent advances in marine-based nutraceuticals and their health benefits. Mar. Drugs. 2020;18:627. doi: 10.3390/md18120627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suárez Quintana W.H., García-Rico R.O., García-Martínez J.B., Urbina-Suarez N.A., López-Barrera G.L., Barajas-Solano A.F., Zuorro A. Enhancement of Metabolite Production in High-Altitude Microalgal Strains by Optimized C/N/P Ratio. Appl. Sci. 2022;12:6779. doi: 10.3390/app12136779. [DOI] [Google Scholar]
- 41.Priyadarshini E., Priyadarshini S.S., Pradhan N. Heavy metal resistance in algae and its application for metal nanoparticle synthesis. Appl. Microbiol. Biotechnol. 2019;103:3297–3316. doi: 10.1007/s00253-019-09685-3. [DOI] [PubMed] [Google Scholar]
- 42.Oukarroum A., Bras S., Perreault F., Popovic R. Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Ecotoxicol. Environ. Saf. 2012;78:80–85. doi: 10.1016/j.ecoenv.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 43.Venkatesan J., Kim S.-K., Shim M.S. Antimicrobial, antioxidant, and anticancer activities of biosynthesized silver nanoparticles using marine algae Ecklonia cava. Nanomaterials. 2016;6:235. doi: 10.3390/nano6120235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neethu S., Midhun S.J., Radhakrishnan E., Jyothis M. Green synthesized silver nanoparticles by marine endophytic fungus Penicillium polonicum and its antibacterial efficacy against biofilm forming, multidrug-resistant Acinetobacter baumanii. Microb. Pathog. 2018;116:263–272. doi: 10.1016/j.micpath.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 45.Wang L., Liu C.-C., Wang Y.-Y., Xu H., Su H., Cheng X. Antibacterial activities of the novel silver nanoparticles biosynthesized using Cordyceps militaris extract. Curr. Appl. Phys. 2016;16:969–973. doi: 10.1016/j.cap.2016.05.025. [DOI] [Google Scholar]
- 46.Srivastava S.K., Yamada R., Ogino C., Kondo A. Biogenic synthesis and characterization of gold nanoparticles by Escherichia coli K12 and its heterogeneous catalysis in degradation of 4-nitrophenol. Nanoscale Res. Lett. 2013;8:70. doi: 10.1186/1556-276X-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahn E.-Y., Hwang S.J., Choi M.-J., Cho S., Lee H.-J., Park Y. Upcycling of jellyfish (Nemopilema nomurai) sea wastes as highly valuable reducing agents for green synthesis of gold nanoparticles and their antitumor and anti-inflammatory activity. Artif. Cells Nanomed. Biotechnol. 2018;46((Suppl. S2)):1127–1136. doi: 10.1080/21691401.2018.1480490. [DOI] [PubMed] [Google Scholar]
- 48.Yan N., Chen X. Sustainability: Don’t waste seafood waste. Nature. 2015;524:155–157. doi: 10.1038/524155a. [DOI] [PubMed] [Google Scholar]
- 49.Doron S., Gorbach S. Bacterial infections: Overview. Int. Encycl. Public Health. 2008:273–282. doi: 10.1016/b978-012373960-5.00596-7. [DOI] [Google Scholar]
- 50.Slavin Y.N., Asnis J., Häfeli U.O., Bach H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017;15:65. doi: 10.1186/s12951-017-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shannon E., Abu-Ghannam N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs. 2016;14:81. doi: 10.3390/md14040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nalini S., Richard D.S., Riyaz S.M., Kavitha G., Inbakandan D. Antibacterial macro molecules from marine organisms. Int. J. Biol. Macromol. 2018;115:696–710. doi: 10.1016/j.ijbiomac.2018.04.110. [DOI] [PubMed] [Google Scholar]
- 53.Gram L., Melchiorsen J., Bruhn J.B. Antibacterial activity of marine culturable bacteria collected from a global sampling of ocean surface waters and surface swabs of marine organisms. Mar. Biotechnol. 2010;12:439–451. doi: 10.1007/s10126-009-9233-y. [DOI] [PubMed] [Google Scholar]
