Abstract
Background: Accurately selecting hypertensive candidates for renal denervation (RDN) therapy is required, as one-third of patients who undergo RDN are non-responders. We aimed to systematically review the literature on RDN response prediction using arterial stiffness assessment, optimizing the selection of patients referred for interventional blood pressure lowering procedures. Methods: A literature search was performed in MEDLINE, Embase, Scopus, and Cochrane databases to retrieve potential eligible studies from the inception to 30 June 2022. Results: Ten studies were finally included in this systematic review. Studies consistently documented that invasive pulse wave velocity (PWV) was correlated with RDN’s significant success. Nevertheless, non-invasive ambulatory arterial stiffness index and PWV derived from ambulatory blood pressure monitoring were independent predictors of blood pressure response (p = 0.04 and p < 0.0001). In some studies, magnetic resonance imaging parameters of arterial stiffness (ascending aortic distensibility, total arterial compliance) were correlated with blood pressure reduction (AUC = 0.828, p = 0.006). Conclusions: Assessing arterial stiffness prior to RDN predicted procedural success, since stiffness parameters were strongly correlated with a significant blood pressure response. Our endeavor should be tackled as a step forward in selecting appropriate hypertensive patients scheduled for RDN therapy. Non-invasive measurements could be an alternative to invasive parameters for response prediction.
Keywords: resistant arterial hypertension, renal denervation, responders, non-responders, prediction
1. Introduction
Arterial hypertension (AHT) exerts a significant burden on public health, as it constitutes one of the most critical risk factors for cardiovascular disease (CVD) and death globally [1]. Almost one-third of the population from low- and middle-income countries is diagnosed with AHT, with a slightly lower proportion in high-income countries (28.5%). Moreover, the control rate of hypertension is still low (47.3 ± 1.17%), despite current therapeutic strategies [1,2].
According to the latest European and American guidelines, resistant AHT is defined as persistent increased blood pressure despite optimal drug therapy with at least three antihypertensives (including a diuretic) [3,4]. Although the prevalence of resistant hypertension is lower (15% of subjects treated for AHT), the risk of adverse cardiovascular events is 50% higher than in patients with controlled AHT [5]. A 2018 meta-analysis with a large population sample (n = 3,207,911) reported a 10.3% prevalence of true-resistant hypertension, which was even higher in the presence of chronic kidney disease (CKD, 22.9%) or older age (12.3%) [6].
Clinical trials consistently documented an increased risk of cardiovascular events in patients with resistant AHT. During a follow-up of 3.8 years, these patients expressed an increased risk of death, myocardial infarction, heart failure, stroke, and CKD (HR 1.47, 95% CI, 1.33–1.62, p < 0.001). Compared to patients with controlled AHT, those with resistant AHT exerted a 2.5-fold higher risk of adverse cardiovascular events [7]. Moreover, cardiovascular mortality was increased by 47% compared to participants with non-resistant AHT. Furthermore, all-cause mortality increased by 33% in patients with uncontrolled AHT. Therefore, appropriate treatment of resistant cases is mandatory to improve long-term outcomes [8].
Besides drug therapy, interventional procedures are available for lowering blood pressure. Previous European guidelines on hypertension management recommended renal denervation intervention (RDN) in patients refractory to drug therapy (class IIb recommendation, level of evidence C) [9]. However, the 2018 guidelines did not advocate for the routine use of device-based therapies [3]. Data regarding RDN efficacy are discrepant in the literature. One of the key studies in the field is the SIMPLICITY HTN-3 trial (randomized, single-blind, sham-controlled trial). The authors did not observe any differences in blood pressure reduction between the groups at six months of follow-up (p = 0.98), with similar safety profiles [10]. Using first-generation RDN systems could represent a potential explanation for SIMPLICITY HTN-3 trial neutral results. New-generation RDN systems appear to be consistently efficient and safe for blood pressure reduction [11,12]. A recent meta-analysis of nine randomized sham-controlled trials reported contrasting yet noteworthy results [13]. RDN reduced not only 24 h ambulatory systolic blood pressure (SBP, p < 0.001) but also daytime SBP (p < 0.001), nighttime SBP (p = 0.006), and office SBP (p < 0.001) [13].
The matter might not reside exclusively in trying to confirm the superiority of RDN versus standard drug therapy but in identifying the appropriate subgroup of patients that would benefit from the interventional procedure. This argument is also the main reason for our current paper. The (so-called) markers of RDN response could prove efficient in selecting patients. Higher baseline blood pressure, larger renal artery diameter, and higher baseline office heart rate were associated with a significant blood pressure reduction following RDN [14,15]. Additionally, few trials advocated arterial stiffness as a possible predictor of RDN [16].
The concept of arterial stiffness refers to the arteries’ elasticity, distensibility, and compliance proprieties [17,18]. The balance between arterial wall elastin and collagen constitutes a major determinant of arterial stiffness. Arterial stiffness increases once the balance is disrupted due to elastin degeneration or collagen accumulation [17]. The contribution of arterial rigidity might prevail in AHT patients with increased arterial stiffness. Thus, RDN could fail to reduce blood pressure in this subset of patients [16].
Pulse wave velocity (PWV) is a strong marker of arterial stiffness (though the terms are not synonymous), being related to arterial wall distensibility [17]. Moreover, carotid-femoral PWV is a guidelines’ recommendation class I, level of evidence A for arterial stiffness evaluation [19]. Thereby, it should be explored if arterial stiffness could help to identify patients who are likely or unlikely to respond to RDN therapy.
Therefore, we aimed to systematically review the literature on RDN response prediction using arterial stiffness to optimize the selection of patients referred for interventional blood-pressure-lowering procedures.
2. Materials and Methods
For standardized reporting, the present systematic review was conducted according to the updated Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [20]. The study protocol was registered in the PROSPERO database (CRD42022348207).
2.1. Data Sources and Search Strategy
A comprehensive literature search was performed in MEDLINE (PubMed), Embase, Scopus, and Cochrane databases to retrieve potential eligible studies from the inception to 30 June 2022. No language restrictions or filters were applied in the search process. We also screened references from cited articles, the Google Scholar search engine, and the ClinicalTrials.gov database of clinical trials, as endorsed by the PRISMA checklist. Combinations between the following keywords and MeSH terms (for MEDLINE database) or Emtree terms (for Embase database) were used to build a search strategy: “arterial hypertension”, “resistant hypertension”, “uncontrolled hypertension”, “high blood pressure”, “renal sympathetic denervation”, “renal denervation”, “response”, “responders”, “prediction”, “arterial stiffness”, and “pulse wave velocity”. The search strategy for each database and the retrieved studies were reported in Table S1.
