Abstract
We have identified and characterized an Enterococcus faecalis alkaline phosphatase (AP, encoded by phoZ). The predicted gene product shows homology with alkaline phosphatases from a variety of species; it has especially high similarity with two alkaline phosphatases from Bacillus subtilis. Expression of phoZ in Escherichia coli, E. faecalis, Streptococcus agalactiae (group B streptococcus [GBS]), or Streptococcus pyogenes (group A streptococcus [GAS]) produces a blue-colony phenotype on plates containing a chromogenic substrate, 5-bromo-4-chloro-3-indolylphosphate (XP or BCIP). Two tests were made to determine if the activity of the enzyme is dependent upon the enzyme’s subcellular location. First, elimination of the signal sequence reduced AP activity to 3% of the wild-type activity (or less) in three species of gram-positive bacteria. Restoration of export, using the signal sequence from C5a peptidase, restored AP activity to at least 50% of that of the wild type. Second, we engineered two chimeric proteins in which AP was fused to either a periplasmic domain or a cytoplasmic domain of lactose permease (a membrane protein). In E. coli, the periplasmic fusion had 17-fold-higher AP activity than the cytoplasmic fusion. We concluded that AP activity is export dependent. The signal sequence deletion mutant, phoZΔss, was used to identify random genomic fragments from GBS that encode exported proteins or integral membrane proteins. Included in this set of fragments were genes that exhibited homology with the Rib protein (a cell wall protein from GBS) or with DppB (an integral membrane protein from GAS). AP acts as a reporter enzyme in GBS, GAS, and E. faecalis and is expected to be useful in a variety of gram-positive bacteria.
Alkaline phosphatase is a metalloenzyme that catalyzes the nonspecific hydrolysis of a wide variety of phosphomonoesters (reviewed in reference 12). Alkaline phosphatases have been identified in a wide variety of organisms, including Escherichia coli and humans (5, 22). A conserved set of amino acids has been shown to be critical to catalysis, and both zinc and magnesium ions are essential for function (23). The structure of the E. coli alkaline phosphatase has been refined to a resolution of 2.0 Å (24). Expression is induced in E. coli by phosphate limitation, and control of expression has been extensively investigated (reviewed in reference 39). E. coli alkaline phosphatase (encoded by phoA) is synthesized as a precursor with a cleavable N-terminal signal sequence (20). In E. coli, the cytoplasm is a reducing environment while the periplasm has a disulfide bond-forming enzymatic system. These contrasting redox environments are thought to limit the formation of essential disulfide bonds to the periplasm and may account for the export-dependent activity of alkaline phosphatase (4).
Investigators working with gram-negative bacteria have exploited the location-sensitive activity of E. coli alkaline phosphatase for many different tasks, including the identification of genes encoding secreted proteins and integral membrane proteins (6), the analysis of membrane protein topology (9), and the discovery of insertion-tolerant sites within a membrane protein (28). The E. coli alkaline phosphatase enzyme (encoded by phoA) exhibits reduced activity in a gram-positive background, possibly due to the absence of an extracytoplasmic enzyme with disulfide bond-forming activity (30).
In contrast with the E. coli enzyme, much less is known about the regulation, processing, structure, and activity of alkaline phosphatases from gram-positive bacteria. The most advanced studies have been performed in Bacillus subtilis, where a complex pathway involving three two-component regulatory systems controls the phosphatase loci (reviewed in reference 19).
In this report we describe the identification and characterization of the Enterococcus faecalis alkaline phosphatase protein (AP, encoded by phoZ). Expression of phoZ conferred a blue-colony phenotype in E. coli and three gram-positive species on 5-bromo-4-chloro-3-indolylphosphate (XP or BCIP) agar. Alkaline phosphatase activity was quantified, and in all strains the activity per cell was shown to be minimal when AP was retained in the cytoplasm. The activity per cell increased substantially when AP was exported, suggesting that the activity of AP is export dependent. We exploited these characteristics to identify random group B streptococcus (GBS) chromosomal fragments that encoded signal sequence-like peptides.
MATERIALS AND METHODS
Strains and media.
The gene encoding AP, phoZ, was cloned from the E. faecalis strain 10C1 (ATCC 11700). The E. coli strain DH5αF′ (Promega, Madison, Wis.) was used for the cloning experiments. The E. coli strain CC873 is CC118 (29) with F′ lacIq ΔlacY::cat (2a). Note that CC118 carries ΔphoA. The GBS strain COH31r/s (33), the group A streptococcus (GAS) strain CS101 (21), and E. faecalis OG1SSp (15) were used for colony morphology studies and quantitative AP assays. E. coli strains were grown either in Luria broth (LB) or M9 medium (Difco, Detroit, Mich.) supplemented with 15 g of agar/liter, 100 μg of ampicillin/ml, 10 μg of chloramphenicol/ml, 50 μg of XP (Sigma, St. Louis, Mo.)/ml, or 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (5-Prime-3-Prime Inc., West Chester, Pa.) as required. COH31 r/s was grown in Todd-Hewitt broth (THB) (Difco) supplemented with 10 μg of chloramphenicol/ml as required. Both CS101 and OG1SSp strains were grown in THB supplemented with 0.2% yeast extract (Difco) and 5 μg of chloramphenicol/ml. E. coli and GBS cultures were grown with shaking at 200 rpm. Strains carrying derivatives of the temperature-sensitive vector pVE6007 were grown at 30°C; all others were grown at 37°C.
DNA manipulation and transformation.
Cloning experiments were performed as previously described (2, 32, 34). Restriction enzymes were obtained from New England Biolabs (Beverly, Mass.) or Promega. Sequencing reactions were carried out by using the ABI PRISM dye terminator cycle sequencing kit (Perkin Elmer, Foster City, Calif.). Sequencing products were separated from unincorporated dyes by using Centri-Sep columns (Princeton Separations, Inc., Adelphia, N.J.). Automated sequencing was performed at the Fred Hutchinson Cancer Research Center (Seattle, Wash.). Plasmid preparations were performed by using Qiagen Mini or Maxi preparation kits (Qiagen, Chatsworth, Calif.). GBS and GAS were electroporated as described in references 17 and 35, respectively. E. faecalis was transformed as described in reference 13 except that the SGM17 media contained 3.5% glycine and 0.4% sucrose. Electroporated cells were recovered in a THB–0.25 M sucrose solution. PCR of large segments of DNA (up to 6 kb) was performed by using the Expand High Fidelity system (Boehringer Mannheim, Indianapolis, Ind.).
Plasmids and λ libraries.
