Abstract
The clinical benefits of nootropics in the treatment of cognitive decline has been either limited or controversial. This study aimed to observe the effectiveness of cholinesterase inhibitor (ChEI) and nootropics combination in the treatment of cognitive impairment in dementia. Data were based on electronic medical records in a university health system. Patients with mild-to-moderate dementia and no history of prior cognitive enhancer use were included (n = 583). The subjects were categorized into the ChEI only group and the ChEI and nootropics combination group. The primary outcome measure was the change in cognitive function, as assessed by the mini-mental state examination (MMSE) from baseline to 300–400 days after the first ChEI prescription. Subsequent analyses were conducted in consideration of the dementia type, medical adherence, and type of nootropics. The changes in MMSE scores from baseline to endpoint were not significantly different between the two groups. In Alzheimer’s dementia, the combination group showed significantly less deterioration in MMSE language subscale scores compared to the ChEI only group (F = 6.86, p = 0.009), and the difference was consistent in the highly adherent subjects (F = 10.16, p = 0.002). The choline alfoscerate and the ginkgo biloba extract subgroups in Alzheimer’s dementia showed more significant improvements in the MMSE language subscale scores compared to the other nootropics subgroup (F = 7.04, p = 0.001). The present study showed that the effectiveness of ChEI and nootropics combination on cognition may appear differently according to the dementia type. This emphasizes the need for well-controlled studies to generalize the effectiveness of nootropics across various clinical settings.
Keywords: cholinesterase inhibitors, nootropic agents, dementia, Alzheimer disease, cognitive dysfunction, mental status and dementia test
1. Introduction
Dementia is one of the most rapidly growing syndromes in aging societies, and it is also the leading cause of disability and caregiver dependency among older adults. Currently, more than 55 million people worldwide are affected by dementia, and nearly 10 million new patients are diagnosed with dementia every year [1]. Dementia leads to deterioration in multiple domains of cognitive function, including orientation, memory, language, attention, and visuospatial construction. To date, no curative drugs for dementia have been widely implemented in clinical practice, and most clinicians still consider cholinesterase inhibitors (ChEIs) as the first line of pharmacological treatment for cognitive deficits in dementia [2,3].
Nootropics, which were originally described as compounds with similar pharmacology to piracetam, are now generally accepted as any and all compounds that are expected to improve learning and memory in experimental and clinical paradigms [4]. In Korea, apart from the general over-the-counter supplements, there are certain nootropics that need prescriptions for use; among those most frequently prescribed are choline alfoscerate, ginkgo biloba extract, acetyl-L-carnitine, nicergoline, and oxiracetam. Many clinicians often prefer concomitant use of ChEIs with nootropics, based on fragmentary evidence that augmentation could have certain clinical benefits [5,6]. In fact, the clinical benefits of nootropics in the treatment of cognitive decline are either limited or controversial, with some studies showing improvements in cognitive function with choline alfoscerate [7,8,9], ginkgo biloba extract [10,11], acetyl-L-carnitine [6,12], and nicergoline [13,14], while other studies demonstrated no clinical benefits of such nootropics [15,16,17,18]. Although nootropics alone may not significantly improve cognitive function, nootropics in combination with ChEIs may enhance cholinergic neurotransmission, neurogenesis, and micro-perfusion in the frontal lobe, hippocampus, and striatum, ultimately delaying cognitive decline in dementia, especially in those with mild-to-moderate disease severity [19,20,21].
This study aimed to elucidate whether the concomitant use of nootropics with ChEIs is effective in the treatment of cognitive impairment in patients with dementia. We hypothesized that dementia patients treated with both ChEIs and nootropics would show less deterioration in cognitive function as assessed by mini-mental state examination (MMSE) over a 1-year follow-up period compared to dementia patients treated with ChEIs only. We further hypothesized that this trend would be more evident in the neurodegenerative forms of dementia, particularly Alzheimer’s dementia.
2. Materials and Methods
2.1. Study Population
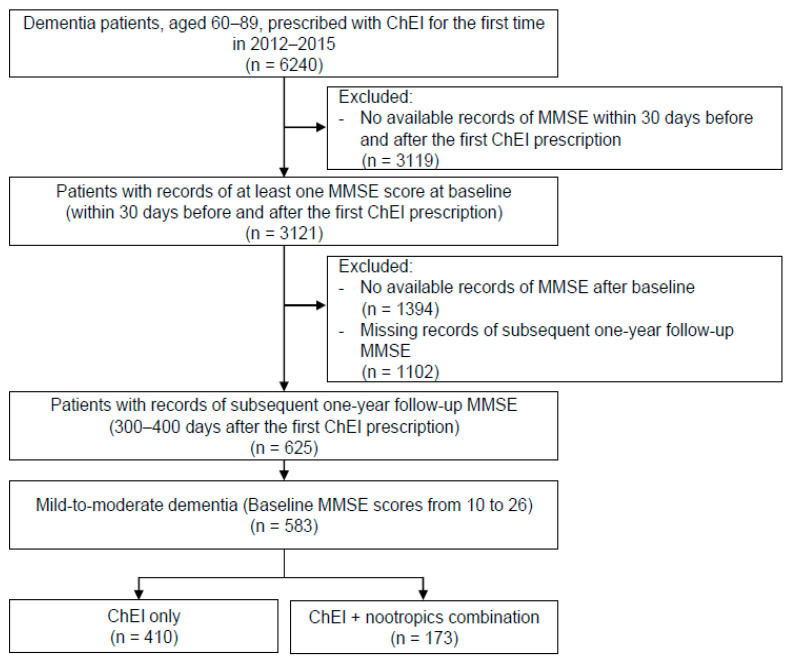
The present study aimed to observe the effectiveness of the concomitant administration of nootropics with ChEIs for the treatment of cognitive deficits in patients with dementia. Data were collected and extracted via the clinical data warehouse of a university health system consisting of three general hospitals with 4050 beds in total. Patients with any diagnosis of dementia, defined according to the International Statistical Classification of Disease and Related Health Problems 10th Revision (ICD-10) diagnostic codes F00, F01, F02, F03, F10.7, G23.1, G30, G31.0, G31.1, and G31.8, between the ages of 60 and 89 years were selected. We extracted all diagnostic codes of the patients regardless of whether they were the main or sub-diagnoses. After screening the entire prescription histories registered on the clinical data warehouse, which was over 7 years of record, only the patients who had their first ChEI prescription between 2012 and 2015 were included in the study. Subjects were further narrowed down to only those with mild-to-moderate dementia, as defined by their baseline MMSE total scores from 10 to 26 (n = 583) (Figure 1). Subjects were categorized into the ChEI only group (n = 410) and the ChEI and nootropics combination group (n = 173). Subjects in the ChEI and nootropics combination group were prescribed at least one of the following nootropics during the follow-up period: choline alfoscerate, ginkgo biloba extract, acetyl-L-carnitine, nicergoline, and oxiracetam. MMSE total scores and its six-subscale scores (orientation, immediate recall, attention and calculation, delayed recall, language, and visuospatial construction) were collected at the baseline (within 30 days of the first ChEI prescription of ChEI) and at the endpoint (300–400 days after the first ChEI prescription), respectively. Baseline data on age, sex, weight, height, body mass index (BMI), blood pressure (BP), education, alcohol use, tobacco use, concomitant psychiatric medications, and comorbid diagnoses were collected during the study period. Subjects were categorized by the type of dementia according to their main diagnosis in the highest order: Alzheimer’s dementia (n = 447), vascular dementia (n = 80), and other dementia (n = 56). For subgroup analysis, the ChEI and nootropics combination group of the Alzheimer’s dementia patients were further classified as follows: choline alfoscerate group (n = 74), ginkgo biloba extract group (n = 39), and other nootropics group (n = 21). Other nootropics included acetyl-L-carnitine, nicergoline, and oxiracetam.