2.2. Eligibility Criteria and Outcomes
Two independent investigators carried out the eligibility assessment of retrieved studies based on pre-established inclusion and exclusion criteria. Studies were considered for inclusion in the present systematic review if they fulfilled the following inclusion criteria: (1) observational studies or randomized clinical trials; (2) participants aged ≥18 years with AHT who underwent RDN were enrolled; (3) arterial stiffness was appraised invasively or non-invasively prior to RDN procedure; and (4) original data were reported concerning the association between arterial stiffness and response to RDN during follow-up (decreased 24 h blood pressure, SBP and diastolic blood pressure—DBP). In addition, critical exclusion criteria were set to guide the eligibility assessment: unpublished data, studies with overlapping populations, editorials, meta-analysis, case reports, and missing data or inability to extract data.
2.3. Data Collection and Synthesis
The following data were extracted from eligible studies that met the inclusion criteria: first author, publication year, population sample size, age of enrolled participants, clinical setting, methods of arterial stiffness measurement, reported outcomes, and follow-up period. Moreover, essential inclusion and exclusion criteria of individual studies that could affect outcome interpretation were extracted and critically analyzed. Data were presented as median or mean values, the area under the curve (AUC), odds ratio (OR), and p-values whenever available.
2.4. Quality Assessment
The quality of included studies was assessed according to their design. The risk of bias in randomized clinical trials was appraised using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) [21]. In the case of observational non-randomized studies, the Newcastle–Ottawa scale (NOS) was applied to judge the overall quality of the studies. It consists of several essential signaling questions, addressing three domains (population sample selection, comparability of groups, and outcomes evaluated) [22].
3. Results
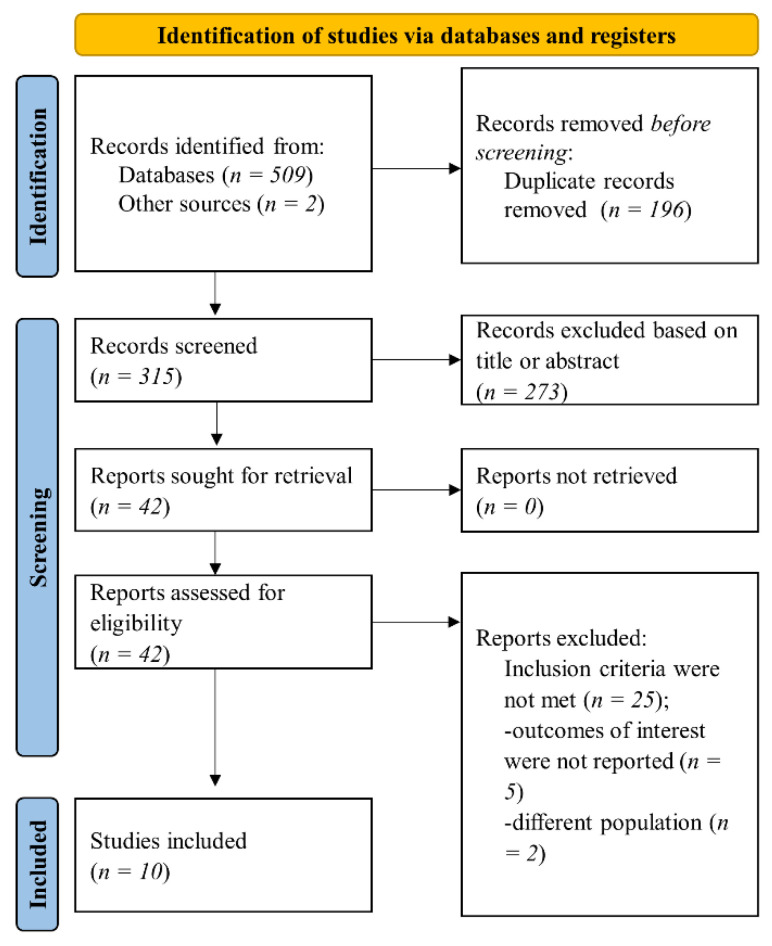
Our search in pre-specified databases and sources retrieved 511 references. Afterward, duplicate records were excluded. The remaining 315 articles were initially screened for eligibility based on title or abstract, and 273 records were excluded. In the next step, 42 records were assessed in full text for inclusion and exclusion criteria. Finally, 10 studies that fulfilled the inclusion criteria were included in the present systematic review (Figure 1).
Figure 1.
Flow diagram of selected studies in the present analysis.
General data from analyzed studies, including population sample size, age of enrolled patients, clinical setting, outcomes, and follow-up duration, are provided in Table 1. Moreover, arterial stiffness measurement methods and RDN response definition used in individual studies (when available) are reported in Table 1. The association between the investigated arterial stiffness parameters (invasive or non-invasive) and the outcomes evaluated in clinical studies is displayed in Table 2.
Table 1.
General characteristics of studies included in the present systematic review.
| First Author, Year | Design | Patients, No | Age, Median/Mean ± SD | Setting | Methods | Outcomes | Follow-Up |
|---|---|---|---|---|---|---|---|
| Ott et al., 2015 [23] | Observational, prospective, single-center | 63 | 56.5 ± 11 (low cPP) | Patients with TRH (office BP ≥ 140/90 mmHg and 24 h ABP ≥ 130/80 mmHg despite treatment with at least 3 AHT drugs, including a diuretic) and eGFR ≥ 15 mL/min/1.73 m2. | Baseline cPP was measured using SphygmoCor. Patients were stratified according to median cPP: low cPP (below 55 mmHg) and high cPP (above 55 mmHg). RDN—radiofrequency technique. |
(a) Office and 24 h systolic and diastolic BP reduction after RDN. (b) Renal function. |
6 months |
| 66.1 ± 8.0 (high cPP) | |||||||
| Okon et al., 2016 [24] | Observational, single-center | 58 | 60.41 ± 10.3 (responders) | Patients with resistant hypertension (24 h ABP: mean daytime systolic BP ≥ 135 mmHg or diastolic BP ≥ 90 mmHg, despite treatment with at least 3 AHT drugs, including a diuretic. Patients with eGFR < 45 mL/min/1.73 m2 were excluded. | PWV was measured invasively. RDN response was defined as reduction with ≥5 mmHg in systolic daytime BP (24 h ABPM). RDN—radiofrequency technique. |
Daytime, night-time, and 24 h BP reduction after RDN. | 6 months |
| 63.1 ± 9.0 (non-responders) | |||||||
| Fengler et al., 2017 [25] | Observational, prospective, single-center | 109 | 60.4 ± 9.0 (combined hypertension) | Patients with resistant hypertension, defined as mean daytime systolic BP > 135 mmHg or diastolic BP > 90 mmHg in ABPM despite treatment with at least 3 AHT drugs, including 1 diuretic unless intolerant. | PWV was measured invasively immediately before renal denervation. Response to RDN was defined as a drop ≥ 5 mmHg in ABPM daytime systolic BP after 3 months. RDN—radiofrequency and ultrasound techniques. |
