Structured Abstract
Purpose of review:
We will highlight the role of ventriculo-arterial (VA) coupling in the pathophysiology of sepsis and how to assess it.
Recent findings:
Most septic patients show a VA uncoupling at the time of diagnosis with arterial elastance (Ea) greater than left ventricle (LV) end-systolic elastance (Ees), often despite arterial hypotension. VA coupling levels predict the cardiovascular response to resuscitation in this heterogeneously responding population.
Summary:
VA coupling is quantified as the ratio of Ea to Ees. The efficiency of the cardiovascular function is optimal when Ea/Ees is near one. When the hydraulic load of the arterial system is excessive either from increased vasomotor tone, decreased LV contractility or both, Ea/Ees becomes >1 (i.e. VA decoupling), and cardiac efficiency decreases leading to heart failure, loss of volume responsiveness and, if sustained, increased mortality. Non-invasive echocardiographic techniques when linked with arterial pressure monitoring allow for the bedside estimates of both Ea and Ees. Studies using this approach have documented the key role VA coupling has defining initial cardiovascular state, response to therapy and outcome from critical illness. Sequential monitoring of VA coupling at the bedside offers a unique opportunity to assess relevant cardiovascular determinants in septic patients requiring resuscitation.
Keywords: Arterial elastance, ventricular elastance, coupling, sepsis, resuscitation
Introduction
Septic shock is a life-threatening disease where the uncontrolled host-response to the infection leads to acute cardiovascular decompensation and severe haemodynamic impairment [1]. Septic shock is characterized as an acute cardiovascular dysfunction where systemic arterial hypotension, vascular dilatation and loss of the vascular tone are responsible for impaired peripheral perfusion, inadequate tissue oxygen delivery relative to metabolic demands with consequent metabolic disturbances and multi-organ dysfunction [2, 3]. Because the cardiovascular function depends on the dynamic interaction between the heart and the circulation, several mechanisms can be involved in the pathologic processes of the haemodynamic instability occurring in septic shock. The concept that ventricular-arterial (VA) coupling is one of the main determinant of the cardiovascular function has been increasingly enforced. As demonstrated many times, cardiovascular function depends on the dynamic interaction between the heart and the circulation with the purpose to provide adequate cardiac output (CO) and organ perfusion [4] by sustaining a high enough arterial pressure to allow for blood flow autoregulation. We and others have documented that the occurrence of VA decoupling can be one of the most important mechanism of the haemodynamic failure in septic patients [2].
Defining VA Coupling
VA coupling is the ratio of the arterial elastance (Ea) to the left ventricle (LV) end-systolic elastance (Ees). Ea/Ees expresses the efficiency of the cardiovascular system in providing adequate peripheral perfusion and oxygen delivery to the organs through the dynamic interaction between the heart and the vascular system. Since LV stroke volume in the steady state is both determined and is limited by arterial pressure, the intersection between stoke volume and arterial pressure represents the end-product of VA coupling in a given subject. As previously demonstrated, the cardiovascular function is optimal when the system is coupled, meaning that Ea/Ees is near the unity (Ea/Ees = 1±0.36) with Ea = 2.2±0.8 mmHg/ml and Ees = 2.3±1 mmHg/ml [2, 5]. This occurs when the continuous modulation of the LV performance to the arterial load provides adequate cardiac output, proper perfusion pressure, and flow distribution to the peripheral organs. In addition, Ea/Ees defines one of the primary determinants of LV energetics, in that mechanical energy needed by the LV to transfer the stoke volume to the arterial system is optimal. This occurs when Ea and Ees are equal each other. When the dynamic interaction between the heart and the vascular system fails, as seen in different acute and chronic pathologic conditions, changes in Ea and Ees occur and the system becomes uncoupled (Ea/Ees >1) [2, 5].
