Abstract
Diaporthe species are endophytes, pathogens, and saprobes with a wide host range worldwide. However, little is known about endophytic Diaporthe species associated with Morinda officinalis. In the present study, 48 endophytic Diaporthe isolates were obtained from cultivated M. officinalis in Deqing, Guangdong Province, China. The nuclear ribosomal internal transcribed spacer (ITS), partial sequences of translation elongation factor 1-α (tef1-α), partial calmodulin (cal), histone H3 (his), and Beta-tubulin (β-tubulin) gene regions were sequenced and employed to construct phylogenetic trees. Based on morphology and combined multigene phylogeny, 12 Diaporthe species were identified, including five new species of Diaporthe longiconidialis, D. megabiguttulata, D. morindendophytica, D. morindae, and D. zhaoqingensis. This is the first report of Diaporthe chongqingensis, D. guangxiensis, D. heliconiae, D. siamensis, D. unshiuensis, and D. xishuangbanica on M. officinalis. This study provides the first intensive study of endophytic Diaporthe species on M. officinalis in China. These results will improve the current knowledge of Diaporthe species associated with this traditional medicinal plant. Furthermore, results from this study will help to understand the potential pathogens and biocontrol agents from M. officinalis and to develop a disease management platform.
Keywords: new species, new host records, Diaporthales, phylogeny, Chinese traditional medicinal plants, taxonomy
1. Introduction
Diaporthe Nitschke (1870, (syn. Phomopsis (Sacc.) Bubák), (Diaporthaceae) [1] includes important plant pathogens, endophytes, and saprobes [2]. Species identification in this genus is based on DNA-based phylogenetic approaches and incorporating sequences from type and voucher specimens to investigate species boundaries. In addition, the incorporation of morphological characters provided a more accurate identification [2]. Diaporthe is identified as a cryptic genus and thus morphology alone with phylogeny is challenging to delineate species [3]. Thus, some studies have shown the importance of incorporating additional approaches such as Genealogical Concordance Phylogenetic Species Recognition (GCPSR) and recombination analysis of incorrect species identification in this genus [3]. Even though polyphasic taxonomy has been recently employed for Diaporthe systematics, like many fungi, there are no consistent criteria for delineating species.
Diaporthe species are widely distributed worldwide and have diverse host associations such as forest trees, vegetables, fruits, and ornamental plants [2,4]. They are compliant and occur as pathogens, endophytes, and saprobes in a wide range of hosts [4]. As plant pathogens, Diaporthe species can cause leaf spots, blights, dieback, scab, decay, stem-end rots, trunk, or even wilt diseases including many economically important plants such as Blueberry [5], Camellia [6], Citrus [7], Coffea [8], Helianthus [9], Lithocarpus [8], Pyrus [10], Senna [11], and Vitis [12]. In addition, Diaporthe species are often reported as endophytes [13,14] that live inside the healthy host tissues, without showing any physical symptoms. They were reported as one of the most isolated and dominant endophytic genera, together with Colletotrichum and Pestaloid-like, while contributing to the hidden diversity of fungi. [15]. Promputtha et al. [16] isolated 24 Diaporthe (as Phomopsis) species from 31 morphospecies of sterile Magnolia liliflora (Magnoliaceae) endophytes. Huang et al. [17] found Diaporthe also as one of the dominant endophytes on Allamanda catharitica, Alstonia scholaris, and other 29 traditional Chinese medicinal plants.
Morinda officinalis F.C. How. (Rutaceae) is a lianoid shrub, which is wildly cultivated in subtropical and tropical areas including China and Vietnam [18,19,20]. In China, this species is cultivated in Guangdong, Guangxi, Fujian, and Hainan Provinces as a traditional medicinal plant. The roots of these plants have been used as tonic or nutrient supplements to treat impotence, osteoporosis, fatigue, rheumatoid arthritis, infertility, and depression [18]. Up until today, several fungal diseases have been reported from M. officinalis in China. The black root rot disease caused by Lasiodiplodia pseudotheobromae in China [19] and Fusarium root rot caused by Fusarium proliferatum in Vietnam [20] are mostly reported diseases. However, there are no studies on endophytes associated with M. officinalis.
Following these facts, the objectives of this study were to isolate endophytic Diaporthe species associated with healthy M. officinalis collected in Deqing city Guangdong province China and to identify species based on multi-locus DNA sequence data. In total, 12 Diaporthe species were identified from 48 isolates including five novel species. Species identification and characterisation were performed based on morphology, molecular phylogeny, and pairwise homoplasy index (PHI) analysis. For all identified fungal taxa species, descriptions and illustrations are provided.
2. Materials and Methods
2.1. Sampling and Isolation of Endophytic Fungi
Healthy M. officinalis leaves, stems, and roots were collected from Deqin city Guangdong province in China (Figure 1). From the collecting site, 10 plants were selected, and from each tree, 10 samples were collected. The collected samples were taken to the laboratory, and endophytic strains were isolated. The samples were washed using running tap water and then sterile water. After that, the samples were cut into 3 × 3 mm segments. Each cutting was surface sterilized in 75% ethanol for 40 s (leaves for 40 s, stems for 60 s, and roots for 90 s), 2.5% sodium hypochlorite for 40 s (leaves for 40 s, stems for 60 s, roots for 70 s), sterile water three times, and then dried on sterile filter paper. The samples were cultured separately on Potato Dextrose Agar (PDA), 2% PDA, Lignocellulose Agar (LCA), Maltose yeast powder medium (M + J), Martin Agar Medium (MD), and P + J medium (100 g healthy and fresh M. officinalis tissues boiled for 20 min, filtered with gauze, and fixed the volume to 200 mL. Add 10% volume fraction of decocting juice to PDA medium) plates and incubated at 25 °C following Zhang et al. [21]. Fungi growing at the edges of the tissue were transferred to a new PDA plate. Pure cultures were obtained by following the single mycelium isolation three times [14]. All cultures obtained in this study are deposited in the culture collection of Zhongkai University of Agriculture and Engineering (ZHKUCC). Herbarium materials (as dry cultures) were deposited at Zhongkai University of Agriculture and Engineering (ZHKU).
Figure 1.
Morinda officinalis F.C. How. (Rutaceae) (a). The whole tree. (b). The root of the tree. (c). The stem of the tree. (d). The leaf of the tree.
2.2. DNA Extraction and PCR Amplification
For DNA extraction, mycelia were scraped from about seven-day-old cultures on PDA. Total genomic DNA was extracted using the MagPure Plant DNA AS Kit (D6351-AS-06, Guangzhou Magen Biotechnology Co., Ltd., Guangzhou, China) using an Acid purification system (Auto-Pure 32A, Allsheng instruments Co., Ltd., Hangzhou, China). For preliminary species confirmation, the ITS gene region was amplified using the ITS1/ITS4 [22]. The sequences were aligned in GenBank by using the BLAST tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 1 July 2021). Then, four additional gene regions of tef-1α [22], cal [23], β-tubulin [24], and his [24,25] were amplified under each PCR conditions (Table 1) and sequenced. PCR amplicons were visualized on 1% agarose electrophoresis gel. The sequencing was performed by Tianyi Huiyuan Biotechnology Co., Ltd., Guangzhou, China. Initial sequence quality was checked using BioEdit 7.25 [26]. The sequence data generated in this study have been deposited in GenBank (Supplementary Table S1).
Table 1.
Gene regions, respective primer pairs, processes, and references used in the study.
| Gene Region | Primers | Optimized PCR Protocols | References |
|---|---|---|---|
| ITS | ITS1 | (94 °C: 30 s, 55 °C: 50 s, 72 °C: 1 min) × 35 cycles | [22] |
| ITS4 | |||
| tef1-α | EF1-728F | (95 °C: 30 s, 58 °C: 30 s, 72 °C: 1 min) × 35 cycles | [23] |
| EF1-986R | |||
| cal | CAL-228F | (95 °C: 30 s, 55 °C: 50 s, 72 °C: 1 min) × 35 cycles | [23] |
| CAL-737R | |||
| his | CYLH4F | (95 °C: 30 s, 58 °C: 30 s, 72 °C: 1 min) × 35 cycles | [24] |
| H3-1b | [25] | ||
| β-tubulin | BT2a | (94 °C: 30 s, 58 °C: 50 s, 72 °C: 1 min) × 35 cycles | [24] |
| Bt2b |
2.3. Phylogenetic Analysis
For the phylogenetic analysis, sequences of reference Diaporthe species and related taxa were downloaded from NCBI GenBank following [14,27] (Supplementary Table S1). Downloaded sequences were aligned together with the sequences obtained in the present study using MAFFT version 7 (http://www.ebi.ac.uk/Tools/msa/mafft/, accessed on 20 January 2022) [28] and adjusted manually using BioEdit 7.25 [26] if necessary. Combined sequence data set of ITS, tef1-α, cal, his, and β-tubulin was used following Guarnaccia et al. [9] and Yang et al. [27]. Phylogenetic relationships were inferred using maximum likelihood (ML) in RAxML [29] and Bayesian analyses (BI) in MrBayes (v. 3.0b4) [30].
The evolutionary models for each locus used in Bayesian analysis were selected using MrModeltest v. 2.3 [31]. The ML analyses were accomplished using RAxML-HPC2 on XSEDE (8.2.8) [32] in the CIPRES Science Gateway platform [33] using the GTR + I +G model of evolution with 1000 non-parametric bootstrap iterations. Bayesian analysis was performed in MrBayes v. 3.0b4 [30] for the portioned data set. The posterior probabilities (PPs) were determined by Markov chain Monte Carlo sampling (MCMC). Four simultaneous Markov chains were run for 20,000,000 generations, sampling the trees at every 100th generation. From the 200,000 trees obtained, the first 5000 representing the burn-in phase were discarded. The remaining 150,000 trees were used to calculate posterior probabilities (PPs) in a majority rule consensus tree.
Taxonomic novelties were submitted to the Faces of Fungi database [34] and Index Fungorum (http://www.indexfungorum.org, accessed on 24 May 2022). New species were described following Jayawardena et al. [35] and Manawasinghe et al. [36].
2.4. Morphological Characterisation
Agar plugs (5 mm diam.) were taken from the edges of actively growing cultures on PDA and transferred onto the middle of PDA plates and incubated at 25 °C equal hours of alternative dark and fluorescent light for over a month to induce sporulation [13]. Colony characters and pigmentation on PDA were recorded after 4–7 d, 15 d, and 30 d. Colony colours (upper and reverse) were described as in Rayner [37]. Cultures were examined periodically for the development of ascomata and conidiomata. Colony diameters were measured after 3–7 days. The shapes, sizes, and colours of at least 15 pycnidia were recorded with an Eclipse 80i photographic microscope (Nikon, Tokyo, Japan). Pycnidia were cut into 30 µm thin sections by a freezing sliding microtome (Bio-Key science and technology Co., LTD, LEICA CM1860, Weztlar, Germany) for photographing and measuring. Forty conidiophores, alpha conidia, and beta conidia were measured using NISElements BR 3.2, and mean sizes were calculated with their standard deviations (SDs).
2.5. Pairwise Homoplasy Index (PHI)
The PHI test was performed using SplitsTree4 v. 4.17.1, [38] to determine the recombination level within closely phylogenetically related species. The concatenated five-locus dataset (ITS + tef1-α + cal + his + β-tubulin) was used for the analyses. PHI test results (Fw) > 0.05 indicated no significant recombination within the dataset. The relationships between closely related taxa were visualized in split graphs with both the Log-Det transformation and splits decomposition options.
3. Results
3.1. Isolation
In total, 48 endophytic Diaporthe strains were obtained (eight from roots and 40 from stems). All media used in this study were able to grow Diaporthe species. From 48 isolates, 9 isolates were obtained from LCA, 4 isolates were obtained from M + J medium, 10 isolates were obtained from MD, 6 isolates were obtained from CMA, 7 isolates from PDA, 8 were obtained from 2% PDA, and 4 isolates were obtained from P + J medium.