(a) BP reduction after RDN at 3 months. (b) BP response in relation to PWV tertiles. |
3 months |
| 66.5 ± 9.8 (isolated systolic hypertension) | |||||||
| Fengler et al., 2018 [26] | Observational, single-center, study sub-analysis | 32 | 64.5 ± 9.9 | Patients treated for resistant hypertension, defined as mean daytime systolic ≥135 mmHg or diastolic BP ≥ 90 mmHg in ABPM, despite intake of at least 3 AHT drugs, including a diuretic. Patients with eGFR < 45 mL/min/1.73 m2 were excluded. | Arterial stiffness measured using MRI (ascending aortic distensibility, total arterial compliance, systemic vascular resistance) versus invasive PWV. Response to RDN was defined as a drop ≥5 mmHg in ABPM daytime systolic BP after 3 months. RDN—radiofrequency technique. |
(a) BP reduction after RDN using ABPM. (b) Invasive and non-invasive parameters of arterial stiffness as predictors for the response after RDN. |
3 months |
| Fengler et al., 2022 [16] | Observational, prospective, single-center | 79 | 62.6 ± 8.8 | Patients with resistant hypertension defined as systolic daytime BP > 135 mmHg, despite treatment with 3 or more different classes of AHT drugs, including one diuretic, unless intolerant to diuretics. | Arterial stiffness was measured invasively (PWV) or non-invasively (CMR-derived ascending aortic distensibility, PWV, and total arterial compliance). Response to RDN was defined as a drop ≥ 5 mmHg in ABPM daytime systolic BP after 3 months. RDN—ultrasound and radiofrequency (in validation cohort) techniques. |
(a) Change in systolic daytime BP on ABPM at 3 months in different arterial stiffness subgroups. (b) RDN response predicting power of non-invasive arterial stiffness parameters compared to invasive PWV measurement. |
3 months |
| Fengler et al., 2018 [27] | Observational, retrospective, single-center | 190 | 62.2 ± 9.9 | Patients with TRH defined as office systolic BP > 160 mmHg and 24 h BP > 135/90 mmHg, despite treatment with 3 or more classes of AHT drugs, including one diuretic, unless intolerant to diuretics. | PWV measured invasively and non-invasive pulse pressure. Response to RDN was defined as a drop ≥ 5 mmHg in ABPM daytime average BP after 3 months. The profound response was defined as a drop ≥ 20 mmHg in ABPM daytime average BP. RDN—radiofrequency and ultrasound techniques. |
Change in BP on ABPM, including a profound response, in relation to arterial stiffness. | 3 months |
| Peters et al., 2017 [28] | Substudy of a randomized, sham-controlled, double-blind trial | 53 | 59 ± 9 (sham) | Patients with therapy-resistant hypertension, with daytime ABPM systolic >145 mmHg and 1 month of stable treatment with at least 3 AHT drugs, including a diuretic. Patients with eGFR < 30 mL/min/1.73 m2 were excluded. |
Carotid-femoral PWV was measured non-invasively at baseline and after 6 months (SphygmoCor). RDN—radiofrequency technique. |
Changes in 24 h AMBP and PWV after RDN. | 6 months |
| 54 ± 8 (RDN) | |||||||
| Sata et al., 2018 [29] | Observational, retrospective | 111 | 63.2 ± 10.3 | Patients with resistant hypertension are defined as having office BP > 140/90 mmHg, despite prescribed treatment with three or more AHT drugs. | The ambulatory arterial stiffness index was derived from 24 h ABPM monitoring. Response to RDN was defined as a reduction of 5% in systolic BP on ABPM. RDN—radiofrequency technique. |
(a) Reduction in systolic BP on ABPM after 6 months from RDN. (b) The predictive value of RDN response attributed to ambulatory arterial stiffness index. |
12 months |
| Stoiber et al., 2018 [30] | Observational, prospective, multicenter | 58 | 64.4 ± 9.6 | Resistant hypertension was defined as office systolic BP ≥ 140 mmHg or mean ambulatory 24 h systolic BP > 135 mmHg despite using≥ 3 AHT drugs, including a diuretic. | Aortic distensibility was derived from MRI. Response to RDN was defined as reduction with at least 10 mmHg in systolic BP. RDN—radiofrequency technique. |
(a) Office systolic and diastolic BP at 6 months after RDN in relation to aortic distensibility. (b) Aortic distensibility response to RDN. |
6 months |
| Weber et al., 2022 [31] | A post hoc analysis of a randomized, sham-controlled clinical trial | 222 | 53.0 ± 11.0 (RDN) | Patients with average systolic BP ≥ 140 mmHg and <170 mmHg on 24 h ABPM, office systolic BP ≥ 150 mmHg and <180 mmHg, and office diastolic BP ≥ 90 mmHg. | Augmentation index, augmentation pressure, backward and forward wave amplitude, estimated aortic PWV, measured non-invasively. RDN—radiofrequency technique. |
Predictive value of RDN response in relation to non-invasive arterial stiffness parameters. | 3 months |
| 51.6 ± 11.0 (sham) |
ABPM = ambulatory blood pressure monitoring; AHT = antihypertensive; BP = blood pressure; cPP = central pulse pressure; eGFR = estimated glomerular filtration rate; MRI = magnetic resonance imaging; PWV = pulse wave velocity; RDN = renal denervation; TRH = treatment resistant hypertension.
Table 2.
Results reported in clinical studies included in the present systematic review.