VA decoupling can lead to severe cardiovascular dysfunction and haemodynamic impairment leading on circulatory failure, cardiac dysfunction or both. Recent studies demonstrated that in sepsis, despite hypotension, VA decoupling commonly occurs. The concept that VA coupling plays a key role in the cardiovascular function in not new, but our ability to assess in at the bedside is reality new. VA decoupling plays a major role in defining altered haemodynamic states and response to therapy.
VA decoupling in septic shock
Septic shock is characterized as an acute cardiovascular dysfunction where circulatory and cardiac failure often coexist leading to severe haemodynamic impairment [2, 5]. Systemic arterial hypotension, vascular dilatation and loss of the vascular tone often despite a relatively high or preserved CO are responsible for low peripheral perfusion, inadequate tissue oxygen delivery with consequent metabolic disturbances and multi-organ dysfunction. For these reasons, the current Surviving Sepsis Guidelines recommend an early treatment aimed at restoring the circulation in order to create an adequate CO and mean arterial pressure to provide tissue perfusion [6]. Despite prompt initial fluid resuscitation, many septic patients remain hypotensive with impaired organ perfusion, defining them as in septic shock. Not surprising because the haemodynamic instability occurring in septic shock may occur from different pathophysiological mechanisms and in patients with varying degrees of cardiovascular reserve, the initial response to fluid resuscitation is quite variable across patients. We hypothesized that VA decoupling was a main determinant of these response differences across septic patient populations. Thus, treatments aimed at restoring VA coupling may improve cardiovascular state independent of their effect of either CO or arterial pressure [2, 3].
Both Ea and Ees may change in septic shock. Myocardial depression impairing both diastolic compliance and contractility are well described. Pathological vasodilation, referred to as vasoplegia, often is a hall mark of septic shock. Presumably this is due to primary alterations in signally between the vascular endothelium and smooth muscle cells because endogenous catecholamine levels are usually high. Both adrenergic receptor down regulation. Smooth muscle cell hyperpolarization and loss of endogenous vasopressin have bene implemented in the pathophysiological process of septic vasoplegia. However, the most common hemodynamic profile of septic shock in treated critically ill patients is an increase in Ea, usually consequently to the pharmacological vasoconstriction induced by the infusion of exogenous vasoactive drugs, and an associated decrease in Ees, due to septic cardiomyopathy. Although in the majority of the septic shock patients Ea/Ees is >1 and the system is uncoupled, some