Abstract
Aim: We investigated whether a difference exists between TSHR, PTEN and RASSF1A methylation status in plasma of subjects with papillary thyroid cancer (PTC). Methods: Peripheral blood samples were collected from 68 patients with PTC and 86 healthy controls (HC). Thyroid cancer tissue and corresponding adjacent normal tissue methylation levels were analyzed. DNA methylation level changes in TSHR, PTEN and RASSF1A genes were analyzed by quantitative methylation-sensitive polymerase chain reaction. Results: We observed that the methylation level of TSHR was significantly higher in the thyroid cancer tissue compared to adjacent normal tissue (p = 0.040). TSHR methylation levels in the PTC group plasma samples were significantly higher compared to HC (p = 0.022). After surgery, PTC plasma samples showed lower TSHR and PTEN methylation levels compared to the levels before surgery (p = 0.003, p = 0.031, respectively). The TSHR methylation level was significantly higher in PTC with larger tumor size (>2 cm) (p < 0.001), and lymph node metastases (p = 0.01), lymphovascular invasion (p = 0.02) and multifocality (p = 0.013) 0ROC analysis revealed that the TSHR methylation level provides high accuracy in distinguishing PTC from HC (p = 0.022, AUC of 0.616). Conclusion: TSHR methylation in peripheral blood samples is expected to be a sensitive and specific minimally invasive tool for the diagnosis of PTC, especially in combination with other diagnostic means.
Keywords: papillary thyroid carcinoma, PTC, methylation, TSHR, PTEN, RASSF1A
1. Introduction
Thyroid cancer is the most frequent endocrine neoplasm. Based on global cancer statistics in 2020, 586,202 new cases of thyroid cancer worldwide were diagnosed. Papillary thyroid carcinoma (PTC) is the most common thyroid cancer type, accounting for approximately 80–85% of all cases and predominantly occurring in middle-aged adults (45–54 years old) [1]. It carries the best overall prognosis. The 10-year survival rate in PTC patients after indicated treatment is approximately 90% [2,3]. However, approximately 10% of cases may present with metastatic disease at diagnosis/initial presentation, and local or regional PTC recurrences occur in up to 30% of patients [4,5].
Fine needle aspiration biopsy (FNAB) cytology by ultrasonography is still the most widely used method for diagnosing PTC, but it is improbable in up to 15–30% of cases [6,7]. Over the past few decades, a minimally invasive diagnostic test that can accurately diagnose the onset and prognosis of PTC has been the subject of research. DNA methylation status changes have been shown to be related to gene expression alterations. The methylation of the gene promoter is often found in cancer-specific genes and can therefore be used as a cancer marker [8].
Thyroid-stimulating hormone receptor (TSHR) is a specific protein on the surface of the thyroid cell membranes, encoded on chromosome 14q31.1 by a gene containing 12 exons. The receptors play an important role in the control of thyroid function and in the pathogenesis of thyroid diseases [9]. Epigenetic research has proven TSHR’s role in thyroid carcinogenesis. TSHR gene promoter is relatively rich in CpG dinucleotides, and it is believed that the changes in DNA methylation determine TSHR gene silencing in thyroid tumors [8]. Activation of the signaling cascades through TSHR is the pathway for carcinogenesis and a tumor growth promoter for thyroid cancer. TSH stimulates cytokine production in tumor cells, which could affect the tumor microenvironment [10]. In addition, TSH binds to extrathyroidal cells, including fibroblasts or endothelial cells, and may have the potential to directly modulate the tumor microenvironment. The methylation of the gene promoter is often found in tumor-specific genes and can therefore be used as a cancer marker [11,12].
Phosphatase and tensin homolog gene (PTEN) is a tumor suppressor gene located in the 10q23.31 chromosomal region. PTEN methylation is generally reported to be closely related to genetic alterations of the PI3K–AKT pathway in thyroid tumors. Changes in the PI3K–AKT signaling pathway may affect normal thyroid cell growth and proliferation, activate tumorigenesis and indicate thyroid cancer progression [13]. Aberrant promoter silencing of PTEN was detected in PTC, follicular thyroid carcinoma and anaplastic thyroid carcinoma cases. Therefore, previous studies have shown that PTEN methylation frequency is higher than mutations and loss of heterozygosity in thyroid neoplasms [14].
Ras association domain family 1A (RASSF1A) is located on chromosome 3p21.3 and plays an important role in the Ras/PI3K/AKT, Ras/RAF/MEK/ERK and Hippo signaling pathways. RASSF1A can be inactivated by hypermethylation of its promoter in 20–32% of PTC [15,16]. RASSF1A is one of the most common epigenetically inactivated tumor suppressor genes in human cancers. Gene inactivation is caused by altered promoter methylation that can lead to loss of expression of RASSF1A. These changes can increase the risk of lung cancer, breast cancer, prostate cancer, ovarian cancer, colorectal cancer, hepatocellular carcinoma, and gastric cancer. Moreover, RASSF1A promoter methylation may be a significant prognostic factor for many human cancers, but the relationship between RASSF1A promoter methylation changes, and disease pathogenesis is still controversial [17].
The aim of the present study was to determine whether a difference exists between selected genes’ (TSHR, PTEN, and RASSF1A) methylation status in plasma of subjects with PTC before and after surgery. Moreover, we correlated the methylation levels of selected genes with clinicopathological features and analyzed possible diagnostic value for PTC.
2. Materials and Methods
2.1. Study Group
Patients with PTC and healthy controls (HC) were involved in our study. Tissue samples were obtained from patients diagnosed with PTC and treated at the Hospital of Lithuanian University of Health Sciences Kaunas Clinics between 2020 and 2022. Plasma samples were obtained from patients with PTC, before and 4–6 weeks after surgery. Surgically resected thyroid tumor tissue and adjacent normal tissue were collected during the surgery. All surgically removed thyroid tissue samples underwent histological examination. The histopathological diagnosis of PTC was confirmed after surgery. Classification of patients with PTC was performed according to the 8th edition of the AJCC/UICC staging system [18]. Histopathological PTC was divided into aggressive (diffuse sclerosing variant and tall cell carcinoma) and non-aggressive (classical and follicular variant) subtypes. The PTC group had no benign nodes of the thyroid and no previous history of any other cancer.