3.2. Phylogenetic Analyses
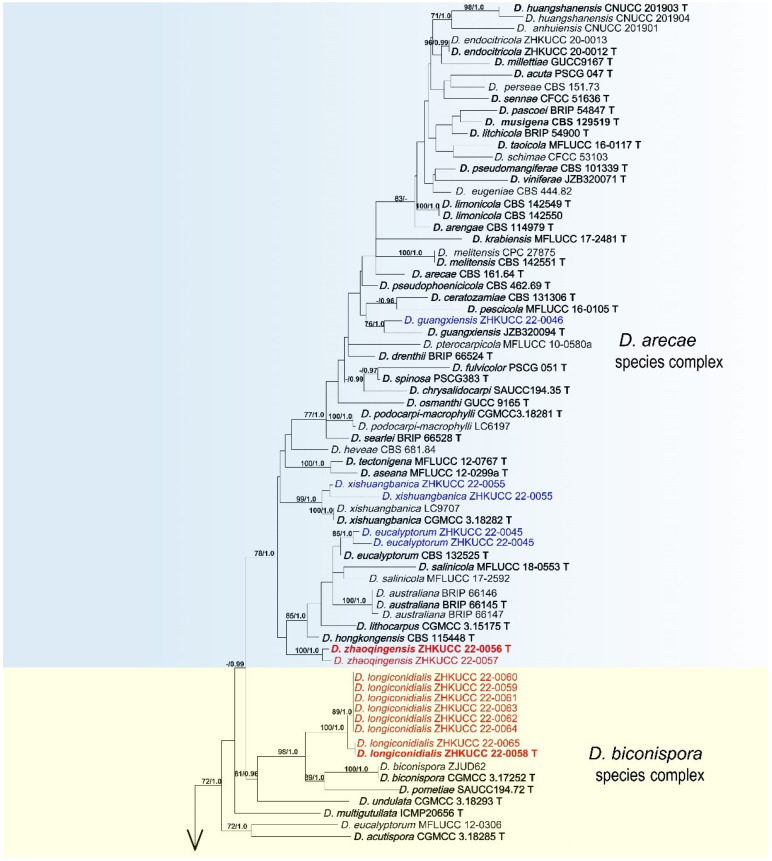
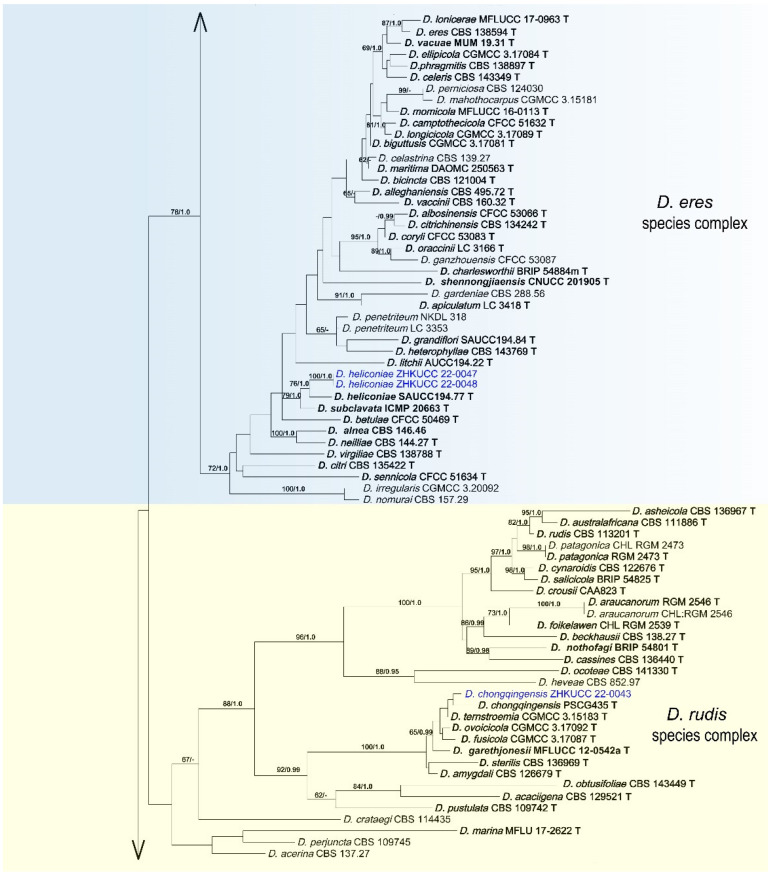
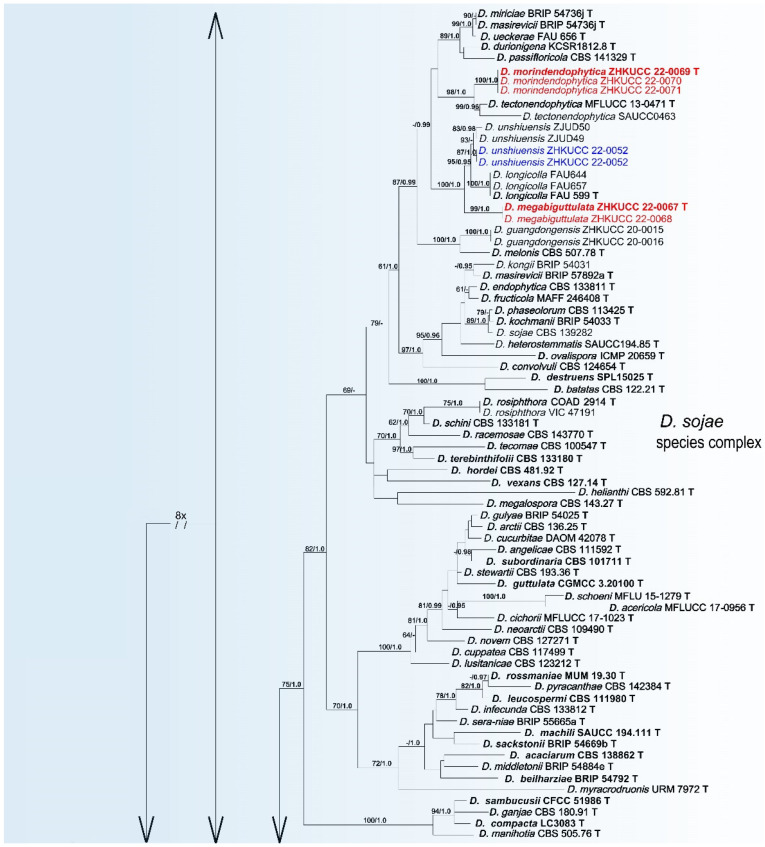
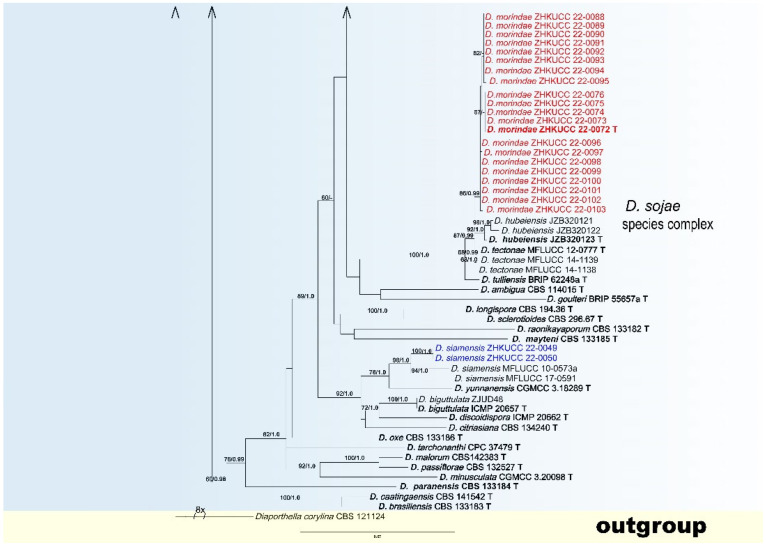
In the present study, we followed Norphanphoun et al. [39] for the taxonomic treatments of Diaporthe. As given in the methods, phylogenetic analyses were arranged in two steps. At first, we developed a genus tree including all species belonging to this genus as given in Guarnaccia et al. [9] and Yang et al. [27]. Then, following Norphanphoun et al. [39], species complexes belonging to isolates from this study were identified, and the final tree was developed. The final analyses were conducted using 271 Diaporthe strains (including types strains) with a combined ITS, tef1-α, cal, his, and β-tubulin sequence alignment. The phylogenetic tree was rooted in Diaporthella corylina (CBS 121124). The final maximum likelihood tree topology was similar to Bayesian analysis. The best scoring RAxML tree with a final likelihood value of −32,402.374230 is given in Figure 2. The matrix consisted of 1065 distinct alignment patterns, with 20.40% of undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.220036, C = 0.307463, G = 0.247268, T = 0.225234; substitution rates AC = 1.189728, AG = 3.654106, AT = 1.419097, CG = 0.937185, CT = 6.230555, GT = 1.000000; gamma distribution shape parameter α = 0.789923. For the Bayesian inference, GTR + I + G model was selected for ITS, TrN + I + G for tef1-α, TIM2 + I + G for cal, TIM1 + I + G for his, and TPM3uf + I + G for β-tubulin. The Bayesian analyses generated 200,000 trees (average standard deviation of split frequencies: 0.032560), from which 150,000 were sampled after 25% of the trees were discarded as burn-in. The alignment contained 1073 unique site patterns. In this resulted tree, isolates belonging to this study were clustered together with seven known Diaporthe species and five novel phylogenetic lineages. They belong to five Diaporthe complexes: D. arecae (D. guangxiensis, D. eucalyptorum, D. xishuangbanica and D. zhaoqingensis), D. biconispora (D. longiconidialis), D. eres (D. heliconiae), D. rudis (D. chongqingensis), and D. sojae (D. megabiguttulata, D. morindendophytica, D. morindae, D. siamensis, D. unshiuensis) species complex. Species descriptions and illustrations of these taxa are given below.
Figure 2.
The best scoring RAxML tree obtained using the combined dataset of ITS, tef1-α, cal, his, and β-tubulin sequences. Diaporthella corylina (CBS 121124) was used to root the tree. Bootstrap support values equal to or greater than 60% in ML and BYPP equal to or greater than 0.95 are shown as ML/BYPP above the respective node. The isolates belonging to the current study are given in blue for known species, and novel taxa are shown in red. Ex-type strains are bold, with T at the end of the strains numbers. The expected number of nucleotide substitutions per site is represented by the scale bar.
3.3. PHI Analysis
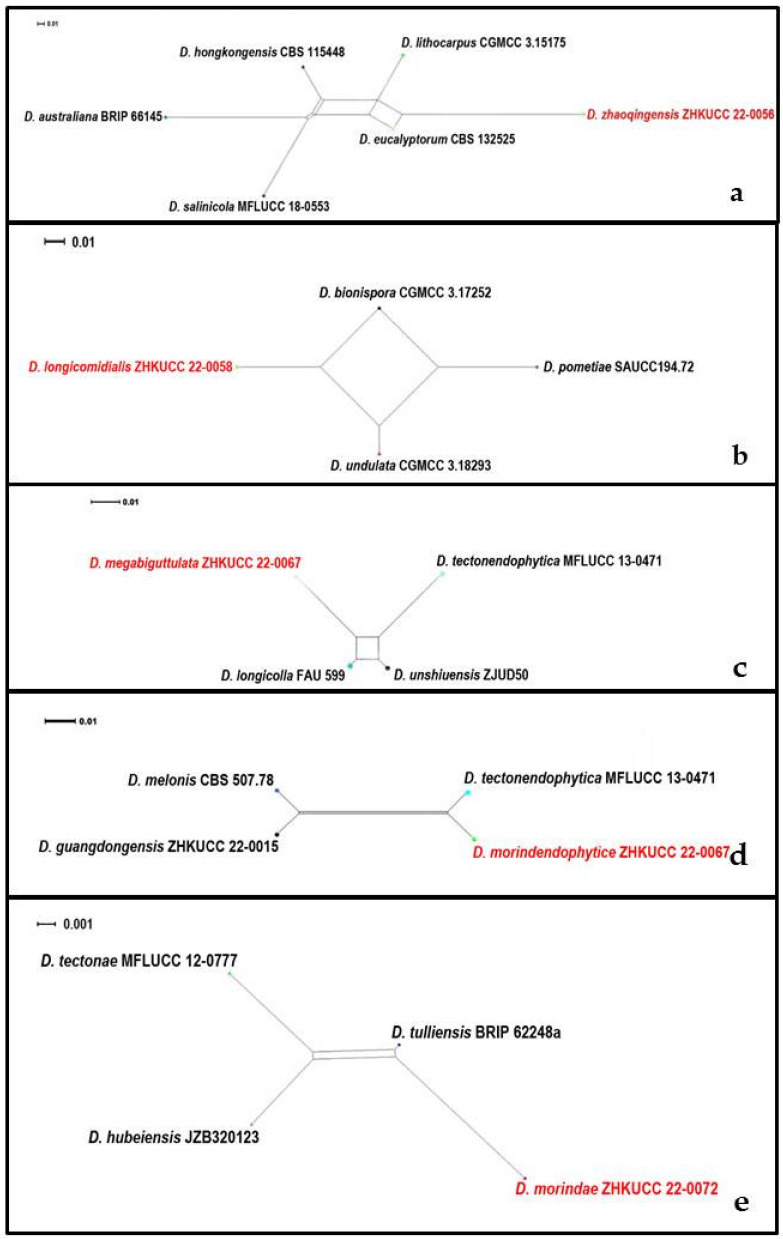
In the phylogenetic analysis of Diaporthe species, our isolates developed five distinct clades within the genus with significant tree lengths and low statistical support. To confirm species, we conducted PHI analysis for these five clades. There was no evidence of significant genetic recombination (Fw > 0.05) between these novel species of Diaporthe and closely related species (Figure 3). These results confirmed that these taxa were significantly different from the existing species of Diaporthe.
