Abstract
The flagellar hook of Salmonella is a filamentous polymer made up of subunits of the protein FlgE. Hook assembly is terminated when the length reaches about 55 nm. After our recent study of the effect of cellular levels of the hook length control protein FliK, we have now analyzed the effect of cellular levels of FlgE itself. When FlgE was overproduced in a wild-type strain, a fliC (flagellin) mutant, or a fliD (hook-associated protein 2 [HAP2], filament capping protein) mutant, the hooks remained at the wild-type length. In a fliK (hook length control protein) mutant, which produces long hooks (polyhooks), the overproduction of FlgE resulted in extraordinarily long hooks (superpolyhooks). In a flgK (HAP1, first hook-filament junction protein) mutant or a flgL (HAP3, second hook-filament junction protein) mutant, the overproduction of FlgE also resulted in longer than normal hooks. Thus, at elevated hook protein levels not only FliK but also FlgK and FlgL are necessary for the proper termination of hook elongation. When FlgE was severely underproduced, basal bodies without hooks were often observed. However, those hooks that were seen were of wild-type length, demonstrating that FlgE underproduction decreases the probability of the initiation of hook assembly but not the extent of hook elongation.
In the pathway of flagellar morphogenesis in Salmonella and Escherichia coli, the hook protein FlgE is assembled after completion of the basal body structure (13). The subunits are exported from the cytoplasm through a central channel in the basal body and assemble at the distal end of the growing hook. Hook assembly is terminated when the hook length reaches about 55 nm (1), and flagellar filament assembly follows.
Several proteins are known to be necessary for hook assembly and termination. FlgD is the hook capping protein (21), located at the tip of the hook during hook elongation. flgD mutants cannot assemble hook subunits, although they can still export them. After the completion of hook assembly, FlgD is displaced from the hook and another protein, FlgK, is assembled at the hook tip. FlgK is one of the hook-filament junction proteins (hook-associated proteins [HAPs]) (4). flgK mutants retain the FlgD cap and cannot assemble the filament (21). The hook length distribution in flgK mutants is similar to that in the wild-type strain (1).
FliK is a soluble protein that is involved in hook length control (7, 22, 25) but has not been found in the flagellar structure. Mutations in fliK result in (i) an abnormal elongation of the hook (the length now ranging from 40 to 900 nm) and (ii) a failure to assemble filament (polyhook phenotype) (1). fliK mutations can be partially suppressed by mutations in flhB (11, 27), which encodes a membrane protein component of the apparatus for exporting external flagellar proteins like hook protein and flagellin (16, 17). fliK-flhB double mutants restore filament assembly, although they are still defective in hook length control (polyhook-filament phenotype).
In a recent study, Koroyasu et al. measured the hook length distribution in fliK mutants (8). They found that, even though the polyhook distribution extended far further out than the wild-type distribution, the peak length was still about 55 nm, i.e., the same as the wild-type hook length. This result suggests that hook elongation in fliK mutants has two different stages—a fast initial elongation stage until a length of 55 nm is achieved and a slow elongation stage thereafter. This result suggests that the mechanism of hook length control must have a FliK-independent component.
We recently demonstrated that the termination of hook assembly correlated with the cellular level of FliK (18). The wild-type level was quite low, about 20 to 80 molecules per cell. When the level was decreased, the number of filaments decreased and the length of the hooks increased. When the level was increased, the hook length was only slightly shorter than the wild-type value. We proposed a model of hook length control in which FliK changed the specificity of the export process by interacting with the export apparatus when the latter received a signal of hook length completion. In this model, the export of hook proteins and their diffusion within the central channel of the hook were assumed to affect the state of the export apparatus.
We have now overexpressed and underexpressed the flgE gene in wild-type cells and various flagellar mutants and examined the effects on the hook length control process.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The Salmonella flagellar mutants and plasmids used in this study are listed in Table 1. All mutants are derivatives of SJW1103, the wild type for flagellation (29). E. coli XL1-Blue (Stratagene) and Salmonella strain JR501 (r− m+) (23) were used as recipients in cloning experiments. Luria medium (LM) contained 1% Bacto Tryptone (Difco), 0.5% yeast extract, and 0.5% NaCl. Soft tryptone motility plates contained 1.0% Bacto Tryptone, 0.7% NaCl, and 0.35% Bacto Agar. Ampicillin (50-μg/ml final concentration) was added to the medium as needed.
TABLE 1.
