Abstract
The bacteriophage Mu strong gyrase site (SGS) is required for efficient replicative transposition and functions by promoting the synapsis of prophage termini. To look for other sites which could substitute for the SGS in promoting Mu replication, we have replaced the SGS in the middle of the Mu genome with fragments of DNA from various sources. A central fragment from the transposing virus D108 allowed efficient Mu replication and was shown to contain a strong gyrase site. However, neither the strong gyrase site from the plasmid pSC101 nor the major gyrase site from pBR322 could promote efficient Mu replication, even though the pSC101 site is a stronger gyrase site than the Mu SGS as assayed by cleavage in the presence of gyrase and the quinolone enoxacin. To look for SGS-like sites in the Escherichia coli chromosome which might be involved in organizing nucleoid structure, fragments of E. coli chromosomal DNA were substituted for the SGS: first, repeat sequences associated with gyrase binding (bacterial interspersed mosaic elements), and, second, random fragments of the entire chromosome. No fragments were found that could replace the SGS in promoting efficient Mu replication. These results demonstrate that the gyrase sites from the transposing phages possess unusual properties and emphasize the need to determine the basis of these properties.
Bacteriophage Mu is one of the largest and most efficient bacterial transposons known. Its 37-kb linear genome is approximately 1% the size of the Escherichia coli chromosome, and during the lytic cycle, approximately 100 replicative transposition events can occur within 40 min (for a general review, see references 10 and 21).
Studies with Mu have been instrumental to our understanding of the mechanism of transposition. Analysis of transposition in an in vitro system with purified components has led to a detailed description of the steps in the transposition pathway (reviewed in references 15 and 17). The in vitro system, however, uses as a substrate a small plasmid containing short segments of the Mu DNA termini to approximate the 37-kb genome which normally undergoes replicative transposition within the bacterial nucleoid. Hence, some aspects of Mu biology may not be addressed by the in vitro system.
We have been interested in synapsis of the prophage termini by transposase, an obligate early step in the transposition pathway, which, in vivo, involves long-range DNA interactions within the constraints imposed by the structure of the nucleoid. Transposase monomers bind to three sites at each end of the prophage and to an enhancer region near the left end (35). Following synapsis of the prophage ends, the transposase monomers rearrange to form a stable tetramer bound to two sites at the right end and one site at the left end. Synapsis is essential for transposition, because transposase acts in trans to generate the required cleavage and ligation reactions (i.e., transposase bound at the left end cleaves at the DNA junction at the right end and vice versa) (1, 27, 37). We have proposed that a site located in the center of the prophage promotes synapsis of the termini and that it does so by organizing the structure of the prophage DNA into a plectonemically interwound supercoiled loop with the site at the apex of the loop and the termini to be synapsed at the base (24). We found a site in the center of the genome that is required for efficient Mu replicative transposition and identified it as a strong DNA gyrase cleavage site (SGS) (24). Deletion of the SGS apparently inhibits transposition at the step of synapsis of the prophage termini (25). Several experimental approaches were used to demonstrate that the SGS has to be symmetrically located between the termini to be synapsed to allow for optimal rates of Mu replication (22, 23).
If the Mu SGS is capable of organizing the topology of prophage DNA as suggested by the model, then an important question that arises is whether this use of a strong gyrase site is unique to the Mu site or whether it is a more general property of a class of sites. For example, the E. coli nucleoid is thought to be composed of 50 to 100 independently supercoiled domains (13, 30, 36), and one could ask whether gyrase sites might be involved in the formation of individual domains. Detection of gyrase sites in the E. coli chromosome that are preferentially cleaved in the presence of a quinolone (4, 31), possibly associated with certain repetitive DNA elements (7, 38), has been cited to support the notion of the involvement of gyrase sites in nucleoid structure. To approach the question, we have chosen to search for sites which could replace the Mu SGS in allowing efficient replication of Mu DNA. The approach used was to clone fragments of DNA from various sources into the center of a prophage lacking the SGS and to examine the effects of the new site on Mu replication.
MATERIALS AND METHODS
Standard cloning procedures were used throughout. Ligations of restriction fragments with incompatible ends were done after end filling with Klenow polymerase or T4 polymerase. The coordinates given for restriction sites in Mu DNA are in kilobases from the left end of the 37.2-kb genome.
Construction of the Δ100 plasmid pMP1856.
In a previous publication (23), we described the construction of the plasmid pMP1812, which contains (i) the BamHI-EcoRI (17.2 to 23.7 kb) fragment of Mu DNA cloned at the BamHI site of a pBR322 plasmid with deletion of its ampicillin resistance (Apr) gene; (ii) a 147-bp MluI-ScaI (18.0 to 18.15 kb) deletion removing the SGS and the 3′ end of the upstream G gene; (iii) an inserted oligonucleotide restoring the end of the G gene and introducing sites for BglII, BstII, and StuI; and (iv) a Knr cassette at the StuI site. To construct pMP1856, the Knr cassette was deleted from p1812 and an Apr cassette was introduced at the BamHI junction of Mu and plasmid DNA, resulting in a net deletion of about 100 bp from 18.05 to 18.15 kb (Table 1).
TABLE 1.