| Study, Year | Parameters | Outcomes | Results | ||
|---|---|---|---|---|---|
| Ott, 2015 [23] | Pre-RDN | Post-RDN | |||
| Low cPP | Office SBP, mmHg | 160 ± 16 | 137 ± 16 | p < 0.001 | |
| Office DBP, mmHg | 95 ± 13 | 82 ± 11 | p < 0.001 | ||
| 24 h SBP, mmHg | 155 ± 15 | 144 ± 15 | p < 0.001 | ||
| 24 h DBP, mmHg | 93 ± 12 | 86 ± 10 | p < 0.001 | ||
| eGFR, mL/min/1.73 m2 | 76.4 ± 21 | 76.0 ± 22 | p = 0.846 | ||
| High cPP | Office SBP, mmHg | 166 ± 20 | 154 ± 26 | p = 0.003 | |
| Office DBP, mmHg | 85 ± 16 | 80 ± 14 | p = 0.049 | ||
| 24 h SBP, mmHg | 157 ± 16 | 154 ± 23 | p = 0.326 | ||
| 24 h DBP, mmHg | 84 ± 11 | 81 ± 12 | p = 0.059 | ||
| eGFR, mL/min/1.73 m2 | 72.1 ± 28 | 70.1 ± 30 | p = 0.243 | ||
| cPP | Office SBP reduction, mmHg | −22 ± 19 in low cPP vs.−12 ± 20 in high cPP | p = 0.038 | ||
| Office DBP reduction, mmHg | −13 ± 11 in low cPP vs.−5 ± 13 in high cPP | p = 0.014 | |||
| 24 h SBP reduction, mmHg | −11 ± 13 in low cPP vs.−3 ± 18 in high cPP | p = 0.07 | |||
| 24 h DBP reduction, mmHg | −8 ± 10 in low cPP vs.−4 ± 10 in high cPP | p = 0.112 | |||
| Okon, 2016 [24] | iPWV | RDN response | OR 1.15 (95% CI, 1.014–1.327) | p = 0.03 | |
| AUC 0.79 (95% CI, 0.658–0.882) | p < 0.0001 | ||||
| 13.7 m/s cut-off: sensitivity 71%, specificity 83%, positive predictive value 85.7% | |||||
| Fengler, 2017 [25] | iPWV | Daytime BP response | Patients with iPWV < 14.4 m/s had a better BP response vs. those with iPWV > 14.4 m/s (11.7 ± 12.7 mmHg vs. 7.2 ± 10.4 mmHg) | p = 0.047 | |
| Patients with isolated systolic hypertension in the lowest iPWV tertile had the best BP response vs. those in the middle iPWV tertile | p = 0.012 | ||||
| Patients with isolated systolic hypertension in the lowest iPWV tertile had the best BP response vs. those in high iPWV tertile | p = 0.013 | ||||
| Responder rate | 77% in low iPWV tertile, 50% in middle iPWV tertile and 23% in high iPWV tertile | p = 0.001 | |||
| BP response | Per 1 m/s of iPWV: OR 0.91, 95% CI, 0.83–0.99) | p = 0.037 | |||
| Fengler, 2018 [30] | iPWV | BP response | Patients with iPWV < 13.6 m/s had better BP response than those with iPWV > 13.6 m/s (−13.0 ± 8.7 mmHg vs. −4.1 ± 5.5 mmHg) | p = 0.002 | |
| AUC 0.849, 95% CI, 0.713–0.985 | p = 0.004 | ||||
| AAD | BP response | Patients with AAD above the median (2.0 × 10−3 mmHg−1) had a better BP response than those with AAD below the median (−11.9 ± 6.9 mmHg vs. −5.6 ± 8.8 mmHg) | p = 0.034 | ||
| AUC 0.828, 95% CI, 0.677–0.979 | p = 0.006 | ||||
| Multivariate analysis: OR 6.8, 95% CI, 1.4–34.2—AAD the only predictor for BP response | p = 0.019 | ||||
| cTAC, TAC | BP response | Patients with cTAC or TAC above the median had a better BP response than those with parameters below the median (−11.6 ± 6.8 mmHg vs. −5.5 ± 9.1 mmHg) | p = 0.041 | ||
| cTAC | BP response | AUC 0.776, 95% CI, 0.563–0.989 | p = 0.021 | ||
| TAC | BP response | AUC 0.753, 95% CI, 0.576–0.929 | p = 0.035 | ||
| Fengler, 2022 [16] | iPWV | Daytime BP reduction | β 0.242, 95% CI, 0.054–0.430 | p = 0.012 | |
| 24 h BP reduction | β = 0.232, 95% CI, 0.046–0.419, AUC 0.695 | p = 0.015 | |||
| AAD | 24 h BP reduction | β = −0.243, 95% CI, −0.428 to −0.058, AUC 0.714 | p = 0.011 | ||
| AAD (logarithmic) | 24 h BP reduction | Β = −0.306, 95% CI, −0.484 to −0.128 | p = 0.001 | ||
| TAC | 24 h BP reduction | β = −0.058 | p = 0.61 | ||
| PWV (MRI) | 24 h BP reduction | β = 0.207 | p = 0.07 | ||
| Carotid-femoral PWV | 24 h BP reduction | β = 0.109 | p = 0.34 | ||
| Fengler, 2018 [27] | iPWV | BP reduction | Lower iPWV was associated with a higher rate of profound BP response (per m/s: OR 0.834, 95% CI, 0.724–0.961) | p = 0.012 | |
| Non-invasive pulse pressure | BP reduction | No differences were observed between no or regular BP response as compared to those with profound BP response | p = 0.16 | ||
| Peters, 2017 [28] | PWV | SBP 24 h response | r2 = 0.002 | p = NS | |
| MAP reduction | r2 = 0.001 | p = NS | |||
| Sata, 2018 [29] | AASI | BP response | Responders had lower AASI compared to non-responders (0.47 ± 0.12 vs. 0.54 ± 0.15) | p = 0.031 | |
| 84% of patients from the highest AASI tertile were non-respondent, compared to 42% in the lowest AASI tertile | |||||
| AASI < 0.51 | BP response | OR 2.62, 95% CI, 1.05–6.79 (univariate analysis) | p = 0.038 | ||
| OR 3.46, 95% CI, 1.0–13.3 (multivariate adjustment) | p = 0.04 | ||||
| AASI < 0.64 | BP response | OR 14.0, 95% CI, 2.57–261.37 | p = 0.001 | ||
| Stoiber, 2018 [30] | Aortic distensibility | SBP reduction | −24.0 ± 26.5 mmHg (low distensibility group) vs. −18.5 ± 16.1 mmHg (high distensibility group) | p = 0.770 | |
| DBP reduction | −8.4 ± 14.7 mmHg (low distensibility group) vs. −6.9 ± 9.6 mmHg (high distensibility group) | p = 0.570 | |||
| Weber, 2022 [31] | Augmentation index | 24 h SBP reduction | −8.4 mmHg in the low augmentation index group vs. −0.6 mmHg in the high augmentation index group | p < 0.001 | |
| AUC 0.70, 95% CI, 0.61–0.79 | p < 0.0001 | ||||
| Augmentation pressure | 24 h SBP reduction | −8.5 mmHg in the low augmentation pressure group vs. −0.5 mmHg in the high augmentation pressure group | p < 0.001 | ||
| AUC 0.74, 95% CI, 0.64–0.82 | p < 0.0001 | ||||
| BWA | 24 h SBP reduction | −7.9 mmHg in low BWA group vs. −1.1 mmHg in high BWA group | p < 0.001 | ||
| AUC 0.70, 95% CI, 0.61–0.79 | p < 0.0001 | ||||
| FWA | 24 h SBP reduction | −7.4 mmHg in low FWA group vs. −1.7 mmHg in high FWA group | p = 0.004 | ||
| AUC 0.65, 95% CI, 0.55–0.74 | p = 0.004 | ||||
| ePWV | 24 h SBP reduction | −8.4 mmHg in low ePWV group vs. −0.6 mmHg in high ePWV group | p < 0.001 | ||
| AUC 0.62, 95% CI, 0.53–0.71 | p = 0.03 | ||||
AAD = ascending aortic distensibility; AASI = ambulatory arterial stiffness index; AUC = area under the curve; BP = blood pressure; BWA = backward wave amplitude; cPP = central pulse pressure; cTAC = central pressure total arterial compliance; DBP = diastolic blood pressure; ePWV = estimated aortic pulse wave velocity; FWA = forward wave amplitude; iPWV = invasive pulse wave velocity; MAP = mean arterial blood pressure; MRI = magnetic resonance imaging; NS = nonsignificant; RDN = renal denervation; SBP = systolic blood pressure; TAC = total arterial compliance.
The majority of included studies had an observational design [16,23,24,25,26,27,29,30], while only two studies were performed as secondary analyses from randomized clinical trials [28,31].