The HC group had no thyroid disease, autoimmune illness, or previous history of any cancer. Thyroid ultrasound, hormone (TSH and fT4) assessments, and anti-thyroid peroxidase (anti-TPO) antibody assessments were performed on all prospective subjects before inclusion in the study.
Plasma thyroglobulin (Tg) was performed on all PTC patients 12 weeks after surgery, before radioiodine therapy. A Tg value of <0.1 ng/mL was considered suppressed.
The study was approved by the Kaunas Regional Committee of Biomedical Research (Lithuania, approval No. BE-2-64; 7 February 2020). Written informed consent was obtained from each participant in the study after a full explanation of the purpose and nature of all procedures used. This study was conducted in accordance with the Declaration of Helsinki.
2.2. DNA Samples
Venous blood was drawn from PTC and HC patients. All peripheral venous blood samples (10 mL) were collected in EDTA (BD Vacutainer PPT™ Plasma Preparation Tube; 13 × 100 mm/5 mL) tubes and separated by the ugation 1900× g for 10 min at 4 °C. Supernatant was then transferred to a new 15 mL conical tube and centrifuged at 16,000× g for 10 min at 4 °C. Purified plasma was transferred to 1.5 mL aliquots and stored at −80 °C until nucleic acid purification.
Thyroid cancer tissue and corresponding adjacent normal tissue were snap-frozen and stored at −80 °C in liquid nitrogen before DNA extraction. Corresponding adjacent normal tissue was removed at least 5 mm away from the primary tumor. Afterward, an experienced pathologist reviewed tissue samples of thyroid cancer tissues consisting of at least 80% cancer cells. Cancer cells were absent in adjacent normal tissue.
2.3. DNA Extraction
Plasma cfDNA was extracted from 5 mL blood plasma using QIAamp Circulating Nucleic Acid Kit (Qiagen, Hildigen, Germany), according to the manufacturer’s protocol. Eluted cfDNA was transferred into 0.2 mL Eppendorf tubes and stored at −80 °C.
Genomic DNA was extracted from 25 to 40 mg of frozen tissues using the All Prep DNA/RNA Kit (Qiagen, Hildigen, Germany), according to the manufacturer’s recommendation. DNA concentration was measured using NanoDrop1000 (Thermo Scientific, Waltham, MA, USA).
2.4. Bisulfite Conversion
For quantitative methylation-specific PCR (QMSP) analysis, up to 400 ng of purified DNA was modified using the EZ DNA Methylation™ Kit (Zymo Research, Irvine, CA, USA), according to the manufacturer’s protocol; the samples were incubated at 42 °C for 15 min.
2.5. Quantitative Methylation-Specific PCR
Target-specific QMSP primers and hydrolysis probes were selected from the previous studies (Supplementary Table S1) [19,20] and ordered from Metabion (Martinsried, Germany). In each assay, ACTB was included and was used for normalization [21]. The QMSP was performed in duplicates (analysis of tissue samples) or triplicates (analysis of blood plasma samples) for each set of primers in separate wells. The 20 μl reaction mix consisted of 1× TaqMan® Universal Master Mix II, no UNG (Applied Biosystems™, Waltham, MA, USA), 300 nM of each primer, 50 nM of the probe, and 10 ng bisulfite-converted DNA. All assays were performed under the following conditions: 95 °C for 10 min followed by 45–50 cycles of 95 °C for 15 s and 60 °C for 1 min, using the QuantStudio 5 Real-Time PCR System (Applied Biosystems™, Waltham, MA, USA). A run was considered valid when methylated controls provided a positive signal, and the NTC gave no amplification product. The methylation level of a particular target was estimated based on the ΔΔCq algorithm and expressed as a percentage of the methylation-positive control.
2.6. Statistical Analysis
Analysis of the Mann–Whitney U test and Kruskal–Wallis H test criteria for abnormal distribution was used to determine the differences in quantitative traits between the comparison groups. The association between qualitative values in comparative groups was assessed by the chi-square (χ2) test. The relationships between the DNA methylation level in the plasma and quantitative parameters were determined by Pearson’s correlation. The predictive capability (diagnostic performance) of each biomarker was investigated by means of the area under the ROC (receiver operating characteristic) curve (AUC). Statistical analyses were performed using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). The results were considered statistically significant at p < 0.05.
3. Results
3.1. Study Population
Demographic and clinicopathological characteristics of the study population with PTC and HC are shown in Table 1.
Table 1.
Demographic and clinicopathological characteristics of the study population with PTC and HC.
| Characteristic | PTC n = 68 |
HC n = 86 |
p-Value |
|---|---|---|---|
| Gender | |||
| Male | 8 (11.8%) | 11 (12.79%) | |
| Female | 60 (88.2%) | 75 (87.2%) | |
| Age at initial surgery (years) | 48.19 (14.9) | 45.30 (12.07) | p = 0.221 |
| T (TNM), n (%) | - | - | |
| pT1a | 27 (39.7) | ||
| pT1b | 7 (10.3) | ||
| pT2 | 4 (5.9) | ||
| pT3a | 19 (27.9) | ||
| pT3b | 11 (16.2) | ||
| Tumor size (cm) | - | - | |
| ≤2 | 48 (70.6) | ||
| >2 | 20 (29.4) | ||
| Lymph node metastases at initial surgery | - | - | |
| Yes | 16 (23.5) | ||
| No | 52 (76.5) | ||
| Variant of PTC, n (%) | - | - | |
| The classical variant | 29 (42.6) | ||
| The follicular variant | 18 (26.5) | ||
| The diffuse sclerosing variant | 17 (25.0) | ||
| The tall cell carcinoma | 4 (5.9) | ||
| Extrathyroidal extension | - | - | |
| Yes | 30 (44.1) | ||
| No | 38 (55.9) | ||
| Lymphovascular invasion | - | - | |
| Yes | 36 (52.9) | ||
| No | 32 (47.1) | ||
| Multifocality | - | - | |
| Yes | 16 (23.5) | ||
| No | 52 (76.5) |
A total of 154 patients were included in the study: 68 patients with a histologically confirmed diagnosis of PTC after surgical treatment and 86 healthy controls. Cases and controls were matched for gender and age. The majority of patients were diagnosed with pT1a (39.7%), tumor size ≤ 2 cm (70.6%), the classical variant (42.6%), and with lymphovascular invasion (52.9%) PTC. Table 1. Characteristics of the population with papillary thyroid cancer (PTC) and healthy controls (HC).