Salmonella flagellar mutants and plasmids used in this study
| Strain or plasmid | Relevant genotype or property | Reference |
|---|---|---|
| Strains | ||
| SJW1103 | Wild type for flagellation | 29 |
| SJW1446 | flgA | 21 |
| SJW1353 | flgEa | 21 |
| SJW1469 | flgH | 9, 21 |
| SJW1351 | flgI | 21 |
| SJW2177 | flgK | 1, 21 |
| SJW2172 | flgL | 21 |
| SJW6017 | ΔfliC | This study |
| SJW2149 | fliD | 21 |
| SJW108 | fliK | 18, 27 |
| MY3018 | fliK flhB | 27 |
| Plasmids | ||
| pKM1500 | pTrc99A/wild-type flgE | This study |
| pKM1501 | pTrc99A/flgE-W208opal | This study |
| pKM1505 | pBR322/flgE-W208opal | This study |
| pKM1508 | pET22b/flgE-W208opal | This study |
Contains a 12-codon deletion (corresponding to residues 10 to 21 of the 408-residue FlgE protein [19]).
Cloning of the wild-type and mutant alleles of flgE.
The cloning of flgE alleles was carried out as described for fliK (18). Chromosomal DNA (28) isolated from Salmonella strain SJW1103 was used as the template for PCR. Synthetic outside primer A for PCR contained a sequence upstream of the putative ribosome binding site for flgE and a BamHI restriction site. Outside primer B contained a complementary sequence downstream of flgE and a HindIII site.
Site-directed mutagenesis of flgE.
Site-directed mutagenesis on flgE was carried out by mutagenic PCR as described previously (18) with minor modifications. Pfu DNA polymerase (Stratagene) was used for the first round of PCR. The mutation sites were confirmed by DNA sequencing (Sequenase; U.S. Biochemicals).
Observation of swarming, free-swimming motility, and flagellar morphology.
For swarm assays, cells were grown overnight at 37°C on LM–1.5% agar plates containing ampicillin, and then single colonies were spotted onto a soft tryptone plate containing ampicillin and incubated at 30°C for the time indicated.
For the observation of free swimming, cells were grown overnight with shaking in LM plus ampicillin at 37°C. They were then diluted 50-fold into LM plus ampicillin, grown with shaking at 37°C till late log phase, and observed by dark-field microscopy.
Flagellar morphology was observed by electron microscopy as described previously (1).
SDS-PAGE and immunoblotting.
Cells were cultured under the same conditions as for free swimming and flagellar morphology observations. Cells were collected by centrifugation and suspended in a sample buffer (50 mM Tris-HCl [pH 8.0], 0.4% NaCl, 0.9% sodium dodecyl sulfate [SDS], 6% glycerol, 0.005% bromophenol blue, and 8% 2-mercaptoethanol) at a concentration of 8 × 109 cells/ml. A suspension of 108 cells was boiled and subjected to SDS–12.5% polyacrylamide gel electrophoresis (PAGE). Immunoblotting was carried out as described previously (18) with a polyclonal rabbit anti-FlgE antibody.
RESULTS
Overproduction of hook protein.
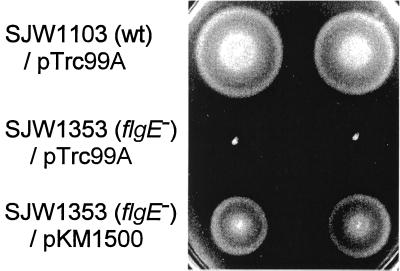
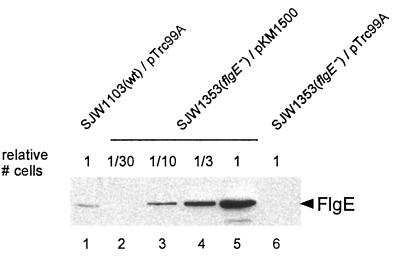
For the overproduction of hook protein, we used the wild-type flgE gene inserted downstream of the trc promoter of plasmid pTrc99A. The resulting plasmid (pKM1500) was used to transform the flgE mutant SJW1353, resulting in the restoration of swarming to close to wild-type levels (Fig. 1). Even in the absence of the inducer IPTG (isopropyl-β-d-thiogalactopyranoside), the cellular level of FlgE was much higher (approximately 20-fold) than the wild-type chromosomal level (Fig. 2). Unless otherwise stated, overproduction results refer to such noninducing conditions.
FIG. 1.
Swarming assay for complementation of the flgE mutant SJW1353. Top row, strain SJW1103(wild type [wt]) transformed with the vector pTrc99A. Middle row, SJW1353(flgE) transformed with pTrc99A. Bottom row, SJW1353 transformed with the pTrc99A-based plasmid pKM1500, containing the wild-type flgE gene. Swarms are shown in duplicate and after incubation at 30°C for 5 h.
