Abstract
Background: Lifestyle interventions, such as fasting, diet, and exercise, are increasingly used as a treatment option for patients with metabolic syndrome (MS). This study assesses the efficacy and safety of fasting followed by lifestyle modification in patients with MS compared to lifestyle modification only. Methods: Single-blind, multicenter, parallel, randomized controlled trial in two German tertiary referral hospitals in metropolitan areas. Interventions: (a) 5-day fasting followed by 10 weeks of lifestyle modification (modified DASH diet, exercise, mindfulness; n = 73); (b) 10 weeks of lifestyle modification only (n = 72). Main outcomes and measures: Co-primary outcomes were ambulatory systolic blood pressure and the homeostasis model assessment (HOMA) index at week 12. Further outcomes included anthropometric, laboratory parameters, and the PROCAM score at weeks 1, 12, and 24. Results: A total of 145 patients with metabolic syndrome (62.8% women; 59.7 ± 9.3 years) were included. No significant group differences occurred for the co-primary outcomes at week 12. However, compared to lifestyle modification only, fasting significantly reduced HOMA index (Δ = −0.8; 95% confidence interval [CI] = −1.7, −0.1), diastolic blood pressure (Δ = −4.8; 95% CI = −5.5, −4.1), BMI (Δ = −1.7; 95% CI = −2.0, −1.4), weight (Δ = −1.7; 95% CI = −2.0, −1.4), waist circumference (Δ = −2.6; 95% CI = −5.0, −0.2), glucose (Δ = −10.3; 95% CI = −19.0, −1.6), insulin (Δ = −2.9; 95% CI = −5.3, −0.4), HbA1c (Δ = −0.2; 95% CI = −0.4, −0.05;), triglycerides (Δ = −48.9; 95% CI = −81.0, −16.9), IL−6 (Δ = −1.2; 95% CI = −2.5, −0.005), and the 10-year risk of acute coronary events (Δ = −4.9; 95% CI = −9.5, −0.4) after week 1. Fasting increased uric acid levels (Δ = 1.0; 95% CI = 0.1, 1.9) and slightly reduced eGRF (Δ = −11.9; 95% CI = −21.8, −2.0). Group differences at week 24 were found for weight (Δ = −2, 7; 95% CI = −4.8, −0.5), BMI (Δ = −1.0; 95% CI = −1.8, −0.3), glucose (Δ = −7.7; 95% CI = −13.5, −1.8), HDL (Δ = 5.1; 95% CI = 1.5, 8.8), and CRP (Δ = 0.2; 95% CI = 0.03, 0.4). No serious adverse events occurred. Conclusions: A beneficial effect at week 24 was found on weight; fasting also induced various positive short-term effects in patients with MS. Fasting can thus be considered a treatment for initializing lifestyle modification for this patient group; however, it remains to be investigated whether and how the multilayered effects of fasting can be maintained in the medium and longer term.
Keywords: fasting, metabolic syndrome, modified DASH diet, Mediterranean diet, lifestyle, relaxation, MICOM (mind–body medicine in integrative and complementary medicine)
1. Introduction
Modern lifestyle leads to an increasing prevalence of type 2 diabetes, metabolic syndrome, and cardiovascular risk constellations [1]. Most risk factors for cardiovascular disease can be influenced by patients’ behavior; this applies above all to poor nutrition, being overweight, lack of exercise, and psychological distress [1]. Epidemiological studies also underline the role of psychological risk factors, such as psychosocial distress, depression, and anxiety, in cardiac health [2,3]. Most coronary patients do not achieve their blood pressure, low-density lipoprotein (LDL) cholesterol, and glucose targets [4]. Moreover, cardiovascular prevention requires advanced preventive cardiological programs delivered by interdisciplinary teams of healthcare professionals, which address all aspects of lifestyle and risk factor management [4].
Lifestyle modification targeting physical inactivity, diet, and psychosocial stress have thus been associated with significant reductions in blood pressure and improvements of other cardiovascular risk factors in risk groups [5,6,7,8,9,10]. There also are hints that combinations of multiple lifestyle modifications might be superior to interventions only targeting a single health behavior [11,12,13]. There is increasing interest and evidence that fasting might substantially reduce cardiovascular risk factors [14,15,16].
There is an increasing number of randomized studies on intermittent fasting in cardiometabolic endpoints, generally lasting 16 to 48 h [16]. Adults who practiced TRE typically lost 1% to 4% of their body weight within several weeks [17,18,19,20]. Furthermore, TRE can improve cardiometabolic endpoints, such as insulin sensitivity and blood pressure [16,21]. Fasting over a longer period, normally from 3 to 21 or more days, has been less studied in humans, although it has a long-standing history in Europe [20]. Fasting is commonly defined as the daily nutritional energy intake of 200 to 500 kcal for up to four weeks [22]. It has been shown to lower blood pressure, blood lipid levels, and other cardiovascular risk factors at least in the short term and appears to be associated with only few adverse events even in diseased populations [22,23]. Animal models of repeated cycles of fasting suggest reductions in mortality and age-associated morbidity, altered signaling, e.g., in signaling pathways of insulin, IGF-1, AMPK, or mTor, as well as enhanced autophagy and ketone body production [24]. Most of these effects have been confirmed in the first human studies on prolonged, periodic, and intermittent fasting as well as fasting-mimicking diets in the meantime [16,24,25,26].
This study aimed to assess the effects of fasting in patients with metabolic syndrome, followed by a multimodal lifestyle modification intervention named MICOM (mind–body medicine in integrative and complementary medicine), compared to lifestyle modification intervention only. We hypothesized a priori that a 5-day fast followed by 10 weeks of lifestyle modification would be more effective for reducing ambulatory systolic blood pressure and the homeostasis model assessment (HOMA) index than lifestyle modification alone.
2. Materials and Methods
2.1. Design
This single-blind, multicenter, parallel, randomized controlled trial was conducted at the Department of Internal and Integrative Medicine, Evang. Kliniken Essen-Mitte, Essen, Germany and the Department of Internal and Integrative Medicine, Immanuel Hospital Berlin and Charité Outpatient Center for Complementary and Integrative Medicine, Berlin, Germany. The study had been approved by the ethics committees of the Charité-Universitätsmedizin Berlin (approval number: EA4/141/13) and the University of Duisburg-Essen (approval number: 14-5733 BO) and registered at ClinicalTrials.gov (registration number: NCT02099968) prior to patient recruitment. The study was conducted and reported in accordance with the CONSORT (CONsolidated Standards of Reporting Trials) 2010 guidelines [27]. No important changes were made to the study protocol after trial commencement.
2.2. Participants
Patients were recruited from study centers and through local newspaper announcements, screened by a research assistant to assess eligibility, and, if apparently eligible, assessed by a study physician. If patients met all inclusion criteria and did not meet any exclusion criteria, informed consent was obtained, and they were included in the trial.
Patients (male/female) with a metabolic syndrome according to the National Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (NCEP-ATP-III) criteria were included. Patients were further required to be diagnosed with systolic hypertension and/or with an additional subclinical atherosclerosis (<50% coronary artery stenosis, <50% carotid artery stenosis, or peripheral artery disease stage 1).
