INTRODUCTION
In recent years, treatment options for Opioid Use Disorder (OUD) have grown to encompass a host of medications with various treatment formulations. Buprenorphine, a mainstay of treatment of OUD since approval from the U.S. Food and Drug Administration (FDA) in 2002, has benefited from a variety of treatment formulations including sublingual, buccal formulations and long-acting subcutaneous injection.1 In recent years, buprenorphine extended-release injections have grown popular, providing patients and clinicians with another tool to treat the debilitating symptoms of addiction.
Buprenorphine, while generally well tolerated in its older formulations, has had less research in its newer extended-release form.2 The novelty of the extended-release formulation of buprenorphine explains the dearth of research on adverse events specifically related to its subcutaneous route. In this case study, a particular case of post-injection cellulitis is discussed and briefly potential causes explored. As extended-release formulations of buprenorphine continue to increase in use, cases studies like these will help identify more uncommon adverse events and help broaden practitioners' differentials.
CASE REPORT
A 35-year-old female with severe opioid use disorder on buprenorphine injection (Sublocade®) therapy presented to the addiction psychiatry treatment center with reported injection site pain, erythema, swelling, and a rash of two days duration. Fourteen days prior to presentation, she had received her second buprenorphine injection in the right lower quadrant of her abdomen. She initially did not develop redness or pain, but while reporting withdrawal symptoms eleven days post-injection, she mentioned unintentionally scratching the site with an acrylic nail the previous day. She denied bleeding but experienced slight pain when this occurred. Of note, she had tolerated her initial injection well with only slight soreness and redness lasting two days. Between her first and second subcutaneous doses, supplemental sublingual buprenorphine/naloxone 8–2 mg daily had been prescribed to ease irritability and cravings.
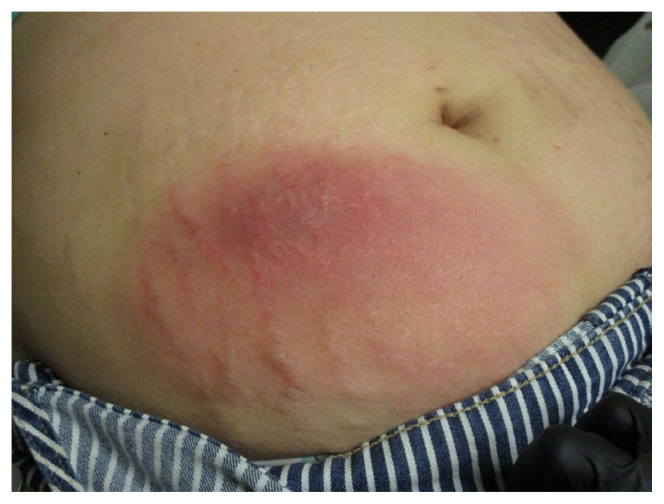
On evaluation, she emphasized the scratch was unintentional. The pain was significant enough that she needed to wear loose clothing over the area. She denied fevers, chills, or expression of material from the site. Vitals were stable with a temperature of 36.4 degrees Celsius, blood pressure of 136/93 mmHg, heart rate of 85 beats/minute, respiratory rate of 17 breaths/minute, and blood oxygen saturation of 100%. Physical exam demonstrated a 3 × 5 cm indurated injection site with slight protuberance from her abdomen. Erythema extended into the surrounding skin involving a total area of 10 × 15 cm, including her striae gravidarum (Figure 1). The site was tender and warm to the touch, but no disruptions in the skin barrier were noted. The induration and surrounding erythema were outlined with a marking pen, and due to concern for cellulitis, the patient was escorted directly to the emergency department. After a seven-day course of cephalexin and trimethoprim-sulfamethoxazole, the cellulitis successfully resolved (Figure 2).
Figure 1.
Injection site cellulitis at the time of presentation.
Figure 2.
Resolved cellulitis seven days post-treatment.