patients show a normal Ea/Ees because of either an appropriate therapeutic approach restored this coupling or the presence of a normal cardiac function despite sepsis [2]. In this context, the assessment of VA coupling in septic shock is useful not only to evaluate the underlying pathophysiology of the haemodynamic failure, but to also predict the response therapy and to assess the effectiveness of the therapeutic strategies once given.
How to assess VA Coupling
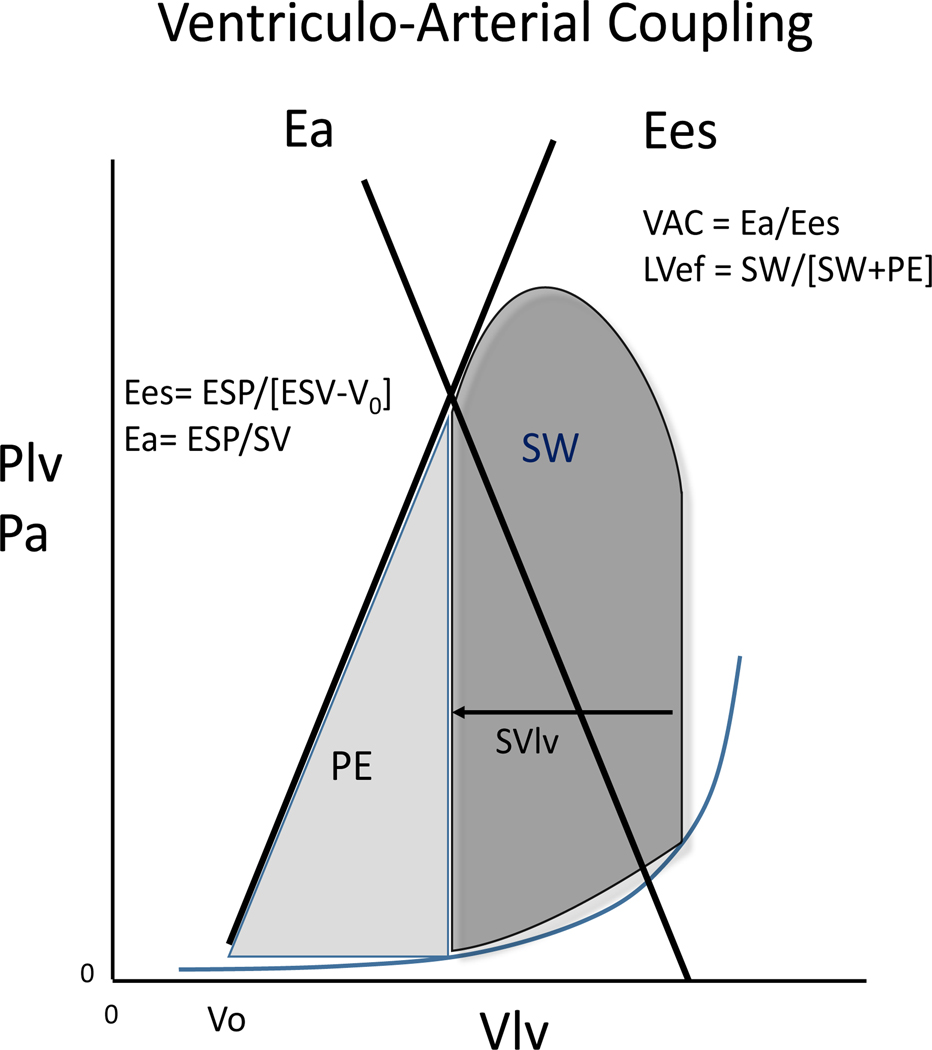
To assess VAC in the critically ill patients, requires using diagnostic tools that can be brought to the bedside and then used to measure function repeatedly if necessary. Since VA coupling is defined by the ratio of Ea to Ees, the tools need to be able to measure both Ea and Ees. Figure one describes the relation between arterial pressure and LV performance of one heart beat using the LV pressure-volume relationship as its basic structure.
Ea is the net afterload imposed to the LV at end-ejection. Ejecting LV stroke volume into the central aorta increases arterial pressure primarily as a function of the rate of ejection, central aortic compliance, mean vascular impedance and initial diastolic arterial pressure of for that beat. Ea can be defined as the capability of the arterial system to increase pressure when stroke volume (SV) increases; Ea is the slope of the line running from the LV end-diastolic volume (LVEDV) to the LV end-systolic pressure (LVESP) end-systolic volume point on the LV pressure/volume (PV) loop [Fig. 1]. Ees is the slope of the end-systolic pressure-volume relationship (ESPVR) created by a series of LV beats as stroke volume is varied by rapidly changing LVEDV. LV Ees is a load independent index of LV contractile function exploring both the intrinsic myocardial contractility and the LV inotropic efficiency. Actually, although the intrinsic myocardial contractility is the main determinant of the LV function, other parameters such as global myocardial contraction synchrony, LV geometry and the biochemical properties of the cardiac cells contribute to the global ventricular performance. For purposes of this illustration we drew an ESPVR line without changing preload for Figure 1. However, below we describe how this can be indirectly measured using the single beat approach.
Fig 1. Ventriculo-arterial coupling.
Left Ventricle elastance (Ees) is the slope of the line running from End Systolic Pressure to V0 on the pressure/volume loop (blue line); Arterial elastance (Ea) is the slope of the line running from the Left Ventricle End-Diastolic Volume to the LV End Systolic Pressure (LVESP) on the pressure/volume loop (red line).
Plv: left ventricular pressure; Vlv: left ventricular volume; ESP: end-systolic pressure; SV: stroke volume; SW: stroke work; PE: potential energy; LVef: left ventricle ejection fraction.
