Abstract
Sea cucumbers are considered a luxury food item and used locally in traditional medication due to their impressive nutritional profile and curative effects. Sea cucumbers contain a wide range of bioactive compounds, namely phenolics, polysaccharides, proteins (collagen and peptides), carotenoids, and saponins, demonstrating strong antioxidant and other activities. In particular, phenolic compounds, mainly phenolic acids and flavonoids, are abundant in this marine invertebrate and exhibit antioxidant activity. Protein hydrolysates and peptides obtained from sea cucumbers exhibit antioxidant potential, mainly dependent on the amino acid compositions and sequences as well as molecular weight, displayed for those of ≤20 kDa. Moreover, the antioxidant activity of sea cucumber polysaccharides, including fucosylated chondroitin sulfate and fucan, is a combination of numerous factors and is mostly associated with molecular weight, degree of sulfation, and type of major sugars. However, the activity of these bioactive compounds typically depends on the sea cucumber species, harvesting location, food habit, body part, and processing methods employed. This review summarizes the antioxidant activity of bioactive compounds obtained from sea cucumbers and their by-products for the first time. The mechanism of actions, chemical structures, and factors affecting the antioxidant activity are also discussed, along with the associated health benefits.
Keywords: sea cucumber, antioxidants, phenolics and polyphenolics, protein hydrolysates and peptides, polysaccharides, carotenoids
1. Introduction
Sea cucumbers are marine invertebrates that have been used for food, cosmetics, and traditional medicine. Around 100 species of sea cucumbers are harvested for commercial purposes, which have been widely consumed in Asian countries, including China, Indonesia, Japan, Korea, and Malaysia. Traditionally, sea cucumbers are a luxurious and nutritious food, and have been used to cure rheumatism, kidney problems, reproductive disorders, impotence, asthma, joint pain, back pain, hypertension, cuts and burns, wound injuries, and constipation [1]. In the contemporary market, various products originating from different body parts of the sea cucumbers are available. This mainly includes dry tablets made from the body wall, liquid extract prepared from whole sea cucumbers, and extracts obtained from the skin of sea cucumbers [2]. Nutritionally, sea cucumbers contain high levels of protein (40–60%), and low levels of lipids (mainly polyunsturated fatty acids (PUFAs)), minerals (e.g., calcium, zinc, iron, and magnesium), and vitamins (e.g., A, B1, B2, and B3) [3]. Apart from these, sea cucumbers contain a series of bioactive compounds, including triterpene glycosides (saponins), phenolics (flavonoids and phenolic acids), polysaccharides (fucosylated chondroitin sulfate), proteins (collagen and peptides), cerebrosides, and sphingoids, which demonstrate potential antioxidant, anticancer, anti-hypertension, anti-inflammatory, antithrombotic, anti-diabetic, anti-obesity, and antimicrobial activities [2]. However, biological activities mainly depend on the species, chemical structures, molecular weights, and testing methods. For example, fucosylated chondroitin sulfate of different sea cucumbers (Stichopus tremulus, Pearsonothuria graeffei, Isostichopus badionotus, and Holothuria vagabunda) exhibited potent anticoagulant activity, which could be associated with the sulfation pattern of the fucose branch [4]. Moreover, Hossain et al. [5] suggested that the antioxidant activity of sea cucumber phenolics is mostly linked with the nature of phenolic compounds, sea cucumber body parts, pre-treatment, and the assays used to determine the activity.
Antioxidants can prevent or slow down oxidative stress in cells and therefore play an important role in controlling various diseases, mainly cardiovascular ailments, cancer, and inflammatory diseases. Human bodies generate more reactive oxygen species (ROS) (e.g., hydroxyl radical, superoxide radical anion, and hydrogen peroxide) under stress. Therefore, a lack of enzymatic (e.g., glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase) and non-enzymatic antioxidants (e.g., α-tocopherol (vitamin E), ascorbic acid (vitamin C), carotenoids, glutathione, and phenols) ultimately leads to cell damage [6]. Apart from this, antioxidants are commonly used to improve the oxidative stability of food and food products, mainly those that are rich in lipids. As a result, the dietary inclusion of natural antioxidants is receiving growing interest and demand due to the potential carcinogenic effect of synthetic antioxidants. Sea cucumbers are one of the major marine animals containing various bioactive compounds with antioxidant activity. The purpose of this review is, for the first time, to summarize the antioxidant activity of bioactive compounds obtained from sea cucumbers and their by-products in order to categorize this echinoderm as a potential candidate in the functional food and nutraceutical sectors. Therefore, we report on the antioxidant activities and detailed mechanisms and chemical structures of the bioactive compounds of sea cucumbers along with their beneficial effects. The relevant data were collected from Scopus, PubMed, and ScienceDirect after searching different keywords such as “sea cucumber”, “antioxidant activity”, “bioactive compounds”, and “health benefits”, among others.
2. Bioactive Compounds of Sea Cucumbers and Their Antioxidant Activity
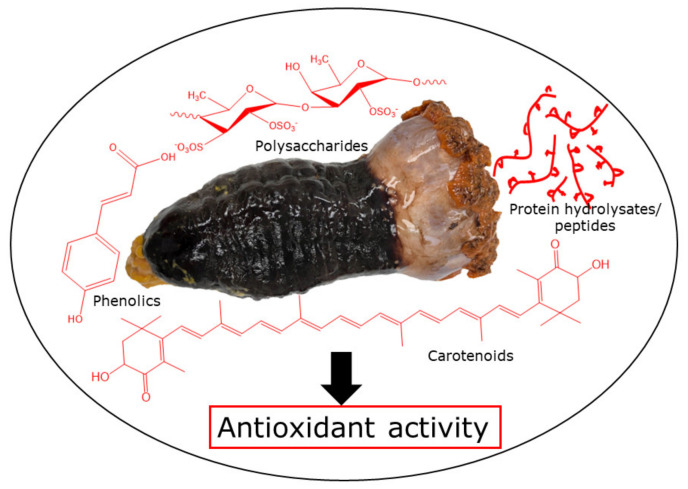
Sea cucumbers are a highly marketable echinoderm, which contains numerous bioactive compounds. These include proteins (collagen and peptides), polysaccharides, saponins, carotenoids, and phenolics with multiple biological and pharmacological properties, mainly antioxidant activity [2,7]. Antioxidants are substances that scavenge free radicals and hence prevent oxidation. The main mechanisms involved are hydrogen atom transfer (HAT), single electron transfer (SET), metal chelation, and reducing power. Therefore, the effectiveness of antioxidants in a specific medium is mainly dependent on the number and arrangement of the hydroxyl groups in the molecules of interest [8]. For example, phenolic antioxidants can donate hydrogen atoms from the hydroxyl groups to lipid radicals in order to neutralize the oxidation reaction, but the phenoxyl radicals that are produced are resonance stabilized and are therefore not involved in further oxidation, thus breaking the cycle of the generation of new radicals. Antioxidants with specific structures can also chelate metal ions (e.g., ferrous and copper), where metal ions can no longer act as an initiator of lipid oxidation due to the formation of a complex between the metal ions and antioxidants. Besides, synergistic effects can be observed among various antioxidants such as phenolics, α-tocopherol, β-carotene, and ascorbic acid [9,10]. Numerous techniques are available for determining antioxidant activity, including radical scavenging assays that include SET (e.g., ferric-reducing antioxidant power (FRAP), Trolox equivalent antioxidant capacity (TEAC), and the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay) and HAT (e.g., total radical-trapping antioxidant parameter (TRAP) and oxygen radical absorbance capacity (ORAC)) mechanisms [11,12]. However, each assay has a different mechanism of action, thus providing varied results for the antioxidant potential of the same sample [13]. On the other hand, due to lipid oxidation, the quality attributes of food, including flavor, color, and texture, deteriorate, which ultimately decreases the shelf life and nutritional value of food. Thus, antioxidants are widely used to control the rate and extent of lipid oxidation in foods. One of the main assays to measure the degree of lipid oxidation is the thiobarbituric acid (TBA) test that measures the TBA reactive substances (TBARS), which are then used to determine the secondary oxidation products, mainly the aldehydes, from among the others that are believed to produce rancid flavors and aromas [14]. Autoxidation is one of the main pathways of lipid oxidation in which PUFAs are involved in a free radical chain reaction under heat, light, or metal ions. Synthetic antioxidants such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), tert-butylhydroquinone (TBHQ), and propyl gallate (PG) have been used as antioxidants in foods to prevent oxidation and off-flavor development. Nevertheless, due to the carcinogenic and toxicity characteristics of some synthetic antioxidants, researchers have shown much attention towards natural antioxidants [15,16]. For instance, sea cucumbers and their by-products are a good source of phenolic acids and flavonoids, which show strong antioxidant activity. The specific bioactive compounds in most common sea cucumbers and their antioxidant activities are detailed below (Figure 1).
Figure 1.
Sea cucumber antioxidants.
2.1. Antioxidant Potential of Sea Cucumber Phenolics and Their Beneficial Effects on Human Health
Phenolic compounds are secondary metabolites that contain one or more aromatic rings and hydroxyl groups. Moreover, phenolic compounds play a key role in protecting plants by engaging in defense mechanisms against ultraviolet radiation, herbivory, and pathogen attacks. Phenolics are also involved in the plants for growth regulation and are responsible for the color, flavor, bitterness, and astringency of foods. Plant phenolics are mainly derived from phenylalanine and, in some cases, tyrosine. The formation of trans-cinnamic acid from phenylalanine is catalyzed by phenylalanine ammonia-lyase (PAL), whereas p-hydroxycinnamic acid from tyrosine is catalyzed by tyrosine ammonia-lyase (TAL) [17]. Phenolics can be categorized into different groups, namely phenolic acids, flavonoids, tannins, stilbenes, lignans, and coumarins [18]. Due to their antioxidant, antimicrobial, and coloring properties, phenolic compounds have received significant attention from several industries, especially from the food, pharmaceutical, cosmetics, packaging, and textile industries. In the food industry, phenolics are used as preservatives to inhibit the oxidation process and microbial growth of food products.
Plant- and marine-based phenolic compounds are receiving increased attention due to their potential health benefits and multiple biological activities. Most of the phenolics have so far been researched from the terrestrial environment, but less attention has been paid to the marine environment, even though it provides many healthy foods due to its biodiversity. Sea cucumbers are one of the marine invertebrates that serves as a possible source of phenolic compounds with strong antioxidant activity. This could be due to the absorption of phenolics from phytoplankton, the primary food source for sea cucumbers. Phytoplankton is a rich source of phenolic compounds, including phenolic acids, flavonoids, and tannins [2,19]. For example, Ceesay et al. [20] reported that sea cucumbers contain catechins and flavonols as they feed mainly on seaweeds, rich sources of flavonoids. Various species of sea cucumbers have different levels of phenolic compounds and varied antioxidant activities. This might be due to the different geographic locations, food habits, and harvesting times. Therefore, suspension-feeding species, such as Cucumaria frondosa, may have more phenolics when compared to the deposit-feeding species. Table 1 shows the phenolic compounds of different sea cucumbers and their antioxidant activities.
Table 1.