The construction of plasmids is diagrammed in Fig. 1. E. faecalis chromosomal DNA was sheared by repeated passage through a narrow-gauge needle, size selected (3 to 8 kb), and joined with EcoRI linkers before ligating the fragments into phosphatase-treated λgt11 arms. After in vitro packaging, recombinants were isolated at a frequency of 8 × 105 per μg of streptococcal DNA. An isolate exhibiting APase activity was purified, and the gene was subcloned, initially into pUC13.
FIG. 1.
Construction of plasmids used to characterize AP activity. The phoZ gene was subcloned from a λ library on a 3.2-kb EcoRI fragment into pUC13 to form pAP01 (not depicted). A 1.9-kb EcoRI-SalI fragment containing phoZ was subcloned from pAP01 into pBluescript to make pAP03. To characterize phoZ activity in E. faecalis, the phoZ gene was joined to a constitutive promoter from the NADH peroxidase gene (Pnpr) (unpublished data) and carried on the pAM401 vector in a three-part ligation producing pAP09. We planned to fuse the mature region of AP (′AP, encoded by ′phoZ) to the integral membrane protein lac permease (encoded by lacY) as part of our characterization of AP activity. Consequently, the phoZ gene on pAP09 was moved on an XbaI-SalI fragment into the broad-host-range vector pGBS1 to make pGBS1phoZ (not depicted). Two BamHI sites found in pJS3phoZ were eliminated by digestion, Klenow filling, and ligase treatment of the blunt-ended fragment to produce pMHL101. Inside-out PCR was used to amplify the entire pJS3phoZ plasmid except the signal sequence codons. (The primers used in the amplification, oML101B and oML107, had 5′ noncomplementary sequences that included a BamHI site [Table 2].) The linear PCR product was digested with BamHI and then ligase treated to form pMHL102, in which the codons encoding the signal sequence were replaced with two codons containing a BamHI site (phoZΔss). The BamHI-AflII fragment from pMHL102 encodes ′AP. Plasmid pCM701 has an insertion carrying a BamHI site within the region of lacY encoding the first periplasmic domain, lacY(P1) (28). Fusion of ′AP to lactose permease was created by introducing the BamHI-AflII fragment from pMHL102 into BamHI-AflII-digested pCM701 to produce pMHL103. To enhance the utility of phoZ (see the Materials and Methods section) the recognition site for PstI was removed by site-directed mutagenesis from pAP03 to form pAP010, while the HindIII site was removed from pAP09 to produce pAP011 (product plasmids not depicted). A HincII fragment from pAP011, bearing the HindIII site point mutation, was cloned into HincII-digested pAP010 to produce the (neither HindIII nor PstI site) double mutant termed phoZ1, on pAP12. An SphI linker was inserted at the unique Eco57I site in phoZ1 on pAP12 to create an allele with wild-type activity, termed phoZ2, on pDC110 (not depicted). The phoZ2 gene was isolated from pDC110 on a HaeII-XmnI fragment and cloned into pDC111 (a pJS3 derivative) to produce pDC113 (10). The codons encoding the hydrophobic core of AP were eliminated from pDC113 by using inside-out PCR with the primers used to create pMHL102. However, the resulting plasmid, pMHL108, was shown to contain a number of PCR-induced mutations. These mutations were reversed by cloning in the BamHI-AflII fragment from pMHL102 to create pMHL109, encoding phoZΔss. Restoring the original signal sequence by inserting a PCR fragment in the BamHI site failed to restore full activity (not shown). Consequently, a PCR fragment placing the BamHI site further upstream and containing all the sequence differences, termed ‘phoZ’, was amplified from pDC113 and cloned into pMHL109 to form pAN200 (see the Materials and Methods section and Table 2).
The phoZ sequences derived from the phage library had recognizable −10 promoter sequences, but the sequences at the −35 position were not included on the cloned chromosomal fragment. Several promoter and broad-host-range vector combinations were constructed in the course of optimizing expression. Plasmid pAP09 contains a 241-bp fragment in which the npr promoter is fused to phoZ and exhibits high AP activity in E. faecalis but not in GBS. Plasmid pGBS1 is composed of the broad-host-range plasmid pJS3 (3) with the multiple cloning site from pUC19 (40) inserted at the HindIII site. When the Pnpr-phoZ construct was cloned into pGBS1, the resulting vector (pMHL101) also failed to produce high levels of AP expression in GBS. When phoZ was cloned in front of the cat promoter in pDC111, the resulting plasmid (pDC113) caused GBS transformant colonies to exhibit a striking blue color on XP media (10).
In the course of cloning phoZ and evaluating expression schemes, we also made a number of alterations to its primary structure. We anticipated that phoZ could be used in cloning vectors that have a multiple cloning site within phoZ. Insertions of cloned DNA should disrupt phoZ and could be identified on the basis of whether colonies were blue or white. Consequently, the sites for the commonly used enzymes HindIII and PstI were removed from phoZ by site-directed mutagenesis to form phoZ1 on pAP12 (see Table 2 for complete definitions for oligonucleotides PX and HX). Linkers carrying SphI were inserted at a number of restriction sites in phoZ1 as a means of detecting insertion-tolerant sites. One such mutation introduced a linker into the unique Eco57I site to form phoZ2, which proved to be phenotypically silent (plasmid pDC113 [10]). All three alterations are detailed in Table 1.
TABLE 2.
Plasmids and PCR primers used to generate various phoZ mutations
| Final plasmid | Template | Oligonucleotides | Effect and/or oligonucleotide sequencea |
|---|---|---|---|
| pAP010 | pAP03 | PX | To remove the PstI site from phoZ; GTC CTG AAA ACG CTT CTG C |
| pAP011 | pAP03 | HX | To eliminate the HindIII site from phoZ; GAA TGA TCA GCA GTT GTA AC |
| pMHL102 | pMHL101 (phoZ1) | To delete the AP hydrophobic core codons in pGBS1phoZ | |
| oML101B | CCT AGC GGA TCC CTT TTC TTC ATT TCC TTG T (reverse primer) | ||
| oML107 | CAC AGC GGA TCC GGG TTG TAC AAA TTT ATC TGA ACA AAA AAG (forward primer) | ||
| (Changes N terminus of AP from MKKRALLGVTLLTFTTLAGCTNLSEQKS to MKKRDPGCTNLSEQKS) | |||
| pMHL108 | pDC113 (phoZ2) | oML101B | Same purpose and oligonucleotides as used to make |
| oML107 | pMHL102, but makes the deletion of the hydrophobic core from AP encoded by pDC113. Note: this PCR gave rise to mutations 3′ to the deletion site | ||
| pMHL109 | No PCR | To correct the mutations in pMHL108. The BamHI-AflII fragment from pMHL102 was cloned into pMHL108 | |
| pAN200 | pDC113 | To remove the AP polar region codons from pMHL109. Amplifies the region beginning with the codons encoding EQKS to the NdeI site in the middle of phoZ. | |
| ANML121 | ACAAAGGATCCCGAACAAAAAAGCGGCG (forward) | ||
| ANML122 | TAG TCA TCG GCG ATT TCT GC (reverse) | ||
| (Changes the N terminus of AP from MKKRDPGCTNLSEQKS to MKKRDPEQKS) | |||
| pAN202 | GBS genomic DNA | Puts the scpB signal sequence into APΔss in pMHL109 | |
| oML119 | TTGCGGGATCCACAGAAACTACCATTTG (forward) | ||
| oML120 | GCTTTGGGATCCGATTGTGCATTGAGCAAG (reverse) | ||
| (Changes the N terminus from MKKRDPEQKS to MKKRDPQKLPFDKLAIALMSTSILLNAQSDPEQKS) |
Italicized bases indicate noncomplementary overhangs containing BamHI sites (GGATCC). Amino acids within the putative hydrophobic core of the AP signal sequence are underlined. Amino acids in the putative polar region are double underlined.