- 54.Qi S.-H., Xu Y., Gao J., Qian P.-Y., Zhang S. Antibacterial and antilarval compounds from marine bacterium Pseudomonas rhizosphaerae. Ann. Microbiol. 2009;59:229–233. doi: 10.1007/BF03178321. [DOI] [Google Scholar]
- 55.Cox S., Abu-Ghannam N., Gupta S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int. Food Res. J. 2010;17:205–220. [Google Scholar]
- 56.Sahoo C.R., Maharana S., Mandhata C.P., Bishoyi A.K., Paidesetty S.K., Padhy R.N. Biogenic silver nanoparticle synthesis with cyanobacterium Chroococcus minutus isolated from Baliharachandi sea-mouth, Odisha, and in vitroantibacterial activity. Saudi J. Biol. Sci. 2020;27:1580–1586. doi: 10.1016/j.sjbs.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trivedi S., Alshehri M.A., Aziz A.T., Panneerselvam C., Al-Aoh H.A., Maggi F., Sut S., Dall’Acqua S. Insecticidal, antibacterial and dye adsorbent properties of Sargassum muticum decorated nano-silver particles. S. Afr. J. Bot. 2021;139:432–441. doi: 10.1016/j.sajb.2021.03.002. [DOI] [Google Scholar]
- 58.Stalin Dhas T., Sowmiya P., Parthasarathy K., Natarajan A., Narendrakumar G., Kumar R., Samrot A.V., Riyaz S.U.M., Ganesh V.K., Karthick V., et al. In vitro antibacterial activity of biosynthesized silver nanoparticles against gram negative bacteria. Inorg. Nano-Met. Chem. 2022:1–10. doi: 10.1080/24701556.2022.2034014. [DOI] [Google Scholar]
- 59.Selvaraj P., Neethu E., Rathika P., Jayaseeli J.P.R., Jermy B.R., AbdulAzeez S., Borgio J.F., Dhas T.S. Antibacterial potentials of methanolic extract and silver nanoparticles from marine algae. Biocatal. Agric. Biotechnol. 2020;28:101719. doi: 10.1016/j.bcab.2020.101719. [DOI] [Google Scholar]
- 60.Thiruchelvi R., Jayashree P., Mirunaalini K. Synthesis of silver nanoparticle using marine red seaweed Gelidiella acerosa—A complete study on its biological activity and its characterisation. Mater. Today Proc. 2021;37:1693–1698. doi: 10.1016/j.matpr.2020.07.242. [DOI] [Google Scholar]
- 61.Al-Dhabi N.A., Mohammed Ghilan A.-K., Arasu M.V. Characterization of Silver Nanomaterials Derived from Marine Streptomyces sp. Al-Dhabi-87 and Its In Vitro Application against Multidrug Resistant and Extended-Spectrum Beta-Lactamase Clinical Pathogens. Nanomaterials. 2018;8:279. doi: 10.3390/nano8050279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajeshkumar S., Nandhini N.T., Manjunath K., Sivaperumal P., Krishna Prasad G., Alotaibi S.S., Roopan S.M. Environment friendly synthesis copper oxide nanoparticles and its antioxidant, antibacterial activities using Seaweed (Sargassum longifolium) extract. J. Mol. Struct. 2021;1242:130724. doi: 10.1016/j.molstruc.2021.130724. [DOI] [Google Scholar]
- 63.Pacheco-Quito E.M., Ruiz-Caro R., Veiga M.D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs. 2020;18:583. doi: 10.3390/md18110583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campo V.L., Kawano D.F., Silva D.B.D., Carvalho I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009;77:167–180. doi: 10.1016/j.carbpol.2009.01.020. [DOI] [Google Scholar]
- 65.Vijayakumar S., Saravanakumar K., Malaikozhundan B., Divya M., Vaseeharan B., Durán-Lara E.F., Wang M.-H. Biopolymer K-carrageenan wrapped ZnO nanoparticles as drug delivery vehicles for anti MRSA therapy. Int. J. Biol. Macromol. 2020;144:9–18. doi: 10.1016/j.ijbiomac.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 66.Khan F., Manivasagan P., Lee J.-W., Pham D.T.N., Oh J., Kim Y.-M. Fucoidan-stabilized gold nanoparticle-mediated biofilm inhibition, attenuation of virulence and motility properties in Pseudomonas aeruginosa PAO1. Mar. Drugs. 2019;17:208. doi: 10.3390/md17040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halder U., Roy R.K., Biswas R., Khan D., Mazumder K., Bandopadhyay R. Synthesis of copper oxide nanoparticles using capsular polymeric substances produced by Bacillus altitudinis and investigation of its efficacy to kill pathogenic Pseudomonas aeruginosa. Chem. Eng. J. Adv. 2022;11:100294. doi: 10.1016/j.ceja.2022.100294. [DOI] [Google Scholar]