Phenolics in sea cucumbers and their antioxidant activity.
| Species | Body Parts | TPC (mg GAE/g) | TFC (mg RE/g) | Antioxidant Assays | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| DPPH (%) | ABTS (mg TE/g) | HRSA (mg TE/g) | MCA (mg EDTAE/g) | ORAC (mmol TE/g) | |||||
| Holothuria forskali | Dried sea cucumber (extract) | 3.19–5.21 | NA | NA | NA | NA | NA | NA | [34] |
| Holothuria forskali | Dried sea cucumber (hydroethanolic and aqueous extracts) | 0.48 | NA | 1.06 f | 18.83 b | NA | NA | NA | [35] |
| Holothuria arguinensis | Dried sea cucumber (hydroethanolic and aqueous extracts) | 0.84 | NA | 0.13 f | 22.34 b | NA | NA | NA | [35] |
| Holothuria mammata | Dried sea cucumber (hydroethanolic and aqueous extracts) | 0.79 | NA | 0.31 f | 30.89 b | NA | NA | NA | [35] |
| Holothuria atra | Body wall (phosphate buffer extract) | NA | NA | 82 to 95 | NA | NA | NA | NA | [31] |
| Holothuria atra | Dried sea cucumber (extract) | Detected | Detected | NA | NA | NA | NA | NA | [36] |
| Holothuria arenicola | Body wall (phosphate buffer extract) | NA | NA | 82–95 | NA | NA | NA | NA | [32] |
| Holothuria scabra | Dried sea cucumber (hexanes, ethyl acetate, and n-butanol extracts) | 20–46.54 | NA | NA | NA | NA | NA | NA | [37] |
| Holothuria scabra | Dried sea cucumber (methanol extract) | 30.52 | NA | 33.77 c | NA | NA | NA | NA | [22] |
| Holothuria scabra | Sea cucumber without viscera (aqueous and organic extracts) | 1.53–4.85 | NA | NA | NA | NA | NA | NA | [21] |
| Holothuria scabra | Dried sea cucumber (extracts) | 2.02–2.86 | 0.35–2.49 e | [23] | |||||
| Holothuria leucospilota | Dried body wall (methanol, acetone, and water extracts) | 4.58 | 0.84 | NA | NA | NA | NA | [20] | |
| Holothuria leucospilota | Sea cucumber without viscera (aqueous and organic extracts) | 2.91–9.7 | NA | 3.91–5.44 | NA | NA | NA | NA | [21] |
| Cucumaria frondosa | Body wall (acetone extract) | 3.05–3.98 | 1.22–1.55 | 4.98–5.04 d | 7.51–8.01 | 10.47–10.65 | 0.41–0.53 | NA | [5] |
| Cucumaria frondosa | Viscera (acetone extract) | 2.32–3.02 | 1.01–1.24 a | 4.37–4.62 d | 7.36–7.87 | 9.57–9.85 | 0.29–0.44 | NA | [24] |
| Cucumaria frondosa | Tentacles/flowers (acetone extract) | 3.09 | 1.61 | 6.67 d | NA | NA | 0.55 | NA | [38] |
| Cucumaria frondosa | Fresh and dried sea cucumber with/without viscera (methanol extract) | 0.88–1.08 | NA | 4.51–7.48 b | NA | NA | NA | 2.09–2.6 | [19] |
| Cucumaria frondosa | Dried digestive tract, gonads, muscles, and respiratory apparatus (extract) | 0.22–2.36 | 0.029–0.59 | NA | NA | NA | 140–800 b | [25] | |
| Stichopus variegatus | Dried sea cucumber without viscera (aqueous extract) | 10.55–10.9 | NA | 1.67–2.3 c | NA | NA | NA | NA | [27] |
| Apostichopus japonicus | Dried internal organs (extract) | 13.6–116.90 | NA | NA | NA | NA | NA | NA | [39] |
| Apostichopus japonicus | Dried body wall (water and ethanol extracts) | 18.65–40.99 | 5.92–30.38 | 3.2–16.37 b | 0.83–1.5 b | NA | NA | NA | [28] |
| Apostichopus japonicus | Dried sea cucumber (methanol extract) | 3.53–20.37 | NA | NA | NA | NA | NA | NA | [40] |
| Stichopus chloronotus | Sea cucumber without viscera (aqueous and organic extracts) | 1.66–8.27 | NA | 2.13 c | NA | NA | NA | NA | [21] |
Abbreviations are: NA, not available; HRSA, hydroxyl radical-scavenging activity; MCA, metal chelation activity; EDTAE, ethylenediaminetetraacetic acid equivalents; TE, Trolox equivalents; a data expressed as mg catechin equivalents/g; b data expressed as µmol TE/g; c data expressed as IC50 in mg extract/mL DPPH; d data expressed as mg TE/g; e data expressed as mg quercetin equivalents/g; and f data expressed as mg ascorbic acid equivalents/g.
It has been reported that the different body parts of sea cucumbers, such as body wall, tentacles/flower, and viscera, contain a significant amount of phenolics with strong antioxidant activity. For example, Althunibat et al. [21] compared the antioxidant activity of three Malaysian sea cucumber species (Holothuria leucospilota, Holothuria scabra, and Stichopus chloronotus) without viscera, and reported that the extracts of H. leucospilota had higher total phenolic contents (TPC, 9.7 mg gallic acid equivalents (GAE)/g), but H. scabra contained a lower amount of TPC (1.53 mg GAE/g). S. chloronotus extracts showed a higher DPPH radical-scavenging capacity (80.58%) compared to the H. scabra (77.46%) and H. leucospilota (64.03%) extracts. Likewise, methanol extracts of H. scabra were found to be a good source of phenolics (30.52 mg GAE/g), dominated by 3- and 4-hydroxybenzaldehyde [22]. Wulandari et al. [23] cultured Holothuria scabra in an open pond system and found that antioxidant activity such as ABTS and hydroxyl radical-scavenging activities as well as ferric-reducing antioxidant power (FRAP) were related to the total flavonoid content (TFC). Besides, TPC and TFC were determined in the body wall of H. leucospilota, which contained 2,4-bis(1,1-dimethylethyl)-phenol [20]. Pre-treatments also affect the content of sea cucumber phenolic and their antioxidant activities. For example, free, esterified, and insoluble-bound phenolics from the body wall and internal organs of Atlantic sea cucumber (Cucumaria frondosa) were determined using high-pressure processing (HPP) pre-treatment [5,24]. Results demonstrated that HPP significantly improved the TPC, TFC, and antioxidant activity. TPC varied between 3.05 and 3.98 mg GAE/g for the body wall and 2.32–3.02 mg GAE/g for internal organs, while the TFC was 1.22–1.55 mg catechin equivalents (CE)/g and 1.01–1.24 mg CE/g for body wall and internal organs, respectively. Additionally, phenolic extracts obtained from the body wall and internal organs exhibited strong antioxidant activity in terms of DPPH, ABTS, and hydroxyl radical scavenging as well as metal chelation activities, which showed a strong positive correlation with TPC and TFC. Especially, TFC had a strong correlation with antioxidant activity, suggesting that sea cucumber phenolics are mostly flavonoids, which are responsible for the antioxidant effect. On the other hand, the antioxidant activity, TPC, and TFC were determined in extracts from different body parts (digestive tract, gonad, muscle, and respiratory apparatus) of C. frondosa [25]. The TPC varied from 0.22 to 2.36 mg GAE/g, while TFC ranged from 0.029 to 0.59 mg rutin equivalents (RE)/g and the oxygen radical absorbance capacity (ORAC) varied from 140 to 800 µmol Trolox equivalents (TE)/g. A higher TPC was also observed in the digestive tract when considering acetonitrile-rich fractions and ethyl acetate extracts, while the maximum TFC was obtained from the gonads using water-rich and acetonitrile-rich fractions. Similarly, Mamelona and Pelletier [26] determined the antioxidant activity (ORAC) of the viscera of C. frondosa using pressure liquid extraction (PLE) and observed that the ethanol extracts had higher ORAC values when compared to methanol, isopropanol, and water extracts at 60 °C extraction. Additionally, PLE allowed better extraction of TPC, total carotenoids, and α-tocopherol using ethanol followed by isopropanol, methanol, and water. In another study, the antioxidant property of fresh and processed C. frondosa with/without internal organs was evaluated [19]. The processed (rehydrated) samples, mainly those with internal organs, exhibited higher ORAC and DPPPH radical-scavenging activity, whereas fresh samples contained a significant amount of phenolics when compared to their rehydrated counterparts. However, Ridhowati et al. [27] reported that dried Stichopus variegatus contained a higher content of TPC (10.55–10.9 mg GAE/g) with strong DPPH radical-scavenging activity. Similarly, Husni et al. [28] stated that the TPC of Apostichopus japonicus body wall extract had a good correlation with antioxidant activity than the TFC, suggesting that phenolic compounds play an important role in exhibiting antioxidant activity.
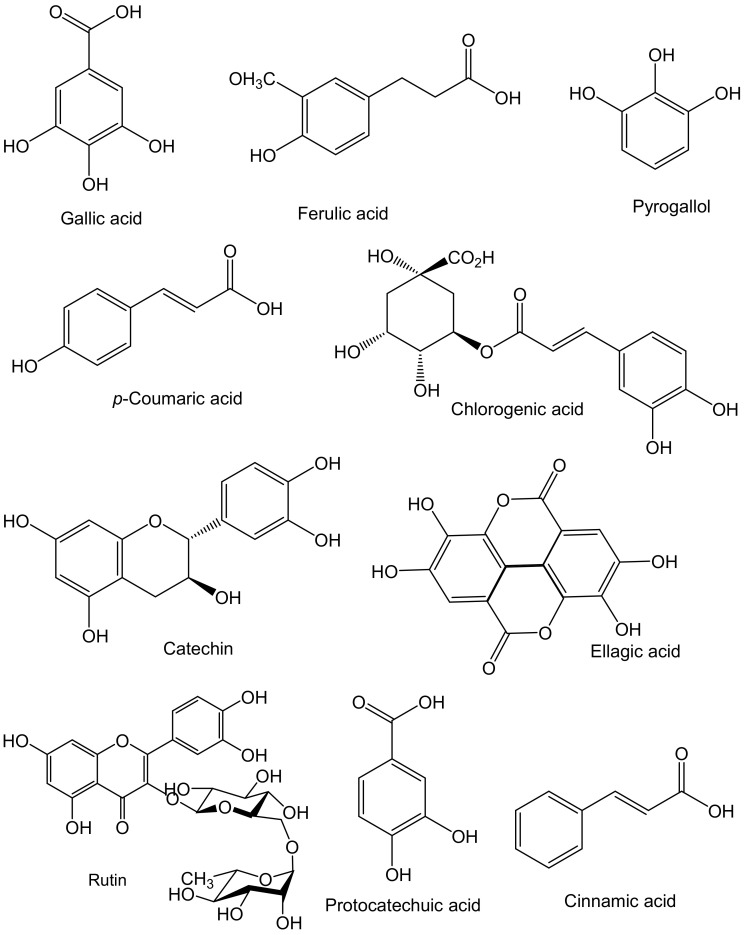
Sea cucumber phenolics are mainly phenolic acids and flavonoids. The most common phenolic compounds found in sea cucumbers are chlorogenic acid, gallic acid, p-coumaric acid, protocatechuic acid, ferulic acid, ellagic acid, cinnamic acid, catechin, rutin, quercetin, and pyrogallol (Table 2 and Figure 2).
Table 2.