Figure 3.
Split graphs showing the results of Pairwise homoplasy index test of (a) Diaporthe zhaoqingensis (Fw = 0.550), (b) Diaporthe longiconidialis (Fw = 0.300), (c) Diaporthe megabiguttulata (Fw = 1.000), (d) Diaporthe morindendophytica (Fw = 0.205), (e) Diaporthe morindae (Fw = 0.238) with their most closely related species using Log-Det transformation and splits decomposition options. The new taxon in each graph is shown in bold font.
3.4. Taxonomy
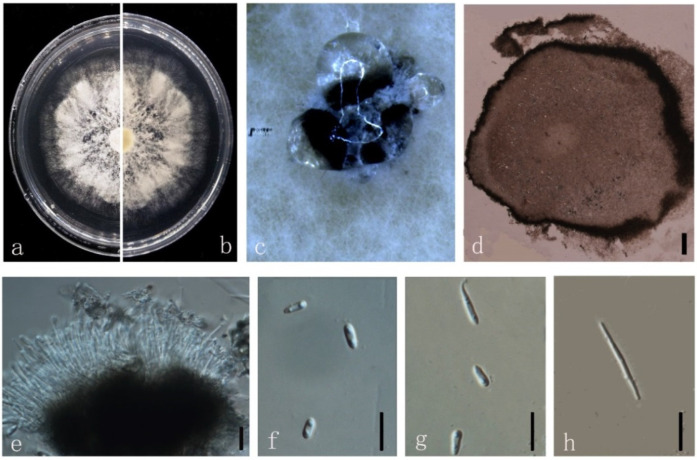
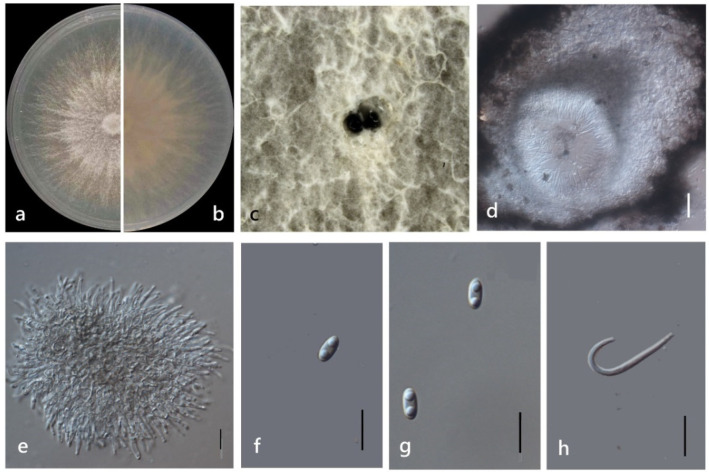
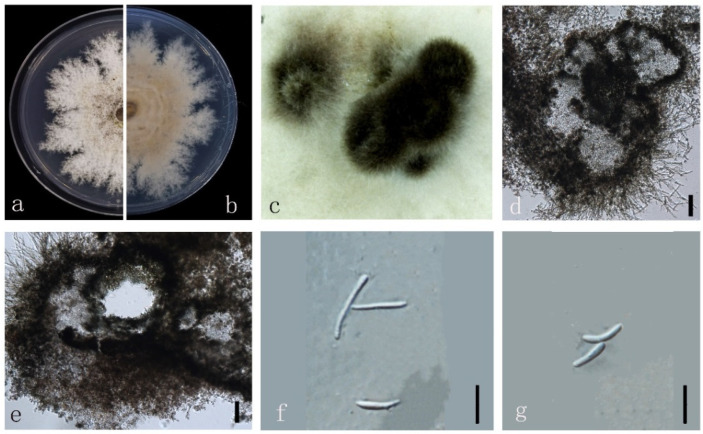
Diaporthe chongqingensis Y.S. Guo & G.P. Wang, in Guo, Crous, Bai, et al., Persoonia 45: 146 (2020) (Figure 4).
Figure 4.
Diaporthe chongqingensis (ZHKUCC 22-0043) (a) upper view of colonies on PDA; (b) Reverse view of colonies on PDA; (c) Pycnidia with conidial droplets on PDA; (d) a vertical Section through a pycnidia; (e,f) Conidiogenous cells; (g,h) Alpha conidia. Scale bars: (d) =100 µm; (e–h) =10 µm.
Index Fungorum number: IF830656
Endophytic on M. officinalis stems. Sexual morph: not observed. Asexual morph: Pycnidia 130–1400 μm × 120–900 μm ( = 542 ± 415 μm × 388 ± 267 μm) oblate or subglobose, grey to black, single or multiple cavities, translucent to black conidial drops exuded from the ostioles. Pycnidia wall thick, exuding creamy to black conidial droplets from ostioles. Conidiophores hyaline, smooth, septate, densely aggregated, cylindrical, straight to sinuous, swelling at the base, tapering towards the apex. Conidiogenous cell hyaline, cylindrical, straight, inner wall buds produce sporulation in bottle form. Alpha conidia 5–10 μm × 2–3 μm ( = 7 ± 0.5 μm × 3 ± 0.2 μm) hyaline, aseptate, fusiform or ellipsoid, biguttulate or multi-guttulate. Beta and gamma conidia not observed.
Culture characteristics: Colonies on PDA reach 85 mm diam. After 5 days. White cotton flocculent aerial mycelium, while the perimeter edge sparse hyphae, then ochre brown mycelium with several black conidiomata for 15 days and a lot of darker black conidiomata at 30 days. Reverse white and become reddish-brown.
Material examined: China, Guangdong Province, Zhaoqing, isolated from a healthy stem of M. officinalis. June 2020, W. Guo (dried culture (ZHKU 22-0032) and living culture (ZHKUCC 22-0043).
Habitat and host: Pear pyrifolia [10].
Known distribution: China [10].
Note: A single isolate (ZHKUCC 22-0043) obtained in this study clustered with D. chongqingensis (PSCG435) with 37% ML. Morphologically our isolates are similar to D. chongqingensis [10]. This species was introduced as a shoot canker pathogen on pear (Pear pyrifolia) in China [10]. This is the first report of D. chongqingensis as an endophyte on the M. officinalis stem.
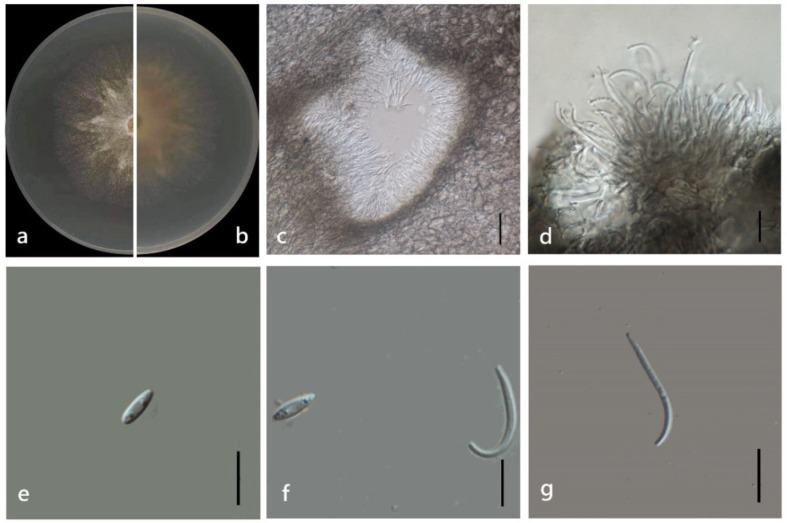
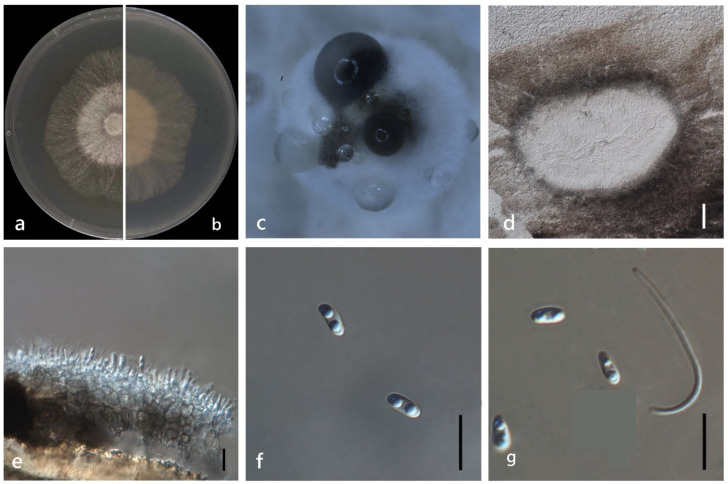
Diaporthe eucalyptorum Crous & R.G. Shivas, in Crous, Summerell, Shivas, et al., Persoonia 28: 153 (2012) (Figure 5).
Figure 5.
Diaporthe eucalyptorum (ZHKUCC 22-0044) (a) upper view of colonies on PDA; (b) Reverse view of colonies on PDA; (c) Pycnidia with conidial droplets on PDA; (d) Section of pycnidia; (e) Conidiogenous cells; (f–h) Alpha conidia; (h) Beta conidia. Scale bars: (d) =100 µm; (e–h) =10 µm.
Index Fungorum number: IF800374
Endophytic on M. officinalis stem. Sexual morph: not observed. Asexual morph: Pycnidia on PDA 360–770 μm × 330–600 μm ( = 573 ± 128 μm × 478 ± 81 µm) in diam., black, erumpent; white to black conidial droplets exuding from central ostioles. Pycnidia wall with black pseudoparenchymatous, exuding transparent to black conidial droplets from ostioles. Conidiophores hyaline, smooth, densely aggregated, cylindrical, straight to sinuous, swelling at the base, tapering towards the apex. Conidiogenous cell phialidic, cylindrical, terminal and lateral, with slight taper towards apex. Alpha conidia 5–10 × 2–4 µm ( = 5 ± 0.6 × 3 ± 0.4 µm), aseptate, hyaline, smooth, guttulate, fusoid, tapering towards ends, straight, apex subobtuse, base subtruncate. Beta conidia 15–30 × 1–3 µm ( = 20 ± 3 μm × 2 ± 0.4 µm), aseptate, hyaline, hamate, filiform, tapering toward both ends.
Culture characteristics: Colonies on PDA reach 85 mm diam. after 4 days. Cottony and radially with abundant aerial mycelium, sparse in the margin, white on surface, then turn to grey. Reverse white to pale yellow.
Material examined: China, Guangdong Province, Zhaoqing, isolated from a healthy stem of M. officinalis. June 2020, W. Guo, (dry culture (ZHKU 22-0033), and living culture (ZHKUCC 22-0044, 22-0045).
Habitat and host: Eucalyptus sp. [40].
Known distribution: Australia [40].
Note: In the multigene phylogenetic tree, two isolates (ZHKUCC 22-0044 and ZHKUCC 22-0045) from this study clustered together with the D. eucalyptorum (CBS 132525) with 85% ML and 1.00 BYPP values. Morphologically, the ZHKUCC 22-0044 isolate is similar to those in the original description of D. eucalyptorum [40]. The ZHKUCC 22-0044 strain developed both alpha and beta conidia while the D. eucalyptorum type strain (CBS 132525) develops alpha conidia only. Diaporthe eucalyptorum was introduced from diseased leaves of Eucalyptus sp. in Australia [40]. This is the first report of D. eucalyptorum as an endophyte on M. officinalis.
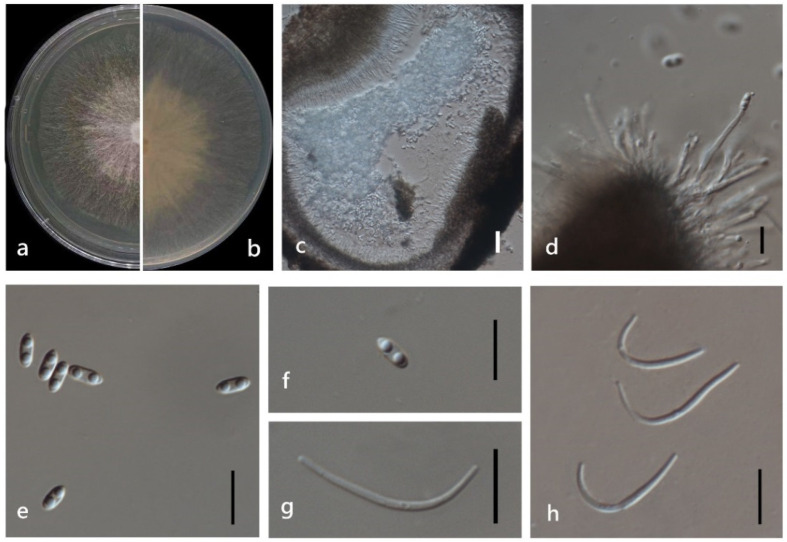
Diaporthe guangxiensis Dissanayake, X.H. Li & K.D. Hyde, in Manawasinghe, Dissanayake, Li, et al., Frontiers in Microbiology 10 (no. 1936): 14 (2019) (Figure 6).