FIG. 2.
Cellular amount of hook protein (FlgE), assayed by immunoblotting with polyclonal anti-FlgE antibody. Lane 1, SJW1103(wild-type [wt]) cells transformed with pTrc99A. Lanes 2 to 5, SJW1353(flgE) cells transformed with pKM1500 (wild-type flgE on pTrc99A). Lane 6, SJW1353(flgE) cells transformed with pTrc99A. Whole-cell lysates were loaded into lanes 1 to 6 as follows: lane 1, 108 cells; lane 2, 3 × 106 cells; lane 3, 107 cells; lane 4, 3 × 107 cells; lane 5, 108 cells; lane 6, 108 cells. Cells were cultured at 37°C in LM plus ampicillin.
Flagellar morphologies in cells overproducing hook protein.
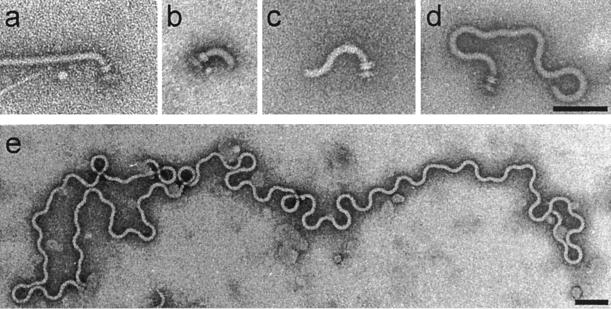
Mutants defective in several flagellar genes retain the ability to assemble hooks. We examined flagellar morphologies of wild-type and mutant strains under conditions of FlgE overproduction. Electron micrographs illustrating these morphologies are given in Fig. 3, and the results are summarized in Table 2.
FIG. 3.
Electron micrographs of hook-filament structures seen in cells overproducing the hook protein from the pTrc99A-based plasmid pKM1500. Types of structures and the specific strain or plasmid used in this figure to illustrate each type of structure are as follows (a summary of the structures seen with these and other strains is shown in Table 2): normal hook-filament (SJW1103[wt]) (a), normal hook with no filament (SJW2149[fliD]) (b), elongated hook with no filament (SJW2172[flgL]) (c), polyhook with no filament (SJW2177[flgK]) (d), and superpolyhook with no filament (SJW108[fliK]) (e). Bars, 100 nm.
TABLE 2.
Flagellar morphology in various mutants under conditions of overproduction and underproduction of the hook protein FlgE
| Host strain and genotype | Plasmid | Flagellar morphologya |
|---|---|---|
| FlgE overproductionb | ||
| SJW1103(wt) | None | Normal hook and filament |
| SJW1103(wt) | pKM1500 | Normal hook and filament |
| SJW1353(flgE) | pKM1500 | Normal hook and filament |
| SJW6017(ΔfliC) | pKM1500 | Normal hook; no filament |
| SJW2149(fliD) | pKM1500 | Normal hook; no filament |
| SJW2172(flgL) | pKM1500 | Normal hook; no filament. Polyhook; no filament |
| SJW2177(flgK) | pKM1500 | Normal hook and polyhook; no filament. Superpolyhook; no filament |
| SJW108(fliK) | pKM1500 | Superpolyhook; no filament |
| MY3018(fliK flhB) | pKM1500 | Polyhook; normal filament |
| SJW1469(flgH) | pKM1500 | Basal body lacking L ring; normal hook; no filament. Basal body lacking L ring; polyhook; filament. |
| SJW1351(flgI) or SJW1446(flgA) | pKM1500 | Basal body lacking L and P rings; normal hook; no filament. Basal body lacking L and P rings; polyhook; filament. |
| FlgE underproductionc | ||
| SJW1103(wt) | None | Normal hook and filament |
| SJW1353(flgE) | pKM1501 | Normal hook and filament. Normal hook; no filament |
| SJW1353(flgE) | pKM1508 | Normal hook; no filament. No hook or filament |
| SJW1353(flgE) | pKM1505 | No hook or filament |
The predominant morphology is shown in boldface.
pKM1500 is a pTrc99A-based plasmid carrying the wild-type flgE gene.
pKM1501, pKM1508, and pKM1505 are pTrc99A-based, pET22b-based, and pBR322-based plasmids, respectively, all carrying the W208opal allele of the flgE gene.
(i) Wild-type strain.