Exclusion criteria included: (i) diabetes mellitus type 1 or insulin bolus therapy (c-peptide < 1.2 ng/mL), (ii) coronary artery disease, myocardial infarction, pulmonary embolism, or stroke within the past 3 months, (iii) heart failure ≥ stage I NYHA, (iv) peripheral artery disease ≥ stage 2, (v) chronic kidney disease > stage 2 (GFR < 60 mL/min), (vi) eating disorder, dementia, or psychosis, or (vii) other severe internal diseases.
2.3. Interventions
Both interventions were group-based and conducted by a multidisciplinary team of healthcare professionals (nutritional counselors, mind–body therapists, or sport therapists) with board certified education. They incorporated aspects of the mind–body program, which was originally developed by the Benson-Henry Mind/Body Medical Institute at Harvard Medical School and further developed and adapted to the German needs, as described in the MICOM (mind–body medicine in integrative and complementary medicine) program [28]. This program focuses on mindfulness and specific group training that are rooted in psychoneuroendocrinology and use formal meditation and gentle yoga exercises. Nutritional education included counseling, comprehensive lectures, and cooking classes.
2.3.1. Fasting and Lifestyle Modification (F + LM)
Patients in this group started with two calorie-restricted vegan days (max 1200 kcal/day), followed by a 5-day modified fasting intervention (intake of 300–350 kcal/day, obtained from vegetable juices and vegetable broth). Thereafter, a stepwise reintroduction of food according to published guidelines of fasting was performed, followed by a group dietary and lifestyle modification intervention [29] (weekly 6-h multimodal sessions for a total of 10 weeks). This included lectures and teaching kitchens on a vegetarian whole-food diet with a focus on a plant-based Mediterranean diet and a modified DASH diet [30,31,32], intermittent fasting (rice only days once/week) [33], and recommendations for specific cardioprotective foods, such as beetroot, nuts, and olive oil.
Each session started with 20–30 min of activating exercises, and moderate aerobic exercise, i.e., walking, was introduced in a 45-min supervised training at one of the first sessions. Each session closed with a supervised training in stress reduction using progressive muscle relaxation, mindfulness meditation, or yoga. Mindfulness was further incorporated in a 90-min supervised training at one of the first sessions. The mindfulness session included a theoretical introduction into the concept of mindfulness combined with practical exercises of meditation, yoga, qigong, body scan, etc. Mindfulness in everyday life was specifically targeted during homework, where participants are taught to be mindful during routine activities, such as eating, talking, or working.
Home practice outside the session was encouraged for both aerobic exercise and relaxation/mindfulness training.
After the end of the program, in weeks 13 to 24, monthly group sessions were offered to ensure adherence.
2.3.2. Lifestyle Modification (LM)
The lifestyle intervention was similar to the one performed for the fasting and lifestyle modification group, without the fasting intervention. The program consisted of 55 h of group intervention over a period of 10 weeks. After the end of the program, in months 13 to 24, monthly group sessions were offered to ensure adherence.
2.4. Randomization
Patients were randomly allocated 1:1 to F + LM or LM by block-randomization with randomly varying block lengths, stratified by (a) study center and (b) the intake/nonintake of antihypertensive medication. The randomization list was created by a biometrician not involved in patient recruitment or assessment, using the Random Allocation Software [34]. The list was password-secured, and no person other than the biometrician was able to access it. Based on these results, the sealed, sequentially numbered opaque envelopes containing the treatment assignments were prepared. After obtaining written informed consent and baseline assessment, the study physician opened the lowest numbered envelope to reveal that patient’s assignment.
2.5. Outcome Measures
Outcomes were assessed at baseline and at 1, 12, and 24 weeks after randomization by a blinded outcome assessor who was not involved in patient recruitment, allocation, or treatment. Two primary outcome measures were defined: ambulatory systolic blood pressure and the homeostasis model assessment (HOMA) index at week 12. Herein, primary outcomes and further clinical parameters are reported. Further explorative experimental variables (immune function, microbiome) are reported elsewhere [35]. Further psychometric parameters will be reported elsewhere.
2.5.1. Physician-Assessed Outcomes
Twenty-four-hour ambulatory systolic and diastolic blood pressure were measured using an validated digital blood pressure monitor (Mobil-O-Graph® PWA, I.E.M., Stolberg, Germany) [36]. Measurements at week 0 were made within a week before the start, those at week 12 within a week after the end of the intervention at the same time of day at each of the three measurement time points. The monitoring software automatically removed incorrect measurements using built-in algorithms. Clinical blood pressure was measured in the hospital by a sphygmomanometer, using the average of three consecutive measurements. Clinical blood pressure was measured at each time point and ambulatory blood pressure only at baseline at weeks 12 and 24.
Body weight was measured using the Omron BF 511 bioelectrical impedance device [37]. BMI was calculated as the weight in kilograms divided by the square of height in meters. Waist circumference was measured by two research assistants using a measuring tape in the horizontal plane exactly midway between the iliac crest and the costal arch. Measures were repeated twice, and the mean of both measures was used; if the two measures differed by more than 1 cm, both measurements were repeated. Hip circumference was measured in the horizontal plain at the maximal circumference of the hips or buttock region above the gluteal fold, whichever is larger, using the same approach as for waist circumference. Waist–hip ratio was calculated as the quotient of waist circumference and hip circumference [38]. Body fat percentage and muscle mass percentage were measured by bioelectrical impedance analysis using the Omron BF 511 bioelectrical impedance device [37].
2.5.2. Laboratory Measures
Blood samples were collected from the antecubital vein into vacutainer tubes and analyzed using the Modular P analyzer (Roche, Mannheim, Germany). Metabolic parameters included blood glucose levels, blood insulin levels, and HbA1c, and were analyzed using standard procedures. HOMA index was calculated as blood insulin level (µU/mL) × blood glucose level (mmol/L)/22.5 [39]. Further laboratory parameters included blood lipid levels (total cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride), uric acid, blood creatinine level, estimated glomerular filtration rate (eGFR), C-reactive protein (CRP), insulin-like growth factor 1 (IGF-1), and interleukin 6 (IL-6).
2.5.3. PROCAM Score
Cardiovascular risk was calculated by the PROCAM (Prospective Cardiovascular Münster Study) score considering clinical (age, smoking status, diagnosis of diabetes mellitus type 2, systolic blood pressure, intake of antihypertensive medication, myocardial infarction, and/or stroke within the close family) and laboratory parameters (HDL, LDL, triglyceride, and fasting glucose level) [40]. Based on this score, the 10-year risk of an acute coronary event was calculated [40].
2.6. Safety
All adverse events occurring during the study period were recorded. Patients experiencing such adverse events were asked to see the study physician to assess their import and initiate any necessary response. Open-ended questions were used at week 1, 12, and week 24 to assess any adverse events not previously mentioned by the patients. Patients were asked to indicate any adverse events during the study period regardless of their severity or potential relationship to the study intervention.