3.2. DNA Methylation in Thyroid Cancer Tissue and Adjacent Normal Tissue Groups
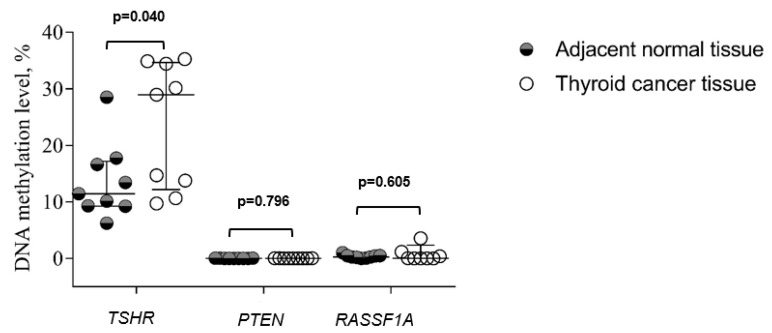
We examined methylation of TSHR, PTEN, and RASSF1A in 20 thyroid cancer tissue samples and compared it to adjacent normal tissue. The methylation level of TSHR was significantly higher in the thyroid cancer tissue compared to adjacent normal tissue (23.61 vs. 13.62%, p = 0.040), while there was no significant difference in PTEN and RASSF1A methylation between these groups (Figure 1).
Figure 1.
The comparison of TSHR, PTEN, and RASSF1A methylation levels in thyroid cancer tissue and adjacent normal tissue groups. Analysis of the Kruskal–Wallis test criterion was used to determine the differences in quantitative traits between the comparison groups. Data are expressed in whisker plots for mean and standard deviation (SD).
3.3. DNA Methylation in Plasma from PTC and HC Groups
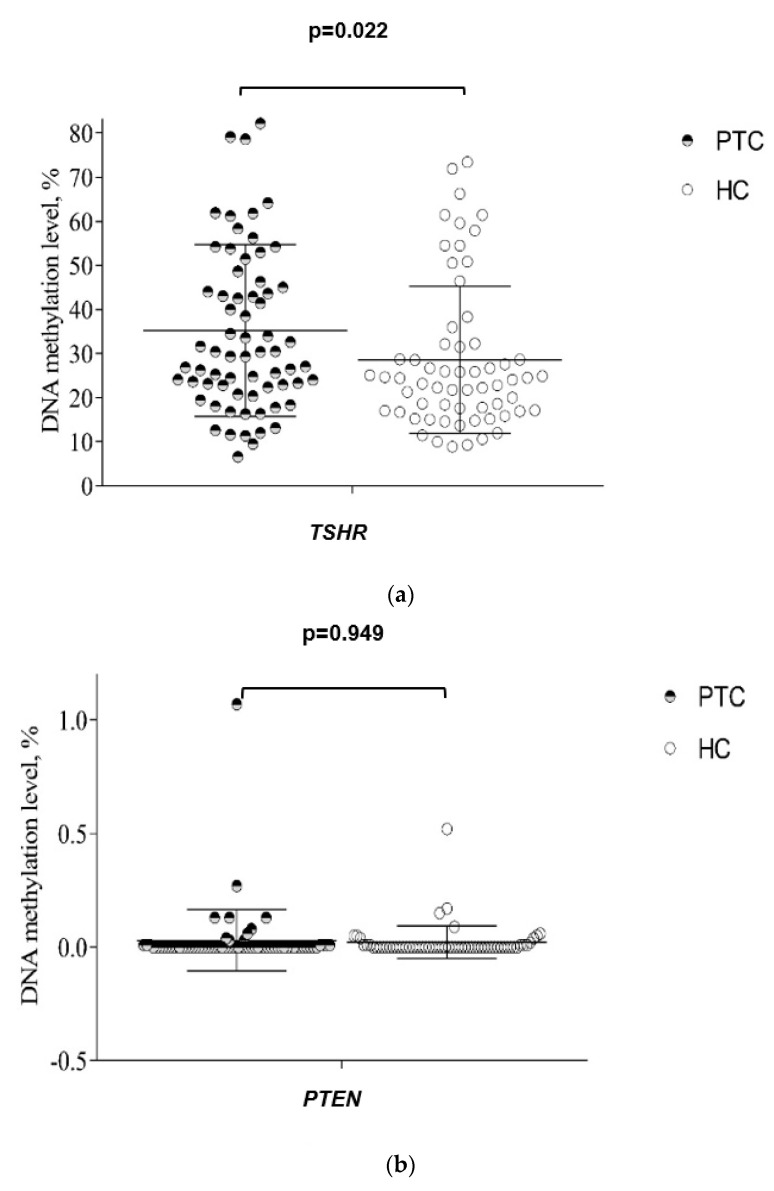
The methylation level of TSHR was significantly higher in plasma of the PTC patients compared to HC (35.25% vs. 28.57%, p = 0.022), while there was no significant difference in PTEN and RASSF1A methylation between these groups (Figure 2a–c).
Figure 2.
(a) The comparison of TSHR methylation level in papillary thyroid cancer (PTC) and healthy control (HC) patients. Analysis of the Mann–Whitney U test criterion was used to determine the differences in quantitative traits between the comparison groups. Data are expressed in whiskers plot for mean and standard deviation (SD). (b) The comparison of PTEN methylation level in papillary thyroid cancer (PTC) and healthy control (HC) patients. Analysis of the Mann–Whitney U test criterion was used to determine the differences in quantitative traits between the comparison groups. Data are expressed in whisker plots for mean and standard deviation (SD). (c) The comparison of RASSF1A methylation level in papillary thyroid cancer (PTC) and healthy control (HC) patients. Analysis of the Mann–Whitney U test criterion was used to determine the differences in quantitative traits between the comparison groups. Data are expressed in whiskers plot for mean and standard deviation (SD).