TABLE 1.
Phenotypically silent mutations introduced in phoZ to make phoZ2 (a high-activity allele with enhanced utility as a reporter gene)
| Wild-type sequence | Ending sequencea | Result |
|---|---|---|
| ACT GCA GAT | ACT GCt GAT | Eliminates the PstI siteb |
| Thr ala asp | Thr ala asp | |
| GAA GCT TTT | GAA GgT TTT | Eliminates the HindIII siteb |
| Glu ala phe | Glu gly phe | |
| GTT GCT GAA GCG | GTT GCa tgc gct GAA GCG | Inserts an SphI siteb |
| Val ala glu ala | Val ala cys ala glu ala |
Modified bases are shown in lowercase.
Shown in italics.
Two constructs were used to determine the activities of AP in different subcellular locations. The first construct simply removed the signal sequence from AP, and the second fused ′AP (mature AP) to lac permease, either within a periplasmic domain or to a cytoplasmic domain. Oligonucleotides oML101B and oML107 were used for the purpose of removing the hydrophobic core of the signal sequence (to create the first construct) while inserting two new codons with an embedded BamHI site (to facilitate construction of the lac permease fusions). These primers are complementary to sequences flanking the signal sequence (Fig. 1; the primers are depicted with plasmids pMHL101 and pDC113), with noncomplementary overhangs containing the new BamHI site. The sequences of the primers and a description of the changes they create in producing APΔss (AP lacking its signal sequence) are shown in Table 2. The PCR that produced phoZΔss on pMHL108 also introduced several mutations 3′ to the new BamHI site. To remove these mutations the BamHI-AflII fragment from pMHL102 carrying ′phoZ (encoding ′AP) was inserted into pMHL108 to create pMHL109. This construct exhibited decreased AP activity. However, activity was not fully rescued when the deleted signal sequence codons were PCR amplified (with flanking BamHI sites) and inserted into the BamHI site in phoZΔss (data not shown). We speculated that the codons encompassing the BamHI sites were distorting the polar region of the signal sequence. Consequently, oligonucleotides ANML121 and ANML122 were used to amplify a region of phoZ that did not include putative polar region codons (Table 2 includes exact descriptions of the oligonucleotides and the changes to AP). The PCR product, termed ‘phoZ’ (Fig. 1), was cloned into pMHL109 to produce pAN200. The phoZ2Δss construct on pAN200 could be fully rescued by exogenous signal sequences (see the Results section).
Plasmids pCM701 and pCM702 (28) were used to create the fusions of lacY and phoZ. Both plasmids carry lacY mutants in which an insertion of 31 codons had been created by TnlacZ/in mutagenesis. In pCM701 the inserted bases are in the region of lacY that encodes the first periplasmic domain. In pCM702 the insertion is in the region encoding the second cytoplasmic domain. The insertions each contain a BamHI site, and there is an AflII site downstream of the lacY sequences. The BamHI-AflII fragment from pMHL102 was cloned into pCM701 to create a gene encoding LacYP1::AP on pMHL103 (the periplasmic fusion). The same fragment was cloned into pCM702 to create a gene encoding LacYC2::AP on pMHL104 (the cytoplasmic fusion, which is not depicted in Fig. 1).
Oligonucleotides oML119 and oML120 were used to amplify the hydrophobic region and polar region of the signal sequence from C5a peptidase (encoded by scpB) with flanking BamHI sites. This scpB signal sequence fragment was inserted at the BamHI site of pAN200 to form phoZsss on pAN202 (confirmed by sequencing but not depicted on Fig. 1). Table 2 provides details of oligonucleotide sequences and the alterations produced in AP.
AP assays.
AP assays in E. coli were performed as described in reference 8 except that iodoacetamide was added to lysed cells (14). Experiments with and without 1 mM iodoacetamide revealed that the reagent had no effect on wild-type AP (data not shown). The AP assay for gram-positive species was very similar to the E. coli assay, but no cell lysis step was performed (there is no outer membrane to slow substrate diffusion) and iodoacetamide was omitted. Briefly, overnight cultures were grown in rich media (see “Strains and media,” above). The cultures were diluted and allowed to grow to mid-log phase (an optical density at 600 nm [OD600] of ∼0.6 for GBS), and the cells were pelleted, washed, and resuspended to their original volume in MOPS (morpholinepropane sulfonic acid) salts (14). Cell density was determined by optical absorbance (OD600 for GBS and E. faecalis and OD680 for GAS). A small volume of cells (5 to 100 μl, depending on the activity per cell) was added to AP buffer (1 M Tris [pH 8.0]–0.1 mM ZnCl2) to a final volume of 0.9 ml, 100 μl of 0.4% p-nitrophenyl phosphate (pNPP) was added, and the reaction was started by incubation at 37°C. Reactions were stopped by adding 120 μl of stop solution (a 1:5 mix of 0.5 M EDTA:1 M KH2PO4). The activity per cell was calculated in Miller units. The numbers of CFU per absorbance unit vary from species to species, so only relative changes can be compared across species. Experiments were run to verify that the measured activity per milliliter was proportional to the number of cells per milliliter (data not shown), to control against saturation of the assay.
Nucleotide sequence accession number and sequence analysis.
The sequence for phoZ has been submitted to GenBank and deposited under accession no. AF154110. Homologs were identified by using a FASTA search on Biology Workbench (38). Alignments were established with the PILEUP or Gap modules of the Genetics Computer Group’s Wisconsin Package (18).