Bacterial strains, plasmids, phages, and lysogens used in this study
| Phage, plasmid, strain, or lysogen |
Characteristics |
|---|---|
| Phages | |
| Mu cts62 | |
| D108 cts10 | |
| Plasmids | |
| pMP1812 | pBR322 Δ(3.2–4.3 kb); + Mu BamHI-EcoRI (17.2–23.7 kb) at BamHI site; Δ100 (Mu Δ18.05–18.15 kb); Knr cassette at site of Δ100 deletion (see reference 19 for details). |
| pMP1852 | As pMP1812, except Apr cassette at site of Δ100 deletion |
| pMP1856 | As pMP1812, except Apr cassette at BamHI site at Mu-pBR322 junction. |
| Bacterial strains | |
| AB1157 | |
| DH5α | |
| Lysogens | |
| X20 | AB1157 recB recC sbcB malF::Mu cts62 |
| MP1928 | X20 Δ100 (Mu Δ18.05–18.15 kb) + Sac Kn insert |
| MP1998 | MP1928 − Sac Kn insert |
| MP2013 | MP1928 − Sac Kn insert + Mu SGS insert (+ orientation) |
| MP2015 | MP1928 − Sac Kn insert + Mu SGS insert (− orientation) |
| MP2014 | MP1928 − Sac Kn insert + D108 insert |
| MP1983 | MP1928 − Sac Kn insert + pSC101 insert |
| MP1987 | MP1928 − Sac Kn insert + pBR322 insert |
| MP1996 | MP1928 − Sac Kn insert + nrd BIME insert |
| MP2001 | MP1928 − Sac Kn insert + asl BIME insert |
| MP2033 | X20 Δ200 (Mu Δ17.95–18.15 kb) + Kn insert |
Construction of Δ100 and Δ200 prophages.
A 3.5-kb DNA fragment containing the sacBR genes and a Knr cassette, derived from pSUP104-sac (29), was cloned into the BglII site in pMP1856. The resulting plasmid was linearized and transformed into strain X20 (W3110 recB recC sbcB malF::Mu cts62). A Knr recombinant was selected which had recombined the Δ100 deletion and the sac Knr fragment into the resident prophage to generate strain MP1928. Then to construct prophages containing DNA fragments to be tested for their ability to replace the SGS, the fragments were cloned into the BglII site on pMP1856, and the resulting plasmids were linearized and transformed into MP1928. Recombinants which had replaced the sac Knr fragment in the prophage with the desired DNA fragment were selected for sucrose resistance on L-agar plates with no salt and with 5% sucrose and screened for kanamycin sensitivity, and the substitution with the desired fragment was verified by restriction analysis and by PCR.
To construct a prophage that lacks the SGS and is G− (the original 147-bp deletion removed the terminal seven amino acids of G and was slightly leaky), a larger deletion was created (BglI-ScaI; bp 17.95 to 18.15) which removed approximately 200 bp; this prophage is referred to as the Δ200 prophage and is in the lysogen MP2033.
Construction of chromosomal libraries in pMP1856.
Total E. coli chromosomal DNA was isolated by phenol extraction and fragmented by complete or partial cleavage with Sau3A or partial digestion with DNase I in the presence of 10 mM MnCl2. The DNA was size fractionated on 1.2% agarose gels, and fragments in the range of 0.5 to 1.5 kb were isolated (use of larger inserts could have resulted in loss of the right end of the genomes containing the inserts due to the “headful” packaging mechanism of Mu DNA). Sau3A-generated fragments were cloned directly into the BglII site of pMP1856 after calf intestinal phosphatase treatment of the digested plasmid DNA. To assess the efficiency of the cloning procedure, plasmid DNAs from 10 independent Apr transformants were isolated and checked for insertions; greater than 80% contained insertions of the expected sizes. The ends of DNase I-generated fragments were filled in with T4 DNA polymerase, BamHI linkers were added, and the fragments were cloned into the BglII site of pMP1856.
Growth, lysis, and replication.
Cultures of lysogens were grown in L broth at 30°C to a density of about 108 cells/ml, diluted threefold with L broth, and induced by transferring the culture to 42°C. In the absence of dilution, some cultures reached densities which were sufficiently high to delay lysis beyond the times seen at lower densities. Culture density was determined with Klett readings to monitor growth and lysis.
Replication was monitored by a semiquantitative PCR procedure. Two hundred microliters of induced cultures was added to tubes with 0.04 g of Chelex and 2 μl of 0.1 M EDTA and boiled for 10 min. Ten microliters or less of the extracts (the volume of extract used was corrected for the amount of growth during the sampling regime) was used in PCRs designed to yield results in a linear range. Pairs of PCR primers were used to simultaneously amplify a fragment of Mu DNA and a fragment of chromosomal DNA (from a gene adjacent to the prophage at malF). The Mu primers 5′-TGATGAGGGTACACTTGCTGG and 5′-GCACAGATGCTGTAATGGTCG yield a 335-bp product from the Mu B gene. The chromosomal primers 5′-TTAAGCCATCTCCTGATGACG and 5′-TTTCTGCTACTGACAGGTGGG yield a 364-bp fragment produced from the malK gene. Following 20 cycles of amplification, the amplified DNA fragments were separated on 2% agarose gels, stained with ethidium bromide, and quantitated with NIH Image software v. 155. Control experiments performed by mixing different ratios of the two products showed that linearity was observed up to a ratio of about 5 to 1. The ratio of Mu to mal is close to 1.0 in uninduced cells and increases after the onset of Mu DNA replication.
Gyrase cleavage in vitro and in vivo.
In vitro gyrase cleavage assays were carried out as described by Pato et al. (24) on plasmid DNA linearized with PvuII digestion. In vivo cleavage assays were modified from the method of Scheirer and Higgins (28). A relevant lysogen was grown in L broth at 30°C to a Klett density of 50, and enoxacin was added to 300 μg/ml. After 5 min of incubation, 5 ml of culture was rapidly lysed by transfer to a tube at 80°C containing 0.5 ml of L broth plus 2.5% sodium dodecyl sulfate (SDS), and incubation was continued for 15 min. The sample was cooled to room temperature, proteinase K was added to 20 μg/ml, and incubation was continued for 1 h at 65°C. DNA was purified by phenol-chloroform extractions and ethanol precipitation and was digested with the appropriate restriction enzyme. A single oligonucleotide primer, complementary to a sequence chosen so that the gyrase site is between the primer site and the restriction site, was 5′ end labeled with 32P and used for 30 cycles of one-directional PCR. The extension products terminated either at the cleaved gyrase site (for templates cleaved by gyrase in vivo) or at the cleaved restriction site (for templates not cleaved by gyrase). The resulting labelled fragments were separated on a sequencing gel and quantitated with a phosphorimager.