Figure 6.
Diaporthe guangxiensis (ZHKUCC 22-0046) (a) upper view of colonies on PDA; (b) Reverse view of colonies on PDA; (c) Pycnidia with conidial droplets on PDA; (d,e) cross section of pycnidia; (f–h) Beta conidia; Scale bars: (d,e) =100 µm; (f–h) =10 µm.
Index Fungorum number: IF552578
Endophytic on M. officinalis stem. Sexual morph: not observed. Asexual morph: Pycnidia on PDA 260–670 μm × 200–580 μm ( = 465 ± 92 μm × 412 ± 91 µm) in diam., superficial, scattered on PDA, dark brown to black, globose, solitary, or clustered in groups. Pycnidia walls pseudoparenchymatous, conidial cirrus extruding from ostiole appearing as white to black droplets. Conidiophores hyaline, smooth, densely aggregated, unbranched. Beta conidia 10–30 × 1–2 µm ( = 16 ± 5 μm × 1 ± 0.5 µm) aseptate, hyaline, hamate, filiform, guttulate, tapering toward both ends. Alpha and gamma conidia not found.
Culture characteristics: Colonies on PDA reach 85 mm diam. after 25 days. White aerial mycelia and then turn to yellowish-brown. Reverse first white, then dark brownish-green.
Material examined: China, Guangdong Province, Zhaoqing, isolated from a healthy stem of M. officinalis. June 2020, W. Guo, dried culture, ZHKU 22-0034, and living culture (ZHKUCC 22-0046)
Habitat and host: Vitis vinifera [12].
Known distribution: China [12].
Note: In the phylogenetic analyses, a single isolate (ZHKUCC 22-0046) from this study clustered together with the D. guangxiensis strain (JZB320094), 76% ML and 1.0 BYPP values. Morphologically, the present isolate is similar to those in the original description of D. guangxiensis [12]. Diaporthe guangxiensis was introduced from diseased V. vinifera trunks [12]. The type strain (JZB320094) of D. guangxiensis developed both alpha and beta conidia while the ZHKUCC 22-0046 strain develop beta conidia only. This is the first report of D. guangxiensis as an endophyte on M. officinalis.
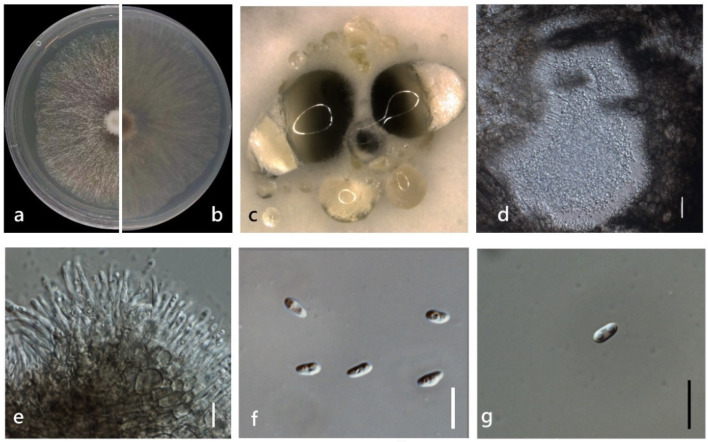
Diaporthe heliconiae S.T. Huang, J.W. Xia, X.G. Zhang & Z. Li, in Sun, Huang, Xia, et al., MycoKeys 77: 76 (2021) (Figure 7).
Figure 7.
Diaporthe heliconiae (ZHKUCC 22-0047) (a) upper view of colonies on PDA; (b) Reverse view of colonies on PDA; (c) Pycnidia with conidial droplets on PDA; (d) vertical section of pycnidia; (e) Conidiogenous cells; (f,g) Alpha conidia; (h) Beta conidia. Scale bars: (d,e) =100 µm; (f–h) =10 µm.
Index Fungorum number: IF 837592
Endophytic on M. officinalis stem. Sexual morph: not observed. Asexual morph: Pycnidia 400–1680 μm × 230–1620 μm ( = 1268 ± 325 μm × 1152 ± 354 µm), solitary or aggregated in groups, erumpent, thin-walled, superficial to embedded on PDA, dark brown to black, globose or subglobose, exuding transparent to dark brown conidial cirrus from the ostioles. Pycnidia wall pseudoparenchymatous, conidial cirrus extruding from ostiole appearing as transparent droplets. Conidiophores hyaline, smooth, densely aggregated, unbranched. Conidiogenous cell 15–40 × 1–3 µm ( = 27 ± 5 μm × 2 ± 0.4 μm), hyaline, aseptate, cylindrical, straight to sinuous, branched. Alpha conidia 5–10 × 2–5 µm ( = 6 ± 0.7 μm × 3 ± 0.4 μm), hyaline, smooth, aseptate, ellipsoidal, 2–4 guttulate, apex subobtuse, base subtruncate. Beta conidia 20–30 × 1–2 µm ( = 26 ± 3 μm × 2 ± 0.3 μm), hyaline, aseptate, filiform, mostly straight and sometimes slightly curved, tapering towards the apex. Gamma conidia not observed.
Culture characteristics: Colonies on PDA reach 85 mm diam. after 15 days. Aerial mycelium abundant, cottony, white, dense in the center, sparse near the margin. White on surface side, then yellowish-brown; reverse white to brown.
Material examined: China, Guangdong Province, Zhaoqing, isolated from a healthy stem of Morinda officinalis. June 2020, W. Guo (dried culture (ZHKU 22-0035), and living culture (ZHKUCC 22-0047, 22-0048).
Habitat and host: Heliconia metallica [41].
Known distribution: China [41].
Note: In the multigene phylogenetic analysis, two isolates (ZHKUCC 22-0047 and ZHKUCC 22-0048) from the present study clustered together with the D. heliconiae (SAUCC194.77) with 100% ML and 1.00 BYPP values. Morphologically, the present isolate is similar to those in the original description of D. heliconiae [41]. Diaporthe heliconiae was introduced as a pathogen on H. metallica petiole [41]. This is the first report of D. heliconiae as an endophyte on M. officinalis.
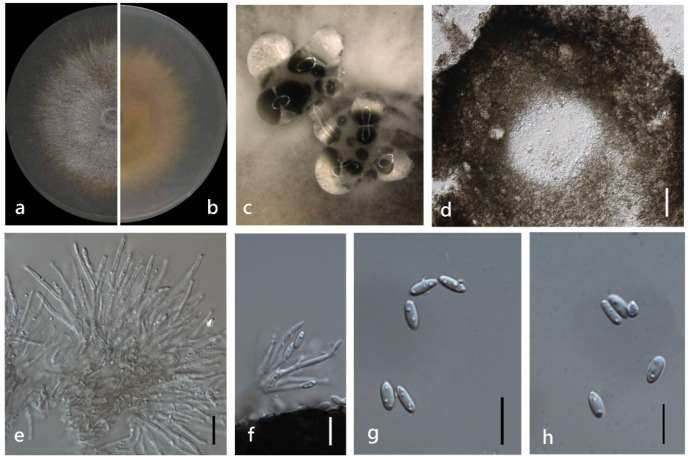
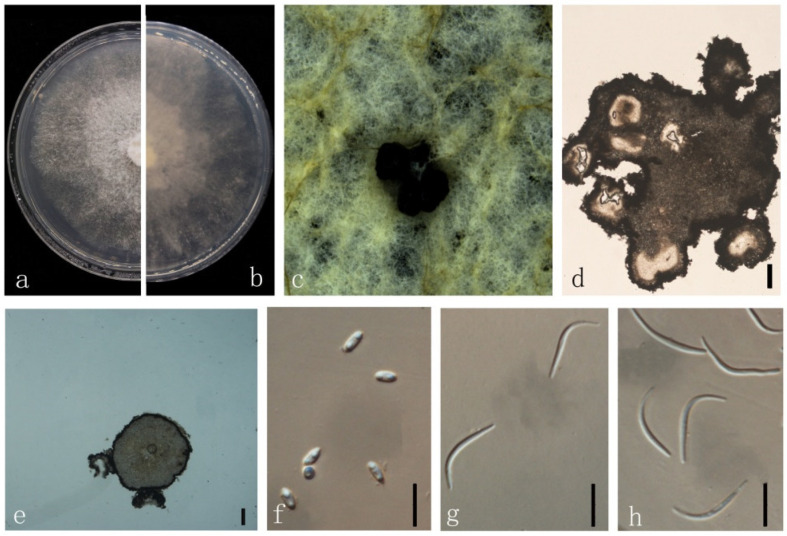
Diaporthe longiconidialis M. Luo, W. Guo, Manawas., M. P. Zhao, K. D. Hyde & C. P. You, sp. nov. (Figure 8).
Figure 8.
Diaporthe longiconidialis (ZHKUCC 22-0058) (a) upper view of colonies on PDA; (b) Reverse view of colonies on PDA; (c) Horizontal section through a pycnidia; (d) Conidiogenous cells; (e,f) Alpha conidia; (f,g) Beta conidia. Scale bars: (c) =100 µm; (d–g) =10 µm.
Index Fungorum number: IF553402
Etymology: In reference to its long spores.
Holotype: ZHKUCC 22-0040
Endophytic on M. officinalis root and stem. Sexual morph: not observed. Asexual morph: Pycnidia 40–400 × 30–160 μm ( = 150 ± 100 μm × 70 ± 40 μm), subglobose, flask or irregularly shaped, single or multiple cavities. Pycnidial wall consisting of sevearal layers of medium transparent textura globosa-angularis. Conidiophores hyaline, unbranched, densely aggregated, cylindrical-filiform, straight to sinuous, 1-septate. Conidiogenous cell phialidic, cylindrical, terminal and lateral, with slight taper towards apex. Alpha conidia 6–10 × 2–4 μm ( = 8 ± 1 μm × 3 ± 0.3 μm), hyaline, fusiform, ellipsoid, long ellipsoid or lanceolate, contains at least one guttulate. Beta conidia 15–30 × 1–3 μm ( = 20 ± 3 μm × 2 ± 0.3 μm), hyaline, linear, curved.
Culture characteristics: Colonies on PDA reach 85 mm diam. after 5 days. White cotton with some polygon mycelium as stars in the center, then with purple pigmentation. Fruiting body occurred from about 15 days, grey to dark black with orange surroundings. Reverse white with some purple pigmentation.
Material examined: China, Guangdong Province, Zhaoqing, isolated from a healthy root of M. officinalis. June 2020, W. Guo, dried culture (ZHKU 22-0040), and living culture (ZHKUCC 22-0058, ZHKUCC 22-0059–0066).
Habitat and host: Healthy stem and root of M. officinalis.
Known distribution: China (Zhaoqing, Guangdong Province).
Note: In the polygenic analysis, eight isolates from the present study clustered together with the Diaporthe biconispora and Diaporthe pometiae ex-type strain with 100% ML and 1.00 BYPP values. Morphologically, Alpha conidia of strain ZHKUCC 22-0058 ( = 8 ± 1 × 3 ± 0.3 μm) were similar to those in the original description of D. biconispora [13]. The colony morphology of our isolates was different to those in the original description of D. biconispora, the surface of the colony is white and light yellow, the center of the back is black, and the sides are light yellow [13]. Alpha conidia of D. pometiae ( = 6.7 × 3.1 μm) were smaller than the ZHKUCC 22-0058 strain. The colony morphology of our isolate was different to those in the original description of D. pometiae. The surface of the colony is white, and the back is white to light grey [42]. Diaporthe biconispora has 3% nucleotide differences in ITS (497 nucleotides), 3% differences in tub2 (353 nucleotides), 4% differences and 4% gaps in tef1-α (355 nucleotides). Diaporthe pometiae has 5% differences and 3% gaps in ITS (541 nucleotides), 6% differences, and 1% gaps in tub2 (471 nucleotides). In addition, there is no evidence of significant genetic recombination (Fw = 0.300) in the PHI analysis. Considering both morphological and molecular data, these isolates were identified as a new species.