RESULTS
A λgt11 library carrying E. faecalis inserts was constructed with the intention of isolating various enterococcal genes by immunological techniques. Plaques were screened by hybridizing with a secondary antibody conjugated with alkaline phosphatase and incubating the blot in the presence of a chromogenic alkaline phosphatase substrate. Unexpectedly, certain plaques were able to cleave the substrate even in the absence of this conjugated antibody, indicating that the recombinant phage encoded an alkaline phosphatase.
These plaques were isolated, and subsequent cloning experiments identified a 1.9-kb EcoRI-SalI fragment with a 471-codon open reading frame that encodes a predicted protein of 51 kDa. A ribosome-binding site (GGAGG) was found 10 bp upstream of the initiator methionine codon. The −10 sequence (TATAGT) typical of ς70-controlled transcription was found further upstream, but the corresponding −35 sequences were not included on the fragment cloned from E. faecalis.
Database searches revealed significant homology to alkaline phosphatases from a variety of organisms. One-on-one Gap sequence analysis indicated that AP residues were identical to 56% of the residues in B. subtilis alkaline phosphatase A and to 63% of the residues in B. subtilis alkaline phosphatase B. In contrast, there were 30 to 40% identities between residues when AP was compared to phosphatases from gram-negative bacteria or eukaryotes. The catalytic core of the E. coli alkaline phosphatase is composed of residues Asp 101, Ser 102, Ala 103, and Arg 166 (of the mature E. coli sequence). These residues are well conserved in an alignment of phoZ with the phosphatase genes from B. subtilis, Bacillus licheniformis, Saccharomyces cerevisiae, Thermus sp. FD3041, and Halocynthia roretzi (not shown). The Thermus alkaline phosphatase has a similar Glu-Ser-Ser sequence in place of the canonical Asp-Ser-Ala. The eight metal-ion-coordinating residues are almost perfectly conserved in this alignment (a histidine expected at position 419 of the Halocynthia sequence appears at position 417 instead).
The full-length AP protein includes a signal sequence composed of an N-terminal region containing three positively charged residues and a core region with 14 hydrophobic residues. The protein appears be exceptionally stable, as it retains phosphatase activity after sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotting onto nitrocellulose (data not shown).
Signal sequence deletions.
A strategy of removing and replacing the signal sequence was adopted to compare the activities of AP in different subcellular locations. The codons encoding the hydrophobic core and putative polar region (underlined), MKKRALLGVTLLTFTTLAGCTNLSEQKS, were replaced with two new codons containing a BamHI site. The predicted N-terminal sequence expressed from the phoZΔss mutant was MKKRDPEQKS. E. coli MC1061 was transformed with pJS3 (does not express AP), pDC113 (expresses AP), or pAN200 (expresses APΔss), and the transformants were subjected to quantitative alkaline phosphatase assays. The negative control, MC1061(pJS3), exhibited AP activity of 2 Miller units. The phoZ+ strain, MC1061(pDC113), exhibited activity of 770 units. In contrast, the phoZΔss strain, MC1061(pAN200), exhibited activity of only 54 units. The 14-fold difference in AP activity between strains expressing phoZ and phoZΔss supports the hypothesis that AP activity is export dependent in E. coli. Although the APΔss strain had relatively low activity, it is notable that it exhibited significantly greater activity than that associated with the AP− strain, MC1061(pJS3). It is possible that APΔss was released from the cytoplasm through a slow process of cell lysis, where it acquired an active conformation.
Restoring signal sequence activity.
Deletion of the signal sequence decreased AP activity. This may be due to the altered subcellular location of the mutant enzyme. However, it is also possible that the mutation may have disrupted the enzyme’s catalytic mechanism. To distinguish between these hypotheses, a PCR fragment encoding the signal sequence from the GBS C5a peptidase (scpB) was inserted at the BamHI site of phoZΔss. The new plasmid was designated pAN202 and encodes phoZsss (scpB signal-sequence). The predicted N-terminal sequence of APsss was MKKRDPQKLPFDKLAIALMSTSILLNAQSDPEQKS. Both of the aspartic acid-proline peptide sequences (double underlined) are encoded by the DNA sequences that contain the BamHI sites. The underlined amino acids are native to C5a peptidase.
Test of export-dependent activity in gram-positive bacteria.
We compared AP activities in strains expressing either no phoZ (negative control) or phoZ2 (positive control), phoZΔss, and phoZsss. The plasmids carrying these genes are pJS3, pDC113, pAN200, and pAN202, respectively. The four plasmids were electroporated into the GBS strain COH31r/s, the GAS strain CS101, and E. faecalis OG1SSp. The colony phenotypes on XP media are shown in Fig. 2 (bottom). In all three species, the control strain without phoZ produced white colonies. The strains that expressed phoZΔss were white or very pale blue. In all three species, however, the strains that expressed either phoZ or phoZsss were blue.
FIG. 2.
Color phenotypes of strains expressing phoZ derivatives and grown as colonies on XP agar. (Top) E. coli CC873 expressing the periplasmic chimera LacYP1::AP, the cytoplasmic chimera LacYC2::AP, or the unfused LacYC2 or LacYP1 proteins (no AP). Strains were grown on Luria agar containing the chromogenic substrate XP. (Bottom). Gram-positive species deficient in AP (NONE) or expressing wild-type AP (WT), AP deleted of its signal sequence (NO S.S), or AP with the C5A peptidase signal sequence (scpB S.S). Strains were grown on Todd-Hewitt agar with XP. On this medium, the GAS strains (insert) grew relatively slowly and had to be photographed 24 h after the other species.
These observations suggested that the enzymatic activity of APΔss could be rescued by insertion of a heterologous signal sequence. Unfortunately, it is difficult to distinguish a partial rescue of AP activity from a complete rescue of AP activity by using plate assays. These two types of rescue can be distinguished with a colorimetric liquid AP assay. The results of such an assay are shown in Fig. 3. In GBS, GAS, and E. faecalis, strains deficient for AP exhibited activity of 7 Miller units or less. Strains expressing wild-type AP exhibited activity of at least 1,500 Miller units. Strains expressing the signal sequence deletion, APΔss, exhibited less than 3% of the wild-type AP activity. Strains expressing the chimeric protein, APsss, exhibited more than 50% of the wild-type AP activity. The substantial restoration of AP activity by the heterologous signal sequence in APsss supports the hypothesis that AP activity is export dependent.
FIG. 3.
Quantitative assay of AP activity in GBS, E. faecalis, or GAS strains expressing various AP constructs. None indicates a strain that does not express phoZ, w.t. indicates a strain expressing phoZ2, no s.s. indicates a strain expressing phoZ2Δss (phoZ lacking its signal sequence codons), and scpB s.s. indicates a strain expressing ′phoZ2 fused to the signal sequence encoded by scpB. Error bars represent standard deviations (n ≥ 6) from experiments run on at least two separate days.