If the cellular amount of hook protein was to contribute to hook length control, overproduction in wild-type cells might cause longer hooks. However, such cells had ordinary flagella (i.e., normal hooks and filaments) (Fig. 3a), with a normal hook length of around 55 nm.
(ii) Mutants defective in flagellin and HAP synthesis.
The second hook-filament junction protein (HAP3, FlgL), the filament capping protein (HAP2, FliD), and flagellin (FliC) all assemble after hook assembly is complete and the hook capping protein FlgD has been displaced by the first hook-filament junction protein (HAP1, FlgK). Therefore, when hook protein was overproduced in fliC, fliD, and flgL mutants, we expected to see essentially the same hook phenotype as with the wild-type strain. The fliC and fliD mutants did in fact produce normal hooks (lacking, of course, filaments) (Fig. 3b).
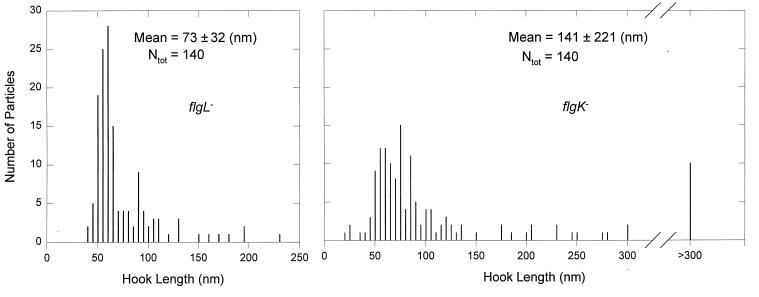
flgL mutants, although they mostly produced normal hooks, also produced some longer hooks (Fig. 3c). This resulted in a length distribution ranging up to 230 nm with a mean of 73 ± 32 nm (Fig. 4, left panel), significantly longer and broader than the wild type. Thus, FlgL, even though it is not physically adjacent to the hook, appears to exert some effect on hook assembly. The peak value in the distribution was still essentially that of the wild type, i.e., around 55 nm.
FIG. 4.
Distribution of hook lengths in mutants overproducing FlgE. SJW2172(flgL) (left) or SJW2177(flgK) (right) cells were transformed with pKM1500. After isolation of hook-basal bodies, their hook lengths were measured from electron micrographs. Ntot, total number of particles measured.
FlgK is assembled after completion of the hook but before the addition of FlgL, FliD, and flagellin. Since the hook in flgK mutants retains FlgD at its tip (21), it might continue to be assembled at elevated hook protein levels. Consistent with this suggestion, we found that flgK produced not only normal hooks (Fig. 3b) but also polyhooks (Fig. 3d). The hook length distribution had a mean value of 141 ± 221 nm (Fig. 4, right panel), significantly longer and broader than the distribution with the flgL mutant. There were some exceedingly long particles, up to 1,700 nm (Fig. 3e). Again, however, the peak value was similar to that for wild-type cells.
(iii) fliK (polyhook) mutant.
Even at normal hook protein levels, fliK mutations result in abnormally long hooks (polyhooks) (1, 7, 22, 25), and so FliK is believed to function in the termination of hook assembly at the appropriate point. Since the polyhook structure retains FlgD at its tip (21), the overproduction of hook protein might result in even greater hook elongation. fliK cells did indeed produce extremely long polyhooks (superpolyhooks; Fig. 3e), with a length distribution ranging to well over 1,000 nm. Again, however, the peak of the distribution remained at 55 nm.
Extragenic suppressor mutations of fliK mutations have been found to be located exclusively in flhB (11, 27). These pseudorevertants still produce an elongated hook but have filament assembly restored (polyhook-filament phenotype) (1). fliK-flhB cells overproducing hook protein produced polyhook-filaments whose polyhooks, though somewhat longer than those in untransformed cells, did not approach the length of those in the single fliK mutant (data not shown).
(iv) L ring and P ring mutants.
The products of the flgH and flgI genes are the structural components of the outer membrane L ring and the periplasmic P ring, respectively (3, 24); flgA is also (for unknown reasons) needed for P ring assembly (9). Although the L and P rings are normally formed before the completion of hook assembly, flgH, flgI, and flgA mutants do produce a very short hook structure at the tip of the rod (9, 26) and were shown under special conditions (transformation of an E. coli flgE mutant with a plasmid carrying the Salmonella flgE gene) to produce complete hooks to which a filament can attach (6, 20).