Figure 1.
Flow diagram of study sample selection. ChEI: cholinesterase inhibitor; MMSE: mini-mental state examination.
This research protocol was exempt from an ethics review and informed consent was waived by the Institutional Review Board of Yongin Severance Hospital (9-2020-0108). This study adhered to the doctrine of the Declaration of Helsinki for Biomedical Research.
2.2. Measures
The main outcome measure was the change in cognitive function from baseline to endpoint, as assessed by the MMSE total score and its six subscale scores. Since the primary analysis did not differentiate the dementia type, subsequent analysis was conducted by comparing the changes in cognitive function between the ChEI only and ChEI and nootropics combination groups for each type of dementia separately. Further analysis was conducted in consideration of adherence to ChEI and nootropics. We defined the proportion of days covered (PDC = (Number of days in period “covered” by prescription) × 100/(Number of days in period)) ≥ 0.7 to be highly adherent, based on previous studies on adherence [22]. In the subgroup analysis, MMSE score changes were analyzed among the subgroups (i.e., choline alfoscerate vs. ginkgo biloba extract vs. other nootropics) to observe the effectiveness of each nootropic type.
2.3. Statistical Analysis
Baseline demographic and clinical characteristics between the two groups were compared by independent t-test for continuous variables and χ2 test or Fisher’s exact test for categorical variables. Two-sample t-tests were utilized to compare the two treatment groups at baseline and endpoint, as well as the change from baseline to endpoint. In order to adjust for critical covariates such as time and other relevant factors, including sex, age, hypertension, diabetes mellitus, stroke, Parkinsonism, visual disturbance/hearing loss, hemiplegia/paraplegia, mood/anxiety disorder, we further used linear mixed-effect model analyses with time, treatment group, and their interaction to test the difference of the change in MMSE total score and its six-subscale scores from baseline to endpoint between the two treatment groups. The analysis of variance (ANOVA) with Bonferroni multiple comparisons test was conducted to compare the changes in MMSE total score and its six-subscale scores among the three ChEI and nootropics combination subgroup of Alzheimer’s dementia (i.e., choline alfoscerate vs. ginkgo biloba extract vs. other nootropics).
All statistical analyses were conducted using IBM SPSS ver. 26 for Windows (IBM Corp: Armonk, NY, USA), and statistical significance was determined at p < 0.05 (two-tailed).
3. Results
3.1. Demographic and Clinical Characteristics
Table 1 shows the comparison of demographic and clinical characteristics of the 583 subjects included in this study. The mean ages were similar in both groups (75.5 ± 6.5 years vs. 75.5 ± 6.6 years in the ChEI only group and the ChEI and nootropics combination group, respectively). Females outnumbered males in both groups (266 (64.9%) females vs. 144 (35.1%) males in the ChEI only group; 117 (67.6%) females vs. 56 (32.4%) males in the ChEI and nootropics combination group). Regarding dementia diagnoses, Alzheimer’s dementia was the most frequent in both groups (313 (76.3%) in the ChEI only group, 134 (77.5%) in the ChEI and nootropics combination group), and vascular dementia was the second most frequent (49 (12.0%) in the ChEI only group, 31 (17.9%) in the ChEI and nootropics combination group). Alcohol use was more frequent in the ChEI only group (85 (20.7%)) than in the ChEI and nootropics combination group (23 (13.3%)). There was no difference in the distribution of the type of ChEI prescribed between the two groups (p = 0.446 for donepezil, p = 0.334 for rivastigmine, p = 0.092 for galantamine) (Supplementary Table S1).
Table 1.