- 68.Barakat K.M., Abouelwafa A., El-Sayed H.S., Beltagy E.A. Silver Nanoparticles Mediated-Marine Fungal Amylase Improving Dicentrarchus Labrax Larvae Growth Performance. Research Square; Durham, NC, USA: 2022. [DOI] [Google Scholar]
- 69.Khan F., Lee J.-W., Manivasagan P., Pham D.T.N., Oh J., Kim Y.-M. Synthesis and characterization of chitosan oligosaccharide-capped gold nanoparticles as an effective antibiofilm drug against the Pseudomonas aeruginosa PAO1. Microb. Pathog. 2019;135:103623. doi: 10.1016/j.micpath.2019.103623. [DOI] [PubMed] [Google Scholar]
- 70.Khan F., Manivasagan P., Pham D.T.N., Oh J., Kim S.-K., Kim Y.-M. Antibiofilm and antivirulence properties of chitosan-polypyrrole nanocomposites to Pseudomonas aeruginosa. Microb. Pathog. 2019;128:363–373. doi: 10.1016/j.micpath.2019.01.033. [DOI] [PubMed] [Google Scholar]
- 71.Khoury M.K., Heid C.A., Cripps M.W., Pickett M.L., Nagaraj M.B., Johns M., Lee F., Hennessy S.A. Antifungal Therapy in Fungal Necrotizing Soft Tissue Infections. J. Surg. Res. 2020;256:187–192. doi: 10.1016/j.jss.2020.06.013. [DOI] [PubMed] [Google Scholar]
- 72.Kainz K., Bauer M.A., Madeo F., Carmona-Gutierrez D. Fungal infections in humans: The silent crisis. Microb. Cell. 2020;7:143–145. doi: 10.15698/mic2020.06.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.El-Hossary E.M., Cheng C., Hamed M.M., Hamed A.N.E.-S., Ohlsen K., Hentschel U., Abdelmohsen U.R. Antifungal potential of marine natural products. Eur. J. Med. Chem. 2017;126:631–651. doi: 10.1016/j.ejmech.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 74.Cheung R.C.F., Wong J.H., Pan W.L., Chan Y.S., Yin C.M., Dan X.L., Wang H.X., Fang E.F., Lam S.K., Ngai P.H.K. Antifungal and antiviral products of marine organisms. Appl. Microbiol. Biotechnol. 2014;98:3475–3494. doi: 10.1007/s00253-014-5575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lopes G., Pinto E., Andrade P.B., Valentao P. Antifungal activity of phlorotannins against dermatophytes and yeasts: Approaches to the mechanism of action and influence on Candida albicans virulence factor. PLoS ONE. 2013;8:e72203. doi: 10.1371/journal.pone.0072203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cassilly C.D., Maddox M.M., Cherian P.T., Bowling J.J., Hamann M.T., Lee R.E., Reynolds T.B. SB-224289 antagonizes the antifungal mechanism of the marine depsipeptide papuamide A. PLoS ONE. 2016;11:e0154932. doi: 10.1371/journal.pone.0154932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu T., Feng Q., Jacob M.R., Avula B., Mask M.M., Baerson S.R., Tripathi S.K., Mohammed R., Hamann M.T., Khan I.A. The marine sponge-derived polyketide endoperoxide plakortide F acid mediates its antifungal activity by interfering with calcium homeostasis. Antimicrob. Agents Chemother. 2011;55:1611–1621. doi: 10.1128/AAC.01022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Algotiml R., Gab-Alla A., Seoudi R., Abulreesh H.H., El-Readi M.Z., Elbanna K. Anticancer and antimicrobial activity of biosynthesized Red Sea marine algal silver nanoparticles. Sci. Rep. 2022;12:2421. doi: 10.1038/s41598-022-06412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aziz A.T. Toxicity of Ulva lactuca and green fabricated silver nanoparticles against mosquito vectors and their impact on the genomic DNA of the dengue vector Aedes aegypti. IET Nanobiotechnol. 2022;16:145–157. doi: 10.1049/nbt2.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rajashree R., Gayathri S., Gobalakrishnan M. Marine Biomolecule Mediated Synthesis of Selenium Nanoparticles and Their Antimicrobial Efficiency against Fish and Crustacean Pathogens. Research Square; Durham, NC, USA: 2022. [Google Scholar]