RESULTS
Replacing the SGS.
To replace the SGS in the center of a Mu prophage with DNA fragments from various sources, two procedures were developed—one to insert specific, known fragments and the other to insert random DNA fragments from cloned libraries.
In the first procedure, the DNA fragments to be tested were cloned into a central fragment of Mu DNA from which the SGS was deleted and then recombined into a prophage inserted at a unique site in the E. coli chromosome. For this procedure, a plasmid was constructed which carries a central fragment of the Mu genome from BamHI to EcoRI (17.2 to 23.7 kb). Approximately 100 bp was deleted from the fragment, removing the SGS, and replaced with an oligonucleotide introducing a BglII site (pMP1856; see Materials and Methods). Selected DNA fragments were then cloned into the BglII site. The plasmid was linearized and transformed into a recB recC sbcB lysogen carrying a Mu prophage with the Δ100 deletion and a 3.5-kb fragment containing the genes for sucrose sensitivity and kanamycin resistance (sac Knr) at the site of the deletion (MP1928). A recombinant was then selected which replaced the sac Knr genes with the fragment carried on the plasmid. The structure of the resulting recombinant was verified by PCR analysis and, when desired, sequencing. The resulting prophages are all at the same chromosomal site (malF) and do not contain drug-resistant gene cassettes (which were present in some earlier constructs).
To test the system, a 147-bp fragment containing the Mu SGS (MluI-ScaI; 18.0 to 18.15 kb) was cloned into the BglII site of pMP1856 in both orientations and recombined into the prophage in MP1928. Also, pMP1856 without an insert was used, resulting in a prophage with only the Δ100 deletion. The lysogens were grown at 30°C and then induced by shifting to 42°C, and growth of the culture and Mu DNA replication were monitored (Fig. 1). The lysis times for lysogens with the Mu inserts in either orientation were essentially identical to that for wild-type Mu, as were the kinetics of Mu DNA replication. The lysogen carrying a prophage lacking the SGS showed long delays before lysis and the onset of Mu DNA replication. A strict correlation between the kinetics of lysis and Mu DNA replication has been observed in all of our work with modified Mu prophages (with obvious exceptions, such as prophages deleted for the lys genes). For rapid screening of many prophage constructions, lysis curves alone were used, because they have the advantage of ease of performance and less experimental fluctuation.
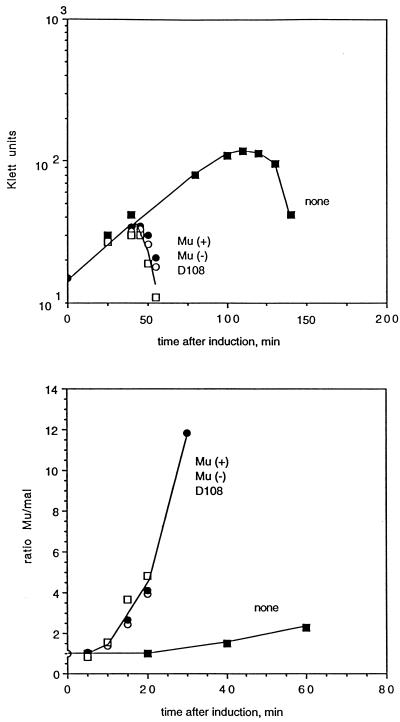
FIG. 1.
Lysis and replication without a central gyrase site (■), with the Mu SGS in the positive (○) or negative (●) orientation, or with the D108 gyrase site (□). Cultures of lysogens were grown in L broth at 30°C to a density of about 108 cells/ml, diluted threefold in L broth, and then induced by shifting to 42°C. Growth was monitored by Klett readings, and samples were taken for quantitative PCR analysis to measure DNA replication. Replication is expressed as the ratio of the amount of an amplified Mu DNA fragment to the amount of a chromosomal malF fragment. (Top panel) Growth and lysis of the cultures. (Bottom panel) Mu DNA replication.
Bacteriophage D108.
The DNA of the transposing bacteriophage D108, a close relative of Mu (6), was examined, because it seemed likely that D108 would have a functional central gyrase site. Initially, a 1.4-kb central fragment of D108 (BamHI-ClaI; corresponding to Mu 17.2 to 18.4 kb) was cloned into pBR322, and about 250 bp of sequence were obtained in the region corresponding to the Mu SGS. The sequence (Fig. 2) was identical to that of Mu, except for 4 base changes, 3 of which were in or near the presumed gyrase site. The MluI and ScaI sites used for cloning the Mu SGS are also present in D108, and hence the corresponding 147-bp fragment from D108 was cloned into pMP1856 and recombined into the Mu prophage in MP1928. Induction of the lysogen with the hybrid prophage gave essentially the same results as those obtained with wild-type Mu (Fig. 1), showing that the Mu SGS could be successfully replaced with the corresponding site from D108.
FIG. 2.
Sequence of a region of the D108 genome containing the central strong gyrase site. Mismatches with the Mu sequence are noted, and the locations of the gyrase site consensus sequence (denoted as SGS) and the restriction sites for MluI and ScaI are underlined. (The GenBank accession no. is Bank It 253889 AF 128885.)