Demographic and clinical characteristics of the cholinesterase inhibitor only group and the nootropics combination group.
| Variable | ChEI Only (n = 410) |
ChEI + Nootropics (n = 173) |
p Value |
|---|---|---|---|
| Age (years) | 75.5 ± 6.5 | 75.5 ± 6.6 | 0.880 |
| Weight (kg) 1 | 56.4 ± 9.8 | 56.7 ± 12.2 | 0.843 |
| Height (cm) 2 | 157.3 ± 10.1 | 156.1 ± 11.5 | 0.274 |
| BMI (kg/m2) 3 | 22.8 ± 3.8 | 23.3 ± 3.8 | 0.265 |
| Blood pressure (mmHg) 4 | |||
| Systolic | 130.3 ± 20.8 | 129.1 ± 22.1 | 0.576 |
| Diastolic | 75.6 ± 12.9 | 75.6 ± 12.9 | 0.962 |
| Sex | |||
| Male | 144 (35.1) | 56 (32.4) | 0.523 |
| Female | 266 (64.9) | 117 (67.6) | |
| Education (years) 5 | |||
| No education | 55 (13.4) | 20 (11.6) | 0.651 |
| Elementary school | 94 (22.9) | 43 (24.9) | |
| Middle/high school | 90 (21.9) | 31 (7.9) | |
| Bachelor’s/master’s degree | 64 (15.6) | 30 (17.3) | |
| Alcohol use 6 | |||
| Yes | 85 (20.7) | 23 (13.3) | 0.011 |
| No | 203 (49.5) | 107 (61.8) | |
| Tobacco use 7 | |||
| Yes | 23 (5.6) | 7 (4.0) | 0.245 |
| No | 224 (54.6) | 114 (65.9) | |
| Psychiatric medication | |||
| Antipsychotics | 73 (17.8) | 34 (19.7) | 0.598 |
| Benzodiazepines, hypnotics | 119 (29.0) | 45 (26.0) | 0.460 |
| Anticholinergics | 83 (20.2) | 43 (24.9) | 0.217 |
| Dementia diagnoses | |||
| Alzheimer’s dementia | 313 (76.3) | 134 (77.5) | 0.771 |
| Vascular dementia | 49 (12.0) | 31 (17.9) | 0.056 |
| Unspecified dementia | 17 (4.1) | 6 (3.5) | 0.701 |
| Frontotemporal dementia | 8 (2.0) | 0 (0) | 0.113 |
| Progressive supranuclear palsy | 16 (3.9) | 0 (0) | 0.004 |
| Alcohol induced persisting dementia | 1 (0.2) | 0 (0) | 1.000 |
| Other specified degenerative disease of nervous system | 6 (1.5) | 2 (1.2) | 1.000 |
| Comorbid diagnoses | |||
| Diabetes mellitus | 81 (19.8) | 50 (28.9) | 0.016 |
| Hypertension | 138 (33.7) | 70 (40.5) | 0.117 |
| Hyperlipidemia | 55 (13.4) | 29 (16.8) | 0.293 |
| Stroke 8 | 36 (8.8) | 36 (20.8) | <0.001 |
| Parkinsonism | 69 (16.8) | 12 (6.9) | 0.002 |
| Visual disturbance/hearing loss | 26 (6.3) | 20 (11.6) | 0.033 |
| Hemiplegia/paraplegia | 3 (0.7) | 6 (3.5) | 0.023 |
| Asthma/COPD | 18 (4.4) | 11 (6.4) | 0.318 |
| Renal insufficiency | 25 (6.1) | 14 (8.1) | 0.378 |
| Mood/anxiety disorder | 141 (34.4) | 75 (43.4) | 0.041 |
| Other psychiatric disorder 9 | 26 (6.3) | 12 (6.9) | 0.790 |
| Any malignancy | 26 (6.3) | 10 (5.8) | 0.797 |
Values are presented as mean ± standard deviation or number (%). Independent t-test was used for continuous variables, and χ2 test or Fisher’s exact test was used for categorical variables. ChEI: cholinesterase inhibitor; BMI: body mass index; COPD: chronic obstructive pulmonary disease. 1 Missing: ChEI only = 132, ChEI + nootropics = 51. 2 Missing: ChEI only = 136, ChEI + nootropics = 53. 3 Missing: ChEI only = 81, ChEI + nootropics = 33. 4 Missing: ChEI only = 102, ChEI + nootropics = 40. 5 Missing: ChEI only = 107, ChEI + nootropics = 49. 6 Missing: ChEI only = 122, ChEI + nootropics = 43. 7 Missing: ChEI only = 163, ChEI + nootropics = 52. 8 Cerebral hemorrhage and ischemic stroke. 9 Psychotic disorder and substance use disorder.
3.2. Change in Cognitive Function: ChEI Only vs. ChEI and Nootropics Combination Group
Results for the primary outcome measure are shown in Table 2. Two-sample t-tests at baseline and endpoint showed no difference between the two groups in terms of the MMSE total score and any of its six subscale scores. A linear mixed-effects model analysis with the MMSE total score as the dependent variable also revealed no significant treatment-by-time interaction (F = 1.48, df = 1581, p = 0.224). None of the six-subscale scores showed any significant treatment-by-time interaction, albeit the change in language subscale score showed a trend of less deterioration in the nootropics combination group than in the ChEI only group (−0.27 for the ChEI only group and −0.03 for the ChEI and nootropics combination group; F = 3.43, df = 1581, p = 0.065).
Table 2.
Results of significance testing and mixed model analyses for mini mental state examination (MMSE): Cholinesterase Inhibitor Only vs. Cholinesterase Inhibitor and Nootropics Combination 1.