Source: adapted from Guarracino et al. Critical Care (2019) 23:118. https://doi.org/10.1186/s13054-019-2414-9
VA coupling, or Ea/Ees, is calculated by the pressure/volume loop analysis as was first demonstrated by Suga and Sugawa and later Suganawa et al. [7, 8]. Because this method requires invasive ventricular catheterization, several non-invasive approaches to the assessment of the VA coupling have been explored. Among the several proposed non-invasive methods, the modified single-beat method proposed by Chen et al. was the first to be validated against the invasive measurement of the VA coupling [9, 10] and remains the most accurate method available. During contraction, LV elastance (or stiffness) increases progressively until end-systole independent of the loading conditions. Chen et al. assumed that Ees can be estimated through the analysis of the LV PV loop in a single beat. Echocardiographic investigation of the LV end diastolic and end systolic areas allows the estimation of the Ees at a single beat through the measurement of the LV ejection fraction (LVEF), SV, pre-ejection time and systolic time interval when coupled with systolic and diastolic arterial pressure measurements. The Chen et al. method is based on the assumption that the end-systolic PV relation is linear over the range of measured values and that a constant minimal LV volume at zero end-systolic pressure (V0) exists as end-systolic pressure varies [9, 11]. With the increasing use of bedside echocardiography in critical care, several single-beat methods are available for the measurement of Ees [11] because all the elements needed in its calculation can be made from the ECG, arterial pressure and routine echocardiographic recordings. Although the Chen method remains the clinical reference non-invasive method, Ees estimation can be simplified as in some other single-beat methods. Ees can be calculated from the ratio of the end-systolic pressure (ESP) to end-systolic volume (ESV). But this assumes that V0 equals 0 mL, which is often not the case. In a recent retrospective, single-center study on 86 consecutive critically ill patients admitted in intensive care units (ICUs), two non-invasive methods that estimated Ees from the LV ESP/EVP was compared to the Chen et al. method [11]. Ees was calculated as 0.9 × systolic arterial pressure (SAP)/ESV in one method and as Ea/(1/LVEF) - 1 in the other method. Ea was calculated as 0.9 × SAP/SV (mmHg ml−1). Although the recent guidelines [6] support either the Chen method either the ESP/ESV-based methods to evaluate VAC in critical care, the authors conclude that the ESP/ESV-based methods cannot substitute the Chen et al. method both for the assessment and for the evaluation of the changes induced by the therapeutic intervention of VAC.
In the recent years we have released a mobile application (iElastance) [3] suited for the bedside calculation of VA coupling using the Chen et al. method. The software employs echocardiographic measures (SV, Ejection Fraction, Total Ejection Time and Pre Ejection Time) and haemodynamic parameters (blood diastolic and systolic pressure) to calculate Ea, Ees and VA coupling. The application is easy to use and, even if it cannot substitute the clinical evaluation of the collected data, it is helpful, especially in the critically ill patients where the rapidity of the clinical assessment of VAC is extremely helpful in both diagnosis and therapeutic intervention. This is particularly true in the sepsis scenario, where a rapid assessment and prompt treatment are required.
Existing evidence on the role of VA Coupling in sepsis
The bedside, non-invasive echocardiographic measurement of VA coupling adds insight into the pathophysiology of the haemodynamic impairment in septic shock. In addition to being an advanced, dynamic haemodynamic monitoring tool, the non-invasive, bedside transthoracic echocardiographic approach to evaluate Ea and Ees and, therefore, Ea/Ees has allowed a better comprehension of the underlying pathophysiological mechanisms of the haemodynamic instability in sepsis. Guarracino et al. (2) measured Ees and Ea in critically ill septic patients using the method of Chen et al. to assess Ees. Ees was calculated by using the single beat method proposed by Chen et al. Ea was calculated as 0.9 × (systolic arterial pressure/SV), and the Ea/Ees ratio has been then calculated. In the initial study they measured VA coupling in septic patients after admission to the ICU and compared the estimated VA coupling of septic patients to other critically ill non-septic patients. They found that most septic patients had an Ea/Ees >1.2 whereas only one in 20 non-septic patients had similar levels of decoupling.