- 81.Raja S., Anjali G., Anitha J., Ephsy D., Sahana K., Mohan K. Optimization of Silver Nanocrystals Reduced from the Functional Molecules Enriched Spirulina—A Potent Antibiotic against Human and Marine Pathogen Vibrio parahaemolyticus. Research Square; Durham, NC, USA: 2022. [Google Scholar]
- 82.Vikneshan M., Saravanakumar R., Mangaiyarkarasi R., Rajeshkumar S., Samuel S.R., Suganya M., Baskar G. Algal biomass as a source for novel oral nano-antimicrobial agent. Saudi J. Biol. Sci. 2020;27:3753–3758. doi: 10.1016/j.sjbs.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yılmaz Öztürk B., Yenice Gürsu B., Dağ İ. Antibiofilm and antimicrobial activities of green synthesized silver nanoparticles using marine red algae Gelidium corneum. Process Biochem. 2020;89:208–219. doi: 10.1016/j.procbio.2019.10.027. [DOI] [Google Scholar]
- 84.Kaidi S., Belattmania Z., Bentiss F., Jama C., Reani A., Sabour B. Synthesis and Characterization of Silver Nanoparticles Using Alginate from the Brown Seaweed Laminaria ochroleuca: Structural Features and Antibacterial Activity. Biointerface Res. Appl. Chem. 2021;12:6046–6057. [Google Scholar]
- 85.Alam A., Tanveer F., Khalil A.T., Zohra T., Khamlich S., Alam M.M., Salman M., Ali M., Ikram A., Shinwari Z.K., et al. Silver nanoparticles biosynthesized from secondary metabolite producing marine actinobacteria and evaluation of their biomedical potential. Antonie Van Leeuwenhoek. 2021;114:1497–1516. doi: 10.1007/s10482-021-01616-5. [DOI] [PubMed] [Google Scholar]
- 86.Babu B., Palanisamy S., Vinosha M., Anjali R., Kumar P., Pandi B., Tabarsa M., You S., Prabhu N.M. Bioengineered gold nanoparticles from marine seaweed Acanthophora spicifera for pharmaceutical uses: Antioxidant, antibacterial, and anticancer activities. Bioprocess Biosyst. Eng. 2020;43:2231–2242. doi: 10.1007/s00449-020-02408-3. [DOI] [PubMed] [Google Scholar]
- 87.Pugazhendhi A., Prabakar D., Jacob J.M., Karuppusamy I., Saratale R.G. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb. Pathog. 2018;114:41–45. doi: 10.1016/j.micpath.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 88.Pugazhendhi A., Prabhu R., Muruganantham K., Shanmuganathan R., Natarajan S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. B Biol. 2019;190:86–97. doi: 10.1016/j.jphotobiol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 89.Bishoyi A.K., Sahoo C.R., Sahoo A.P., Padhy R.N. Bio-synthesis of silver nanoparticles with the brackish water blue-green alga Oscillatoria princeps and antibacterial assessment. Appl. Nanosci. 2021;11:389–398. doi: 10.1007/s13204-020-01593-7. [DOI] [Google Scholar]
- 90.Dhanaraj S., Thirunavukkarasu S., Allen John H., Pandian S., Salmen S.H., Chinnathambi A., Alharbi S.A. Novel marine Nocardiopsis dassonvillei-DS013 mediated silver nanoparticles characterization and its bactericidal potential against clinical isolates. Saudi J. Biol. Sci. 2020;27:991–995. doi: 10.1016/j.sjbs.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al-Dhabi N.A., Ghilan A.-K.M., Arasu M.V., Duraipandiyan V. Green biosynthesis of silver nanoparticles produced from marine Streptomyces sp. Al-Dhabi-89 and their potential applications against wound infection and drug resistant clinical pathogens. J. Photochem. Photobiol. B Biol. 2018;189:176–184. doi: 10.1016/j.jphotobiol.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 92.Alharthi S.S., Gomathi T., Joseph J.J., Rakshavi J., Florence J.A.K., Sudha P.N., Rajakumar G., Thiruvengadam M. Biological activities of chitosan-salicylaldehyde schiff base assisted silver nanoparticles. J. King Saud Univ. Sci. 2022;34:102177. doi: 10.1016/j.jksus.2022.102177. [DOI] [Google Scholar]