Definitions of variables and methods used were different across studies. Of the included studies, five measured arterial stiffness exclusively by non-invasive methods: PWV, central pulse pressure, ambulatory arterial stiffness index (AASI), and magnetic resonance-derived parameters [23,28,29,30,31]. Definition of blood pressure response to RDN also varied in clinical studies; some used a 5 mmHg cut-off [16,24,25,26], while the others used a 10 mmHg cut-off or 5% decrease to delineate between responders and non-responders [29,30].
Data on non-invasive PWV measurement were available from three studies [16,28,31]. Moreover, five reported data on invasive PWV assessment [16,24,25,26]. Non-invasive PWV was measured using magnetic resonance imaging only in one study [16], while the other two investigated classic non-invasive PWV [28,31].
In one of the two studies investigating classic non-invasive PWV, the authors reported that estimated PWV from ambulatory blood pressure monitoring was independently associated with blood pressure response at multivariate analysis (OR 0.031, 95% CI, 0.006–0.167, p < 0.0001) [31]. Although estimated PWV was independently associated with RDN response, the proportion of responders stratified according to PWV values was not reported. Moreover, estimated PWV, augmentation pressure, backward wave amplitude, and forward wave amplitude had modest predictive power for RDN response, with AUC ranging from 0.62 (95% CI, 0.53–0.71) to 0.74 (95% CI, 0.64–0.82) [31]. However, classic non-invasive PWV measurement was not associated with RDN response at follow-up in the second study. Nevertheless, the small sample size (n = 53) limits the application of the results in all AHT patients [28].
The most recent trial investigated response prediction to RDN using invasive PWV compared to non-invasive markers (ascending aortic distensibility, PWV, and total arterial compliance derived from magnetic resonance imaging) [16]. Invasive PWV and ascending aortic distensibility measured non-invasively were documented as independent predictors of blood pressure response to RDN (p = 0.019 and p = 0.006). However, PWV measured by magnetic resonance imaging was not correlated with blood pressure drop at univariate analysis (p = 0.07) [16].
In terms of cut-off values, invasive PWV < 14.4 m/s was linked to a better blood pressure response than invasive PWV above the established value (p < 0.01) [16]. Notably, the predictive power of ascending aortic distensibility (measured non-invasively) was somewhat better than for invasive PWV (AUC 0.714 and 0.695, respectively). Furthermore, integrating arterial stiffness variables in a bivariate model (logarithmic ascending aortic distensibility and baseline 24 h SBP) or a multivariable model significantly improved the predictive accuracy of blood pressure response to RDN (bivariate model: AUC 0.740; multivariate model: AUC 0.791) [16].
Another study compared non-invasive arterial stiffness measurement using magnetic resonance imaging with invasive PWV [26]. Invasive PWV and ascending aortic distensibility had good predictive power (AUC 0.849 and AUC 0.828). Noteworthy, in multivariate analysis, only ascending aortic distensibility assessed by magnetic resonance imaging was linked to RDN response (OR 6.8, 95% CI, 1.4–34.2, p = 0.019). Other parameters failed to prove significant in multivariate analysis [26]. The authors from the other two studies documented that low PWV measured invasively was associated with blood pressure response to RDN [24,25]. Moreover, a 13.7 m/s cut-off for invasive PWV had 71% sensibility, 83% specificity, and 85.7% positive predictive value for RDN response [24].
Another study investigated the association between invasive PWV or non-invasive pulse pressure with blood pressure drop following RDN [26]. The authors reported a statistically significant association with blood pressure reduction only in the case of invasive PWV at multivariate analysis (OR 0.834, 95% CI, 0.724–0.961, p = 0.012). Pulse pressure measured non-invasively was not associated with blood pressure response (p = 0.16). However, response to RDN was defined as a drop ≥ 20 mmHg in ambulatory daytime average blood pressure, which differed from other analyzed studies. Therefore, the study’s methodology might affect the results and should be considered in case of extrapolation to other patients [26].
Unconvincing results regarding pulse pressure and blood pressure response were obtained in another study [23]. Low central pulse pressure measured non-invasively was associated with a reduction in office blood pressure values compared to high central pulse pressure (p = 0.038 for SBP and p = 0.014 for DBP). Although 24 h blood pressure drop was slightly bigger in subgroup with low central pulse pressure, it did not reach statistical significance (p = 0.07 for 24 h SBP and p = 0.112 for 24 h DBP) [23].
AASI derived from 24 h ambulatory blood pressure monitoring was associated with RDN response, as was documented in one study [29]. AASI lower than 0.51 was linked to blood pressure drop even after adjustment for multiple variables (OR 3.46, 95% CI, 1.0–13.3, p = 0.04). When the AASI cut-off was set at 0.64, it had a 100% sensitivity, 29% specificity, 32% positive predictive value, and 13% negative predictive value for RDN response [29].
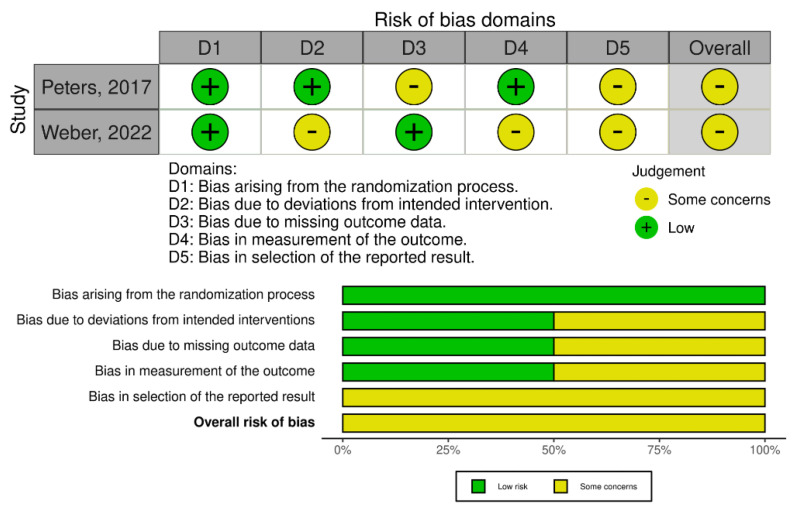
The overall quality of analyzed observational studies was modest to good, as appraised using the NOS scale (Table S2). There were some concerns regarding the risk of bias in the case of randomized trials (post hoc analyses) evaluated by the RoB 2 tool (Figure 2).
Figure 2.
The overall risk of bias assessment using the revised Cochrane risk-of-bias tool [28,31].
4. Discussion
To the best of our knowledge, this systematic review is the first to investigate reported data in the literature on the validity of arterial stiffness for blood pressure response prediction following RDN.
Our endeavor should be perceived as a step forward in selecting those patients with resistant hypertension who most likely will respond to RDN therapy. Moreover, it certainly is a background for future studies and clinical models to increase the discriminatory capacity between responders and non-responders to RDN. Arterial stiffness could improve cardiovascular risk stratification in AHT patients who are candidates for RDN, as it is associated with adverse cardiovascular events and all-cause mortality [32]. In addition, arterial stiffness could be measured early and late after RDN to identify the potential (arterial) ‘destiffening’ with subsequent impact on overall cardiovascular risk [33].