2.7. Sample Size Calculation and Statistical Analysis
The required sample size was calculated a priori using G*Power software [41]. Based on prior research on multimodal lifestyle interventions [6,7], such as yoga [42], mindfulness [43], and Mediterranean diet [44], a between-group effect size of d = 0.5 was expected. A level 2.5% t-test requires a total of 64 patients per group to detect a respective group difference with a statistical power of 80%. Accounting for a potential loss in power because of a maximum of 10% dropouts, it was planned to include at least 142 patients in this trial.
All analyses were based on an intention-to-treat basis, including all participants being randomized, regardless of whether they provided a full set of data or adhered to the study protocol. Missing data were imputed by multiple means using Markov chain Monte Carlo methods [45,46], yielding a total of 50 complete datasets.
Group differences were analyzed by univariate analyses of covariance (ANCOVA), which modeled the outcome at week 1, 12, or 24 as a function of the treatment group (classified factor), the stratification factors study center (classified covariate), baseline antihypertensive medication intake (classified covariate), and the respective baseline value (linear covariate). Afterward, the 50 estimates of group differences were combined to produce overall effect size estimates, 95% confidence intervals, and p-values.
Thus, the analysis accounted for potential baseline differences in medication and in the respective outcomes. Inferential statistical tests for baseline differences between groups were not conducted because the CONSORT statement explicitly discourages such tests, given that baseline differences in a randomized trial are generally considered to be random.
For the primary and secondary outcomes, p-values ≤ 0.025 and ≤0.050, respectively, were considered significant.
All analyses were performed using the Statistical Package for Social Sciences software (IBM SPSS Statistics for Windows, release 22.0, IBM Group, Armonk, NY, USA).
3. Results
3.1. Patients
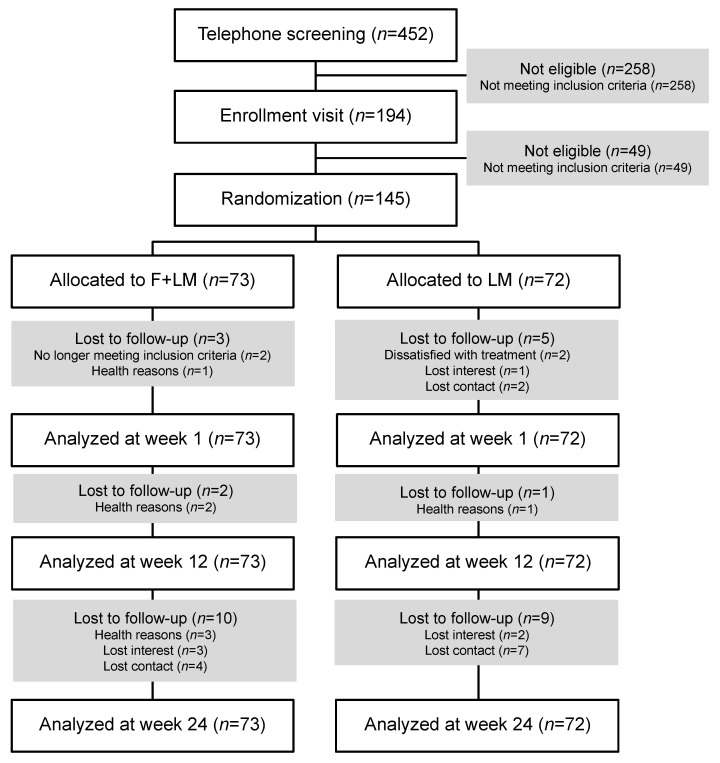
A total of 452 patients were telephone screened, and 258 were excluded because they did not meet the inclusion criteria (Figure 1). A total of 49 patients were excluded after medical assessment. A total of 145 patients were enrolled after providing informed consent and were randomized to the F + LM group (n = 73) or the LM group (n = 72). During the study period, 15 patients each were omitted during follow-up in the F + LM and LM groups (Figure 1). Participants’ mean age was 59.7 ± 9.3 in the whole study population (Table 1) and about two-thirds of the participants were female, one-third of those having a university degree. Baseline characteristics were balanced between groups. Patients had a BMI of 33.3 ± 4.5 kg/m2 (Table 1). Patients in the F + LM group attended a mean of 8.2 ± 2.3 (82.0%) out of 10 possible intervention sessions; patients in the LM group attended 7.1 ± 3.5 (71%) sessions (p = 0.124).
Figure 1.
Study flow chart. Abbreviations: F + LM, fasting and lifestyle modification; LM, lifestyle modification.
Table 1.
Baseline sociodemographic and clinical characteristics. If not otherwise denoted, values are reported as mean ± standard deviation. Abbreviations: F + LM, fasting and lifestyle modification; LM, lifestyle modification.
| Total (n = 145) |
F + LM (n = 73) |
LM (n = 72) |
|
|---|---|---|---|
| Sociodemographic characteristics | |||
| Gender female n (%) | 91 (62.8%) | 48 (65.8%) | 43 (59.7%) |
| Age years | 59.7 ± 9.3 | 58.6 ± 10.8 | 60.8 ± 7.5 |
| Marital status n (%) | |||
| Single | 15 (10.3%) | 6 (8.2%) | 9 (12.5%) |
| Married | 98 (67.6%) | 49 (67.1%) | 49 (68.1%) |
| Divorced | 21 (14.5%) | 12 (16.4%) | 9 (12.5%) |
| Widowed | 5 (3.4%) | 3 (4.1%) | 2 (2.8%) |
| Education n (%) | |||
| Secondary modern school (“Hauptschule”) qualification | 25 (17.2%) | 9 (12.3%) | 16 (22.2%) |
| High school (“Realschule”) qualification | 41 (28.3%) | 19 (26.0%) | 22 (30.6%) |
| A level (“Abitur”) | 18 (12.4%) | 12 (16.4%) | 6 (8.3%) |
| University degree | 53 (36.6%) | 28 (38.3%) | 25 (34.7%) |
| Employment n (%) | |||
| Employed full-time | 41 (28.3%) | 20 (27.4%) | 21 (29.2%) |
| Employed part-time | 19 (13.1%) | 11 (15.1%) | 8 (11.1%) |
| Occasionally | 5 (3.4%) | 3 (4.1%) | 2 (2.8%) |
| On sick leave | 3 (2.1%) | 2 (2.7%) | 1 (1.4%) |
| Unemployed | 3 (2.1%) | 2 (2.7%) | 1 (1.4%) |
| Retired age-related | 44 (30.3%) | 20 (27.4%) | 24 (33.3%) |
| Retired health-related | 15 (10.3%) | 7 (9.6%) | 8 (11.1%) |
| Homekeeper | 10 (6.9%) | 6 (8.2%) | 4 (5.6%) |
| Clinical characteristics | |||
| Weight kg | 97.0 ± 15.8 | 98.1 ± 16.1 | 95.9 ± 15.5 |
| Body Mass Index kg/m2 | 33.3 ± 4.5 | 33.7 ± 4.5 | 32.84.5 |
| Diagnosis of obesity since months | 65.0 ± 123.6 | 73.3 ± 140.7 | 56.5 ± 103.8 |
| Diagnosis of hypertension since months | 119.5 ± 112.4 | 119.3 ± 113.8 | 119.7 ± 111.8 |
| Antihypertensive drugs since months | 106.0 ± 108.6 | 98.7 ± 101.7 | 113.4 ± 115.4 |
| Diagnosis of coronary artery disease n (%) | 5 (3.4%) | 2 (2.7%) | 3 (4.2%) |
| Diagnosis of peripheral artery disease n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Diagnosis of hyperlipidemia since months | 62.2 ± 83.1 | 63.7 ± 91.9 | 60.7 ± 73.9 |
| Lipid-lowering drugs since months | 30.4 ± 59.0 | 28.3 ± 56.9 | 32.6 ± 61.4 |
| Diagnosis of hyperglycemia since months | 11.5 ± 39.0 | 10.2 ± 32.6 | 12.7 ± 44.8 |
| Anti-hyperglycemic drugs since months | 17.2 ± 36.1 | 9.0 ± 24.8 | 25.6 ± 43.3 |
| Bariatric surgery n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
3.2. Primary Outcome Measures
The two primary outcome measures, 24-h ambulatory systolic blood pressure and the HOMA index at week 12, showed reductions in both groups and were not significantly different between the groups (Table 2 and Table 3).