- 93.Mostafa E.M., Abdelgawad M.A., Musa A., Alotaibi N.H., Elkomy M.H., Ghoneim M.M., Badawy M.S.E.M., Taha M.N., Hassan H.M., Hamed A.A. Chitosan Silver and Gold Nanoparticle Formation Using Endophytic Fungi as Powerful Antimicrobial and Anti-Biofilm Potentialities. Antibiotics. 2022;11:668. doi: 10.3390/antibiotics11050668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dixit S., Vishnoi N., Tripathi N.M., Singh D.P., Sharma Y.K. Biosynthesis of nanostructured silver by green algae and evaluation of its microbicidal property against pathogenic microbes. Environ. Sustain. 2022;5:197–206. doi: 10.1007/s42398-022-00223-y. [DOI] [Google Scholar]
- 95.RathnaKumari P., Kolanchinathan P., Siva D., Abirami B., Masilamani V., John G., Achiraman S., Balasundaram A. Antibacterial efficacy of seagrass Cymodocea serrulata-engineered silver nanoparticles against prawn pathogen Vibrio parahaemolyticus and its combative effect on the marine shrimp Penaeus monodon. Aquaculture. 2018;493:158–164. doi: 10.1016/j.aquaculture.2018.04.061. [DOI] [Google Scholar]
- 96.Elrefaey A.A.K., El-Gamal A.D., Hamed S.M., El-belely E.F. Algae-mediated biosynthesis of zinc oxide nanoparticles from Cystoseira crinite (Fucales; Sargassaceae) and it’s antimicrobial and antioxidant activities. Egypt J. Chem. 2022;65:231–240. doi: 10.21608/ejchem.2021.87722.4231. [DOI] [Google Scholar]
- 97.Younis N.S., Mohamed M.E., El Semary N.A. Green Synthesis of Silver Nanoparticles by the Cyanobacteria Synechocystis sp.: Characterization, Antimicrobial and Diabetic Wound-Healing Actions. Mar. Drugs. 2022;20:56. doi: 10.3390/md20010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hamouda R.A., Hussein M.H., Abo-elmagd R.A., Bawazir S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019;9:13071. doi: 10.1038/s41598-019-49444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ajarem J.S., Maodaa S.N., Allam A.A., Taher M.M., Khalaf M. Benign Synthesis of Cobalt Oxide Nanoparticles Containing Red Algae Extract: Antioxidant, Antimicrobial, Anticancer, and Anticoagulant Activity. J. Clust. Sci. 2022;33:717–728. doi: 10.1007/s10876-021-02004-9. [DOI] [Google Scholar]
- 100.El-Sheekh M.M., Shabaan M.T., Hassan L., Morsi H.H. Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herps Simplex (HSV-1) virus in vitro using cell-line culture technique. Int. J. Environ. Health Res. 2022;32:616–627. doi: 10.1080/09603123.2020.1789946. [DOI] [PubMed] [Google Scholar]
- 101.Khalid M., Khalid N., Ahmed I., Hanif R., Ismail M., Janjua H.A. Comparative studies of three novel freshwater microalgae strains for synthesis of silver nanoparticles: Insights of characterization, antibacterial, cytotoxicity and antiviral activities. J. Appl. Phycol. 2017;29:1851–1863. doi: 10.1007/s10811-017-1071-0. [DOI] [Google Scholar]
- 102.El-Enain I.A., Abed N., Helal E., Abdelkhalek E., Suleiman W. Ecofriendly Biosynthesis of Ag-NPs by Streptomyces Griseus Eradicating Candida albicans associated with Different Types of Dysspermatism. Research Square; Durham, NC, USA: 2022. [Google Scholar]
- 103.Sodimalla T., Yalavarthi N. Biosynthesis of silver nanoparticles from Pseudomonas fluorescens and their antifungal activity against Aspergillus niger and Fusarium udum. Ann. Appl. Biol. 2022:1–11. doi: 10.1111/aab.12761. [DOI] [Google Scholar]
- 104.Kailasam S., Sundaramanickam A., Tamilvanan R., Kanth S.V. Macrophytic waste optimization by synthesis of silver nanoparticles and exploring their agro-fungicidal activity. Inorg. Nano-Met. Chem. 2022:1–10. doi: 10.1080/24701556.2022.2034013. [DOI] [Google Scholar]
- 105.Kitazato K., Wang Y., Kobayashi N. Viral infectious disease and natural products with antiviral activity. Drug Discov. Ther. 2007;1:14–22. [PubMed] [Google Scholar]