Phenolic compounds of sea cucumber.
| Species | Body Parts | Identified Compounds (mg/100 g) | References |
|---|---|---|---|
| Cucumaria frondosa | Body wall | Protocatechuic acid (8.86), gallic acid (7.34),catechin (5.19), p-coumaric acid (5.11), epigallocatechin gallate (4.87), ellagic acid (4.55), hydroxygallic acid (3.9), p-hydroxybenzoic acid (3.66), p-coumaroyl glycolic acid (3.56), isoferulic acid (3.4), quercetin (3.35), p-hydroxybenzaldehyde (3.12), vanillic acid (3.04), cinnamic acid (2.9), syringic acid (2.51), myricetin (1.04), phlorizin (0.96), sinapinic acid (0.94), p-hydroxycoumarin (0.93), and caffeic acid (0.7) | [5] |
| Cucumaria frondosa | Viscera | Catechin (9.33), p-coumaric acid (7.15), protocatechuic acid (7.13), hydroxygallic acid (6.2), quercetin (5.48), gallic acid (5.66), chlorogenic acid (5.53), cinnamic acid (4.78), ellagic acid (4.33), syringic acid (4.23), p-hydroxybenzaldehyde (3.34), sinapinic acid (3.07), vanillic acid (3.05), caffeoyl glucoside (2.47), p-hydroxybenzoic acid (1.89), scopoletin (1.56), homovanillic acid (1.03), caffeic acid (1.01), p-coumaroyl glycolic acid (0.91), p-hydroxycoumarin (0.8), isoferulic acid (0.76), chicoric acid (0.73), and leachianol F (0.63) | [24] |
| Cucumaria frondosa | Tentacles/flower | Protocatechuic acid (6.91), catechin (6.32), gallic acid (6.14), p-coumaric acid (4.9), gallic acid monohydrate (4.46), quercetin (4.07), ellagic acid (3.86), cinnamic acid (3.35), sinapinic acid (2.56), syringic acid (2.51), p-hydroxybenzaldehyde (2.41), vanillic acid (2.4), chicoric acid (2.32), chlorogenic acid (2.25), caffeic acid (1.91), isoferulic acid (1.74), fraxin (1.64), kaempferol 3-O-glucoside (1.46), p-hydroxybenzoic acid (1.42), quercetin-3-O-arabinose (1.34), caffeoyl glucoside (1.05), epigallocatechin gallate (1.05), rosmarinic acid (1.05), scopoletin (1.04), homoveratric acid (1), ferulic acid (0.97), sinapine (0.97), homovanillic acid (0.97), p-coumaroyl glycolic acid (0.73), ferulic acid hexoside (0.72), and myricetin (0.2) | [38] |
| Holothuria atra | Body wall | Chlorogenic acid (80.34%), coumaric acid (2.43), pyrogallol (2.25%), and rutin (0.82) | [31] |
| Holothuria atra | Body wall | Chlorogenic acid (92.86%), pyrogallol (2.99%), rutin (1.83%), coumaric acid (1.55%), and catechin (0.51) | [30] |
| Holothuria arenicola | Body wall | Chlorogenic acid (89.66%), pyrogallol (1.88%), coumaric acid (1.23%), and rutin (1.06%) | [32] |
| Holothuria scabra | Dried sea cucumber | 3-Hydroxybenzaldehyde and 4-hydroxybenzaldehyde | [22] |
| Holothuria leucospilota | Body wall | 2,4-bis(1,1-dimethylethyl)-phenol | [20] |
| Holothuria tubulosa | Body wall | Epicatechin (790 µg/g), 2,5-dihydroxybenzoic acid (130.54–158.89 µg/g), ellagic acid (109.25–558.67 µg/g), gallic acid (133.16–205.87 µg/g)), chlorogenic acid, 3,4-dihydroxybenzoic acid, 4-hydroxybenzoic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, cinnamic acid, rutin, naringin, and quercetin | [33] |
| Holothuria forskali | Digestive tract, muscle, body wall, gonad, and respiratory tree | Quinic acid (0.39–0.47 μg/mL), salvianolic acid (0.039–0.057 μg/mL), caffeoylquinic acid (0.13–0.14 μg/mL), caffeic acid, syringic acid, trans ferulic acid, o-coumaric acid, rosmarinic acid, and gallic acid | [29] |
Figure 2.
Major phenolic compounds found in sea cucumbers.
For instance, high-performance liquid chromatography coupled with a mass spectroscopy (HPLC-MS) was used to identify the phenolic compounds of Holothuria forskali extracts, which were mostly phenolic acids such as quinic, gallic, rosmarinic, and salvianolic acids. The same study reported that quinic acid was abundant in different body parts, including the digestive tract, muscle, body wall, gonad, and respiratory tree, whereas only gallic acid and caffeoylquinic acid were present in the gonads of this sea cucumber [29]. Moreover, the body wall of Holothuria atra was an excellent source of chlorogenic acid (up to 92 wt%), and it also contained pyrogallol, coumaric acid, rutin, and catechin [30]. These phenolics demonstrated strong antioxidant activity, including DPPH radical-scavenging and metal-chelating activities. The presence of phenolics in the body wall could be due to the eating of phenolic-rich ingredients, mainly phytoplankton and particles derived from degrading marine macroalgae [5]. Likewise, phenolic compounds of Holothuria atra were identified and quantified using HPLC, and major compounds were chlorogenic acid (80.34%), pyrogallol, rutin, and coumaric acid [31]. Similarly, chlorogenic acid was the major component (~90%) in the body wall of Holothuria arenicola, whereas pyrogallol, rutin, and coumaric acid were also identified from this species [32]. On the other hand, Alper and Günes [33] prepared aqueous and methanolic extracts from Holothuria tubulosa, and HPLC analysis found epicatechin and 2.5 dihydroxybenzoic acid as the most abundant components, among others. 3-Hydroxybenzaldehyde and 4-hydroxybenzaldehyde were identified from the methanol extract of Holothuria scabra, which demonstrated strong antioxidant activity [22]. However, Hossain et al. [5] found that sea cucumber (C. frondosa) phenolics primarily existed in the free form followed by esterified and insoluble-bound phenolics. Moreover, HPP pre-treatment not only improved the content of individual phenolic compounds but also increased the number of phenolic compounds in their profile. UHPLC-QTOF-MS/MS identified 20 phenolic compounds from the body wall of C. frondosa, which are mainly phenolic acids and flavonoids such as protocatechuic acid, gallic acid, p-coumaric acid, ellagic acid, catechin, and epigallocatechin gallate. Similarly, 23 phenolic compounds were identified and quantified from the processing discards of C. frondosa; among them, 11 of which were identified for the first time in any species of sea cumber [24]. These were protocatechuic acid, homovanillic acid, gallic acid monohydrate, isoferulic acid, p-coumaroyl glycolic acid, sinapinic acid, caffeoyl glucoside, chicoric acid, p-hydroxycoumarin, scopoletin, and leachianol F.
Sea cucumbers have been used as traditional medicine in Asian countries to treat hypertension, anemia, asthma, stomach ulcers, reproductive disorder, rheumatism, wound injuries, and constipation, among others [2]. However, various cell lines and animal studies have assessed the potential health benefits of bioactive compounds, mainly phenolics, of the sea cucumber. So far, the phenolics of the sea cucumber have been studied for their anticancer, antidiabetic, anti-inflammatory, antihypertension, anti-fibrotic, anti-aging, cardiovascular, and antioxidant properties. A summary of the potential health-promoting properties of sea cucumber phenolics is provided in Table 3.
Table 3.
Potential health promoting properties and mechanisms of action of sea cucumber phenolics.
| Health Effects | Species | Body Parts | Responsible Compounds/Extracts | Results/Mechanisms | References |
|---|---|---|---|---|---|
| Anticancer | Holothuria tubulosa | Body wall | Aqueous and methanolic extracts rich in epicatechin and ellagic acid | Inhibited the growth of cancer cell lines and induced apoptosis in A549 (human non-small lung carcinoma) and HeLa (cervix adenocarcinoma) cells | [33] |
| Holothuria scabra, Holothuria leucospilota, and Stichopus chloronotus | Sea cucumber without viscera | Aqueous extracts | Inhibited the growth of C33A (human cervical cancer) and A549 cancer cells | [21] | |
| Stichopus variegatus | Dried sea cucumber | Aqueous extracts | Possessed cytotoxicity on colon cancer cells WiDr, breast cancer cells T47D, and normal cells Vero | [27] | |
| Holothuria scabra | Dried sea cucumber | Extracts | Exhibited cytotoxic activity against human breast cancer cells (MDA-MB 231) | [23] | |
| DNA oxidation inhibition | Cucumaria frondosa | Dried body wall and internal organs | Acetone extracts rich in phenolic acids and flavonoids | Inhibited hydroxyl and peroxyl radical-induced DNA oxidation | [5,24] |
| Anti-inflammatory | Apostichopus japonicus | Fresh sea cucumber | Ethyl acetate extract. | Inhibited the productions of NO (nitric oxide) and PGE2 (prostaglandin E2) by inhibiting iNOS (inducible nitric oxide synthase) and COX-2 (cycloxygenase-2) | [40] |
| Holothuria scabra | Dried sea cucumber | Hexanes, ethyl acetate, and n-butanol extracts | Inhibited pro-inflammatory cytokine synthesis | [37] | |
| LDL oxidation inhibition | Cucumaria frondosa | Dried body wall and internal organs | Acetone extracts rich in phenolic acids and flavonoids | Inhibited primary oxidation products, conjugated dienes (CD) | [5,24] |
| Hepatoprotective and curative | Holothuria atra | Body wall | Phosphate buffer extracts rich in chlorogenic acid | Alleviated the hepatorenal toxicity resulting from DMBA (7,12-dimethylbenz[a]anthracene) hydrocarbon exposure | [31] |
| Holothuria atra | Body wall | Organic and aqueous extracts rich in chlorogenic acid | Exhibited hepatoprotective activity against thioacetamide-induced liver fibrosis in a rat model | [30] | |
| Anti-cholestatic | Holothuria arenicola | Body wall | Phosphate buffer extracts rich in chlorogenic acid | Prevented liver damage following cholestasis | [32] |
| Antibacterial | Holothuria atra | Dried sea cucumber | Hexane, ethyl acetate, and butanol extracts | Showed inhibitory activity against Pseudomonas aeruginosa | [36] |
| Holothuria forskali | Digestive tract, muscle, body wall, gonad, and respiratory tree | Ethyl-acetate extracts rich in quinic acid | Escherichia coli and Bacillus subtilis were inhibited | [29] | |
| α-Glucosidase inhibition | Apostichopus japonicus | Dried internal organs | Organic extracts | Showed potential to inhibit α-glucosidase enzyme | [39] |
| Cucumaria frondosa | Body wall | Acetone extracts rich in phenolic acids and flavonoids | Slowed down the activity of α-glucosidase enzyme | [5] | |
| Antiglycation | Cucumaria frondosa | Dried body wall and internal organs | Acetone extracts rich in phenolic acids and flavonoids | Controlled the formation of advanced glycation end products(AGEs) | [5,24] |
| Anti-tyrosinase | Cucumaria frondosa | Dried internal organs | Acetone extracts rich in phenolic acids and flavonoids | Inhibited tyrosinase enzyme | [24] |
For example, the anticancer properties of three Malaysian sea cucumbers (H. leucospilota, H. scabra, and S. chloronotus) extracts rich in phenolics were studied [21]. It was found that sea cucumber, mainly S. chloronotus, extracts prepared with organic solvents inhibited the growth of A549 (human non-small lung carcinoma) and C33A (human cervical cancer) cancer cells. Similarly, Alper and Günes [33] prepared aqueous and methanolic extracts of phenolics from Holothuria tubulosa in order to evaluate their cytotoxic effects. They suggested that phenolic extracts which were rich in epicatechin and 2,5-dihydroxybenzoic acid might inhibit the growth of cancer cells and induce apoptosis in A549 (human non-small lung carcinoma) and HeLa (cervix adenocarcinoma) cells. However, the phenolic compounds of the Atlantic sea cucumber’s (C. frondosa) body wall and internal organs were used in various in vitro biological assays [5,24]. Results suggested that phenolic compounds had the potential to inhibit DNA oxidation, low-density lipoprotein (LDL) oxidation, formation of advanced glycation end products (AGEs), and α-glucosidase and tyrosinase enzymes. The free phenolic fraction contained a significant number of phenolic acids and flavonoids, which showed strong inhibitory activity. On the other hand, Himaya et al. [40] suggested that phenolic compounds in ethyl acetate extracts prepared from S. japonicus may mediate the anti-inflammatory action via blocking of the MAPK (ERK and p38 MAPK) signaling pathway in murine macrophages. Likewise, the ethyl acetate fraction of H. scabra extracts, rich in phenolic compounds, inhibited pro-inflammatory cytokine synthesis at both the transcriptional and translational levels, mainly inducible nitric oxide synthase (iNOS), nitric oxide (NO), interleukin-1β (IL-1β), prostaglandin E2 (PGE2), and tumor necrosis factor-α (TNF-α) [37]. Carletti et al. [35] demonstrated that polyphenol-rich sea cucumber (H. forskali, H. arguinensis, and H. mammata) extracts, mainly hydroethanolic extracts, exhibited anti-inflammatory activity via COX-2 inhibition and osteogenic activity in a zebrafish (Danio rerio) model. However, phenolic-rich Holothuria atra extracts were used to determine the antioxidant efficacy against 7,12-dimethylbenz[a]anthracene (DMBA)-induced hepatorenal dysfunction [31]. It was found that the DMBA increased the level of liver malondialdehyde (MDA), decreased the level of glutathione-S-transferase (GST), and reduced glutathione (GSH), catalase (CAT), and SOD in the liver tissue in a rat model. Moreover, Fahmy [32] claimed that Holothuria arenicola extract was a good source of phenolic compounds with the potential to decrease the levels of total conjugated and unconjugated bilirubin and MDA as well as the activities of alkaline phosphatase and serum aminotransferases, and to increase GSH and serum albumin. Sea cucumber phenolics also showed antibacterial activities against various bacteria. For instance, Telahigue et al. [29] prepared ethyl acetate extracts from Holothuria forskali and tested them against Bacillus cereus, Bacillus subtilis, Pseudomonas aeruginosa, and Escherichia coli. It was found that ethyl acetate extracts are a good source of phenolic acids, which had the potential to inhibit E. coli and B. subtilis. Similarly, methanolic extracts of Holothuria atra contained phenolics with demonstrated antibacterial activities against Pseudomonas aeruginosa [36].