Our results are in general agreement with those of Ohnishi et al. (20) and Jones et al. (6). In swarming assays, flgH, flgI, and flgA mutants formed small colonies with tiny satellite colonies (data not shown). The overproduction of hook protein improved swarming somewhat, suggesting that the efficiency of hook assembly had been increased. For flgH cells, basal bodies lacking the L ring but with a normal hook attached were the major product; basal bodies lacking the L ring but with a polyhook-filament attached were a minor product (data not shown). Thus, there appears to be an inverse relationship between the control of hook length and the ability to add filament. flgI and flgA cells gave similar results, except that in these cases both the L and P rings were missing from the basal bodies.
Underproduction of hook protein.
In a previous study with the hook length protein FliK (18), we had successfully underproduced it by the replacement of a natural UGG (Trp) codon by a UGA (opal) codon, taking advantage of the low but significant frequency of UGA readthrough by wild-type tRNATrp. We have adopted the same strategy with flgE, replacing its natural Trp208 UGG codon by UGA. The flgE-W208opal gene was cloned into three vectors, pTrc99A (to give pKM1501), pET22b (pKM1508), and pBR322 (pKM1505). These vectors result in progressively decreasing expression levels, as was confirmed by immunoblotting estimates based on plasmids carrying the wild-type gene (data not shown). The underproduced FlgE levels resulting from the use of the flgE-W208opal mutation were at or below the limit of detection by immunoblotting; however, we assume the same progression applies here also, an assumption that is supported by the motility and morphology phenotypes that are described next.
Motility under conditions of hook protein underproduction.
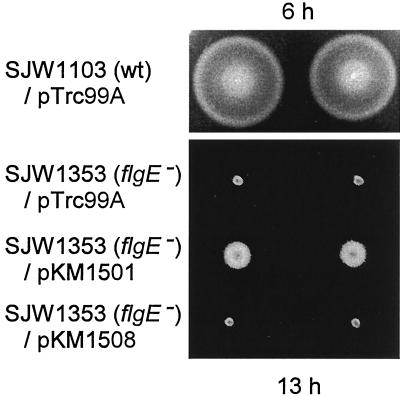
pKM1501 restored weak swarming to SJW1353 cells (Fig. 5, row 3). In a liquid medium, a small fraction of cells were motile, and the motility was greatly improved in the presence of IPTG. Neither pKM1508 (Fig. 5, row 4) nor pKM1505 (data not shown) complemented either swarming or swimming at all.
FIG. 5.
Swarming ability of cells underproducing hook proteins. Row 1, wild-type (wt) SJW1103 transformed with pTrc99A. Rows 2 to 4, SJW1353(flgE) cells transformed with the following plasmids: pTrc99A, pKM1501 (pTrc99A flgE-W208opal), and pKM1508 (pET22b flgE-W208opal). Swarms are shown in duplicate and after incubation at 30°C for the times indicated.
Flagellar morphology in cells underproducing hook protein.
flgE cells transformed with pKM1501 (flgE-W208opal on pTrc99A) produced mostly normal hook filaments (Fig. 3a) as well as some normal hooks lacking filaments (Fig. 3b). Transformation with pKM1508 (flgE-W208opal on pET22b) resulted mostly in normal hooks lacking filaments (Fig. 3b) as well as some basal bodies lacking both hooks and filaments. With pKM1505 (flgE-W208opal on pBR322), cells produced only basal bodies lacking both hooks and filaments, the same phenotype observed with the untransformed SJW1353(flgE) mutant. It is noteworthy that abnormally short hooks were very seldom seen with any of these plasmids; where they were seen, they may have simply represented particles caught during the process of hook assembly.
DISCUSSION
Hook protein concentration does not affect control of hook length in wild-type cells.
This study was designed to ask the following two questions. (i) To what extent does variation of the concentration of the substrate, hook protein or FlgE, perturb the control process in the wild-type cell? (ii) How do various mutants in flagellar assembly respond to variation in hook protein concentration and what do their responses tell us about the assembly process?
We did not imagine that in wild-type cells the control of hook length would be highly sensitive to hook protein concentration; if it were, control could easily fail under different conditions of metabolism or gene expression. Nonetheless, we found it remarkable how robust the control process was (Table 2). Overproduction by a factor of 20 had no apparent effect on either hook assembly or hook length control. Moderate underproduction (flgE-W208opal expressed from pTrc99A) also did not affect hook assembly or hook length control, though it did decrease somewhat the probability of filament initiation. As underproduction was made more severe (flgE-W208opal expressed from pET22b), there was a decrease in the number of hooks seen, but for those that were seen, the control of hook length was normal; the initiation of filament assembly, however, was essentially abolished. Under the most severe underproduction conditions tested (flgE-W208opal expressed from pBR322), the system appeared to be totally starved for its substrate, since essentially the only particles seen were simple basal bodies.