Membrane-protein fusions.
The hypothesis that AP functions with different levels of efficiency in different subcellular locations was further tested by fusing ′phoZ at different sites along a gene encoding an integral membrane protein. AP fused to a cytoplasmic site should be retained in the cytoplasm, while a fusion to an extracytoplasmic site should cause AP to be exported.
Lactose permease is an E. coli integral membrane protein encoded by lacY. ISlacZ/in mutagenesis has previously been used to introduce BamHI sites at various points along the length of lacY (28). Mutant lacY(P1) has an insertion just after the codon for asparagine 38, in the region of lacY encoding the first periplasmic domain (Fig. 4A). A ′phoZ cassette was cloned into the BamHI site in lacY(P1), producing lacY(P1)::phoZ on pMHL103 (Fig. 1). This fusion encodes a protein in which the mature region of AP is fused to the first periplasmic domain in lactose permease (Fig. 4B). Similarly, mutant lacY(C2) has a BamHI site inserted after the codon for glycine 71, in the region of lacY encoding the second cytoplasmic domain of lactose permease (Fig. 4C). The ′phoZ cassette was cloned into the BamHI site in lacY(C2), producing lacY(C2)::phoZ on pMHL104. This fusion encodes a protein in which the mature region of AP is fused to the second cytoplasmic domain in lactose permease (Fig. 4D).
FIG. 4.
Proteins used to study AP export dependence in E. coli. (A) Topology of the lac-permease mutant LacYP1, which contains a 31-amino-acid insertion (zigzag lines) in the first periplasmic domain. The insertion was produced by ISlacZ/in mutagenesis (28). The underlying 31-codon insertion contains a BamHI site, which was used to fuse the lacY(P1) to ′phoZ. The two parallel lines represent the cytoplasmic membrane of E. coli. (B) Topology of the chimeric protein LacYP1::AP. The AP sequences are diagrammed as a dark spiral. (C) The lactose permease mutant LacYC2 contains a 31-residue insertion in a cytoplasmic domain, and the underlying BamHI site was used to fuse lacY(C2) to ′phoZ. (D) Topology of the chimeric protein LacYC2::AP.
The plasmids encoding these chimeras were transformed into E. coli strain CC873, and the transformants were plated on XP agar. The colony phenotypes are shown in Fig. 2 (top). Control strains expressing unfused LacYP1 or LacYC2 produced white colonies on media containing XP. Colonies expressing LacYP1::AP (the periplasmic chimera) were blue on XP media. Colonies expressing LacYC2::AP (the cytoplasmic chimera) were pale blue. The faint color of the latter colonies may be due to low levels of AP export or possibly to cell lysis and release of AP. The faint blue color has also been observed when E. coli alkaline phosphatase was fused to cytoplasmic sites in lactose permease.
The AP activities of these strains were analyzed in a quantitative assay, and the results are shown in Fig. 5. The strain expressing unfused LacYP1 exhibited activity of less than 1 Miller unit. The strain expressing the periplasmic fusion exhibited activity of 166 Miller units. In contrast, the cytoplasmic AP fusion exhibited activity of only 10 Miller units. This 17-fold difference in activity is consistent with the hypothesis that AP requires export before it can act efficiently as a phosphatase.
FIG. 5.
AP assays of E. coli CC873 expressing lactose permease-AP chimeras. No AP denotes a strain expressing unfused lacY(P1). P1 denotes a strain expressing the periplasmic fusion lacY(P1)::phoZ. C2 denotes a strain expressing the cytoplasmic fusion lacY(C2)::phoZ. Error bars indicate standard deviations (n = 3).
Transformation of fusions into GBS.
The fusion studies showed that AP exhibits higher phosphatase activity when exported in E. coli. However, ultrastructural differences between gram-negative and gram-positive organisms may cause fusions to behave differently in gram-positive bacteria. Both lacY::phoZ fusion genes were cloned into a shuttle vector, pVE6007. Both of the new constructs exhibited the expected AP activities when transformed into E. coli (data not shown). Three attempts were made to electroporate the plasmids into GBS. The control plasmid pVE6007 transformed GBS at approximately 103 transformants per μg; however, no colonies were isolated when plasmids encoding the fusion proteins were used. The reason for our inability to transform GBS with these plasmids remains unclear. Plasmids encoding wild-type AP have been transformed into GBS previously (here, and see reference 10), and functional lactose permease has been expressed in the gram-positive organism Corynebacterium glutamicum (7). It is possible that transcription from the fusion gene interfered with plasmid maintenance functions or that the fusion unexpectedly produced a toxic protein in GBS.
Cloning genes encoding exported proteins.
The phoA gene from E. coli is widely used in gram-negative bacteria to identify genes that encode secreted proteins or to characterize the topology of integral membrane proteins. We hypothesized that phoZ could be used for the same purpose in GBS. COH1 chromosomal DNA was partially digested with Sau3A, and fragments in the range from 0.5 to 4 kb were cloned into the BamHI site of the phoZΔss construct (pAN200). The library was electroporated into E. coli ER2566 and plated on media containing XP. After 24 h of growth, 0.6% of the transformed colonies were blue. Electroporation of the ligation products into the GBS strain COH31 r/s did not produce transformants, presumably due to the low efficiency of electroporation in GBS. Instead, the library established in E. coli was recovered, and the amplified library was electroporated into GBS. After 24 h of growth, 0.5% of the transformed GBS colonies were blue. The number of blue colonies increased with longer incubation, although the background levels of alkaline phosphatase activity made it difficult to distinguish low-activity fusions after growth for three days.
We had already shown that the lactose permease::AP fusions could not be moved from E. coli into GBS, suggesting that there may be significant differences in the expression and processing mechanisms utilized by these two organisms. To test this hypothesis, we isolated 12 plasmids that conferred a blue-colony phenotype in E. coli and transformed these plasmids into GBS strain COH31r/s. All 12 transformations produced GBS colonies that were blue on XP media. These observations suggest that translational fusions exhibiting AP activity in E. coli will also function in GBS.