| Baseline | Endpoint | Difference in Scores | Group-Time Interaction 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MMSE | ChEI Only | ChEI + Nootropics | p Value 3 | ChEI Only | ChEI + Nootropics | p Value 3 | ChEI Only | ChEI + Nootropics | p Value 3 | F | p Value 4 |
| Total | 20.00 ± 4.30 |
20.27 ± 4.26 |
0.487 | 18.87 ± 5.43 |
19.56 ± 5.13 |
0.152 | −1.13 ± 3.98 |
−0.71 ± 3.47 |
0.199 | 1.48 | 0.224 |
| Orientation | 6.81 ± 2.33 |
6.94 ± 2.23 |
0.533 | 6.28 ± 2.70 |
6.54 ± 2.57 |
0.276 | −0.53 ± 2.21 |
−0.40 ± 2.15 |
0.504 | 0.45 | 0.504 |
| Immediate recall | 2.83 ± 0.47 |
2.84 ± 0.47 |
0.925 | 2.74 ± 0.63 |
2.77 ± 0.59 |
0.526 | −0.10 ± 0.66 |
−0.06 ± 0.63 |
0.594 | 0.28 | 0.594 |
| Attention & calculation | 1.99 ± 1.58 |
2.01 ± 1.57 |
0.881 | 1.81 ± 1.58 |
1.95 ± 1.62 |
0.327 | −0.18 ± 1.53 |
−0.06 ± 1.29 |
0.364 | 0.83 | 0.364 |
| Delayed recall | 0.92 ± 1.00 |
1.10 ± 1.11 |
0.064 | 0.90 ± 1.06 |
0.93 ± 1.10 |
0.715 | −0.03 ± 1.02 |
−0.17 ± 1.16 |
0.150 | 2.29 | 0.131 |
| Language | 6.97 ± 1.22 |
6.87 ± 1.41 |
0.387 | 6.70 ± 1.46 |
6.84 ± 1.55 |
0.295 | −0.27 ± 1.45 |
−0.03 ± 1.43 |
0.065 | 3.43 | 0.065 |
| Visuospatial construction |
0.47 ± 0.50 |
0.50 ± 0.50 |
0.561 | 0.44 ± 0.50 |
0.51 ± 0.50 |
0.111 | −0.03 ± 0.56 |
0.01 ± 0.58 |
0.375 | 0.79 | 0.375 |
Values are presented as mean ± standard deviation. MMSE: mini-mental state examination; ChEI: cholinesterase inhibitor. 1 Cholinesterase inhibitor only [n = 410] and cholinesterase inhibitor and nootropics combination [n = 173]. 2 The results for the linear mixed-effects model were adjusted for covariates of sex, age, hypertension, diabetes mellitus, stroke, Parkinsonism, visual disturbance/hearing loss, hemiplegia/paraplegia, and mood/anxiety disorder. 3 p value for t-test. 4 p value for linear mixed-effects model.
3.3. Change in Cognitive Function for Each Type of Dementia: Alzheimer’s Dementia, Vascular Dementia, and Other Dementia
Comparison of the change in cognitive function for each of the three types of dementia is shown in Table 3. Linear mixed-effects model analyses using the MMSE total score as the dependent variable revealed no significant treatment-by-time interaction in all three types of dementia (F = 2.10, p = 0.148 for Alzheimer’s dementia, F = 0.38, p = 0.540 for vascular dementia, F = 0.04, p = 0.906 for other dementia). Within the subjects diagnosed with Alzheimer’s dementia, the ChEI and nootropics combination group showed a significantly less deterioration in the MMSE language subscale score compared to the ChEI only group, revealing a significant treatment-by-time interaction (−0.34 for the ChEI only group and 0.04 for the ChEI and nootropics combination group; F = 6.86, p = 0.009), while in the vascular dementia subjects, the ChEI and nootropics combination group showed a significantly less deterioration in the MMSE attention and calculation subscale score compared to the ChEI only group, with a significant treatment-by-time interaction (−0.59 for the ChEI only group and 0.06 for the ChEI and nootropics combination group; F = 4.44, p = 0.038). None of the six-subscale scores showed any significant treatment-by-time interaction in the other dementia patients.
Table 3.
Results of mixed model analyses 1 for mini-mental state examination (MMSE) among the three dementia subgroups.
| Alzheimer’s Dementia (n = 447) |
Vascular Dementia (n = 80) |
Others (n = 56) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difference in Scores | Group-Time Interaction | Difference in Scores | Group-Time Interaction | Difference in Scores | Group-Time Interaction | |||||||
| MMSE | ChEI Only (n = 313) |
ChEI + Nootropics (n = 134) | F | p Value | ChEI Only (n = 49) |
ChEI + Nootropics (n = 31) | F | p Value | ChEI Only (n = 48) |
ChEI + Nootropics (n = 8) | F | p Value |
| Total | −1.30 ± 3.87 |
−0.75 ± 3.27 |
2.10 | 0.148 | −0.88 ± 3.46 |
−0.35 ± 4.06 |
0.38 | 0.540 | −0.27 ± 5.04 |
−1.38 ± 4.66 |
0.04 | 0.906 |
| Orientation | −0.62 ± 2.16 |
−0.45 ± 1.91 |
0.66 | 0.417 | −0.33 ± 2.04 |
−0.16 ± 3.03 |
0.09 | 0.771 | −0.15 ± 2.67 |
−0.50 ± 1.93 |
0.14 | 0.707 |
| Immediate recall | −0.08 ± 0.65 |
−0.09 ± 0.62 |
0.01 | 0.922 | −0.12 ± 0.81 |
0.10 ± 0.70 |
1.55 | 0.217 | −0.15 ± 0.62 |
−0.25 ± 0.46 |
0.01 | 0.937 |
| Attention & calculation | −0.18 ± 1.50 |
−0.11 ± 1.28 |
0.21 | 0.651 | −0.59 ± 1.38 |
0.06 ± 1.32 |
4.44 | 0.038 | 0.25 ± 1.77 |
0.38 ± 1.41 |
1.13 | 0.293 |
| Delayed recall | −0.02 ± 1.05 |
−0.19 ± 1.15 |
2.46 | 0.117 | 0.00 ± 0.94 |
−0.13 ± 1.15 |
0.30 | 0.584 | −0.10 ± 0.99 |
0.00 ± 1.51 |
1.35 | 0.252 |
| Language | −0.34 ± 1.45 |
0.04 ± 1.39 |
6.86 | 0.009 | 0.14 ± 1.04 |
−0.10 ± 1.11 |
0.96 | 0.331 | −0.23 ± 1.72 |
−1.00 ± 2.67 |
1.90 | 0.175 |
| Visuospatial construction |
−0.05 ± 0.57 |
0.05 ± 0.58 |
3.28 | 0.071 | −0.04 ± 0.54 |
−0.16 ± 0.53 |
0.97 | 0.327 | 0.56 ± 0.08 |
0.76 ± 0.27 |
0.18 | 0.677 |
Values are presented as mean ± standard deviation. MMSE, mini-mental state examination; ChEI, cholinesterase inhibitor. 1 The results for the linear mixed-effects model were adjusted for covariates of sex, age, hypertension, diabetes mellitus, stroke, Parkinsonism, visual disturbance/hearing loss, hemiplegia/paraplegia, and mood/anxiety disorder.