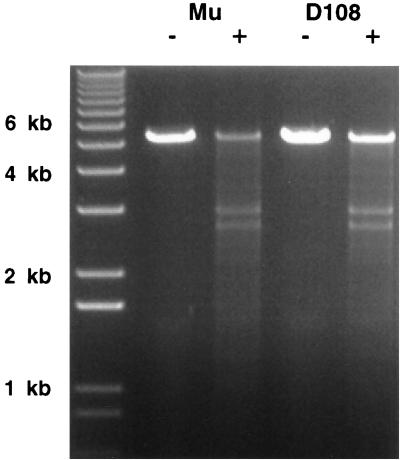
To determine if the D108 site is indeed a gyrase site, cleavage of the D108 DNA in the presence of gyrase and the quinolone enoxacin was examined. Quinolones inhibit the gyrase reaction after the enzyme creates a double-strand break in the bound DNA, and the protein is covalently linked to the cleaved DNA ends (9, 33). Hence treatment with gyrase and a quinolone followed by deproteination can reveal a double-strand break. For the analysis, the 1.4-kb central fragments from Mu and D108 were cloned into pBR322. The plasmids were linearized by restriction enzyme digestion and cleaved with gyrase in the presence of enoxacin (see Materials and Methods). After treatment with SDS and proteinase K, the resulting fragments were separated on an agarose gel, stained with ethidium bromide, and photographed. The results in Fig. 3 show that gyrase cleavage at the D108 site occurred with approximately the efficiency observed at the Mu site.
FIG. 3.
Gyrase cleavage of Mu and D108 DNA. pBR322 Δ(EcoRI-BamHI) plasmids with cloned 1.4-kb central fragments from Mu (lanes 2 and 3) and D108 (lanes 4 and 5) were linearized by PvuII restriction digestion and then incubated with DNA gyrase in the presence of enoxacin. Following treatment with SDS and proteinase K, the DNA fragments were separated on a 1% agarose gel and stained with ethidium bromide. The expected fragment sizes for cleavage at the Mu gyrase site are ∼2.9 and 2.6 kb. Lane 1, molecular size markers. −, no gyrase; +, with gyrase.
To determine the effect on D108 of deleting the gyrase site, the MluI-ScaI fragment was deleted from the 1.4-kb central fragment described above and replaced with a 1.1-kb Knr cassette, and the deletion was recombined into a D108 cts prophage. Induction of the resulting lysogen resulted in delays in lysis and D108 replication equivalent to those seen with the corresponding Mu ΔSGS prophage (data not shown).
pSC101 and pBR322 gyrase sites.
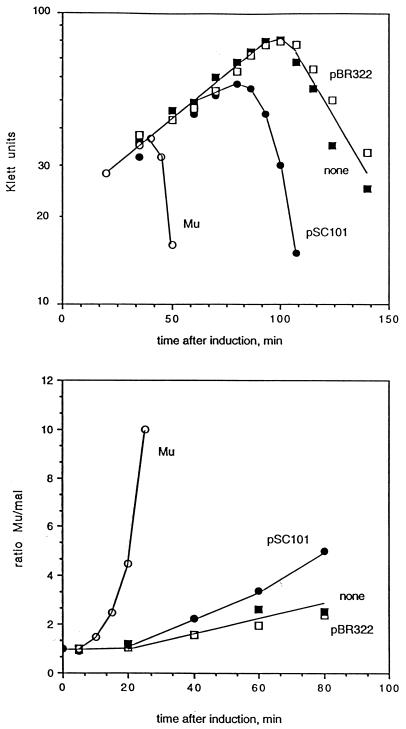
We wished to examine the effect of replacing the Mu SGS with known gyrase sites and began by selecting two well-studied sites from the plasmids pBR322 and pSC101. A large number of gyrase sites on pBR322 have been described (16), and the major site centered on base 991 has been used as substrate in much of the work with gyrase (e.g., see references 5 and 19). A strong gyrase site has been observed on pSC101, where it was originally identified as part of the par region of the plasmid (34). A 775-bp fragment of pBR322 and a 400-bp fragment of pSC101 carrying the gyrase sites were separately cloned into pMP1856 and recombined into the prophage in MP1928. Induction curves for the resulting lysogens show that neither of the fragments was capable of restoring normal replication (Fig. 4). However, the pSC101 fragment did have a measurable effect, reducing the lysis time from 110 min to about 90 min and allowing some increase in the rate of Mu replication over that seen in the absence of a gyrase site.
FIG. 4.
Lysis and replication without a central gyrase site (■), with the Mu SGS in the positive orientation (○), with the pSC101 gyrase site (●), or with the pBR322 gyrase site (□). Growth and replication were monitored as in Fig. 1. (Top panel) Growth and lysis of the cultures. (Bottom panel) Mu DNA replication.
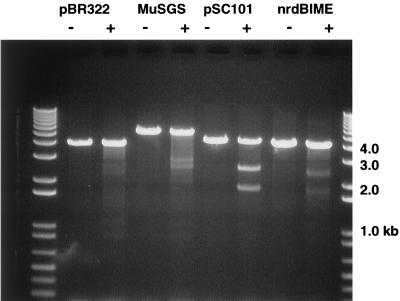
The inability of the plasmid gyrase sites to replace the Mu SGS in promoting efficient Mu replication could be due to their being weaker sites than the SGS. To assess the relative strengths of the three gyrase sites, we performed both in vitro and in vivo cleavage assays with the quinolone enoxacin. For the in vitro analysis, small fragments with the different gyrase sites were cloned into pBR322. The plasmids were linearized by restriction enzyme digestion, cleaved with gyrase in the presence of enoxacin, and processed as described above. The first three pairs of lanes in Fig. 5 show results with pBR322, without and with the Mu and pSC101 gyrase sites. Even within the context of the greater than 40 weak gyrase sites in pBR322, specific cleavages were readily observed at the Mu and pSC101 gyrase sites. The pSC101 site was cleaved more efficiently than the Mu site, and under the conditions used, no clear cleavage at the major pBR322 site was observed.
FIG. 5.
In vitro gyrase cleavage. pBR322 Δ(EcoRI-BamHI) plasmids without (lanes 2 and 3) or with cloned fragments carrying the Mu SGS (lanes 4 and 5), the pSC101 gyrase site (lanes 6 and 7), or the nrdAB BIME (lanes 8 and 9) were linearized with PvuII restriction digestion and then incubated with DNA gyrase in the presence of enoxacin. Following treatment with SDS and proteinase K, the DNA fragments were separated on a 1% agarose gel and stained with ethidium bromide. The expected fragment sizes for cleavage at the gyrase site in question were as follows: pBR322, 2.8 + 1.2 kb; Mu, 2.9 + 2.6 kb; pSC101, 2.5 + 1.9 kb; nrd BIME, 2.4 + 1.8 kb. Lanes 1 and 10, molecular size markers. −, no gyrase; +, with gyrase.