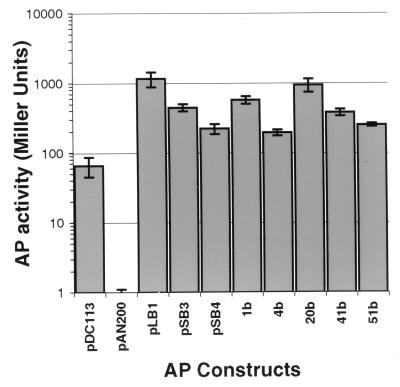
It was possible that the translational fusions identified in E. coli had a significantly different range of activity than those identified in GBS. To test this hypothesis, five plasmids identified in E. coli and three plasmids identified in GBS were transformed into GBS strain COH31r/s. AP activity was tested by using the colorimetric liquid assay. The plasmid encoding wild-type AP was used as a positive control (pDC113), and the plasmid encoding the signal sequence deletion was used as a negative control (pAN200). The results of quantitative liquid AP assays for each of these strains are shown in Fig. 6. The APΔss strain exhibited activity of 1 Miller unit, and the strain expressing wild-type AP exhibited activity of 70 Miller units. All eight of the chimeric proteins produced higher activity than the strain expressing wild-type XP. However, there was no support for the hypothesis that fusions identified in GBS exhibit activity significantly different from that of fusions identified in E. coli (P > 0.20, t test for two groups of unpaired observations). The high activity of the fusions was not surprising, as we had screened the library of phoZ fusions for those that conferred a dark-blue-colony phenotype within the first 24 h of growth. Variations in activity could be attributed to changes in plasmid copy number, increases in levels of expression due to the introduction of new promoters or ribosome-binding sites, or improved export due to fusion of AP with a more efficient export signal.
FIG. 6.
Activities of ′phoZ fusions with genomic fragments in COH31r/s. Plasmid pDC113 encodes full-length AP as a positive control, while pAN200 encodes APΔss and is used as a negative control. Plasmids pBL1, pSB3, and pSB4 were originally identified in GBS, while 1b, 4b, 20b, 41b, and 51b were identified in E. coli and then moved into GBS. Error bars represent standard deviations (n ≥ 6) from experiments run on at least 2 separate days.
Protein export via the sec-dependent pathway in E. coli requires that the exported protein contain a contiguous segment of uncharged residues with a summed hydrophobicity of −23 kcal/mol or less (26). In this analysis, each residue in a signal sequence is assigned a hydrophobicity value by using the GES scale (16). The peptide sequence is broken into contiguous segments delimited by charged residues, and the hydrophobicities of all residues within a segment are summed. Export in gram-positive bacteria is less well characterized than export in gram-negative bacteria, and we were interested in determining if the phoZ fusions identified in GBS had segments with summed hydrophobicities exceeding the E. coli threshold.
All eight phoZ fusions were sequenced to determine if the chimeric proteins had segments of uncharged residues that met the summed-hydrophobicity criteria. The results are summarized in Table 3. All five chimeras identified in E. coli contained nonpolar peptide segments with summed hydrophobicities ranging from −33 to −52 kcal/mol. Similarly, the inserts identified in GBS all had hydrophobic segments with summed hydrophobicities ranging between −39 and −43 kcal/mol. Thus, all of the chimeric proteins characterized in this study conformed to the hydrophobicity threshold established for efficient export in E. coli. A similar threshold may exist for efficient export in GBS.
TABLE 3.
Hydrophobic sequences identified in the N termini of ′AP chimeras that produce blue-colony phenotype on XP media
| Insert | Backgrounda | No. of amino acids addedb | Hydrophobic sequence | Hydrophobic sequence lengthc | Summed hydrophobicitiesd (kcal/mol) |
|---|---|---|---|---|---|
| pSB3 | GBS | 104 | AALGAFIIFLLAIFGLFY | 18 | −43 |
| pSB4 | GBS | 286 | LTFVLWVLLIGSVGFGI | 17 | −39 |
| pBL1 | GBS | 189 | LLVASLAFGMAVSPVTPIAFAA | 22 | −43 |
| 1b | E. coli | 32 | ITTLSTIALTLMLCVG | 16 | −33 |
| 4b | E. coli | 73 | LLIIVMMFMVVFLSGLA | 17 | −46 |
| 20b | E. coli | 139 | MIILIIALATILTFVTWMLI | 21 | −52 |
| 41b | E. coli | 56 | ISVPTFWIGLIFLLIFSV | 18 | −42 |
| 51b | E. coli | 16 | IIIPIITILLIAL | 13 | −33 |
Bacterial species in which the insert was first identified as having export activity.
Number of amino acids added by the insert to the chimeric ′AP protein.
Number of contiguous, nonpolar residues in the hydrophobic core of the putative export signal.
Summed hydrophobicities (GES scale) of the amino acids in the putative hydrophobic core (25).
BLAST searches were used to compare the N-terminal sequence from each of the AP chimeras against the database of proteins, as a final means of determining if the AP reporter was fused to an exported peptide. Chimeric protein 20b has a 25-residue segment with 100% homology to a repeated motif in Rib, a GBS cell wall protein (37). Similarly, chimeric protein 41 has a 48-residue segment that shows 100% identity to an internal segment of DppB, a GAS integral membrane protein encoded as part of the dipeptide transporter operon (1). None of the other six N-terminal sequences showed significant similarity to proteins on the database. We did not find any chimera in which the N terminus appeared to be a cytoplasmic enzyme, while both of the identified homologs were exported.
DISCUSSION
We have identified and characterized phoZ, an E. faecalis gene that encodes AP. AP activity is reduced when the peptide is retained in the cytoplasm of gram-positive bacteria, and changes in activity can be easily screened by using plate assays based on the cleavage of a chromogenic substrate. These features were exploited to identify GBS genes that encode exported proteins.
An active phosphatase with low specificity could be deleterious if retained in the cytoplasm. Indeed, when E. coli alkaline phosphatase is retained in the cytoplasm the enzyme is inactive, possibly due to slow rates of disulfide bond formation in the cytoplasm (4). E. faecalis AP activity was expected to be similarly export dependent. In GAS, GBS, and E. faecalis, the signal sequence deletion mutant exhibited reduced activity. When export was restored by using an exogenous signal sequence, however, AP activity was restored. The exogenous signal sequence was unlikely to have the conformation and side chains needed to restore a disrupted catalytic site, so mutational inactivation is an implausible cause for low AP functionality in the cytoplasm. It is interesting that the deletion removed the only cysteine codon found on the phoZ open reading frame. Thus, unlike the E. coli alkaline phosphatase, disulfide bond formation cannot be essential for AP activity and cannot contribute to the different activities of the enzyme in different subcellular locations.
In an independent test, AP was placed in opposing subcellular locations by fusing the enzyme to a cytoplasmic domain or to a periplasmic domain in lactose permease. We could predict the subcellular location of AP for both of these chimeras, because E. coli alkaline phosphatase has been fused to the same two lactose permease domains and those chimeras placed alkaline phosphatase in opposing locations (9). The periplasmic AP chimera produced 17-fold-greater activity than the cytoplasmic chimera. In this test the native AP signal sequence is missing from both chimeras, obviating concerns that the signal sequence has an integral role in the mechanism of catalysis. Clearly, E. faecalis AP activity is suppressed in the cytoplasm and/or activated by export from the cytoplasm.