This patient’s history was significant for buprenorphine/naloxone diversion, which served as the indication for her treatment to be converted to the injectable formulation. While accepting her history of diversion and its implications on her treatment options, she initially desired to discontinue the injections and resume sublingual treatment on follow-up. She was given the option of converting to methadone treatment, but she ultimately chose to continue the injections due to the significant functional benefit that this treatment modality awarded her. Although guidelines recommended subcutaneous buprenorphine to be dosed at 300 mg for the first two injections and 100 mg thereafter,3 this patient likely will continue to receive doses of 300 mg due to continued cravings and withdrawal symptoms.
DISCUSSION
Opioid use disorder is a public health emergency that has become a serious epidemic in the United States, specifically the high risk of overdose with opioids such as heroin, prescription opioids, and illicit fentanyl.4 Moderate to severe forms of OUD requires continuing sustained care for effective treatment and achieving long-term opioid abstinence. There are three medications currently approved by FDA used for OUD treatment that targets opioid receptors: methadone, buprenorphine, and long-acting naltrexone. These medications have been shown to reduce relapse to opioid use, overdose, and HIV and HCV transmission, as well as improve other health outcomes.4–7
Buprenorphine is a partial μ-opioid agonist used for the maintenance treatment of opioid use disorder.2 Buprenorphine has potential merits, including a lower overdose risk and “low ceiling effect” for respiratory depression, fewer pharmaceutical interactions, and less risk of QTc-prolongation when compared to methadone.8 Buprenorphine is available in three formulations: sublingual films and tabs and buprenorphine extended-release injection (Sublocade®).4,6,7,9 The extended-release injectable formulation was approved by FDA in November 2017. This formulation is a monthly subcutaneous injection administered only by a healthcare provider. It is available in two doses of 300 mg/1.5 ml and 100/0.5 ml. Per current guidelines, the first two doses recommended are 300 mg each with the following monthly maintenance dose of 100 mg each.4,6,7 The monthly formulation of extended-release buprenorphine produces a sufficient steady-state blood level and may be more favorable for persons who have difficulty adhering to daily sublingual forms.4,5,10
The most common adverse drug reactions of extended-release buprenorphine injection are injection site pain, injection site pruritus, constipation, sweating, nausea, vomiting, headache, fatigue, and sedation (more than 5%).3,4,11–14 The majority of injection-site adverse reactions are mild to moderate in severity and include pain, hives, and erythema.3,4,12–13 These side effects are usually temporary and appear most commonly with the first injection and decrease in frequency with subsequent injections.4,15
Our case report was unique as an injection site reaction appeared in a patient 14 days after her second injection of extended-release buprenorphine, following unintentional scratching of the injection site with an acrylic nail while in the shower. Looking specifically at the Phase 3 trial data, only 1 of the 201 patients in the initial trial experienced injection site cellulitis, and 2 others of a total of 412 participants have experienced injection site cellulitis in the ongoing open-label long-term safety study.15 Possible explanations that may have predisposed our patient to an injection site reaction may have included improper injection of the medication, as adverse reactions are more likely to occur with intradermal or intramuscular injection.3 Both of these considerations were difficult to reconcile given the delayed onset from the injection. Our patients' acrylic nails may have acted as a nidus that seeded an infection over time as well.
It is important to consider that the cellulitis could have resulted from the patient attempting to remove the Sublocade®dose in an attempt to divert depot buprenorphine. The manufacturer suggested providers continue to monitor injection sites for evidence of tampering or attempts to remove the depot.3 In a clinical setting, removal of the depot can be performed through surgical excision under anesthesia within 14 days of injection. Given that the depot formulation is meant to decrease the risk of diversion,10 this finding would be of considerable interest. However, the patient denied intentional tampering and it cannot be confirmed if this patient actually attempted to remove the medication.
ACKNOWLEDGEMENTS
The patient has given written consent for publication of the figures and the details of the case in the literature.