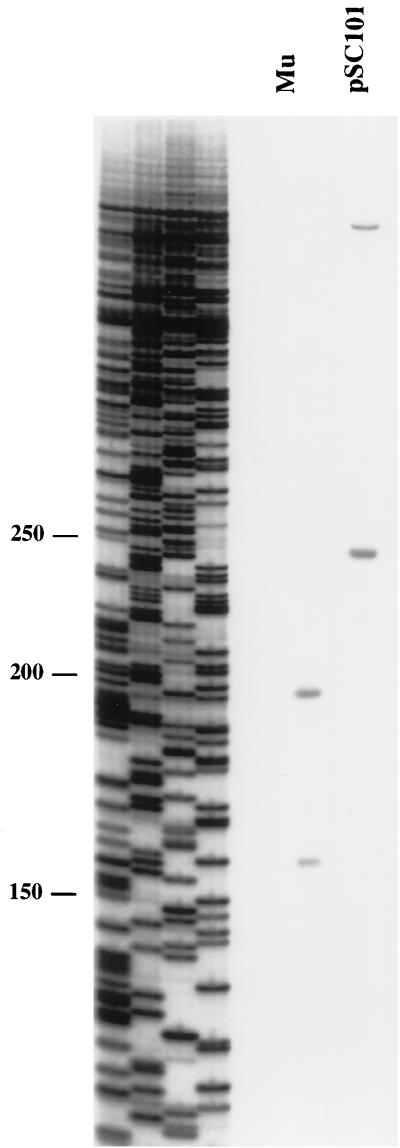
For the in vivo analysis, lysogens with different central gyrase sites were used. Exponentially growing cultures were treated with enoxacin for 5 min, followed by rapid lysis in SDS at 80°C. DNA was isolated, deproteinized with proteinase K, and cleaved with a selected restriction enzyme. One-directional PCR using a primer near each of the gyrase cleavage sites was performed. If gyrase cleavage had occurred on a template molecule, primer extension could proceed up to the gyrase site; if gyrase cleavage had not occurred, extension could proceed past the gyrase site up to the restriction site, yielding a longer fragment. The ratios of the amount of the shorter fragment to the total of the two fragments were determined and were ∼40% with the Mu gyrase site and ∼60% with the pSC101 gyrase site (Fig. 6). No clear gyrase cleavage was observed with the pBR322 site, consistent with the lack of observable cleavage in the in vitro assay. By this assay, the pSC101 gyrase site is even stronger than the Mu SGS, and the pBR322 site is very weak relative to the other two. Because the pSC101 gyrase site was unable to efficiently promote Mu replication (Fig. 4), it appears that replacing the function of the Mu SGS in Mu replication requires more than just the introduction of a strong gyrase site.
FIG. 6.
In vivo gyrase cleavage. Lysogens with prophages carrying either the Mu SGS or the pSC101 gyrase site were grown in L broth, enoxacin was added to 300 μg/ml for 5 min, and the cells were rapidly lysed in hot SDS. DNA was isolated, treated with proteinase K, and digested with the appropriate restriction enzyme. One-directional PCR with primers near the gyrase sites yielded shorter fragments from templates that were cleaved by gyrase and longer fragments from templates not cleaved by gyrase. A sequencing ladder provided size markers (base pairs).
The E. coli chromosome—BIME sites.
The E. coli nucleoid is thought to be composed of 50 to 100 independently supercoiled domains (30, 36). Since the Mu SGS can promote the organization of the Mu prophage DNA into a supercoiled domain, one wonders if there are chromosomal sites which could promote domain formation in an analogous manner. To seek chromosomal sites capable of replacing the Mu SGS, we have used two approaches. The first involved replacing the SGS with known chromosomal sites called bacterial interspersed mosaic elements (BIMEs). These elements are composed of combinations of repetitive extragenic palindrome (REP) sequences, which are scattered throughout the genome, are often associated with gyrase binding, and have been proposed as organizing elements for the nucleoid (reviewed in reference 2). Two classes of BIMEs have been defined: BIME-1 sites contain integration host factor (IHF) binding sites flanked by REPs; BIME-2 sites contain a particular pattern of REPs without an IHF binding site. We chose to examine the nrdAB BIME-2, because it contains the strongest gyrase site of the several sites examined by Espeli and Boccard (7), and the aslAB BIME-1. A 250-bp fragment containing the nrdAB BIME-2 and a 240-bp fragment containing the aslAB BIME-1 (generous gifts from F. Boccard) were separately cloned into pMP1856 and recombined into the Mu prophage in MP1928. Induction of the resulting lysogens showed that neither of the BIMEs was capable of replacing the Mu SGS in supporting efficient Mu replication, because the lysis times were essentially identical with and without the BIME insertions (data not shown).
The nrdAB BIME contained the strongest gyrase site of several BIMEs examined by Espeli and Boccard (7). In vitro gyrase cleavage was performed with pBR322 with a cloned nrdAB BIME insert and showed a degree of cleavage similar to that observed with the plasmid containing the Mu SGS insert (Fig. 5). Interestingly, no preferential cleavage was observed with the in vivo gyrase cleavage assay (data not shown).
The E. coli chromosome—random fragments.
The second procedure used to search for chromosomal sites capable of replacing the Mu SGS was to construct libraries of total E. coli DNA cloned into the BglII site in the Δ100 plasmid pMP1856 and then to recombine the random fragments into the center of a Mu genome lacking the SGS. For these experiments, strain MP2033 was constructed, which carries a Mu prophage with a deletion of approximately 200 bp, removing both the SGS and the 3′ end of the upstream G gene. The G gene product is required for proper assembly of phage heads and tails (12), and G− phage lysates do not produce plaque-forming particles. In contrast, phage with the Δ100 deletion which lack the SGS but are G+ are capable of forming pinpoint plaques on some hosts (E. coli DH5α) while incapable of forming plaques on other hosts (E. coli AB1157).