Several aspects of AP remain to be characterized. Highly homologous B. subtilis genes are known to be regulated by a complicated circuitry of signaling pathways, possibly because of the bacteria’s critical need for phosphate. The control of phoZ expression in E. faecalis has not yet been addressed. The assays described here have shown that AP can function in a highly alkaline environment, but the optimal conditions for activity have not been established. Finally, it would be useful to quantify the rates of expression for AP fusions, since per-cell AP activity can be affected by the rates of expression as well as the chimeric protein’s subcellular location. The most commonly used methods for quantifying AP expression would require anti-AP antibodies, which are not available.
A number of alternative reporter enzymes have been described for use in gram-positive bacteria. The secreted nuclease (Nuc) from Staphylococcus aureus has been used to identify Lactococcus lactis genes encoding secreted proteins (31). Similarly, the α-amylase of B. licheniformis has been used to identify B. subtilis genes that encode secreted proteins (36). Like AP, these reporter enzymes are active in gram-positive bacteria, there are plate assays that permit identification of active chimeras, and the activity of these reporter enzymes can be quantified by using spectrophotometric assays. The AP system presents a number of advantages. The AP plate assay is based on colony color, while the nuclease and amylase plate assays both rely on a zone of clearing around colonies grown on indicator media. Consequently, AP chimeras can be readily evaluated even when plates are crowded with colonies, phoZ+ sectors arising within colonies can be visualized, reversion events can be readily identified, and the AP colony color phenotype can be distinguished for days after the initial plating. Chimeras formed with other reporter enzymes (although not the S. aureus nuclease) seem biased toward short insertions (31). This bias is not seen in the AP fusions, since the chimeras described here included N-terminal peptides ranging in length from 16 to 286 residues (Table 3). Finally, the AP reporter enjoys some procedural advantages over other reporter systems. First, many labs already have experience in running and evaluating alkaline phosphatase assays. Second, the BamHI site engineered into ′phoZ makes it fairly simple to replace the reporter with either lacZ or phoA (from E. coli), should either of these alternative reporter systems be advantageous (27).
We utilized E. faecalis AP to identify genes encoding exported proteins in GBS. Eight AP chimeras were identified and sequenced. All eight fusions encoded proteins with signal sequence-like segments, and none of the eight chimeras had significant homology with cytoplasmic proteins. Additionally, two had homology with proteins known to be exported in either GAS or GBS. These results demonstrate that ′AP can be used as a means of identifying genes that encode exported proteins in GBS.
Some bacterial strains have high constitutive levels of alkaline phosphatase activity, limiting the utility of AP as a reporter enzyme. This may not be a general problem. The phoZ gene has been used as a reporter enzyme in E. coli, E. faecalis, GAS, GBS, and Streptococcus gordonii (10). In our investigations, only S. aureus RN4220 has exhibited a prohibitively high level of endogenous phosphatase activity (9a). Furthermore, a study of 54 strains isolated from sheep rumina revealed that none of 21 gram-positive strains exhibited detectable levels of phosphatase activity (11). In contrast, 9 of 33 gram-negative species had constitutive phosphatase expression. Thus, the breadth of the range of gram-positive species in which AP can be used is expected to be comparable to that of gram-negative species in which E. coli alkaline phosphatase has been used. If a particular strain exhibits high levels of phosphatase activity, it may be possible to suppress the background by growth on media supplemented with inorganic phosphate. Alternatively, it may be possible to work with derivative strains lacking the gene that encodes their endogenous alkaline phosphatase.
This report identifies the E. faecalis phoZ gene and presents a characterization of its gene product. The export-dependent functionality of the enzyme was used to identify eight genes that encode exported proteins in GBS, using the broad-host-range vector pAN200. This vector can be introduced into GBS, GAS, and E. faecalis and should be useful in identifying exported proteins in a variety of gram-positive bacteria.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI25152 (C.E.R.), The Streptococcus Initiative, and by NIH grant GM35394 (A.C.).
We thank Colin Manoil for providing several of the E. coli strains, the lacY mutants, and advice. Thanks to Donald Chaffin for strains, plasmids, and help with GBS manipulations and to Scott Winram for a critical reading of the manuscript.
REFERENCES
- 1.Abouhamad W N, Manson M D. The dipeptide permease of Escherichia coli closely resembles other bacterial transport systems and shows growth-phase-dependent expression. Mol Microbiol. 1994;14:1077–1092. doi: 10.1111/j.1365-2958.1994.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 2a.Bailey, J., and C. Manoil. Personal communication.
- 3.Ballester S, Lopez P, Alonso J C, Espinosa M, Lacks S A. Selective advantage of deletions enhancing chloramphenicol acetyltransferase gene expression in Streptococcus pneumoniae plasmids. Gene. 1986;41:153–163. doi: 10.1016/0378-1119(86)90094-6. [DOI] [PubMed] [Google Scholar]
- 4.Bardwell J C, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;67:581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 5.Berg P E. Cloning and characterization of the Escherichia coli gene coding for alkaline phosphatase. J Bacteriol. 1981;146:660–667. doi: 10.1128/jb.146.2.660-667.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bina J E, Nano F, Hancock R E. Utilization of alkaline phosphatase fusions to identify secreted proteins, including potential efflux proteins and virulence factors from Helicobacter pylori. FEMS Microbiol Lett. 1997;148:63–68. doi: 10.1111/j.1574-6968.1997.tb10268.x. [DOI] [PubMed] [Google Scholar]
- 7.Brabetz W, Liebl W, Schleifer K-H. Lactose permease of Escherichia coli catalyzes active β-galactoside transport in a gram-positive bacterium. J Bacteriol. 1993;175:7488–7491. doi: 10.1128/jb.175.22.7488-7491.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brickman E, Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and φ80 transducing phages. J Mol Biol. 1975;96:307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- 9.Calamia J, Manoil C. lac permease of Escherichia coli: topology and sequence elements promoting membrane insertion. Proc Natl Acad Sci USA. 1990;87:4937–4941. doi: 10.1073/pnas.87.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Chaffin, D. Unpublished observations.