The guidelines describe and endorse several methods to evaluate arterial stiffness [19]. Non-invasive assessment strategies usually include pulsed-wave velocity evaluation and parameters derived from magnetic resonance imaging.
Non-invasive PWV is a ‘marker of arterial stiffness’ referred to as the ‘gold standard’ of arterial stiffness measurement [18]. Notably, carotid-femoral non-invasively PWV received a guidelines’ recommendation class I, level of evidence A for arterial stiffness evaluation. Moreover, PWV could be measured in other arterial places, including ankle-brachial index or cardiac-ankle stiffness index (class I, level of evidence B). Devices and approaches to evaluate PWV are also provided by the guidelines: e.g., devices using a tonometer, oscillometric devices, or those using an ultrasound probe [19].
Magnetic resonance measurement could also acquire non-invasive data regarding blood flow velocity as well as arterial distensibility and compliance, with good technical reproducibility [19]. Moreover, a good correlation between magnetic-resonance-derived PWV and invasive PWV was reported [34]. Data suggested similar values of non-invasive parameters compared to invasive methods [34].
Invasive aortic PWV assessment constitutes an accurate and reproducible tool to evaluate arterial stiffness. Nevertheless, this ‘old-fashioned’ intravascular PWV assessment might not provide additional (and clinically significant) information compared to non-invasive assessment. In addition, invasive PWV evaluation has limited applicability in the general population due to its invasiveness and potential complications [19].
Though randomized clinical trials documented a benefit in blood pressure reduction after RDN, almost one-third of patients who underwent RDN were non-responders [35,36]. In other words, up to a third of patients with hypertension who had renal denervation did not respond to the procedure. In the era of targeted therapies, non-invasive markers of RDN response are particularly interesting. It has been suggested that baseline ambulatory daytime DBP, number of antihypertensive drugs administered, or orthostatic hypertension could predict the response to RDN [36].
However, none of the investigated predictive parameters could accurately identify all patients who would benefit from RDN [36]. New markers were developed and validated to increase the predictive accuracy, such as higher baseline blood pressure, larger renal artery diameter, or higher baseline office heart rate [14,15]. In this regard, arterial stiffness measurement prior to RDN attracted interest in the last decade. Several clinical studies reported that arterial stiffness measurements could improve the selection of patients responding to RDN [23,24]. Patients with lower arterial stiffness parameters were prone to respond, as 10 patients out of 13 were responders, while in the subgroup with increased arterial stiffness, only 3 patients out of 13 responded to RDN. Consequently, measuring arterial stiffness could identify 77% of patients who would probably respond to RDN [25]. In this case, arterial stiffness had a modest to good predictive power, with AUC up to 0.849 [26].
On another note, arterial stiffness could also distinguish that one-third of non-responder patients were unlikely to exhibit a blood-pressure reduction. Most patients with AHT and increased arterial stiffness were non-responders (84%), as highlighted in one study [29]. Consequently, a significant proportion of patients who are unlikely to respond could be spared from worthless complications and exposure to an invasive and radiating procedure. RDN is cost-effective when performed only in a certain subgroup of high-risk patients [37]. Assessment of arterial stiffness prior to RDN could identify 84% of patients who would be unlikely to respond, avoiding a futile invasive procedure [29]. Thus, optimizing the selection of patients for RDN (also by arterial stiffness measurement) improves the cost-efficiency ratio.
As arterial stiffness could be evaluated non-invasively, it represents a feasible marker that could be implemented in clinical practice. Ascending aortic distensibility measured non-invasively by magnetic resonance imaging performed slightly better in predicting RDN response than invasively PWV (AUC 0.714 vs. AUC 0.695). Accordingly, ascending aortic distensibility could accurately distinguish between responders and non-responders in 71% of cases, which was improved by integrating non-invasive markers in different prediction models [16]. In addition, augmentation pressure had similar discrimination power, allowing an accurate response prediction in 74% of patients (p < 0.0001) [31].
In combination with other clinical and paraclinical parameters, arterial stiffness could be included in a multivariate prediction model further to refine the selection of patients [16]. Proposed bivariate/multivariate models significantly improved the ability to discriminate between responders and non-responders (AUC 0.740 and 0.791, respectively). Subsequently, a multivariate model could predict blood pressure response in almost 80% of cases. It seems reasonable to integrate additional response markers rather than perform or preclude RDN based on a single marker approach [16].
A potential explanation for the association between arterial stiffness and RDN response relies on AHT and RDN pathophysiology [16]. RDN influences the neurohormonal component of AHT, including sympathetic nervous system activation [16,38]. However, arterial stiffness increases with age; thus, a biomechanical component of AHT could prevail over sympathetic activity in this subgroup of patients. Moreover, aortic stiffness is usually caused by the destruction of elastin in the aortic wall and substitution with fibrosis. At that stage, it could probably be too late to intervene, as the rigidity of fibrotic arteries perpetuates AHT rather than sympathetic-induced vascular smooth muscle cell contraction [16,17,39,40]. In consequence, the effect of RDN on the biomechanical part of AHT and, subsequently, on blood pressure reduction could be limited in patients with increased arterial stiffness [16].
Nevertheless, non-invasive measurement of arterial stiffness is susceptible to different physiological and methodological confounders, which should be considered when implementing arterial stiffness in clinical practice or research [19]. Mean arterial pressure constitutes a vital confounder, as stated in the American Heart Association (AHA) scientific statement on improving and standardizing vascular research on arterial stiffness [19]. In addition, the increased heart rate could be linked to higher arterial stiffness, especially in patients with increased mean arterial pressure. Moreover, the lack of PWV measurement standardization across different healthcare centers could limit the discrimination power between responders and non-responders to RDN. Therefore, arterial stiffness should be assessed in a standardized context and environment, in line with AHA recommendations [19]. Adopting a protocol for arterial stiffness measurement, especially for the purpose of research, could enhance the reproducibility and robustness of obtained results.
Notably, sodium–glucose co-transporter-2 (SGLT2) inhibitors could also alleviate arterial stiffness parameters and the blood pressure reduction effect [41,42]. Therefore, SGLT2 inhibitors could affect the arterial stiffness measurement prior to RDN. The possibility of influencing outcomes following RDN by SGLT2 inhibitors therapy should be investigated in clinical trials.
Another limitation derives from the RDN techniques performed. RDN could be achieved by applying three distinct ablation types: radiofrequency, ultrasound, and alcohol-mediated ablation. In our systematic review, radiofrequency ablation was used in most clinical studies [23,24,26,28,29,30,31], while only three used radiofrequency and ultrasound techniques [16,25,27]. Moreover, none of the studies explored alcohol-mediated RDN. Thus, caution is required when extrapolating the results across all RDN techniques.