Table 2.
Effects of the study interventions on physician-assessed outcomes, the PROCAM score, and the 10-year risk of acute coronary events based on the PROCAM score. Values are expressed as mean ± standard deviation. Bold p-values indicate significant group differences (p < 0.05). Abbreviations: BP, blood pressure; CI, confidence interval; F + LM, fasting and lifestyle modification; LM, lifestyle modification; NA, not assessed.
| Outcome | Group | Week 0 | Week 1 | Week 1 | Week 12 | Week 12 | Week 24 | Week 24 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group Difference (95% CI) | p | Group Difference (95% CI) | p | Group Difference (95% CI) | p | ||||||
| Ambulatory systolic BP | F + LM | 131.1 ± 9.1 | NA | NA | NA | 126.9 ± 8.9 | −0.5 (−5.0, 3.9) | 0.813 | 130.0 ± 9.0 | −1.2 (−5.6, 2.1) | 0.366 |
| LM | 132.5 ± 11.0 | NA | 129.3 ± 10.8 | 130.7 ± 9.7 | |||||||
| Ambulatory diastolic BP | F + LM | 80.1 ± 8.2 | NA | NA | NA | 78.4 ± 8.2 | −1.3 (−4.8, 2.1) | 0.450 | 79.1 ± 7.6 | −1.0 (−4.2, 2.3) | 0.501 |
| LM | 82.0 ± 8.3 | NA | 80.2 ± 8.7 | 80.6 ± 8.5 | |||||||
| Clinical systolic BP | F + LM | 138.9 ± 14.4 | 130.9 ± 16.1 | −4.1 (−11.3, 3.2) | 0.270 | 133.7 ± 13.5 | 1.6 (−4.4, 7.5) | 0.609 | 138.3 ± 14.4 | 2.5 (−3.6, 8.5) | 0.417 |
| LM | 141.2 ± 19.0 | 136.2 ± 14.5 | 134.5 ± 12.3 | 135.2 ± 10.8 | |||||||
| Clinical diastolic BP | F + LM | 88.3 ± 10.6 | 81.5 ± 9.7 | −4.8 (−9.6, −0.06) | 0.047 | 86.5 ± 11.2 | 3.4 (−0.7, 7.5) | 0.106 | 88.7 ± 10.3 | −2.7 (−6.8, 1.4) | 0.200 |
| LM | 89.5 ± 11.2 | 87.0 ± 11.1 | 86.7 ± 8.7 | 87.7 ± 8.8 | |||||||
| Weight | F + LM | 98.1 ± 16.1 | 93.2 ± 15.2 | −4.8 (−5.5, −4.1) | <0.001 | 92.8 ± 15.4 | −3.5 (−5.1, −1.8) | <0.001 | 93.3 ± 15.5 | −2.7 (−4.8, −0.5) | 0.014 |
| LM | 95.9 ± 15.5 | 95.8 ± 15.3 | 94.3 ± 15.1 | 93.8 ± 15.1 | |||||||
| Body Mass Index | F + LM | 33.7 ± 4.5 | 32.1 ± 4.3 | −1.7 (−2.0, −1.4) | <0.001 | 31.9 ± 4.3 | −1.3 (−1.9, −0.7) | <0.001 | 32.1 ± 4.4 | −1.0 (−1.8, −0.3) | 0.007 |
| LM | 32.8 ± 4.5 | 32.8 ± 4.4 | 32.3 ± 4.4 | 32.1 ± 4.3 | |||||||
| Waist circumference | F + LM | 114.1 ± 10.5 | 110.7 ± 11.2 | −2.6 (−5.0, −0.2) | 0.035 | 108.2 ± 11.5 | −3.5 (−5.8, −1.1) | 0.004 | 108.9 ± 10.9 | −1.2 (−6.3, 3.9) | 0.633 |
| LM | 112.1 ± 11.1 | 111.3 ± 11.1 | 109.4 ± 10.9 | 107.2 ± 16.5 | |||||||
| Waist/hip Ratio | F + LM | 1.0 ± 0.5 | 1.0 ± 0.1 | −0.01 (−0.05, 0.03) | 0.486 | 1.0 ± 0.1 | −0.03 (−0.06, 0.01) | 0.192 | 1.0 ± 0.1 | −0.01 (−0.06, 9.04) | 0.658 |
| LM | 1.0 ± 0.2 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | |||||||
| Body fat percentage | F + LM | 41.5 ± 8.9 | 42.0 ± 9.1 | 0.6 (−0.8, 1.9) | 0.420 | 39.4 ± 8.9 | −2.2 (−3.4, 0.9) | 0.001 | 40.1 ± 9.2 | −1.0 (−2.6, 0.6) | 0.214 |
| LM | 39.4 ± 8.6 | 39.9 ± 8.6 | 39.2 ± 8.7 | 39.1 ± 8.8 | |||||||
| PROCAM Score | F + LM | 48.4 ± 13.6 | 45.4 ± 13.2 | −2.4 (−5.6, 0.8) | 0.139 | 45.8 ± 13.4 | −3.4 (−6.7, −0.2) | 0.048 | 47.3 ± 12.7 | −2.0 (−5.5, 1.4) | 0.242 |
| LM | 50.1 ± 11.8 | 50.9 ± 13.2 | 49.7 ± 12.9 | 50.0 ± 11.6 | |||||||
| 10-year coronary risk | F + LM | 14.9 ± 12.6 | 12.1 ± 11.2 | −4.9 (−9.5, −0.4) | 0.033 | 12.6 ± 11.8 | −6.2 (−10.3, −2.0) | 0.004 | 13.7 ± 12.0 | −3.8 (−8.2, 0.5) | 0.080 |
| LM | 16.0 ± 13.6 | 17.4 ± 15.2 | 15.5 ± 12.9 | 15.8 ± 12.5 | |||||||
Table 3.