REFERENCES
- 1.Wakhlu S. Buprenorphine: A review. J Opioid Manag. 2009;5(1):59–64. doi: 10.5055/jom.2009.0007. [DOI] [PubMed] [Google Scholar]
- 2.Welsh C, Valadez-Meltzer A. Buprenorphine: A (relatively) new treatment for opioid dependence. Psychiatry (Edgmont) 2005;2(12):29–39. [PMC free article] [PubMed] [Google Scholar]
- 3.Highlights of Prescribing Information. SUBLOCADE (buprenorphine extended-release) injection. 2022. [Accessed May 13, 2022]. No author. https://www.sublocade.com/Content/pdf/prescribing-information.pdf .
- 4.Knopf A. SAMHSA publishes TIP 63, focusing on medications for OUD treatment. Alcoholism & Drug Abuse Weekly. 2018;30(10):6. [Google Scholar]
- 5.Lofwall MR, Fanucchi LC. Long-acting buprenorphine injectables: Opportunity to improve opioid use disorder treatment among rural populations. Prev Med. 2021;152(Pt 2):106756. doi: 10.1016/j.ypmed.2021.106756. [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell JK, Gladden RM, Seth P. Trends in deaths involving heroin and synthetic opioids excluding methadone, and law enforcement drug product reports, by census region - United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(34):897–903. doi: 10.15585/mmwr.mm6634a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: Available evidence to inform clinical practice and research. J Subst Abuse Treat. 2005;28(4):321–329. doi: 10.1016/j.jsat.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Substance Abuse and Mental Health Services Administration. TIP 63: Medications for Opioid Use Disorder. 2021. [Accessed May 24, 2022]. https://store.samhsa.gov/sites/default/files/SAMHSA_Digital_Download/PEP21-02-01-002.pdf .
- 9.World Health Organization. WHO/UNODC/UNAIDS position paper: Substitution maintenance therapy in the management of opioid dependence and HIV/AIDS prevention. 2004. [Accessed May 13, 2022]. https://apps.who.int/iris/handle/10665/42848 .
- 10.Ling W, Shoptaw S, Goodman-Meza D. Depot buprenorphine injection in the management of opioid use disorder: From development to implementation. Subst Abuse Rehabil. 2019;10:69–78. doi: 10.2147/SAR.S155843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganetsky VS. Clinical Updates on the Use of Injectable Medications for Opioid Use Disorder (MOUD) May 7, 2021. [Accessed May 13, 2022]. https://sites.rutgers.edu/mat-coe/wp-content/uploads/sites/473/2021/05/Clinical-Updates-on-the-Use-of-Injectable-Medications-for-Opioid-Use-Disorders-MOUD-05072021.pdf .
- 12.Indivior. Indivior's RECOVER™ Study Finds Three-quarters of Patients with Moderate to Severe Opioid Use Disorder Who Received 12 monthly Injections of SUBLOCADE™ (Buprenorphine Extended-Release) Injection were Abstinent from Illicit Opioids One Year Later. Apr 5, 2019. [Accessed May 16, 2022]. https://www.prnewswire.com/news-releases/indiviors-recover-study-finds-three-quarters-of-patients-with-moderate-to-severe-opioid-use-disorder-who-received-12-monthly-injections-of-sublocade-buprenorphine-extended-release-injection-were-abstinent-from-illicit-opioids-300825177.html .
- 13.Independence Blue Cross. Drugs Used for the Maintenance Treatment of Opioid or Alcohol Use Disorder (e.g., Naltrexone Implants, Probuphine Implant, Sublocade Injection, Vivitrol Injection) Policy #: 08.01.37a. Aug 11, 2021. [Accessed May 18, 2022]. https://medpolicy.ibx.com/ibc/Commercial/Pages/Policy/1f4283fc-5e91-48fe-a0ec-deae8a129738.aspx .
- 14.Chappuy M, Trojak B, Nubukpo P, et al. Prolonged-release buprenorphine formulations: Perspectives for clinical practice. Therapie. 2020;75(5):397–406. doi: 10.1016/j.therap.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: A multi-centre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778–790. doi: 10.1016/S0140-6736(18)32259-1. [DOI] [PubMed] [Google Scholar]