5. Conclusions
Arterial stiffness constitutes a significant part of the solution for selecting appropriate hypertensive patients for renal denervation intervention. Accurately selecting candidates for RDN is required, as almost one-third of patients who undergo RDN are non-responders. The solution provided by assessing arterial stiffness is attractive, as it could be measured non-invasively—a standing alternative to invasive parameters for response prediction. As reported in clinical studies, arterial stiffness parameters were strongly correlated with a greater blood pressure response to RDN. The ability of arterial stiffness to discriminate between responders and non-responders was good in all analyzed studies. Arterial stiffness could be integrated with other (clinical and paraclinical) parameters as part of a multivariate prediction model to refine further the selection of patients who would benefit from RDN. Therefore, this systematic review should be tackled as a step forward in selecting appropriate AHT patients scheduled for RDN therapy. More standardized and robust data is required before introducing arterial stiffness as a major predictor of RDN response, due to heterogeneity in the methodology, RDN techniques, and center experience across studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11164837/s1. Table S1: Databases and search strategy; Table S2: Quality assessment.
Author Contributions
Conceptualization, A.B. and A.C. (Adrian Covic); methodology, A.B., M.F., and C.B.; writing—original draft preparation A.B., C.B., M.F., A.E.S. and A.C. (Andreea Covic); writing—review and editing, A.B., C.B., M.F. and A.C. (Andreea Covic); supervision, A.B. and A.C. (Adrian Covic). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mills K.T., Stefanescu A., He J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020;16:223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim K.-I., Ji E., Choi J.-Y., Kim S.-W., Ahn S., Kim C.-H. Ten-year trends of hypertension treatment and control rate in Korea. Sci. Rep. 2021;11:6966. doi: 10.1038/s41598-021-86199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M., Clement D.L., Coca A., de Simone G., Dominiczak A., et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 4.Unger T., Borghi C., Charchar F., Khan N.A., Poulter N.R., Prabhakaran D., Ramirez A., Schlaich M., Stergiou G.S., Tomaszewski M., et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 5.Pimenta E., Calhoun D.A. Resistant hypertension: Incidence, prevalence, and prognosis. Circulation. 2012;125:1594–1596. doi: 10.1161/CIRCULATIONAHA.112.097345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noubiap J.J., Nansseu J.R., Nyaga U.F., Sime P.S., Francis I., Bigna J.J. Global prevalence of resistant hypertension: A meta-analysis of data from 3.2 million patients. Heart. 2019;105:98–105. doi: 10.1136/heartjnl-2018-313599. [DOI] [PubMed] [Google Scholar]
- 7.Daugherty S.L., Powers J.D., Magid D.J., Tavel H.M., Masoudi F.A., Margolis K.L., O’Connor P.J., Selby J.V., Ho P.M. Incidence and Prognosis of Resistant Hypertension in Hypertensive Patients. Circulation. 2012;125:1635–1642. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaczmarski K.R., Sozio S.M., Chen J., Sang Y., Shafi T. Resistant hypertension and cardiovascular disease mortality in the US: Results from the National Health and Nutrition Examination Survey (NHANES) BMC Nephrol. 2019;20:138. doi: 10.1186/s12882-019-1315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancia G., Fagard R., Narkiewicz K., Redón J., Zanchetti A., Böhm M., Christiaens T., Cifkova R., De Backer G., Dominiczak A., et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J. Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt D.L., Kandzari D.E., O’Neill W.W., D’Agostino R., Flack J.M., Katzen B.T., Leon M.B., Liu M., Mauri L., Negoita M., et al. A Controlled Trial of Renal Denervation for Resistant Hypertension. N. Engl. J. Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 11.Santos-Gallego C., Badimón J. Catheter-based Renal Denervation as a Treatment for Pulmonary Hypertension: Hope or Hype? Rev. Española Cardiol. 2015;68:551–553. doi: 10.1016/j.recesp.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Versaci F., Sciarretta S., Scappaticci M., Calcagno S., di Pietro R., Sbandi F., Dei Giudici A., Del Prete A., de Angelis S., Biondi-Zoccai G. Renal arteries denervation with second generation systems: A remedy for resistant hypertension? Eur. Heart J. Suppl. 2020;22:L160–L165. doi: 10.1093/eurheartj/suaa158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogoyama Y., Tada K., Abe M., Nanto S., Shibata H., Mukoyama M., Kai H., Arima H., Kario K. Effects of renal denervation on blood pressures in patients with hypertension: A systematic review and meta-analysis of randomized sham-controlled trials. Hypertens. Res. 2022;45:210–220. doi: 10.1038/s41440-021-00761-8. [DOI] [PubMed] [Google Scholar]
- 14.Rosa J., Kvasnicka J., Lambert L., Waldauf P., Zelinka T., Petrak O., Strauch B., Holaj R., Indra T., Kratka Z., et al. PREDICTION OF LONG-TERM RENAL DENERVATION EFFICACY. J. Hypertens. 2018;36:e48. doi: 10.1097/01.hjh.0000539091.97172.94. [DOI] [Google Scholar]
- 15.Böhm M., Tsioufis K., Kandzari D.E., Kario K., Weber M.A., Schmieder R.E., Townsend R.R., Kulenthiran S., Ukena C., Pocock S., et al. Effect of Heart Rate on the Outcome of Renal Denervation in Patients with Uncontrolled Hypertension. J. Am. Coll. Cardiol. 2021;78:1028–1038. doi: 10.1016/j.jacc.2021.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Fengler K., Rommel K.P., Kriese W., Kresoja K.P., Blazek S., Obradovic D., Feistritzer H.J., Lücke C., Gutberlet M., Desch S., et al. Assessment of arterial stiffness to predict blood pressure response to renal sympathetic denervation. EuroIntervention: J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2022 doi: 10.4244/eij-d-21-01036. Online ahead of print . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurent S., Boutouyrie P. Arterial Stiffness and Hypertension in the Elderly. Front. Cardiovasc. Med. 2020;7:544302. doi: 10.3389/fcvm.2020.544302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salvi P., Valbusa F., Kearney-Schwartz A., Labat C., Grillo A., Parati G., Benetos A. Non-Invasive Assessment of Arterial Stiffness: Pulse Wave Velocity, Pulse Wave Analysis and Carotid Cross-Sectional Distensibility: Comparison between Methods. J. Clin. Med. 2022;11:2225. doi: 10.3390/jcm11082225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Townsend R.R., Wilkinson I.B., Schiffrin E.L., Avolio A.P., Chirinos J.A., Cockcroft J.R., Heffernan K.S., Lakatta E.G., McEniery C.M., Mitchell G.F., et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.-Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 22.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 23.Ott C., Schmid A., Toennes S.W., Ditting T., Veelken R., Uder M., Schmieder R.E. Central pulse pressure predicts BP reduction after renal denervation in patients with treatment-resistant hypertension. EuroIntervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2015;11:110–116. doi: 10.4244/EIJV11I1A19. [DOI] [PubMed] [Google Scholar]