Effects of the study interventions on laboratory parameters. Values are expressed as mean ± standard deviation. Bold p-values indicate significant group differences (p < 0.05). Abbreviations: BP, blood pressure; CI, confidence interval; CRP, C-reactive Protein; eGFR, estimated glomerular filtration rate; F + LM, fasting and lifestyle modification; HbA1c, glycated hemoglobin; HOMA, homeostasis model assessment; IGF, insulin-like growth factors; IL, interleukin; LM, lifestyle modification.
| Outcome | Group | Week 0 | Week 1 | Week 1 | Week 12 | Week 12 | Week 24 | Week 24 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group Difference (95% CI) | p | Group Difference (95% CI) | p | Group Difference (95% CI) | p | ||||||
| HOMA index | F + LM | 3.5 ± 2.5 | 2.0 ± 1.6 | −0.8 (−1.7, −0.1) | 0.046 | 3.2 ± 2.2 | 0.2 (−0.7, 1.1) | 0.676 | 3.0 ± 1.9 | −0.2 (−0.9, 0.6) | 0.639 |
| LM | 3.7 ± 2.4 | 3.4 ± 2.3 | 3.4 ± 2.2 | 3.6 ± 2.0 | |||||||
| Blood glucose | F + LM | 113.3 ± 18.9 | 107.0 ± 18.3 | −10.3 (−19.0, −1.6) | 0.022 | 106.2 ± 13.4 | −7.7 (−17.2, 1.9) | 0.113 | 110.5 ± 12.8 | −7.7 (−13.5, −1.8) | 0.011 |
| LM | 110.1 ± 22.0 | 114.4 ± 26.9 | 109.3 ± 24.4 | 111.5 ± 20.3 | |||||||
| Blood insulin | F + LM | 12.4 ± 7.3 | 8.0 ± 5.4 | −2.9 (−5.3, −0.4) | 0.024 | 11.6 ± 6.6 | 0.9 (−1.4, 3.3) | 0.428 | 10.9 ± 5.9 | −0.5 (−2.7, 1.7) | 0.641 |
| LM | 13.0 ± 7.5 | 12.4 ± 7.0 | 11.9 ± 5.9 | 12.4 ± 5.7 | |||||||
| HbA1c | F + LM | 5.9 ± 0.5 | 5.8 ± 0.5 | −0.2 (−0.4, −0.05) | 0.010 | 5.8 ± 0.5 | −0.08 (−0.3, 0.1) | 0.485 | 5.9 ± 0.4 | −0.2 (−0.4, 0.04) | 0.122 |
| LM | 5.9 ± 0.7 | 6.0 ± 0.7 | 5.9 ± 0.7 | 6.0 ± 0.7 | |||||||
| Total cholesterol | F + LM | 224.4 ± 50.0 | 208.4 ± 47.6 | −6.9 (−25.3, 11.5) | 0.458 | 214.3 ± 40.6 | −4.0 (−19.7, 11.7) | 0.616 | 227.7 ± 39.9 | 9.5 (−9.7, 27.9) | 0.339 |
| LM | 224.3 ± 48.3 | 224.9 ± 47.5 | 212.8 ± 42.2 | 216.8 ± 42.0 | |||||||
| HDL cholesterol | F + LM | 53.4 ± 16.0 | 49.2 ± 12.0 | −1.1 (−4.4, 2.3) | 0.531 | 53.4 ± 14.7 | 3.3 (−1.0, 7.7) | 0.134 | 55.3 ± 13.3 | 5.1 (1.5, 8.8) | 0.007 |
| LM | 56.6 ± 19.0 | 54.1 ± 16.0 | 53.1 ± 15.7 | 53.5 ± 15.1 | |||||||
| LDL cholesterol | F + LM | 140.2 ± 37.3 | 134.1 ± 40.3 | −0.9 (−16.3, 14.5) | 0.904 | 135.2 ± 34.5 | −3.6 (−17.1, 9.9) | 0.598 | 144.9 ± 32.1 | 7.1 (−7.7, 21.9) | 0.344 |
| LM | 139.6 ± 43.5 | 142.3 ± 42.6 | 132.8 ± 37.4 | 137.8 ± 37.5 | |||||||
| Triglyceride | F + LM | 188.0 ± 210.6 | 116.4 ± 53.9 | −48.9 (−81.0, −16.9) | 0.003 | 157.4 ± 89.5 | −23.0 (−58.2, 12.1) | 0.197 | 157.3 ± 93.9 | −21.9 (−59.4, 15.6) | 0.250 |
| LM | 175.5 ± 111.1 | 169.9 ± 93.4 | 175.3 ± 101.9 | 161.0 ± 78.4 | |||||||
| Uric acid | F + LM | 6.3 ± 1.7 | 8.0 ± 2.2 | 1.0 (0.1, 1.9) | 0.026 | 6.3 ± 1.6 | −0.1 (−0.9, 0.6) | 0.710 | 6.2 ± 1.6 | 0.1 (−0.5, 0.7) | 0.650 |
| LM | 6.6 ± 1.5 | 6.7 ± 1.7 | 6.2 ± 1.5 | 6.2 ± 1.2 | |||||||
| Creatinine | F + LM | 0.9 ± 0.2 | 1.0 ± 0.2 | 0.04 (−0.05, 0.1) | 0.383 | 0.9 ± 0.2 | −0.03 (−0.1, 0.04) | 0.354 | 0.8 ± 0.2 | −0.04 (−0.1, 0.02) | 0.187 |
| LM | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | |||||||
| eGFR | F + LM | 83.5 ± 15.7 | 73.4 ± 17.5 | −11.9 (−21.8, −2.0) | 0.019 | 85.8 ± 13.6 | 1.4 (−6.4, 9.1) | 0.728 | 86.6 ± 14.6 | 2.4 (−6.1, 11.0) | 0.577 |
| LM | 82.4 ± 14.5 | 81.5 ± 14.5 | 82.9 ± 13.6 | 82.1 ± 12.2 | |||||||
| CRP | F + LM | 0.4 ± 0.4 | 0.5 ± 0.4 | 0.03 (−0.1, 0.2) | 0.677 | 0.4 ± 0.5 | 0.04 (−0.1, 0.2) | 0.628 | 0.4 ± 0.5 | 0.2 (0.03, 0.4) | 0.024 |
| LM | 0.3 ± 0.3 | 0.4 ± 0.3 | 0.3 ± 0.3 | 0.4 ± 0.3 | |||||||
| IGF-1 | F + LM | 119.9 ± 38.1 | 104.1 ± 40.6 | −13.6 (−28.8, 1.5) | 0.077 | 123.7 ± 38.6 | −7.6 (−20.2, 5.0) | 0.235 | 126.5 ± 40.6 | 2.4 (−11.7, 16.6) | 0.736 |
| LM | 126.2 ± 48.2 | 120.0 ± 44.2 | 129.9 ± 43.7 | 128.4 ± 43.7 | |||||||
| IL-6 | F + LM | 3.1 ± 2.0 | 2.8 ± 2.7 | −1.2 (−2.5, −0.005) | 0.049 | 3.4 ± 4.7 | −1.5 (−3.5, 0.5) | 0.149 | 2.9 ± 1.7 | −0.5 (−1.5, 0.6) | 0.358 |
| LM | 2.8 ± 2.2 | 3.1 ± 2.8 | 3.7 ± 4.2 | 3.2 ± 1.6 | |||||||
3.3. Physician-Assessed Outcomes and PROCAM Score
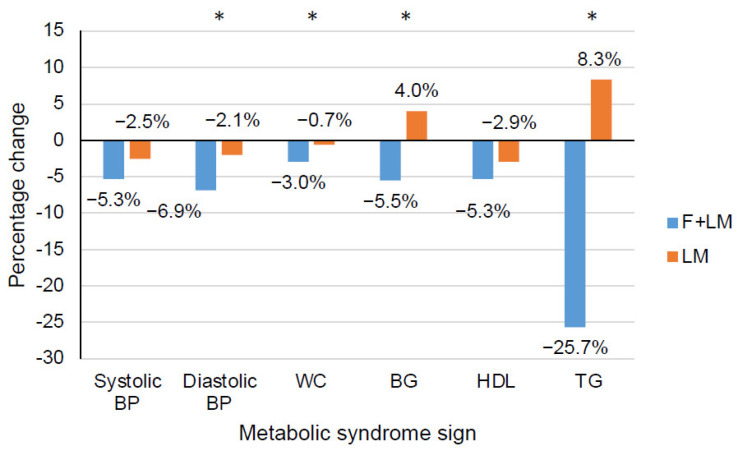
At week 1, after the fasting week, the clinical diastolic blood pressure, weight, BMI, waist circumference, and the 10-year risk of acute coronary events (based on the PROCAM score) were significantly lower in the F + LM group than in the LM group (Table 2, Figure 2). While these effects were maintained at week 12, along with further effects on body fat percentage and the PROCAM score, only weight and BMI were lower in the F + LM group at week 24 (Table 2).
