Abstract
Acylhomoserine lactones, which serve as quorum-sensing signals in gram-negative bacteria, are produced by members of the LuxI family of synthases. LuxI is a Vibrio fischeri enzyme that catalyzes the synthesis of N-(3-oxohexanoyl)-l-homoserine lactone from an acyl-acyl carrier protein and S-adenosylmethionine. Another V. fischeri gene, ainS, directs the synthesis of N-octanoylhomoserine lactone. The AinS protein shows no significant sequence similarity with LuxI family members, but it does show sequence similarity with the Vibrio harveyi LuxM protein. The luxM gene is required for the synthesis of N-(3-hydroxybutyryl)-l-homoserine lactone. To gain insights about whether AinS and LuxM represent a second family of acylhomoserine lactone synthases, we have purified AinS as a maltose-binding protein (MBP) fusion protein. The purified MBP-AinS fusion protein catalyzed the synthesis of N-octanoylhomoserine lactone from S-adenosylmethionine and either octanoyl-acyl carrier protein or, to a lesser extent, octanoyl coenzyme A. With the exception that octanoyl coenzyme A served as an acyl substrate for the MBP-AinS fusion protein, the substrates for and reaction kinetics of the MBP-AinS fusion protein were similar to those of the several LuxI family members previously studied. We conclude that AinS is an acylhomoserine lactone synthase and that it represents a second family of such enzymes.
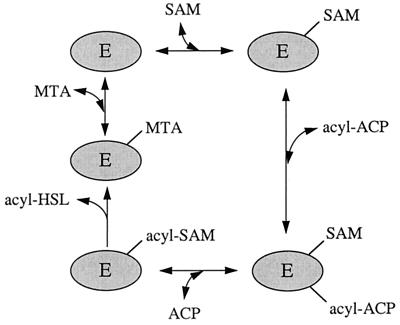
Quorum sensing in gram-negative bacteria is often mediated by diffusible acylhomoserine lactone (acyl-HSL) signal molecules. The LuxI family of acyl-HSL synthases constitutes a group of enzymes from different species. These enzymes require S-adenosylmethionine (SAM) and an acyl-acyl carrier protein (acyl-ACP) as substrates (6, 10, 11, 15, 18). A model of the reaction catalyzed by LuxI family members is shown in Fig. 1. Different LuxI homologs show selectivity for different acyl-ACPs and synthesize different acyl-HSLs. The acyl groups of acyl-HSLs range from 4 to 14 carbons in length; they can have either a carbonyl group, a hydroxyl group, or a lack of substitution on the third carbon; and they are often, but not always, saturated (for reviews, see references 3, 4, and 17).
FIG. 1.
A model for the synthesis of acyl-HSLs by the LuxI protein family. This model is based primarily on information about LuxI (15) and the LuxI homolog, RhlI (11). SAM binds to the enzyme, followed by acyl-ACP. An amide bond is formed between SAM and the acyl group, and holo-ACP is released. The acyl-SAM intermediate cyclizes, releasing the acyl-HSL and methylthioadenosine (MTA).
LuxI is from the marine luminescent bacterium Vibrio fischeri. An analysis of a V. fischeri luxI mutant revealed that it no longer produced the luminescence quorum-sensing signal N-(3-oxohexanoyl)-HSL, but N-octanoyl-HSL was produced by this mutant (9). A gene responsible for octanoyl-HSL production was identified; the sequence of the product of this gene, ainS, shows no similarity with members of the LuxI family of acyl-HSL synthases. AinS does show similarity with the luxM gene product from the marine luminescent bacterium Vibrio harveyi (5, 9). LuxM is thought to be involved in the synthesis of N-(3-hydroxybutyryl)-HSL (1). Thus, ainS and luxM appear to represent a second family of genes that direct gram-negative bacteria to synthesize acyl-HSLs (5).
Although ainS directs octanoyl-HSL synthesis in vivo, there is no information about how octanoyl-HSL is synthesized. AinS might possess enzymatic activity similar to that of the LuxI family, or it might catalyze acyl-HSL synthesis via a different route. Alternatively, AinS might lack enzymatic activity but direct other cellular machinery to synthesize octanoyl-HSL. To distinguish between these possibilities, we overexpressed and purified a maltose-binding protein (MBP)-AinS fusion protein. The ability of the purified fusion protein to catalyze the synthesis of acyl-HSLs was examined. Our results indicate that AinS is an acyl-HSL synthase with an enzymatic activity similar to the activities of LuxI family members.