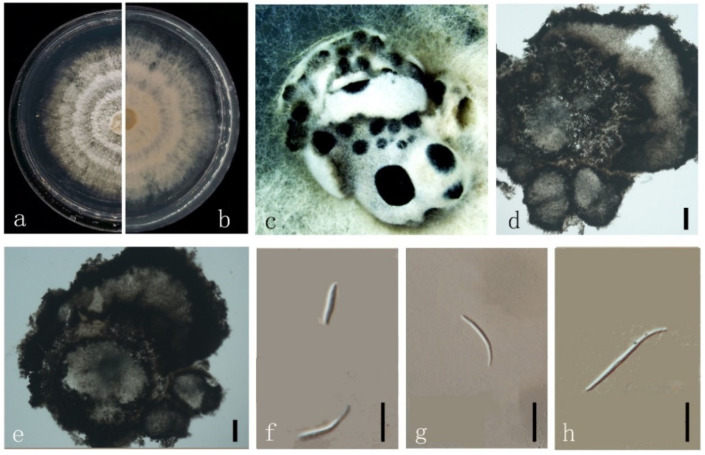
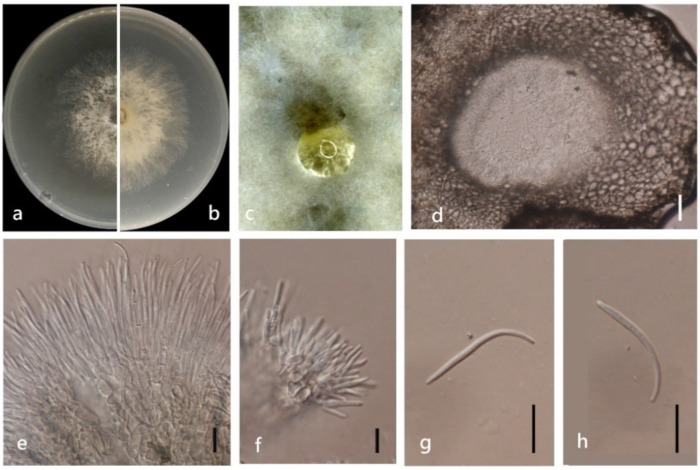
Diaporthemegabiguttulata M. Luo, W. Guo, Manawas., M. P. Zhao, K. D. Hyde, & C. P. You, sp. nov. (Figure 9).
Figure 9.
Diaporthe megabiguttulata (ZHKUCC 22-0067) (a) upper view of colonies on PDA; (b) Reverse view of colonies on PDA; (c) Horizontal section through a pycnidia; (d) Conidiogenous cells; (e,f) Alpha conidia; (g,h) Beta conidia. Scale bars: (c) =100 µm; (d–h) =10 µm.
Index Fungorum number: IF553404
Etymology: In reference to its spores with two big guttulates.
Holotype: ZHKUCC 22-0067
Description: Endophytic on M. officinalis root. Sexual morph: not observed. Asexual morph: Pycnidia 50–440 × 40–270 μm ( = 180 ± 120 × 120 ± 70 μm), oblate or hemispherical, single, or multiple cavity or rotary cavity. Pycnidial wall consisting of several layers of medium transparent textura globosa-angularis. Conidiogenus cells hyaline, phialidic. Conidiogenous cell hyaline, phialidic. Alpha conidia 5–10 × 2–3 μm ( = 6 ± 0.5 × 20 ± 0.2 μm), hyaline, ellipsoid or lanceolate, biguttulate, with one end obtuse and the other acute. Beta conidia 20–30 × 1–2 μm ( = 30 ± 3 × 1 ± 0.3 μm), hyaline, hamate or curved, base truncate.
Culture characteristics: Colonies on PDA reach 85 mm diam. after 4 days. Aerial mycelium, white or pale white, with fruiting body after 20 days. Reverse white, then turns to pale.
Material examined: China, Guangdong Province, Zhaoqing, isolated from a healthy stem of M. officinalis. June 2020, W. Guo, dried culture (ZHKU 22-0041), and living culture (ZHKUCC 22-0067 ex-type and ZHKUCC 22-0068 ex-paratype).
Habitat and host: Healthy stem and root of M. officinalis.
Known distribution: China (Zhaoqing, Guangdong Province).
Note: In the polygenic phylogenetic tree, two isolates (ZHKUCC 22-0067 and 22-0068) from the present study clustered together with the D. unshiuensis [13] and D. longicolla [42] ex-type strain, 99% ML, and 1.00 BYPP values. Morphologically, alpha conidia of D. unshiuensis ( = 6.5 ± 0.6 × 2.8 ± 0.4 µm) [13] were wider than the ZHKUCC 22-0067 ( = 6 ± 0.5 × 20 ± 0.2 μm), the colony gradually turns gray on the front and dark gray in the middle on the back. Alpha conidia of D. longicolla ( = 6.4 ± 0.3 × 2.3 ± 0.1 μm) [43] were longer, and both of them were not found beta conidia. Compared between D. unshiuensis, D. longicolla and strain ZHKUCC 22-0067, D. unshiuensis has 1% differences in ITS (491 nucleotides), while D. longicolla has 4% differences and 2% gaps in ITS (550 nucleotides). For the tub2 gene, there are 1% differences (446 nucleotides) with D. longicolla and 1% differences (373 nucleotides) with D. unshiuensis. In addition, there is no evidence of significant genetic recombination (Fw = 1.000) in the PHI analysis. Considering both morphological and molecular data, these isolates were identified as novel species.
Diaporthe morindae M. Luo, W. Guo, M. P. Zhao, Manawas., K. D. Hyde & C. P. You, sp. nov. (Figure 10).
Figure 10.
Diaporthe morindae (ZHKUCC 22-0072) (a) upper view of colonies on PDA; (b) Reverse view of colonies on PDA; (c) Conidiomata sporulating on PDA; (d) longitudinal section of Pycnidium; (e) Conidiogenous cells; (f,g) Alpha conidia. Scale bars: (d) =100 µm; (e–g) =10 µm.
Index Fungorum number: IF553409
Etymology: In reference to its host of M. officinalis.
Holotype: ZHKUCC 22-0072
Endophytic on Morinda officinalis root and stem. Sexual morph: not observed. Asexual morph: Pycnidia 50–380 × 30–160 μm ( = 170 ± 90 μm × 90 ± 40 μm), oblate, subglobose, flask or irregularly shaped, multiple cavity or rotary cavity. Pycnidial wall consisting of sevearal layers of medium transparent textura globosa-angularis. Conidiogenus cells hyaline, phialidic. Conidiogenous cell hyaline, phialidic. Alpha conidia 6–7 × 2–4 μm ( = 6 ± 0.3 μm × 3 ± 0.3 μm), hyaline, ellipsoid or torque circular, blunt at ends, mono- or biguttulate. Beta and gamma conidia not observed.
Culture Characteristics: Colonies on PDA reach 85 mm diam. after 5 days. White cotton flocculent aerial hyphae. At 15 days, the central part with a diameter of 10 mm was black on the back of the colony, and the part beyond the diameter of 40 mm began to turn black, then the black deepened, and oil droplets appeared. At 30 days, black dots appeared in the colony, and the fruiting body was gradually produced.
Material examined: China, Guangdong Province, Zhaoqing, isolated from a healthy stem of M. officinalis. June 2020, W. Guo, dried culture (ZHKU 22-0043), and living culture (ZHKUCC 22-0072 ex-type and ZHKUCC 22-0073–0076).
Habitat and host: healthy stem and root of M. officinalis.
Known distribution: China (Zhaoqing, Guangdong Province).
Note: In the polygenic phylogenetic tree five isolates in the present study clustered together with the D. hubeiensis (JZB320121 and JZB320122) and D. tectoane (MFLUCC 14.1139, MFLUCC 12.0777, and MFLUCC 14.1138) strains with 86% ML and 0.99 BYPP. Morphologically, Alpha conidia of D. hubeiensis ( = 6.1 × 1.8 μm) [12] and D. tectonae ( = 5.5 × 2.6 μm) [43] are smaller. In pairwise nucleotide comparisons, Diaporthe hubeiensis has 19% differences and 18% gaps in ITS (543 nucleotides), 2% differences in tub2 (522 nucleotides), 10% differences and 7% gaps in cal (510 nucleotides), and 5% differences and 4% gaps in tef1-ɑ (339 nucleotides). D. tectonae has 3% differences and 2% gaps in ITS (547 nucleotides), 7% differences and 4% gaps in tub2 (485 nucleotides), 3% differences and 1% gaps in cal (476 nucleotides), and 2% differences in tef1-ɑ (315 nucleotides). In addition, there is no evidence of significant genetic recombination (Fw = 0.238) in the PHI analysis. Considering both morphological and molecular data, these isolates were identified as a novel species.
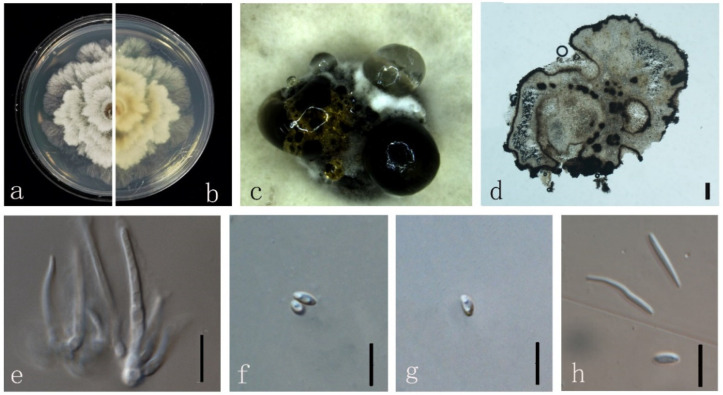
Diaporthemorindendophytica M. Luo, W. Guo, Manawas., M. P. Zhao, K. D. Hyde, & C. P. You, sp. nov. (Figure 11).
Figure 11.
Diaporthe morindendophytica (ZHKUCC 22-0069) (a) Upper view of colonies on PDA; (b) Reverse view of colonies on PDA; (c) Pycnidia sporulating on PDA; (d) Section of pycnidia; (e) Conidiogenous cells; (f,g) Alpha conidia; (h) Beta conidia. Scale bars: (d) =100 µm; (e–h) =10 µm.
Index Fungorum number: IF553423
Etymology: In reference to its endophytic nature in the host of Morinda officinalis.
Holotype: ZHKUCC 22-0069
Endophytic on M. officinalis. Sexual morph: not observed. Asexual morph: Pycnidia 40–200 × 30–130 μm ( = 120 ± 450 μm × 70 ± 20 μm), oblate, subglobose or irregularly shaped, single or multiple cavities. Pycnidial wall consisting of sevearal layers of medium transparent textura globosa-angularis. Conidial masses produced as black droplets extruding through the ostioles. Conidiogenus cells hyaline, phialidic. Alpha conidia 5–10 × 2–3 μm ( = 6 ± 0.5 μm × 3 ± 0.3 μm), hyaline, ellipsoid, torque circular or lanceolate, blunt at both ends or slightly pointed at one end, biguttulate. Beta conidia 20–30 × 1–2 μm ( = 20 ± 3 μm × 2 ± 0.2 μm), hyaline, linear, and curved.
Culture characteristics: Colonies on PDA reach 85 mm diam. after 4 days. White cotton flocculent aerial hyphae, turned grayish-black or yellow. Reverse yellowish-brown, with a large number of black dots.
Material examined: China, Guangdong Province, Zhaoqing, isolated from a healthy stem of M. officinalis. June 2020, W. Guo, dried culture (ZHKU 22-0042), and living culture (ZHKUCC 22-0069 ex-type and ZHKUCC 22-0070, 22-0071 ex-paratype).
Habitat and host: Healthy stem of M. officinalis.
Known distribution: China (Zhaoqing, Guangdong Province).
Note: In the polygenic phylogenetic tree three isolates (ZHKUCC 22-0069–0071) from the present study cluster together with the Diaporthe tectonendophytica (MFKUCC 13.0471 and SAUCC0463), 100% ML and 1.00 BYPP values. Morphologically, D. tectonendophytica develops bigger pycnidia ( = 542 × 660 μm) [3] than our isolates. Alpha conidia ( = 5 × 2.2 μm) and Beta conidia ( = 23 × 1.3 μm) are smaller than the ZHKUCC 22-0069 isolate. The culture characteristics are different. Diaporthe tectonendophytica was first white and turn to yellowish grey, while the ZHKUCC 22-0069 strain turned grayish-black or yellow. There are 3% differences in ITS (547 nucleotides), 3% differences in tub2 (487 nucleotides), 3% differences in tef1-α (307 nucleotides) between D. tectonendophytica and strain ZHKUCC 22-0069. In addition, there is no evidence of significant genetic recombination (Fw = 0.205) in the PHI analysis. Considering both morphological and molecular data, these isolates were identified as novel species.
Diaporthe siamensis Udayanga, Xing Z. Liu & K.D. Hyde, in Udayanga, Liu, Mckenzie et al., Cryptog. Mycol. 33(3): 298 (2012) (Figure 12).
Figure 12.
Diaporthe siamensis (ZHKUCC 22-0049) (a) upper view of colonies on PDA; (b) Reverse view of colonies on PDA; (c) Pycnidia with conidial droplets on PDA; (d) Section of pycnidia; (e) Conidiogenous cells; (f,g) Alpha and beta conidia. Scale bars: (d) =100 µm; (e–g) =10 µm.