Figure 2.
Percentage change in diagnostic signs of the metabolic syndrome after the 5-day fasting period (F + LM) or 5-day waiting period (LM). Abbreviations: BG, blood glucose level; BP, blood pressure; F + LM, fasting and lifestyle modification; HDL, high-density lipoprotein cholesterol; LM, lifestyle modification; TG, triglycerides; WC, waist circumference; *, p < 0.05.
3.4. Laboratory Parameters
Group differences favoring the F + LM group over the LM group were found after the fasting week for HOMA index, blood glucose level, blood insulin level, HbA1c, triglyceride levels, and IL-6 (Table 3, Figure 2). Uric acid and eGFR significantly worsened in the F + LM group compared to the LM group after the fasting week. None of these group differences persisted at week 12. However, further positive effects favoring F + LM over LM at week 24 were found for blood glucose level and HDL cholesterol (Table 2). CRP was higher in F + LM compared to LM at week 24.
3.5. Safety
One serious adverse event each occurred in both groups. In the F + LM group, an acute diverticulitis occurred in a patient with a known diverticulosis; a causal relationship to the study intervention was rated as unlikely. The patient completely recovered after sigmoid resection and stationary treatment. In the LM group, a patient had suffered a “trauma” (physical and psychological), resulting in a hospitalization; further details could not be elicited as the patient did not want to give further information. A total of 73 minor adverse events occurred in 43 patients in the F + LM group, and a total of 51 minor adverse events occurred in 32 patients in the LM group (Table S1 in the Supplementary Material).
4. Discussion
No significant between-group differences were found in the two primary outcomes, 24-h systolic blood pressure and HOMA index, at week 12 in patients with metabolic syndrome. However, there were interesting exploratory findings suggesting benefits from fasting in this patient population. In patients initially fasting for 5 days, lower body weight and BMI values were found at all three assessment points compared to the non-fasting group along with further, mainly short-term, anthropometric and laboratory differences. Markedly, all parameters of the metabolic syndrome except for HDL cholesterol showed significant improvement after the fasting period compared to the non-fasting period. Both interventions were safe.
Trial participants were demographically heterogeneous. More than 60% were women. The distributions of educational attainment were broad but slightly skewed toward persons with higher education. The inclusion criteria of the study included a large proportion of patients who were German adults with metabolic syndrome. These aspects of the study suggest that the study results should be applicable to a large proportion of the German population.
The beneficial augmenting cardiometabolic effects of fasting are in line with previous studies [22,23,26]. Calorie restriction and fasting have been argued to reduce cardiovascular risk; however, this was predominantly demonstrated in animal models [47,48], uncontrolled studies [23], and studies including time-restricted eating, intermittent fasting, and fasting-mimicking diets [26]. In a large observational study [23], weight loss increased and abdominal circumference decreased with the length of the fasting period. Beneficial effects on blood pressure, blood lipids, and glycaemia were also shown.
At least in the short-term, the 6% absolute risk reduction in cardiovascular events in this study corroborates these findings. Proposed mechanisms for risk reduction include reductions in age-associated changes in the heart and vessels as well as reduced levels of apoptosis, insulin and IGF-1 signaling, enhanced autophagy, and ischemic preconditioning [24,47].
Minor reduction in calculated glomerular filtration was not persistent and might be attributed to mild protein catabolism [23]. Likewise, increased uric acid levels during fasting are known in the literature but have not been associated with symptomatic gout [22,23]. On the one hand, the increased concentration of uric acid is probably due to a slight initial increase in protein catabolism. On the other hand, once the levels of ketone bodies rise in the serum, uric acid is being retained in the kidneys due to both substances competing for tubular secretion and ketone bodies being preferentially secreted during fasting [49]. Prior studies found no deterioration of renal function [23]. Nevertheless, the effect of fasting on kidney function must be further investigated.
Our study has some important limitations. First, the main limitation is the lack of effects at week 24. Since the primary assessment time point was the end of the lifestyle modification programs, the short-term effects of fasting can be regarded as preliminary only. While the interventions included dietary advice, fasting was used only once and no approaches to maintain the effects of fasting in the medium and longer term, such as regular intermittent fasting or repeated cycles of fasting or fasting-mimicking diets, as recently suggested, were used [15,26]. It seems likely that the mean difference between groups expected in the sample size calculation was chosen too optimistically in view of the relatively strong control group. Accordingly, the lack of effects at week 24 could also be attributed to a lack of power. Second, adherence to the (dietary) interventions was not assessed in detail (e.g., via nutritional protocols). Third, the time intensity of the two programs was slightly different, as more elements were implemented in the F + LM group due to the fasting component.
Against the background of the above-mentioned limitations, future studies should undertake efforts to maintain the fasting effect by repeating fasting cycles or the additive use of intermittent fasting. Hereby, relevant and delineated mechanisms behind the metabolic switch of fasting, such as endocrine and neurobiological effects, autophagy and microbiome-related effects should be considered and translated to clinical protocols [47,50]. Future studies will likely require substantially larger sample sizes to detect group differences in e.g., coronary risk scores, if such effects do exist.
Most health insurance companies do not cover multimodal lifestyle modification interventions for the prevention and treatment of metabolic syndrome. Given the substantial health benefits of lifestyle modification interventions to improve cardiovascular parameters, it is time to consider how such programs might be implemented, particularly for patients at increased cardiovascular risk. The costs of such programs should be weighed against the benefits of preventing heart disease, hypertension, diabetes, and other conditions, thereby avoiding the need for potentially costly medical treatments.