The basis of the selection is the following: recombination between the Δ200 genome and a Δ100 fragment on a plasmid yields a Δ100 genome which can at least give rise to a pinpoint plaque on DH5α. If an E. coli fragment capable of substituting for the Mu SGS has been recombined into the center of the phage genome, then the recombinant phage could form a plaque on an AB1157 host. Hence, the plasmid libraries were transformed into the Mu Δ200 lysogen, large numbers of individual transformants were pooled, and the pooled culture was induced for Mu growth. Lysates collected several hours after induction were plated on the appropriate indicator strains, and plaques were counted.
To test the system, a size-fractionated library of Mu DNA fragments, approximately 1 kb in size, was cloned in pMP1856 and electroporated into the Δ200 lysogen. Colonies of plasmid-containing (ampicillin-resistant) transformants were scrapped from plates and pooled. Cultures of the pooled cells were induced, and lysates collected after ∼3 h were plated in top agar on the following different indicators: a, DH5α, to determine the number of G+ recombinants; b, DH5α carrying a plasmid supplying G protein in trans (pMP1852), to determine the total number of phage in the lysate; and c, AB1157, to determine the number of recombinants carrying the SGS or SGS substitute (“Jackpots”).
The numbers of pinpoint plaques on indicators a and b allowed estimates of approximately 10 phage produced per induced cell after 150 min, of which about 1/104 was a G+ recombinant. Approximately 1% of these recombinants gave normal-size plaques on both DH5α and AB1157. PCR and restriction digestion analyses of the DNA of these “Jackpot” phage revealed inserts corresponding to the central, SGS-containing fragment. No fragments from other portions of the genome were observed in Jackpot phage. These results demonstrated that the system could be used in a search for sites capable of replacing the SGS in Mu replication.
Libraries of E. coli DNA were constructed in pMP1856 by several techniques, including Sau3AI partial and complete digests and digestion with DNase in the presence of Mn2+. In numerous independent experiments, 0.5- to 1.0-kb fragments were cloned into the BglII site of the Δ100 plasmid and electroporated into the Δ200 lysogen. The plasmids from numerous independent transformants were analyzed, and most or all plasmids were found to contain inserts. Colonies of cells containing the plasmid were pooled—generally about 5,000 colonies per pool and 20,000 colonies total in each experiment. The pooled cells were grown to ∼108 cells/ml and induced, and sufficient amounts of lysates were plated on AB1157 to contain at least 50,000 G+ recombinants. An experiment using E. coli chromosomal DNA from a Mu lysogen revealed the presence of a small number of Jackpot phage which were shown to carry the Mu SGS, but no Jackpot phage were found with E. coli DNA from the nonlysogen. Jackpot phage were observed with lysogen DNA at a frequency of approximately 1/104 G+ recombinants, and greater than 105 G+ recombinants were processed with nonlysogen DNA; hence the selection technique would likely have detected chromosomal sites capable of substituting for the SGS if they were present. The failure to find such sites emphasizes the unusual properties of the Mu SGS.
DISCUSSION
Previous studies in this laboratory have demonstrated that there is a site in the center of the Mu genome that is required for efficient replicative transposition. The site was shown to contain a strong gyrase site, which may be both necessary and sufficient to fulfill the role of the SGS in promoting Mu replication. To test whether other sites, in particular other gyrase sites, could replace the Mu SGS in promoting Mu replication, we replaced the SGS with known gyrase sites or with random fragments of the E. coli chromosome and evaluated the effects on Mu replication. A central fragment from the related transposing bacteriophage D108 successfully replaced the Mu SGS in promoting Mu replication, and we demonstrated that the D108 site is indeed a strong gyrase site comparable to the Mu SGS.
The gyrase site from the plasmid pSC101 was found to be a stronger site than the Mu SGS, as measured by cleavage in the presence of gyrase and the quinolone enoxacin, both in vitro and in vivo. However, it was not able to promote efficient Mu replication, ruling out the hypothesis that any strong gyrase site can replace the Mu SGS. A slight increase in the level of Mu replication was observed with the pSC101 site over that seen in the absence of any central site. The major gyrase site from pBR322 was much weaker than the Mu or pSC101 sites in the cleavage assay and showed no ability to promote Mu replication. We found that the E. coli chromosome has no sites which can replace the Mu SGS in promoting efficient replication, even though sites such as the nrdAB BIME exhibit activity in the cleavage assay comparable to that seen with the Mu SGS.
It may be useful to review the evidence that a gyrase site is required for efficient Mu replication. (Please note that the requirement for the central site is not an absolute one; replication in the absence of the site does occur, but is delayed by approximately an hour.)
(i) A strong gyrase site is present, as defined by the quinolone cleavage assay (24). (ii) Deletion of 100 bp of noncoding sequence at the site inhibits replication (24). (iii) Insertion of a short oligonucleotide linker at the gyrase cleavage site inhibits replication (25). (iv) Replication of Mu is inhibited in gyrase mutants, and two mutants of Mu (nuB) which were selected for their ability to grow on a gyrase mutant (39) contain single base changes in the central gyrase site which increase the strength of the site, as measured by the quinolone cleavage assay (24). More recently isolated nuB mutants contain the same mutation as one of the original mutants (3). (v) Replacement of the SGS with the strong pSC101 site did allow a discernible improvement of Mu replication over that observed in the absence of any central site, although considerably less than normal replication (this work).
It is therefore likely that a central gyrase site is required for efficient Mu replication. However, the inability of strong sites such as the pSC101 or nrdAB BIME sites to substitute for the Mu SGS suggests that some additional property of the SGS is required.