2.2. Antioxidant Potential of Protein Hydrolysates and Peptides and Their Health Benefits
Marine products and by-products are protein-rich and can be used to prepare protein hydrolysates, collagens, or peptides. The most common ways of producing protein hydrolysates include enzymatic (Flavourzyme, Alcalase, Protamex, papain, trypsin, chymotrypsin, and pepsin, among others), non-enzymatic (high-pressure processing, ultrasound, and supercritical fluids), organic solvents, and fermentation methods [41,42]. Among them, enzymatic procedures have received growing attention due to their higher efficiency, green nature, and lesser destruction than other techniques in order to produce value-added products for disease risk reduction and health promotion. In particular, microbial proteases, including Flavourzyme, Alcalase, and Corolase are favorable in industrial use due to their promising operational conditions [43]. However, the functionality of protein hydrolysates/peptides is mainly dependent on their amino acid compositions and sequences, molecular weight, and hydrophobicity/hydrophilicity, among others. Generally, bioactive peptides contain 3–20 amino acid units and show antioxidant activity [44]. Notably, the antioxidant activity of the bioactive peptides can improve in the presence of amino acids such as tyrosine, phenylalanine, proline, glutamic acid, histidine, and arginine. For example, proline is very common in collagen, shielding cells from the oxidation induced by free radicals [45].
Numerous studies have been performed on the preparation of protein hydrolysates from various marine sources, including sea cucumber, which demonstrate several health benefits. Protein hydrolysates of different sea cucumbers and their antioxidant activities are presented in Table 4.
Table 4.
Protein hydrolysates of sea cucumber and their antioxidant activity.
| Species | Body Parts | Protein Hydrolysates/Collagens/Peptides | Antioxidant Assays | References | ||||
|---|---|---|---|---|---|---|---|---|
| DPPH (%) | ABTS (µmol TE/g) | HRSA (%) | MCA (µmol EDTAE/g) | ORAC (µmol TE/g) | ||||
| Cucumaria frondosa | Body wall, tentacles, and internal organs | Protein hydrolysates using Alcalase, Corolase, and Flavourzyme | 7–14 a | 17.79–79.08 | NA | 16.5–37.43 | NA | [43] |
| Cucumaria frondosa | Viscera | Protein hydrolysates using Alcalase, Neutrase, trypsin, papain, bromelain, and Flavourzyme | 14.42 | NA | 27.04 | NA | NA | [46] |
| Cucumaria frondosa | Viscera | Protein hydrolysates using Alcalase | NA | NA | NA | NA | 421 | [48] |
| Isostichopus fuscus | Body wall | Protein hydrolysates and peptides using proteases | NA | NA | NA | NA | 0.00072 | [60] |
| Holothuria parvula | Dried sea cucumber | Protein hydrolysates using Neutrase | 5.25 b | NA | NA | NA | NA | [59] |
| Holothuria leucospilat | Whole animal | Protein hydrolysates using Alcalase and Flavourzyme | 35.3–68.27 | NA | NA | NA | NA | [41] |
| Holothuria scabra | Dried sea cucumber | Protein hydrolysates using papain, Alcalase, and Flavourzyme | 0.34–3.82 b | 1.28–1.65 b | NA | NA | NA | [57] |
| Acaudina molpadioides | Body wall | Protein hydrolysates using papain, pepsin, trypsin, and Neutrase | ~32 | NA | NA | NA | NA | [45] |
| Apostichopus japonicus | Egg | Protein hydrolysates using papain and Flavourzyme | NA | NA | 37–89.82 | NA | NA | [55] |
| Apostichopus japonicus | Body wall | Collagen using pepsin | 45.58 | NA | ~90 | NA | NA | [53] |
| Apostichopus japonicus | Body wall | Protein hydrolysates Flavourzyme | NA | NA | 0.28 b | NA | NA | [54] |
Abbreviations are: NA, not available; HRSA, hydroxyl radical-scavenging activity; MCA, metal-chelation activity; EDTAE, ethylenediaminetetraacetic acid equivalents; TE, Trolox equivalents; a data expressed as µmol TE/g; b data expressed as IC50 in mg/mL.
The physicochemical and antioxidant properties of the protein hydrolysates of C. frondosa and its processing discards were evaluated using Alcalase, Flavourzyme, and Corolase as well as their combination [43]. The hydrolysates prepared with combination of enzymes were predominant in glutamic acid and displayed the highest radical-scavenging activity against ABTS and DPPH radicals as well as metal-chelation activity. In addition, hydrolysates were able to inhibit TBARS production in a meat model system and beta-carotene bleaching in an oil-in-water emulsion. They also noted that the level of free amino acids after hydrolysis, the degree of hydrolysis, amino acid sequence, and molecular weight played the main role in rendering radical-scavenging activity. Similarly, Yan et al. [46] prepared enzymatic hydrolysates from C. frondosa viscera using Alcalase, Flavourzyme, Neutrase, trypsin, papain, and bromelain and observed that Alcalase, Flavourzyme, and trypsin were major enzymes that resulted in strong antioxidant activity, possibly related to a high amount of hydrophobic amino acids in the hydrolysates. The choice of proteases in the preparation of protein hydrolysates plays a significant role in the resultant antioxidant potency and bioactivity of peptides. For example, Alcalase-produced protein hydrolysates obtained from C. frondosa exhibited up to 35% higher in vitro antioxidant activity (e.g., DPPH radical, hydroxyl radical, and superoxide radical anion-scavenging properties) than the trypsin-produced hydrolysates, suggesting that the amino acid composition and structural conformation of the peptides played main roles in determining antioxidant activity [47]. This is because trypsin-produced peptide fractions were not the same as Alcalase-produced peptide fractions, mainly when the sizes of the peptides were small (≤10 kDa). Moreover, in silico docking for in vivo function prediction demonstrated a better inhibitory activity in myeloperoxidase (a marker of in vivo oxidative stress) by Alcalase than by trypsin. Likewise, protein hydrolysates obtained from Atlantic sea cucumber viscera showed antioxidant activities in lipid oxidation tests and the ORAC assay, which could be related to the releasing of antioxidative peptides upon hydrolysis [48]. The findings of Wang et al. [49] suggest that C. frondosa internal organs hydrolysates have the potential to show anti-diabetic activity through insulin resistance and lipid metabolism syndromes. Besides, enzymatic hydrolysates prepared from C. frondosa by-products (aquapharyngeal bulb and internal organs) using nine different proteases, including Alcalase, bromelain, Flavourzyme, fungal protease, neutral protease, papain, peptidase AM (Aspergillus melleus), peptidase AO (Aspergillus oryzae), and Protamex, were tested against Herpes Simplex virus 1 (HSV-1) [50]. Results suggested that papain was the most efficient enzyme in demonstrating antiviral activities. Lin et al. [51] reported the anti-aging effect of sea cucumber (C. frondosa) hydrolysates, which was mainly linked to the low-molecular-weight (~<3 kDa) peptides. The anti-aging mechanism could be related to the up-regulated Klotho expression, down-regulated acetylcholinesterase activity, increased SOD and GSH-Px activities, and lipid and protein oxidation inhibition.
Antioxidant peptides were isolated from Apostichopus japonicus using papain, pepsin, trypsin, neutral protease, and acid protease, and it was found that trypsin-produced peptides exhibited the highest in vitro antioxidant activity (e.g., hydroxyl radical and superoxide radical anion-scavenging activities) when compared to the other enzymes [52]. Moreover, TP2b-1 was isolated as a novel antioxidative peptide showing radical-scavenging activity. Similarly, Zhu et al. [53] produced pepsin-solubilized collagen (PSC) from the body wall of Apostichopus japonicus and reported that PSC was more active in scavenging hydroxyl and DPPH radicals than the vitamins C and E, respectively. Likewise, low-molecular-weight gelatin hydrolysate was prepared from Apostichopus japonicus using Flavourzyme, which showed antioxidant activity against superoxide and hydroxyl radicals as well as inhibitory activity against melanin synthesis, tyrosinase enzyme, and melanogenesis [54]. However, Zhang et al. [55] purified peptides obtained from Apostichopus japonicus egg using ultra-filtration, gel filtration, and high-speed counter-current chromatography (HSCCC). The purified peptide was estimated to be about 30 kDa, which showed strong antioxidant activity against hydroxyl radicals. Recently, Guo et al. [56] determined the antioxidant and anti-aging effects of protein hydrolysate obtained from Apostichopus japonicus in vivo and characterized the function of peptides using a bioinformatics tool such as in silico. Results suggested that protein hydrolysate scavenged DPPH radicals directly and was able to inhibit ROS accumulation, reduce MDA content, and upregulate SOD activity in an animal model (C. elegans) under increased oxidative stress.