Index Fungorum number: IF800826
Endophytic on M. officinalis. Sexual morph: not observed. Asexual morph: Pycnidia 100–700 μm × 40–500 μm ( = 320 ± 160 μm × 220 ± 130 μm) subglobose, flask or irregularly shaped, single or multiple cavities. Pycnidia wall pseudoparenchymatous, conidial cirrus extruding from ostiole appearing as transparent to yellowish-brown droplets. Conidiophores cylindrical, wide at the base, hyaline, simple, densely aggregated. Conidiogenus cells hyaline, phialidic, cylindrical. Alpha conidia 5–10 × 2–3 μm ( = 7 ± 0.4 μm × 3 ± 0.3 μm), hyaline, fusiform, ellipsoid, torque circular or lanceolate, blunt at both ends or slightly pointed at one end, mostly biguttulate. Beta conidia 20–40 × 1–2 μm ( = 30 ± 5 μm × 2 ± 0.2 μm), hyaline, linear, curved.
Culture characteristics: Colonies on PDA reach 85 mm diam. after 5 days. White cotton flocculent aerial mycelium in the center, surrounding sparse hyphae, and then become yellowish brown, margin lobate. Reverse white and then purple grey due to pigment formation.
Material examined: China, Guangdong Province, Zhaoqing, isolated from a healthy stem of M. officinalis. June 2020, W. Guo, dried culture (ZHKU 22-0036), and living culture (ZHKUCC 22-0049 and ZHKUCC 22-0050).
Habitat and host: Dasymaschalon trichophorum [13,43], Pandanus tectorius [44].
Known distribution: China [13,43], Thailand [44].
Note: In the phylogenetic analysis, two isolates (ZHKUCC 22-0049 and ZHKUCC 22-0050) obtained in this study clustered with Diaporthe siamensis (MFLUCC 17.0591 and MFLUCC 10.0573a) with 100% ML and 1.00 BYPP values. The ZHKUCC 22-0049 isolate from this study is morphologically similar to the D. siamensis type description [3] by conidia with similar shapes and dimensions. This species was first reported on necrotic leaves of Dasymaschalon in China by Udayanga et al. [3]. This is the first report of D. siamensis as an endophyte on M. officinalis.
Diaporthe unshiuensis F. Huang, K.D. Hyde & Hong Y. Li, in Huang, Udayanga, Mei, et al., Fungal Biology 119(5): 344 (2015) (Figure 13).
Figure 13.
Diaporthe unshiuensis (ZHKUCC 22-0051) (a) upper view of colonies on PDA; (b) Reverse view of colonies on PDA; (c) Conidiomata sporulating on PDA; (d,e) Section of pycnidia; (f) Alpha conidia; (g,h) Beta conidia. Scale bars: (d,e) =100 µm; (f–h) =10 µm.
Index Fungorum number: IF810845
Endophytic on M. officinalis stem. Sexual morph: not observed. Asexual morph: Pycnidia 350 × 300 μm, globose, subglobose or irregular, bark brown, pseudoparenchymatous walls. Conidial masses produced as black droplets extruding through the ostioles. Pycnidia wall consisting of several layers of medium transparent textura globosa-angularis. Pycnidia wall consisting of several layers of medium transparent textura globosa-angularis, conidial cirrus extruding from ostiole appearing as black droplets. Conidiophores cylindrical, wide at the base, hyaline, simple. Alpha conidia 5–10 × 2–3 μm ( = 6 ± 0.8 × 3 ± 0.3 μm), ellipsoidal or clavate, base truncate, aseptate, smooth, biguttulate. Beta conidia 20–30 × 1–3 μm ( = 25 ± 3 × 2 ± 0.4 μm), hyaline, linear, curved, hook-shaped.
Culture characteristics: Colonies on PDA reach 85 mm diam. after 9 days. Surface white and turning to grey with ageing, reverse white to grey with dark grey at the centre.
Material examined: China, Guangdong Province, Zhaoqing, isolated from healthy root of M. officinalis. June 2020, W. Guo, dried culture (ZHKU 22-0037), and living culture (ZHKUCC 22-0051–53).
Habitat and host: Carya illinoinensis, Citrus sp., Citrus unshiu, Fortunella margarita, Glycine max and Vitis vinifera [45].
Known distribution: China, Louisiana [45].
Note: In the multigene phylogenetic analysis, three isolates (ZHKUCC 22-0051–53) from the present study cluster together with the D. unshiuensis strains (ZJUD49 and ZJUD50), 87% ML and 1.00 BYPP values. Morphologically, the ZHKUCC 22-0051 isolate is similar to those in the original description of D. unshiuensis [13]. Diaporthe unshiuensis was introduced as an endophyte on the Citrus unshiu fruits, on branches and twigs of F. margarita [13]. Our strains develop both alpha and beta conidia while the D. unshiuensis type strain (ZJUD52) develops only alpha conidia [13]. This is the first report of D. unshiuensis as an endophyte on M. officinalis.
Diaporthe xishuangbanica Y.H. Gao & L. Cai, in Gao, Liu, Duan, et al., IMA Fungus 8(1): 179 (2017) (Figure 14).
Figure 14.
Diaporthe xishuangbanica (ZHKUCC 22-0054) (a) upper view of colonies on PDA; (b) Reverse view of colonies on PDA; (c) Pycnidia with conidial droplets on PDA; (d) Section of pycnidia; (e,f) Conidiogenous cells; (g,h) Beta conidia. Scale bars: (d) =100 µm; (e–h) =10 µm.
Index Fungorum number: IF820685
Endophytic on M. officinalis root and stem. Sexual morph: not observed. Asexual morph: Pycnidia 30–360 × 20–230 μm ( = 119 ± 90 μm × 72 ± 60 μm), oblate, subglobose or irregularly shaped, single or multiple cavities. Conidial masses produced as transparent droplets extruding through the ostioles. Pycnidia wall consisting of several layers of medium transparent textura globosa-angularis. Conidial masses produced as transparent droplets extruding through the ostioles. Conidiophores hyaline, branched, septate. Conidiogenus cells grow from the conidia, hyaline, phialidic. Beta conidia 20–30 × 1–2 μm ( = 27 ± 3 μm × 2 ± 0.3 μm), hyaline, linear, and curved. Alpha conidia and gamma conidia not observed.
Culture characteristics: Colonies on PDA reach 85 mm diam. after 5 days. White cotton flocculent aerial mycelium in the center, surrounding sparse hyphae. At 15 days, white cotton flocculent aerial hyphae and a few black dots appear, reddish pigments are produced on the back side of the colony. At 30 days, the black dots increased, and the fruiting body was produced.
Material examined: China, Guangdong Province, Zhaoqing, isolated from healthy root of M. officinalis. June 2020, W. Guo, dried culture (ZHKU 22-0038), and living culture (ZHKUCC 22-0054, 22-0055).
Habitat and host: Camellia sinensis [8].
Known distribution: China [8].
Note: In the multigene phylogenetic tree two isolates (ZHKUCC 22-0054, 22-0055) from the present study cluster together with the D. xishuangbanica strains (LC9707 and CGMCC 3.18282) with 99% ML and 1.0 BYPP. Morphologically, the ZHKUCC 22-0054 isolate is similar to those in the original description of D. xishuangbanica [8]. However, D. xishuangbanica developed alpha conidia while the ZHKUCC 22-0054 isolate developed only beta conidia. Diaporthe xishuangbanica was introduced from Camellia sinensis diseased leaves. This is the first report of D. xishuangbanica as an endophyte on M. officinalis.
Diaporthe zhaoqingensis M. Luo, M. P. Zhao, W. Guo, Manawas., K. D. Hyde & C. P. You, sp. nov. (Figure 15).
Figure 15.
Diaporthe zhaoqingensis (ZHKUCC 22-0056) (a) upper view of colonies on PDA; (b) Reverse view of colonies on PDA; (c) Pycnidia with conidial droplets on PDA; (d,e) Pycnidium; (f,g) gamma conidia; Scale bars: (d,e) =100 µm; (f,g) =10 µm.
Index Fungorum number: IF553427
Etymology: In reference to the Zhaoqing city, Guangdong Province, from where the samples were collected.
Holotype: ZHKUCC 22-0039
Endophytic on M. officinalis root and stem. Sexual morph: not observed. Asexual morph: Pycnidia 200–1000 × 170–1000 μm ( = 574 ± 272 × 520 ± 277 μm), subglobose, flask or irregularly shaped, single or multiple cavities. Pycnidial wall consisting of several layers of medium transparent textura globosa-angularis. Conidiophores hyaline, unbranched, densely aggregated, cylindrical-filiform, straight to sinuous. Conidiogenus cells phialidic, cylindrical, terminal, and lateral, with slight taper towards apex. Gamma conidia 10–30 × 0.5–2 μm ( = 15 ± 4 μm × 2 ± 0.5 μm), fusiform, hyaline. Alpha and Beta conidia not observed.
Culture characteristics: Colonies on PDA reach 50 mm diam. After 7 days. White cotton flocculent aerial mycelium in the center, surrounding sparse hyphae, with lobate margin. First white, then turn to grey and yellow grey.
Material examined: China, Guangdong Province, Zhaoqing, isolated from healthy stem and root of Morinda officinalis. June 2020, W. Guo, dried culture ZHKU 22-0039, and living culture ZHKUCC 22-0039 and 22-0040 ex-type.
Habitat and host: Healthy stem and root of M. officinalis.
Known distribution: China (Zhaoqing, Guangdong Province).
Note: In the polygenic phylogenetic tree two isolates (ZHKUCC 22-0039 and 22-0040) from the present study clustered as an independent clade with 100% ML and 1.00 BYPP support. In the PHI analysis, there was no evidence of significant genetic recombination (Fw = 0.550) among our isolates (ZHKUCC 22-0039) and its closely related taxa (D. australiana (BRIP 66145), D. eucalyptorum (CBS 132525), D. hongkongensis (CBS 115448), D. lithocarpus (CGMCC 3.15175), and D. salinicola (MFLUCC 18.0553)). Morphologically, ZHKUCC 22-0039 strain develops a significantly higher amount of gamma conidia while other closely related species D. australiana [46], D. eucalyptorum [41], D. hongkongensis [2], D. lithocarpus [11], and D. salinicola [47] are not developing. Considering both morphological and molecular data, here we introduce our isolates as a novel Diaporthe species.
4. Discussion
Endophytic fungi live inside the healthy host tissues [48,49]. They are a common and diverse group of fungi [12,49]. Diaporthe species are ubiquitous endophytes on numerous hosts. Skaltsas et al. [50] isolated endophytic Diaporthe from Hevea brasiliensis, H. guianensis, and Micandra spp. in Cameroon, Mexico. Rhoden [51] isolated 97 strains from Trichilia elegans (Meliaceae) in Brazil, while the Diaporthe were the most frequently isolated genus. This reflects the species richness of Diaporthe species as endophytes in many hosts. In the present study, 48 endophytic Diaporthe isolates were obtained from M. officinalis in China from stems and roots. Based on the multigene phylogeny, all 48 isolates from this study were grouped in 12 distinct clades within the Diaporthe phylogenetic tree. Among them, seven new host records were identified: Diaporthe chongqingensis, D. guangxiensis, D. heliconiae, D. siamensis, D. unshiuensis, and D. xishuangbanica. The remaining five species were identified as novel species: Diaporthe longiconidialis, D. megabiguttulata, D. morindendophytica, D. morindae, and D. zhaoqingensis. Our study is the first comprehensive study on endophytic fungi associated with M. officinalis in China.
During the last few years, many Diaporthe species were introduced. For example, in 2020, 34 new species were introduced, and in 2021, 37 new species were introduced (Index Fungorum 2022, accessed on 15 June 2022). Almost all these species were introduced based on molecular phylogeny following the phylogenetic species concept [13,27,46]. However, Diaporthe species do not have enough morphologies to distinguish; thus, the phylogenetic tree with five loci became the key tool to define the species. This resulted in over 200 species which are genetically closer and define Diaporthe as cryptic species. What is missing here is that there are no discussions on the different species or different genotypes of the existing species. In the phylogenetic analysis of the present study, D. morindae, a new species from our study, was clustered together with D. hubeiensis, D. tectonae, and D. tulliensis. These four species develop a distinct clade from other Diaporthe species in the tree. Within this clade, these species develop independent lineages, yet their morphologies are overlapping. However, it is quite interesting as this clustering might be based on the geography and host in which, D. morindae and D. hubeiensis introduced from China are associated with Citrus (this study) and Vitis [12]. Diaporthe tectonae was introduced from m Tectona grandis from Thailand [52]. In contrast, D. tulliensis was introduced from the rotted stem end of the fruit of Theobroma cacao in Australia [53].
Additional analyses such as recombination tests and phylogenetic incompatibility are employed, but the species delineation enigma of Diaporthe is unresolved. Therefore, in the present study, we followed polyphasic taxonomic approaches which included phylogeny, morphology, and recombination analysis to identify the species. For existing species, species definition was based on phylogeny and morphology. Furthermore, we emphasise that taxon sampling is critical to establishing a primary tree to void the possible false introduction of species. However, it is necessary to discuss “what is the species” in Diaporthe.