MATERIALS AND METHODS
Construction of an MBP-AinS expression vector.
A 1.2-kbp fragment of V. fischeri MJ-1 DNA containing the ainS coding region was generated by PCR with the ainS plasmid pAI009 as the template DNA (5). The forward PCR primer was complementary to the first 22 bases of ainS and contained a 5′ overhang that included an EcoRI restriction sequence adjacent to the ainS translational start codon. The reverse primer was complementary to a region 52 to 69 bases beyond the ainS translational stop codon and contained a 5′ overhang with a HindIII restriction site. The PCR product was digested with EcoRI and HindIII and ligated with EcoRI-HindIII-digested pMAL-c2 (New England Biolabs, Beverly, Mass.). The ligation mixture was used to transform Escherichia coli XL1-Blue. Transformants were screened for acyl-HSL production by coculturing with E. coli VJS533(pHV200I−), which produces light only when provided with exogenous acyl-HSLs, and selecting transformants which induced light production (7). Plasmids from several acyl-HSL-producing transformants contained 1.2-kbp EcoRI-HindIII inserts and directed the production of a recombinant polypeptide with an apparent molecular weight of 87,000. This polypeptide was shown to be an MBP fusion protein by Western immunoblotting with anti-MBP serum (New England Biolabs). The plasmid from one transformant was chosen for further studies and designated pMA100. To confirm the presence of ainS, the cloned DNA was sequenced by using the chain termination method (13).
Purification of the MBP-AinS fusion.
A culture of E. coli XL1-Blue(pMA100) was grown in 1 liter of Luria broth containing glucose (0.2 g/ml), tetracycline (10 μg/ml), and ampicillin (200 μg/ml) with shaking at 30°C. The ptac-malE-ainS gene was activated by the addition of 1 mM isopropyl β-d-thiogalactanoside (IPTG) to the culture in mid-logarithmic phase (optical density at 600 nm, 0.5). After 2 h in the presence of IPTG, cells were harvested by centrifugation at 5,000 × g for 30 min. The cell pellet was stored at −70°C. The cell pellet was then resuspended (1 g of wet cell paste per 5 ml) in a buffer containing 50 mM sodium phosphate (pH 7.0), 200 mM sodium chloride, 1 mM EDTA, 1 mM dithiothreitol (DTT), glycerol (100 mg/ml), phenylmethylsulfonyl fluoride (100 μg/ml), leupeptin (0.5 μg/ml), and pepstatin A (0.7 μg/ml). Lysozyme (1 mg/ml), DNase (10 μg/ml), and RNase (10 μg/ml) were added to the cell suspension, and it was incubated on ice for 20 min. The cells were lysed in a French pressure cell (two passes at 6.9 kPa). The cell extract was clarified by centrifugation at 9,000 × g at 4°C for 30 min, and the MBP-AinS fusion protein was purified from the clarified cell extract by amylose affinity chromatography according to the manufacturer’s instructions (New England Biolabs). The purified protein was stored at −70°C.
Acyl-HSL synthase activity assays.
Unless otherwise specified, the standard reaction buffer contained 50 mM sodium chloride, 2 mM DTT, 800 μM SAM, either 25 μM octanoyl-ACP or 200 μM octanoyl-coenzyme A (CoA), and 50 mM Tris · Cl (pH 8.5). The activity assays were in 100-μl volumes. Reactions were started by the addition of 1 μg of MBP-AinS. The incubation temperature was 25°C. After an incubation time of 40 min, the reactions were stopped by the addition of 4 μl of 1 N HCl.
The amount of octanoyl-HSL in ethyl acetate extracts of acyl-HSL synthase reactions was measured either by a bioassay (14) or by a radiometric assay (11) as indicated. For the radiometric assay, reaction mixtures included 600 μM S-adenosyl-l-[carboxyl-14C]methionine (1.3 to 3.8 mCi/mmol).
Chromatographic analysis of reaction product.