3.4. DNA Methylation in Plasma PTC Patients before and after Surgery
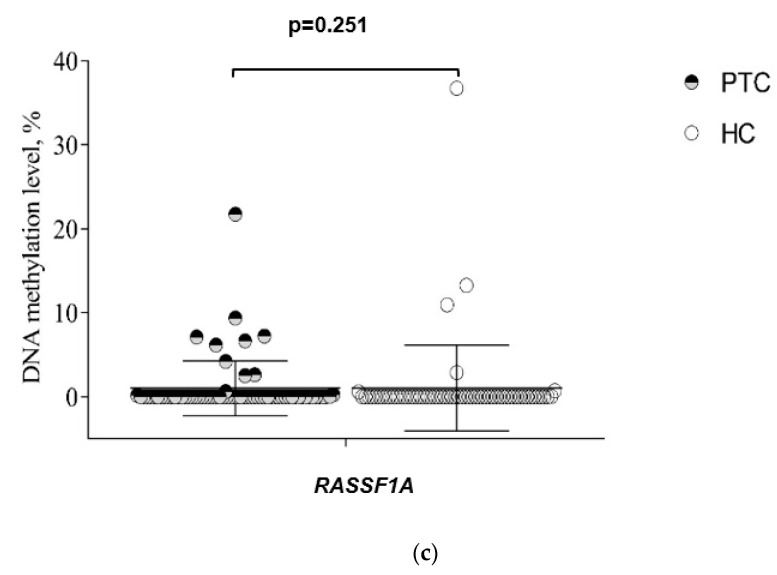
Plasma TSHR, PTEN, and RASSF1A methylation were compared before and after thyroid surgery. After surgery, samples showed significantly lower levels of TSHR and PTEN methylation than before surgery. Moreover, the methylation level of TSHR and PTEN after surgery significantly decreased in PTC patients with suppressed Tg concentrations (p < 0.001 and p = 0.038, respectively) (Figure 3b). However, after surgery, the level of RASSF1A methylation decrease was not statistically significant (Table 2).
Figure 3.
(a) The comparison of TSHR methylation level dynamics after surgery in PTC patients with suppressed and not suppressed thyroglobulin. * p < 0.001. A chi-square independence test was used to determine the differences between the comparison groups. (b) The comparison of PTEN methylation level dynamics after surgery in PTC patients with suppressed and not suppressed thyroglobulin. * p = 0.038. A chi-square independence test was used to determine the differences between the comparison groups.
Table 2.
Plasma TSHR, PTEN, and RASSF1A methylation in papillary thyroid cancer (PTC) patients before and after thyroidectomy. Analysis of Wilcoxon signed-rank test was used. * p < 0.05.
| Marker | Methylation Level: MEAN (SD) | p-Value | |
|---|---|---|---|
| Pre-Operative PTC (n = 68) |
Post-Operative PTC (n = 62) |
||
| TSHR | 34.105 (17.790) | 26.901 (11.617) | 0.003 * |
| PTEN | 0.029 (0.134) | 0.003 (0.014) | 0.031 * |
| RASSF1A | 1.010 (3.248) | 0.816 (4.072) | 0.903 |
3.5. Association of DNA Methylation Level in Plasma with Clinicopathological Features of PTC
We analyzed the association of TSHR, PTEN, and RASSF1A methylation levels with demographic and clinicopathological characteristics of the study population. The TSHR methylation level was significantly higher in PTC with larger tumor size (>2 cm) compared to smaller (≤2 cm) tumor size (p < 0.001). Patients with lymph node metastases, lymphovascular invasion and multifocality had significantly higher methylation levels of TSHR (p = 0.010, p = 0.020 and p = 0.013, respectively). Meanwhile, no statistically significant relationship was found in extrathyroidal extension groups (p = 0.621). The methylation levels of TSHR, PTEN, and RASSF1A in aggressive histology variants subtypes of PTC to other non-aggressive subtypes of PTC were compared, but no statistically significant relationship was found (Table 3).
Table 3.
Correlation between clinicopathological features of papillary thyroid cancer (PTC) and methylation level of TSHR, PTEN, and RASSF1A in plasma samples. * p < 0.05.
| Methylation Level: MEAN (Minimum–Maximum) | |||
|---|---|---|---|
| Characteristic | TSHR | PTEN | RASSF1A |
| 35.24 (6.60–100.00) | 0.02 (0.00–1.07) | 1.18 (0.00–36.74) | |
| % | % | % | |
| p-Value | p-Value | p-Value | |
| p-ValueGender | 0.505 | 0.456 | 0.059 |
| Male | 33.06 (11.35–78.60) | 0.01 (0.00–0.06) | 0.94 (0.00–6.66) |
| Female | 35.54 (6.60–100.00) | 0.03 (0.00–1.07) | 1.02 (0.00–21.74) |
| Age at initial surgery (years) | 0.599 | 0.204 | 0.061 |
| ≤55 years | 35.95 (11.63–100.00) | 0.04 (0.00–1.07) | 0.79 (0.00–21.74) |
| >55 years | 33.95 (6.60–79.13) | 0.01 (0.00–0.13) | 1.41 (0.00–9.36) |
| pT (TNM) | 0.422 | 0.148 | 0.627 |
| pT1 | 37.15 (6.60–100.00) | 0.04 (0.00–1.07) | 1.35 (0.00–21.74) |
| pT2–3 | 33.22 (11.35–79.13) | 0.01 (0.00–0.13) | 0.65 (0.00–7.10) |
| Tumor size (cm) | <0.001 * | 0.743 | 0.164 |
| ≤ 2 | 28.84 (6.60–58.32) | 0.04 (0.00–1.07) | 1.28 (0.00–21.74) |
| > 2 | 50.62 (12.04–100.00) | 0.01 (0.00–0.13) | 0.36 (0.00–7.22) |
| Lymph node metastases at initial surgery | 0.010 * | 0.368 | 0.487 |
| Yes | 47.01 (12.62–82.19) | 0.04 (0.00–0.27) | 1.04 (0.00–9.36) |
| No | 31.62 (6.60–100.00) | 0.03 (0.00–1.07) | 1.00 (0.00–21.74) |
| Variant of PTC | 0.300 | 0.791 | 0.824 |
| Aggressive histology of PTC | 31.32 (12.62–64.15) | 0.01 (0.00–0.08) | 0.95 (0.00–9.36) |