Sea cucumber-derived peptides were also reported to demonstrate a protective effect against UV-B-induced skin cell damage. Doungapai et al. [57] used papain, Alcalase, and Flavourzyme to prepare protein hydrolysate from Holothuria scabra; papain-derived hydrolysate had both hydrophilic and hydrophobic amino acids and showed strong UV-B protective activity in a HaCaT cell model. Rathnayake et al. [58] prepared Holothuria spinifera enzymatic hydrolysates (HSEA) using Alcalase, papain, Neutrase, α-chymotrypsin, and trypsin, where trypsin hydrolysate showed the greatest β-secretase inhibitory properties. RP-HPLC fraction of HSEA contained four bioactive peptides which reduced cellular levels of the β-secretase enzyme, amyloid-beta, and soluble amyloid precursor protein beta proteins as well as protecting SH-SY5Y (neuro-blastoma cell) from oxidation. On the other hand, microwave-assisted enzymatic (papain, Neutrase, pepsin, and trypsin) hydrolysis of collagen obtained from Acaudina molpadioides demonstrated antioxidant activity [45]. In particular, Neutrase-derived hydrolysate produced four bioactive peptides and showed strong DPPH radical-scavenging activity, suggesting that it was related to the presence of hydrophobic amino acids in their sequences and low molecular weights (<1 kDa). Similarly, collagen hydrolysates (molecular weight 14.4 to 25 kDa) obtained from golden sea cucumber revealed strong DPPH radical scavenging activity, where Neutrase was used to start the hydrolysis [59]. García et al. [34] designed novel functional food products using protein hydrolysate (Protamex and Alcalase) obtained from Parastichopus tremulus and Holothuria forskali, which demonstrated antioxidant, antihypertensive, and anti-inflammatory properties, especially those obtained from H. forskali. Furthermore, Safari and Yaghoubzadeh [41] suggested that Holothuria leucospilata protein hydrolysate with a molecular weight < 30 kDa exhibited strong antioxidant activity in in terms of the DPPH radical-scavenging assay and ferric reducing-antioxidant power, mainly those prepared from Alcalase rather than those from Flavourzyme. In addition, proteases were isolated from tentacles of the sea cucumber (Isostichopus fuscus) and then used to produce bioactive peptides from the body wall of I. fuscus [60]. It was found that the 3 kDa fraction obtained from the protein hydrolysate displayed a strong ORAC when compared to the high-molecular-weight (>3 kDa) fractions.
2.3. Antioxidant Potential of Sea Cucumber Polysaccharides
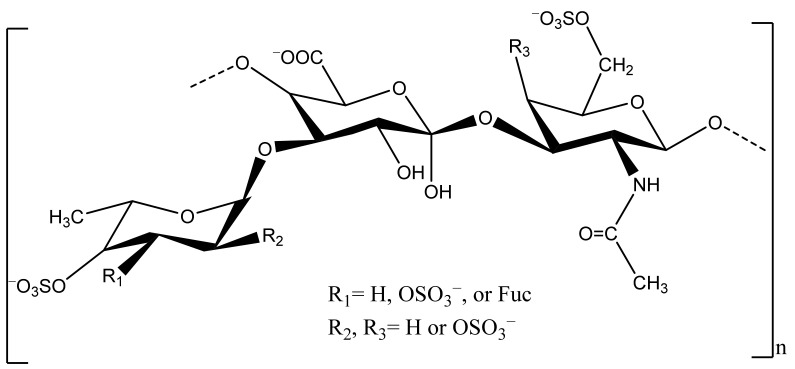
Sea cucumber, mainly the body wall, is a good source of polysaccharides, including sulfated polysaccharides (fucosylated chondroitin sulfate, FCS) and fucan (Figure 3).
Figure 3.
Chemical structure of FCS of sea cucumber.
The chain conformation of polysaccharides mainly affects their antioxidant activity. In particular, the antioxidant activity of sulfated polysaccharides is associated with the molecular weight, degree of sulfation, type of main sugars, and glycosidic bonds. Hence, the antioxidant activity of sea cucumber polysaccharides is not related to a single factor but rather a combination of several factors. For example, Liu et al. [61] reported that the polysaccharides of Apostichopus japonicus were mainly composed of glucosamine, glucuronic acid, galactosamine, mannose, galactose, glucose, and fucose, which showed potent hydroxyl, DPPH, and superoxide radical-scavenging activities as well as reducing power. These could be due to the ability of free radicals to abstract anomeric hydrogen from polysaccharides [62]. Similarly, polysaccharides obtained from Phyllophorus proteus exhibited hydroxyl, DPPH, ABTS, and superoxide radical-scavenging activities, which may closely be linked to their structural features, including monosaccharide compositions and contents of their sulfate and carboxyl groups [63]. In particular, sulfate and carboxyl groups are extremely nucleophilic and may chelate metal ions (e.g., Cu2+ and Fe2+) and show hydroxyl radical-scavenging activity. Moreover, the sulfate group in sulfated polysaccharides could initiate superoxide radical-scavenging activity due to its electron-donating substituents in a saccharide ring [64]. However, Gao et al. [65] claimed that Holothuria fuscopunctata polysaccharides (fucan sulfate) had potent antioxidant activity for superoxide radicals, while exhibiting almost no scavenging effect for hydroxyl, DPPH, and ABTS radicals, which is related to the structural characteristics of sulfated polysaccharides. Moreover, Li et al. [66] found that Stichopus chloronotus fucoidan mainly consists of L-fucose and sulfate esters, which demonstrate significant inhibition of lipid peroxidation and immunoregulatory properties. The sulfate content, sulfate patterns, and molecular weight of fucoidan especially affect the inhibitory activities of superoxide radicals. Likewise, ultrasound treatment was found to slightly improve the antioxidant activity (DPPH radical-scavenging activity and ORAC) of fucoidan obtained from Isostichopus badionotus [67] which could be related to its lower molecular weight. Furthermore, Li et al. [68] stated that the sulfated polysaccharides of Holothuria fuscogliva show strong hydroxyl and superoxide radical scavenging activities as well as anticoagulant properties. On the other hand, the sulfation patterns of fucose branches of FCS obtained from Stichopus chloronotus, Apostichopus japonicus, and Acaudina molpadioidea were 4-O-, 2,4-di-O, and 3,4-di-O-sulfation, respectively, which inhibited DPPH and nitric oxide radicals as well as lipid peroxidation. The inhibitory activity may be affected by the sulfation patterns of the fucose branches. In this regards, 4-O sulfation residues exhibit the strongest antioxidant properties, while the opposite scenario is seen for 3,4-O-sulfated fucose residues [69]. Yu et al. [70] prepared fucoidan from Thelenota ananas and found a significant inhibitory effect of polysaccharides for superoxide radicals, closely related to its sulfate groups. Similarly, FCS isolated from Acaudina molpadioidea and Holothuria nobilis showed moderate antioxidant properties against hydroxyl, DPPH, and superoxide radicals in a dose-dependent manner. The presence of sulfate groups in polysaccharides with the ability to bind metal ions was found to be responsible [71]. In addition, sea cucumber (Apostichopus japonicus) gonadal polysaccharide exhibited DPPH and hydroxyl radical-scavenging activities as well as reducing power. The activity was greater in the presence of a higher content of sulfate groups and maintaining a lower molecular weight [72]. Besides, Qi et al. [73] suggested that sea cucumber processing liquor is mainly composed of mannose, glucose, and fucose, which showed strong DPPH, hydroxyl, and superoxide radical anion-scavenging activities. Moreover, in an in vivo model, these polysaccharides were able to increase the activity of catalase and SOD.
2.4. Antioxidant Potential of Carotenoids and Physiological Effects of PUFAs
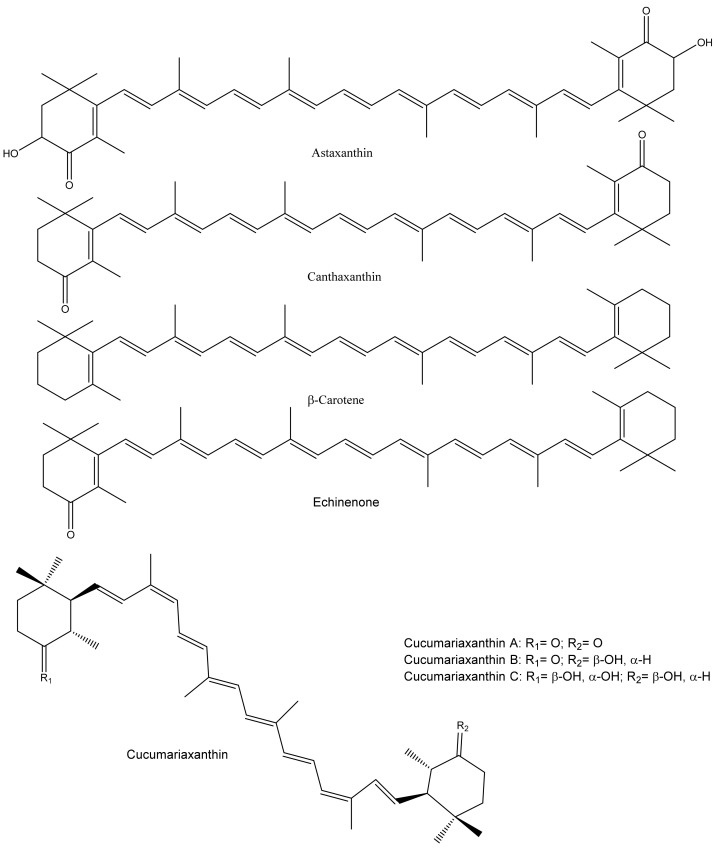
Marine animals, including sea cucumbers, are a good source of carotenoids that show structural diversity. Sea cucumbers, mainly their gonads and aquapharyngeal bulb/tentacles, contain various carotenoids (Figure 4).
Figure 4.
Major carotenoids found in sea cucumbers.
The distinctive color of their internal organs is due to carotenoids that contain a series of conjugated C=C. Sea cucumbers, mostly passive feeder species, use algal or other carotenoid-rich materials as their primary food source, resulting in carotenoid accumulation in their tissues. The major carotenoids in sea cucumbers are astaxanthin and canthaxanthin, and their composition varies with species, geographical location, and body part. For example, Matsuno and Tsushima [74] investigated the carotenoid composition of seven sea cucumber species (Stichopus japonicus, Holothuria moebi, Holothuria pervicax, Holothuria leucospilota, Cucumaria echinata, Cucumaria japonica, and Pentacta australis), and β-echinenone, β-carotene, phoenicoxanthin, canthaxanthin, and astaxanthin were found in all of them. However, cucumariaxanthin A, B, and C were only present in C. echinata, C. japonica, and P. australis. Furthermore, Tsushima et al. [75] identified carotenoids (5,6,5′,6′-tetrahydro-carotenoids with 9Z,9′Z configurations) termed as cucumariaxanthins A, B, and C from Cucumaria japonica gonads, where cucumariaxanthin C showed antiviral activity on Epstein-Barr virus activation. Similarly, Maoka et al. [76] identified a new carotenoid (9Z,9′Z-tetrahydroastaxanthin) along with echinenone, canthaxanthin, β-carotene, astaxanthin, adonirubin, and cucumariaxanthin A from Plesiocolochirus minutusalong. Recently, 14 carotenoids were identified from the Cucumaria frondosa japonica Semper using supercritical CO2 extraction, where cucumariaxanthin and canthaxanthin were abundant [77]. In addition, the composition of fatty acids and carotenoids were analyzed from 12 sea cucumbers, and their effect on cancer cells was tested [78]. It was found that PUFAs, mainly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and carotenoids significantly contributed to the cytotoxic activity against cervical (HeLa), colon (WiDR), and breast (T47D and MCF-7) cancer cells. However, little is known about the antioxidant activity of sea cucumber carotenoids and their detailed mechanisms. The antioxidant activity of these carotenoids could be related to the scavenging of ROS, mainly singlet oxygen (1O2) and peroxyl radicals, due to their special chemical features (conjugated C=C) [79].