The ethyl acetate extracts from acyl-HSL synthase reactions in the presence of S-adenosyl-l-[carboxyl-14C]methionine were fractionated by C18 reverse-phase high-performance liquid chromatography (HPLC) in a 20 to 100% (vol/vol) methanol-in-water gradient as described elsewhere (12). One-milliliter fractions were collected and the 14C-acyl-HSLs in each fraction were measured as described previously (11).
Chemicals.
SAM was obtained from Fluka Chemical Corp. (Ronkonkoma, N.Y.) and S-adenosyl-l[carboxyl-14C]methionine was obtained from Amersham Life Sciences, Inc. (Arlington Heights, Ill.). Holo-ACP and apo-ACP were prepared from an E. coli strain that overproduces the protein (8). Octanoyl-HSL (2) and acyl-ACPs (16) were synthesized as previously described. All other chemicals were purchased from Sigma Chemical Co. (St. Louis, Mo.).
RESULTS
Purification of the MBP-AinS fusion protein.
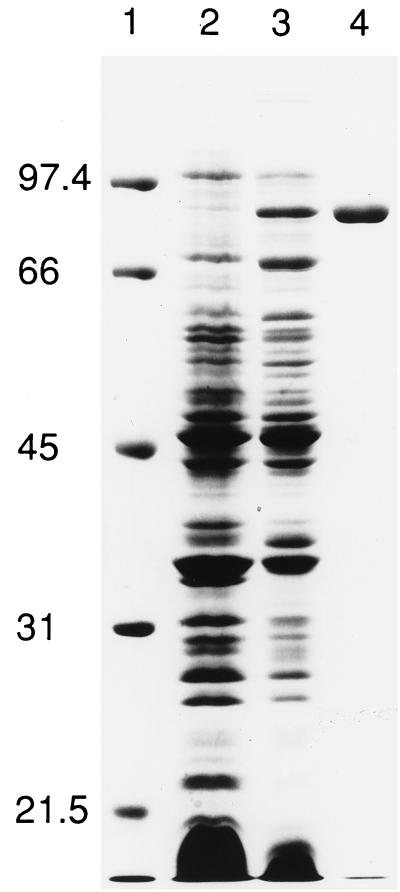
Production of a polypeptide with an apparent molecular weight of 87,000 was induced by the growth of E. coli XL1-Blue(pMA100) in the presence of IPTG. Western immunoblotting demonstrated that this polypeptide contained a MalE epitope (see Materials and Methods). This MBP-AinS fusion protein was purified by amylose affinity chromatography (Fig. 2).
FIG. 2.
Purification of the MBP-AinS fusion protein from clarified cell extracts. Lane 1, molecular mass standards (prestained low-range markers; Bio-Rad, Hercules, Calif.), with molecular masses indicated in kilodaltons; lane 2, clarified cell extract from an uninduced culture (60 μg of protein); lane 3, clarified cell extract from an IPTG-induced culture (60 μg of protein); lane 4, amylose affinity column-purified MBP-AinS (5 μg of protein).
Acyl-HSL synthase activity of the purified MBP-AinS protein.
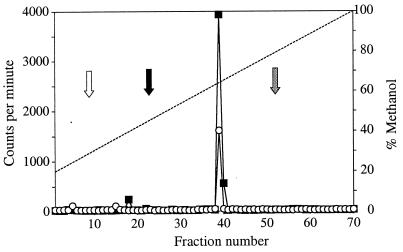
The purified MBP-AinS catalyzed the synthesis of octanoyl-HSL when incubated with SAM and octanoyl-ACP (Table 1; Fig. 3). A product was also detected in reaction mixtures when MBP-AinS was incubated with SAM and octanoyl-CoA (Table 1; Fig. 3). Octanoyl-HSL was the only product detected by HPLC fractionation of acyl-HSLs synthesized from octanoyl-ACP or octanoyl-CoA and SAM. The production of octanoyl-HSL from SAM and octanoyl-ACP or octanoyl-CoA was linear for at least 60 min, and the amount of octanoyl-HSL synthesized was dependent on protein concentration over the range tested, 0.5 and 2 μg of MBP-AinS per 100 μl of reaction mixture (data not shown). Synthesis of octanoyl-HSL was dependent on the presence of protein, SAM, and either octanoyl-ACP or octanoyl-CoA (Table 1). From these results, we concluded that AinS is a synthase that catalyzes the production of octanoyl-HSL from SAM and either octanoyl-ACP or octanoyl-CoA.