5. Conclusions
While the beneficial effects of fasting were not preserved in week 24, fasting induced relevant short-term effects in patients with metabolic syndrome. Fasting can thus be considered as a starting point for treating metabolic syndrome. However, it remains to be investigated how the effects of fasting can be maintained in the medium and longer term. Ultimately, a population-wide adoption of a healthy lifestyle as implemented in the study interventions may reduce the societal burden of cardiovascular diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11164751/s1, Table S1: Type and number of adverse events in the two intervention groups.
Author Contributions
Conceptualization, H.C., C.H., R.L., K.-E.C., N.S. (Nico Steckhan), A.P., C.v.S., T.O., G.D. and A.M.; data curation, N.S. (Nadia Schneider), N.S. (Nico Steckhan), F.R. and D.A.; formal analysis, H.C. and T.O.; investigation, C.H., N.S. (Nadia Schneider), F.R., E.S. and M.J.; project administration, H.C., C.H., R.L. and A.M.; supervision, H.C., C.H., R.L., N.S. (Nico Steckhan) and A.M.; writing—original draft, H.C. and M.J.; writing—review and editing, H.C., C.H., R.L., K.-E.C., N.S. (Nadia Schneider), N.S. (Nico Steckhan), F.R., D.A., A.P., C.v.S., T.O., D.A.K.-L., C.S.K., G.D., A.M. and M.J.; guarantor of article: H.C. and M.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The trial was registered at clinicaltrials.gov under NCT02099968 and was approved by the respective ethics committee (Charité-Universitätsmedizin Berlin EA4/141/13, 16 January 2014) and the University of Duisburg-Essen (approval number: 14-5733 BO, 26 February 2014). It followed the Declaration of Helsinki and Good Clinical Practice guidelines for trial conduct.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest related to this publication.
Funding Statement
This work was supported by Corona-Foundation, Essen, Germany (funding number: S199/10056/2012). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. We acknowledge financial support from the Open Access Publication Fund of Charité-Universitätsmedizin Berlin and the German Research Foundation (DFG).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Donnell M.J., Xavier D., Liu L., Zhang H., Chin S.L., Rao-Melacini P., Rangarajan S., Islam S., Pais P., McQueen M.J., et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 3.Rosengren A., Hawken S., Ounpuu S., Sliwa K., Zubaid M., Almahmeed W.A., Blackett K.N., Sitthi-amorn C., Sato H., Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 4.Kotseva K., De Backer G., De Bacquer D., Ryden L., Hoes A., Grobbee D., Maggioni A., Marques-Vidal P., Jennings C., Abreu A., et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: Results from the European Society of Cardiology ESC-EORP EUROASPIRE V registry. Eur. J. Prev. Cardiol. 2019;26:824–835. doi: 10.1177/2047487318825350. [DOI] [PubMed] [Google Scholar]
- 5.Appel L.J., Champagne C.M., Harsha D.W., Cooper L.S., Obarzanek E., Elmer P.J., Stevens V.J., Vollmer W.M., Lin P.H., Svetkey L.P., et al. Effects of comprehensive lifestyle modification on blood pressure control: Main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 6.Ornish D., Brown S.E., Scherwitz L.W., Billings J.H., Armstrong W.T., Ports T.A., McLanahan S.M., Kirkeeide R.L., Brand R.J., Gould K.L. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336:129–133. doi: 10.1016/0140-6736(90)91656-U. [DOI] [PubMed] [Google Scholar]
- 7.Ornish D., Scherwitz L.W., Billings J.H., Brown S.E., Gould K.L., Merritt T.A., Sparler S., Armstrong W.T., Ports T.A., Kirkeeide R.L., et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001–2007. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- 8.Michalsen A., Grossman P., Lehmann N., Knoblauch N.T., Paul A., Moebus S., Budde T., Dobos G.J. Psychological and quality-of-life outcomes from a comprehensive stress reduction and lifestyle program in patients with coronary artery disease: Results of a randomized trial. Psychother. Psychosom. 2005;74:344–352. doi: 10.1159/000087781. [DOI] [PubMed] [Google Scholar]
- 9.Bray G.A., Fruhbeck G., Ryan D.H., Wilding J.P. Management of obesity. Lancet. 2016;387:1947–1956. doi: 10.1016/S0140-6736(16)00271-3. [DOI] [PubMed] [Google Scholar]
- 10.Lean M.E., Leslie W.S., Barnes A.C., Brosnahan N., Thom G., McCombie L., Peters C., Zhyzhneuskaya S., Al-Mrabeh A., Hollingsworth K.G., et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet. 2018;391:541–551. doi: 10.1016/S0140-6736(17)33102-1. [DOI] [PubMed] [Google Scholar]
- 11.Elmer P.J., Obarzanek E., Vollmer W.M., Simons-Morton D., Stevens V.J., Young D.R., Lin P.H., Champagne C., Harsha D.W., Svetkey L.P., et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann. Intern. Med. 2006;144:485–495. doi: 10.7326/0003-4819-144-7-200604040-00007. [DOI] [PubMed] [Google Scholar]
- 12.Cramer H., Lauche R., Paul A., Langhorst J., Michalsen A., Dobos G. Mind-Body Medicine in the Secondary Prevention of Coronary Heart Disease. Dtsch. Arztebl. Int. 2015;112:759–767. doi: 10.3238/arztebl.2015.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziv A., Vogel O., Keret D., Pintov S., Bodenstein E., Wolkomir K., Doenyas K., Mirovski Y., Efrati S. Comprehensive Approach to Lower Blood Pressure (CALM-BP): A randomized controlled trial of a multifactorial lifestyle intervention. J. Hum. Hypertens. 2013;27:594–600. doi: 10.1038/jhh.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malinowski B., Zalewska K., Węsierska A., Sokołowska M.M., Socha M., Liczner G., Pawlak-Osińska K., Wiciński M. Intermittent Fasting in Cardiovascular Disorders-An Overview. Nutrients. 2019;11:673. doi: 10.3390/nu11030673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei M., Brandhorst S., Shelehchi M., Mirzaei H., Cheng C.W., Budniak J., Groshen S., Mack W.J., Guen E., Di Biase S., et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 2017;9:eaai8700. doi: 10.1126/scitranslmed.aai8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamshed H., Steger F.L., Bryan D.R., Richman J.S., Warriner A.H., Hanick C.J., Martin C.K., Salvy S.-J., Peterson C.M. Effectiveness of Early Time-Restricted Eating for Weight Loss, Fat Loss, and Cardiometabolic Health in Adults with Obesity: A Randomized Clinical Trial. JAMA Intern. Med. 2022 doi: 10.1001/jamainternmed.2022.3050. Online ahead of print . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adafer R., Messaadi W., Meddahi M., Patey A., Haderbache A., Bayen S., Messaadi N. Food Timing, Circadian Rhythm and Chrononutrition: A Systematic Review of Time-Restricted Eating’s Effects on Human Health. Nutrients. 2020;12:3770. doi: 10.3390/nu12123770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J.H., Lu L.W., Ge Q., Feng D., Yu J., Liu B., Zhang R., Zhang X., Ouyang C., Chen F. Missing puzzle pieces of time-restricted-eating (TRE) as a long-term weight-loss strategy in overweight and obese people? A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021:1–17. doi: 10.1080/10408398.2021.1974335. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson M.J., Manoogian E.N.C., Zadourian A., Lo H., Fakhouri S., Shoghi A., Wang X., Fleischer J.G., Navlakha S., Panda S., et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020;31:92–104.e105. doi: 10.1016/j.cmet.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattson M.P., Longo V.D., Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017;39:46–58. doi: 10.1016/j.arr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchison A.T., Regmi P., Manoogian E.N.C., Fleischer J.G., Wittert G.A., Panda S., Heilbronn L.K. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity. 2019;27:724–732. doi: 10.1002/oby.22449. [DOI] [PubMed] [Google Scholar]