In a follow on study Guarracino et al. recruited septic shock patients prior to initial resuscitation and measured Ea, Ees and a variety of other dynamic hemodynamic parameters sequentially during the initial course of sepsis resuscitation, based on the Surviving Sepsis Guidelines. Those guidelines treat all patients using a common protocol of 30 ml∙kg−1 crystalloids, followed by norepinephrine (NE), if still hypotensive, and dobutamine if still unstable on norepinephrine. Although these current guidelines focus on the early resuscitation in order to restore haemodynamic stability providing an adequate CO and a sufficient mean arterial pressure to provide tissue perfusion, less is known about the treatment of the septic patients who are not responsive to the volume expansion. Since multifactorial pathophysiologic mechanisms underlying the haemodynamic instability occur in septic shock, using a common approach, even in the initial resuscitation period may not be as effective as one guided by known pathophysiologic state and the degree of volume responsiveness with respect to the conventional functional haemodynamic monitoring [12]. Not surprisingly, many studies have documented a wide variability in the cardiovascular response to the volume expansion in septic shock patients. Guarracino et al. hypothesized that the pretreatment cardiovascular state (reserve, functionality) would accurately predict subsequent response to protocolized therapy [3**]. They submitted 55 septic shock patients to advanced haemodynamic monitoring and bedside echocardiographic assessment of Ea, Ees and the associated cardiovascular derived dynamic parameters, like pulse pressure variation (PPV), stroke volume variation (SVV) and dynamic arterial elastance (Eadyn), in order to achieve a deeper understanding of the underlying mechanisms of the haemodynamic instability and to investigate the further response to the recommended therapy. Ees was calculated by the method of Chen et al. and Ea calculated as 0.9 × (systolic arterial pressure/SV). To get a further understanding of the role VA coupling would have on myocardial energetics they also calculated LV efficiency estimated as the ratio of external work to total cardiac work during cardiac cycle, one of the main determinants of the cardiac performance, as shown in Figure 1. Impressively, VA coupling was tightly correlated to LV efficiency, such that inefficiency was associated with VA uncoupling. The results of the study confirmed the wide variability of the cardiovascular system response to the treatment in the septic shock patients according to the Surviving Sepsis campaign recommendation. The majority of the septic patients increased their CO in response to the initial 30 ml∙kg−1 fluid bolus and also increased their mean arterial pressure (MAP) in proportion to the pre-resuscitation pulse pressure variation value, confirming the predictive value of the baseline PPV and SVV, as previously [13]. In these patients, the restoration of the circulating volume was sufficient to increase cardiac output and, although Ees is a load-independent determinant of LV contractility, both Ees and VAC improved. The reason why Ees improved is unclear but probably due to reversing hypotension, increasing coronary perfusion pressure. Furthermore, the assessment of pretreatment VAC was correlated with the patients’ response to the use of NE in those patients who remained hypotensive after volume expansion. Regrettably, the increase in Ea induced by the NE administration lead to a restoration of VA uncoupling [14]. While, patients with high Ees and normal VA coupling tolerated the increase in LV afterload induced by NE infusion resulting in higher cardiac output [15]. The infusion of dobutamine induced an increase in cardiac output and improved VA coupling, with an effect on MAP emphasizing the role of inotropic support in septic shock patients. The conclusion was that the cardiovascular function and reserve of the critically septic shock patients examined prior to treatment can guide an individualized management of volume expansion in order to predict the response to the fluid resuscitation and the therapeutic approach to septic shock. VA decoupling occurring in septic shock can depend on both Ea and Ees changes, and Guarracino et al. [3] demonstrated that VA decoupling can occur even in patients with normal LVEF, although the majority of the enrolled septic patients showed a low LVEF. Either preexisting cardiac dysfunction or sepsis-induced myocardial depression can affect the hemodynamic course in septic shock [16, 17].