TABLE 1.
Substrate requirements for acyl-HSL synthesis by purified MBP-AinSa
| Amino donor | Acyl donor | Acyl-HSL produced (μmol · min−1 · mg−1) inb:
|
|
|---|---|---|---|
| Octanoyl-HSL bioassay | Radiometric assay | ||
| SAM | Octanoyl-ACP | 12.5 | 15.4 |
| Octanoyl-CoA | 2.6 | 2.2 | |
| Sodium octanoate | <0.02 | ||
| Methionine | Octanoyl-ACP | <0.02 | |
| Octanoyl-CoA | <0.02 | ||
| HSL | Octanoyl-ACP | <0.02 | |
| Octanoyl-CoA | <0.02 | ||
| Homoserine | Octanoyl-ACP | <0.02 | |
| Octanoyl-CoA | <0.02 | ||
| S-Adenosylhomocysteine | Octanoyl-ACP | <0.02 | |
| Octanoyl-CoA | <0.02 | ||
| Cystathionine | Octanoyl-ACP | <0.02 | |
| Octanoyl-CoA | <0.02 | ||
| S-Adenosylethionine | Octanoyl-ACP | 0.47 | |
| Octanoyl-CoA | 0.045 | ||
| SAM | Butyryl-ACP | 0.46 | |
| Butyryl-CoA | 0.06 | ||
| Hexanoyl-ACP | 5.8 | ||
| Hexanoyl-CoA | 0.47 | ||
| Decanoyl-ACP | 12.2 | ||
| Decanoyl-CoA | 0.43 | ||
Concentrations: Acyl-ACP, 25 μM; acyl-CoA, 100 μM; sodium octanoate, 200 μM; amino donors, 800 μM for reactions analyzed with the bioassay and 600 μM for reactions assayed radiometrically. No octanoyl-HSL synthesis was detected from either octanoyl-ACP or octanoyl-CoA and SAM if the MBP-AinS was omitted from the reaction mixture.
Values are means from two to five independent experiments, each performed in duplicate. The ranges were from ±10 to ±38% of the mean.
FIG. 3.
HPLC analysis of radioactive acyl-HSLs produced in vitro by the MBP-AinS fusion. The ethyl acetate extracts from the reaction mixtures were dried under a stream of N2 gas, dissolved in 20% methanol–80% water, and loaded onto the column. Products from SAM and octanoyl-ACP (■) and from SAM and octanoyl-CoA (○) are shown. The methanol gradient is indicated by the dashed line. Synthetic octanoyl-HSL, measured by using the bioassay, elutes in the same fraction as the enzyme product. The white arrow shows where butyryl-HSL elutes, the black arrow shows where hexanoyl-HSL elutes, and the gray arrow shows where 3-oxododecanoyl-HSL elutes.
To examine the substrate specificity of AinS, we tested a range of compounds in the acyl-HSL synthase assays. The only compound other than SAM that served as an amino donor was S-adenosyl-l-ethionine, and activity with this amino donor was less than 10% of that with SAM as the amino donor (Table 1). There was no detectable activity when sodium octanoate was provided as the acyl substrate (Table 1). This indicates that the acyl-thioester bond in octanoyl-ACP or octanoyl-CoA is required for enzyme activity. To determine the selectivity of the enzyme for specific acyl-substrates, we measured the activity of MBP-AinS with acyl-CoAs and acyl-ACPs containing acyl groups 4 to 10 carbons in length. The highest specific activity was detected with octanoyl-ACP, and activities with decanoyl-ACP and hexanoyl-ACP were 80 and 38% of that with octanoyl-ACP, respectively (Table 1). With butyryl-ACP, activity was only 3% of that with octanoyl-ACP. Acyl-CoA substrates were less active then acyl-ACP substrates with acyl side chains of the same length. Octanoyl-CoA had about 14% of the activity of octanoyl-ACP, even though octanoyl-CoA was present at a fourfold-higher concentration than octanoyl-ACP (Table 1). Activity with the acyl-CoA substrates decreased dramatically as the acyl side chain was increased or decreased from eight carbons in length (Table 2).
TABLE 2.