Medicinal plants have a high economical and important role in human cultures worldwide. Morinda officinalis is a famous traditional medicinal plant, which has various biological effects such as anti-inflammatory activities [53], antiosteoporotic [54], antifatigue [55], anti-rheumatoid arthritis [56], and anti-oxidant [57]. It has been mentioned that endophytes live inside the host might affect the phenotype of the host in many ways, including providing resistance to pathogens [58,59], promoting seed germination or/and plant growth [60], herbivores [61], weed control [62,63], resistance to abiotic stresses, or even in litter decomposition [64]. In addition, some studies have revealed that secondary metabolites produced by endophytic fungi could be a novel source of medicinal compounds [64]. Though two new metabolites from M. officinalis endophytic Alternaria sp. A744 [63] and two polyketide compounds from the M. officinalis endophytic Trichoderma spirale A725 [65] were found. However, little is known about the endophytic fungi of M. officinalis. Therefore, further studies are necessary to explore the relationship between medicinal properties and a plant’s endophytic biota.
One endophytic species might occur in different tissues in the same host [13]. In the present study, endophytes Diaporthe were successfully isolated from M. officinalis’s roots and stems. Diaporthe evcalyptorum, D. longiconidialis, D. morindae and D. xishuangbanica were isolated from roots and stems of M. officinalis, while other species were isolated from only one tissue type. However, we were not able to isolate any Diaporthe species from leaves. Gond et al. [66] obtained only two endophytic Diaporthe strains from leaves of Aegle marmelos Correae (Rutaceae). Dong et al. [14] identified two endophytic Diaporthe species from Citrus grandis cv. Tomentosa while 22 strains were from fruits or twigs. Huang et al. [13] did not find any endophytes Diaporthe from Citrus leaves. These variations might be a result of differences in the tissue organization structure and the different nutrition content of each tissue type [13,67]. However, the exact underlying reasons and mechanisms for these variations are not known. At the same time, further studies are needed to compare the variations in endophytic colonization according to different seasons or different stages of maturity of the plant.
Optimization of cultural conditions is the most common and simple method to obtain different fungal diversity. Zhou et al. [68] found that the addition of vitamins in the media significantly increased the diversities of isolated fungi, and the increment reached 207% and 81% at the generic level at both 4 °C and 25 °C, respectively, conditions. In the present study, D. morindendophytica was isolated from three media (MD, CMA, and P++J medium), while two species of them including D. longiconidialis, and D. morindae were isolated from all the media tested (PDA, 2% PDA, LCA, M + J, MD and P + J medium). In addition, these four species were isolated from both roots and stems. Therefore, these species might be the dominant endophytic Diaporthe species in M. officinalis. Even though Diaporthe species can grow in different media, D. biguttulata, D. chongqingensis, D. heliconiae, D. heterophyllae, D. guangxiensis, and D. megabiguttulata were only isolated in one medium. Thus, optimizing and employing several media to isolate endophytes will enhance the diversity of endophytes obtained from a particular host.
Several endophytes such as Colletotrichum and Botryosphaeriaceae have been considered latent pathogens or opportunistic pathogens [69,70]. Diaporthe is also known to be opportunistic pathogens. Huang et al. [13] observed that some Diaporthe species associated with Citrus in China may act as opportunistic pathogens. Dong et al. [14] found Diaporthe limonicola as endophyte in C. grandis cv. Tomentosa, while it has also been reported as pathogenic on Citrus sp. In China [13] and as a dieback pathogen of lemon trees in Europe [7]. In the present study, all species isolated are known pathogenic species from a different host [8,10,11,12,13,41,42,43]. Diaporthe unshiuensis was also reported as an endophyte of Citrus in China [13]. It was reported as a dieback disease associated with Carya illinoinensisin [71], peach [72], and grapevine [12] in China and as Citrus disease in Europe [7]. Therefore, further studies are necessary to understand the pathogenicity of these endophytic strains and the exact role of endophytic fungal taxa in the medicinal properties of these host plants.
In conclusion, in the present study, 12 endophytic species Diaporthe species were isolated from a traditional medicinal plant in China. To delineate these isolated taxa employing polyphasic approaches are necessary. Either morphology alone or phylogeny is difficult to delineate species in Diaporthe. Fungal growth media might have a significant effect on the number of different species isolated. Moreover, species occurrence is varied based on the plant tissue type as well. All previously known species obtained in this study have been reported as pathogens on various hosts in China and other countries. Therefore, future studies are necessary to understand the pathogenicity of these species on M. officinalis. The present study will be a baseline to understand the endophytic fungal diversity of Chinese Traditional Medicinal plants and thus to understand the effects of endophytes on medical properties.
Acknowledgments
We would like to thank Shaun Pennycook for his guidance on the species names. Mei Luo, I.S.M., and C.P.Y. would like to thank the Research Project of the Innovative Institute for Plant Health (KA21031H101). Mei Luo would like to thank the Guangdong rural science and Technology Commissioner project (KTP20210313). Zhangyong Dong would like to thank the Key Realm R&D Program of Guangdong Province (2018B020205003). Ishara S. Manawasinghe would like acknowledges grant from Zhongkai University of Agriculture and Engineering, Guangzhou, China (KA210319288).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8080806/s1, Table S1: Collection details and GenBank accession numbers of isolates included in this study.
Author Contributions
Conceptualization, M.L. and C.Y.; methodology, W.G.; software, Z.D., J.L. and I.S.M.; validation, I.S.M., V.G. and C.Y.; formal analysis, M.L.; resources, M.L. and W.G.; data curation, W.G. and M.Z.; writing—original draft preparation, M.L.; writing—review and editing, I.S.M., V.G., K.D.H. and C.Y.; project administration, C.Y.; funding acquisition, M.L. and C.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The sequence data generated in this study are deposited in NCBI GenBank (https://www.ncbi.nlm.nih.gov/genbank, accessed on 1 April 2022). All accession numbers are given in Table S1.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This project was funded by the Guangdong rural science and Technology Commissioner project (KTP20210313), the Research Project of the Innovative Institute for Plant Health (KA21031H101) and the Key Realm R&D Program of Guangdong Province (2018B020205003).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Senanayake I.C., Crous P.W., Groenewald J.Z., Maharachchikumbura S.S., Jeewon R., Phillips A.J.L., Bhat J.D., Perera R.H., Li Q.R., Li W.J., et al. Families of Diaporthales based on morphological and phylogenetic evidence. Stud. Mycol. 2017;86:217–296. doi: 10.1016/j.simyco.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes R.R., Glienke C., Videira S.I.R., Lombard L., Groenewald J.Z., Crous P.W. Diaporthe: A genus of endophytic, saprobic and plant pathogenic fungi. Persoonia. 2013;31:1–41. doi: 10.3767/003158513X666844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Udayanga D., Liua X., Mckenzie E.H.C., Chukeatirote E., Hyde K.D. Multi-locus Phylogeny Reveals Three new Species of Diaporthe from Thailand. Cryptogam. Mycol. 2012;33:295–309. doi: 10.7872/crym.v33.iss3.2012.295. [DOI] [Google Scholar]
- 4.Udayanga D., Castlebury L.A., Rossman A.Y., Hyde K.D. Species limits in Diaporthe: Molecular reassessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Persoonia. 2014;32:83–101. doi: 10.3767/003158514X679984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guarnaccia V., Martino I., Tabone G., Brondino L., Gullino M.L. Fungal pathogens associated with stem blight and dieback of blueberry in northern Italy. Phytopathol. Mediterr. 2020;59:229–245. [Google Scholar]
- 6.Ariyawansa H.A., Tsai I., Wang J.Y., Withee P., Tanjira M., Lin S.R., Suwannarach N., Kumla J., Elgorban A.M., Cheewangkoon R. Molecular Phylogenetic Diversity and Biological Characterization of Diaporthe Species Associated with Leaf Spots of Camellia sinensis in Taiwan. Plants. 2021;10:1434. doi: 10.3390/plants10071434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarnaccia V., Crous P.W. Emerging citrus diseases in Europe caused by species in Diaporthe. IMA Fungus. 2017;8:317–334. doi: 10.5598/imafungus.2017.08.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Y., Liu F., Duan W., Crous P.W., Cai L. Diaporthe is paraphyletic. IMA Fungus. 2017;8:153–187. doi: 10.5598/imafungus.2017.08.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guarnaccia V., Crous P.W. Species of Diaporthe on Camellia and Citrus in the Azores Islands. Phytopathol. Medit. 2018;57:307–319. [Google Scholar]
- 10.Guo Y.H., Crous P.W., Bai Q., Fu M., Yang M.M., Wang X.H., Du Y.M., Hong N., Xu W.X., Wang G.P. High diversity of Diaporthe species associated with pear shoot canker in China. Persoonia. 2020;45:132–162. doi: 10.3767/persoonia.2020.45.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Q., Fan X.L., Du Z., Tian C.M. Diaporthe species occurring on Senna bicapsularis in southern China, with descriptions of two species. Phytotaxa. 2017;302:145–155. doi: 10.11646/phytotaxa.302.2.4. [DOI] [Google Scholar]
- 12.Manawasinghe I.S., Dissanayake A.J., Li X., Liu M., Wanasinghe D.N., Xu J., Zhao W., Zhang W., Zhou Y., Hyde K.D., et al. High genetic diversity and species complexity of Diaporthe associated with grapevine dieback in China. Front. Microbiol. 2019;10:1936. doi: 10.3389/fmicb.2019.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang F., Udayanga D., Wang X.H., Hou X., Mei X.F., Fu Y.S., Hyde K.D., Li H. Endophytic Diaporthe associated with Citrus: A phylogenetic reassessment with seven new species from China. Fungal Biol. 2015;119:331–347. doi: 10.1016/j.funbio.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Dong Z., Manawasinghe I.S., Huang Y., Shu Y., Phillips A.J.L., Dissanayake A.J., Hyde K.D., Xiang M.M., Luo M. Endophytic Diaporthe Associated With Citrus grandis cv. Tomentosa in China. Front. Microbiol. 2021;11:609387. doi: 10.3389/fmicb.2020.609387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crous P.W., Groenewald J.Z. Hosts, species and genotypes: Opinions versus data—Presented as a keynote address at the 15th biennial conference of the Australasian plant pathology society, Geelong, Australia, 26–29 September 2005. Australas. Plant Pathol. 2005;34:463–470. doi: 10.1071/AP05082. [DOI] [Google Scholar]
- 16.Promputtha I., Jeewon R., Lumyong S., Mckenzie E., Hyde K. Ribosomal DNA fingerprinting in the identification of non sporulating endophytes from Magnolia liliifera (Magnoliaceae) Fungal Divers. 2005;20:167–186. [Google Scholar]
- 17.Huang W., Cai Y., Hyde K., Corke H., Sun M. Biodiversity of endophytic fungi associated with 29 Traditional Chinese Medicinal plants. Fungal Divers. 2008;33:61–75. [Google Scholar]
- 18.Zhang J.H., Xin H.L., Xu Y.M., Shen Y., He Y.Q., Lin B., Song H.T., Yang H.Y., Qin L.P., Zhang Q.Y., et al. Morinda officinalis How.—A comprehensive review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2018;213:230–255. doi: 10.1016/j.jep.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 19.Dong Z., Shu Y., Luo M., Zhang W., Xiang M.M. First report of black root rot disease on Morinda officinalis caused by Lasiodiplodia pseudotheobromae in China. Plant Dis. 2019;103:2693. doi: 10.1094/PDIS-04-19-0857-PDN. [DOI] [Google Scholar]
- 20.Nguyen D.T., Hieu N.C., Hung N.V., Hoat T.X. Biological control of Fusarium root rot of indian mulberry (Morinda officinalis how.) with consortia of agriculturally important microorganisms in vietnam. Chem. Biol. Technol. Agric. 2019;6:27. doi: 10.1186/s40538-019-0168-x. [DOI] [Google Scholar]
- 21.Zhang Y.X., Yang J., Huang J.H., Xiang M.M. Study on biodiversity of endophytic fungi of Amomum villosum. J. Zhongkai Univ. Agric. Eng. 2010;23:15–17. (In Chinese) [Google Scholar]
- 22.White T., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: MInnis A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols, a Guide to Methods and Applications. Academic Press; New York, NY, USA: 1990. pp. 315–322. [Google Scholar]
- 23.Carbone I., Kohn L. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. doi: 10.1080/00275514.1999.12061051. [DOI] [Google Scholar]
- 24.Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crous P.W., Groenewald J.Z., Risède J., Simoneau P., Hywel-Jones N.L. Calonectria species and their cylindrocladium anamorphs species with sphaeropedunculate vesicles. Stud. Mycol. 2004;50:415–430. doi: 10.3114/sim.55.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall T. Bioedit. Department of Microbiology, North Carolina State University. 2006. [(accessed on 1 July 2021)]. Available online: http://www.mbio.ncsu.edu/BioEdit/page2.html.