For highly-adherent Alzheimer’s dementia patients, the MMSE language subscale score consistently revealed a significant treatment-by-time interaction, showing favorable results for the ChEI and nootropics combination group (−0.38 for the ChEI only group and 0.12 for the ChEI and nootropics combination group; F = 10.16, p = 0.002) (Table 4). Apart from the language subscale score, a linear mixed-effects model with the MMSE visuospatial construction subscale score as the dependent variable also showed a significant treatment-by-time interaction (−0.06 for the ChEI only group and 0.06 for the ChEI and nootropics combination group; F = 4.00, p = 0.046) (Table 4).
Table 4.
Results of mixed model analyses 1 for mini-mental state examination (MMSE) for highly-adherent 2 Alzheimer’s Dementia Patients.
| Alzheimer’s Dementia (n = 412) |
Vascular Dementia (n = 72) |
Others (n = 53) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difference in Scores | Group-Time Interaction | Difference in Scores | Group-Time Interaction | Difference in Scores | Group-Time Interaction | |||||||
| MMSE | ChEI Only (n = 300) |
ChEI + Nootropics (n = 112) | F | p Value | ChEI Only (n = 45) |
ChEI + Nootropics (n = 27) | F | p Value | ChEI Only (n = 45) |
ChEI + Nootropics (n = 8) | F | p Value |
| Total | −1.36 ± 3.87 |
−0.77 ± 2.85 |
2.18 | 0.141 | −0.78 ± 3.51 |
−0.59 ± 4.17 |
0.04 | 0.841 | −0.36 ± 5.19 |
−1.38 ± 4.66 |
0.27 | 0.606 |
| Orientation | −0.64 ± 2.16 |
−0.54 ± 1.82 |
0.22 | 0.640 | −0.38 ± 1.84 |
−0.22 ± 3.23 |
0.07 | 0.795 | −0.18 ± 2.76 |
−0.50 ± 1.93 |
0.10 | 0.753 |
| Immediate recall | −0.08 ± 0.66 |
−0.07 ± 0.57 |
0.03 | 0.865 | −0.13 ± 0.84 |
0.11 ± 0.75 |
1.54 | 0.219 | −0.16 ± 0.64 |
−0.25 ± 0.46 |
0.16 | 0.692 |
| Attention & calculation | −0.18 ± 1.49 |
−0.12 ± 1.32 |
0.16 | 0.689 | −0.58 ± 1.42 |
0.04 ± 1.22 |
0.04 | 0.841 | 0.13 ± 1.75 |
0.38 ± 1.41 |
0.14 | 0.714 |
| Delayed recall | −0.01 ± 1.03 |
−0.22 ± 1.11 |
3.35 | 0.068 | 0.07 ± 0.92 |
−0.19 ± 1.15 |
1.06 | 0.307 | −0.07 ± 0.99 |
0.00 ± 1.51 |
0.03 | 0.872 |
| Language | −0.38 ± 1.46 |
0.12 ± 1.26 |
10.16 | 0.002 | 0.20 ± 1.04 |
−0.14 ± 1.11 |
2.21 | 0.142 | −0.22 ± 1.74 |
−1.00 ± 2.67 |
1.14 | 0.291 |
| Visuospatial construction |
−0.06 ± 0.56 |
0.06 ± 0.59 |
4.00 | 0.046 | −0.02 ± 0.54 |
−0.15 ± 0.53 |
0.92 | 0.341 | 0.13 ± 0.55 |
0.00 ± 0.76 |
0.36 | 0.552 |
Values are presented as mean ± standard deviation. MMSE, mini-mental state examination; ChEI, cholinesterase inhibitor. 1 The results for the linear mixed-effects model were adjusted for covariates of sex, age, hypertension, diabetes mellitus, stroke, Parkinsonism, visual disturbance/hearing loss, hemiplegia/paraplegia, and mood/anxiety disorder. 2 Highly-adherent subjects are defined as proportion of days covered ≥ 0.7; Proportion of days covered = (Number of days in the period “covered” by prescription) × 100/(Number of days in period).
3.4. Subgroup Analysis within the ChEI and Nootropics Combination Group in Alzheimer’s Dementia: Choline Alfoscerate vs. Ginkgo Biloba Extract vs. Other Nootropics
When the three types of nootropics within the ChEI and nootropics combination group of the Alzheimer’s dementia subjects were compared, choline alfoscerate and ginkgo biloba extract combination with ChEI showed significantly less declines in the MMSE total scores (p = 0.006) compared to other nootropics combination (−0.28 for the choline alfoscerate group, −0.51 for the ginkgo biloba extract group, −2.81 for the other nootropics group) (Supplementary Table S2). Highly significant between-group differences, in favor of the choline alfoscerate combination and the ginkgo biloba extract combination, were shown in the MMSE language subscale score (p = 0.001) as well (0.22 for the choline alfoscerate group, 0.26 for the ginkgo biloba group, −0.95 for the other nootropics group) (Supplementary Table S2).
4. Discussion
Our study findings suggest that the effectiveness of nootropics combination with ChEI may be different according to the type of dementia. The difference in change in the MMSE total scores from baseline to endpoint between the ChEI only group and the ChEI and nootropics combination group was not evident, but the results implied a positive impact of ChEI augmentation with nootropics on some domains of cognitive function in certain types of dementia, particularly Alzheimer’s dementia. The impact of nootropics augmentation on ChEI in Alzheimer’s dementia was consistently depicted on the language domain after consideration of medical adherence and nootropics type.
Previous studies on nootropics revealed nootropics to have minimal, if any, effects on the prevention of cognitive decline. Most studies were either examined over a relatively short follow-up time or conducted with a limited number of subjects, and many of them focused on nootropic monotherapy rather than concomitant use with ChEI [6,9,10,17,23,24,25]. This study aimed to compare the nootropics and ChEI combination with ChEI monotherapy in an attempt to validate the effectiveness of nootropics in association with ChEI. We examined the change in not only MMSE total scores but also in its six subscale scores over a long period of exposure (300–400 days after the first prescription), which was considered long enough to elucidate the effect of the ChEI and nootropics combination. We categorized the subjects by dementia types in order to clarify the impact of nootropics combination in each type of dementia separately, and mainly focused on Alzheimer’s dementia, which is the most common neurodegenerative form of dementia. We did not limit nootropics to only one type of drug, but included multiple types of nootropics that are most frequently prescribed by clinicians in Korea, to speculate the effectiveness of different types of nootropics, particularly focusing on the two drugs that were most commonly used: choline alfoscerate and ginkgo biloba extract.