Inhibitors of MBP-AinS synthase activity
| Inhibitor | Concn | % MBP-AinS activitya |
|---|---|---|
| Methylthioadenosine | 5 μM | 49 |
| 60 μM | 8 | |
| S-Adenosyl-d-homocysteine | 250 μM | 50 |
| 2.5 mM | 15 | |
| S-Adenosyl-l-homocysteine | 250 μM | 84 |
| 2.5 mM | 55 | |
| Holo-ACPb | 250 μM | 74 |
| 500 μM | 57 |
Activity is given as a percentage of the activity in controls with no inhibitor added.
Reaction mixtures with holo-ACP also contained 10 mM DTT. The DTT alone did not affect MBP-AinS activity.
With SAM and octanoyl-ACP as substrates, the purified MBP-AinS showed a temperature optimum at 32°C. The activity was greater than 60% of the maximum at temperatures between 25 to 37°C and was less than 15% of the maximum at 15 and 42°C. With either octanoyl-ACP or octanoyl-CoA as the acyl donor, MBP-AinS showed a broad peak in activity over a pH range of 8 to 10. The activity decreased sharply above pH 10 and below pH 8 and was undetectable at pH 6 or 11. Octanoyl-HSL has reduced stability under alkaline conditions (14). Furthermore, the substrates may be unstable in the reaction mixtures at pH 11.
Kinetics of octanoyl-HSL synthase activity.
We determined the Michaelis constant (Km) of MBP-AinS for SAM in the presence of either octanoyl-CoA or octanoyl-ACP and for octanoyl-ACP and octanoyl-CoA in the presence of SAM. The Km and maximum velocity (Vmax) values for SAM were determined by varying the concentration of SAM over a range of 20 to 600 μM in the presence of either 75 μM octanoyl-ACP or 200 μM octanoyl-CoA. The Km and Vmax values for octanoyl-ACP were determined by varying the octanoyl-ACP concentration over a range of 10 to 100 μM in the presence of 600 μM SAM. The Km and Vmax values for octanoyl-CoA were determined by varying the octanoyl-CoA concentration between 0.5 to 100 μM in the presence of 600 μM SAM. The Vmax value of octanoyl-HSL synthesis was about 10 times higher with octanoyl-ACP (2.37 mol of octanoyl-HSL · min−1 · mol of protein−1) than with octanoyl-CoA (0.21 mol of octanoyl-HSL · min−1 · mol of protein−1). The apparent Km value for octanoyl-CoA was 4 μM and the apparent Km value for octanoyl-ACP was 15 μM, nearly four times higher. However, the apparent Km value for SAM in the presence of octanoyl-CoA was 61 μM, whereas the apparent Km value for SAM in the presence of octanoyl-ACP was 23 μM, nearly threefold lower.
Inhibition of octanoyl-HSL synthase activity by substrate analogs and potential reaction products.
For an initial screen of potential inhibitors, reaction mixtures contained SAM and octanoyl-ACP at concentrations near their Km (25 μM radioactive SAM and 15 μM octanoyl-ACP). The inhibitors were included at concentrations of 250 μM, and the reactions were stopped after 10 min. The following compounds did not serve as inhibitors of octanoyl-HSL synthesis (activity in the presence of the inhibitor was at least 90% of the control): methionine, homoserine (HS), HSL, homocysteine thiolactone, adenosine, apo-ACP (an acyl carrier protein lacking the 4′-phosphopantethine prosthetic group), or HS-CoA. Octanoyl-HSL did not inhibit the reaction, even at concentrations as high as 2,500 μM. Inhibition was detected with the putative reaction product, 5′-deoxymethylthioadenosine, and the SAM analog S-adenosylhomocysteine (Table 2). Interestingly, S-adenosyl-d-homocysteine (possessing the opposite chirality of SAM in the reactions) was a stronger inhibitor than S-adenosyl-l-homocysteine. Holo-ACP, an expected reaction product, was a weak inhibitor and inhibition required high concentrations of DTT (10 mM), presumably to reduce the intermolecular disulfide bonds between the 4′-phosphopantethine prosthetic groups.