- 22.Michalsen A., Li C. Fasting therapy for treating and preventing disease—Current state of evidence. Forsch. Komplement. 2013;20:444–453. doi: 10.1159/000357765. [DOI] [PubMed] [Google Scholar]
- 23.Wilhelmi de Toledo F., Grundler F., Bergouignan A., Drinda S., Michalsen A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS ONE. 2019;14:e0209353. doi: 10.1371/journal.pone.0209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longo V.D., Mattson M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fontana L. Calorie restriction and cardiometabolic health. Eur. J. Cardiovasc. Prev. Rehabil. 2008;15:3–9. doi: 10.1097/HJR.0b013e3282f17bd4. [DOI] [PubMed] [Google Scholar]
- 26.Fanti M., Mishra A., Longo V.D., Brandhorst S. Time-Restricted Eating, Intermittent Fasting, and Fasting-Mimicking Diets in Weight Loss. Curr. Obes. Rep. 2021;10:70–80. doi: 10.1007/s13679-021-00424-2. [DOI] [PubMed] [Google Scholar]
- 27.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010;152:726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 28.Dobos G., Paul A. Mind-Body-Medizin—Integrative Konzepte zur Ressourcenstärkung und Lebensstilveränderung. Urban & Fischer Verlag/Elsevier; Amsterdam, The Netherlands: 2019. [Google Scholar]
- 29.Paul A., Lauche R., Cramer H., Altner N., Dobos G. An Integrative Day-Care Clinic for chronically ill patients: Concept and case presentation. Eur. J. Integr. Med. 2012;4:E455–E459. doi: 10.1016/j.eujim.2012.07.980. [DOI] [PubMed] [Google Scholar]
- 30.de Lorgeril M., Renaud S., Mamelle N., Salen P., Martin J.L., Monjaud I., Guidollet J., Touboul P., Delaye J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343:1454–1459. doi: 10.1016/S0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 31.De Lorgeril M., Salen P., Martin J.L., Mamelle N., Monjaud I., Touboul P., Delaye J. Effect of a mediterranean type of diet on the rate of cardiovascular complications in patients with coronary artery disease. Insights into the cardioprotective effect of certain nutriments. J. Am. Coll. Cardiol. 1996;28:1103–1108. doi: 10.1016/S0735-1097(96)00280-X. [DOI] [PubMed] [Google Scholar]
- 32.Esposito K., Marfella R., Ciotola M., Di Palo C., Giugliano F., Giugliano G., D’Armiento M., D’Andrea F., Giugliano D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 33.Kempner W. Treatment of hypertensive vascular disease with rice diet. Arch. Intern. Med. 1974;133:758–790. doi: 10.1001/archinte.1974.00320170040005. [DOI] [PubMed] [Google Scholar]
- 34.Saghaei M. Random allocation software for parallel group randomized trials. BMC Med. Res. Methodol. 2004;4:26. doi: 10.1186/1471-2288-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maifeld A., Bartolomaeus H., Löber U., Avery E.G., Steckhan N., Markó L., Wilck N., Hamad I., Šušnjar U., Mähler A., et al. Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients. Nat. Commun. 2021;12:1970. doi: 10.1038/s41467-021-22097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westhoff T.H., Straub-Hohenbleicher H., Schmidt S., Tolle M., Zidek W., van der Giet M. Convenience of ambulatory blood pressure monitoring: Comparison of different devices. Blood Press. Monit. 2005;10:239–242. doi: 10.1097/01.mbp.0000172711.82287.7f. [DOI] [PubMed] [Google Scholar]
- 37.Bosy-Westphal A., Later W., Hitze B., Sato T., Kossel E., Gluer C.C., Heller M., Muller M.J. Accuracy of bioelectrical impedance consumer devices for measurement of body composition in comparison to whole body magnetic resonance imaging and dual X-ray absorptiometry. Obes. Facts. 2008;1:319–324. doi: 10.1159/000176061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization . Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation. World Health Organization; Geneva, Switzerland: 2011. [Google Scholar]
- 39.Rudenski A.S., Matthews D.R., Levy J.C., Turner R.C. Understanding “insulin resistance”: Both glucose resistance and insulin resistance are required to model human diabetes. Metab. Clin. Exp. 1991;40:908–917. doi: 10.1016/0026-0495(91)90065-5. [DOI] [PubMed] [Google Scholar]
- 40.Assmann G., Cullen P., Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation. 2002;105:310–315. doi: 10.1161/hc0302.102575. [DOI] [PubMed] [Google Scholar]
- 41.Faul F., Erdfelder E., Lang A.G., Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 42.Cramer H., Lauche R., Haller H., Steckhan N., Michalsen A., Dobos G. Effects of yoga on cardiovascular disease risk factors: A systematic review and meta-analysis. Int. J. Cardiol. 2014;173:170–183. doi: 10.1016/j.ijcard.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Hughes J.W., Fresco D.M., Myerscough R., van Dulmen M.H., Carlson L.E., Josephson R. Randomized controlled trial of mindfulness-based stress reduction for prehypertension. Psychosom. Med. 2013;75:721–728. doi: 10.1097/PSY.0b013e3182a3e4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esposito K., Ciotola M., Giugliano D. Mediterranean diet and the metabolic syndrome. Mol. Nutr. Food Res. 2007;51:1268–1274. doi: 10.1002/mnfr.200600297. [DOI] [PubMed] [Google Scholar]
- 45.Rubin D.B. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons; New York, NY, USA: 1987. [Google Scholar]
- 46.Schafer J.L. Analysis of Incomplete Multivariate Data. Chapman and Hall; New York, NY, USA: 1997. [Google Scholar]
- 47.Di Francesco A., Di Germanio C., Bernier M., de Cabo R. A time to fast. Science. 2018;362:770–775. doi: 10.1126/science.aau2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li N., Guenancia C., Rigal E., Hachet O., Chollet P., Desmoulins L., Leloup C., Rochette L., Vergely C. Short-term moderate diet restriction in adulthood can reverse oxidative, cardiovascular and metabolic alterations induced by postnatal overfeeding in mice. Sci. Rep. 2016;6:30817. doi: 10.1038/srep30817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.National Kidney Foundation Estimated Glomerular Filtration Rate (eGFR) [(accessed on 12 June 2022)]. Available online: https://www.kidney.org/atoz/content/gfr.
- 50.de Cabo R., Mattson M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019;381:2541–2551. doi: 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.