Hook protein concentration affects hook length in certain mutant classes.
With mutants defective in the synthesis of either the filament protein (FliC) or the capping protein (FliD) that is needed for filament assembly, hook length control was again unaffected by hook protein overproduction. Thus, the events surrounding filament assembly appear to be effectively isolated from those surrounding hook assembly, and so fliC and fliD mutants behave like wild-type cells with regard to the latter process.
When hook protein was overproduced, abnormally long hooks (polyhooks), in some cases with filaments attached (polyhook-filaments), were observed in flgK, flgL, fliK, fliK-flhB, flgH, flgI, and flgA mutants.
By far the longest hook structures were seen in the fliK mutant (and also, less frequently, in the flgK mutant), leading us to create the term superpolyhook for these extraordinary structures (Fig. 3e). The contrast between the tight control of hook length in wild-type cells (Fig. 3a) or cells defective in filament assembly (Fig. 3b) and this total loss of length control—in both cases in the face of overproduction of hook protein—was striking.
The situation with the fliK mutant seems fairly easy to understand. Spontaneous fliK polyhook mutants (including the one used in this study) have all proved to be nulls resulting from frameshift or nonsense mutations (27); the total lack of FliK results in a complete failure of the export apparatus to undergo its substrate specificity switch. The family of filament-type proteins (FlgK, FlgL, FliD, and FliC—and also the regulatory protein FlgM [5, 10]) never gets exported, the hook cap stays in place, and elongation continues indefinitely. Increased hook protein therefore would be expected to increase mean hook length, as is illustrated dramatically in Fig. 3e. The failure to see these extremely long hook structures with fliK-flhB pseudorevertants presumably reflects the fact that filament initiation intervenes and halts the elongation process.
Our data indicate that under conditions of FlgE overproduction not only FliK but also FlgK and FlgL are necessary to ensure the termination of hook assembly at the proper length. The effect was especially pronounced with flgK mutants where some particles were long enough to justify the term superpolyhooks that we originally coined for fliK mutants. FlgD, the hook capping protein, is still located at the tip of the hook in flgK mutants (21), in principle permitting the addition of hook subunits. Apparently the high hook protein levels defeat the FliK control mechanism and allow such addition to take place. The fact that hook assembly in the flgL mutant did not terminate at the wild-type length is less easily explained. Although FlgK can replace FlgD in the absence of FlgL (4, 21), we suggest that the assembly and stability of the FlgK zone and hence its ability to efficiently and completely displace the FlgD hook cap must be to some degree dependent on FlgL.
To summarize the above discussion, we propose that the roles of FliK, FlgK, and FlgL are as follows. (i) FliK initiates the assembly of HAPs and flagellin by interacting with the export apparatus and switching its export specificity. (ii) FlgK replaces FlgD at the tip of the hook and terminates hook assembly. (iii) FlgL ensures that the assembled FlgK zone is stable and permanent.
The flgH and the flgI and flgA mutants produced polyhook-filaments lacking the L ring and the L and P rings, respectively, when the hook protein was overproduced. While the loss of the rings is expected, we are not sure of the cause of the increase in hook length. Gene expression could be a factor. The lack of the outer ring structures can affect the export of flagellar proteins (17), and the failure to export FlgM at the appropriate time could result in an abnormally low expression of FlgK and FlgL, with a consequent delay in terminating hook assembly.
Peak hook length is maintained even when hook length control has failed.
In a previous study, Koroyasu et al. found that although fliK mutants produced polyhooks, the peak of the hook length distribution did not change from the wild-type value of 55 nm (8). They proposed that hook elongation has two phases, the rate in the first phase decreasing with increasing hook length up to 55 nm and the rate in the second phase being constant at a much lower value. Since these were null mutants (27), it appeared that the peak hook length was determined independently of FliK function.
We have now extended this observation to conditions of hook protein overproduction, not only with fliK mutants but also with mutants defective in the hook-associated proteins FlgK and FlgL (Fig. 4). Thus, under a number of different conditions, length per se appears to play a role that is independent of the amount of substrate or switching of export specificity.
Underproduction of hook protein affects the probability of filament initiation.
Although the underproduction of hook protein did not interfere with the length control process, it did decrease the probability of hook assembly; thus, the initiation of hook assembly appears to be the factor most directly affected. Hook protein underproduction also decreased the probability of filament assembly onto those hooks that were produced. This may well be a consequence of underexpression of the filament-type genes, including those for the HAPs, since a cell with a low-level complement of hooks will have a low-level capacity for export (and hence inactivation) of the anti-sigma factor FlgM.