Holo-ACP and HS-CoA were also tested for the ability to inhibit octanoyl-HSL synthesis with SAM and octanoyl-CoA as substrates. In these experiments, SAM and octanoyl-CoA were present at concentrations near their Km (60 μM radioactive SAM and 4 μM octanoyl-CoA), holo-ACP or HS-CoA was included at a concentration as high as 500 μM in the presence of 10 mM DTT, and the reactions were stopped after 30 min. Neither holo-ACP nor HS-CoA was a strong inhibitor of the reaction.
DISCUSSION
We have purified the V. fischeri AinS polypeptide in the form of an MBP fusion protein. This protein can catalyze the synthesis of acyl-HSLs from SAM and either acyl-ACP or acyl-CoA (Table 1). Thus, we conclude that the AinS protein possesses acyl-HSL synthase activity. We know from previous investigations that ainS directs V. fischeri to synthesize octanoyl-HSL and that it encodes a polypeptide that shows no resemblance to members of the LuxI family of acyl-HSL synthases. Thus, we propose that AinS represents a family of acyl-HSL synthases, distinct from the LuxI family, and that the AinS family also includes the V. harveyi LuxM protein, which shows sequence similarity with AinS (5). LuxM is required for the production of N-(3-hydroxybutyryl)-HSL by V. harveyi (1).
How does the activity of the MBP-AinS fusion protein compare to that of LuxI family members? Three LuxI family members have been purified: LuxI was purified as an MBP fusion protein (15), Agrobacterium tumefaciens TraI was purified as a His-tagged protein (10), and Pseudomonas aeruginosa RhlI was purified in its native form (11). Like the MBP-AinS fusion, all of these LuxI family members use SAM rather than HSL, HS, or methionine as an amino donor (Table 1). All of the enzymes use acyl-ACPs as acyl substrates and, as in the MBP-AinS fusion protein (Table 1; Fig. 3), the greatest activity is with acyl-ACPs containing an acyl group equal in length to the side chain on the primary acyl-HSL produced in vivo. In contrast to the MBP-AinS fusion protein, acyl-CoAs are used poorly, if at all, as acyl group donors by the LuxI family members (10, 11, 15). The kinetics of octanoyl-HSL synthesis from octanoyl-ACP and SAM are quite similar to the kinetics reported for the purified LuxI family members. The Km values are in the micromolar range. The Vmax value for the MBP-AinS fusion protein is slightly higher than the Vmax values reported for the purified LuxI (15) and TraI (10) fusion proteins and somewhat lower than the value reported for the native RhlI protein (11).
A recent detailed kinetic analysis of the P. aeruginosa RhlI protein has led to a model for the enzymatic steps in the synthesis of acyl-HSLs by LuxI family members (11) (Fig. 1). A study of end products and dead-end inhibitors led to the conclusion that acyl-HSL synthesis proceeds by a sequentially ordered reaction. The first proposed intermediate is an enzyme-SAM complex to which the acyl-ACP binds. After both substrates are bound to the enzyme, amide bond synthesis occurs to form an enzyme-bound butyryl-SAM intermediate. Holo-ACP is then released, the amino acid portion of butyryl-SAM cyclizes, butyryl-HSL is released, and finally methylthioadenosine is released (11). Methylthioadenosine is a very strong competitive inhibitor of SAM binding and thus of the enzyme, ACP is a weak inhibitor of the enzyme, and the acyl-HSL product does not inhibit RhlI activity. In addition, the SAM analog S-adenosylhomocysteine is a strong inhibitor of RhlI. This was also the case for AinS (Table 2). Our kinetic analysis and inhibitor studies were not detailed but are consistent with the RhlI results, suggesting that acyl-HSL synthesis by AinS involves a mechanism similar to RhlI.
The MBP-AinS fusion protein was able to use octanoyl-CoA as an acyl group donor in a relatively efficient manner (Table 1). This is not the case for the LuxI family members that have been studied (see above). It is tempting to speculate that there are physiological conditions under which V. fischeri uses octanoyl-CoA as a substrate for ainS-directed octanoyl-HSL synthesis. Because evidence indicated that LuxI does not use acyl-CoA substrates (15), one must consider the notion that AinS can synthesize octanoyl-HSL in V. fischeri in some environments where LuxI does not synthesize N-(3-oxohexanoyl)-HSL. This could explain, at least in part, the occurrence of two different families of acyl-HSL synthases in V. fischeri; however, this possibility remains to be studied.