Choline alfoscerate is a derivative of phosphatidylcholine that enhances cholinergic transmission by upregulating acetylcholine (ACh) synthesis or release in the hippocampus [26,27]; thus, it ultimately facilitates learning and memory and decreases age-dependent structural changes, as seen in the frontal cortices and hippocampi of rats [7,28]. Previous studies reported that the combination of choline alfoscerate with ChEI significantly increased ACh concentrations and prevented volume loss in the frontal and temporal lobe, hippocampus, and striatum in both rats [20] and in Alzheimer’s dementia patients [21]. One study also showed that choline alfoscerate increased hippocampal neurogenesis and provided protection against seizure-induced neuronal death and cognitive impairment [29]. A study on the supplementary effect of choline alfoscerate on speech detection and recognition among hearing aid users revealed that in the aging brain, nicotinic acetylcholine receptor activation in the medial geniculate body decreases, which contributes to deterioration in speech recognition and comprehension and, in such cases, choline precursor supplements could improve language functioning [30]. Ginkgo biloba extract, on the other hand, is hypothesized to enhance amyloid β-induced hippocampal neuron dysfunction and death, amyloid β aggregation, and neurogenesis [31,32,33], and induce a significant decrease in the density of β-adrenoreceptors in the frontal cortex and hippocampus [34]. Studies have shown that the hippocampus is closely associated with language production and verbal communication, either by contributing semantic memory to spoken language, processing the mismatch between the expected sensory consequences of speaking and perceived speech feedback, or via coupling between the hippocampal/supplementary motor area and the auditory cortex, anterior cingulate cortex, and cerebellum [30,35]. These findings all suggest that nootropics, such as choline alfoscerate and ginkgo biloba extract, may enhance the cholinergic pathway in brain areas including the hippocampus, frontal lobes, and auditory cortex, which are critical in language processing and utilization, and enhance language function in association with ChEI administration. Language function is not only highly sensitive in detecting cognitive impairment in older age groups [36], but is also a biological marker that differentiates Alzheimer’s dementia from normal aging [37]. To our knowledge, this study is the first to elucidate the effect of nootropics co-administered with ChEIs on the attenuation of language domain deterioration in Alzheimer’s dementia using clinical data. The possible effect of nootropics on delaying the deterioration of language function may be of great value, considering the association between cognitive decline and language function in the aging population and dementia patients.
The strength of this study lies in the methodological aspects of real-world data utilization. Many studies in Korea are actively conducted using the national claims database in an attempt to perform large-scale research [38]. However, imperative baseline characteristics of each patient, including medical histories, medication, and laboratory findings, are missing when using the national claims databases. On the other hand, the clinical data warehouse from our university healthcare system is a composition of each patient’s clinical and demographic data. In this study, we were able to extract and collect critical information regarding each patient’s clinical and demographic backgrounds such as, but not limited to, the education level and BMI, specific MMSE subscale scores, and detailed dementia diagnosis. As a result, we were able to make corrections for many variables that could have affected the MMSE results, including other relevant diagnoses and medical history, such as hypertension and diabetes mellitus, Parkinsonism, and visual/hearing loss. Furthermore, this study is meaningful in that until now, there have only been a few, if any, studies on the effect of nootropics on ChEI efficacy outside of the only actively researched country, Italy [19,20,39].
The study had some limitations. Due to its retrospective nature, we were not able to control the treatment settings in terms of medication dosage, treatment duration, the overlapping periods of concomitant nootropic and ChEI use, and adherence monitoring. In order to compensate for these factors, we calculated and considered PDC, the number of days covered by the prescriptions in the total prescription periods, to reflect and consider drug adherence. Moreover, we only included subjects with MMSE scores within 300–400 days after the baseline, which might have excluded certain patients who could not take the annual MMSE test either due to a deleterious progression of dementia or because they no longer sought help or changed hospitals. In the near future, it seems that these limitations can be solved by conducting research based on a large database that combines real-world data from numerous hospitals (i.e., common data model). Finally, analyzing the six subscale domains of the MMSE may raise the issue of multiple comparison. However, through subsequent analyses, we induced consistent results at least in the Alzheimer’s dementia group, of which nootropics combination could have a subtle positive impact on the language domain. Surely, further studies that more deliberately focus on the separate cognitive domains should be performed with other more qualified neuropsychological tests. Still, this study would be of best effort to observe the effectiveness of nootropics utilizing the accumulated real-world data.
5. Conclusions
In conclusion, our results demonstrated partial effectiveness of nootropics with ChEIs on some cognitive domains in Alzheimer’s dementia. The reliability of the current study was further strengthened by its 1-year follow-up period, consideration of treatment adherence, and adjustments for confounding comorbid diagnoses. This study emphasizes the need for future well-controlled studies to generalize the effect of nootropics on cognitive function across various clinical settings or cause of dementia.
Acknowledgments
This study was supported by a research grant of Yongin Severance Hospital, Yonsei University College of Medicine.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11164661/s1, Table S1: Comparison of the distribution of the three types of cholinesterase inhibitor in the cholinesterase inhibitor only group and the cholinesterase inhibitor and nootropics combination group; Table S2: Results of ANOVA for mini-mental state examination (MMSE) total score and its six-subscale scores among the three cholinesterase and nootropics combination subgroups in Alzheimer’s dementia.
Author Contributions
Conceptualization, W.J.K.; methodology, W.J.K., E.L. and M.K.; validation, W.J.K. and E.L.; investigation, W.J.K.; resources, W.J.K., D.B.L. and M.K.; software, S.K.; data curation, M.K. and D.B.L.; formal analysis, M.K. and S.K.; writing—original draft preparation, M.K.; writing—review and editing, M.K., E.L. and W.J.K.; visualization, M.K. and W.J.K.; supervision, W.J.K. and E.L. All authors contributed to the development and revision of the final manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Yongin Severance Hospital (9-2020-0108).