Commonly, LVEF is used as an index of LV systolic function and, indirectly, of myocardial contractility. An index of intrinsic LV performance should depend on myocardial changes in contractility without being influenced by changes in loading conditions. Regrettably, LVEF depends on not only myocardial contractility but also on other determinants of LV function, such as loading conditions, limiting its effectiveness on expressing global LV performance [18–21]. The dependence of LVEF on loading changes, especially afterload variation, has been well documented in septic shock patients [17]. In a recent paper, Guarracino et al. remarked that in septic shock patients LVEF cannot be considered as an index of global cardiac performance but mostly as an index of cardiovascular performance [21]. Our conclusion was that the main determinants of LVEF are Ees and Ea and that LVEF depends more on VA coupling than on vascular loading changes and heart rate. According to the result of the study, LVEF is an index of cardiovascular performance rather than solely of LV contractility.
Conclusions
The great variability of the cardiovascular response to the resuscitation strategies in the septic shock patients makes the use of standardized interventional protocols hard to be applied. The better understanding of the complex process responsible for the haemodynamic instability in sepsis can be helpful either for the diagnosis either to predict the cardiovascular response to treatment. There is strong evidence that non-invasive echocardiographic bedside assessment of VA coupling is helpful to evaluate the intrinsic mechanisms of the haemodynamic impairment occurring in human septic shock and to monitor the response to the therapeutic interventions. As the cardiovascular reserve is often impaired in septic shock patients, modifying the response to the therapeutic strategies recommended by the international Surviving Sepsis Guidelines, the more personalized approach to tailoring therapy based on volume responsiveness and VA coupling should be useful to directing an effective and efficient therapeutic approach to resuscitation from severe sepsis. Such a multi-modality approach that links the bedside assessment of Ea and Ees with the available dynamic indexes of fluid responsiveness are helpful to a deeper understanding of pathophysiology, and to guide personalized management of the severe haemodynamic instability of sepsis and septic shock.
Key points:
Changes in either arterial, ventricular elastance or both can occur in sepsis and determine the cardiovascular profile.
Both arterial and ventricular elastances and their ratio (ventriculo-arterial coupling) can be assessed non-invasively at the bedside.
The assessment of ventriculo-arterial coupling allows predicting the response to treatments in addition to tailoring the resuscitation by timely administering volume and/or vasoactive drugs.
Acknowledgements:
Thanks to Rubia Baldassarri, MD for her valuable input and review.
Footnotes
Conflicts of interest
None
References and recommended reading:
- 1. Singer M, Deutschman CS, Seymour CW. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:801–10. **The task force has defined sepsis as the association between infection and organ dysfunction, emphasizing the role of the uncontrolled host’s response to infection as the main pathologic mechanism of organ dysfunction. According to the new definition of sepsis, the Systemic Inflammatory Response Syndrome (SIRS) can be considered a physiologic response to infection rather than a feature of sepsis. Therefore, this consensus has changed the approach to sepsis based on a better knowledge of the intrinsic pathogenic mechanisms of the organ dysfunction
- 2. Guarracino F, Ferro B, Morelli A, et al. Ventriculo-arterial decoupling in human septic shock. Crit Care 2014;18(2): R80. *In this study the authors reported that the majority of septic shock patients show ventriculo-arterial decoupling and highlighted the importance of ventriculo-arterial coupling in the pathogenesis of septic hemodynamic derangement.
- 3. Guarracino F, Bertini P, Pinsky MR. Cardiovascular determinants of resuscitation from sepsis and septic shock. Crit Care 2019. Apr 15;23(1):118. **In this study, the authors demonstrate that the multifactorial pathophysiologic mechanisms underlying the haemodynamic instability occurring in septic shock require a different approach with respect to the conventional functional haemodynamic monitoring. A multimodality approach that links the bedside assessment of Ea and Ees with the available dynamic indexes of fluid responsiveness can be helpful to a deeper understanding of pathophysiology, and to guide the management of the severe haemodynamic instability of sepsis and septic shock.