Sea cucumbers, mostly their internal organs, contain around 3.5% lipids, mainly PUFAs (up to 50%) such as EPA (up to 30%) and DHA. Apart from this, sea cucumbers are rich in ether-phospholipids such as 1-alkenyl-2-acyl-phosphoethanolamine and 1-alkyl-2-acyl-phosphocholine [80]. They also reported that the most dominant PUFAs esterified to phospholipids were EPA and arachidonic acid (AA). Liu et al. [81] reported that freeze- and air-dried viscera of C. frondosa produced a similar composition of total fatty acid as fresh viscera, including a higher level of PUFAs (30–31%), mainly EPA (27–28%), and a lower content of omega-6 fatty acids (~1%). Therefore, the effect of sea cucumber PUFAs on various physiological functions is of interest. For example, EPA-enriched phospholipids (EPL) liposomes obtained from C. frondosa exhibited antitumor activity via the activation of caspase 9 and caspase 3 against S180 ascitic tumor-bearing mice [82]. Similar to this study, the impact of EPL isolated from C. frondosa on oxidative injury in rat pheochromocytoma (PC12) cells stimulated by tert-butylhydroperoxide and hydrogen peroxide was investigated [83]. Results suggested that the EPL was able to demonstrate a neuroprotective effect that could be related to inhibiting the mitochondria-dependent apoptotic pathway. Likewise, EPL obtained from C. frondosa could serve as a rapid regulator of fat burning due to its ability to increase the expression of genes related to β-oxidation in the epididymal adipose tissue and liver [84]. Furthermore, Hu et al. [85] stated that EPA-enriched phosphatidylcholine (EPC) isolated from C. frondosa significantly increased glycogen synthesis and insulin secretion as well as decreased blood glucose levels in diabetic rats. Moreover, reverse transcription-polymerase chain reaction (RT-PCR) analysis showed that EPC exhibited anti-hyperglycemic activities via up-regulating phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB) signal pathway. On the other hand, long-chain bases from C. frondosa have the potential to regulate lipid metabolism via activation of the adenosine monophosphate-activated protein kinase (AMPK) pathway and inhibit adipogenesis via activation of WNT/β-catenin signaling [86]. Besides, Jia et al. [87] reported that glucocerebrosides of C. frondosa exhibit inhibitory effects on cell proliferation (HepG2 cells), which could be linked to the degree of saturation and/or hydroxylation of long-chain bases and fatty acids. In addition, fatty acids, mainly cis-9-octadecenoic acid and 1,3-dipalmitolein, of internal organs of Apostichopus japonicus showed potent α-glucosidase inhibitory activity [39].
2.5. Antioxidant Potential of Other Bioactive Compounds of Sea Cucumber
Apart from phenolics, protein hydrolysates/peptides, polysaccharides, and carotenoids, other bioactive compounds such as the saponins and cerebrosides of the sea cucumber also exhibit potential antioxidant activity. For example, the antioxidant and cytotoxic activities of organic and aqueous extracts of Stichopus horrens and Holothuria edulis were investigated and both extracts showed strong antioxidant activity against DPPH and linoleate (β-carotene bleaching assay) radicals. Meanwhile the organic extract showed higher cytotoxic effects against A549 (lung cancer) and TE1 (esophageal cancer) cells [88]. Moreover, four sulfated holostan-type triterpene glycosides (echinoside B 12-O-methyl ether, echinoside B, 24-dehydroechinoside B, and holothurin B) were isolated from the Saudi Red Sea cucumber Holothuria atra, which demonstrated antioxidant activity (reducing power and DPPH radical-scavenging activity) and cytotoxic effects against Ehrlich ascites carcinoma cells [89]. Furthermore, processing methods have a significant effect on antioxidant and physicochemical properties of sea cucumber products. For instance, sea cucumber powder (Holothuria scabra) was made using microwave heating, smoking, and steaming; the microwave treatment rendered a stronger DPPH radical-scavenging activity than the other two methods, possibly related to the heating via direct interaction with microwaved materials [90]. The antioxidant activity of microwave-treated powder could be linked to the presence of phenolics, steroids, and alkaloids. In addition, 21 Indonesian sea cucumber extracts were tested for their biological activities; H. atra and S. vastus extracts showed strong antioxidant (IC50 = 14.22 µg/µL) and antiviral activities, respectively [91]. Similarly, Rasyid et al. [92] analyzed the ABTS radical-scavenging activity of five Indonesian sea cucumber extracts, where H. leucospilota, H. lessoni, and Stichopus quadrifasciatus extracts had the strongest scavenging activity. In another study, Wulandari et al. [93] stated that H. scabra cultivated in the pond for 12 months demonstrated strong antioxidant and antibacterial activities. However, Wu et al. [94] determined the effect of Acaudina molpadioides cerebrosides on the tert-butyl hydroperoxide and hydrogen peroxide-induced oxidative damage in PC12 cells. The sea cucumber cerebrosides had a positive effect against oxidative damage by inhibiting mitochondria-mediated apoptosis, which may be related to their antioxidant effect.
3. Conclusions
Sea cucumbers, mainly Holothuria atra, Holothuria scabra, Cucumaria frondosa, and Apostichopus japonicus, are an excellent source of antioxidants such as phenolic acids, flavonoids, peptides, fucosylated chondroitin sulfate (FCS), fucoidan, and triterpene glycosides. Moreover, these compounds have the potential to show multiple biological activities, including anticancer, anti-inflammatory, anti-glycation, anti-tyrosinase, anti-hypertension, antithrombotic, anti-diabetic, and antimicrobial activities. Hence, sea cucumber antioxidants could serve as potential candidates in nutraceuticals, pharmaceuticals, cosmeceuticals, and functional foods. Nevertheless, further studies are required to understand their detailed chemical structures, mechanisms of action, and bioaccessibility and bioavailability through in vivo analysis and clinical trials to support the health claims and commercialize sea cucumber-derived value-added products.
Author Contributions
Conceptualization, A.H., D.D. and F.S.; resources A.H., D.D. and F.S.; data curation, A.H.; visualization, A.H., D.D. and F.S.; writing initial draft, A.H.; writing review and final editing, A.H., D.D. and F.S.; supervision, D.D. and F.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Natural Sciences and Engineering Research Council (NSERC) of Canada (RGPIN-2015-06121 and RGPIN-2016-04468).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wen J., Hu C., Fan S. Chemical composition and nutritional quality of sea cucumbers. J. Sci. Food Agric. 2010;90:2469–2474. doi: 10.1002/jsfa.4108. [DOI] [PubMed] [Google Scholar]
- 2.Hossain A., Dave D., Shahidi F. Northern sea cucumber (Cucumaria frondosa): A potential candidate for functional food, nutraceutical, and pharmaceutical sector. Mar. Drugs. 2020;18:274. doi: 10.3390/md18050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gajdosechova Z., Palmer C.H., Dave D., Jiao G., Zhao Y., Tan Z., Chisholm J., Zhang J., Stefanova R., Hossain A., et al. Arsenic speciation in sea cucumbers: Identification and quantitation of water-extractable species. Environ. Pollut. 2020;266:1–10. doi: 10.1016/j.envpol.2020.115190. [DOI] [PubMed] [Google Scholar]
- 4.Chen S., Xue C., Yin L., Tang Q., Yu G., Chai W. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 2011;83:688–696. doi: 10.1016/j.carbpol.2010.08.040. [DOI] [Google Scholar]
- 5.Hossain A., Dave D., Shahidi F. Effect of high-pressure processing (HPP) on phenolics of North Atlantic sea cucumber (Cucumaria frondosa) J. Agric. Food Chem. 2022;70:3489–3501. doi: 10.1021/acs.jafc.2c00140. [DOI] [PubMed] [Google Scholar]
- 6.Krishnaiah D., Sarbatly R., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011;89:217–233. doi: 10.1016/j.fbp.2010.04.008. [DOI] [Google Scholar]
- 7.Bordbar S., Anwar F., Saari N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs. 2011;9:1761–1805. doi: 10.3390/md9101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahidi F., Naczk M. Phenolics in Food and Nutraceuticals. 2nd ed. CRC Press; Boca Raton, FL, USA: 2004. [Google Scholar]
- 9.Pereira D.M., Valentão P., Pereira J.A., Andrade P.B. Phenolics: From Chemistry to Biology. Molecules. 2009;14:2202–2211. doi: 10.3390/molecules14062202. [DOI] [Google Scholar]
- 10.Shahidi F., Zhong Y. Measurement of antioxidant activity. J. Funct. Foods. 2015;18:757–781. doi: 10.1016/j.jff.2015.01.047. [DOI] [Google Scholar]
- 11.Hossain A., Moon H.K., Kim J.-K. Antioxidant properties of Korean major persimmon (Diospyros kaki) leaves. Food Sci. Biotechnol. 2018;27:177–184. doi: 10.1007/s10068-017-0195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahidi F., Ho C.T. Antioxidant Measurement and Applications. American Chemical Society; Washington, DC, USA: 2007. Antioxidant measurement and applications: An overview; pp. 2–7. [Google Scholar]
- 13.Shahidi F., Hossain A. Bioactives in spices, and spice oleoresins: Phytochemicals and their beneficial effects in food preservation and health promotion. J. Food Bioact. 2018;3:8–75. doi: 10.31665/JFB.2018.3149. [DOI] [Google Scholar]
- 14.Shahidi F., Hossain A. Preservation of aquatic food using edible films and coatings containing essential oils: A review. Crit. Rev. Food Sci. Nutr. 2020;62:66–105. doi: 10.1080/10408398.2020.1812048. [DOI] [PubMed] [Google Scholar]
- 15.Balasundram N., Sundram K., Samman S. Phenolic compounds in plants and agri-industrial by-products : Antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- 16.Shahidi F. Antioxidants: Principles and applications. In: Shahidi F., editor. Handbook of Antioxidants for Food Preservation. Woodhead Publishing; Cambridge, UK: 2015. pp. 1–14. [Google Scholar]
- 17.Shahidi F., Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods. 2015;18:820–897. doi: 10.1016/j.jff.2015.06.018. [DOI] [Google Scholar]
- 18.Shahidi F., Varatharajan V., Oh W.Y., Peng H. Phenolic compounds in agri-food by-products, their bioavailability and health effects. J. Food Bioact. 2019;5:57–119. doi: 10.31665/JFB.2019.5178. [DOI] [Google Scholar]
- 19.Zhong Y., Khan M.A., Shahidi F. Compositional characteristics and antioxidant properties of fresh and processed sea cucumber (Cucumaria frondosa) J. Agric. Food Chem. 2007;55:1188–1192. doi: 10.1021/jf063085h. [DOI] [PubMed] [Google Scholar]