Informed Consent Statement
Patient consent was waived by the Institutional Review Board of Yongin Severance Hospital (9-2020-0108) due to use of anonymous samples.
Data Availability Statement
Data are not available according to the policy of Yonsei University College of Medicine.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization Dementia. [(accessed on 18 September 2021)];2021 Available online: https://www.who.int/news-room/fact-sheets/detail/dementia.
- 2.O’Brien J.T., Holmes C., Jones M., Jones R., Livingston G., McKeith I., Mittler P., Passmore P., Ritchie C., Robinson L., et al. Clinical practice with anti-dementia drugs: A revised (third) consensus statement from the British association for psychopharmacology. J. Psychopharmacol. 2017;31:147–168. doi: 10.1177/0269881116680924. [DOI] [PubMed] [Google Scholar]
- 3.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006;1:CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholson C.D. Pharmacology of nootropics and metabolically active compounds in relation to their use in dementia. Psychopharmacology. 1990;101:147–159. doi: 10.1007/BF02244119. [DOI] [PubMed] [Google Scholar]
- 5.Moreno M.D. Cognitive improvement in mild to moderate Alzheimer’s dementia after treatment with the acetylcholine precursor choline alfoscerate: A multicenter, double-blind, randomized, placebo-controlled trial. Clin. Ther. 2003;25:178–193. doi: 10.1016/S0149-2918(03)90023-3. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery S.A., Thal L.J., Amrein R. Meta-analysis of double blind randomized controlled clinical trials of acetyl-L-carnitine versus placebo in the treatment of mild cognitive impairment and mild Alzheimer’s disease. Int. Clin. Psychopharmacol. 2003;18:61–71. doi: 10.1097/00004850-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Parnetti L., Amenta F., Gallai V. Choline alphoscerate in cognitive decline and in acute cerebrovascular disease: An analysis of published clinical data. Mech. Ageing Dev. 2001;122:2041–2055. doi: 10.1016/S0047-6374(01)00312-8. [DOI] [PubMed] [Google Scholar]
- 8.Parnetti L., Abate G., Bartorelli L., Cucinotta D., Cuzzupoli M., Maggioni M., Villardita C., Senin U. Multicentre study of l-alpha-glyceryl-phosphorylcholine vs. ST200 among patients with probable senile dementia of Alzheimer’s type. Drugs Aging. 1993;3:159–164. doi: 10.2165/00002512-199303020-00006. [DOI] [PubMed] [Google Scholar]
- 9.Parnetti L., Mignini F., Tomassoni D., Traini E., Amenta F. Cholinergic precursors in the treatment of cognitive impairment of vascular origin: Ineffective approaches or need for re-evaluation? J. Neurol. Sci. 2007;257:264–269. doi: 10.1016/j.jns.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 10.DeKosky S.T., Williamson J.D., Fitzpatrick A.L., Kronmal R.A., Ives D.G., Saxton J.A., Lopez O.L., Burke G., Carlson M.C., Fried L.P., et al. Ginkgo biloba for prevention of dementia: A randomized controlled trial. JAMA. 2008;300:2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gschwind Y.J., Bridenbaugh S.A., Reinhard S., Granacher U., Monsch A.U., Kressig R.W. Ginkgo biloba special extract LI 1370 improves dual-task walking in patients with MCI: A randomised, double-blind, placebo-controlled exploratory study. Aging Clin. Exp. Res. 2017;29:609–619. doi: 10.1007/s40520-016-0699-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spagnoli A., Lucca U., Menasce G., Bandera L., Cizza G., Forloni G. Long-term acetyl-L-carnitine treatment in Alzheimer’s disease. Neurology. 1991;41:1726–1732. doi: 10.1212/WNL.41.11.1726. [DOI] [PubMed] [Google Scholar]
- 13.Zang G., Fang L., Chen L., Wang C. Ameliorative effect of nicergoline on cognitive function through the PI3K/AKT signaling pathway in mouse models of Alzheimer’s disease. Mol. Med. Rep. 2018;17:7293–7300. doi: 10.3892/mmr.2018.8786. [DOI] [PubMed] [Google Scholar]
- 14.Nappi G., Bono G., Merlo P., Borromei A., Caltagirone C., Lomeo C., Martucci N., Fabbrini G., Annoni K., Battaglia A. Long-Term nicergoline treatment of mild to moderate senile dementia. Clin. Drug Investig. 1997;13:308–316. doi: 10.2165/00044011-199713060-00003. [DOI] [PubMed] [Google Scholar]
- 15.Vellas B., Coley N., Ousset P.-J., Berrut G., Dartigues J.-F., Dubois B., Grandjean H., Pasquier F., Piette F., Robert P., et al. Long-term use of standardised ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): A randomised placebo-controlled trial. Lancet Neurol. 2012;11:851–859. doi: 10.1016/S1474-4422(12)70206-5. [DOI] [PubMed] [Google Scholar]
- 16.Snitz B.E., O’Meara E.S., Carlson M.C., Arnold A.M., Ives D.G., Rapp S.R., Saxton J., Lopez O.L., Dunn L.O., Sink K.M., et al. Ginkgo biloba for preventing cognitive decline in older adults: A randomized trial. JAMA. 2009;302:2663–2670. doi: 10.1001/jama.2009.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson S., Tabet N. Acetyl-L-carnitine for dementia. Cochrane Database Syst. Rev. 2003;2003:CD003158. doi: 10.1002/14651858.CD003158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fioravanti M., Flicker L. Efficacy of nicergoline in dementia and other age associated forms of cognitive impairment. Cochrane Database of Syst. Rev. 2001;4:CD003159. doi: 10.1002/14651858.CD003159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amenta F., Carotenuto A., Fasanaro A.M., Rea R., Traini E. The ASCOMALVA trial: Association between the cholinesterase inhibitor donepezil and the cholinergic precursor choline alphoscerate in Alzheimer’s disease with cerebrovascular injury: Interim results. J. Neurol. Sci. 2012;322:96–101. doi: 10.1016/j.jns.