| Non-aggressive subtypes of PTC | 37.12 (6.60–100.00) | 0.04 (0.00–1.07) | 1.04 (0.00–21.74) |
| Extrathyroidal extension | 0.621 | 0.608 | 0.501 |
| Yes | 38.12 (13.13–100.00) | 0.05 (0.00–1.07) | 0.65 (0.00–7.22) |
| No | 32.97 (6.60–78.60) | 0.01 (0.00–0.27) | 1.24 (0.00–21.74) |
| Lymphovascular invasion | 0.020 * | 0.726 | 0.578 |
| Yes | 40.96 (12.62–100.00) | 0.04 (0.00–1.07) | 0.72 (0.00–7.22) |
| No | 28.81 (6.60–61.79) | 0.02 (0.00–0.27) | 0.42 (0.00–21.74) |
| Multifocality | 0.013 * | 0.094 | 0.437 |
| Yes | 47.19 (13.13–100.00) | 0.08 (0.00–0.27) | 0.86 (0.00–7.10) |
| No | 31.57 (6.60–82.19) | 0.01 (0.00–1.07) | 1.06 (0.00–21.74) |
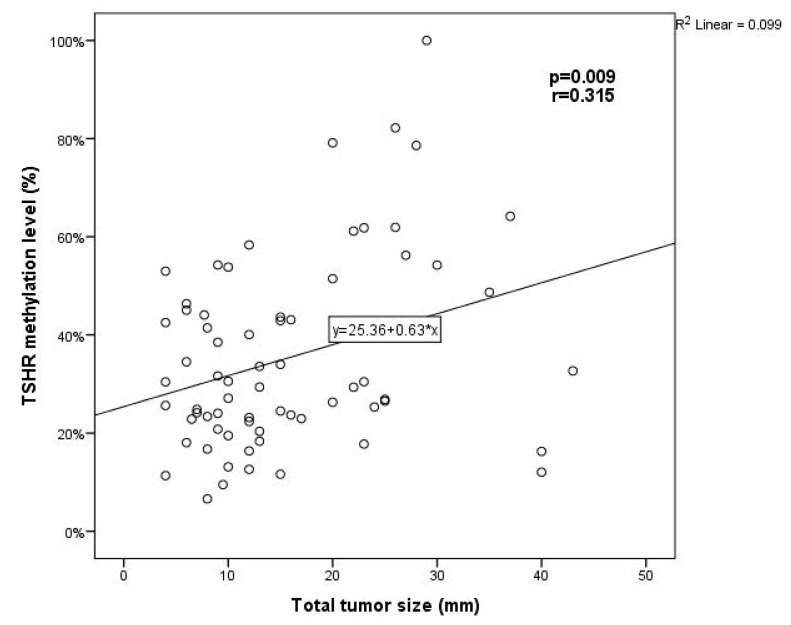
The total tumor size was calculated as the sum of the diameters of all tumors in PTC multifocal cases. The analysis showed a moderate positive correlation between the methylation level of TSHR with the total size of PTC tumors (p = 0.009, r = 0.315) (Figure 4).
Figure 4.
The correlation between TSHR methylation level and the total tumor size. The Pearson correlation coefficient was used to measure the strength of a linear association between two variables.
3.6. The Diagnostic Value of Plasma TSHR, PTEN, and RASSF1A Methylation
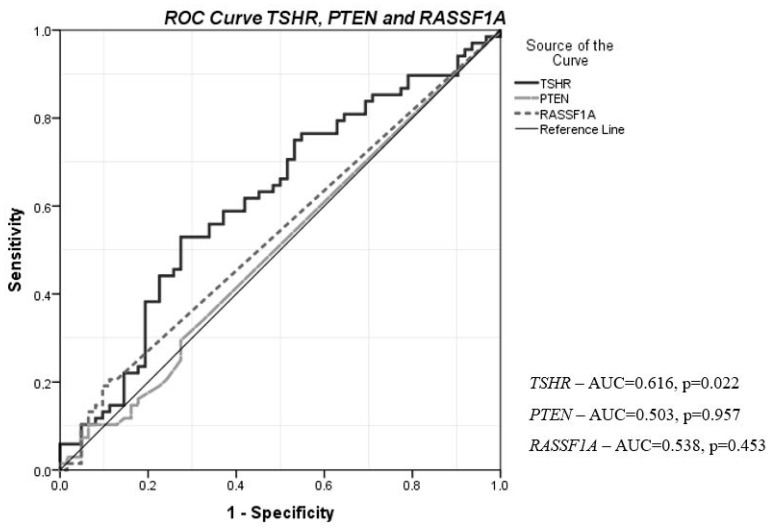
To evaluate the possible diagnostic value of TSHR, PTEN, and RASSF1A, ROC analysis was performed. The methylation level of TSHR was statistically significant and satisfactory to differentiate PTC patients from HC (p = 0.022). TSHR had the highest AUC of 0.616 (95% CI = 0.519–0.714), with 83.8% sensitivity and 71.0% specificity. This might be useful for differentiating PTC from HC, especially in combination with other PTC-specific biomarkers. The methylation of PTEN and RASSF1A did not show statistically significant differences between PTC and HC (Figure 5).
Figure 5.
Diagnostic value of plasma TSHR, PTEN, and RASSF1A methylation levels in discriminating PTC from HC. ROC curves were used to distinguish the groups. ROC, receiver operating characteristic; AUC, area under the curve; PTC, papillary thyroid cancer; HC, healthy control.
4. Discussion
The methylation of DNA promoter is an important and well-known mechanism for carcinogenesis reported in many types of cancers. The promoter CpG islands of genes in healthy cells are generally protected from hypermethylation, but this protection may be lost early in carcinogenesis. This has led to the use of methylation of tumor suppressor genes as biomarkers for the early diagnosis of cancers [22].
The aim of our study was to evaluate the level of promoter methylation of a set of three independent genes (TSHR, PTEN and RASSF1A) and to assess their diagnostic and prognostic values in papillary thyroid tumors. We examined snap-frozen thyroid cancer and adjacent normal tissue samples. The methylation level of TSHR was significantly higher in the thyroid cancer tissue compared to adjacent normal tissue, while there was no significant difference in PTEN and RASSF1A methylation between these groups. The methylation levels of TSHR, PTEN, and RASSF1A promoters were compared in peripheral blood samples of PTC and HC. We observed that TSHR methylation levels in the PTC group were significantly higher compared to HC, while the levels of PTEN and RASSF1A methylation did not differ significantly. After surgery, PTC plasma samples showed lower TSHR and PTEN methylation levels compared to the levels before surgery. The TSHR methylation level was significantly higher in PTC with larger tumor size (>2 cm) compared to a smaller tumor size (≤2 cm) and in patients with lymph node metastases, lymphovascular invasion and multifocality. The methylation level of TSHR correlated with total tumor size. ROC analysis revealed that TSHR methylation level provides higher accuracy in distinguishing PTC from HC and may be used as a potential tumor marker. The specificity, sensitivity, and accuracy of PTEN and RASSF1A in the diagnosis of PTC showed no significant value.