The structures of a few gyrase sites have been probed with footprinting techniques, and a model has evolved in which about 120 to 140 bp of DNA is wrapped around the enzyme (reviewed in reference 26). A central core of DNA, estimated to be about 30 to 40 bp with DNase I (8, 14, 18) or about 13 bp with hydroxyl radicals (20), is strongly protected in the footprinting analyses and is thought to be bound at the active site of the enzyme. This core roughly corresponds to the 20-bp consensus sequence derived from a large number of weak gyrase sites on pBR322 (16) and includes the actual cleavage sites. Flanking the core are two regions, or arms, which show enhanced sensitivity in the footprinting analyses at intervals of 10 to 11 bp, suggesting that these arms are wrapped around the enzyme (8, 14, 18, 20). As with nucleosomal DNA, the arms may require inherent flexibility rather than specific DNA sequences.
Mutation and deletion analyses of the Mu SGS and the pSC101 gyrase site are in progress and should allow us to determine whether the unusual properties of the SGS are due to specific regions of the gyrase site or, for example, are due to the presence of a second site. Various possibilities can be envisioned, such as the presence of a site responsible for increased processivity of gyrase action at the SGS. Completion of these studies will hopefully provide an understanding of the unusual properties of the central gyrase sites of the transposing phages.
One of the reasons for undertaking the present studies was the hope of learning something about the structure of bacterial nucleoids. Although the model for the structure of the E. coli nucleoid was proposed over 20 years ago, we still do not know if the structure is a static or a dynamic one, in the sense of whether the independently supercoiled loops are fixed structures or ones that are constantly in flux. Experiments such as those of Higgins and coworkers (11, 32) point toward an interpretation of a dynamic structure. The failure to find chromosomal sites which can replace the Mu SGS perhaps can best be understood in terms of a dynamic structure for the nucleoid. That is, the purpose of the Mu SGS is to promote the formation of a fixed structure—a single domain containing the Mu prophage with its termini synapsed at the base of a loop by a transposase tetramer. Analogous chromosomal sites might restrict the movement of the DNA within the nucleoid. If the nucleoid is a dynamic structure, with alternative plectonemically interwound loops continually being formed and dissipated, then it is still possible that weaker gyrase sites are involved in initiating the formation of the alternative domainal loops.
ACKNOWLEDGMENTS
This work was supported by NSF grant MCB-9727991.
We thank Katherine Scheirer and Pat Higgins for assistance with the in vivo gyrase cleavage assay and Frederich Boccard for the BIME clones.
REFERENCES
- 1.Aldaz H, Schuster E, Baker T A. The interwoven architecture of the Mu transposase couples DNA synapsis to catalysis. Cell. 1996;85:257–269. doi: 10.1016/s0092-8674(00)81102-2. [DOI] [PubMed] [Google Scholar]
- 2.Bachellier S, Gilsen E, Hofnung M, Hill C W. Repeated sequences. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznickoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C: American Society for Microbiology; 1996. pp. 2012–2040. [Google Scholar]
- 3.Baxa C A, Chiang L, Howe M M. DNA sequence characterization of the G gene region of bacteriophage Mu. J DNA Sequencing Mapping. 1992;2:329–333. doi: 10.3109/10425179209030967. [DOI] [PubMed] [Google Scholar]
- 4.Condamine G, Smith C L. Transcription regulates oxolinic acid-induced DNA gyrase cleavage at specific sites on the E. coli chromosome. Nucleic Acids Res. 1990;18:7389–7396. doi: 10.1093/nar/18.24.7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cove E C, Tingey A P, Maxwell A. DNA gyrase can cleave short DNA fragments in the presence of quinolone drugs. Nucleic Acids Res. 1997;25:2716–2722. doi: 10.1093/nar/25.14.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DuBow M. Transposable Mu-like phages. In: Symonds N, Toussaint A, van de Putte P, Howe M M, editors. Phage Mu. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1987. pp. 201–214. [Google Scholar]
- 7.Espeli O, Boccard F. In vivo cleavage of Escherichia coli BIME-2 repeats by DNA gyrase: genetic characterization of the target and identification of the cut site. Mol Microbiol. 1997;26:767–777. doi: 10.1046/j.1365-2958.1997.6121983.x. [DOI] [PubMed] [Google Scholar]
- 8.Fisher L M, Mizuuchi K, O’Day M H, Ohmori H, Gellert M. Site-specific interactions of DNA gyrase with DNA. Proc Natl Acad Sci USA. 1981;78:4165–4169. doi: 10.1073/pnas.78.7.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gellert M, Mizuuchi K, O’Day M, Itoh T, Tomizawa J. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci USA. 1977;74:4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harshey R. Phage Mu. In: Calender R, editor. The bacteriophages. New York, N.Y: Plenum Press; 1988. pp. 193–224. [Google Scholar]
- 11.Higgins N P, Yang X, Fu Q, Roth J R. Surveying a supercoil domain by using the γδ resolution system in Salmonella typhimurium. J Bacteriol. 1996;178:2825–2835. doi: 10.1128/jb.178.10.2825-2835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howe M. Late genes, particle morphogenesis, and DNA packaging. In: Symonds N, Toussaint A, van de Putte P, Howe M M, editors. Phage Mu. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1987. pp. 63–74. [Google Scholar]
- 13.Kavenoff R, Ryder O. Electron microscopy of membrane-associated folded chromosomes of Escherichia coli. Chromosoma. 1976;55:13–23. doi: 10.1007/BF00288323. [DOI] [PubMed] [Google Scholar]