- 10.Chaffin D O, Rubens C E. Blue/white screening of recombinant plasmids in Gram-positive bacteria by interruption of alkaline phosphatase gene (phoZ) expression. Gene. 1998;219:91–99. doi: 10.1016/s0378-1119(98)00396-5. [DOI] [PubMed] [Google Scholar]
- 11.Cheng K J, Costerton J W. Alkaline phosphatase activity of rumen bacteria. Appl Environ Microbiol. 1977;34:586–590. doi: 10.1128/aem.34.5.586-590.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman J E, Gettins P. Alkaline phosphatase, solution structure and mechanisms. Adv Enzymol Relat Areas Mol Biol. 1993;55:381–452. doi: 10.1002/9780470123010.ch5. [DOI] [PubMed] [Google Scholar]
- 13.Cruz-Rodz A L, Gilmore M S. Electroporation of glycine-treated Enterococcus faecalis. In: Dunny G M, Cleary P P, McKay L L, editors. Genetics and molecular biology of streptococci, lactococci, and enterococci. Washington, D.C: American Society for Microbiology; 1991. p. 300. [Google Scholar]
- 14.Derman A I, Beckwith J. Escherichia coli alkaline phosphatase localized to the cytoplasm slowly acquires enzymatic activity in cells whose growth has been suspended: a caution for gene fusion studies. J Bacteriol. 1995;177:3764–3770. doi: 10.1128/jb.177.13.3764-3770.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunny G, Funk C, Adsit J. Direct stimulation of the transfer of antibiotic resistance by sex pheromones in Streptococcus faecalis. Plasmid. 1981;6:270–278. doi: 10.1016/0147-619x(81)90035-4. [DOI] [PubMed] [Google Scholar]
- 16.Engelman D M, Steitz T A, Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- 17.Framson P E, Nittayajarn A, Merry J, Youngman P, Rubens C E. New genetic techniques for group B streptococci: high-efficiency transformation, maintenance of temperature-sensitive pWV01 plasmids, and mutagenesis with Tn917. Appl Environ Microbiol. 1997;63:3539–3547. doi: 10.1128/aem.63.9.3539-3547.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genetics Computer Group. Wisconsin package, version 9. Madison, Wis: Genetics Computer Group; 1999. [Google Scholar]
- 19.Hulett F M. The signal-transduction network for Pho regulation in Bacillus subtilis. Mol Microbiol. 1996;19:933–939. doi: 10.1046/j.1365-2958.1996.421953.x. [DOI] [PubMed] [Google Scholar]
- 20.Inouye H, Barnes W, Beckwith J. Signal sequence of alkaline phosphatase of Escherichia coli. J Bacteriol. 1982;149:434–439. doi: 10.1128/jb.149.2.434-439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacks W J, Kim Y, Cleary P P. Restricted deposition of C3 on M+ group A streptococci: correlation with resistance to phagocytosis. J Immunol. 1982;128:1897–1902. [PubMed] [Google Scholar]
- 22.Kam W, Clauser E, Kim Y S, Kan Y W, Rutter W J. Cloning, sequencing, and chromosomal localization of human term placental alkaline phosphatase cDNA. Proc Natl Acad Sci USA. 1985;82:8715–8719. doi: 10.1073/pnas.82.24.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim E E, Wyckoff H W. Reaction mechanism of alkaline phosphatase based on crystal structures. Two-metal ion catalysis. J Mol Biol. 1991;218:449–464. doi: 10.1016/0022-2836(91)90724-k. [DOI] [PubMed] [Google Scholar]
- 24.Kim E E, Wyckoff H W. Structure of alkaline phosphatases. Clin Chim Acta. 1990;186:175–187. doi: 10.1016/0009-8981(90)90035-q. [DOI] [PubMed] [Google Scholar]
- 25.Lee E, Manoil C. Mutations eliminating the protein export function of a membrane-spanning sequence. J Biol Chem. 1994;269:28822–28828. [PubMed] [Google Scholar]
- 26.Lee M, Manoil C. Molecular genetic analysis of membrane protein topology. In: Konigs W N, Kaback H R, Lolkema J S, editors. Transport processes in eukaryotic and prokaryotic organisms. Amsterdam, The Netherlands: Elsevier Science BV; 1996. pp. 189–201. [Google Scholar]
- 27.Manoil C. Analysis of protein localization by use of gene fusions with complementary properties. J Bacteriol. 1990;172:1035–1042. doi: 10.1128/jb.172.2.1035-1042.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manoil C, Bailey J. A simple screen for permissive sites in proteins: analysis of Escherichia coli lac permease. J Mol Biol. 1997;267:250–263. doi: 10.1006/jmbi.1996.0881. [DOI] [PubMed] [Google Scholar]
- 29.Manoil C, Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci USA. 1985;82:8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearce B J, Yin Y B, Masure H R. Genetic identification of exported proteins in Streptococcus pneumoniae. Mol Microbiol. 1993;9:1037–1050. doi: 10.1111/j.1365-2958.1993.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 31.Poquet I, Ehrlich S D, Gruss A. An export-specific reporter designed for gram-positive bacteria: application to Lactococcus lactis. J Bacteriol. 1998;180:1904–1912. doi: 10.1128/jb.180.7.1904-1912.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross R P, Claiborne A. Cloning, sequence and overexpression of NADH peroxidase from Streptococcus faecalis 10C1. Structural relationship with the flavoprotein disulfide reductases. J Mol Biol. 1991;221:857–871. doi: 10.1016/0022-2836(91)80180-3. [DOI] [PubMed] [Google Scholar]
- 33.Rubens C E, Wessels M R, Heggen L M, Kasper D L. Transposon mutagenesis of type III group B streptococcus: correlation of capsule expression with virulence. Proc Natl Acad Sci USA. 1987;84:7208–7212. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Simon D, Ferretti J J. Electrotransformation of Streptococcus pyogenes with plasmid and linear DNA. FEMS Microbiol Lett. 1991;66:219–224. doi: 10.1016/0378-1097(91)90336-9. [DOI] [PubMed] [Google Scholar]
- 36.Smith H, de Jong A, Bron S, Venema G. Characterization of signal-sequence-coding regions selected from the Bacillus subtilus chromosome. Gene. 1988;70:351–361. doi: 10.1016/0378-1119(88)90207-7. [DOI] [PubMed] [Google Scholar]
- 37.Stalhammar-Carlemalm M, Stenberg L, Lindahl G. Protein rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J Exp Med. 1993;177:1593–1603. doi: 10.1084/jem.177.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramaniam S, Unwin R, Fenton J, Whitsitt M, Bhagavan M, Stephens A. Biology Workbench 3.0. Computational Biology Group. National Center for Supercomputing Applications, University of Illinois at Urbana-Champaign; 1999. [Google Scholar]
- 39.Wanner B L. Phosphorus assimilation and control of the phosphate regulon. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Samonella. 2nd ed. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 1357–1381. [Google Scholar]
- 40.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]