- 27.Yang Q., Jiang N., Tian C.M. New species and records of Diaporthe from Jiangxi Province, China. MycoKeys. 2021;77:41–64. doi: 10.3897/mycokeys.77.59999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katoh K., Toh H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 2010;9:286–298. doi: 10.1093/bib/bbn013. [DOI] [PubMed] [Google Scholar]
- 29.Silvestro D., Michalak I. RaxmlGUI: A Graphical Front-End for RAxML. 2010. [(accessed on 25 February 2021)]. Available online: http://sourceforgenet/projects/raxmlgui/
- 30.Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 31.Nylander J.A.A. MrModeltest 2.0. Program Distributed by the Author. Evolutionary Biology Centre Uppsala University; Uppsala, Sweden: 2004. [Google Scholar]
- 32.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller M.A., Pfeiffer W., Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees; Proceedings of the Gateway Computing Environments Workshop (GCE); New Orleans, LA, USA. 14 November 2010; New Orleans, LA, USA: Institute of Electrical and Electronics Engineers; 2010. [Google Scholar]
- 34.Jayasiri S.C., Hyde K.D., Ariyawansa H.A., Bhat J., Buyck B., Cai L., Dai Y.C., Abd-Elsalam K.A., Ertz D., Hidayat I., et al. The faces of fungi database: Fungal names linked with morphology, molecular and human attributes. Fungal Divers. 2015;74:3–18. doi: 10.1007/s13225-015-0351-8. [DOI] [Google Scholar]
- 35.Jayawardena R.S., Hyde K.D., de Farias A.R.G., Bhunjun C.S., Ferdinandez H.S., Manamgoda D.S., Udayanga D., Herath I.S., Thambugala K.M., Manawasinghe I.S., et al. What is a species in fungal plant pathogens? Fungal Divers. 2021;109:239–266. doi: 10.1007/s13225-021-00484-8. [DOI] [Google Scholar]
- 36.Manawasinghe I.S., Phillips A.J.L., Xu J., Balasuriya A., Hyde K.D., Tępień L., Harischandra D.L., Karunarathna A., Yan J., Weerasinghe J., et al. Defining a species in plant pathology: Beyond the species level. Fungal Divers. 2021;109:267–282. doi: 10.1007/s13225-021-00481-x. [DOI] [Google Scholar]
- 37.Rayner R.W. A Mycological Colour Chart. Commonwealth Mycological Institute; Kew, UK: 1970. [Google Scholar]
- 38.Huson D.H., Bryant D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- 39.Norphanphoun C., Gentekaki E., Hongsanan S., Jayawardena R., Senanayake C., Manawasinghe I., Abeywickrama P., Bhunjun C.S., Hyde K.D. Diaporthe: Formalizing species-group 26 concepts. Mycosphere. 2022 unpublished . [Google Scholar]
- 40.Crous P.W., Summerell B.A., Shivas R.G., Burgess T.I., Decock C.A., Dreyer L.L., Granke L.L., Guest D.I., Hardy G.E.S.J., Hausbeck M.K., et al. Fungal Planet description sheets: 107–127. Persoonia. 2012;28:138–182. doi: 10.3767/003158512X652633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun W., Huang S., Xia J., Zhang X., Li Z. Morphological and molecular identification of Diaporthe species in south-western China, with description of eight new species. MycoKeys. 2021;77:65–95. doi: 10.3897/mycokeys.77.59852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang S.T., Xia J.W., Zhang X.G., Sun W.X. Morphological and phylogenetic analyses reveal three new species of Diaporthe from Yunnan, China. MycoKeys. 2021;78:49–77. doi: 10.3897/mycokeys.78.60878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santos J.M., Vrandecic K., Cosic J., Duvnjak T., Phillips A.J.L. Resolving the Diaporthe species occuring on soybean. Persoonia. 2011;27:9–19. doi: 10.3767/003158511X603719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hyde K.D., Norphanphoun C., Chen J., Dissanayake A.J., Doilom M., Hongsan S., Jayawardena R.S., Jeewon R., Perera R.H., Thongbai B., et al. Thailand’s amazing diversity: Up to 96% of fungi in northern Thailand may be novel. Fungal Divers. 2018;93:215–239. doi: 10.1007/s13225-018-0415-7. [DOI] [Google Scholar]
- 45.Farr D.F., Rossman A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. [(accessed on 20 January 2022)]; Retrieved 27 March 2022. Available online: https://nt.ars-grin.gov/fungaldatabases/
- 46.Wrona C.J., Mohankumar V., Schoeman M.H., Tan Y.P., Shivas R.G., Jeff-Ego O.S., Akinsanmi O.A. Phomopsis husk rot of macadamia in Australia and South Africa caused by novel Diaporthe species. Plant Pathol. 2020;69:911–921. doi: 10.1111/ppa.13170. [DOI] [Google Scholar]
- 47.Dayarathne M.C., Jones E.B.G., Maharachchikumbura S.S.N., Devadatha B., Sarma V.V., Khongphinitbunjong K., Chomnunti P., Hyde K.D. Morpho-molecular characterization of microfungi associated with marine based habitats. Mycosphere. 2020;11:1–188. doi: 10.5943/mycosphere/11/1/1. [DOI] [Google Scholar]
- 48.Hyde K.D., Soytong K. The fungal endophyte dilemma. Fungal Divers. 2008;33:163–173. [Google Scholar]
- 49.Ghimire S.R., Charlton N.D., Bell J.D., Krishnamurthy Y.L., Craven K.D. Biodiversity of fungal endophyte communities inhabiting switchgrass (Panicum virgatum L.) growing in the native tall grass prairie of northern Oklahoma. Fungal Divers. 2011;47:19–27. doi: 10.1007/s13225-010-0085-6. [DOI] [Google Scholar]
- 50.Skaltsas D., Castlebury L., Chaverri P. Delimitation of tropical endophytic Phomopsis species from three euphorbiaceous hosts: Hevea brasiliensis, H. guianensis, and Micandra sp. Inoculum. 2011;62:41. [Google Scholar]
- 51.Rhoden S.A., Garcia A., Rubin Filho C.J., Azevedo J.L., Pamphile J.A. Phylogenetic diversity of endophytic leaf fungus isolates from the medicinal tree Trichilia elegans (meliaceae) Genet. Mol. Res. GMR. 2012;11:2513–2522. doi: 10.4238/2012.June.15.8. [DOI] [PubMed] [Google Scholar]
- 52.Doilom M., Dissanayake A.J., Wanasinghe D.N., Boonmee S., Liu J.K., Bhat D.J., Taylor J.E., Bahkali A.H., McKenzie E.H.C., Hyde K.D. Microfungi on Tectona grandis (teak) in Northern Thailand. Fungal Divers. 2016;82:107–182. doi: 10.1007/s13225-016-0368-7. [DOI] [Google Scholar]
- 53.Choi J., Lee K.T., Choi M.Y., Nam J.H., Jung H.J., Park S.K., Park H.J. Antinociceptive antiinflammatory effect of monotropein isolated from the root of Morinda officinalis. Biol. Pharm. Bull. 2005;28:1915–1918. doi: 10.1248/bpb.28.1915. [DOI] [PubMed] [Google Scholar]
- 54.Wu Y.B., Zheng C.J., Qin L.P., Sun L.N., Han T., Jiao L., Zhang Q.Y., Wu J.Z. Antiosteoporotic activity of anthraquinones from Morinda officinalis on osteoblasts and osteoclasts. Molecules. 2009;14:573–583. doi: 10.3390/molecules14010573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang H.L., Li J., Li G., Wang D.M., Zhu L.P., Yang D.P. Structural characterization and anti-fatigue activity of polysaccharides from the roots of Morinda officinalis. Int. J. Biol. Macromol. 2009;44:257–261. doi: 10.1016/j.ijbiomac.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 56.Wang F., Wu L., Li L., Chen S. Monotropein exerts protective effects against IL-1β-induced apoptosis and catabolic responses on osteoarthritis chondrocytes. Int. Immunopharmacol. 2014;23:575–580. doi: 10.1016/j.intimp.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Wu Z.Q., Chen D.L., Lin F.H., Lin L., Shuai O., Wang J.Y., Qi L.K., Zhang P. Effect of bajijiasu isolated from Morinda officinalis F. C. How on sexual function in male mice and its antioxidant protection of human sperm. J. Ethnopharmacol. 2015;164:283–292. doi: 10.1016/j.jep.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 58.Carvalho C.R., Gonalves V.N., Pereira C.B., Johann S., Galliza I.V., Alves T.M.A., Rabello A., Sobral M.E.G., Zani C., Rosa C.A., et al. The diversity, antimicrobial and anticancer activity of endophytic fungi associated with the medicinal plant Stryphnodendron adstringens (mart.) coville (fabaceae) from the brazilian savannah. Symbiosis. 2012;57:95–107. doi: 10.1007/s13199-012-0182-2. [DOI] [Google Scholar]
- 59.Carrión V.J., Perez-Jaramillo J., Cordovez V., Tracanna V., de Hollander M., Ruiz-Buck D., Mendes L.W., Van Ijcken W.F.J., Gomez-Exposito R., Elsayed S.S., et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science. 2019;366:606–612. doi: 10.1126/science.aaw9285. [DOI] [PubMed] [Google Scholar]
- 60.Wonglom P., Ito S.I., Sunpapao A. Volatile organic compounds emitted from endophytic fungus Trichoderma asperellum t1 mediate antifungal activity, defense response and promote plant growth in lettuce (Lactuca sativa) Fungal Ecol. 2020;43:100867. doi: 10.1016/j.funeco.2019.100867. [DOI] [Google Scholar]
- 61.Estrada C., Wcislo W.T., Van Bael S.A. Symbiotic fungi alter plant chemistry that discourages leaf-cutting ants. New Phytol. 2013;198:241–251. doi: 10.1111/nph.12140. [DOI] [PubMed] [Google Scholar]
- 62.Kusari S., Hertweck C., Spiteller M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012;19:792–798. doi: 10.1016/j.chembiol.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 63.Ying W., Liu H.X., Chen Y.C., Sun Z.H., Li H.H., Li S.N., Yan M.L., Zhang W.M. Two new metabolites from the endophytic fungus Alternaria sp. A744 derived from Morinda officinalis. Molecules. 2017;22:765. doi: 10.3390/molecules22050765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Purahong W., Hyde K.D. Effects of fungal endophytes on grass and non-grass litter decomposition rates. Fungal Divers. 2011;47:1–7. doi: 10.1007/s13225-010-0083-8. [DOI] [Google Scholar]
- 65.Chen S., Liu H., Liu Z., Li S., Chen Y., Li H., Li D., Zhang W. Two New Polyketide Compounds from the Endophytic Fungus Trichoderma spirale A725 of Morinda officinalis. Chin. J. Org. Chem. 2020;40:209–214. doi: 10.6023/cjoc201907041. [DOI] [Google Scholar]
- 66.Gond S.K., Verma V.C., Kumar A., Kumar V., Kharwar R.N. Study of endophytic fungal community from different parts of Aegle marmelos correae (rutaceae) from varanasi (India) World J. Microbiol. Biotechnol. 2007;23:1371–1375. doi: 10.1007/s11274-007-9375-x. [DOI] [Google Scholar]
- 67.Rana B.K., Singh U.P., Taneja D. Antifungal activity and kinetics of inhibition by essential oil isolated from leaves of Aegle marmelos. J. Ethnopharmacol. 1997;57:29–34. doi: 10.1016/S0378-8741(97)00044-5. [DOI] [PubMed] [Google Scholar]
- 68.Zhou S.Y., Zhou X., Zhang Z.F., Liang J.M., Cai L. Attempt to isolate hitherto uncultured fungi. Mycosystema. 2020;39:766–776. [Google Scholar]
- 69.Begoude B.A.D., Slippers B., Wingfield M.J., Roux J. The pathogenic potential of endophytic Botryosphaeriaceous fungi on Terminalia species in Cameroon. For. Pathol. 2011;41:281–292. doi: 10.1111/j.1439-0329.2010.00671.x. [DOI] [Google Scholar]
- 70.de Silva D.D., Ades P.K., Crous P.W., Taylor P.W.J. Colletotrichum species associated with chili anthracnose in Australia. Plant Pathol. 2017;66:254–267. doi: 10.1111/ppa.12572. [DOI] [Google Scholar]
- 71.Yang Q., Fan X.L., Guarnaccia V., Tian C.M. High diversity of Diaporthe species associated with dieback diseases in China, with twelve new species described. MycoKeys. 2018;39:97–149. doi: 10.3897/mycokeys.39.26914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dissanayake A.J., Zhang W., Liu M., Hyde K.D., Zhao W.S., Li X.H., Yan J.Y. Diaporthe species associated with peach tree dieback in Hubei, China. Mycosphere. 2017;8:533–549. doi: 10.5943/mycosphere/8/5/2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequence data generated in this study are deposited in NCBI GenBank (https://www.ncbi.nlm.nih.gov/genbank, accessed on 1 April 2022). All accession numbers are given in Table S1.