- 20.Ceesay A., Nor Shamsudin M., Aliyu-Paiko M., Ismail I.S., Nazarudin M.F., Mohamed Alipiah N. Extraction and characterization of organ components of the Malaysian sea cucumber Holothuria leucospilota yielded bioactives exhibiting diverse properties. BioMed Res. Int. 2019;2019:2640684. doi: 10.1155/2019/2640684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Althunibat O.Y., Hashim R.B., Taher M., Daud J.M., Ikeda M.-A., Zali B.I. In vitro antioxidant and antiproliferative activities of three Malaysian sea cucumber species. Eur. J. Sci. Res. 2009;37:376–387. [Google Scholar]
- 22.Nobsathian S., Tuchinda P., Sobhon P., Tinikul Y., Poljaroen J., Tinikul R., Sroyraya M., Poomton T., Chaichotranunt S. An antioxidant activity of the whole body of Holothuria scabra. Chem. Biol. Technol. Agric. 2017;4:17–21. doi: 10.1186/s40538-017-0087-7. [DOI] [Google Scholar]
- 23.Wulandari D.A., Gustini N., Murniasih T., Bayu A., Sari M., Syahputra G., Harahap I.A., Rasyid A., Moria S.B., Rahmawati S.I., et al. Nutritional value and biological activities of sea cucumber Holothuria scabra cultured in the open pond system. J. Aquat. Food Prod. Technol. 2022;31:599–614. doi: 10.1080/10498850.2022.2082902. [DOI] [Google Scholar]
- 24.Hossain A., Yeo J.D., Dave D., Shahidi F. Phenolic compounds and antioxidant capacity of sea cucumber (Cucumaria frondosa) processing discards as affected by high-pressure processing (HPP) Antioxidants. 2022;11:337. doi: 10.3390/antiox11020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mamelona J., Pelletier É., Girard-Lalancette K., Legault J., Karboune S., Kermasha S. Quantification of phenolic contents and antioxidant capacity of Atlantic sea cucumber, Cucumaria frondosa. Food Chem. 2007;104:1040–1047. doi: 10.1016/j.foodchem.2007.01.016. [DOI] [Google Scholar]
- 26.Mamelona J., Pelletier É. Producing high antioxidant activity extracts from echinoderm by products by using pressured liquid extraction. Biotechnology. 2010;9:523–528. doi: 10.3923/biotech.2010.523.528. [DOI] [Google Scholar]
- 27.Ridhowati S., Zakaria F.R., Syah D., Chasanah E. Anticancer and antioxidant activities from sea cucumber (Stichopus variegatus) flour dried vacuum oven. Pertanika J. Trop. Agric. Sci. 2018;41:1125–1138. [Google Scholar]
- 28.Husni A., Shin I.S., You S.G., Chung D. Antioxidant properties of water and aqueous ethanol extracts and their crude saponin fractions from a far-eastern sea cucumber, Stichopus japonicus. Food Sci. Biotechnol. 2009;18:419–424. [Google Scholar]
- 29.Telahigue K., Ghali R., Nouiri E., Labidi A., Hajji T. Antibacterial activities and bioactive compounds of the ethyl acetate extract of the sea cucumber Holothuria forskali from Tunisian coasts. J. Mar. Biol. Assoc. U. K. 2020;100:229–237. doi: 10.1017/S0025315420000016. [DOI] [Google Scholar]
- 30.Esmat A.Y., Said M.M., Soliman A.A., El-Masry K.S.H., Badiea E.A. Bioactive compounds, antioxidant potential, and hepatoprotective activity of sea cucumber (Holothuria atra) against thioacetamide intoxication in rats. Nutrition. 2013;29:258–267. doi: 10.1016/j.nut.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Dakrory A.I., Fahmy S.R., Soliman A.M., Mohamed A.S., Amer S.A.M. Protective and curative effects of the sea cucumber Holothuria atra extract against DMBA-induced Hepatorenal diseases in rats. BioMed Res. Int. 2015;2015:563652. doi: 10.1155/2015/563652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fahmy S.R. Anti-fibrotic effect of Holothuria arenicola extract against bile duct ligation in rats. BMC Complement. Altern. Med. 2015;15:14. doi: 10.1186/s12906-015-0533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alper M., Günes M. Evaluation of cytotoxic, apoptotic effects and phenolic compounds of sea cucumber Holothuria tubulosa (Gmelin, 1791) extracts. Turkish J. Vet. Anim. Sci. 2020;44:641–655. doi: 10.3906/vet-1909-80. [DOI] [Google Scholar]
- 34.García J., Méndez D., Álvarez M., Sanmartin B., Vazquez Sobrado R., Regueiro L., Atanassova M. Design of novel functional food products enriched with bioactive extracts from holothurians for meeting the nutritional needs of the elderly. LWT Food Sci. Technol. 2019;109:55–62. doi: 10.1016/j.lwt.2019.03.097. [DOI] [Google Scholar]
- 35.Carletti A., Cardoso C., Lobo-Arteaga J., Sales S., Juliao D., Ferreira I., Chainho P., Dionísio M.A., Gaudêncio M.J., Afonso C., et al. Antioxidant and anti-inflammatory extracts from sea cucumbers and tunicates induce a pro-osteogenic effect in zebrafish larvae. Front. Nutr. 2022;9:833. doi: 10.3389/fnut.2022.888360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sukmiwati M., Ilza M., Putri A.E., Sidauruk S.W. Antibacterial activity of sea cucumber (Holothuria atra) against Pseudomonas aeruginosa. IOP Conf. Ser. Earth Environ. Sci. 2019;404:012047. doi: 10.1088/1755-1315/404/1/012047. [DOI] [Google Scholar]
- 37.Pranweerapaiboon K., Apisawetakan S., Nobsathian S., Itharat A., Sobhon P., Chaithirayanon K. An ethyl-acetate fraction of Holothuria scabra modulates inflammation in vitro through inhibiting the production of nitric oxide and pro-inflammatory cytokines via NF-κB and JNK pathways. Inflammopharmacology. 2020;28:1027–1037. doi: 10.1007/s10787-019-00677-3. [DOI] [PubMed] [Google Scholar]
- 38.Hossain A., Senadheera R.L.T., Dave D., Shahidi F. Phenolic profiles of Atlantic sea cucumber tentacles and their biological properties. Food Res. Int. 2022 doi: 10.1016/j.foodres.2022.112262. under revision . [DOI] [PubMed] [Google Scholar]
- 39.Nguyen T.H., Kim S.M. α-Glucosidase inhibitory activities of fatty acids purified from the internal organ of sea cucumber Stichopus japonicas. J. Food Sci. 2015;80:H841–H847. doi: 10.1111/1750-3841.12810. [DOI] [PubMed] [Google Scholar]
- 40.Himaya S.W.A., Ryu B.M., Qian Z.J., Kim S.K. Sea cucumber, Stichopus japonicus ethyl acetate fraction modulates the lipopolysaccharide induced iNOS and COX-2 via MAPK signaling pathway in murine macrophages. Environ. Toxicol. Pharmacol. 2010;30:68–75. doi: 10.1016/j.etap.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 41.Safari R., Yaghoubzadeh Z. Antioxidant activity of bioactive peptides extracted from sea cucumber (Holothuria leucospilata) Int. J. Pept. Res. Ther. 2020;26:2393–2398. doi: 10.1007/s10989-020-10031-9. [DOI] [Google Scholar]
- 42.Senadheera T.R.L., Dave D., Shahidi F. Sea cucumber derived type I collagen: A comprehensive review. Mar. Drugs. 2020;18:471. doi: 10.3390/md18090471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senadheera T.R.L., Dave D., Shahidi F. Antioxidant potential and physicochemical properties of protein hydrolysates from body parts of North Atlantic sea cucumber (Cucumaria frondosa) Food Prod. Process. Nutr. 2021;3:3. doi: 10.1186/s43014-020-00049-3. [DOI] [Google Scholar]
- 44.Shahidi F., Zhong Y. Bioactive peptides. J. AOAC Int. 2008;91:914–931. doi: 10.1093/jaoac/91.4.914. [DOI] [PubMed] [Google Scholar]
- 45.Jin H.-X., Xu H.-P., Li Y., Zhang Q.-W., Xie H. Preparation and evaluation of peptides with potential antioxidant activity by microwave assisted enzymatic hydrolysis of collagen from sea cucumber Acaudina molpadioides obtained from Zhejiang province in China. Mar. Drugs. 2019;17:169. doi: 10.3390/md17030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan M., Tao H., Qin S. Effect of enzyme type on the antioxidant activities and functional properties of enzymatic hydrolysates from sea cucumber (Cucumaria frondosa) viscera. J. Aquat. Food Prod. Technol. 2016;25:940–952. doi: 10.1080/10498850.2014.994083. [DOI] [Google Scholar]
- 47.Zhang Y., He S., Bonneil É., Simpson B.K. Generation of antioxidative peptides from Atlantic sea cucumber using alcalase versus trypsin: In vitro activity, de novo sequencing, and in silico docking for in vivo function prediction. Food Chem. 2020;306:125581. doi: 10.1016/j.foodchem.2019.125581. [DOI] [PubMed] [Google Scholar]
- 48.Mamelona J., Saint-Louis R., Pelletier É. Nutritional composition and antioxidant properties of protein hydrolysates prepared from echinoderm byproducts. Int. J. Food Sci. Technol. 2010;45:147–154. doi: 10.1111/j.1365-2621.2009.02114.x. [DOI] [Google Scholar]
- 49.Wang T., Zheng L., Wang S., Zhao M., Liu X. Anti-diabetic and anti-hyperlipidemic effects of sea cucumber (Cucumaria frondosa) gonad hydrolysates in type II diabetic rats. Food Sci. Hum. Wellness. 2022;11:1614–1622. doi: 10.1016/j.fshw.2022.06.020. [DOI] [Google Scholar]
- 50.Tripoteau L., Bedoux G., Gagnon J., Bourgougnon N. In vitro antiviral activities of enzymatic hydrolysates extracted from byproducts of the Atlantic holothurian Cucumaria frondosa. Process Biochem. 2015;50:867–875. doi: 10.1016/j.procbio.2015.02.012. [DOI] [Google Scholar]
- 51.Lin L., Yang K., Zheng L., Zhao M., Sun W., Zhu Q., Liu S. Anti-aging effect of sea cucumber (Cucumaria frondosa) hydrolysate on fruit flies and d-galactose-induced aging mice. J. Funct. Foods. 2018;47:11–18. doi: 10.1016/j.jff.2018.05.033. [DOI] [Google Scholar]
- 52.Zhou X., Wang C., Jiang A. Antioxidant peptides isolated from sea cucumber Stichopus japonicus. Eur. Food Res. Technol. 2012;234:441–447. doi: 10.1007/s00217-011-1610-x. [DOI] [Google Scholar]
- 53.Zhu B.-W., Dong X.-P., Zhou D.-Y., Gao Y., Yang J.-F., Li D.-M., Zhao X.-K., Ren T.-T., Ye W.-X., Tan H., et al. Physicochemical properties and radical scavenging capacities of pepsin-solubilized collagen from sea cucumber Stichopus japonicus. Food Hydrocoll. 2012;28:182–188. doi: 10.1016/j.foodhyd.2011.12.010. [DOI] [Google Scholar]
- 54.Wang J., Wang Y., Tang Q., Wang Y., Yaoguang C., Qin Z., Changhu X. Antioxidation Activities of Low-Molecular-Weight Gelatin Hydrolysate Isolated from the Sea Cucumber Stichopus japonicus. J. Ocean Univ. China. 2010;9:94–98. doi: 10.1007/s11802-010-0094-9. [DOI] [Google Scholar]
- 55.Zhang J., Liu S., Zhang Y., Lu Y., Wang M., Wang G., Liu X. Purification and antioxidant ability of peptide from egg in sea cucumber Apostichopus japonicus. Int. J. Food Prop. 2017;20:306–317. doi: 10.1080/10942912.2016.1160409. [DOI] [Google Scholar]
- 56.Guo K., Su L., Wang Y., Liu H., Lin J., Cheng P., Yin X., Liang M., Wang Q., Huang Z. Antioxidant and anti-aging effects of a sea cucumber protein hydrolyzate and bioinformatic characterization of its composing peptides. Food Funct. 2020;11:5004–5016. doi: 10.1039/D0FO00560F. [DOI] [PubMed] [Google Scholar]
- 57.Doungapai C., Siriwoharn T., Malila Y., Autsavapromporn N., Makkhun S., Yarnpakdee S., Jantanasakulwong K., Regenstein J.M., Wangtueai S., Brück W., et al. UV-B Protective and Antioxidant Activities of Protein Hydrolysate from Sea Cucumber (Holothuria scabra) Using Enzymatic Hydrolysis. Front. Mar. Sci. 2022;9:892255. doi: 10.3389/fmars.2022.892255. [DOI] [Google Scholar]