A growing body of research shows that blood plasma DNA methylation status is associated with cancer in several tissues, which is why methylation status in peripheral blood may be a potential source of non-invasive cancer biomarkers.
There are many studies of DNA methylation analysis in peripheral blood samples for detecting hepatic, colon, lung, breast, stomach, and endometrial tumors [23,24,25]. Meanwhile, there is only one study analyzing global DNA methylation in peripheral blood from PTC patients and control individuals, without significant differences detected [26]. Feng Wei et al. analyzed the methylation status of PTEN and DAPK in blood and tissue samples of patients with thyroid cancer [27].
Our study shows that TSHR methylation levels in the PTC group were significantly higher compared to HC. Moreover, the methylation level of TSHR was significantly higher in the thyroid cancer tissue compared to adjacent normal tissue. ROC curve analysis confirmed that the plasma TSHR methylation level might be quite a reliable biomarker in discriminating PTC from HC (AUC of 0.616 (95% CI = 0.519–0.714)). J. K. Stephen et al. also demonstrated that methylation of TSHR distinguished PTC from normal thyroid tissue [28]. This finding may support the methylation of TSHR as a promising biomarker in differentiating PTC patients from healthy individuals, especially when used in combination with other PCT-specific biomarkers.
In most PTC patients with suppressed Tg, the TSHR and PTEN methylation levels were significantly decreased after surgery. The decrease in Tg levels may take approximately 1 year. In our study, we observed significant differences only 3 months after surgery. Our findings suggest that the plasma methylation levels of TSHR and PTEN changed significantly after surgery and therefore might be indicators of radical removal of the tumor and useful prognostic markers after thyroidectomy. However, it would be useful to assess the changes after 1 year.
TSHR plays a key role in the regulation of thyroid cell proliferation, differentiation and function. Hypermethylation of TSHR gene, which leads to TSHR expression silencing, plays an important role in the pathogenesis of thyroid cancer. Studies by Wang and Zheng et al. found that the methylation of TSHR gene in PTC patients in later stages was higher than that in earlier stages, which provided evidence that TSHR gene inactivation promotes the progression of PTC. [29,30] Liu T et al. demonstrated that TSHR inhibits metastasis through regulating epithelial-mesenchymal transition (EMT) in vitro, and that a lack of expression of TSHR is a significant independent factor affecting distant metastasis and poor prognosis in DTC [31].
We performed the analysis to explore the relationship between the methylation level of TSHR promoter in plasma samples and clinicopathological features of PTC. Previous studies reported that the incidence of TSHR promoter methylation in patients with lymph node metastasis is significantly higher than that in PTC with no lymph node metastasis [12]. On the contrary, Dai et al.’s study results showed that the TSHR gene methylation rate in patients with no lymph node metastasis was higher than that in patients with lymph node metastasis [32]. In contrast, our results showed that the level of TSHR promoter methylation in PTC patients with lymph node metastasis was significantly higher than in patients with no lymph node metastasis. A significant relationship between TSHR methylation and tumor diameter was also described in the meta-analysis, as the TSHR promoter methylation occurrence in patients with tumor diameter >2 cm was higher than in patients with tumor diameter ≤2 cm [12]. Our findings also suggest that the TSHR methylation level was significantly higher in patients with larger tumor size. Moreover, we observed that the TSHR methylation level was significantly higher in the presence of lymphovascular invasion and multifocality. On the contrary, Mohammadi-asl J. et al. did not find a significant relationship between TSHR methylation and lymphovascular invasion [33]. In Lucieli Ceolin et al.’s study, no significant relationship was observed between DNA methylation levels and tumor size or presence of metastatic disease in thyroid cancer [26].
Feng Wei et al. analyzed the methylation status of PTEN in peripheral blood and tissue samples. In both samples, the rate of PTEN methylation was significantly higher in PTC patients compared with controls. PTEN methylation status was not affected by tumor size but significantly correlated with metastasis in lymph nodes. Moreover, there was no significant difference in the specificity, sensitivity, or accuracy of PTEN in the diagnosis of PTC [27]. On the contrary, in our study, there was no significant difference in the PTEN methylation level in PTC patients compared with the control group and no relationship between the clinicopathological features of PTC. On the other hand, the plasma methylation level of PTEN changed significantly after surgery. Therefore, we support the further investigation of PTEN methylation in a large cohort.
The epigenetic value of the RASSF1A in thyroid carcinoma has been highlighted. RASSF1A methylation can be used as a diagnostic marker in thyroid malignancies [34,35]. On the contrary, in some studies, no significant relationship between RASSF1A methylation and thyroid cancer was detected [36,37]. We also did not find any significant relationship between RASSF1A methylation and clinicopathological features in PTC and the control group.
Our study has several limitations that restrict TSHR, PTEN, and RASSF1A methylation to be suggested as markers for PTC detection. Due to a short patient follow-up period after the surgery, it is difficult to determine the prognostic value of these genes’ methylation in PTC. Another limitation is the relatively small sample size.
Altogether, our findings indicate that TSHR methylation in peripheral blood samples is expected to be quite a sensitive and specific minimally invasive parameter for the diagnosis of PTC. However, further investigation is required to confirm the diagnostic and prognostic TSHR methylation value for PTC. Further insight on epigenetic biomarkers for the non-invasive detection of PTC might be provided by epigenomic techniques in large, independent cohorts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11164917/s1, Table S1. Primers used for quantitative methylation-specific PCR.