- 106.Riccio G., Ruocco N., Mutalipassi M., Costantini M., Zupo V., Coppola D., de Pascale D., Lauritano C. Ten-year research update review: Antiviral activities from marine organisms. Biomolecules. 2020;10:1007. doi: 10.3390/biom10071007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang W., Wang S.-X., Guan H.-S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs. 2012;10:2795–2816. doi: 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Conte R., Marturano V., Peluso G., Calarco A., Cerruti P. Recent advances in nanoparticle-mediated delivery of anti-inflammatory phytocompounds. Int. J. Mol. Sci. 2017;18:709. doi: 10.3390/ijms18040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Assali M., Shawahna R., Shareef M., Alhimony I.-A. Dexamethasone-diclofenac loaded polylactide nanoparticles: Preparation, release and anti-inflammatory activity. Eur. J. Pharm. Sci. 2018;122:179–184. doi: 10.1016/j.ejps.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 110.Kaulmann A., Bohn T. Carotenoids, inflammation, and oxidative stress—implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014;34:907–929. doi: 10.1016/j.nutres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 111.Yatoo M., Gopalakrishnan A., Saxena A., Parray O.R., Tufani N.A., Chakraborty S., Tiwari R., Dhama K., Iqbal H. Anti-inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders-a review. Recent Pat. Inflamm. Allergy Drug Discov. 2018;12:39–58. doi: 10.2174/1872213X12666180115153635. [DOI] [PubMed] [Google Scholar]
- 112.David L., Moldovan B., Vulcu A., Olenic L., Perde-Schrepler M., Fischer-Fodor E., Florea A., Crisan M., Chiorean I., Clichici S. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surf. B Biointerfaces. 2014;122:767–777. doi: 10.1016/j.colsurfb.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 113.Azeem M.N.A., Ahmed O.M., Shaban M., Elsayed K.N.M. In vitro antioxidant, anticancer, anti-inflammatory, anti-diabetic and anti-Alzheimer potentials of innovative macroalgae bio-capped silver nanoparticles. Environ. Sci. Pollut. Res. 2022:1–18. doi: 10.1007/s11356-022-20039-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Grewal S.S. Why should cancer biologists care about tRNAs? tRNA synthesis, mRNA translation and the control of growth. Biochim. Et Biophys. Acta (BBA)-Gene Regul. Mech. 2015;1849:898–907. doi: 10.1016/j.bbagrm.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 115.Rajeshkumar S., Malarkodi C., Vanaja M., Annadurai G. Anticancer and enhanced antimicrobial activity of biosynthesizd silver nanoparticles against clinical pathogens. J. Mol. Struct. 2016;1116:165–173. doi: 10.1016/j.molstruc.2016.03.044. [DOI] [Google Scholar]
- 116.Maharani V., Sundaramanickam A., Balasubramanian T. In vitro anticancer activity of silver nanoparticle synthesized by Escherichia coli VM1 isolated from marine sediments of Ennore southeast coast of India. Enzym. Microb. Technol. 2016;95:146–154. doi: 10.1016/j.enzmictec.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 117.Hassabo A.A., Ibrahim E.I., Ali B.A., Emam H.E. Anticancer effects of biosynthesized Cu2O nanoparticles using marine yeast. Biocatal. Agric. Biotechnol. 2022;39:102261. doi: 10.1016/j.bcab.2021.102261. [DOI] [Google Scholar]
- 118.Saeed A.F., Su J., Ouyang S. Marine-derived drugs: Recent advances in cancer therapy and immune signaling. Biomed. Pharmacother. 2021;134:111091. doi: 10.1016/j.biopha.2020.111091. [DOI] [PubMed] [Google Scholar]
- 119.Zhang D., Ramachandran G., Mothana R.A., Siddiqui N.A., Ullah R., Almarfadi O.M., Rajivgandhi G., Manoharan N. Biosynthesized silver nanoparticles using Caulerpa taxifolia against A549 lung cancer cell line through cytotoxicity effect/morphological damage. Saudi J. Biol. Sci. 2020;27:3421–3427. doi: 10.1016/j.sjbs.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shunmugam R., Renukadevi Balusamy S., Kumar V., Menon S., Lakshmi T., Perumalsamy H. Biosynthesis of gold nanoparticles using marine microbe (Vibrio alginolyticus) and its anticancer and antioxidant analysis. J. King Saud Univ. Sci. 2021;33:101260. doi: 10.1016/j.jksus.2020.101260. [DOI] [Google Scholar]