Export switching model for hook and filament assembly.
In previous studies, an export switching model was proposed for hook assembly termination and filament assembly initiation (11, 18, 27). In this model, FliK changes the substrate specificity of the export apparatus from hook to flagellin (and other proteins like the HAPs) after the hook has reached its mature length. In the wild-type cell, displacement of the FlgD cap by FlgK is not necessary for hook growth to stop, supporting the assumption that the export of hook subunits has been stopped by export switching; direct measurements of the export of hook-type and filament-type proteins in flgD and fliC mutants further support this assumption (15). However, it may be that the hook subunits can to some limited degree (or for some limited time) be exported after the initiation of the HAP and flagellin export, since flgK and flgL mutants are still able to export and assemble the hook proteins after the hook length has reached its normal value of 55 nm; this is especially noteworthy with the flgL mutant, where one might have expected FlgK to terminate hook assembly. flgK and flgL mutants have undergone the specificity switch, since they can make flagellin and excrete it into the culture medium (2, 12). If the HAPs and flagellin are to any degree exported with the hook protein, they might compete with each other at the export or assembly stage and decrease the rate of hook elongation.
In a previous study, we proposed a mechanism of hook length control in which hook subunit diffusion through the central channel of the hook-basal body abruptly changed at the point of mature hook length (18). We assumed that the abrupt shift of diffusion rate caused a change in the status of the export apparatus and caused FliK-mediated export switching to occur. Based on the results of the present study, we have to reconsider this model. Hook overproduction increased polyhook length in the fliK mutant, and so the diffusion rate after the mature hook length has been reached is apparently increased under these conditions.
A surprising recent finding is that FliK, although not a component of the assembled flagellum, is exported via the flagellum-specific export apparatus during the process of hook assembly (14). Thus for the first time, the possibility arises that FliK is exercising control in situ, rather than remotely from the cytoplasm as has been believed until now.
ACKNOWLEDGMENTS
We thank May Kihara and Kenta Yamaguchi for technical assistance.
This work was supported by NIH grant AI12202 (to R.M.M.).
REFERENCES
- 1.Hirano T, Yamaguchi S, Oosawa K, Aizawa S-I. Roles of FliK and FlhB in determination of flagellar hook length in Salmonella typhimurium. J Bacteriol. 1994;176:5439–5449. doi: 10.1128/jb.176.17.5439-5449.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Homma M, Fujita H, Yamaguchi S, Iino T. Excretion of unassembled flagellin by Salmonella typhimurium mutants deficient in the hook-associated proteins. J Bacteriol. 1984;159:1056–1059. doi: 10.1128/jb.159.3.1056-1059.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Homma M, Komeda Y, Iino T, Macnab R M. The flaFIX gene product of Salmonella typhimurium is a flagellar basal body component with a signal peptide for export. J Bacteriol. 1987;169:1493–1498. doi: 10.1128/jb.169.4.1493-1498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Homma M, Kutsukake K, Iino T, Yamaguchi S. Hook-associated proteins essential for flagellar filament formation in Salmonella typhimurium. J Bacteriol. 1984;157:100–108. doi: 10.1128/jb.157.1.100-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes K T, Gillen K L, Semon M J, Karlinsey J E. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science. 1993;262:1277–1280. doi: 10.1126/science.8235660. [DOI] [PubMed] [Google Scholar]
- 6.Jones C J, Homma M, Macnab R M. Identification of proteins of the outer (L and P) rings of the flagellar basal body of Escherichia coli. J Bacteriol. 1987;169:1489–1492. doi: 10.1128/jb.169.4.1489-1492.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawagishi I, Homma M, Williams A W, Macnab R M. Characterization of the flagellar hook length control protein FliK of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1996;178:2954–2959. doi: 10.1128/jb.178.10.2954-2959.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koroyasu S, Yamazato M, Hirano T, Aizawa S-I. Kinetic analysis of the growth rate of the flagellar hook in Salmonella typhimurium by the population balance method. Biophys J. 1998;74:436–443. doi: 10.1016/S0006-3495(98)77801-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubori T, Shimamoto N, Yamaguchi S, Namba K, Aizawa S-I. Morphological pathway of flagellar assembly in Salmonella typhimurium. J Mol Biol. 1992;226:433–446. doi: 10.1016/0022-2836(92)90958-m. [DOI] [PubMed] [Google Scholar]
- 10.Kutsukake K. Excretion of the anti-sigma factor through a flagellar structure couples flagellar gene expression with flagellar assembly in Salmonella typhimurium. Mol Gen Genet. 1994;243:605–612. doi: 10.1007/BF00279569. [DOI] [PubMed] [Google Scholar]
- 11.Kutsukake K, Minamino T, Yokoseki T. Isolation and characterization of FliK-independent flagellation mutants from Salmonella typhimurium. J Bacteriol. 1994;176:7625–7629. doi: 10.1128/jb.176.24.7625-7629.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutsukake K, Ohya Y, Iino T. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J Bacteriol. 1990;172:741–747. doi: 10.1128/jb.172.2.741-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macnab R M. Flagella and motility. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 123–145. [Google Scholar]
- 14.Minamino, T., S.-I. Aizawa, and R. M. Macnab. Unpublished data.