Author Contributions
Conceptualization, A.D. and B.Ž.; methodology, M.K., D.D., A.D., K.Ž., R.S. and S.J.; validation, R.K. and A.D.; formal analysis, R.K., A.K. and M.K.; investigation, A.K., R.K., M.K, K.Ž. and A.D.; resources, B.Ž. and A.D.; data curation, A.K., M.K., R.K., A.D. and D.D.; writing—original draft preparation, R.K., M.K. and K.Ž.; supervision, D.D., A.D. and B.Ž.; writing—review and editing, A.D., D.D., B.Ž., R.S. and S.J.; visualization, R.K., M.K., D.D. and A.D.; project administration, A.D., B.Ž. and S.J.; funding acquisition, B.Ž. and A.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee Kaunas Regional Committee of Biomedical Research (Lithuania, approval No. BE-2-64; 7 February 2022) for studies involving humans.
Informed Consent Statement
Written informed consent was obtained from each participant of the study after a full explanation of the purpose and nature of all procedures used.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding Statement
This study was supported by the Lithuanian Research Council (Grant No. S-SEN-20-14 ”The role of epigenetic markers for early detection of papillary thyroid carcinoma and prognostic significance in long-term outcome in elderly patients”).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Limaiem F., Rehman A., Mazzoni T. Papillary Thyroid Carcinoma. StatPearls Publishing LLC; Treasure Island, FL, USA: 2022. [PubMed] [Google Scholar]
- 2.Fugazzola L., Elisei R., Fuhrer D., Jarzab B., Leboulleux S., Newbold K., Smit J. 2019 European Thyroid Association Guidelines for the Treatment and Follow-Up of Advanced Radioiodine-Refractory Thyroid Cancer. Eur. Thyroid J. 2019;8:227–245. doi: 10.1159/000502229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito Y., Miyauchi A., Kihara M., Fukushima M., Higashiyama T., Miya A. Overall Survival of Papillary Thyroid Carcinoma Patients: A Single-Institution Long-Term Follow-Up of 5897 Patients. World J. Surg. 2018;42:615–622. doi: 10.1007/s00268-018-4479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medas F., Canu G.L., Boi F., Lai M.L., Erdas E., Calo P.G. Predictive Factors of Recurrence in Patients with Differentiated Thyroid Carcinoma: A Retrospective Analysis on 579 Patients. Cancers. 2019;11:1230. doi: 10.3390/cancers11091230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowalska A., Walczyk A., Kowalik A., Palyga I., Trybek T., Kopczynski J., Kajor M., Chrapek M., Pieciak L., Chlopek M., et al. Increase in Papillary Thyroid Cancer Incidence Is Accompanied by Changes in the Frequency of the BRAF V600E Mutation: A Single-Institution Study. Thyroid. 2016;26:543–551. doi: 10.1089/thy.2015.0352. [DOI] [PubMed] [Google Scholar]
- 6.Lewinski A., Adamczewski Z. Papillary thyroid carcinoma: A cancer with an extremely diverse genetic background and prognosis. Pol. Arch. Intern. Med. 2017;127:388–389. doi: 10.20452/pamw.4058. [DOI] [PubMed] [Google Scholar]
- 7.Ward L.S., Kloos R.T. Molecular markers in the diagnosis of thyroid nodules. Arq. Bras. Endocrinol. Metabol. 2013;57:89–97. doi: 10.1590/S0004-27302013000200001. [DOI] [PubMed] [Google Scholar]
- 8.Alsina J., Alsina R., Gulec S. A Concise Atlas of Thyroid Cancer Next-Generation Sequencing Panel ThyroSeq v.2. Mol. Imaging Radionucl. Ther. 2017;26:102–117. doi: 10.4274/2017.26.suppl.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mon S.Y., Riedlinger G., Abbott C.E., Seethala R., Ohori N.P., Nikiforova M.N., Nikiforov Y.E., Hodak S.P. Cancer risk and clinicopathological characteristics of thyroid nodules harboring thyroid-stimulating hormone receptor gene mutations. Diagn. Cytopathol. 2018;46:369–377. doi: 10.1002/dc.23915. [DOI] [PubMed] [Google Scholar]
- 10.Chu Y.D., Yeh C.T. The Molecular Function and Clinical Role of Thyroid Stimulating Hormone Receptor in Cancer Cells. Cells. 2020;9:1730. doi: 10.3390/cells9071730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kartal K., Onder S., Kosemehmetoglu K., Kilickap S., Tezel Y.G., Kaynaroglu V. Methylation status of TSHr in well-differentiated thyroid cancer by using cytologic material. BMC Cancer. 2015;15:824. doi: 10.1186/s12885-015-1861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu M., Wan S., Ren B., Wu H., Liu L., Shen H. Association between TSHR gene methylation and papillary thyroid cancer: A meta-analysis. Endocrine. 2020;69:508–515. doi: 10.1007/s12020-020-02284-7. [DOI] [PubMed] [Google Scholar]
- 13.Beg S., Siraj A.K., Jehan Z., Prabakaran S., Al-Sobhi S.S., Al-Dawish M., Al-Dayel F., Al-Kuraya K.S. PTEN loss is associated with follicular variant of Middle Eastern papillary thyroid carcinoma. Br. J. Cancer. 2015;112:1938–1943. doi: 10.1038/bjc.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y.M., Cheng F., Teng L.S. The association between phosphatase and tensin homolog hypermethylation and patients with breast cancer, a meta-analysis and literature review. Sci. Rep. 2016;6:32723. doi: 10.1038/srep32723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S.J., Lee M.H., Kim D.W., Lee S., Huang S., Ryu M.J., Kim Y.K., Kim S.J., Kim S.J., Hwang J.H. Cross-regulation between oncogenic BRAFV600E kinase and the MST1 pathway in papillary thyroid Carcinoma. PLoS ONE. 2011;6:e16180. doi: 10.1371/journal.pone.0016180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khatami F., Larijani B., Heshmat R., Nasiri S., Haddadi-Aghdam M., Teimoori-Toolabi L., Tavangar S.M. Hypermethylated RASSF1 and SLC5A8 promoters alongside BRAF(V600E) mutation as biomarkers for papillary thyroid carcinoma. J. Cell. Physiol. 2020;235:6954–6968. doi: 10.1002/jcp.29591. [DOI] [PubMed] [Google Scholar]