- 4.Guarracino F, Baldassarri R, Pinsky MR: Ventriculo-arterial decoupling in acutely altered hemodynamic states. Crit Care 2013, 17:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chantler PD, Lakatta EG, Najjar SS: Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol 2008, 105:1342–51. [A published erratum appears in J Appl Physiol 2009, 106:1027.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017, 43(3):304–77. [DOI] [PubMed] [Google Scholar]
- 7.Sunagawa K, Maughan WL, Sagawa K: Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ Res 1985, 56:586–95. [DOI] [PubMed] [Google Scholar]
- 8.Sunagawa K, Maughan WL, Burkhoff D: Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol 1983, 245:H773–80. [DOI] [PubMed] [Google Scholar]
- 9. Chen CH, Fetics B, Nevo E, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol 2001, 38:2028–34. **In this study Chen and colleagues validated the single-beat method assessment of left ventricular end-systolic elastance. This method has allowed the bedside, non-invasive assessment of the ventriculo-arterial coupling.
- 10.Takeuchi M, Igarashi Y, Tomimoto S. Single-beat estimation of the slope of the end-systolic pressure-volume relation in the human left ventricle. Circulation 1991, 83:202–12. [DOI] [PubMed] [Google Scholar]
- 11. Nguyen M, Berhoud V, Bartamian L. Agreement between different non-invasive methods of ventricular elastance assessment for the monitoring of ventricular-arterial coupling in intensive care. J Clin Monit Comput 2019. Oct 10. doi: 10.1007/s10877-019-00397-7. *This study highlights the superiority of the Chen et al.’s method for single-beat determination of ventriculo-arterial coupling in critically ill patients.
- 12.Guarracino F, Bertini P, Pinsky MR. Novel applications of bedside monitoring to plumb patient hemodynamic state and response to therapy. Minerva Anestesiol 2018, 84(7):858–64. [DOI] [PubMed] [Google Scholar]
- 13.Monge Garcia MI, Gil Cano A, Gracia Romero M. Dynamic arterial elastance to predict arterial pressure response to volume loading in preload dependent patients. Crit Care 2011, 15:R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monge-Garcia MI, Jian Z, Settles JJ, et al. Performance comparison of ventricular and arterial dP/dtmax for assessing left ventricular systolic function during different experimental loading and contractile conditions. Crit Care 2018, 22(1): 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guinot PG, Longrois D, Kamel S. Ventriculo-arterial coupling analysis predicts the hemodynamic response to norepinephrine in hypotensive postoperative patients: a prospective observational study. Crit Care Med 2018, 46:e17–25. [DOI] [PubMed] [Google Scholar]
- 16.Boissier F, Razazi K, Seemann A. Left ventricular systolic dysfunction during septic shock: the role of loading conditions. Intensive Care Med 2017, 43(5):633–42. [DOI] [PubMed] [Google Scholar]
- 17.Repesse X, Charron C, Vieillard-Baron A. Evaluation of left ventricular systolic function revisited in septic shock. Crit Care 2013, 17(4):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konstam MA, Abboud FM. Ejection fraction: misunderstood and overrated (changing the paradigm in categorizing heart failure). Circulation 2017, 135(8):717–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cikes M, Solomon SD. Beyond ejection fraction: an integrative approach for assessment of cardiac structure and function in heart failure. Eur Heart J 2016, 37(21):1642–50. [DOI] [PubMed] [Google Scholar]
- 20.Cecconi M, De Backer D, Antonelli M, et al. Consensus on Circulatory Shock and Hemodynamic Monitoring, Task Force of the European Society of Intensive Care Medicine, Intensive Care Med 2014, 40(12):1795–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Monge Garcia MI, Jian Z, Settles JJ, et al. Determinants of left ventricular ejection fraction and a novel method to improve its assessment of myocardial contractility. Ann Crit Care 2019, 9(1):48. **In this paper the authors have both revisited the role of the LVEF as an index of LV contractility and emphasized the tight connection between LVEF and ventriculo-arterial coupling.