- 14.Kirkegaard K, Wang J. Mapping the topography of DNA wrapped around gyrase by nucleolytic and chemical probing of complexes of unique DNA sequences. Cell. 1981;23:721–729. doi: 10.1016/0092-8674(81)90435-9. [DOI] [PubMed] [Google Scholar]
- 15.Lavoie B D, Chaconas G. Transposition of phage Mu DNA. Curr Topics Microbiol Immunol. 1995;204:83–99. doi: 10.1007/978-3-642-79795-8_4. [DOI] [PubMed] [Google Scholar]
- 16.Lockshon D, Morris D R. Sites of reaction of Escherichia coli DNA gyrase on pBR322 in vivo as revealed by oxolinic acid-induced plasmid linearization. J Mol Biol. 1985;181:63–74. doi: 10.1016/0022-2836(85)90324-9. [DOI] [PubMed] [Google Scholar]
- 17.Mizuuchi K. Transpositional recombination: mechanistic insights from studies of Mu and other elements. Annu Rev Biochem. 1992;60:1011–1051. doi: 10.1146/annurev.bi.61.070192.005051. [DOI] [PubMed] [Google Scholar]
- 18.Morrison A, Cozzarelli N R. Contacts between DNA gyrase and its binding site on DNA: features of symmetry and asymmetry revealed by protection from nucleases. Proc Natl Acad Sci USA. 1981;78:1416–1420. doi: 10.1073/pnas.78.3.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison A, Higgins N P, Cozzarelli N C. Interaction between DNA gyrase and its cleavage site on DNA. J Biol Chem. 1980;255:2211–2219. [PubMed] [Google Scholar]
- 20.Orphanides G, Maxwell A. Evidence for a conformational change in the DNA gyrase-DNA complex from hydroxyl radical footprinting. Nucleic Acids Res. 1994;22:1567–1575. doi: 10.1093/nar/22.9.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pato M L. Bacteriophage Mu. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C: American Society for Microbiology; 1989. pp. 23–52. [Google Scholar]
- 22.Pato M L. Central location of the Mu strong gyrase binding site is obligatory for optimal rates of replicative transposition. Proc Natl Acad Sci USA. 1994;91:7056–7060. doi: 10.1073/pnas.91.15.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pato M L, Banerjee M. The Mu strong gyrase-binding site promotes efficient synapsis of the prophage termini. Mol Microbiol. 1996;22:283–292. doi: 10.1046/j.1365-2958.1996.00115.x. [DOI] [PubMed] [Google Scholar]
- 24.Pato M L, Howe M M, Higgins N P. A DNA gyrase-binding site at the center of the bacteriophage Mu genome is required for efficient replicative transposition. Proc Natl Acad Sci USA. 1990;87:8716–8720. doi: 10.1073/pnas.87.22.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pato M L, Karlok M, Wall C, Higgins N P. Characterization of Mu prophage lacking the central strong gyrase binding site: localization of the block in replication. J Bacteriol. 1995;177:5937–5942. doi: 10.1128/jb.177.20.5937-5942.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reece R J, Maxwell A. DNA gyrase: structure and function. Crit Rev Biochem Mol Biol. 1991;26:335–375. doi: 10.3109/10409239109114072. [DOI] [PubMed] [Google Scholar]
- 27.Savilahti H, Mizuuchi K. Mu transpositional recombination: donor DNA cleavage and strand transfer in trans by the Mu transposase. Cell. 1996;85:271–280. doi: 10.1016/s0092-8674(00)81103-4. [DOI] [PubMed] [Google Scholar]
- 28.Scheirer K E, Higgins N P. The DNA cleavage reaction of DNA gyrase: comparison of stable ternary complexes formed with enoxacin and CcdB protein. J Biol Chem. 1997;272:27202–27209. doi: 10.1074/jbc.272.43.27202. [DOI] [PubMed] [Google Scholar]
- 29.Simon R, Hötte B, Klauke B, Kosier B. Isolation and characterization of insertion sequence elements from gram-negative bacteria by using new broad-host-range positive selection vectors. J Bacteriol. 1991;173:1502–1508. doi: 10.1128/jb.173.4.1502-1508.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinden R R, Pettijohn D. Chromosomes in living E. coli cells are segregated into domains of supercoiling. Proc Natl Acad Sci USA. 1981;78:223–228. doi: 10.1073/pnas.78.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder M, Drlica K. DNA gyrase on the bacterial chromosome: DNA cleavage induced by oxolinic acid. J Mol Biol. 1979;131:287–302. doi: 10.1016/0022-2836(79)90077-9. [DOI] [PubMed] [Google Scholar]
- 32.Staczek P, Higgins N P. Gyrase and Topo IV modulate chromosome domain size in vivo. Mol Microbiol. 1998;29:1435–1446. doi: 10.1046/j.1365-2958.1998.01025.x. [DOI] [PubMed] [Google Scholar]
- 33.Sugino A, Peebles C L, Kreuzer K N, Cozzarelli N R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci USA. 1977;74:4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wahle E, Kornberg A. The partition locus of plasmid pSC101 is a specific binding site for DNA gyrase. EMBO J. 1988;7:1889–1895. doi: 10.1002/j.1460-2075.1988.tb03022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson M A, Chaconas G. Three-site synapsis during Mu DNA transposition: a critical intermediate preceding engagement of the active site. Cell. 1996;85:435–445. doi: 10.1016/s0092-8674(00)81121-6. [DOI] [PubMed] [Google Scholar]
- 36.Worcel A, Burgi E. On the structure of the chromosome of Escherichia coli. J Mol Biol. 1972;71:127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- 37.Yang J-Y, Jayaram M, Harshey R. Positional information within the Mu transposase tetramer: catalytic contributions of individual monomers. Cell. 1996;85:447–455. doi: 10.1016/s0092-8674(00)81122-8. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Ames G F-L. DNA gyrase binds to the family of prokaryotic repetitive extragenic palindromic sequences. Proc Natl Acad Sci USA. 1988;85:8850–8854. doi: 10.1073/pnas.85.23.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida R K, Miller J K, Miller H I, Friedman D L, Howe M M. Isolation and mapping of Mu nu mutants which grow in him mutants of E. coli. Virology. 1982;120:269–272. doi: 10.1016/0042-6822(82)90027-7. [DOI] [PubMed] [Google Scholar]