- 121.Aboeita N.M., Fahmy S.A., El-Sayed M.M.H., Azzazy H.M.E.-S., Shoeib T. Enhanced Anticancer Activity of Nedaplatin Loaded onto Copper Nanoparticles Synthesized Using Red Algae. Pharmaceutics. 2022;14:418. doi: 10.3390/pharmaceutics14020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vijayakumar S., Chen J., Kalaiselvi V., Tungare K., Bhori M., González-Sánchez Z.I., Durán-Lara E.F. Marine polysaccharide laminarin embedded ZnO nanoparticles and their based chitosan capped ZnO nanocomposites: Synthesis, characterization and in vitro and in vivo toxicity assessment. Environ. Res. 2022;213:113655. doi: 10.1016/j.envres.2022.113655. [DOI] [PubMed] [Google Scholar]
- 123.Mistry H., Thakor R., Bariya H. Biogenesis and Characterization of Proficient Silver Nanoparticles Employing Marine Procured Fungi Hamigera Pallida and Assessment of Their Antioxidative, Antimicrobial and Anticancer Potency on Human Breast Cancer (MCF-7) Cell Line. Research Square; Durham, NC, USA: 2022. [DOI] [PubMed] [Google Scholar]
- 124.Acharya D., Satapathy S., Yadav K.K., Somu P., Mishra G. Systemic Evaluation of Mechanism of Cytotoxicity in Human Colon Cancer HCT-116 Cells of Silver Nanoparticles Synthesized Using Marine Algae Ulva lactuca Extract. J. Inorg. Organomet. Polym. Mater. 2022;32:596–605. doi: 10.1007/s10904-021-02133-8. [DOI] [Google Scholar]
- 125.Ameen F., Al-Maary K.S., Almansob A., AlNadhari S. Antioxidant, antibacterial and anticancer efficacy of Alternaria chlamydospora-mediated gold nanoparticles. Appl. Nanosci. 2022:1–18. doi: 10.1007/s13204-021-02047-4. [DOI] [Google Scholar]
- 126.González-Ballesteros N., Diego-González L., Lastra-Valdor M., Grimaldi M., Cavazza A., Bigi F., Rodríguez-Argüelles M.C., Simón-Vázquez R. Immunomodulatory and Antitumoral Activity of Gold Nanoparticles Synthesized by Red Algae Aqueous Extracts. Mar. Drugs. 2022;20:182. doi: 10.3390/md20030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen X., Han W., Zhao X., Tang W., Wang F. Epirubicin-loaded marine carrageenan oligosaccharide capped gold nanoparticle system for pH-triggered anticancer drug release. Sci. Rep. 2019;9:6754. doi: 10.1038/s41598-019-43106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Patil M.P., Kang M.-J., Niyonizigiye I., Singh A., Kim J.-O., Seo Y.B., Kim G.-D. Extracellular synthesis of gold nanoparticles using the marine bacterium Paracoccus haeundaensis BC74171T and evaluation of their antioxidant activity and antiproliferative effect on normal and cancer cell lines. Colloids Surf. B Biointerfaces. 2019;183:110455. doi: 10.1016/j.colsurfb.2019.110455. [DOI] [PubMed] [Google Scholar]
- 129.Vijayakumar M., Priya K., Ilavenil S., Janani B., Vedarethinam V., Ramesh T., Arasu M.V., Al-Dhabi N.A., Kim Y.-O., Kim H.-J. Shrimp shells extracted chitin in silver nanoparticle synthesis: Expanding its prophecy towards anticancer activity in human hepatocellular carcinoma HepG2 cells. Int. J. Biol. Macromol. 2020;165:1402–1409. doi: 10.1016/j.ijbiomac.2020.10.032. [DOI] [PubMed] [Google Scholar]
- 130.Kumar P., Senthamilselvi S., Govindaraju M. Phloroglucinol-encapsulated starch biopolymer: Preparation, antioxidant and cytotoxic effects on HepG2 liver cancer cell lines. RSC Adv. 2014;4:26787–26795. doi: 10.1039/c4ra02621g. [DOI] [Google Scholar]