- 24.Okon T., Röhnert K., Stiermaier T., Rommel K.P., Müller U., Fengler K., Schuler G., Desch S., Lurz P. Invasive aortic pulse wave velocity as a marker for arterial stiffness predicts outcome of renal sympathetic denervation. EuroIntervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2016;12:e684–e692. doi: 10.4244/EIJV12I5A110. [DOI] [PubMed] [Google Scholar]
- 25.Fengler K., Rommel K.P., Hoellriegel R., Blazek S., Besler C., Desch S., Schuler G., Linke A., Lurz P. Pulse Wave Velocity Predicts Response to Renal Denervation in Isolated Systolic Hypertension. J. Am. Heart Assoc. 2017;6:e005879. doi: 10.1161/JAHA.117.005879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fengler K., Rommel K.P., Blazek S., Von Roeder M., Besler C., Lücke C., Gutberlet M., Steeden J., Quail M., Desch S., et al. Cardiac magnetic resonance assessment of central and peripheral vascular function in patients undergoing renal sympathetic denervation as predictor for blood pressure response. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2018;107:945–955. doi: 10.1007/s00392-018-1267-6. [DOI] [PubMed] [Google Scholar]
- 27.Fengler K., Rommel K.P., Blazek S., von Roeder M., Besler C., Hartung P., Desch S., Thiele H., Lurz P. Predictors for profound blood pressure response in patients undergoing renal sympathetic denervation. J. Hypertens. 2018;36:1578–1584. doi: 10.1097/HJH.0000000000001739. [DOI] [PubMed] [Google Scholar]
- 28.Peters C.D., Mathiassen O.N., Vase H., Bech Nørgaard J., Christensen K.L., Schroeder A.P., Rickers H., Opstrup U.K., Poulsen P.L., Langfeldt S., et al. The effect of renal denervation on arterial stiffness, central blood pressure and heart rate variability in treatment resistant essential hypertension: A substudy of a randomized sham-controlled double-blinded trial (the ReSET trial) Blood Press. 2017;26:366–380. doi: 10.1080/08037051.2017.1368368. [DOI] [PubMed] [Google Scholar]
- 29.Sata Y., Hering D., Head G.A., Walton A.S., Peter K., Marusic P., Duval J., Lee R., Hammond L.J., Lambert E.A., et al. Ambulatory arterial stiffness index as a predictor of blood pressure response to renal denervation. J. Hypertens. 2018;36:1414–1422. doi: 10.1097/HJH.0000000000001682. [DOI] [PubMed] [Google Scholar]
- 30.Stoiber L., Mahfoud F., Zamani S.M., Lapinskas T., Böhm M., Ewen S., Kulenthiran S., Schlaich M.P., Esler M.D., Hammer T., et al. Renal sympathetic denervation restores aortic distensibility in patients with resistant hypertension: Data from a multi-center trial. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2018;107:642–652. doi: 10.1007/s00392-018-1229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber T., Wassertheurer S., Mayer C.C., Hametner B., Danninger K., Townsend R.R., Mahfoud F., Kario K., Fahy M., DeBruin V., et al. Twenty-Four-Hour Pulsatile Hemodynamics Predict Brachial Blood Pressure Response to Renal Denervation in the SPYRAL HTN-OFF MED Trial. Hypertension. 2022;79:1506–1514. doi: 10.1161/HYPERTENSIONAHA.121.18641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonarjee V.V.S. Arterial Stiffness: A Prognostic Marker in Coronary Heart Disease. Available Methods and Clinical Application. Front. Cardiovasc. Med. 2018;5:64. doi: 10.3389/fcvm.2018.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berukstis A., Navickas R., Neverauskaite-Piliponiene G., Ryliskyte L., Misiura J., Vajauskas D., Misonis N., Laucevicius A. Arterial Destiffening Starts Early after Renal Artery Denervation. Int. J. Hypertens. 2019;2019:3845690. doi: 10.1155/2019/3845690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grotenhuis H.B., Westenberg J.J.M., Steendijk P., van der Geest R.J., Ottenkamp J., Bax J.J., Jukema J.W., de Roos A. Validation and reproducibility of aortic pulse wave velocity as assessed with velocity-encoded MRI. J. Magn. Reson. Imaging. 2009;30:521–526. doi: 10.1002/jmri.21886. [DOI] [PubMed] [Google Scholar]
- 35.Azizi M., Schmieder R.E., Mahfoud F., Weber M.A., Daemen J., Davies J., Basile J., Kirtane A.J., Wang Y., Lobo M.D., et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): A multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–2345. doi: 10.1016/S0140-6736(18)31082-1. [DOI] [PubMed] [Google Scholar]
- 36.Saxena M., Schmieder R.E., Kirtane A.J., Mahfoud F., Daemen J., Basile J., Lurz P., Gosse P., Sanghvi K., Fisher N.D.L., et al. Predictors of blood pressure response to ultrasound renal denervation in the RADIANCE-HTN SOLO study. J. Hum. Hypertens. 2022;36:629–639. doi: 10.1038/s41371-021-00547-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chowdhury E.K., Reid C.M., Zomer E., Kelly D.J., Liew D. Cost-Effectiveness of Renal Denervation Therapy for Treatment-Resistant Hypertension: A Best Case Scenario. Am. J. Hypertens. 2018;31:1156–1163. doi: 10.1093/ajh/hpy108. [DOI] [PubMed] [Google Scholar]
- 38.Schlaich M.P., Lambert E., Kaye D.M., Krozowski Z., Campbell D.J., Lambert G., Hastings J., Aggarwal A., Esler M.D. Sympathetic augmentation in hypertension: Role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension. 2004;43:169–175. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- 39.Boutouyrie P., Chowienczyk P., Humphrey J.D., Mitchell G.F. Arterial Stiffness and Cardiovascular Risk in Hypertension. Circ. Res. 2021;128:864–886. doi: 10.1161/CIRCRESAHA.121.318061. [DOI] [PubMed] [Google Scholar]
- 40.Sethi S., Rivera O., Oliveros R., Chilton R. Aortic stiffness: Pathophysiology, clinical implications, and approach to treatment. Integr. Blood Press. Control. 2014;7:29–34. doi: 10.2147/IBPC.S59535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Requena-Ibáñez J.A., Santos-Gallego C.G., Rodriguez-Cordero A., Vargas-Delgado A.P., Mancini D., Sartori S., Atallah-Lajam F., Giannarelli C., Macaluso F., Lala A., et al. Mechanistic Insights of Empagliflozin in Nondiabetic Patients with HFrEF: From the EMPA-TROPISM Study. JACC Heart Fail. 2021;9:578–589. doi: 10.1016/j.jchf.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 42.Bosch A., Ott C., Jung S., Striepe K., Karg M.V., Kannenkeril D., Dienemann T., Schmieder R.E. How does empagliflozin improve arterial stiffness in patients with type 2 diabetes mellitus? Sub analysis of a clinical trial. Cardiovasc. Diabetol. 2019;18:44. doi: 10.1186/s12933-019-0839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.