- 58.Rathnayake A.U., Abuine R., Palanisamy S., Lee J.K., Byun H.G. Characterization and purification of β−secretase inhibitory peptides fraction from sea cucumber (Holothuria spinifera) enzymatic hydrolysates. Process Biochem. 2021;111:86–96. doi: 10.1016/j.procbio.2021.10.007. [DOI] [Google Scholar]
- 59.Fawzya Y.N., Putra N.A., Witarto A.B., Patantis G. Golden sea cucumber: Identification and the antioxidant activity of its collagen hydrolysates. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2020;15:119–129. doi: 10.15578/squalen.v15i3.511. [DOI] [Google Scholar]
- 60.Hernndez-Smano A.C., Hernndez-Ledesma B. Release of antioxidant peptides from the body wall proteins of the sea cucumber Isostichopus fuscus. Nat. Prod. Commun. 2015;10:1427–1430. [PubMed] [Google Scholar]
- 61.Liu X., Sun Z., Zhang M., Meng X., Xia X., Yuan W., Xue F., Liu C. Antioxidant and antihyperlipidemic activities of polysaccharides from sea cucumber Apostichopus japonicus. Carbohydr. Polym. 2012;90:1664–1670. doi: 10.1016/j.carbpol.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 62.Zhu B.W., Zhou D.Y., Li T., Yan S., Yang J.F., Li D.M., Dong X.P., Murata Y. Chemical composition and free radical scavenging activities of a sulphated polysaccharide extracted from abalone gonad (Haliotis Discus Hannai Ino) Food Chem. 2010;121:712–718. doi: 10.1016/j.foodchem.2010.01.010. [DOI] [Google Scholar]
- 63.Qin Y., Yuan Q., Zhang Y., Li J., Zhu X., Wen J., Liu J., Zhao L., Zhao J., Zhao L. Enzyme-assisted extraction optimization, characterization and antioxidant activity of polysaccharides from sea cucumber Phyllophorus proteus. Molecules. 2018;23:590. doi: 10.3390/molecules23030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie J.H., Wang Z.J., Shen M.Y., Nie S.P., Gong B., Li H.S., Zhao Q., Li W.J., Xie M.Y. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocoll. 2016;53:7–15. doi: 10.1016/j.foodhyd.2015.02.018. [DOI] [Google Scholar]
- 65.Simal-Gandara J., Gao L., Xu C., Tao X., Zuo Z., Ning Z., Wang L., Gao N., Zhao J. Structure elucidation of fucan sulfate from sea cucumber Holothuria fuscopunctata through a bottom-up strategy and the antioxidant activity analysis. Int. J. Mol. Sci. 2022;23:4488. doi: 10.3390/ijms23094488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Q., Jiang S., Shi W., Qi X., Song W., Mou J., Yang J. Structure characterization, antioxidant and immunoregulatory properties of a novel fucoidan from the sea cucumber Stichopus chloronotus. Carbohydr. Polym. 2020;231:115767. doi: 10.1016/j.carbpol.2019.115767. [DOI] [PubMed] [Google Scholar]
- 67.Guo X., Ye X., Sun Y., Wu D., Wu N., Hu Y., Chen S. Ultrasound effects on the degradation kinetics, structure, and antioxidant activity of sea cucumber fucoidan. J. Agric. Food Chem. 2014;62:1088–1095. doi: 10.1021/jf404717y. [DOI] [PubMed] [Google Scholar]
- 68.Li R., Huahua Y.U., Yang Y., Song L., Rong’e X., Xiaolin C., Li P. Sulfated polysaccharides with antioxidant and anticoagulant activity from the sea cucumber Holothuria fuscogliva. Chin. J. Oceanol. Limnol. 2017;35:763–769. doi: 10.1007/s00343-017-5339-7. [DOI] [Google Scholar]
- 69.Mou J., Li Q., Qi X., Yang J. Structural comparison, antioxidant and anti-inflammatory properties of fucosylated chondroitin sulfate of three edible sea cucumbers. Carbohydr. Polym. 2018;185:41–47. doi: 10.1016/j.carbpol.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 70.Yu L., Xue C., Chang Y., Xu X., Ge L., Liu G., Wang Y. Structure elucidation of fucoidan composed of a novel tetrafucose repeating unit from sea cucumber Thelenota ananas. Food Chem. 2014;146:113–119. doi: 10.1016/j.foodchem.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 71.Zou S., Pan R., Dong X., He M., Wang C. Physicochemical properties and antioxidant activities of two fucosylated chondroitin sulfate from sea cucumber Acaudina molpadioidea and Holothuria nobilis. Process Biochem. 2016;51:650–658. doi: 10.1016/j.procbio.2016.02.009. [DOI] [Google Scholar]
- 72.Wang J., Shi S., Li F., Du X., Kong B., Wang H., Xia X. Physicochemical properties and antioxidant activity of polysaccharides obtained from sea cucumber gonads via ultrasound-assisted enzymatic techniques. LWT Food Sci. Technol. 2022;160:113307. doi: 10.1016/j.lwt.2022.113307. [DOI] [Google Scholar]
- 73.Qi H., Ji X., Liu S., Feng D., Dong X., He B., Srinivas J., Yu C. Antioxidant and anti-dyslipidemic effects of polysaccharidic extract from sea cucumber processing liquor. Electron. J. Biotechnol. 2017;28:1–6. doi: 10.1016/j.ejbt.2017.04.001. [DOI] [Google Scholar]
- 74.Matsuno T., Tsushima M. Comparative biochemical studies of carotenoids in sea cucumber. Comp. Biochem. Physiol. 1995;111:597–605. doi: 10.1016/0305-0491(95)00028-7. [DOI] [PubMed] [Google Scholar]
- 75.Tsushima M., Fujiwara Y., Matsuno T. Novel marine di-Z-carotenoids: Cucumariaxanthins A, B, and C from the sea cucumber Cucumaria japonica. J. Nat. Prod. 1996;59:30–34. doi: 10.1021/np960022s. [DOI] [PubMed] [Google Scholar]
- 76.Maoka T., Nakachi S., Kobayashi R., Mori M., Sakagami Y. A new carotenoid, 9Z,9′Z-tetrahydroastaxanthin, from the sea cucumber Plesiocolochirus minutus. Tetrahedron Lett. 2015;56:5954–5955. doi: 10.1016/j.tetlet.2015.09.060. [DOI] [Google Scholar]
- 77.Zakharenko A., Romanchenko D., Thinh P.D., Pikula K., Thuy Hang C.T., Yuan W., Xia X., Chaika V., Chernyshev V., Zakharenko S., et al. Features and advantages of supercritical CO2 extraction of sea cucumber Cucumaria frondosa japonica semper, 1868. Molecules. 2020;25:4088. doi: 10.3390/molecules25184088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chasanah E., Fawzya Y.N., Tarman K., Januar H.I., Nursid M. Fatty acid profile, carotenoid content, and in vitro anticancer Activity of Karimunjawa and Lampung sea cucumber. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2016;11:117. doi: 10.15578/squalen.v11i3.269. [DOI] [Google Scholar]
- 79.Young A.J., Lowe G.L. Carotenoids—antioxidant properties. Antioxidants. 2018;7:28. doi: 10.3390/antiox7020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang X., Cong P., Chen Q., Li Z., Xu J., Xue C. Characterizing the phospholipid composition of six edible sea cucumbers by NPLC-Triple TOF-MS/MS. J. Food Compos. Anal. 2020;94:103626. doi: 10.1016/j.jfca.2020.103626. [DOI] [Google Scholar]
- 81.Liu Y., Dave D., Trenholm S., Ramakrishnan V.V., Murphy W. Effect of drying on nutritional composition of atlantic sea cucumber (Cucumaria frondosa) viscera derived from Newfoundland fisheries. Processes. 2021;9:703. doi: 10.3390/pr9040703. [DOI] [Google Scholar]
- 82.Du L., Yang Y.H., Wang Y.M., Xue C.H., Kurihara H., Takahashi K. Antitumour activity of EPA-enriched phospholipids liposomes against S180 ascitic tumour-bearing mice. J. Funct. Foods. 2015;19:970–982. doi: 10.1016/j.jff.2015.06.061. [DOI] [Google Scholar]
- 83.Wu F.J., Xue Y., Liu X.F., Xue C.H., Wang J.F., Du L., Takahashi K., Wang Y.M. The protective effect of eicosapentaenoic acid-enriched phospholipids from sea cucumber Cucumaria frondosa on oxidative stress in PC12 cells and SAMP8 mice. Neurochem. Int. 2014;64:9–17. doi: 10.1016/j.neuint.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 84.Zhang L., Wang D., Wen M., Du L., Xue C., Wang J., Xu J., Wang Y. Rapid modulation of lipid metabolism in C57BL/6J mice induced by eicosapentaenoic acid-enriched phospholipid from Cucumaria frondosa. J. Funct. Foods. 2017;28:28–35. doi: 10.1016/j.jff.2016.10.022. [DOI] [Google Scholar]
- 85.Hu S., Xu L., Shi D., Wang J., Wang Y., Lou Q., Xue C. Eicosapentaenoic acid-enriched phosphatidylcholine isolated from Cucumaria frondosa exhibits anti-hyperglycemic effects via activating phosphoinositide 3-kinase/protein kinase B signal pathway. J. Biosci. Bioeng. 2014;117:457–463. doi: 10.1016/j.jbiosc.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 86.Tian Y., Hu S., Xu H., Wang J., Xue C., Wang Y. Long-chain bases from Cucumaria frondosa inhibit adipogenesis and regulate lipid metabolism in 3T3-L1 adipocytes. Food Sci. Biotechnol. 2016;25:1753–1760. doi: 10.1007/s10068-016-0267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jia Z., Song Y., Tao S., Cong P., Wang X., Xue C., Xu J. Structure of sphingolipids from sea cucumber Cucumaria frondosa and structure-specific cytotoxicity against human hepg2 cells. Lipids. 2016;51:321–334. doi: 10.1007/s11745-016-4128-y. [DOI] [PubMed] [Google Scholar]
- 88.Althunibat O., Ridzwan B., Taher M., Daud J., Jauhari Arief Ichwan S., Qaralleh H. Antioxidant and cytotoxic properties of two sea cucumbers, Holothuria edulis Lesson and Stichopus horrens Selenka. Acta Biol. Hung. 2013;64:10–20. doi: 10.1556/ABiol.64.2013.1.2. [DOI] [PubMed] [Google Scholar]
- 89.Hawas U.W., Abou El-Kassem L.T., Shaher F.M., Ghandourah M., Al-Farawati R. Sulfated triterpene glycosides from the Saudi red sea cucumber Holothuria atra with antioxidant and cytotoxic activities. Thalassas. 2021;37:817–824. doi: 10.1007/s41208-021-00305-4. [DOI] [Google Scholar]
- 90. Ansharullah, Tamrin, Patadjai A.B., Asranuddin. Powder production of sea cucumber (Holothuria scabra): Effect of processing methods on the antioxidant activities and physico-chemical characteristics. IOP Conf. Ser. Earth Environ. Sci. 2020;443:331–334. doi: 10.1088/1755-1315/443/1/012024. [DOI] [Google Scholar]
- 91.Nugroho A., Harahap I.A., Ardiansyah A., Bayu A., Rasyid A., Murniasih T., Setyastuti A., Putra M.Y. Antioxidant and antibacterial activities in 21 species of Indonesian sea cucumbers. J. Food Sci. Technol. 2022;59:239–248. doi: 10.1007/s13197-021-05007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rasyid A., Putra M.Y., Yasman. Free radical scavenging activity of five selected sea cucumbers collected from Lampung waters, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021;890:012014. doi: 10.1088/1755-1315/890/1/012014. [DOI] [Google Scholar]
- 93.Wulandari D.A., Murniasih T., Sari M., Syahputra G., Rasyid A., Septiana E., Untari F., Harahap I.A., Ardiansyah A., Gustini N., et al. Characterization, antioxidant and antibacterial activity of cultivated sea cucumbers from Bali, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021;744:012102. doi: 10.1088/1755-1315/744/1/012102. [DOI] [Google Scholar]
- 94.Wu F.-J., Xue Y., Tang Q.-J., Xu J., Du L., Xue C.-H., Takahashi K., Wang Y.-M. The protective effects of cerebrosides from sea cucumber and starfish on the oxidative damage in PC12 cells. J. Oleo Sci. 2013;62:717–727. doi: 10.5650/jos.62.717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.