ACKNOWLEDGMENTS
Research in the laboratory of E.P.G. is supported by a grant from the National Science Foundation (MCB 9808308). B.L.H. has been supported by a U.S. Public Health Training Grant (732 GM8365). M.R.P. is a National Institutes of Health Postdoctoral Fellow (GM 18740-01A1). Research in the laboratory of P.V.D. is supported by a grant from the National Science Foundation (MCB 9722972). Research in the laboratory of J.E.C. is supported by a grant from the National Institutes of Health (AI15650).
REFERENCES
- 1.Bassler B L, Wright M, Showalter R E, Silverman M R. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 2.Eberhard A, Burlingame A L, Eberhard C, Kenyon G L, Nealson K H, Oppenheimer N J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 3.Fuqua C, Greenberg E P. Self perception in bacteria: quorum sensing with acylated homoserine lactones. Curr Opin Microbiol. 1998;1:183–189. doi: 10.1016/s1369-5274(98)80009-x. [DOI] [PubMed] [Google Scholar]
- 4.Fuqua W C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 5.Gilson L, Kuo A, Dunlap P V. AinS and a new family of autoinducer synthesis proteins. J Bacteriol. 1995;177:6946–6951. doi: 10.1128/jb.177.23.6946-6951.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanzelka B L, Greenberg E P. Quorum sensing in Vibrio fischeri: evidence that S-adenosylmethionine is the amino acid substrate for autoinducer synthesis. J Bacteriol. 1996;178:5291–5294. doi: 10.1128/jb.178.17.5291-5294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanzelka B L, Stevens A M, Parsek M R, Greenberg E P. Mutational analysis of the Vibrio fischeri LuxI polypeptide: critical regions of an autoinducer synthase. J Bacteriol. 1997;179:4882–4887. doi: 10.1128/jb.179.15.4882-4887.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keating D H, Carey M R, Cronan J E., Jr The unmodified (apo) form of Escherichia coli acyl carrier protein is a potent inhibitor of cell growth. J Biol Chem. 1995;270:22229–22235. doi: 10.1074/jbc.270.38.22229. [DOI] [PubMed] [Google Scholar]
- 9.Kuo A, Blough N V, Dunlap P V. Multiple N-acyl-l-homoserine lactone autoinducers of luminescence in the marine symbiotic bacterium Vibrio fischeri. J Bacteriol. 1994;176:7558–7565. doi: 10.1128/jb.176.24.7558-7565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moré M I, Finger L D, Stryker J L, Fuqua C, Eberhard A, Winans S C. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science. 1996;272:1655–1658. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- 11.Parsek M R, Val D L, Hanzelka B L, Cronan J E, Jr, Greenberg E P. Acyl homoserine lactone quorum-sensing signal generation. Proc Natl Acad Sci USA. 1999;96:4360–4365. doi: 10.1073/pnas.96.8.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearson J P, Gray K M, Passador L, Tucker K D, Eberhard A, Iglewski B H, Greenberg E P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaefer, A. L., B. L. Hanzelka, M. R. Parsek, and E. P. Greenberg. Detection, purification and structural elucidation of acylhomoserine lactone inducer of Vibrio fischeri luminescence and other related molecules. Methods Enzymol., in press. [DOI] [PubMed]
- 15.Schaefer A L, Val D L, Hanzelka B L, Cronan J E, Jr, Greenberg E P. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc Natl Acad Sci USA. 1996;93:9505–9509. doi: 10.1073/pnas.93.18.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen Z, Fice D, Byers D M. Preparation of fatty-acylated derivatives of acyl carrier protein using Vibrio harveyi acyl-ACP synthetase. Anal Biochem. 1992;204:34–39. doi: 10.1016/0003-2697(92)90135-t. [DOI] [PubMed] [Google Scholar]
- 17.Swift S, Throup J P, Williams P, Salmond G P C, Stewart G S A B. Quorum sensing: a population-density component in the determination of bacterial phenotype. Trends Biochem Sci. 1996;21:214–219. [PubMed] [Google Scholar]
- 18.Val D L, Cronan J E., Jr In vivo evidence that S-adenosylmethionine and fatty acid synthesis intermediates are the substrates for the LuxI family of autoinducer substrates. J Bacteriol. 1998;180:2644–2651. doi: 10.1128/jb.180.10.2644-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]