- 15.Minamino T, Doi H, Kutsukake K. Substrate specificity switching of the flagellum-specific export apparatus during flagellar morphogenesis in Salmonella typhimurium. Biosci Biotechnol Biochem. 1999;63:1301–1303. doi: 10.1271/bbb.63.1301. [DOI] [PubMed] [Google Scholar]
- 16.Minamino T, Iino T, Kutsukake K. Molecular characterization of the Salmonella typhimurium flhB operon and its protein products. J Bacteriol. 1994;176:7630–7637. doi: 10.1128/jb.176.24.7630-7637.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minamino T, Macnab R M. Components of the Salmonella flagellar export apparatus and classification of export substrates. J Bacteriol. 1999;181:1388–1394. doi: 10.1128/jb.181.5.1388-1394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muramoto K, Makishima S, Aizawa S-I, Macnab R M. Effect of the cellular level of FliK on flagellar hook and filament assembly in Salmonella typhimurium. J Mol Biol. 1998;277:871–882. doi: 10.1006/jmbi.1998.1659. [DOI] [PubMed] [Google Scholar]
- 19.Ohnishi, K. Personal communication.
- 20.Ohnishi K, Homma M, Kutsukake K, Iino T. Formation of flagella lacking outer rings by flaM, flaU, and flaY mutants of Escherichia coli. J Bacteriol. 1987;169:1485–1488. doi: 10.1128/jb.169.4.1485-1488.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohnishi K, Ohto Y, Aizawa S-I, Macnab R M, Iino T. FlgD is a scaffolding protein needed for flagellar hook assembly in Salmonella typhimurium. J Bacteriol. 1994;176:2272–2281. doi: 10.1128/jb.176.8.2272-2281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson-Delafield J, Martinez R J, Stocker B A D, Yamaguchi S. A new fla gene in Salmonella typhimurium—flaR—and its mutant phenotype—superhooks. Arch Mikrobiol. 1973;90:107–120. doi: 10.1007/BF00414513. [DOI] [PubMed] [Google Scholar]
- 23.Ryu J, Hartin R J. Quick transformation in Salmonella typhimurium LT2. BioTechniques. 1990;8:43–44. [PubMed] [Google Scholar]
- 24.Schoenhals G J, Macnab R M. Physiological and biochemical analyses of FlgH, a lipoprotein forming the outer membrane L ring of the flagellar basal body of Salmonella typhimurium. J Bacteriol. 1996;178:4200–4207. doi: 10.1128/jb.178.14.4200-4207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki T, Iino T. Role of the flaR gene in flagellar hook formation in Salmonella spp. J Bacteriol. 1981;148:973–979. doi: 10.1128/jb.148.3.973-979.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki T, Iino T, Horiguchi T, Yamaguchi S. Incomplete flagellar structures in nonflagellate mutants of Salmonella typhimurium. J Bacteriol. 1978;133:904–915. doi: 10.1128/jb.133.2.904-915.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams A W, Yamaguchi S, Togashi F, Aizawa S-I, Kawagishi I, Macnab R M. Mutations in fliK and flhB affecting flagellar hook and filament assembly in Salmonella typhimurium. J Bacteriol. 1996;178:2960–2970. doi: 10.1128/jb.178.10.2960-2970.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woo T H S, Cheng A F, Ling J M. An application of a simple method for the preparation of bacterial DNA. BioTechniques. 1992;13:696–698. [PubMed] [Google Scholar]
- 29.Yamaguchi S, Fujita H, Sugata K, Taira T, Iino T. Genetic analysis of H2, the structural gene for phase-2 flagellin in Salmonella. J Gen Microbiol. 1984;130:255–265. doi: 10.1099/00221287-130-2-255. [DOI] [PubMed] [Google Scholar]