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Amenta F., Tayebati S.K., Vitali D., di Tullio M.A. Association with the cholinergic precursor choline alphoscerate and the cholinesterase inhibitor rivastigmine: An approach for enhancing cholinergic neurotransmission. Mech. Ageing Dev. 2006;127:173–179. doi: 10.1016/j.mad.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Traini E., Carotenuto A., Fasanaro A.M., Amenta F. Volume analysis of brain cognitive areas in Alzheimer’s disease: Interim 3-year results from the ASCOMALVA trial. J. Alzheimers Dis. 2020;76:317–329. doi: 10.3233/JAD-190623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dao N., Lee S., Hata M., Sarino L. Impact of appointment-based medication synchronization on proportion of days covered for chronic medications. Pharmacy. 2018;6:44. doi: 10.3390/pharmacy6020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins J.P., Flicker L. Lecithin for dementia and cognitive impairment. Cochrane Database Syst. Rev. 2003;3:CD001015. doi: 10.1002/14651858.CD001015. [DOI] [PubMed] [Google Scholar]
- 24.Birks J., Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst. Rev. 2009;1:CD003120. doi: 10.1002/14651858.CD003120.pub3. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Y., Xie Y., Qi M., Zhang L., Wang W., Zhang W., Sha L., Wu J., Li W., Wu T. Ginkgo biloba extract is comparable with donepezil in improving functional recovery in Alzheimer’s disease: Results from a multilevel characterized study based on clinical features and resting-state functional magnetic resonance imaging. Front. Pharmacol. 2021;12:721216. doi: 10.3389/fphar.2021.721216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catanesi M., d’Angelo M., Antonosante A., Castelli V., Alfonsetti M., Benedetti E., Desideri G., Ferri C., Cimini A. Neuroprotective potential of choline alfoscerate against β-amyloid injury: Involvement of neurotrophic signals. Cell Biol. Int. 2020;44:1734–1744. doi: 10.1002/cbin.11369. [DOI] [PubMed] [Google Scholar]
- 27.Sigala S., Imperato A., Rizzonelli P., Casolini P., Missale C., Spano P. L-alpha-glycerylphosphorylcholine antagonizes scopolamine-induced amnesia and enhances hippocampal cholinergic transmission in the rat. Eur. J. Pharmacol. 1992;211:351–358. doi: 10.1016/0014-2999(92)90392-H. [DOI] [PubMed] [Google Scholar]
- 28.Tayebati S.K., di Tullio M.A., Tomassoni D., Amenta F. Neuroprotective effect of treatment with galantamine and choline alphoscerate on brain microanatomy in spontaneously hypertensive rats. J. Neurol. Sci. 2009;283:187–194. doi: 10.1016/j.jns.2009.02.349. [DOI] [PubMed] [Google Scholar]
- 29.Lee S.H., Choi B.Y., Kim J.H., Kho A.R., Sohn M., Song H.K., Choi H.C., Suh S.W. Late treatment with choline alfoscerate (l-alpha glycerylphosphorylcholine, α-GPC) increases hippocampal neurogenesis and provides protection against seizure-induced neuronal death and cognitive impairment. Brain Res. 2017;1654:66–76. doi: 10.1016/j.brainres.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Na G., Kwak S.H., Jang S.H., Noh H.E., Kim J., Yang S., Jung J. Supplementary effect of choline alfoscerate on speech recognition in patients with age-related hearing loss: A prospective study in 34 patients (57 ears) Front. Aging Neurosci. 2021;13:684519. doi: 10.3389/fnagi.2021.684519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bastianetto S., Ramassamy C., Doré S., Christen Y., Poirier J., Quirion R. The ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by beta-amyloid. Eur. J. Neurosci. 2000;12:1882–1890. doi: 10.1046/j.1460-9568.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- 32.Luo Y., Smith J.V., Paramasivam V., Burdick A., Curry K.J., Buford J.P., Khan I., Netzer W.J., Xu H., Butko P. Inhibition of amyloid-β aggregation and caspase-3 activation by the ginkgo biloba extract EGb761. Proc. Natl. Acad. Sci. USA. 2002;99:12197–12202. doi: 10.1073/pnas.182425199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tchantchou F., Xu Y., Wu Y., Christen Y., Luo Y. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J. 2007;21:2400–2408. doi: 10.1096/fj.06-7649com. [DOI] [PubMed] [Google Scholar]
- 34.Hadjiivanova C.I., Petkov V.V. Effect of Ginkgo biloba extract on β-adrenergic receptors in different rat brain regions. Phytother. Res. 2002;16:488–490. doi: 10.1002/ptr.933. [DOI] [PubMed] [Google Scholar]
- 35.Van de Ven V., Waldorp L., Christoffels I. Hippocampus plays a role in speech feedback processing. Neuroimage. 2020;223:117319. doi: 10.1016/j.neuroimage.2020.117319. [DOI] [PubMed] [Google Scholar]
- 36.Alegret M., Peretó M., Pérez A., Valero S., Espinosa A., Ortega G., Hernández I., Mauleón A., Rosende-Roca M., Vargas L., et al. The role of verb fluency in the detection of early cognitive impairment in Alzheimer’s disease. J. Alzheimers Dis. 2018;62:611–619. doi: 10.3233/JAD-170826. [DOI] [PubMed] [Google Scholar]
- 37.Salmon D.P., Bondi M.W. Neuropsychological assessment of dementia. Annu. Rev. Psychol. 2009;60:257–282. doi: 10.1146/annurev.psych.57.102904.190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryu D.-R. Introduction to the medical research using national health insurance claims database. Ewha Med. J. 2017;40:66–70. doi: 10.12771/emj.2017.40.2.66. [DOI] [Google Scholar]
- 39.Colucci L., Bosco M., Rosario Ziello A., Rea R., Amenta F., Fasanaro A.M. Effectiveness of nootropic drugs with cholinergic activity in treatment of cognitive deficit: A review. J. Exp. Pharmacol. 2012;4:163–172. doi: 10.2147/JEP.S35326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not available according to the policy of Yonsei University College of Medicine.