- 17.Shou F., Xu F., Li G., Zhao Z., Mao Y., Yang F., Wang H., Guo H. RASSF1A promoter methylation is associated with increased risk of thyroid cancer: A meta-analysis. Onco Targets Ther. 2017;10:247–257. doi: 10.2147/OTT.S124417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuttle R.M., Haugen B., Perrier N.D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid. 2017;27:751–756. doi: 10.1089/thy.2017.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou P., Ji M., Xing M. Association of PTEN gene methylation with genetic alterations in the phosphatidylinositol 3-kinase/AKT signaling pathway in thyroid tumors. Cancer. 2008;113:2440–2447. doi: 10.1002/cncr.23869. [DOI] [PubMed] [Google Scholar]
- 20.Rogeri C.D., Silveira H.C.S., Causin R.L., Villa L.L., Stein M.D., de Carvalho A.C., Arantes L.M.R.B., Scapulatempo-Neto C., Possati-Resende J.C., Antoniazzi M. Methylation of the hsa-miR-124, SOX1, TERT, and LMX1A genes as biomarkers for precursor lesions in cervical cancer. Gynecol. Oncol. 2018;150:545–551. doi: 10.1016/j.ygyno.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann U., Langer F., Feist H., Glockner S., Hasemeier B., Kreipe H. Quantitative assessment of promoter hypermethylation during breast cancer development. Am. J. Pathol. 2002;160:605–612. doi: 10.1016/S0002-9440(10)64880-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith J.A., Fan C.Y., Zou C., Bodenner D., Kokoska M.S. Methylation status of genes in papillary thyroid carcinoma. Arch. Otolaryngol. Head Neck Surg. 2007;133:1006–1011. doi: 10.1001/archotol.133.10.1006. [DOI] [PubMed] [Google Scholar]
- 23.Huang W., Li T., Yang W., Chai X., Chen K., Wei L., Duan S., Li B., Qin Y. Analysis of DNA methylation in plasma for monitoring hepatocarcinogenesis. Genet. Test. Mol. Biomark. 2015;19:295–302. doi: 10.1089/gtmb.2014.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schotten L.M., Darwiche K., Seweryn M., Yildiz V., Kneuertz P.J., Eberhardt W.E.E., Eisenmann S., Welter S., Sisson B.E., Pietrzak M., et al. DNA methylation of PTGER4 in peripheral blood plasma helps to distinguish between lung cancer, benign pulmonary nodules and chronic obstructive pulmonary disease patients. Eur. J. Cancer. 2021;147:142–150. doi: 10.1016/j.ejca.2021.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Miller B.F., Petrykowska H.M., Elnitski L. Assessing ZNF154 methylation in patient plasma as a multicancer marker in liquid biopsies from colon, liver, ovarian and pancreatic cancer patients. Sci. Rep. 2021;11:221. doi: 10.1038/s41598-020-80345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ceolin L., Goularte A.P.P., Ferreira C.V., Romitti M., Maia A.L. Global DNA methylation profile in medullary thyroid cancer patients. Exp. Mol. Pathol. 2018;105:110–114. doi: 10.1016/j.yexmp.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Wei F., Wu Y., Wang Z., Li Y., Wang J., Shao G., Yang Y., Shi B. Diagnostic significance of DNA methylation of PTEN and DAPK in thyroid tumors. Clin. Endocrinol. 2020;93:187–195. doi: 10.1111/cen.14192. [DOI] [PubMed] [Google Scholar]
- 28.Stephen J.K., Chen K.M., Merritt J., Chitale D., Divine G., Worsham M.J. Methylation markers differentiate thyroid cancer from benign nodules. J. Endocrinol. Investig. 2018;41:163–170. doi: 10.1007/s40618-017-0702-2. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z., Feng K. Relationship between methylation of TSHR and NIS gene promoter regions and clinicopathological characteristics in thyroid papillary carcinoma. Shandong Med. J. 2017;57:83–85. doi: 10.3969/j.issn.1002-266X. [DOI] [Google Scholar]
- 30.Zheng C., Lu X., Ling Z., Ge M. Methylation of TSHR gene promoter in papillary thyroid carcinoma and its clinical significance. J. Chin. Oncol. 2017;23:257–261. doi: 10.11735/j.issn.1671-170X.2017.04.B001. [DOI] [Google Scholar]
- 31.Liu T., Men Q., Su X., Chen W., Zou L., Li Q., Song M., Ouyang D., Chen Y., Li Z., et al. Downregulated expression of TSHR is associated with distant metastasis in thyroid cancer. Oncol. Lett. 2017;14:7506–7512. doi: 10.3892/ol.2017.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai Y., Cai D., Chen H., Zhang H., Zhang Z., Li J. The relevance between the promoter hypermethylation of tshr and p16 gene and clinicopathological parameters in human papillary thyroid carcinoma. J. Cap. Med. Univ. 2012;33:361–365. [Google Scholar]
- 33.Mohammadi-asl J., Larijani B., Khorgami Z., Tavangar S.M., Haghpanah V., Kheirollahi M., Mehdipour P. Qualitative and quantitative promoter hypermethylation patterns of the P16, TSHR, RASSF1A and RARβ2 genes in papillary thyroid carcinoma. Med. Oncol. 2011;28:1123–1128. doi: 10.1007/s12032-010-9587-z. [DOI] [PubMed] [Google Scholar]
- 34.Zhang K., Li C., Liu J., Tang X., Li Z. DNA methylation alterations as therapeutic prospects in thyroid cancer. J. Endocrinol. Investig. 2019;42:363–370. doi: 10.1007/s40618-018-0922-0. [DOI] [PubMed] [Google Scholar]
- 35.Niu H., Yang J., Yang K., Huang Y. The relationship between RASSF1A promoter methylation and thyroid carcinoma: A meta-analysis of 14 articles and a bioinformatics of 2 databases (PRISMA) Medicine. 2017;96:e8630. doi: 10.1097/MD.0000000000008630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brait M., Loyo M., Rosenbaum E., Ostrow K.L., Markova A., Papagerakis S., Zahurak M., Goodman S.M., Zeiger M., Sidransky D., et al. Correlation between BRAF mutation and promoter methylation of TIMP3, RARbeta2 and RASSF1A in thyroid cancer. Epigenetics. 2012;7:710–719. doi: 10.4161/epi.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qu F., Xue W. RASSF1A methylation and its clinical roles in papillary thyroid carcinoma. J. Nantong Univ. (Med. Sci.) 2012;32:490–492. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.