- 131.Kumar H., Bhardwaj K., Nepovimova E., Kuča K., Singh Dhanjal D., Bhardwaj S., Bhatia S.K., Verma R., Kumar D. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials. 2020;10:1334. doi: 10.3390/nano10071334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nandi A., Yan L.-J., Jana C.K., Das N. Role of catalase in oxidative stress-and age-associated degenerative diseases. Oxidative Med. Cell. Longev. 2019;2019:9613090. doi: 10.1155/2019/9613090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Munteanu I.G., Apetrei C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021;22:3380. doi: 10.3390/ijms22073380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Valko M., Rhodes C., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 135.Zhong Q., Wei B., Wang S., Ke S., Chen J., Zhang H., Wang H. The antioxidant activity of polysaccharides derived from marine organisms: An overview. Mar. Drugs. 2019;17:674. doi: 10.3390/md17120674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu C., Fu Y., Li C.-E., Chen T., Li X. Phycocyanin-functionalized selenium nanoparticles reverse palmitic acid-induced pancreatic β cell apoptosis by enhancing cellular uptake and blocking reactive oxygen species (ROS)-mediated mitochondria dysfunction. J. Agric. Food Chem. 2017;65:4405–4413. doi: 10.1021/acs.jafc.7b00896. [DOI] [PubMed] [Google Scholar]
- 137.Kumar P., Yuvakkumar R., Vijayakumar S., Vaseeharan B. Cytotoxicity of phloroglucinol engineered silver (Ag) nanoparticles against MCF-7 breast cancer cell lines. Mater. Chem. Phys. 2018;220:402–408. doi: 10.1016/j.matchemphys.2018.08.074. [DOI] [Google Scholar]
- 138.Mahalakshmi M., Kumar P. Phloroglucinol-conjugated gold nanoparticles targeting mitochondrial membrane potential of human cervical (HeLa) cancer cell lines. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019;219:450–456. doi: 10.1016/j.saa.2019.04.060. [DOI] [PubMed] [Google Scholar]
- 139.Khan F., Oh D., Chandika P., Jo D.M., Bamunarachchi N.I., Jung W.K., Kim Y.M. Inhibitory activities of phloroglucinol-chitosan nanoparticles on mono- and dual-species biofilms of Candida albicans and bacteria. Colloids Surf. B Biointerfaces. 2022;211:112307. doi: 10.1016/j.colsurfb.2021.112307. [DOI] [PubMed] [Google Scholar]
- 140.Khan F., Kang M.-G., Jo D.-M., Chandika P., Jung W.-K., Kang H.W., Kim Y.-M. Phloroglucinol-gold and-zinc oxide nanoparticles: Antibiofilm and antivirulence activities towards Pseudomonas aeruginosa PAO1. Mar. Drugs. 2021;19:601. doi: 10.3390/md19110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jena J., Pradhan N., Dash B.P., Panda P.K., Mishra B.K. Pigment mediated biogenic synthesis of silver nanoparticles using diatom Amphora sp. and its antimicrobial activity. J. Saudi Chem. Soc. 2015;19:661–666. [Google Scholar]
- 142.Chandika P., Khan F., Heo S.-Y., Kim Y.-M., Yi M., Jung W.-K. Enhanced wound-healing capability with inherent antimicrobial activities of usnic acid incorporated poly (ε-caprolactone)/decellularized extracellular matrix nanofibrous scaffold. Biomater. Adv. 2022;140:213046. doi: 10.1016/j.bioadv.2022.213046. [DOI] [PubMed] [Google Scholar]
- 143.Abdelwahab G.M., Mira A., Cheng Y.-B., Abdelaziz T.A., Lahloub M.F.I., Khalil A.T. Acetylcholine esterase inhibitory activity of green synthesized nanosilver by naphthopyrones isolated from marine-derived Aspergillus niger. PLoS ONE. 2021;16:e0257071. doi: 10.1371/journal.pone.0257071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dai S., Wang B., Song Y., Xie Z., Li C., Li S., Huang Y., Jiang M. Astaxanthin and its gold nanoparticles mitigate cadmium toxicity in rice by inhibiting cadmium translocation and uptake. Sci. Total Environ. 2021;786:147496. doi: 10.1016/j.scitotenv.2021.147496. [DOI] [PubMed] [Google Scholar]
- 145.Manivasagan P., Khan F., Hoang G., Mondal S., Kim H., Doan V.H.M., Kim Y.-M., Oh J. Thiol chitosan-wrapped gold nanoshells for near-infrared laser-induced photothermal destruction of antibiotic-resistant bacteria. Carbohydr. Polym. 2019;225:115228. doi: 10.1016/j.carbpol.2019.115228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.